Abstract
Aims
Nanoelectrodes are an emerging biomedical technology that can be used to record intracellular membrane potentials from neurons while causing minimal damage during membrane penetration. Current nanoelectrode designs, however, have low aspect ratios or large substrates and thus are not suitable for recording from neurons deep within complex natural structures, such as brain slices.
Materials & methods
We describe a novel nanoelectrode design that uses nanowires grown on the ends of microwire recording electrodes similar to those frequently used in vivo.
Results & discussion
We demonstrate that these nanowires can record intracellular action potentials in a rat brain slice preparation and in isolated leech ganglia.
Conclusion
Nanoelectrodes have the potential to revolutionize intracellular recording methods in complex neural tissues, to enable new multielectrode array technologies and, ultimately, to be used to record intracellular signals in vivo.
Keywords: electrophysiology, intracellular recording, nanoelectrode, nanotechnology, nanowire, neuron
Intracellular recordings are widely used by biomedical researchers to investigate the biophysical dynamics of neurons, and can reveal changes in membrane potentials that show both the synaptic inputs and action potentials of neurons. Typically, a glass micropipette with a tip diameter of the order of 0.1–1 μm is used to perform intracellular recordings [1]. A sharp electrode has an open tip and large shaft that penetrate the neuronal membrane as it gains access to the inside of the cell. This action causes cell damage and ionic leakage that affect the fidelity of the recording and typically limits the duration of recordings to a few hours or less. Intracellular electrodes can also dialyze a cell by replacing the cell’s cytoplasm with the fluid inside the micropipette, limiting recording time across cell membranes and contributing to eventual cell death [2].
Nanoelectrodes with tip diameters considerably less than 1 μm have the potential to minimize membrane damage during cell penetration, thus allowing for long-duration intracellular recordings. Furthermore, multielectrode array devices, which are currently limited to extracellular recordings, can use nanoelectrodes to create new ways of recording chronically across cell populations. Ideally, a nanoelectrode should have a diameter of less than 100 nm, as cells have been shown to have significantly shorter survival times with larger diameter nanowires [3]. Nanoelectrodes able to obtain intracellular membrane potential recordings have been fabricated with silicon nanowires [4], carbon nanopipettes [5], highly polished glass nanopipettes [6] and sharpened silicon needles [7]. However, after insertion, the portion of the electrode in contact with the membrane is often much larger than 100 nm due to the kinked design of the nanowires or the taper of the nanopipettes and needles. To minimize the contact area with a cell membrane, one group introduced a nanoelectrode that had a high aspect ratio using a single multiwalled carbon nanotube to penetrate a cell [8]. Its support structure, however, was large and not suited for recording from neurons deep within complex neural tissues, such as in brain slices or the intact brain, where the electrodes must pass through several tens or hundreds of microns of tissue before reaching their target neurons.
In this article, we present a novel approach to fabricating nanoelectrodes that addresses these challenges and limitations, and demonstrate that our design permits the intracellular recording of action potentials within rat brain slice and a leech ganglion. We developed a threestep process to construct our high aspect ratio nanoelectrodes:
▪ Nanowires were grown on the end of traditional microwire electrodes using electron-beam-induced deposition (EBID);
▪ The nanowires were completely insulated using atomic layer deposition (ALD);
▪ The insulation was removed from the tips with a focused ion beam (FIB).
Materials & methods
Figure 1 shows the fabrication steps used to make the nanoelectrodes. For the support structure, we chose to use traditional microelectrode recording microwires (18 μm in diameter and made of 90% platinum/10% iridium and insulated with polyimide; California Fine Wire, CA, USA). This microwire is routinely used to penetrate through intact rat brains in vivo to perform extracellular recordings of neurons for up to several months [9]. To form the support structure for the nanoelectrode, a segment of microwire with a length of approximately 7 mm was cut with surgical scissors under a stereo microscope. One end was cut at a bias to an angle of approximately 20° from vertical to allow for easier visualization from above in the scanning electron microscope (Figures 2A & B).
Figure 1. Fabrication steps for the nanoelectrode.
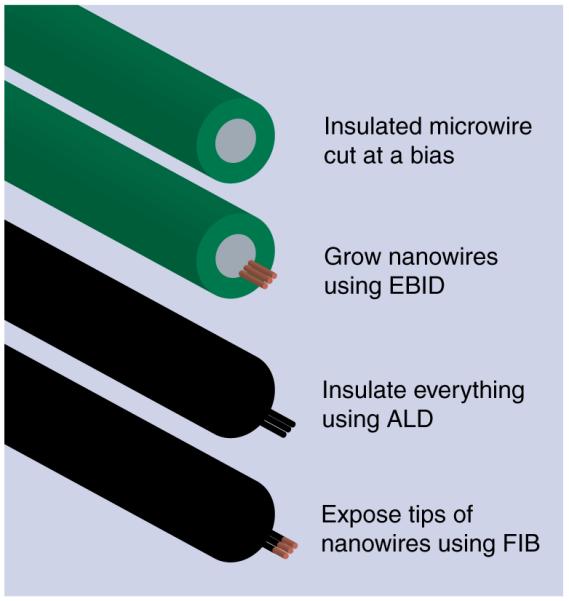
ALD: Atomic layer deposition; EBID: Electron-beam-induced deposition; FIB: Focused ion beam.
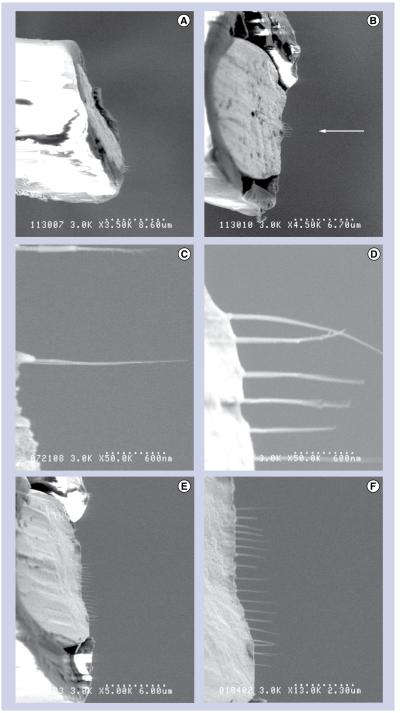
Figure 2. Scanning electron microscope Images of the fabricated nanoelectrode.
(A) A side-view of a nanoelectrode shows the bias cut. (B) Nanoelectrodes were viewed from above during electron-beam-induced deposition growth of nanowires (shown with an arrow). (C) Single nanowires were grown using horizontal line scans. (d) Typically, six nanowires were grown per nanoelectrode. (e) As many as 18 nanowires were grown on a single nanoelectrode. (F) A closer view of the 18-nanowire nanoelectrode.
As mentioned above, nanowires were grown using EBID, where a beam from an ultra-high resolution field-emission scanning electron microscope (S900, Hitachi, Tokyo, Japan) was focused on the end of the microwire and slowly scanned in horizontal lines extending out from the conductive portion of the microwire tip. No lithographic masks were necessary. Each EBID line scan took approximately 1 min and created a wire with a diameter of 10–30 nm and a length of up to 2 μm (Figure 2C). This step was repeated until the desired number of nanowires was obtained. EBID was chosen because of its customizability; the length, number and spacing of nanowires could be controlled for each nanoelectrode. Typically, six parallel nanowires were grown on the end of each nanoelectrode, reducing the impedance to the range of typical extracellular microwire electrodes (Figure 2D). Nanoelectrodes with one to 18 nanowires were also fabricated during development of the process (Figures 2e & F). The spacing between nanowires was chosen so that the distance between the furthest nanowires spanned less than a micron, ensuring that all of the nanowires would fit inside a single neuron. We used the residual hydrocarbons in the scanning electron microscope as the precursor for growth, a process known to create nanowires made of metal nanocrystals surrounded by a carbon matrix [10].
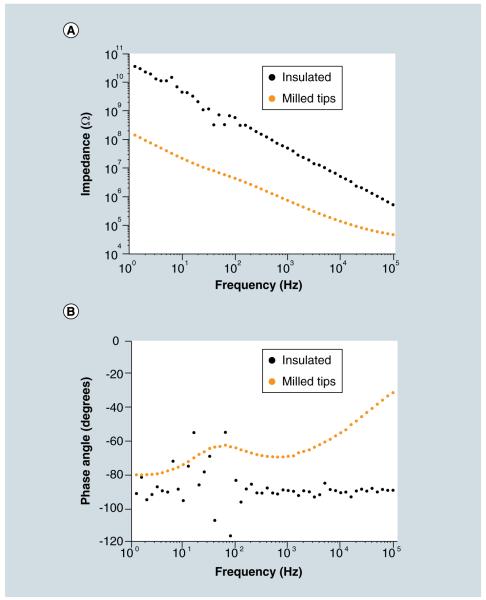
Next, a 30 nm layer of aluminum oxide was applied using ALD (Savannah S100, Cambridge NanoTech, MA, USA). Aluminum oxide is an effective insulator often used in glass microelectrodes [11]. ALD was chosen to ensure high-quality conformal insulation of both the nanowires and the microwire substrate. The electrical properties of a fully insulated nanoelectrode were measured by electrical impedance spectroscopy with a potentiostat (Reference 600, Gamry, PA, USA) (Figure 3). The nanoelectrode had an impedance of 64 MΩ at 1 kHz, the frequency most important for detecting action potentials, and an impedance of 5.7 GΩ at 10 Hz, the frequency important for postsynaptic potentials. The phase angles were measured to be approximately −90° at 1 kHz and −96° at 10 Hz, implying a capacitive electrode.
Figure 3. Impedances of the nanoelectrodes.
(A) The magnitude of impedance was significantly reduced after the nanowire tips were milled, removing the insulation. (B) The fully insulated nanoelectrode was capacitive, but the milled nanoelectrode had more resistive impedances.
Finally, the tips of the nanowires were milled to remove the insulation using a FIB (Quanta 200 3D, FEI, OR, USA). FIB allowed for selective milling of the tips with complete control on how much of the insulated tip was removed. In most cases, approximately 10–20 nm was removed from the tips of the nanowires. After the insulation was removed from the tips of the nanowires of a nanoelectrode, the impedance magnitudes were measured to be 942 kΩ at 1 kHz and 28 MΩ at 10 Hz, a reduction of two orders of magnitude from the fully insulated nanoelectrode. Also, the phase angles increased to −69° at 1 kHz and −74° at 10 Hz, implying a more resistive electrode.
Three biological preparations were used for nanoelectrode intracellular recordings. The first intracellular recording was from CA1 neurons in a rat hippocampal slice preparation [12]. A 45-day-old Long–Evans rat was deeply anesthetized using isoflurane. The brain was extracted and bathed in chilled artificial cerebral spinal fluid (composition in mM: 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 26 NaHCO3, and 10 d-glucose at pH 7.4, 295 mosM) for 3 min. The brain was dissected for transverse slices of the ventral horn of the hippocampal region and sectioned 400 μm thick on a Vibratome 3000 (Leica Microsystems, IL, USA) using slow speed and high oscillation (settings: 0.5 and 9, respectively; maximum of 10 in arbitrary units). Slices were further dissected after sectioning; leaving only the hippocampus and entorhinal cortex. Slices were transferred to a 35°C, oxygenated submersion chamber for 30 min to recover from the slicing procedure. The submersion chamber was then brought to room temperature until used. Neurons were visualized using differential interference contrast optics (Olympus, PA, USA). Nanoelectrode recordings were performed in the pyramidal cell body layer of the CA1 region of the hippocampus. The nanoelectrode was connected to a CV-7B headstage of a Multiclamp 700B amplifier (Molecular Devices, CA, USA) in current clamp mode. The neuron’s membrane potential was amplified and low-pass filtered at 2.4 kHz and digitized and recorded on a desktop computer (NiDAQ 6259, National Instruments, TX, USA). All rat experiments were completed in accordance with a protocol approved by the University of Minnesota Institutional Animal Care and Use Committee.
The second and third intracellular recordings were performed on touch-sensitive sensory neurons (T cells) and Retzius neurons in isolated and desheathed leech ganglion preparations [13]. Medicinal leeches (Hirudo verbana) weighing 2–3 g (Leeches USA Ltd., NY, USA) were housed in 15°C artificial pond water consisting of 0.5% Instant Ocean salts (United Pet Group, Inc., OH, USA) in deionized water (pH adjusted to 7.4). Leeches were anesthetized on ice for 15 min before individual segmental ganglia were extracted and desheathed in 4°C leech saline (composition in mM for T cells: 115 NaCl, 1.8 CaCl2, 4 KCl, 1.5 MgCl2, 10 Tris-maleate at pH 7.4; for Retzius cells: 10.0 dextrose and 10.0 Trizma® preset crystals at pH 7.4 were used to replace the Tris-maleate; Sigma-Aldrich, MO, USA). A nickel solution was added to induce bursting in the T cells [14]. Ganglia were harvested as follows: after exposing the ventral blood sinus, surrounding sinus tissue was cut dorsally to expose several segmental ganglia from the middle body region (M8-M15). The sinus was completely removed from the interganglionic connectives between the exposed ganglia. Single segmental ganglia were removed with adjacent interganglionic connectives and placed in a recording chamber. The ganglion was pinned with the ventral side up and desheathed to facilitate access to either the T cells or Retzius neurons, which were visually identified. Standard T-cell and Retzius neuron recordings were made using an AxoClamp 2B (Molecular Devices) or an IX2-700 amplifier (Dagan Instruments, MN, USA), respectively. Glass microelectrodes (outer diameter: 1.0 mm, inner diameter: 0.75 mm, borosilicate glass, Dagan Instruments) were pulled to a tip resistance of 25–30 MΩ (P-97 microelectrode puller, Sutter Instruments, CA, USA) when filled with 3 M potassium acetate and 20 mM potassium chloride. Once a T cell was identified by its characteristic action potentials, the glass microelectrode was withdrawn and replaced with a nanoelectrode connected to an intracellular recording amplifier (Model 3100, A-M Systems, WA, USA). By contrast, for the Retzius cell recording, the sharp glass microelectrode was not removed when the nanoelectrode was inserted. This configuration allowed for simultaneous recordings from both the nanoelectrode and the sharp glass microelectrode, which were connected to different channels of the intracellular amplifier (IX2-700, Dagan Instruments, MN, USA). Microelectrodes and nanoelectrodes were controlled by either a Huxley-style (MX310, Siskiyou Corporation, OR, USA) or Leitz micromanipulator (Leica, McHenry, IL, USA). Leech somata and electrode manipulation were monitored visually using a boom-mounted stereoscope with 30–80× magnification and dark field illumination.
Electrode penetration of all neurons across the three biological preparations used the ‘buzz’ or ‘ringer’ controls on the respective amplifiers. It is probable that the brief (500 ms or less) oscillating current, introduced to the nanoelectrodes, vibrated the nanowires into the cells. Throughout the recordings, AC coupling was used to eliminate slow voltage drifts encountered while recording. Capacitance compensation was not used.
Results & discussion
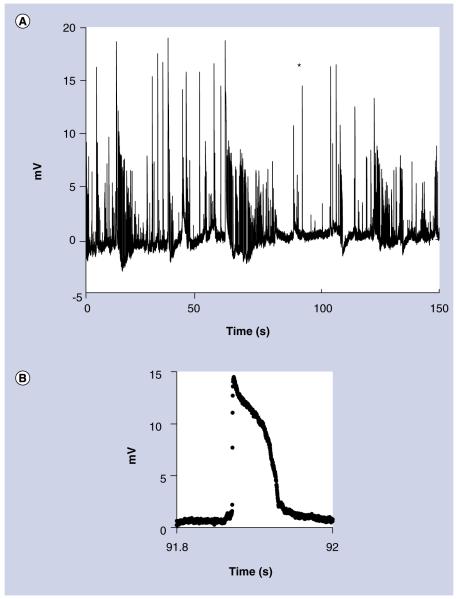
To test the nanoelectrodes in a complex natural structure, intracellular action potentials were recorded in spontaneously active CA1 neurons in a rat hippocampal slice preparation (Figure 4). The action potentials were approximately 15 mV in amplitude and were recorded for several minutes. These action potentials were smaller than those normally obtained with a traditional patch clamp microelectrode because of the large complex impedance of the nanoelectrode. However, the amplitude is consistent with other nanoelectrode recordings of neurons [5,7].
Figure 4. Intracellular recordings In a rat hippocampal slice.
(A) 2.5 min (150 s) of action potentials recorded from spontaneously active CA1 neurons. (B) An expanded view of the action potential marked with an asterisk. The waveform is typical of intracellular action potentials recorded without capacitance compensation.
Intracellular action potentials were also recorded from a touch-sensitive sensory neuron (T cell) of the medicinal leech (Hirudo verbana) in a single isolated ganglion preparation (Figure 5A). The polarity, amplitude and waveform of the action potentials recorded with the nanoelectrode (Figure 5B) were similar to those recorded using a sharp microelectrode. Additionally, extracellular action potentials were observed as the nanoelectrode approached the cell. These extracellular action potentials had a primarily negative polarity, opposite to the positive polarity of the action potentials after cell penetration. It is notable that recordings were maintained for up to 30 min. Following the recording session, the nanoelectrodes were examined using a scanning electron microscope (S900, Hitachi, Tokyo, Japan). We observed that all of the nanowires grown on the nanoelectrodes used during these experiments remained intact, indicating the robustness of the nanoelectrode design.
Figure 5. Intracellular action potential recording from a single T cell neuron from an intact ganglion of a medicinal leech (Hirudo verbana).
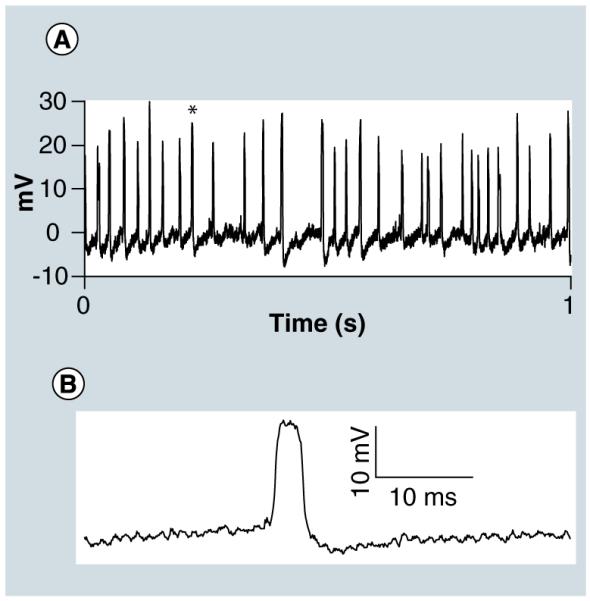
(B) is a zoom-in of the spike in (A) marked with an asterisk.
To directly compare recorded action potentials between the nanoelectrode and a sharp microelectrode, recordings were obtained from the uniquely identifiable Retzius neurons of the leech ganglion. Both the nanoelectrode and traditional sharp electrode remained in the cell to simultaneously record the neuron’s activity (Figures 6A & B). We observed that the polarity and timing of the action potentials recorded with the nanoelectrode matched the traditional sharp micropipette recording. The recordings also showed the well-documented smaller amplitude potentials (Figure 6C) known to stem from the electrically coupled contralateral homolog [15]. The waveform and polarity of these signals in both traces are, indeed, indicative of an intracellular-specific recording.
Figure 6. Simultaneous Intracellular nanoelectrode and sharp microelectrode recording from a single Retzius neuron from an Intact ganglion of a medicinal leech (Hirudo verbana).
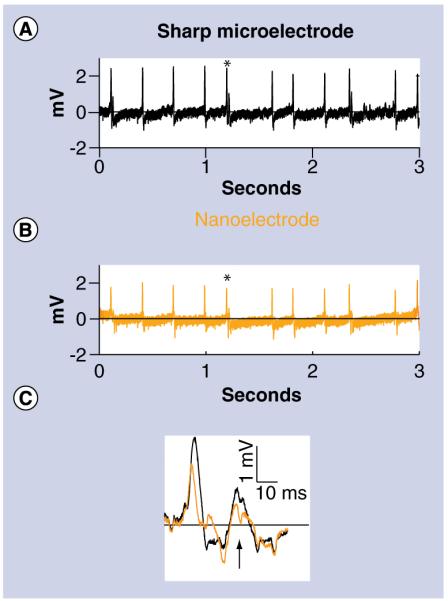
The polarity and timing of the action potentials recorded with the (A) traditional sharp microelectrode matched the (B) nanoelectrode. AC coupling was performed post-recording to remove the baseline drift. (C) An expanded view of the action potential marked with an asterisk. The nanoelectrode was also able to record smaller amplitude potentials known to stem from the electrically coupled contralateral homolog (arrow). An expanded view of the action potential marked with an asterisk.
Conclusion
The results obtained in this study illustrate that intracellular action potentials can be obtained successfully from neurons using our novel nanoelectrode design. These nanoelectrodes not only have high aspect ratios and thin, conformal insulation up to the tip, they are also highly customizable. The diameter, length, spacing, number and material composition of the nanowires can be tailored using different EBID scanning parameters or gas precursors [16]. Also, the thickness of insulation and the area of exposed tips can be controlled with ALD and FIB. Due to its design flexibility, the nanoelectrode described here can be customized to targeted neurons. For example, a nanoelectrode with more or less spacing between nanowires can be used for larger or smaller target cells, respectively. Additionally, because the nanoelectrodes are built on supporting microwire structures that have been used extensively to record chronic activity from intact neural tissues and mammalian brains, these nanoelectrodes are potentially well suited for use in neural tissues in vivo.
Future perspective
Nanoelectrodes are a new technology and must be further developed and tested before being recommended for widespread use. Nevertheless, nanoelectrodes have the potential to supplement or replace intracellular recording microelectrodes. Their customizability, small tip size, and ability to be functionalized and grown on nontraditional substrates (e.g., planar surfaces and complementary metal–oxide–semiconductor pads) are unique advantages. Furthermore, electrode arrays could be developed that combine nanoelectrodes and traditional microwire electrodes to enable simultaneous intracellular and extracellular recordings, especially over long durations. In addition, nanoelectrodes could be adapted for in vivo recordings by using blind intracellular recording techniques, such as locating cells by monitoring changes in impedance or other parameters [17]. Ultimately, growing nanowires on the ends of microwire electrodes may potentially allow for long-term, intracellular recordings in awake and behaving animals.
Executive summary.
Materials & methods
▪ Nanowires were grown using electron-beam-induced deposition on traditional microwire recording electrodes, like those frequently used in vivo to allow for penetration through complex neural tissue.
▪ The nanowires were insulated with atomic layer deposition to ensure thin, conformal and high quality insulation. The tips of the fully insulated nanowires were then exposed using a focused ion beam.
Results & discussion
▪ Intracellular recordings of neuronal action potentials were achieved using nanoelectrodes in rat brain slice preparations and intact leech ganglia.
▪ Nanoelectrodes can be used to record neural signals for a wide range of biomedical applications.
Conclusion
▪ Nanoelectrodes with tip diameters less than 100 nm can minimize membrane damage during penetration and can allow for longduration intracellular recordings.
Acknowledgements
We thank C Fretham at the University of Minnesota Characterization Facility (MN, USA) for his helpful advice and invaluable assistance with electron-beam-induced deposition.
This work was supported by a grant from the University of Minnesota Institute for Engineering in Medicine, a University of Minnesota Interdisciplinary Doctoral Fellowship to JE Ferguson, a NIH T32-EB008389 training grant to JE Ferguson, and a National Science Foundation IOS-0924155 grant to KA Mesce. Parts of this work were carried out in the University of Minnesota Nanofabrication Center and the University of Minnesota Characterization Facility (MN, USA), which receive partial support from the National Science Foundation through the National Nanotechnology Infrastructure Network program.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research The authors state that they have obtained appropriate insti tutional review board approval or have followed the princi ples outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investi gations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
n of interest
nn of considerable interest
- 1.Purves RD. Microelectrode methods for intracellular recording and ionophoresis. Academic Press; London: 1981. [Google Scholar]
- 2.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, Ng JK, Kunitake ME, Conklin BR, Yang P. Interfacing silicon nanowires with mammalian cells. J. Am. Chem. Soc. 2007;129(23):7228–7229. doi: 10.1021/ja071456k. ▪▪ Report showing that cells cultured on vertically aligned nanowire arrays survive longer when penetrated with smaller diameter nanowires.
- 4.Tian B, Cohen-Karni T, Qing Q, Duan X, Xie P, Lieber CM. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science. 2010;329(5993):830–834. doi: 10.1126/science.1192033. ▪ 3D, kinked silicon nanowire probes used for intracellular recording of cultured embryonic chicken cardiomyocytes.
- 5.Schrlau MG, Dun NJ, Bau HH. Cell electrophysiology with carbon nanopipettes. ACS Nano. 2009;3(3):563–568. doi: 10.1021/nn800851d. ▪ Carbon nanopipettes used for intracellular recording of the mouse hippocampal cell line HT-22.
- 6.Sun P, Laforge FO, Abeyweera TP, Rotenberg SA, Carpino J, Mirkin MV. Nanoelectro chemistry of mammalian cells. Proc. Natl Acad. Sci. USA. 2008;105(2):443–448. doi: 10.1073/pnas.0711075105. ▪ Highly polished glass nanopipettes used for electrochemical measurements of cultured human breast cells.
- 7.Hanein Y, Böhringer K, Wyeth R, Willows A. Towards MEMS probes for intracellular recording. Sensors Update. 2002;10:47–75. ▪ Micro-machined silicon needles used for intracellular recording from an isolated brain of a sea slug (Tritonia diomedea).
- 8.de Asis ED, Leung J, Wood S, Nguyen CV. High spatial resolution single multiwalled carbon nanotube electrode for stimulation, recording, and whole cell voltage clamping of electrically active cells. Appl. Phys. Lett. 2009;95(15):153701. ▪▪ Single multiwalled carbon nanotubes mechanically attached to nickel-coated support structures used for intracellular recording of an excised frog sartorius muscle.
- 9.McNaughton BL, O’Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J. Neurosci. Methods. 1983;8(4):391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- 10.Koops HWP, Kretz J, Rudolph M, Weber M, Dahm G, Lee KL. Characterization and application of materials grown by electron-beam-induced deposition. Jpn. J. Appl. Phys. 1994;33:7099–7107. [Google Scholar]
- 11.Sherman-Gold R. The axon guide. Axon Instruments, Inc.; CA, USA: 1993. [Google Scholar]
- 12.Stigen T, Danzl P, Moehlis J, Netoff T. Controlling spike timing and synchrony in oscillatory neurons. J. Neurophysiol. 2011;105(5):2074–2082. doi: 10.1152/jn.00898.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puhl JG, Mesce KA. Dopamine activates the motor pattern for crawling in the medicinal leech. J. Neurosci. 2008;28(16):4192–4200. doi: 10.1523/JNEUROSCI.0136-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angstadt JD, Friesen WO. Synchronized oscillatory activity in leech neurons induced by calcium channel blockers. J. Neurophysiol. 1991;66(6):1858–1873. doi: 10.1152/jn.1991.66.6.1858. [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara S, Morita H. Electronic transmission between two nerve cells in leech ganglion. J. Neurophysiol. 1962;25:721–731. doi: 10.1152/jn.1962.25.6.721. [DOI] [PubMed] [Google Scholar]
- 16.Botman A, Mulders JJL, Hagen CW. Creating pure nanostructures from electron-beam-induced deposition using purification techniques: a technology perspective. Nanotechnology. 2009;20(37):372001. doi: 10.1088/0957-4484/20/37/372001. ▪▪ Controlling the material composition of nanostructures using electron-beam-induced deposition.
- 17.Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflügers Arch. 2002;444(4):491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]