Abstract
Multiple studies suggest a role for the cerebral cortex in the generation of reflex cough in awake humans. Reflex cough is preceded by detection of an urge to cough; strokes specifically within the cerebral cortex can affect parameters of reflex cough, and reflex cough can be voluntarily suppressed. However, it is not known to what extent healthy, awake humans can volitionally modulate the cough reflex, aside from suppression. The aims of this study were to determine whether conscious humans can volitionally modify their reflexive cough and, if so, to determine what parameters of the cough waveform and corresponding muscle activity can be modified. Twenty adults (18–40 yr, 4 men) volunteered for study participation and gave verbal and written informed consent. Participants were seated and outfitted with a facemask and pneumotacograph, and two surface EMG electrodes were positioned over expiratory muscles. Capsaicin (200 μM) was delivered via dosimeter and one-way (inspiratory) valve attached to a side port between the facemask and pneumotachograph. Cough airflow and surface EMG activity were recorded across tasks including 1) baseline, 2) small cough (cough smaller or softer than normal), 3) long cough (cough longer or louder than normal), and 4) not cough (alternative behavior). All participants coughed in response to 200 μM capsaicin and were able to modify the cough. Variables exhibiting changes include those related to the peak airflow during the expiratory phase. Results demonstrate that it is possible to volitionally modify cough motor output characteristics.
Keywords: airway protection, capsaicin, irritant-induced cough, dystussia
cough is an airway defense mechanism that acts to expel material from the airway. In humans, three types of cough have been classified based on their hypothesized central control mechanisms. This includes type I (reflex cough), type II (voluntary cough), and type III (evoked cough), which is preceded by an urge to cough (5). According to this classification scheme, reflex cough is brain stem mediated, bypassing cortical control completely; voluntary cough is initiated on command to cough (with no cough stimulus); and evoked cough is initiated with a tussigenic stimulus accompanied by a preceding urge-to-cough sensation.
The neural structures involved in voluntary cough and evoked cough have been studied in humans using functional magnetic resonance imaging (fMRI). Voluntary cough is associated with activation of primary motor and somatosensory cortices, supplementary motor area (SMA), operculum, anterior and posterior mid-cingulate cortex (19, 24), insula, thalamus, basal ganglia, precuneus, inferior temporal gyri, amygdala, brain stem, and cerebellum (24). Evoked cough via inhalation of capsaicin is associated with activation of many of the same structures, including primary motor and somatosensory cortices, SMA, operculum, anterior and posterior mid-cingulate cortex, insula, thalamus, brain stem, and cerebellum (18). In addition, Mazzone and colleagues (18) found that evoked cough uniquely activates the rostral and caudal medulla. Thus both overlapping and distinctive features of a cortical control network for generating cough exist between voluntary and evoked cough. These findings support peripheral comparisons between these two types of cough with demonstrable similarities in terms of the phases of cough airflow parameters (inspiratory, compression, expiratory phases) and expiratory muscle activation accompanied by distinct differences in the amount of airflow, amplitudes, and relative timing of muscle activation between the tasks (14, 17).
The distinction between evoked and reflex cough is related to the strength or intensity of the tussigenic stimulus. That is, a relatively weak stimulus evokes an urge to cough that may be followed by an evoked cough (3, 9). An important characteristic of evoked cough is that it can be suppressed (5). With increasing intensity of the stimulus (e.g., higher concentration of capsaicin, for example), cough cannot be suppressed, and in this case a reflex cough is elicited (5). Accordingly, one way to separate these two types of cough in the laboratory is to measure the cough threshold to inhaled tussigenic stimuli with different instructions to the participant: one that evokes a cough with the simple command “cough if you need to,” and one that evokes a cough only when participants can no longer suppress the cough response (5). According to Eccles' model, the former would represent an evoked cough (type III) and the latter a reflex cough (type I).
In humans, reflex cough is suppressed during sleep (15) and is downregulated with neurological disease of the cortex and subcortex (1, 4, 31), suggesting a critical role for suprapontine structures in the generation of reflex cough in humans. Studies of cough suppression begin to provide insight as to cortical influence on the brain stem control of cough, showing that, up to a certain stimulus intensity level, the cortex can completely inhibit a cough response. Hutchings and colleagues (9) were the first to demonstrate this empirically. Their study of healthy young adults showed that the cough threshold to a tussigenic stimulus was significantly higher when participants were instructed not to cough (e.g., to suppress cough). Thus they termed the non-suppressed (“cough if you need to”) stimulus threshold the “natural cough threshold” and the suppressed stimulus threshold the “suppressed threshold.” These differences between natural and suppressed cough thresholds reflect a degree of suprapontine modulation over the brain stem control center for cough. Imaging analysis of a suppressed cough support this hypothesis, showing diffuse cortical activation of many of the same regions activated with evoked cough, with the addition of pre-SMA and basal ganglia activation unique to cough suppression (18).
Thus cortical influence on evoked vs. reflex cough is treated in a binary fashion: cough either can or cannot be suppressed. If not able to be suppressed, the type of cough would fall into the reflex cough category, and presumably the cortex does not play a role in the generation of that cough response (5). However, even though it is not possible to completely suppress a cough, it may be possible to volitionally attenuate or enhance the reflex cough response. If this is the case, it would help to explain the degradation of airway protection related to hypotussic disorders that occur in human neurological patients with intact brain stem control centers (4, 26, 30). The goal of the present study was to determine whether at a suprathreshold level of capsaicin healthy human participants could volitionally modify the reflex cough response. It was hypothesized that the reflex cough response would be volitionally modified in a manner quantifiable with increases and decreases in specific expiratory airflow and muscle EMG parameters, depending on the cough modification task. Vovk et al. (29) found decreased EMG activation and airflow measurements with successive cough expulsive events, indicating a natural decline in power and sheer force as the number of expulsive events increases. Therefore, it was further hypothesized that the effect of the modulation would extend to not only the first cough response in a cough series (Cr1) but also to up to one subsequent cough expulsive event (Cr2).
MATERIALS AND METHODS
Participants.
Twenty adults (18–40 yr, 4 men) volunteered for study participation and gave verbal and written informed consent. All participants denied a history of chronic cough, current or chronic respiratory disease, neurological disorder, head and/or neck cancer, and dysphagia (disordered swallowing). Pulmonary function testing and oral motor examination confirmed forced expiratory volume in 1 s/forced vital capacity of >75% predicted and integrity of cranial nerve function, respectively. The institutional review board at the University of Florida approved the study.
Equipment.
Participants were outfitted with a facemask covering the nose and mouth. The facemask was coupled to a pneumotacograph (Validyne MP45) and had a side delivery port with a one-way inspiratory valve for nebulizer connection. The nebulizer was a DeVilbiss T-piece (DeVilbiss Healthcare) connected to a dosimeter (Koko Dosimeter) that delivered aerosolized solution during inspiration with a delivery duration of 2 s. Participants were administered single inhalations of aerosolized 200 μM capsaicin dissolved in a vehicle solution consisting of 80% physiological saline, 10% Tween 20, and 10% ethanol. The concentration of 200 μm was chosen based on the study by Vovk and colleagues (29) that found 200 μm to be a suprathreshold concentration for eliciting the C5 response in healthy participants. The vehicle solution alone (80% physiological saline, 10% Tween 20, and 10% ethanol) was administered as a control aerosol.
Active surface EMG electrodes (DE 2.1 sensors, Delsys Bagnoli 8 system) were positioned over the right rectus abdominus (RA) muscle and right eighth intercostal space (IC8). These muscles were selected based on the findings of Vovk et al. (29) that showed measuring EMG activity in any of these muscle groups (IC8, RA, or external obliques) is adequate in assessing strength of airway defensive responses in humans. Therefore, we chose to include one abdominal (RA) and one thoracic placement (IC8). The RA electrode was placed such that the medial edge of the electrode was 2 cm lateral to the umbilicus. The eighth rib was palpated, and the IC8 electrode was placed 2 cm lateral to the costal margin, ∼1 cm medial to the anterior axillary line. Electrodes remained in place for the duration of the study. Electromyographic recordings were rectified and band-pass filtered (100–1,000 Hz). Both the airflow and EMG signals were recorded to a desktop computer (Dell Optiplex 745) via PowerLab Data Acquisition System (ADInstruments).
Protocol.
Participants were seated comfortably with the facemask and EMG electrodes in place. Initially, 30 s of quiet breathing were recorded in order for participants to acclimate to the facemask. Participants were given instructions for the cough tasks, including baseline (cough if you need to), and three experimental tasks: 1) small cough (cough smaller or softer than normal), 2) long cough (cough longer or louder than normal), and 3) suppress cough (do not cough). Participants were told which task they would be doing 10–15 s before delivery of the aerosol. The capsaicin or control solution was automatically administered on inspiration. Baseline trials were always completed first, and the order of the capsaicin and control solutions was randomized across participants. Participants were not told which solution was being presented. Three baseline trials were completed for both the capsaicin and control solutions. The three experimental tasks were then completed only if the participant exhibited a minimum of a two-cough (Cr2) response to the capsaicin and/or control solution during all three baseline trials. The experimental tasks (small, long, and suppressed cough) were presented in a randomized block design, with a total of three blocks. Three trials of all tasks were completed. Participants rested for a minimum of 1 min between all trials and were provided with water to drink as needed.
Data analysis and statistics.
For the purpose of this study, cough was defined as inspiration followed by an expiratory effort against a closed glottis (compression phase), followed by glottal opening and rapid expiratory airflow. The first cough response (Cr1) and up to one associated reacceleration (Cr2) in the first 30 s after capsaicin or control solution presentation were used for data analysis (Fig. 1). From those responses, both airflow and surface EMG (sEMG) parameters were derived. Cough airflow parameters are illustrated in Fig. 2 and include compression phase duration (CPD), peak expiratory flow rate (PEFR), peak expiratory flow rise time (PEFRT), cough volume acceleration (CVA; PEFR/PEFRT), post-peak phase duration (PPPD), and post-peak phase integrated area (PPPIA). These expiratory airflow parameters are related to subglottal pressure generation (CPD), rapid expiratory airflow acceleration (PEFR, PEFRT, CVA), and the sustained expiratory airflow plateau phase, which is a function of the airflow velocity (PPPD, PPPIA). sEMG parameters are also illustrated in Fig. 2 and include the duration of activation and maximum and RMS values from the integrated sEMG signals from the RA and IC8. These EMG measures are related to cough intensity, and they correlate well with cough airflow parameters (3). sEMG data was normalized within each participant to baseline sEMG activity and expressed as arbitrary units (AUs).
Fig. 1.
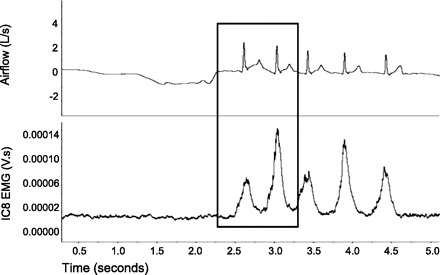
Cough airflow waveform with associated surface EMG (sEMG) from a representative baseline capsaicin trial. The box indicates the first two coughs (Cr1 and Cr2) that were used for data analysis.
Fig. 2.
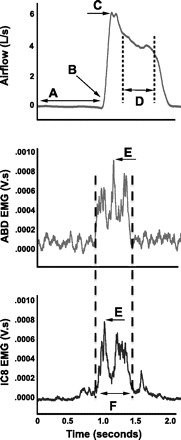
Cough and sEMG parameters for data analysis. A, compression phase duration (CPD); B and C, peak expiratory flow rise time (PEFR); C, peak expiratory flow rate (PEFR); D, post peak plateau duration (PPPD) and integrated area (PPPIA); E, maximum sEMG (RA MAX & IC8 MAX); F, duration of sEMG activation (sEMG DUR) and sEMG RMS (RA RMS & IC8 RMS).
The mean values of measures for Cr1 and Cr2 (cough#) were calculated across the three trials completed for the baseline, small, and long cough tasks. Independent variables were task (baseline, short, long) and cough# (Cr1 and Cr2). Dependent variables included airflow (PEFR, PEFRT, CVA, PPPD, PPPIA) and sEMG (sEMG duration, RA MAX, RA RMS, IC8 MAX, IC8 RMS) measures. A two-factor multivariate ANOVA (MANOVA) with factors task and cough# and the interaction term task × cough# was used to analyze the data. Tukey's honestly significant difference was used for post hoc testing. For the suppressed cough condition, a binary system (yes or no) was used to identify whether participants were able to suppress the cough response. The type of response (if not cough) observed was also reported.
RESULTS
All participants exhibited at least a two-cough (C2) response to each of the three baseline presentations of 200 μM capsaicin. No participants coughed in response to the control vehicle solution. One of the 20 participants chose to withdraw from the study after the baseline trials. One additional participant reported no additional urge to cough from capsaicin as the experimental tasks were initiated; this participant then admitted to regularly smoking cigarettes (although had denied smoking in the last 5 yr upon initial study screening) and was subsequently withdrawn from the study. As such, experimental task data from 18 participants was included in data analysis.
Suppressed cough condition.
All 18 participants responded with multiple behaviors to 200 μM capsaicin in the suppressed cough condition; 17 of 18 coughed as part of that response, and 1 of 18 was able to completely substitute other behaviors for cough. Non-cough behaviors included throat clears (13 of 18), breath holding (7 of 18), expiratory efforts (6 of 18), and swallowing (4 of 18). All participants who coughed (17 of 18) exhibited at least one additional behavior to cough; on average, participants exhibited two additional behaviors. Figure 3 illustrates a representative suppressed cough response that includes multiple behaviors. Alternative behaviors were identified observationally by the researchers in conjunction with participant self-report.
Fig. 3.
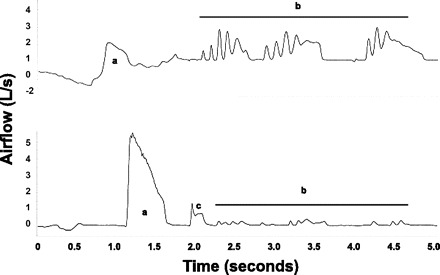
Example of not-cough alternative behavior airflow waveforms from two representative participants. a, Expiratory efforts; b, throat clears; c, cough.
Small and long cough conditions.
Means and standard deviations for baseline, small cough, and long cough are given in Table 1. A significant main effect was found for task and cough#, but there was no significant task by cough# interaction (Table 2). Specifically, effects for both task and cough# were found for CPD, PPPIA, and sEMG duration. An effect for cough# only was found for PEFRT and PEFR, and for task only on PPPD and CVA (Table 2; Figs. 4 and 5). Post hoc testing indicated that small cough was characterized by longer CPD, shorter PPPD, and decreased CVA vs. baseline cough. Long cough was characterized by longer PPPD and sEMG duration vs. short and baseline cough (Fig. 4). For cough#, Cr1 had longer durations (CPD and sEMG) rise time (PEFRT) and higher flow rates (PEFR and PPPIA) vs. Cr2 (Fig. 5).
Table 1.
Mean and standard error data for dependent variables according to task and cough number
| Baseline Cough M (SE) |
Small Cough M (SE) |
Long Cough M (SE) |
||||
|---|---|---|---|---|---|---|
| Cr1 | Cr2 | Cr1 | Cr2 | Cr1 | Cr2 | |
| CPD, s | 0.42 (0.053) | 0.21 (0.036) | 0.61 (0.073) | 0.38 (0.065) | 0.57 (0.079) | 0.23 (0.061) |
| PEFRT, s | 0.07 (0.010) | 0.04 (0.001) | 0.07 (005) | 0.05 (0.004) | 0.05 (0.004) | 0.04 (0.006) |
| PEFR, liters/s | 4.63 (0.373) | 3.01 (0.292) | 3.72 (0.541) | 2.59 (0.241) | 5.63 (0.716) | 2.94 (0.337) |
| CVA, liters · s−1 · s−1 | 85.59 (7.913) | 80.14 (7.733) | 56.47 (5.994) | 51.67 (4.914) | 109.51 (9.785) | 80.89 (8.001) |
| PPPIA, liters · s−1 · s−1 | 0.60 (0.075) | 0.26 (0.032) | 0.26 (0.059) | 0.18 (0.020) | 0.76 (0.126) | 0.28 (0.039) |
| PPPD, s | 0.20 (0.025) | 0.16 (0.009) | 0.11 (0.013) | 0.11 (0.011) | 0.26 (0.036) | 0.21 (0.034) |
| sEMG duration, s | 0.37 (0.026) | 0.28 (0.016) | 0.31 (0.032) | 0.29 (0.024) | 0.47 (0.048) | 0.35 (0.034) |
| Max RA EMG, AU | 5.5 (0.95) | 6.5 (1.184) | 3.7 (0.92) | 6.7 (2.168) | 7.3 (1.711) | 6.3 (0.961) |
| RMS RA EMG, AU | 2.7 (0.52) | 3.4 (0.68) | 1.7 (0.38) | 2.6 (0.551) | 3.2 (0.76) | 3.0 (0.570) |
| Max IC8 EMG, AU | 50.1 (40.2) | 64.7 (48.2) | 93.7 (89.8) | 98.0 (94.9) | 87.3 (81.3) | 73.4 (67.9) |
| RMS IC8 EMG, AU | 24.12 (19.54) | 33.64 (26.12) | 49.50 (47.73) | 59.07 (57.44) | 43.40 (40.64) | 42.03 (39.41) |
Values are means (SE) data for dependent variables according to task and cough number. CPD, compression phase duration; PEFRT, peak expiratory flow rise time; PEFR, peak expiratory flow rate; CVA, cough volume acceleration; PPPIA, post-peak plateau integrated area; PPPD, post-peak plateau duration; Max RA EMG, maximum EMG from the rectus abdominus; RMS RA EMG, root mean square EMG from the rectus abdominus; Max IC8 EMG, maximum EMG from the eighth intercostal space; RMS IC8 EMG, root mean square EMG from the eighth intercostal space; AU, arbitrary units.
Table 2.
Multivariate (MANOVA) and univariate (ANOVA) analyses of variance F ratios for trial x cough# effects for cough measures. Only those measures with significant P values are included
| ANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|
| MANOVA | CPD | PEFRT | PEFR | PPPD | PPPIA | CVA | EMG Duration | |
| T: F(20,130) | T: F(2,74) | T: F(2,74) | T: F(2,74) | T: F(2,74) | T: F(2,74) | T: F(2,74) | T: F(2,74) | |
| C: F(10,65) | C: F(1,74) | C: F(1,74) | C: F(1,74) | C: F(1,74) | C: F(1,74) | C: F(1,74) | C: F(1,74) | |
| Task (T) | 3.74‡ | 3.11* | 1.69 | 3.48 | 7.75‡ | 6.00† | 8.27‡ | 6.70† |
| Cough numbr (C) | 5.49‡ | 6.51‡ | 12.64‡ | 19.62‡ | 0.97 | 22.65‡ | 2.44 | 7.27† |
| T × C | 1.06 | 0.32 | 0.82 | 0.93 | 1.17 | 3.47 | 1.07 | 1.30 |
Multivariate ANOVA (MANOVA) F ratio is Wilk's Lambda approximation of F. T, task; C, cough number. Significant difference:
P < 0.05;
P < 0.01;
P < 0.001.
Fig. 4.

Results of post hoc statistical analysis for the effect of trial (baseline, small, long cough) on airflow and sEMG parameters. Only those parameters with statistically significant differences are shown. Error bars are standard error.
Fig. 5.

Results of post hoc statistical analysis for the effect of cough (Cr1, Cr2) on airflow and sEMG parameters. Only those parameters with statistically significant differences are shown. Error bars are standard error.
DISCUSSION
The results of the present study confirmed the hypothesis that healthy human participants can volitionally modify their cough response to capsaicin at a concentration where complete suppression of cough is not possible. This was demonstrated quantitatively in terms of significant differences between modified cough and baseline cough airflow and sEMG parameters. For the small cough condition, when participants were instructed to cough smaller or softer than normal, they increased the CPD, decreased the PPPD, and decreased the rate of acceleration to peak expiratory airflow (CVA). For the long cough condition, where participants were told to cough longer or louder than normal, they increased both the PPPD and PPPIA vs. the small cough. As well, long cough had significantly longer sEMG duration vs. both baseline and short cough. These findings are important for at least two reasons. First, they lend insight as to the neurological control network governing the generation of cough in humans. Second, these results indicate cough parameters that can be volitionally modified and may thus serve as therapeutic targets for disorders of cough.
A significant role for the cerebral cortex in the control of voluntary and evoked cough has been demonstrated empirically in healthy human participants via fMRI studies (18, 19, 24) as well as via study of cough in patients with neurological disease, including stroke (6, 10, 13, 27, 30) and Parkinson's disease (PD) (4, 10, 22). Unlike previous studies, the present study investigated the cortical control of reflex cough in a nonbinary manner. That is, instead of classifying cough as either able to be suppressed or not able to be suppressed, the present methodology focused specifically on cough generated at a level of capsaicin where suppressing cough was not possible. This allowed for determination of those specific aspects of reflex cough that can be volitionally modulated. Additionally, the types of behaviors associated with “do not cough” were variable within and between subjects, consisting of breath holding, swallowing, expiratory efforts, and throat clearing. This illustrates the broad spectrum of airway protective behaviors that may be employed and modified with stimulation by a tussigenic stimulus. Anecdotally, one can imagine many situations where cough cannot be suppressed, so it is instead modified. A movie theater or testing auditorium are settings where a loud, explosive cough would be socially inappropriate, and most people will decrease the intensity and loudness of their cough, or perhaps substitute coughing with other behaviors, where the urge to cough cannot be completely suppressed. Thus, under normal physiological conditions, humans have a significant degree of cortical input regulating reflex cough allowing for these modifications. Like other functions with brain stem control centers (for example, respiration and the pharyngeal phase of swallowing), it may be possible to generate a cough in the absence of cortical input; however, it likely will not result in normal task execution.
Although the present study did not employ imaging techniques, it can be hypothesized based on previous studies that structures including the primary motor and sensory cortices, SMA, operculum, pre-SMA, anterior and posterior midcingulate cortex, insula, and caudate may be important for modulation of reflex cough due to their activity during evoked cough and cough suppression (18), suggesting that modulation of reflex cough would also result in medullary activation. Without being able to identify specific neural structures involved in reflex cough based on the present study results, it is hypothesized that reflex cough neural control in healthy awake humans includes a cortical pathway that can exert excitatory and/or inhibitory influence on brain stem cough control centers. Thus where cortical structures are damaged, for example in hemispheric stroke or neurodegenerative disease, degradation of normal cough function occurs (4, 16, 22, 27, 30).
The generation of a cough is dependent mechanically on coordination of respiratory and laryngeal structures. Based on the impacts of cough modulation of the present study's dependent variables, it can be hypothesized that both contributed to these results. The increased CPD noted for small coughs may reflect prolonged laryngeal adductor activity that increased time for generation of subglottal pressure and subsequent active dilation of the vocal folds (28). Cough volume acceleration was significantly decreased in small cough, and there was a trend toward a significant increase for CVA in long cough vs. baseline (Table 1). CVA reflects the interaction between the two measures (CVA = PEFR/PEFRT); interestingly, neither PEFR nor PEFRT was significantly different between the three cough tasks. The trend for the small cough task is a decrease in PEFR and increase in PEFRT, whereas the trend for long cough is an increase in PEFR and decrease in PEFRT. Knudson et al. (12) suggest that the time to reach peak expiratory flow (PEFRT) is dependent on glottal function; however, the peak flow itself (PEFR) is due to a combination of airway and pulmonary components, including the intraplural pressure, dynamic compression of the airway, and volume of air in the lungs at cough initiation (2, 25). Lung volume at cough initiation was not controlled in this study, but it was confirmed post hoc to be within the range of tidal volume on examination of the cough airflow signal (i.e., no differences were detected between the volume of inspired air preceding a reflex cough vs. that measured during tidal breathing). Therefore, it is unlikely that significant increases in lung volume at initiation of the long cough account for these data. It is more likely that, for long cough, increased intraplural pressure and subsequent dynamic airway compression contributed to the trend seen for CVA. Although there was not a statistical difference detected in expiratory muscle activation for long cough vs. baseline or small cough, examination of Table 1 reveals a trend toward increased RA and IC8 activation that may indicate muscle modulation for airway compression during the long cough task.
In terms of the post peak phase of cough, a difference was seen for small cough for PPPD vs. baseline, and for PPPD and PPPIA vs. long cough. A trend was seen for long cough for increased PPPD and PPPIA vs. baseline. These measures are thought to be reflective of pulmonary and airway components, as opposed to an active laryngeal component (28). Only a very slight trend was seen for a decrease in expiratory sEMG duration of small cough vs. baseline cough, even with a significant decrease in PPPD duration. It may be that with the small cough task participants relied on active laryngeal adductor muscles to stop the flow of air to attenuate the length of the cough, thereby maintaining the duration of sEMG activation against a closed glottis. For long cough, there was a significant increase in sEMG duration vs. both baseline and short cough, indicating that prolonged activation of expiratory muscles sustained PPPD in this condition. Thus mechanically reflex cough modulation is achieved both at the larynx, via alteration of the timing of initial cough events, and by the respiratory apparatus via increases in pressure and subsequent airflow achieved for each cough task.
In their study of airway defense motor patterns, Vovk et al. (29) reported measures of IC8 and RA muscle activity, airflow acceleration (CVA) and mean airflow (PEFR) were greatest with the initial cough expulsive event (CR1) and declined with subsequent expulsive events. Likewise, measures of rise time (PEFRT) and plateau duration (PPPD) were longest for Cr1 with no appreciable differences or a trend seen for compression phase duration (CPD) between expulsive events. Results of the present study are in agreement with these results regarding greater PEFR, CVA, and longer PPPD. However, the results differ in terms of the amplitude-based EMG measures, and CPD. In the present study, no differences were found for sEMG amplitude for RA or IC8 muscles. A difference was found for sEMG duration where the first cough (Cr1) had a longer duration vs. Cr2. The present study also found longer CPD for Cr1 vs. Cr2, whereas the Vovk et al. study found no CPD differences. One reason for this apparent discrepancy may be the differences in goals of the two studies. The present study sought to identify modification of cough and impact on sEMG and airflow measures, whereas Vovk et al. sought to identify behavioral differences in response to multiple concentration levels of capsaicin. As such, multiple reaccelerations were considered in the data analysis in the former study compared with just the first cough (Cr1) and first reacceleration (Cr2) in the present study.
No interaction was found between cough# and trial; thus the reported results for cough# reflect all data averaged across baseline, long, and short conditions. Applying the statistical model comparing only baseline Cr1 and Cr2 yielded essentially the same results for CPD and no appreciable trends appearing for the sEMG amplitude measures. There were multiple concentration levels of capsaicin in the Vovk et al. study (29), including very low concentrations where cough could likely be suppressed. In the present study, all participants were given 200 μM concentration, and 17 of 18 could not suppress the cough response. (The remaining participant expressed other expiratory behaviors but not cough in the response.) As such, it may be that reflex cough evoked with suprathreshold levels of capsaicin elicited behaviors resulting in subtle differences in cough motor expression vs. cough elicited at subthreshold level of the irritant. The findings of Lasserson and colleagues (14) support this hypothesis since they reported no significant differences in expiratory muscle sEMG activation patterns between the first (Cr1) and second (Cr2) reflex cough response, although the trend in that data was more similar to the results of Vovk et al. (29). Again, the concentration level of the stimulus (for the Lasserson study, L-tartaric acid) was not tested at a suppressed vs. supramaximal (reflex) threshold, so it remains a possibility that the type of cough elicited leads to observed differences in CPD and sEMG muscle activation patterns.
Investigations of voluntary cough also provide interesting data for comparison of cough reaccelerations examined in the present study. Harris and Lawson (8) studied Cr1, 2, and 3 produced voluntarily in healthy participants. Although the analysis used in that study included differing variables and measurements, the results are qualitatively similar in that the peak flows, volume acceleration, and total amount of air expired decreased from the first to the second cough produced. In the former study, the first cough accounted for 53.2% of the total expired air, the second (Cr2) for 28.0%, and the third (Cr3) for 18.8% (expressed as a percent of total volume expired) (8). Using our PPPIA measure as a measure of expired volume, our overall results would indicate the Cr1 accounted for 68% and the Cr2 for 32% of the total air expired. Parsing out the baseline, small, and long tasks, the results yield 69% and 31% (baseline), 58% and 42% (small), and 73% and 27% (long) for Cr1 and Cr2, respectively. These numbers are approximations because our participants were not limited to only two coughs; it was decided post hoc that analysis would be completed on the first two because all participants had a Cr2 response across tasks. Therefore, if an individual participant had a Cr5, for example, the total expired volume and consequent percentage allocation among all responses would change. However, the relative strength in terms of airflow of the first cough (Cr1) relative to subsequent reaccelerations (Cr2) for reflex cough is similar to that reported for voluntary cough. Furthermore, it illustrates that the relative contribution in terms of total cough expiratory airflow for multiple coughs is sensitive to modulation.
The ability to volitionally modify reflex cough has important implications in terms of treatment and rehabilitation of disordered cough (dystussia). Ward and colleagues (30) as well as Smith-Hammond et al. (26) report degradation in the velocity and volume of expiratory airflow in reflexive and voluntary cough, respectively, in stroke patients. As well, in patients with Parkinson's disease, decreases in peak expiratory airflow and prolonged CPD are seen for voluntary cough production (21). These findings are likely related to changes in the strength and coordination of expiratory muscles and laryngeal muscles (2, 7, 20, 30). The compression phase and expiratory flows during cough are important for generating effective shearing forces for airway clearance and, when diminished, are indicative of the inability to effectively eject aspirate material from the airway (23). Therapeutic focus on the volitional enhancement of strength and coordination of the structures and muscles integral to cough production may be a valuable tool in improving the ability to cough for patients who experience dystussia (11, 21).
Putting the present results in the context of classification scheme for cough proposed by Eccles (5), the demarcation between the three types of cough is less absolute than the present classification model suggests. Although the brain stem-only pathway for reflex cough may be relevant and applicable for humans under general anesthesia or during loss of consciousness, under normal physiological conditions separating suprapontine from the brain stem pathway is not physiologically appropriate. Our data suggests the cortex participates in reflex cough not only in terms of detecting a sensory stimulus (as in the case of type III evoked cough) and selecting a response (cough/no cough) but also in terms of the nonsuppressible reflex cough response. Figure 6 presents a revised version of Eccles' classification model that reflects incorporation of the cortex for generation of all types of cough while still acknowledging the possibility that reflex cough can be purely brain stem mediated under specific conditions. This revised model supports cough as a complex sensorimotor behavior and helps to explain why differences in both reflex and voluntary cough appear with neuropathologies primarily affecting the cortex.
Fig. 6.
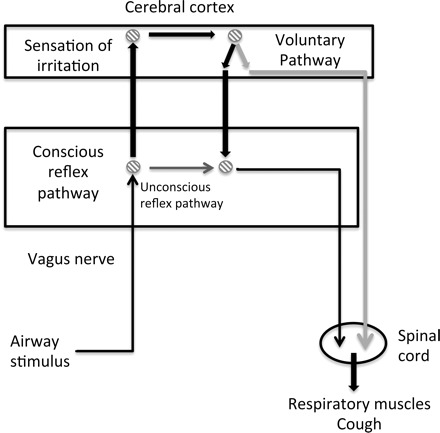
Revised model for the neural control of cough, based on the model proposed by Eccles (5). Thin black arrows indicate common pathway components for conscious and unconscious control; dark gray indicates unconscious control; bolded black indicates conscious control; and light gray indicates voluntary control.
In conclusion, results of the present study indicate that, for the afferents stimulated using capsaicin, healthy participants can exert volitional control over reflex cough. This indicates that, under normal physiological conditions, cough production reflects an interaction between multiple control sites along the neuraxis resulting in mechanical differences reflective of both laryngeal and respiratory muscle modulation. When specific components of control are injured, a subsequent change in cough function occurs. Identification of specific components of cough expiratory airflow (which is essential to clearing material from the airway) that can be modified provides a basis for development of behaviorally based treatments that may lead to effective cough rehabilitation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.W.H., D.C.B., and P.W.D. conception and design of research; K.W.H. and P.W.D. performed experiments; K.W.H. analyzed data; K.W.H., D.C.B., and P.W.D. interpreted results of experiments; K.W.H. prepared figures; K.W.H. drafted the manuscript; K.W.H., D.C.B., and P.W.D. edited and revised the manuscript; D.C.B. and P.W.D. approved the final version of the manuscript.
REFERENCES
- 1. Addington WR, Stephens RE, Gilliland K, Rodriguez M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil 80: 150–154, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Arora NS, Gal TJ. Cough dynamics during progressive expiratory muscle weakness in healthy curarized subjects. J Appl Physiol 51: 494–498, 1981 [DOI] [PubMed] [Google Scholar]
- 3. Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, Danzig M. The effect of codeine on the urge-to-cough response to inhaled capsaicin. Pulm Pharmacol Ther 20: 338–346, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkinson disease. Chest 124: 1009–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Eccles R. Central mechanisms IV: conscious control of cough and the placebo effect. Hand Exp Pharmacol 187: 241–262, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Hammond S, Carol A, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke. Chest 135: 769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harraf F, Ward K, Man W, Rafferty G, Mills K, Polkey M, Moxham J, Kalra L. Transcranial magnetic stimulation study of expiratory muscle weakness in acute ischemic stroke. Neurology 71: 2000, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Harris RS, Lawson TV. The relative mechanical effectiveness and efficiency of successive voluntary coughs in healthy young adults. Clin Sci 34: 569–577, 1968 [PubMed] [Google Scholar]
- 9. Hutchings HA, Eccles R, Smith AP, Jawad MS. Voluntary cough suppression as an indication of symptom severity in upper respiratory tract infections. Eur Respir J 6: 1449–1454, 1993 [PubMed] [Google Scholar]
- 10. Imoto Y, Kojima A, Osawa Y, Sunaga H, Fujieda S. Cough reflex induced by capsaicin inhalation in patients with dysphagia. Acta Otolaryngol (Stockh) 131: 96–100, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Davenport P, Sapienza C. Effect of expiratory muscle strength training on elderly cough function. Arch Gerontol Geriatr 48: 361–366, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Knudson RJ, Mead J, Knudson DE. Contribution of airway collapse to supramaximal expiratory flows. J Appl Physiol 36: 653–667, 1974 [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi H, Hoshino M, Okayama K, Sekizawa K, Sasaki H. Swallowing and cough reflexes after onset of stroke. Chest 105: 1623, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Lasserson D, Mills K, Arunachalam R, Polkey M, Moxham J, Kalra L. Differences in motor activation of voluntary and reflex cough in humans. Thorax 61: 699–705, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee KK, Birring SS. Cough and sleep. Lung 188, Suppl 1: S91–S94, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Leow LP, Beckert L, Anderson T, Huckabee ML. Changes in chemosensitivity and mechanosensitivity in aging and parkinson's disease. Dysphagia 27: 106–114, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Magni C, Chellini E, Lavorini F, Fontana GA, Widdicombe J. Voluntary and reflex cough: similarities and differences. Pulm Pharmacol Ther 24: 308–311, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 31: 2948–2958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 176: 327–332, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Murty GE, Lancaster P, Kelly PJ. Cough intensity in patients with a vocal cord palsy. Clin Otolaryngol Allied Sci 16: 248–251, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Pitts T, Bolser D, Rosenbek J, Troche M, Okun M, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 135: 1301–1308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia 23: 297–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohrer F. The mechanism of coughing. In: Translations in Respiratory Physiology, edited and translated by West JB. Stroudsburg, PA: Dowden, Hutchinson, and Ross, 1975, p. 772 [Google Scholar]
- 24. Simonyan K, Saad ZS, Loucks TM, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage 37: 401–409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh P, Mahajan RP, Murty GE, Aitkenhead AR. Relationship of peak flow rate and peak velocity time during voluntary coughing. Br J Anaesth 74: 714–716, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest 135: 769–777, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology 56: 502–506, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Von Leden, Isshiki N. An analysis of cough at the level of the larynx. Arch Otolaryngol 81: 616–625, 1965 [DOI] [PubMed] [Google Scholar]
- 29. Vovk A, Bolser DC, Hey JA, Danzig M, Vickroy T, Berry R, Martin AD, Davenport PW. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulm Pharmacol Ther 20: 423–432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward K, Seymour J, Steier J, Jolley C, Polkey M, Kalra L, Moxham J. Acute ischaemic hemispheric stroke is associated with impairment of reflex in addition to voluntary cough. Eur J Respir 36: 1383–1390, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Widdicombe J, Singh V. Physiological and pathophysiological down-regulation of cough. Respir Physiol Neurobiol 150: 105–117, 2006 [DOI] [PubMed] [Google Scholar]


