Summary
Autophagy is a process of cellular self-digestion induced by various forms of starvation. The mechanisms by which metabolic deficiencies are sensed by a cell to regulate autophagy remain unclear. While nitrogen deficit is a common trigger, yeast cells induce autophagy upon switch from a rich to minimal media without nitrogen starvation. We show that the amino acid methionine is sufficient to inhibit such non-nitrogen starvation (NNS)-induced autophagy. Methionine boosts synthesis of the methyl donor, S-adenosylmethionine (SAM). SAM inhibits autophagy and promotes growth through the action of the methyltransferase Ppm1p, which methylates the catalytic subunit of PP2A in tune with SAM levels. Methylated PP2A promotes dephosphorylation of Npr2p, a component of a conserved complex that regulates NNS-autophagy and other growth-related processes. Thus, methionine and SAM levels represent a critical gauge of amino acid availability that is sensed via this distinctive methylation modification of PP2A to reciprocally regulate cell growth and autophagy.
INTRODUCTION
Autophagy is an important cellular process that enables the delivery of cytoplasmic contents and organelles to the vacuole or lysosome for recycling or degradation (Levine and Klionsky, 2004; Nakatogawa et al., 2009). An outstanding question in the field concerns how autophagy is regulated in relation to the metabolic state of a cell. Autophagy is commonly thought to be regulated by the nutrient-sensitive Target of Rapamycin Complex 1 (TORC1) (Nakatogawa et al., 2009). In budding yeast, when nutrients are plentiful, TORC1 signaling is activated, leading to the phosphorylation of Atg13p (Kamada et al., 2010). The phosphorylation of Atg13p prevents its interaction with the Atg1p initiator kinase, thus inhibiting autophagy. When nutrients are deficient, TORC1 activity is inhibited. This enables a dephosphorylated Atg13p to interact with Atg1p to form an active autophagy initiation complex (Kamada et al., 2010; Nakatogawa et al., 2009). However, the precise mechanisms by which nutrients and amino acids direct TORC1 kinase activity towards autophagy and other growth regulators remain poorly understood (Laplante and Sabatini, 2012; Rabinowitz and White, 2010). Moreover, the exact nature of the nutritional deficiencies sensed by a cell to promote autophagy induction and cell survival remain to be elucidated.
Upon switch from a rich to a minimal, defined medium with lactate as the carbon source (YPL -> SL), we previously observed that yeast cells induce both mitophagy and general autophagy (Wu and Tu, 2011). Such induction of autophagy was readily evident by standard assays including observation of the translocation of a mitochondrial GFP reporter to the vacuole, release of free GFP resulting from degradation of these reporters in the vacuole, and quantitative measurements with cytosolic or mitochondria targeted alkaline phosphatase reporters (Wu and Tu, 2011). The induction of autophagy under these conditions is somewhat unexpected, because although SL medium is less rich than YPL medium, it contains sufficient nitrogen, phosphate, sulfate, and carbon sources to support the growth of yeast. We termed this form of autophagy, non-nitrogen starvation-induced autophagy (NNS-autophagy), which occurs in response to less severe changes in the growth environment than are typically used to induce autophagy (Wu and Tu, 2011). Importantly, we identified a complex of three proteins, Iml1p/Npr2p/Npr3p, that is required for NNS-autophagy but not typical nitrogen starvation-induced autophagy. Interestingly, Iml1p occasionally co-localizes with markers of pre-autophagosomal structures (PAS) upon switch to NNS-autophagy-inducing conditions, suggesting it may play a role in regulating autophagosome formation (Wu and Tu, 2011). In addition, Npr2p and Npr3p have previously been linked to the negative regulation of TORC1 signaling (Neklesa and Davis, 2009), and all three proteins are members of a conserved coatomer-related complex (Dokudovskaya et al., 2011). However, little else is known regarding their function. Thus, emerging evidence suggests that there exist distinct regulators of autophagy acting in response to different nutritional triggers.
The robust induction of NNS-autophagy upon switch to a defined, minimal medium enabled us to assess whether supplementation of any particular metabolites or nutrients could inhibit this process. A transient deficiency in such a nutrient could represent the triggering event for NNS-autophagy. We observed that unexpectedly, a single amino acid is sufficient to potently and specifically inhibit NNS-autophagy. Subsequent metabolic analysis and characterization revealed that this amino acid and its downstream metabolite represent a critical yet previously unrecognized barometer of cellular metabolic state. In this and the accompanying manuscript (Laxman et al., 2013), we show that the availability of these sentinel metabolites directs a nutritional regulatory pathway that is required for proper cell growth control.
RESULTS
Sulfur-Containing Amino Acids Inhibit NNS-Autophagy
Our previous studies established that yeast cells robustly induce autophagy upon switch from a rich medium containing lactate as the carbon source (YPL) to a minimal medium with lactate (SL). Since SL medium contains ammonium sulfate as a nitrogen source (Sherman, 2002), we termed this form of autophagy, NNS (non-nitrogen starvation)-autophagy (Wu and Tu, 2011). If the core autophagy machinery is disrupted, cell growth upon switch to SL medium is compromised, indicating that NNS-autophagy plays an important role in cellular homeostasis, enabling cells to adapt to the less favorable growth conditions (Wu and Tu, 2011).
What might represent the metabolic trigger of NNS-autophagy? In contrast to YPL which contains yeast extract and peptone, SL is a defined minimal medium which lacks free amino acids, requiring prototrophic yeast cells to synthesize all twenty amino acids from more simple precursors. We added back the twenty amino acids to determine whether one or more could suppress NNS-autophagy (Figure 1). We observed that addition of the sulfur-containing amino acids cysteine and methionine potently suppressed NNS-autophagy as monitored using each of the three standard assays for autophagy: imaging the translocation of a mitochondrial RFP reporter to the vacuole, GFP cleavage assay by Western blot, and the quantitative alkaline phosphatase (ALP) reporter assay (Figures 1A–1C). In striking contrast, the addition of other non-sulfur-containing amino acids either alone or in combination, even at much higher concentrations (30-fold), had virtually no effect on NNS-autophagy (Figure 1 and Figure S1). Tyrosine was not included in the non-sulfur amino acid mixture due to its poor solubility. However, addition of tyrosine alone at maximal soluble concentrations also had no effect on NNS-autophagy (Figure S1). As expected, the addition of either sulfur or non-sulfur amino acids strongly suppressed nitrogen starvation-induced autophagy, since each of these amino acids can be utilized as a nitrogen source (Figure S1).
Figure 1. Inhibition of NNS-autophagy by Sulfur-Containing Amino Acids.
Cells were grown in YPL rich medium to log phase before switching to SL minimal medium to induce NNS-autophagy. As shown using three different standard assays for autophagy (A-C), NNS-autophagy was significantly inhibited by methionine and cysteine, but not other amino acids. Non-S Mix refers to a non-sulfur amino acid mixture which includes all amino acids except Met, Cys, and Tyr (17 total). See also Figure S1.
(A) Imaging: Note the accumulation of the mitochondria-targeted RFP (mtRFP) reporter in the vacuole following switch to SL. Met or Cys addition was sufficient to inhibit translocation of mtRFP to the vacuole. Vph1-GFP is a vacuolar membrane marker.
(B) GFP-cleavage assay: Note the accumulation of free GFP in strains expressing either mitochondria-targeted OM45-GFP or IDH1-GFP following switch to SL medium. Met or Cys addition was sufficient to significantly reduce the amount of free GFP produced as a result of cleavage in the vacuole.
(C) ALP activity assay: Using a mitochondria or cytosolic targeted alkaline phosphatase reporter, mitophagy and general autophagy were monitored using the alkaline phosphatase assay as described previously (Wu and Tu, 2011). Met or Cys, but not other amino acids, was sufficient to potently inhibit NNS-autophagy. *** p<0.001, ** p<0.01, * p<0.05, ns p >0.05
The Effect of Sulfur-Containing Amino Acids Can be Attributed Specifically to Methionine
In multiple experiments, submillimolar concentrations of both cysteine and methionine were highly effective in suppressing both mitophagy and general autophagy (Figure 1). However, upon inclusion of low concentrations of cysteine alone to SL medium, cell proliferation was transiently inhibited. Cysteine can be processed by certain metabolic enzymes in the cell to produce hydrogen sulfide (Singh and Banerjee, 2011), which can inhibit respiration (Kabil and Banerjee, 2010). We therefore accounted for the possibility that inhibition of respiration triggered by cysteine addition compromised the induction of autophagy. We found that respiration uncouplers or inhibitors such as CCCP or Antimycin A potently suppressed NNS-autophagy (Figure S2). Thus, we could not distinguish whether the ability of cysteine to block NNS-autophagy was due specifically to cysteine or its inhibitory effects on respiration and growth. Indeed, mitochondrial function has been observed to be important for autophagy regulation in both yeast and mammalian cells (Graef and Nunnari, 2011; Ma et al., 2011).
To circumvent this issue, we observed that inclusion of cysteine together with all other amino acids except methionine and tyrosine no longer inhibited growth in SL medium. Under these conditions, cysteine no longer inhibited NNS-autophagy as assayed using both the ALP and GFP cleavage assays (Figure 2A), suggesting that the effect of cysteine alone on autophagy was due to its inhibitory effects on respiration. In contrast, the addition of methionine together with the non-sulfur amino acid mixture still potently inhibited NNS-autophagy (Figure 2A), suggesting that methionine, and not cysteine, may be the key amino acid. The addition of other amino acids such as glutamate, glutamine or leucine that have previously been implicated as critical nutrients for TOR signaling and autophagy regulation (Bonfils et al., 2012; Han et al., 2012; Nicklin et al., 2009) had no effect on NNS-autophagy even at ~30-fold higher concentrations (Figures 1, 2 and Figure S1).
Figure 2. Methionine is the Sole Amino Acid Sufficient for Inhibition of NNS-Autophagy.
(A) In the presence of other amino acids, methionine but not cysteine still inhibited NNS-autophagy. Following switch to SL medium plus the indicated amino acids, general autophagy and mitophagy were measured using the ALP assay (left) and the GFP cleavage assay (right). See also Figure S2.
(B) Diagram outlining sulfur utilization and metabolism in budding yeast.
(C) Homocysteine is unable to inhibit NNS-autophagy in met6Δ cells. Following switch to SL plus the indicated metabolites, general autophagy was measured using the ALP assay. In the met6Δ mutant, metabolic flux from homocysteine to methionine is blocked.
(D) Oxidative stress is not involved in the regulation of NNS-autophagy. General autophagy and mitophagy were assayed following switch to SL plus the indicated concentrations of reductants. Glutathione (GSH) inhibited NNS-autophagy only at much higher concentrations compared to Met or Cys. Two other potent anti-oxidants, dithiothreitol (DTT) and β-mercaptoethanol (βME), did not significantly inhibit NNS-autophagy.
(E) Global oxidative stress is not induced upon switch to SL medium. Intracellular levels of GSSG (oxidized GSH) and GSH were determined by multiple reaction monitoring (MRM) using previously established LC-MS/MS methods (Tu et al., 2007). Relative abundance of two daughter fragments targeting GSH and GSSG are shown.
Yeast harbor enzymes that can interconvert methionine and cysteine via the transsulfuration pathway (Figure 2B) (Thomas and Surdin-Kerjan, 1997). To definitively demonstrate that methionine is the key amino acid, we observed that homocysteine also blocked NNS-autophagy (Figure 2C). Similar to cysteine, homocysteine addition alone also inhibited cell growth, likely due to its capacity to produce sulfide (Singh and Banerjee, 2011). Such growth inhibition could also be overcome by adding homocysteine together with the non-sulfur amino acid mixture. Under these conditions, in contrast to cysteine, homocysteine still blocked NNS-autophagy (Figure 2C). Homocysteine can be converted either to cysteine by way of cystathionine, or to methionine by the methionine synthase Met6p (Figure 2B). Homocysteine no longer inhibited NNS-autophagy in a met6Δ mutant (Figure 2C), strongly suggesting that the conversion of homocysteine to methionine or downstream metabolites is critical for inhibition of NNS-autophagy.
Since oxidative stress has been linked to autophagy regulation (Scherz-Shouval and Elazar, 2011), we tested whether the effects of homocysteine or methionine on autophagy might be mediated by some production of cysteine and glutathione (Figure 2B). Cysteine is a precursor for the biosynthesis of glutathione (GSH), which is the primary reductant and redox buffer in eukaryotic cells (Hwang et al., 1992). We added glutathione as well as other thiol-based reducing agents, such as β-mercaptoethanol and DTT, to assess whether they could inhibit NNS-autophagy (Figure 2D). Although very high concentrations of GSH (>15 mM) modestly inhibited autophagy as measured by the ALP assay, the addition of the other exogenous reducing agents had little effect, suggesting that the inhibition of autophagy by sulfur-containing metabolites is not due to an anti-oxidant effect (Figure 2D). Since yeast contain peptidases that degrade the glutathione tripeptide (Ganguli et al., 2007), the modest inhibitory effect of very high concentrations of glutathione is likely due to its degradation to produce cysteine equivalents.
We next measured reduced (GSH) and oxidized glutathione (GSSG) levels by LC-MS/MS to assess cellular redox status before and after switch to SL medium. Upon switch to SL, there was an initial increase in GSH abundance followed by a gradual decrease in both GSH and GSSG (Figure 2E). Notably, there was no significant increase in total intracellular GSSG or the ratio of GSSG to GSH. Collectively, these data suggest that oxidative stress is not the primary trigger of NNS-autophagy, and that the effects of homocysteine and methionine may be separate from their ability to contribute cellular reducing equivalents in the form of glutathione.
Methionine Inhibits Autophagy in Parallel or Downstream of TORC1 and Atg13p
We then investigated whether the effects of methionine on autophagy were mediated through the Target of Rapamycin Complex 1 (TORC1), which is responsive to the amino acid and nutrient status of cells (Kamada et al., 2000; Noda and Ohsumi, 1998). Atg13p is a protein important for autophagy initiation that is phosphorylated by TORC1 (Kamada et al., 2000). When TORC1 is inactivated in response to nutrient deprivation, Atg13p is dephosphorylated and then associates with the Atg1p kinase to initiate autophagosome formation (Kamada et al., 2000; Nakatogawa et al., 2009). To this end, we monitored the phosphorylation status of Atg13p upon induction of NNS-autophagy as a readout for TORC1 activity (Figure 3A). Upon switch to SL medium, Atg13p rapidly became dephosphorylated as expected (Wu and Tu, 2011). Although there was a gradual recovery of Atg13p phosphorylation over time in SL, the addition of methionine did not significantly promote such recovery, despite its inhibitory effects on autophagy (Figure 3A). Non-sulfur amino acids, which did not inhibit NNS-autophagy, also had minimal effect on recovery of Atg13p phosphorylation. Under these nutrient conditions, we observed little correlation between Atg13p phosphorylation state and the extent of NNS-autophagy. These observations indicate that methionine is likely not sensed directly by TORC1, but instead acts in parallel or downstream of TORC1 and Atg13p to inhibit autophagy.
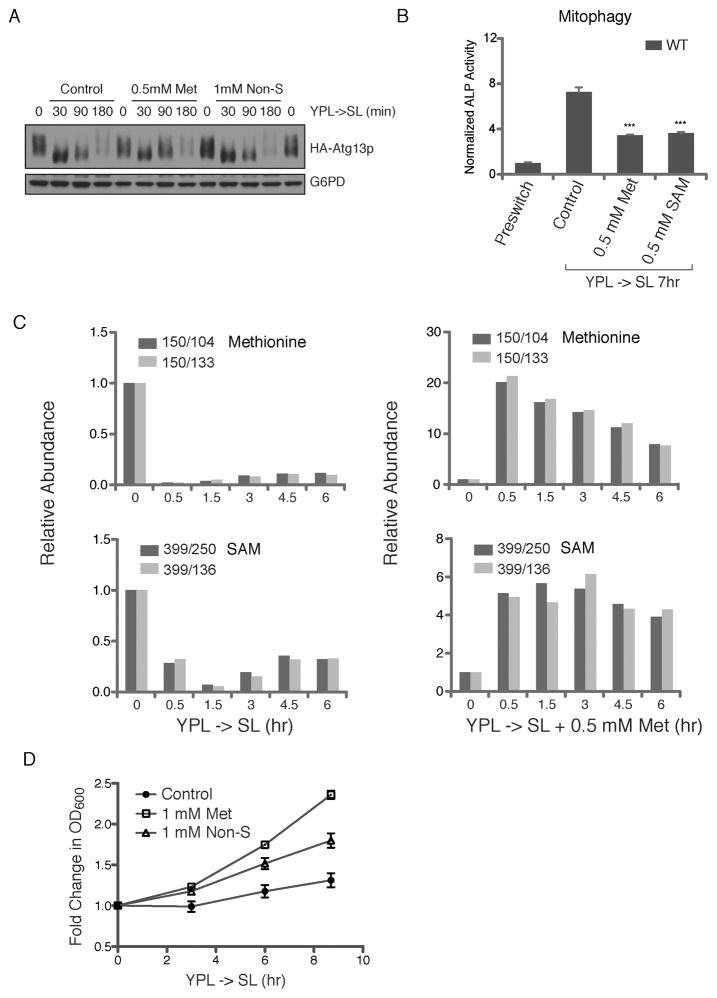
Figure 3. SAM is the Key Metabolite Which Acts in Parallel or Downstream of TORC1 to Regulate Autophagy.
(A) Methionine addition does not promote Atg13p re-phosphorylation following induction of NNS-autophagy. Cells expressing HA-Atg13p were grown in YPL to log phase before switch to SL. At the indicated time points, cells were collected and processed for analysis of Atg13p phosphorylation status by Western blot. Although there was a gradual recovery of Atg13p phosphorylation over time in SL, methionine and non-sulfur amino acids did not promote such recovery. Under these nutrient conditions, there was little correlation between the phosphorylation state of Atg13p and the extent of NNS-autophagy. NNS-autophagy is maximal at ~8 h following switch from YPL to SL medium (Wu and Tu, 2011).
(B) SAM inhibits NNS-autophagy as effectively as methionine. Mitophagy was assayed using the ALP assay after switch to SL plus the indicated concentrations of either methionine or SAM.
(C) Significant decrease in intracellular methionine and SAM levels under conditions of NNS-autophagy induction. Methionine and SAM levels were measured using previously established LC-MS/MS methods (Tu et al., 2007). Relative abundance of two daughter fragments for MRM-based detection of methionine and SAM are shown. Duplicate experiments were performed and the results of one representative experiment are shown. See also Figure S3.
(D) Methionine promotes increased growth rates. Following switch to SL, the addition of methionine alone improved the initial growth rate of cells more significantly than a mixture of 17 non-sulfur amino acids combined.
SAM Inhibits NNS-autophagy through the Action of Specific Methyltransferase Enzymes
Since methionine is the key amino acid monitored by cells to regulate NNS-autophagy and functions separate from regulating TORC1-Atg13p phosphorylation, we asked how methionine functions to inhibit autophagy. Methionine can be converted in one step to S-adenosylmethionine (SAM) by SAM synthetase in an ATP-dependent reaction (Figure 2B). SAM is the methyl donor for the numerous methyltransferase enzymes in the cell that utilize SAM as a substrate. We observed that SAM blocked NNS-autophagy at comparable concentrations as methionine (Figure 3B), indicating that SAM could be responsible for the inhibitory effects of methionine on autophagy.
The ability of methionine and SAM to inhibit autophagy suggests that cells might be starved for these metabolites upon switch to SL medium. We monitored intracellular amounts of methionine and SAM by LC-MS/MS in YPL and in the NNS-autophagy-inducing SL medium. Methionine and SAM abundance decreased significantly upon switch to SL medium over a time period during which NNS-autophagy is induced (Figure 3C). These data reveal that cells have difficulty producing sufficient amounts of methionine and SAM upon switch to SL medium, rendering them starved for these metabolites. Such deficiency in SAM may represent a nutritional trigger of NNS-autophagy. As expected, upon supplementation of methionine to SL medium, intracellular levels of both methionine and SAM became plentiful and were likely no longer limiting for the molecular events that initiate NNS-autophagy (Figure 3C). Although some other amino acids also decreased in abundance upon switch to SL medium, they typically did not decrease as much as methionine (Figure S3). The observed decreases in other amino acids are likely not as important for NNS-autophagy regulation as their re-addition did not block this process (Figure 1). Moreover, we observed that the addition of methionine alone significantly increased the initial growth rate of cells upon switch to SL medium (Figure 3D), more so than the mixture of non-sulfur amino acids combined. These observations highlight methionine as a sentinel amino acid in cell growth control.
We next tested the possibility that the effects of SAM on autophagy might be dependent on particular SAM-consuming methyltransferase enzymes. To this end, we individually knocked out each of ~70 known or predicted methyltransferases in the budding yeast genome (Table S2) (Petrossian and Clarke, 2009). For each single methyltransferase mutant, we tested whether methionine could still block NNS-autophagy upon switch from YPL to SL medium using the GFP cleavage assay (Figure 4). For 47 non-essential methyltransferase deletion mutants, methionine effectively blocked NNS-autophagy, while the non-sulfur amino acid mixture had minimal effect (Figure 4 and Table S3). For only two methyltransferase mutants, ppm1Δ and cho2Δ, methionine was no longer able to inhibit NNS-autophagy (Figure 4). Ppm1p is a methyltransferase that methylates the catalytic subunit of PP2A (Lee and Stock, 1993; Xie and Clarke, 1993, 1994b), while Cho2p is a membrane-bound methyltransferase that catalyzes the first step in the conversion of the phospholipid phosphatidylethanolamine (PE) to phosphatidylcholine (PC) (Kodaki and Yamashita, 1987). We were unable to test 18 remaining methyltransferases for a possible role in mediating the effects of methionine because the knockout mutants were either lethal or too sick when grown in YPL medium (Table S3). Notably, methionine still blocked NNS-autophagy in the absence of various methyltransferases that act on ribosomal subunits or the translation machinery, as well as histone methyltransferases that regulate chromatin structure and gene transcription (Figure 4 and Table S2). These data indicate that the effects of methionine on autophagy are specifically dependent on particular methyltransferase enzymes in the cell. The remainder of the manuscript will be focused on the role of Ppm1p in the regulation of NNS-autophagy.
Figure 4. Identification of Methyltransferases Required for Methionine to Inhibit NNS-Autophagy.
Yeast strains containing individual deletions in each of the indicated methyltransferase enzymes were constructed. These mutants were tested for their ability to support the inhibitory effects of methionine on NNS-autophagy using the GFP-cleavage assay. In the majority of mutants, methionine still effectively inhibited autophagy, while the non-sulfur amino acid mixture had no effect. Note that in two mutants, ppm1Δ and cho2Δ, methionine no longer inhibited NNS-autophagy. A full list of methyltransferase mutants that were tested and their known functions are provided as Tables S2 and S3.
The Methylation State of PP2A is Responsive to SAM Levels and Regulates Cell Growth and Autophagy
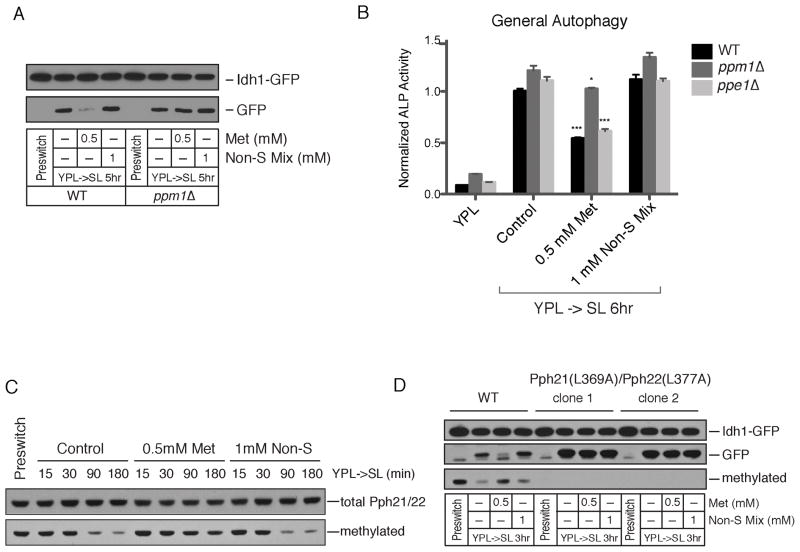
Ppm1p encodes an enzyme that methylates a conserved carboxy terminal leucine residue of the catalytic “C” subunit of Protein Phosphatase 2A (PP2A) (Lee and Stock, 1993; Xie and Clarke, 1993, 1994b). This methylation event is thought to regulate the association of particular “B” regulatory subunits as a means to direct PP2A activity towards particular sets of substrates (Tolstykh et al., 2000; Wei et al., 2001; Wu et al., 2000). The methyltransferase Ppm1p as well as the methylesterase Ppe1p, which catalyzes the demethylation of this leucine residue, are both conserved (Lee et al., 1996; Lee and Stock, 1993; Xie and Clarke, 1993, 1994a,b). Budding yeast harbor an enzyme Ppm2p that exhibits extensive similarity to Ppm1p. However, Ppm2p reportedly does not methylate PP2A (Kalhor et al., 2001; Noma et al., 2006). Methionine no longer inhibited NNS-autophagy in the ppm1Δ mutant, but still inhibited autophagy in the ppm2Δ mutant (Figure 4 and Figure S4). We further confirmed that methionine no longer blocked autophagy in the ppm1Δ mutant strain using both the GFP cleavage and ALP assays (Figure 5A–B). Collectively, these observations suggest that Ppm1p methyltransferase activity negatively regulates NNS-autophagy.
Figure 5. Ppm1p Catalyzes the Methionine/SAM-Responsive Methylation of PP2A to Regulate Autophagy.
(A) Ppm1p is required for methionine to inhibit NNS-autophagy. WT and ppm1Δ strains were switched from YPL to SL plus the indicated amino acids and autophagy was measured using the GFP cleavage assay. See also Figure S4.
(B) The ppe1Δ mutant which lacks the PP2A carboxymethylesterase/demethylase exhibits normal NNS-autophagy induction. The indicated strains were switched from YPL to SL and autophagy was measured using the ALP assay. Methionine was still able to inhibit NNS-autophagy in ppe1Δ but not ppm1Δ mutants.
C) Methylation of the C-terminal leucine residue of the catalytic subunit of PP2A is responsive to methionine and SAM availability. WT cells were grown in YPL and switched to SL. At the indicated times, cells were harvested and proteins were extracted to assay the amount of methylated PP2A using an antibody specifically recognizing the methylated form. Methylated PP2A levels decreased upon switch to SL, conditions under which SAM becomes limiting, and were restored upon addition of methionine but not the non-sulfur amino acid mixture.
(D) Point mutations in the C-terminal leucine residue of the catalytic subunit of PP2A renders cells unresponsive to methionine. WT or Pph21p(L369A)/Pph22p(L377A) cells were switched from YPL to the indicated media and assayed for autophagy using the GFP-cleavage assay. Note methionine no longer blocks autophagy in this mutant.
The inability of methionine to block autophagy in cells lacking Ppm1p suggests that the catalytic subunit of PP2A (Pph21p, Pph22p in yeast) might be differentially methylated in YPL compared to SL medium. Using an antibody specific to the methylated form of the catalytic subunit, we assessed amounts of methylated PP2A in either YPL or SL. The amount of methylated PP2A decreased significantly upon switching to SL medium (Figure 5C). The addition of methionine, but not the non-sulfur amino acid mixture, strongly attenuated this decrease in methylated PP2A, suggesting the methylation status of PP2A is sensitive to the levels of methionine and SAM in the cell. In the ppm1Δ mutant, methylated PP2A could no longer be detected, consistent with previous reports that Ppm1p is likely the sole PP2A methyltransferase in yeast cells (Figure S4) (Kalhor et al., 2001). Moreover, deletion of the Ppe1p methylesterase that demethylates PP2A did not affect NNS-autophagy or the ability of methionine to inhibit NNS-autophagy (Figure 5B).
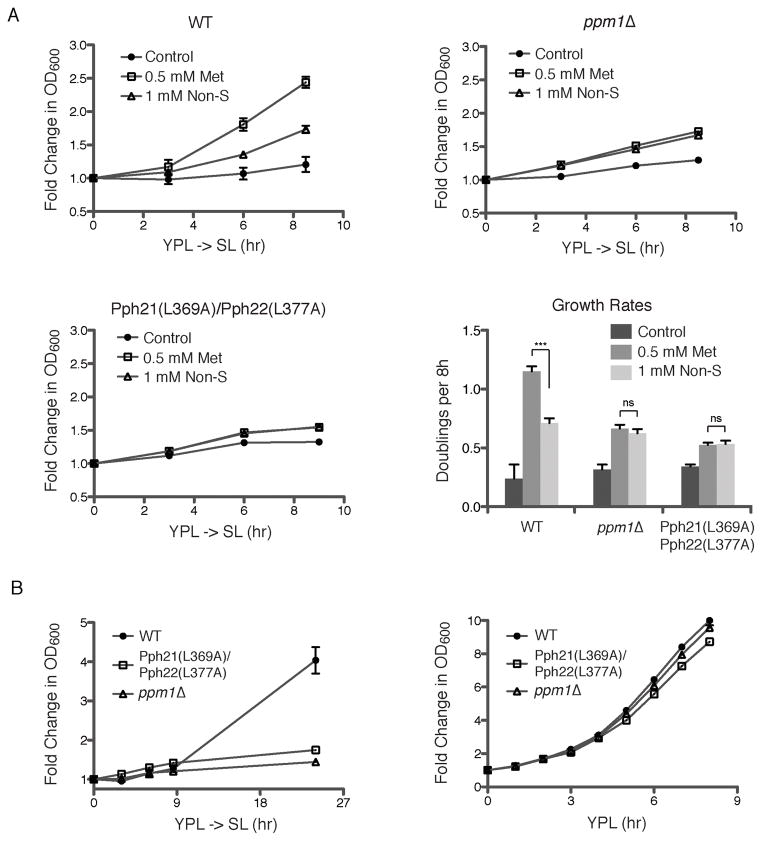
To confirm that the effects of methionine are mediated specifically through Ppm1p, we created point mutations changing the C-terminal leucine residue to alanine in Pph21p and Pph22p (both encode the catalytic subunit of PP2A in yeast). In this mutant, the PP2A catalytic subunit was no longer methylated (Figure 5D). Moreover, these two point mutations rendered cells completely unresponsive to methionine (Figure 5D). Methionine no longer inhibited autophagy in the Pph21p(L369A)/Pph22p(L377A) mutant, despite the presence of Ppm1p. Methionine also no longer improved growth of either this mutant or ppm1Δ cells upon switch to SL medium (Figure 6A). These results firmly establish that methylation of the catalytic subunit of PP2A functions to inhibit NNS-autophagy and promote growth. Notably, this methylation modification is responsive to methionine and SAM levels in the cell, perhaps enabling PP2A to act as a “sensor” of cellular methionine and SAM levels. Moreover, the ppm1Δ mutant itself exhibited a severe growth defect in SL medium over an extended period of time, suggesting that the methylation of PP2A becomes more important for growth regulation under more challenging nutrient environments (Figure 6B). Although much remains unknown regarding the role of PP2A methylation in determining its substrate specificity, our observations suggest that the SAM-regulated methylation of PP2A enables it to dephosphorylate specific substrates that act to negatively regulate autophagy and promote growth.
Figure 6. Methionine Promotes Cell Growth through the Methylation of PP2A.
(A) Growth of the indicated strains (WT, ppm1Δ, Pph21(L369A)/Pph22(L377A)) was monitored following switch from YPL to SL plus the indicated amino acids. Growth rates calculated as doublings per 8 hr are shown. Note that methionine alone improves growth in SL better than a mixture of other amino acids within this time period, via the methylation of PP2A.
(B) The indicated strains were grown in YPL or switched from YPL to SL. Mutants lacking the ability to methylate PP2A grow much more poorly under the more challenging conditions.
The NNS-Autophagy Regulator Npr2p is a Target of Methylated PP2A and Loss of its Function Leads to Unchecked Growth
Having established that methionine and SAM are the limiting metabolites that trigger NNS-autophagy by modulating the methylation status of PP2A, it remains unclear how a subsequent signal is relayed to known regulators of NNS-autophagy (the Iml1p/Npr2p/Npr3p complex) or the autophagy core machinery. We previously reported that Npr2p is a phosphoprotein, and that Iml1p primarily interacts with the phosphorylated form of Npr2p (Wu and Tu, 2011). Upon switch to SL medium, we observed the accumulation of a more phosphorylated form of Npr2p and a decrease in the less phosphorylated form, which was reversed by the addition of methionine (Figure 7A). Notably, the ability of methionine to promote the accumulation of the less phosphorylated form of Npr2p was completely dependent on the methylation of PP2A (Figure 7A). These data suggest that methylated PP2A functions to dephosphorylate Npr2p. Since Iml1p preferentially interacts with the phosphorylated form of Npr2p as part of an active complex that can translocate to pre-autophagosomal structures and the vacuole (Wu and Tu, 2011), methylated PP2A may negatively regulate NNS-autophagy by promoting the dephosphorylation of Npr2p.
Figure 7. The NNS-Autophagy Regulator Npr2p is a Target of Methylated PP2A and Cells Lacking Npr2p Exhibit Uncontrolled Growth.
(A) Methylated PP2A regulates the phosphorylation status of NNS-autophagy regulator Npr2p. The indicated strains were switched from YPL to SL. Npr2p runs as a doublet in YPL and the more phosphorylated form is maintained upon switch to SL. Methionine increases the abundance of the less phosphorylated form of Npr2p in a manner dependent on the methylation of PP2A.
(B) Mutants lacking NNS-autophagy regulators exhibit unchecked growth. Growth curves of WT, iml1Δ, npr2Δ, npr3Δ strains in YPL or following switch from YPL to SL. Rapamycin (20 ng/mL) suppresses the unchecked growth phenotype of these mutants in SL medium, suggesting the Iml1p/Npr2p/Npr3p may act upstream of TORC1. See also Figure S5.
(C) Model depicting the influence of intracellular methionine and SAM levels on cell growth control. The methylation of PP2A is responsive to intracellular methionine/SAM levels and may help direct its phosphatase activity towards particular substrates. Notably, methylated PP2A promotes the dephosphorylation of Npr2p, which functions to inhibit NNS-autophagy. The Iml1p/Npr2p/Npr3p complex also negatively regulates tRNA thiolation as a means to down-regulate translation and growth – see accompanying manuscript (Laxman et al., 2013). This complex also negatively regulates TORC1 under particular conditions as in (B) and (Neklesa and Davis, 2009).
We further examined the phenotype of cells lacking Iml1p, Npr2p, or Npr3p, which regulate NNS-autophagy (Wu and Tu, 2011). Mutants in the core autophagy machinery (e.g., atg1Δ, atg8Δ) grow slower than WT cells upon switch from YPL to SL medium, suggesting that induction of autophagy under these conditions is important for cellular homeostasis and enabling adaptation to the new growth environment (Wu and Tu, 2011). However, instead of exhibiting a phenotype similar to autophagy core machinery mutants, iml1Δ, npr2Δ and npr3Δ mutants all grew significantly faster than WT upon switch to SL (Figure 7B). These three mutants also did not undergo metabolic cycles that are characteristic of WT yeast cells despite their robust growth rates (Figure S5), suggesting they harbor a significant defect in some aspect of metabolic regulation or compartmentalization.
The uncontrolled growth of iml1Δ, npr2Δ and npr3Δ mutants was reversed by addition of rapamycin (Figure 7B). This phenotype is consistent with a model in which Npr2p and Npr3p function to negatively regulate TORC1 signaling under appropriate conditions (Neklesa and Davis, 2009). In the absence of the Iml1p/Npr2p/Npr3p complex, TORC1 may be aberrantly active and directing growth outputs at times when cells normally should not maintain as high a growth rate. These data indicate that the Iml1p/Npr2p/Npr3p complex plays a critical role in cell growth control and that this regulatory pathway is intimately linked to the intracellular availability of the amino acid methionine and its downstream metabolite SAM (Figure 7C). In the accompanying manuscript, we show evidence that the complex also negatively regulates tRNA thiolation abundance upon switch to SL medium as a means to down-regulate cellular translational capacity and spare sulfur equivalents (Laxman et al., 2013). Collectively, our studies reveal how these NNS-autophagy regulators enable cells to modulate key growth-related processes in response to availability of these key sulfur-containing metabolites.
DISCUSSION
In this study, we elucidated the metabolic trigger of NNS-autophagy, which occurs when yeast cells are switched from a rich to minimal medium with a non-fermentable carbon source such as lactate (YPL -> SL), in the absence of nitrogen starvation. NNS-autophagy is more prominent in certain strains of yeast and is important for cellular homeostasis and adaptation to the lower growth rate (Wu and Tu, 2011). By adding back various metabolites to SL medium, we discovered that a single amino acid methionine is sufficient to suppress NNS-autophagy. This effect of methionine is extremely specific as the other 19 amino acids have virtually no effect on NNS-autophagy induction either alone or in combination (Figure 1 and Figure S1).
We traced the effect of methionine to its downstream metabolite SAM and its role as a substrate for particular methyltransferase enzymes within the cell. By screening individual knockout mutants, we identified a methyltransferase specifically required for methionine to inhibit NNS-autophagy. The absence of Ppm1p, which methylates the catalytic subunit of PP2A, eliminates the ability of methionine to block NNS-autophagy, suggesting that this enzyme is central to mediating the effects of methionine and SAM. Our findings suggest the methyltransferase activity of Ppm1p is regulated by the availability of its substrate SAM within the cell. Consistent with this idea, the methylation status of PP2A was highly dynamic depending on whether cells were in YPL medium where SAM is abundant, or in SL medium, where SAM becomes limiting (Figure 5B). Thus, we predict that methylated PP2A preferentially dephosphorylates substrates that lead to growth-promoting functions, while demethylated PP2A might preferentially dephosphorylate substrates that lead to growth-inhibitory and autophagy-promoting functions. One such substrate of methylated PP2A may be Npr2p (Figure 7A), whose phosphorylated form appears to be important for its autophagy-promoting functions (Wu and Tu, 2011). Interestingly, mutants in PPM1 suppress the poor survivability and glucose wasting phenotype of leucine auxotrophs upon leucine starvation (Boer et al., 2008), consistent with the idea that methylation of PP2A plays a critical role in regulating cell growth and nutrient utilization. Since this methylation event is highly responsive to methionine and SAM levels, the PP2A phosphatase can act as a sensor of metabolic state to enact appropriate cellular outputs. Future work will be required to determine the full range of substrates dephosphorylated by either methylated or demethylated PP2A.
We have uncovered growth conditions under which the sulfur-containing metabolites methionine and SAM become sufficiently limiting to induce autophagy. Why might cells choose methionine and SAM as a gauge of their metabolic/nutritional state? Sulfur-containing amino acids are very energetically costly to synthesize (Thomas and Surdin-Kerjan, 1997), requiring four equivalents of NADPH simply to reduce one molecule of sulfate to metabolically active sulfide (Figure 2B). Sulfide is then used to synthesize homocysteine, whose carbon skeleton is ultimately derived from oxaloacetate, a TCA cycle intermediate. The methyl group that is appended to homocysteine to form methionine is derived from one-carbon metabolism and the folate pathway, which also consumes significant NADPH (Thomas and Surdin-Kerjan, 1997). Thus, sulfur amino acids are dependent on multiple important metabolic pathways for their synthesis.
Sulfur and its metabolites also form the basis of a metabolic checkpoint for entry into the cell cycle (Unger and Hartwell, 1976). Upon sulfate starvation, yeast cells conserve nutrients and properly arrest their growth to promote survivability (Boer et al., 2008). They also preferentially express proteins containing a smaller percentage of cysteine and methionine residues as a strategy to spare sulfur (Fauchon et al., 2002). The Met4p transcription factor, which controls the transcription of many genes involved in sulfur utilization, is degraded prior to entry into START (Kaiser et al., 2006; Patton et al., 2000). A recent study showed that met4Δ mutants grow poorly even when supplemented with methionine. However, the addition of SAM restored normal growth rates, suggesting that SAM levels are rate-limiting for supporting maximal growth rates (Hickman et al., 2011). Lastly, ~1% of yeast and mammalian gene products encode SAM-dependent methyltransferases (Petrossian and Clarke, 2011), and this number does not include enzymes that utilize SAM via other mechanisms. Among these enzymes that consume SAM as a substrate are those required for the methylation of lipids and histones, and the synthesis of polyamines and other essential metabolites. Thus, the availability of methionine and SAM may reflect the overall biosynthetic capacity and amino acid status of cells and could be sensed by way of particular methyltransferase enzymes.
It should be noted that NNS-autophagy is potently suppressed by abundant glucose (Wu and Tu, 2011). Many metabolites needed for growth are more easily derived from glucose compared to non-fermentable carbon sources such as lactate. Glucose also supports the facile production of acetyl-CoA, which promotes the acetylation of histones to drive the transcription of a set of over 1,000 growth genes, including virtually all genes important for ribosome biogenesis and the upregulation of translational capacity (Cai et al., 2011). Furthermore, glucose supplies ribose sugars as well as NADPH via the pentose phosphate pathway, which are both important for cell proliferation. Sulfate assimilation and utilization in yeast are major consumers of cellular NADPH equivalents (Thomas and Surdin-Kerjan, 1997). Cells growing in lactate or carbon-poor environments might be challenged in their ability to produce NADPH compared to cells growing in high glucose. An external supply of methionine might help preserve cellular NADPH equivalents that can instead be applied towards other biosynthetic or growth-promoting processes in the cell. In support of this idea, the addition of methionine alone is sufficient to improve growth of yeast cells in SL medium in a manner dependent on the methylation of PP2A (Figure 3D), suggesting that this regulatory modification is responsive to cellular methionine and SAM levels.
In the accompanying manuscript, we show that cells decrease amounts of a thiolation modification on particular tRNAs under the same conditions that induce NNS-autophagy (Laxman et al., 2013). tRNA thiolation enhances translational capacity when sulfur amino acids are abundant, but this sulfur-consuming process is suppressed upon switch to sulfur-challenged SL medium. These observations reveal that an insufficiency of sulfur equivalents represents a physiological trigger of multiple regulatory events aimed towards down-regulating cell growth and translation. Collectively, our findings highlight the importance of regulating cell growth and homeostasis in tune with the nutritional availability of sulfur sources.
It is notable that all three NNS-autophagy regulators Iml1p, Npr2p, and Npr3p are conserved in mammalian cells. However, little is known regarding their function in metazoans. Intriguingly, the mammalian ortholog of Npr2p (NPRL2) lies in the 3p21.3 chromosomal region that is very frequently lost in lung cancer and its re-introduction is sufficient to suppress multiple tumorigenic phenotypes of various lung cancer cell lines (Ji et al., 2002; Li et al., 2004). The unchecked growth of npr2Δ mutant yeast cells in SL medium (Figure 7B) is consistent with the proposed tumor suppressive function of mammalian NPRL2. Mutants lacking these NNS-autophagy regulators do not appear to properly down-regulate various growth-related processes (e.g., induce autophagy, inhibit tRNA thiolation) under less favorable growth environments. The observation that the unchecked growth of these mutants can be reversed by rapamycin is also consistent with a previous study that implicates Npr2p and Npr3p in the negative regulation of TORC1 signaling (Neklesa and Davis, 2009). Thus, it is readily conceivable that loss of NPRL2 might be a key event that promotes tumorigenesis.
Finally, our findings highlight the possibility that methionine could modulate growth and TORC1 signaling through regulation of the methylation status of PP2A. The methylation status of PP2A could direct its phosphatase activity towards particular substrates that are also substrates of TORC1 itself, thus tilting the balance of particular growth or autophagy regulators towards either the phosphorylated or dephosphorylated state. Moreover, the methylation status of PP2A could also influence TORC1 activity through modulating the phosphorylation state of Npr2p, which is a negative regulator of TORC1 (Figure 7C). As this methylation modification of PP2A is responsive to intracellular SAM levels, this feature may enable the phosphatase to function as a sensor of amino acid status that is encoded by methionine availability. Interestingly, certain tumors show increased methionine uptake by positron emission tomography (Singhal et al., 2008). Since the enzymes that catalyze the methylation and demethylation of PP2A are conserved (Lee et al., 1996; Lee and Stock, 1993; Xie and Clarke, 1993, 1994a, b), the methylation of PP2A could represent a widely utilized mechanism to regulate protein phosphorylation status in tune with metabolic state. Taken together, our studies have highlighted the importance of methionine and SAM in cell growth control and revealed regulatory pathways specifically responsive to these sulfur-containing metabolites. Because methionine is an essential amino acid for mammals, dietary methionine intake may impact organismal growth, development, reproduction, and the etiology of metabolic diseases such as cancer and aging, through mechanisms described here.
EXPERIMENTAL PROCEDURES
Yeast Strains and Media
The prototrophic CEN.PK strain background was used in all experiments. Strains used in this study are listed in Table S1. Growth media and strain construction methods are described in Supplemental Experimental Procedures.
Assays to Monitor Autophagy
The alkaline phosphatase assay, GFP-cleavage assay, and imaging assay are described in detail in Supplemental Experimental Procedures.
Whole Cell Extracts Preparation
Cell pellets were resuspended with 300 μL yeast lysis buffer (50 mM NaCl, 50 mM NaF, 100 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1% Triton X-100 or 0.1% Tween 20, 10% Glycerol, 14 mM 2-mercaptoethanol, 1X EDTA-free protease inhibitor cocktail (Roche), 1 mM PMSF, 5 μM Pepstatin A, 10 μM Leupeptin). After adding ~80 μL glass beads, cells were lysed by three rounds of bead-beating: 1min beating with 1 min cooling on ice. Antibodies used were: mouse anti-GFP monoclonal antibody (Roche, clone 7.1 and 13.1), mouse anti-Pgk1 monoclonal antibody (Invitrogen), mouse anti-FLAG M2 antibody (Sigma), mouse anti-HA monoclonal antibody (Roche, clone 12CA5), mouse monoclonal anti-methyl-PP2A, C subunit (Millipore, clone 2A10), mouse monoclonal anti-PP2A, C subunit (Millipore, clone 7A6)
Atg13p phosphorylation
At the indicated time points, 5 OD of cells expressing HA-ATG13 were quenched by mixing with TCA to a final concentration of 5–6% and incubated on ice for at least 5 min before centrifugation. Cell pellets were then subject to whole cell TCA extraction and analyzed by immunoblotting.
Metabolite Quantitation
Metabolites were quantitated by LC-MS/MS using previously established methods (Tu et al., 2007). Sulfur-containing metabolites were detected by MRM in positive mode using 0.1% formic acid. Care was taken to quench cells quickly and maintain metabolites in acid to minimize oxidation.
Supplementary Material
HIGHLIGHTS.
Iml1/Npr2/Npr3 complex regulates autophagy induced in response to limited methionine
Methionine inhibits autophagy and promotes growth through the methylation of PP2A
Methylation of PP2A is responsive to SAM levels and regulates phosphorylation of Npr2
Methionine may impact the phosphostatus of proteins and TORC1 substrates via PP2A
Acknowledgments
We thank members of the Tu lab for helpful discussions. X.W. was supported by a fellowship from the Chilton Foundation and a Med into Grad Initiative sponsored by HHMI. This research was supported by award R01GM094314 from NIGMS, the UTSW Endowed Scholars Program, the Welch Foundation (I-1797), the Burroughs Wellcome Fund, the David and Lucile Packard Foundation, and the Damon Runyon Cancer Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boer VM, Amini S, Botstein D. Influence of genotype and nutrition on survival and metabolism of starving yeast. Proc Natl Acad Sci U S A. 2008;105:6930–6935. doi: 10.1073/pnas.0802601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA Synthetase Controls TORC1 via the EGO Complex. Mol Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokudovskaya S, Waharte F, Schlessinger A, Pieper U, Devos DP, Cristea IM, Williams R, Salamero J, Chait BT, Sali A, et al. A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae. Mol Cell Proteomics. 2011;10:M110 006478. doi: 10.1074/mcp.M110.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchon M, Lagniel G, Aude JC, Lombardia L, Soularue P, Petat C, Marguerie G, Sentenac A, Werner M, Labarre J. Sulfur sparing in the yeast proteome in response to sulfur demand. Mol Cell. 2002;9:713–723. doi: 10.1016/s1097-2765(02)00500-2. [DOI] [PubMed] [Google Scholar]
- Ganguli D, Kumar C, Bachhawat AK. The alternative pathway of glutathione degradation is mediated by a novel protein complex involving three new genes in Saccharomyces cerevisiae. Genetics. 2007;175:1137–1151. doi: 10.1534/genetics.106.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef M, Nunnari J. Mitochondria regulate autophagy by conserved signalling pathways. EMBO J. 2011;30:2101–2114. doi: 10.1038/emboj.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA Synthetase Is an Intracellular Leucine Sensor for the mTORC1-Signaling Pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hickman MJ, Petti AA, Ho-Shing O, Silverman SJ, McIsaac RS, Lee TA, Botstein D. Coordinated regulation of sulfur and phospholipid metabolism reflects the importance of methylation in the growth of yeast. Mol Biol Cell. 2011;22:4192–4204. doi: 10.1091/mbc.E11-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Ji L, Nishizaki M, Gao B, Burbee D, Kondo M, Kamibayashi C, Xu K, Yen N, Atkinson EN, Fang B, et al. Expression of several genes in the human chromosome 3p21.3 homozygous deletion region by an adenovirus vector results in tumor suppressor activities in vitro and in vivo. Cancer Res. 2002;62:2715–2720. [PMC free article] [PubMed] [Google Scholar]
- Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P, Su NY, Yen JL, Ouni I, Flick K. The yeast ubiquitin ligase SCFMet30: connecting environmental and intracellular conditions to cell division. Cell division. 2006;1:16. doi: 10.1186/1747-1028-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhor HR, Luk K, Ramos A, Zobel-Thropp P, Clarke S. Protein phosphatase methyltransferase 1 (Ppm1p) is the sole activity responsible for modification of the major forms of protein phosphatase 2A in yeast. Archives of biochemistry and biophysics. 2001;395:239–245. doi: 10.1006/abbi.2001.2558. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Yoshino K, Kondo C, Kawamata T, Oshiro N, Yonezawa K, Ohsumi Y. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol Cell Biol. 2010;30:1049–1058. doi: 10.1128/MCB.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaki T, Yamashita S. Yeast phosphatidylethanolamine methylation pathway. Cloning and characterization of two distinct methyltransferase genes. J Biol Chem. 1987;262:15428–15435. [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxman S, Sutter BM, Wu X, Kumar S, Guo X, Trudgian DC, Mirzaei H, Tu BP. Sulfur amino acids regulate cellular translational capacity and metabolic homeostasis through modulation of tRNA uridine thiolation. 2013. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Chen Y, Tolstykh T, Stock J. A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc Natl Acad Sci U S A. 1996;93:6043–6047. doi: 10.1073/pnas.93.12.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Stock J. Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J Biol Chem. 1993;268:19192–19195. [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Developmental Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Li J, Wang F, Haraldson K, Protopopov A, Duh FM, Geil L, Kuzmin I, Minna JD, Stanbridge E, Braga E, et al. Functional characterization of the candidate tumor suppressor gene NPRL2/G21 located in 3p21.3C. Cancer Res. 2004;64:6438–6443. doi: 10.1158/0008-5472.CAN-03-3869. [DOI] [PubMed] [Google Scholar]
- Ma X, Jin M, Cai Y, Xia H, Long K, Liu J, Yu Q, Yuan J. Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem Biol. 2011;18:1474–1481. doi: 10.1016/j.chembiol.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- Neklesa TK, Davis RW. A genome-wide screen for regulators of TORC1 in response to amino acid starvation reveals a conserved Npr2/3 complex. PLoS Genet. 2009;5:e1000515. doi: 10.1371/journal.pgen.1000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Noma A, Kirino Y, Ikeuchi Y, Suzuki T. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006;25:2142–2154. doi: 10.1038/sj.emboj.7601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Peyraud C, Rouillon A, Surdin-Kerjan Y, Tyers M, Thomas D. SCF(Met30)-mediated control of the transcriptional activator Met4 is required for the G(1)-S transition. EMBO J. 2000;19:1613–1624. doi: 10.1093/emboj/19.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrossian TC, Clarke SG. Multiple Motif Scanning to identify methyltransferases from the yeast proteome. Mol Cell Proteomics. 2009;8:1516–1526. doi: 10.1074/mcp.M900025-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrossian TC, Clarke SG. Uncovering the human methyltransferasome. Mol Cell Proteomics. 2011;10:M110 000976. doi: 10.1074/mcp.M110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Singh S, Banerjee R. PLP-dependent H(2)S biogenesis. Biochim Biophys Acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2008;10:1–18. doi: 10.1007/s11307-007-0115-2. [DOI] [PubMed] [Google Scholar]
- Thomas D, Surdin-Kerjan Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1997;61:503–532. doi: 10.1128/mmbr.61.4.503-532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL. Cyclic changes in metabolic state during the life of a yeast cell. Proc Natl Acad Sci U S A. 2007;104:16886–16891. doi: 10.1073/pnas.0708365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MW, Hartwell LH. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976;73:1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ashby DG, Moreno CS, Ogris E, Yeong FM, Corbett AH, Pallas DC. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits Cdc55p and Rts1p. J Biol Chem. 2001;276:1570–1577. doi: 10.1074/jbc.M008694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Tolstykh T, Lee J, Boyd K, Stock JB, Broach JR. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tu BP. Selective regulation of autophagy by the Iml1-Npr2-Npr3 complex in the absence of nitrogen starvation. Mol Biol Cell. 2011;22:4124–4133. doi: 10.1091/mbc.E11-06-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Clarke S. Methyl esterification of C-terminal leucine residues in cytosolic 36-kDa polypeptides of bovine brain. A novel eucaryotic protein carboxyl methylation reaction. J Biol Chem. 1993;268:13364–13371. [PubMed] [Google Scholar]
- Xie H, Clarke S. An enzymatic activity in bovine brain that catalyzes the reversal of the C-terminal methyl esterification of protein phosphatase 2A. Biochem Biophys Res Commun. 1994a;203:1710–1715. doi: 10.1006/bbrc.1994.2383. [DOI] [PubMed] [Google Scholar]
- Xie H, Clarke S. Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J Biol Chem. 1994b;269:1981–1984. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.