Abstract
Decades of research have shown that childhood experiences interact with our genetics to change the structure and function of the brain. Within the range of normal experiences, this system enables the brain to be modified during development to adapt to various environments and cultures. Experiences with and attachment to the caregiver appear particularly important, and recent research suggests this may be due, in part, to the attachment circuitry within the brain. Children have brain circuitry to ensure attachment to their caregivers. Attachment depends on the offspring learning about the caregiver in a process that begins prenatally and continues through most of early life. This attachment serves two basic functions. First, attachment ensures the infant remain in the proximity of the caregiver to procure resources for survival and protection. Second, attachment “quality programs” the brain. This programming impacts immediate behaviors, as well as behaviors that emerge later in development. Animal research has uncovered segments of the attachment circuitry within the brain and has highlighted rapid, robust learning to support this attachment. A child attaches to the caregiver regardless of the quality of care received, even if the caregiver is abusive and neglectful. While a neural system that ensures attachment regardless of the quality of care has immediate benefits, this attachment comes with a high cost. Traumatic experiences interact with genetics to change the structure and function of the brain, compromising emotional and cognitive development and initiating a pathway to pathology. Neurobiological research on animals suggests that trauma during attachment is processed differently by the brain, with maternal presence dramatically attenuating the fear center of the brain (amygdala). Thus, the immaturity of the brain combined with the unique processing of trauma may underlie the enduring effects of abuse, which remain largely hidden in early life but emerge as mental health issues in periadolescence.
Introduction
We have known for decades that childhood experiences interact with genetics to change the structure of the brain and cause behavioral change.1 These early life experiences can dramatically alter the number of specialized communication cells within the brain (neurons), and these experiences can then increase or decrease the complexity of the neurons (dendritic branches) and the number of communication sites between them (synapses). The effects of this experience-based sculpting of the brain have profound effects on how the brain functions. In particular, they can determine how emotional centers of the brain communicate with the cortex and its higher functioning to determine our personality, our choices, and how we approach the world. This flexible, experience-based tuning of the brain’s development enables many parenting styles and relationships to produce children who grow into productive, law-abiding citizens that contribute to society. Aberrant experiences, including abuse and neglect from the caregiver, however, can hijack this experience-based system, leading to emotional and cognitive deficits and a view of the world as a dangerous place. These early life traumas go beyond the normal programming of the brain and initiate a pathway to pathology, which can often have a delayed expression until the child approaches periadolescence. Since early life abuse can be associated with brain damage from prenatal and postnatal (that is, via lactation) drug and alcohol abuse,2 the effects of child abuse can be comorbid with additional difficulties. Decades ago, we attributed these deficits to psychological problems as though there was no physical manifestation of the problems, but we now know better—the structure and functioning of the brain contribute to these behavioral traits. This Article reviews the child abuse and neglect neuroscience literature presented within the framework of attachment, because most abuse is from the caregiver. Attachment has two basic functions: (1) Attachment ensures the child remain in proximity of the caregiver, and (2) attachment programs the lifelong structure and function of the brain. Importantly, within this framework the effects of early life abuse can be expressed differently at different ages, with short- and long-term effects showing distinct patterns and the most dramatic effects delayed until later life.3
I. Brain Development Basics
First, it is important to understand some basics about brain development.4 Importantly, the original view of brain development as subject to tight genetic control has been abandoned. Brain development is a constantly changing interaction between genes and the environment, with postnatal experiences altering the structure of the brain. The brain begins as a neural tube, where cells are born (neurogenesis), travel to the proper place in the brain (migration), and sprout branches (axons for input and dendrites for output) that enable proximity to other neurons and build pathways and circuits throughout the brain. Then, finally, the neuron forms the chemical-electrical connections between cells (synapses) to relay information. Some of these processes occur prenatally (particularly neurogenesis and migration), although the later steps continue at high levels during the first two to three years of life as the brain exponentially increases in size. This process continues through adolescence. The scope of this postnatal maturation is enormous, with tens of thousands of new synapses being formed daily during the early years. Much of this growth is dependent upon predetermined events programmed by genetics, but normal experience fine-tunes this process (experience-dependent plasticity). Experience can determine the selective survival of neurons, the relative complexity of the axonal and dendritic branching, and the number of synapses that exist between cells.
Much of this experience-dependent control of brain development relies upon the experiences either increasing or decreasing the neural activity of a cell. For example, unused neurons (neurons with little neural activity) will die, while used neurons will survive. This is a normal process that occurs in the developing brain—too many cells are born and are then pruned. While new neurons are born in the brain throughout life, the enormity of early life growth is never replicated in later life. The implications of this process for custodial decisions in very early life are enormous—early life deprivation fails to activate neurons, which means that a greater number of neurons will die. Equally important, neurons that would typically die under “normal” conditions could be retained under deprivation or conditions of abuse. In either situation, brain function for the typical social environment in our Western culture might be compromised. For example, Romanian orphans reared in extreme physical and social isolation have smaller brains, and adopted orphans from Romania and China have a larger amygdala than their non-adopted counterparts.5 The amygdala is a brain area concerned with emotion and fear, and a larger amygdala would suggest altered emotion and fear processing.
Next, more refined control of brain development is accomplished by changing the activity of specific connections between neurons. Activity patterns between neurons can cause some neurons to grow more dendritic branches and synapses but prune others, and so particular types of information processing are enhanced. Importantly, a specific level of neural activity is needed because both too much and too little activity has been shown to be suboptimal. Equally important, the optimal types and intensities of experiences will vary at each stage of development. For example, while rough and tumble play or watching a video might be appropriate sensory stimulation for a four-year-old child, they are likely inappropriate for an infant or a toddler. A more appropriate pattern and intensity of sensory stimulation for a one-year-old would be socially interacting with a nurturing and interesting caregiver. The implications of experience instructing fine-tuning of brain development are critical for custodial issues. If early life experience does not activate the attachment system, it is likely that the development of future attachment formation will be compromised. This seems to have occurred in some orphans adopted from China and Romania. Or, if early life attachment coexists with fear, then the activity of these systems could be overly coordinated.6 Of course, exploring these issues in the human brain is extremely difficult, but animal research in both rodents and primates certainly supports this view, as discussed below.
Importantly, we also know that no brain area functions in isolation and that brain changes induced by early life experiences are ubiquitous throughout the brain. Thus, information about brain development for a given brain area needs to be interpreted within the context of other neural changes because brain activity is a coordinated process of functional connectivity between areas. Moreover, the contribution of learning and interventions, which can dramatically alter brain activity, needs to be considered as we relate neuroscience to behavior and policy.7
II. The Child’s Brain Is Not an Immature Version of the Adult Brain
In the traditional view of development, the child’s brain was simply an immature version of an adult’s. As the brain matured, the child became more “adult-like” by slowly adding more skills. While this is true in some cases, a view more consistent with general brain development is that the brain functions differently at each stage of development to ensure appropriate behaviors for survival at each of those developmental stages. A simple example of this is eating, where ingestion gradually transitions from infant sucking to independent “adult-like” feeding. These two feeding systems use different brain circuits that control different muscles to control food intake. They have distinct sensory feedback to signal when eating should stop. Importantly, at some point in development, these systems co-exist, and each system can emerge in its proper context.
While developmental transitions can be less obvious in some behaviors, this basic concept holds true for many others—for example, social behavior. Social behavior must transition from the stage of the infant-caregiver social relationship, to the toddler stage that expands social relationships to include other adults and children, to adolescence where the focus is on peers, and finally to the complexities of adult social behavior, reproduction, and child care. As these behaviors change and adapt to the developmental stage, so does the brain because it is the brain that produces these behavioral transitions. When considering child abuse, it is important to place the child’s social behavior within the context of the stage of social behavior and the critical consideration of attachment to the caregiver. Additionally and as discussed in the following Parts of this Article, in early life it is the social interactions with the caregiver and the caregiver’s stimulation of the child’s sensory receptors in the eyes, ears, tongue, nose, and skin that provide the experience-based programming of the brain. In other words, the only possible route for experience to enter the brain is by sensory stimulation. The caregiver is the primary source of this stimulation and is the gateway to other sensory stimulations via access to toys and an interesting and intensity-appropriate environment. The enduring effects of this programming can sometimes lie dormant until a later developmental stage when certain behavioral brain circuits mature or change function.8
III. Why Love and Nurturing Are So Important in Early Life
Even with proper nutrition and perfunctory care, if an infant does not receive affectionate social interaction, her physical development will be stunted and her brain development compromised. The important role of sensory stimulation for brain development—discussed in Part II—is thought to be an important mechanism in an infant’s development. Even moments after birth, the child needs an attachment figure, and the social behavior of the newborn is designed to induce the parent into providing the resources required for her growth.9 In other words, the infant is typically quite effective at convincing her caregivers to relinquish their personal resources of time and money. Of course, the infant’s needed resources include food and protection, but there are other important needs to be filled. The caregiver must also control the infant’s physiological functions, such as temperature regulation. Specifically, the caregiver regulates the child’s temperature by dressing her in an appropriate manner as well as through physical contact and heat exchange. In fact, the caregiver controls many of the infant’s physiological systems and this appears to be a major mechanism for the caregiver to program the infant. This is a critical concept, because it further indicates that sensory stimulation is important and further explains why there is an certain level of sensory stimulation required for optimal development.
Myron Hofer calls the mother’s sensory stimulation of the infant a “hidden regulator” of the infant’s physiology and behavior.10 For example, touch regulates the infant’s levels of growth hormone, and the caregiver’s presence reduces the infant’s levels of stress hormone during stressful events. The regulatory function of the caregiver occurs fairly naturally once an attachment is formed and the caregiver has had the opportunity or has taken the time to learn how to parent (parenting is not an innate skill). This provides stimulation of the appropriate sensory system at the appropriate intensity and patterning to promote healthy development. There is no special receptor for love, a feeling of safety, or any other emotion to enter the child’s brain. The only way information about attachment quality can be transduced to enter the brain is through our five senses. This pattern of sensory stimulation is how experience enters the brain and changes its development via changing chemicals and individual neural activity. Healthy attachment naturally provides the developing brain with the appropriate sensory stimulation and neural activity. As is suggested by the wide range of child rearing approaches in different cultures, a wide range of types and patterning of sensory stimuli can produce a healthy child that matures into a healthy adult.
IV. Specialized Infant Social Behavior: Attachment to the Caregiver
People of all ages, including infants, have a need to belong. Infants’ brains are wired to form attachments to their caregivers and also to form behavioral systems that engage the complementary prewired attachment systems in caregivers—as has been suggested in Bowlby’s Attachment Theory.11 The infant is a social being at birth, and the process of building this social infant begins during the last trimester of pregnancy when the infant’s sensory systems are first functional.12 Sounds from the mother’s voice travel through her bones and tissue and then through the amniotic fluid to stimulate the infant’s auditory system. The mother’s individual olfactory signature and the food she eats also enter the amniotic fluid, wash over the receptors of the chemical senses of taste and smell, and are repeatedly swallowed by the baby. This sensory stimulation provides a unique programming of the infant’s senses in two ways: It produces an experience-dependent connection to the brain that will influence lifelong sensory processing, and it enables the infant to learn about the mother’s characteristics while still in utero. Consequently, at birth the mother’s voice and odor are already learned by the baby. She has the power to soothe her baby and smooth the transition to life outside the womb. After birth, the infant begins to elicit caregiving by responding and orienting to the human face and the voice and scents of the caregiver.
The infant quickly learns about other caregivers. The infant’s behaviors in response to sensory stimuli from caregivers have enormous power to elicit additional caregiving in adults and produce strong emotions, particularly in caregivers prepared to accept a baby and to parent. This begins a finely tuned dance of social behaviors that support bidirectional learning of attachment and bonding in caregivers and babies. The maternal odor is also important for babies’ location of the nipple, and a newborn baby will crawl on the mother’s abdomen to reach the nipple. This odor also produces mouthing which, when combined with the tactile stimulation of the nipple, induces suckling. In fact, maternal odor produces a sequence of behaviors to ensure nipple attachment: First, the odor quiets the crying infant. Next, the infant orients to the odor source (that is, turns her head toward her mother). She then begins mouthing to facilitate nipple placement within the mouth, and nursing begins.
It should be noted that parenting is learned. Parenting combines with the transmission of parenting skills across generations to determine one’s parental abilities and responses to the baby’s behavior. In other words, strong social behaviors in young infants need to be responded to by a sensitive caregiver. Research indicates adults, including abusive parents, can be taught to be sensitive caregivers. The resilient infant is very responsive to improvements in parenting skills.
Earlier research seemed to indicate that the infant’s attachment to the caregiver needed to occur soon after birth, but we now realize that human infants show great resilience, and the effects of brief postnatal separation from their mothers are quickly overcome. More prolonged separations of weeks or a few months may require some intervention to encourage attachment for both partners in the dyad. The success of adoptions and the wide spectrum of individuals that can become excellent attachment figures provide the clearest support of a broad attachment system, especially under normal conditions and an infant with a history of experience with an attachment figure. Inexperience with attachment compromises the functioning of the attachment system. That is, the ability of a child to form strong attachments as the child matures is strongly based on the foundation of a strong early life attachment. Early life deprivation and severe disrupted attachment produce aberrant attachment styles and can hinder the formation of new attachment for the rest of the child’s life.13
V. Sensitive Period for Attachment
With maturation, the infant’s attachment changes as the infant learns about the caregiver and the caregiver’s special role. In the developmental psychology literature, the nine-month-old to one-year-old range has been highlighted as a special time when the infant finally forms a psychological representation of the caregiver and the protracted process of attachment formation is completed. Indeed, while it is impossible to experimentally determine the existence of a mental representation in the infant, at this age, the infants’ behaviors to the caregiver do change. Specifically and most importantly, there appears to be an emergence of exclusivity about who can function as an attachment figure, with infants expressing protest at separation and depression with extended separation.
This new expression of attachment has led some to conclude that the attachment prior to this protest is weaker and remains more plastic—with new attachment figures easily learned and former attachment easily dismissed. This, however, is debatable. A younger child removed from her parent reacts strongly to the separation, although the presence of another attachment figure can alleviate the signs of this. A nurturing stranger can also comfort the child but does not have the control over the child’s physiological functions that is characteristic of the caregiver.
An alternative view of attachment—which is more prevalent in the neurobiology perspective and accepted by some developmental psychologists—is that attachment occurs throughout the child’s life and simply changes in its expression as the ecological demands of life change. This view sees attachment present in the young infant, but the attachment changes due to a new developmental period. For example, a child approaching one year of age has greater locomotion as she crawls, cruises, and transitions to walking. Thus, stronger proximity maintenance may be required. This is also the age when the child becomes more interested in the surrounding world and spends more time watching and imitating activities. At this age, the developing fear system and stranger anxiety also emerge. This developmental difference might reflect a change in attachment expression, not necessarily a change in attachment strength or quality. As discussed in Part IX, it is important to note that some effects of attachment are observable immediately, while others lie dormant and are expressed later in life. The absence of an observable effect in early life might not indicate earlier attachment bonds are less important or strong. Indeed, in animal research sensory stimulation has its major impact on brain development for variation in typical maternal care and produces enduring effects with respect to emotional and cognitive development.14 Especially after the first year of life, once a child forms this strong, biologically determined bond with a caregiver, that bond is difficult to break regardless of the quality of that attachment—that is, even if the caregiver is an abuser. The difficulty in breaking the bond with the caregiver is seen in many other species, including nonhuman primates and other mammals.
VI. Neurobiology of Attachment to Nurturing and Abusive Caregivers
Our understanding of attachment and Bowlby’s Attachment Theory15 is strongly influenced by nonhuman animal research. For this reason, it is not surprising that our concept of attachment is readily applicable to other species. Specifically, many altricial species show attachment and need to learn to identify and remember the attachment figure. There is a specialized biological system that supports infant attachment. After all, the brain is the organ of behavior, and every behavior must have the brain circuitry to permit its expression. For many reasons, we cannot assess this circuitry in humans. Not only are there limitations due to limited brain scanning techniques, but the ethical limits inherent in human research also constrain our ability to assess causation and mechanisms. Due to the use of a multitude of invasive techniques for animal research, however, identifying causation and mechanism is possible. More importantly, animal research permits the use of a very specific question and manipulation of independent variables. For example, we can manipulate attachment quality while measuring and actually controlling the activity of a very specific brain area and function. This type of manipulation has provided great insight into the characteristics and circuitry of attachment, especially abuse related attachment.
Research on infant nonhuman animals provides some clarity for our understanding of an infant’s biological need for proximity seeking of the caregiver. Of course, human attachment is more complex than what is seen in animal models. Still, studying the brain of infant animals can provide insight into basic neural circuits for attachment. However, translational work and applying animal models to humans must first consider the differences between species. For example, while humans rely on vision, audition, and olfaction for attachment, rat pups (the principle example discussed below) only rely on olfaction because they cannot see or hear for the first couple of weeks of life. Importantly, cognition in human attachment is more complex than that seen in rat pups: Children have the cognitive ability to rationalize the abuse they receive, which is unlikely to occur in rat pups because a rat pup’s cortex never reaches the complexity of a child’s. The basic circuitry for attachment in pups, however, is evolutionarily old and parallels many of the characteristics of human attachment.
Similar to human infants, rat pups must learn about their caregivers and attachment. The pups are born without sight or hearing and rely on their senses of smell to interact with their mothers. Also similar to humans, the rat pup has prenatal exposure to the mother’s odor and learns very quickly after birth about new odors. The maternal odor induces pups to approach the mother and permits nipple attachment. In fact, the maternal odors of rats and humans produce a strikingly similar sequence of behaviors: Presenting the odor to pups stops vocalizations, suggesting the odor decreases distress. The pup then approaches the source of the odor (the mother). When contact is made, the pup begins the sequence of behaviors to attach to the nipple. Without the maternal odor, pups do not survive. This is quite similar to the sequence of behaviors, described above, of human infants in response to maternal odors. Of course, human infants that do not have a sense of smell can rely on other sensory systems to interact with their mothers.
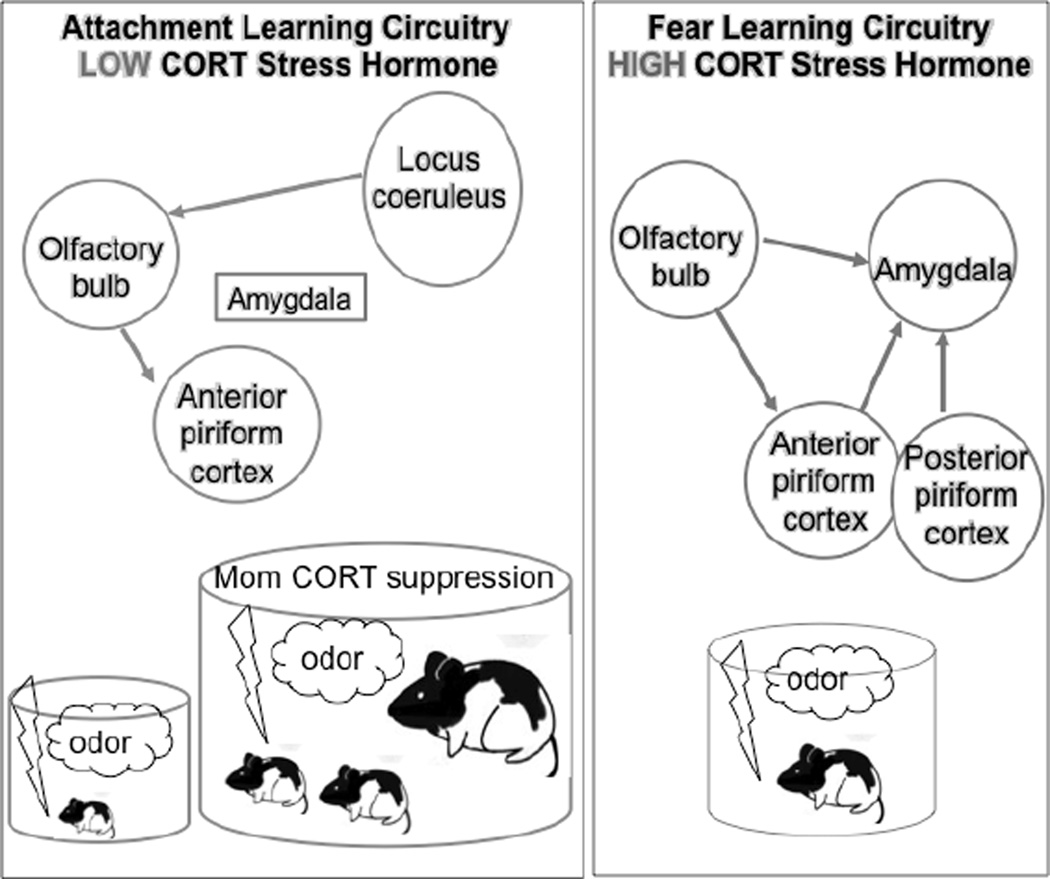
The abuse-related attachment circuitry has been characterized in rat pups. The pups’ attachment system is illustrated in Figure 1. The attachment circuit requires high levels of norepinephrine (“NE”) from the locus coeruleus (“LC”) to produce a new maternal odor through plasticity in the olfactory bulb. Pups will learn an attachment even when pain is experienced, provided corticosterone (“CORT”) levels are low. This occurs at two developmental points: (1) during the sensitive period for attachment when CORT levels are naturally low and (2) in older pups, provided the mother is present to block the CORT elevation typically induced by pain. Blocking CORT prevents amygdala plasticity in pups, a characteristic important for fear learning. On the right, the more adult-like learning system is present where pups readily learn fear via activation of the amygdala by CORT.
Figure 1.
There are some unique features of this attachment circuit. One of them is the myriad of rewards that support this learning, including responses to both nurturing and painful stimuli (Figure 1). This is markedly different from adult learning, where rewards that support approach learning generally involve positive rewards and pleasant stimuli. They also include items such as food, warmth, and other stimuli that address a human need. In the rat pup, however, any sensory stimulation that increases the neurotransmitter NE will function as a reward to activate the brain’s unique attachment circuitry. For example, receiving milk from the mother (normal care) will increase the pups’ NE. Additionally, the mother’s licking her pups (normal care) or stepping on her pups (abusive care) also produces a similar increase in NE. Thus, many types of sensory stimuli readily support attachment learning—even unpleasant stimuli that arise from abuse or neglect. Again, the infant brain is not an immature version of the adult brain and is suited for the needs of the infant—that is, attachment to the caregiver to receive support for survival.
Pups’ brains are wired to produce the rapid and robust release of NE, due to pups’ inability to turn off the brain area that releases NE once it has been turned on by maternal stimulation or experimental manipulation. Human infants also have very high levels of NE during the first year of life and thus NE is correlated with the early life attachment system. Notably, the use of animal research and our ability to manipulate neurotransmitters in very small brain areas have shown that the infant’s abundant release of NE is the causal mechanism that supports attachment.16 Specifically, the ability to learn a maternal odor produces learning-associated changes in the mitral cells of the olfactory bulb,17 and this plasticity requires the abundant release of NE into the olfactory bulb. This abundant NE release is induced by the stimuli that support attachment learning—that is, mother licking or stepping on a pup—we can experimentally prevent the LC (the brain area that is the source of NE) from releasing NE to prevent attachment learning. Thus, pups’ enhanced levels of NE induced by maternal stimuli, which occur during the presentation of a novel odor, are necessary and cause (rather than just correlate with) the plasticity within the olfactory bulb that produces pups’ learning of a new maternal odor.
One of the main reasons the sensitive period for attachment learning ends is because the LC no longer releases sufficient NE to support attachment learning in older pups. This is an example of a brain area changing how it functions during development to support the survival demands of an ecological niche. The sensitive period for NE-dependent attachment learning is supportable because the LC has unique features in infancy that will support rapid learning. A similar role for NE in infant attachment has been found in numerous species, suggesting a phylogenetically conserved system for attachment. Recent work has also suggested that similar attachment circuitry can become active again for mate bonding and for a mother’s learning about her infant in species such as mice and sheep. Oxytocin is also important for attachment throughout the life span, as seen in work in voles, although this neurohormone has received little attention in infant attachment to the caregiver in other animal models.
VII. Fear Learning Is Blocked in Early Life to Support Attachment
There is at least one very perplexing characteristic of attachment in children: Why do children attach to abusive caregivers? Animal research provides clues to the answer to this question. Pain-related attachment is not unique to humans and has been observed in numerous species, including avian species and a myriad of mammalian species. Indeed, Bowlby, the father of Attachment Theory,18 built his model on the combined assessment of clinical work and animal research. First, the newly documented imprinting in birds suggested that attachment to the caregiver is innate or biologically determined. At hatching, chicks quickly learn to “attach” to or “imprint” on the first moving object they see, typically the caregiver, although a human or other animate object can be substituted. This imprinting occurs even when approach to the caregiver is associated with pain. Similar abusive attachment has been demonstrated in nonhuman primate colonies as well as other mammals such as infant dogs and rat pups. For example, shocking chicks during imprinting to the mother supports approach learning, while shock supports avoidance learning just hours after the imprinting sensitive period closes. Similarly, shocking or mistreating an infant dog while interacting with a caregiver still results in a strong attachment to the caregiver. This paradoxical attachment learning has also been demonstrated in nonhuman primates, including Harlow’s monkeys and more recently in other primate colonies, when abused infant monkeys form strong attachments to an abusive caregiver.19 Furthermore, children tolerate considerable abuse while remaining strongly attached to an abusive caretaker. It appears that selection pressure and evolution have produced an attachment system that ensures the infant attaches to the caregiver regardless of the quality of caregiving received.
Next, we describe the infant’s unique brain processing of pain, and the mother’s ability to further change the brain’s response to pain (Figure 1). Rat pups reared by an abusive mother still form an attachment to that mother. Developmental research has carefully manipulated this abuse-related attachment learning away from the mother to explore why pain supports attachment and to understand why pups do not learn to avoid the abusive mother. That is, why and how is learning in the fear system suppressed? Our results showed that the amygdala, which is a brain area required for fear and avoidance learning in adult animals, does not participate in the infant odor-pain learning and prevents the infants from learning fear.20 Thus, while painful and presumably pleasant stimuli both activate the attachment circuitry, pups’ attachment learning with pain also requires suppression of the amygdala’s plasticity, which is normally activated by pain and required for learning fear. It should be noted that electrophysiological studies indicate the pain information does reach the amygdala, yet the amygdala fails to exhibit the plasticity required for fear learning. This activation without learning appears to be critical in programming the amygdala for later life. Research suggests the combined activation of the attachment circuitry and suppression of the fear circuitry might produce a particular vulnerability to later mental health difficulties.21 In rat pups, this infant experience results in later depressive-like behavior and an altered amygdala with suboptimal connectivity connectivity to the prefrontal cortex, a brain area concerned with higher order brain functions. Remarkably, simply experiencing the pain without attachment did not result in changes in the amygdala—indicating that pain within attachment is processed differently than pain without attachment. While the mechanism for this is unclear, we do know that pain with attachment and without attachment produce quantitatively different responses in the amygdala, as measured through gene expression, neurotransmitter release, and neural activity.22
In relation to attachment and custody, attachment learning has unique features that produce a rapid learning regardless of whether the caregiver provides support or pain. This circuitry has been identified in myriad nonhuman animals. Additionally, at least on the behavioral level, this circuitry appears to also exist in humans. It is beyond current technology to unquestionably determine whether this system exists in humans. We cannot ethically or technically place children in brain scanners and make them learn and unlearn an attachment figure. These animal research results enable us to view attachment from a biological approach and to go beyond explaining abuse attachment from a strictly cognitive perspective.
VIII. Stress Hormones and Caregivers’ Control of the Off-Switch for Fear
Short exposures to stress can be beneficial because stress provides our bodies with a rapid method of preparing for an emergency.23 However, more prolonged stress has been documented to negatively impact people and other animals, and its damaging effects are more robust in early life. It is thought that the chronic stress of chaotic homes, divorce, abuse, and other stressors produce prolonged stress responses that are particularly damaging to children. One mechanism that can reduce stress hormone release is social buffering, whereby an attachment figure (or, at later stages of development, a trusted partner) can greatly attenuate the release of stress hormones. Indeed, the attachment figure is a strong social buffering stimulus in children, although this system appears compromised in some abused children. Social buffering can protect a child from the damaging effects of stress. The role of the attachment figure as a regulator of the child’s stress response for social buffering is related to the role of the mother as a “hidden regulator” of physiological functions discussed in Part II.24
Social buffering occurs in many species, including rats. We explored the mother’s ability to socially buffer her pups and assessed how this impacted brain responses to trauma. Surprisingly, we found that the level of the stress hormone CORT can turn on and off the amygdala, the brain area responsible for fear and avoidance learning. In rat pups, the amygdala is always turned off in early life because the stress hormone CORT (cortisol in humans) is present at low levels. However, as pups mature and reach the appropriate age to begin short visits to the world outside the nest, the levels of this hormone are increased if they experience pain or a fear stimulus. Thus, the amygdala is turned on and pups can respond with fear and learn fear and avoidance. Even more amazingly, the mother can control her pups’ levels of this stress hormone via social buffering and thus control their fear. We have recently identified how the mother can control pups’ CORT level: She blocks the release of NE into the paraventricular nucleus of the hypothalamus, the brain areas used to initiate the stress response. If the mother blocks CORT, the amygdala cannot support the plasticity required for learning fear. If the pup is alone outside the nest, it can still learn to avoid dangerous events and learn about the world in preparation for independence. In other words, the simple presence of the mother functions as a biochemical switch to determine whether pups will learn to avoid or prefer odors paired with pain.
IX. Enduring and Sometimes Delayed Effects of Early Life Trauma
The enduring effects of traumatic early life experiences for brain and behavioral development have been demonstrated in clinical studies since the 1950s. These studies showed strong emotional and physical stunting of orphaned and hospitalized infants who had been separated from their mothers. Animal researchers soon mimicked these deprived early experiences in animal models using the maternal separation paradigm (prolonged separation of the infant from the mother) in rodents and primates. This work quickly showed a causal link between early life adversities, elevation in CORT levels, and later, disrupted emotional and cognitive behavior that mirrored the levels found in the orphans in later life. This work also provided insights on the manner in which childhood adversity is associated with later life psychiatric disorders and adverse brain development of the amygdala, hippocampus, prefrontal cortex, and cerebellum. The mechanisms that translate early life experiences are broad and range from learning, changes in neurotransmitters or anatomy, to genetics.25 The long-term effects of early life adversity appear to alter maternal care, which is then transmitted nongenomically to the next generations via learning, brain sculpting, and epigenetics.26
As we integrate more recent research into the maternal separation paradigm, a more refined understanding of the complexity of early life adversity emerges. For example, this research suggests that adversity and stress are detrimental to development and when experienced within the context of attachment they may yield a negative outcome. Specifically, the effects of abuse-related attachment produce the early onset of social behavior problems (pup reaching weanling age), and depressive-like behavior emerges as periadolescence approaches—although aberrant amygdala function appears to contribute to both behavioral deficits at both ages. Thus, the animal research mirrors a critical feature of early life trauma and abuse. Clinically, trauma effects are not always expressed in early life and can be delayed until later childhood, adolescence, or adulthood.27 Additionally, how the trauma is expressed can be quite distinct at each of these ages. While we have seen this effect clinically in children, research on rodents and nonhuman primates has more clearly shown the dynamic effects of early life trauma whose mechanisms we are beginning to understand.
Thus there is now unambiguous evidence from both human and nonhuman animal research that neglect and abuse are detrimental to brain development, although genetics may provide some resilience.28 While we are still not sure how these early life experiences change the brain, animal research suggests that the early life brain processes trauma differently than the adult brain. It also suggests that attachment and the caregiver further change the processing of trauma. Additionally, the specific enduring effects of trauma are dependent on the age and type of trauma received. These unique infant responses are likely to be protective of the brain in very small doses but are detrimental to development in larger doses. Thus far, we have identified ways to help the abused child which rely on heightened caregiving, most notably from the attachment figure, and therapy involving social behaviors that are most effective when involving both the caregiver and offspring. It is our goal to determine brain mechanisms to streamline these interventions in an age-specific manner.
Conclusion
The number of children who experience neglect or abuse is high—about ten out of every thousand children in the United States in 2008.29 Identifying and helping these children is especially difficult unless there are bruises or physical injuries. The effects of early life attachment can lie dormant in the brain until later life. The impact of these hidden effects is that, by adolescence, eighty percent of abused children will be diagnosed with a major psychiatric disorder. Imaging studies of abuse survivors often show that brain areas controlling emotion and cognition are abnormal and underlie these psychiatric disorders and difficulties functioning as a productive citizen. Animal research has provided great insight into how early life caregiving can impact these brain areas and has highlighted unexpected functioning of the brain in early life and the enormous role of the caregiver in controlling the brain’s response to trauma. The comparison of normal attachment formation and pain-related attachment suggests similar behaviors in early life are expressed as attachment to the caregiver, and the activation of different neural substrates may lay the foundation for the enduring effects of early life trauma.
Acknowledgements
Thank you to NIH (NIMH MH091451, NIDCD DC009910) for support.
References
- 1.McEwen Bruce S. Early Life Influences on Life-Long Patterns of Behavior and Health, 9. Mental Retardation & Developmental Disabilities Research Revs. 2003;149:149. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]; Children’s Bureau. Statistics & Research. U.S. Dep’t of Health & Human Servs. http://www.acf.hhs.gov/programs/cb/stats_research/index.htm (last visited Mar. 11, 2012).
- 2.Thompson Barbara L, et al. Prenatal Exposure to Drugs: Effects on Brain Development and Implications for Policy and Education, 10. Nature Revs. Neurosci. 2009;303:303–309. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See generally Toth Sheree L, Cicchetti Dante. Frontiers in Translational Research on Trauma, 23. Dev. & Psychopathology. 2011:353. doi: 10.1017/S0954579411000101. Gunnar Megan R, et al. Bringing Basic Research on Early Experience and Stress Neurobiology to Bear on Preventive Interventions for Neglected and Maltreated Children, 18. Dev. & Psychopathology. 2006:651. Teicher Martin H, et al. The Neurobiological Consequences of Early Stress and Childhood Maltreatment, 27. Neurosci. & Biobehav. Revs. 2003:33. doi: 10.1016/s0149-7634(03)00007-1.
- 4.The following discussion is derived from Stiles Joan, Jernigan Terry L. The Basics of Brain Development, 20. Neuropsychol. Rev. 2010:327. doi: 10.1007/s11065-010-9148-4.
- 5.Mehta Mitul A, et al. Amygdala, Hippocampal and Corpus Callosum Size Following Severe Early Institutional Deprivation: The English and Romanian Adoptees Study Pilot, 50. J. Child Psychol. & Psychiatry. 2009;943:945–948. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]; Tottenham Nim, Sheridan Margaret A. A Review of Adversity, the Amygdala and the Hippocampus: A Consideration of Developmental Timing. Frontiers Hum. Neurosci. 2010 Jan.:1–18. doi: 10.3389/neuro.09.068.2009. at 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilbarger Julia, et al. Sensory Processing in Internationally Adopted Post-Institutionalized Children, 51. J. Child Psychol. & Psychiatry. 2010;1105:1105–1114. doi: 10.1111/j.1469-7610.2010.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnar et al., supra note 3, and accompanying text.
- 8.Romeo Russell D, Tang Akaysha C, Sullivan Regina M. Hormones, Brain & Behavior 1975. 2009. Early Life Experiences: Enduring Behavioral, Neurological and Endocrinological Consequences. [Google Scholar]
- 9.Sullivan Regina M, et al. Infant Bonding and Attachment to the Caregiver: Insights from Basic and Clinical Science, 38. Clinics Perinatology. 2011;643:643–655. doi: 10.1016/j.clp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofer Myron A. The Roots of Human Behavior: An Introduction to the Psychobiology of Early Development. 1981. [Google Scholar]; Hofer Myron A. Hidden Regulators in Attachment, Separation, and Loss, 59. Monographs Soc’y for Research Child Dev. 1994;192:192–207. [PubMed] [Google Scholar]
- 11.Attachment theory suggests that mental health could be attributed to quality of early life care. This evolutionary based theory was influenced by animal behavior and ethology and presented attachment as instinctive. See generally Bowlby John. A Secure Base: Clinical Applications of Attachment Theory. 1988.
- 12.See Hofer supra note 10 and accompanying text.
- 13.See generally Bos Karen, et al. Psychiatric Outcomes in Young Children with a History of Institutionalization, 19. Harv. Rev. Psychiatry. 2011:15. doi: 10.3109/10673229.2011.549773. Kumsta Robert, et al. Deprivation-Specific Psychological Patterns, 75. Monographs Soc’y for Researcj Child Dev. 2010:48. doi: 10.1111/j.1540-5834.2010.00550.x. Ghera Melissa M, et al. The Effects of Foster Care Intervention on Socially Deprived Institutionalized Children’s Attention and Positive Affect: Results from the BEIP Study, 50. J. Child Psychol. & Psychiatry. 2009:246. doi: 10.1111/j.1469-7610.2008.01954.x. Dozier Mary, Bick Johanna. Changing Caregivers: Coping with Early Adversity, 36. Pediatric Annals. 2007:205. doi: 10.3928/0090-4481-20070401-09.
- 14.See generally Raineki Charlis, Moriceau Stephanie, Sullivan Regina M. Developing a Neurobehavioral Animal Model of Infant Attachment to an Abusive Caregiver, 67. Biological Psychiatry. 2010:1137. doi: 10.1016/j.biopsych.2009.12.019. Weaver Ian CG, et al. Epigenetic Programming by Maternal Behavior, 7. Nature Neurosci. 2004:847. doi: 10.1038/nn1276. Caspi Avshalom, et al. Role of Genotype in the Cycle of Violence in Maltreated Children, 297. Sci. 2002:851. doi: 10.1126/science.1072290.
- 15.See generally Bowlby John. Attachment and Loss. 1969.
- 16.Sullivan Regina M, Holman Parker J. Transitions in Sensitive Period Attachment Learning in Infancy: The Role of Corticosterone, 34. Neurosci. & Biobehav. Revs. 2010;835:835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Moriceau Stephanie, et al. Early-Life Stress Disrupts Attachment Learning: The Role of Amygdala Corticosterone, Locus Coeruleus Corticotropin Releasing Hormone, and Olfactory Bulb Norepinephrine, 29. J. Neurosci. 2009;15745:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouly Anne-Marie, Sullivan Regina M. Memory and Plasticity in the Olfactory System: From Infancy to Adulthood. In: Menini Anna., editor. The Neurobiology of Olfaction. 2009. pp. 367–394. [PubMed] [Google Scholar]
- 18.See generally Bowlby, supra note 15.
- 19.McCormack Kai, et al. Serotonin Transporter Gene Variation, Infant Abuse, and Responsiveness to Stress in Rhesus Macaque Mothers and Infants, 55. Hormones & Behav. 2009;538:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roma Peter G, et al. The Kids Are Alright: Maternal Behavioral Interaction and Stress Reactivity in Infants of Differentially Reared Rhesus Monkeys, 1. J. Dev. Processes. 2007;103:103–122. [Google Scholar]
- 20.Moriceau Stephanie, Sullivan Regina M. Maternal Presence Serves as a Switch Between Learning Fear and Attraction in Infancy, 9. Nature Neurosci. 2006;1004:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sullivan Regina M, et al. Good Memories of Bad Events in Infancy, 407. Nature. 2000;38:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.See Sullivan, Transitions, supra note 16 and accompanying text.
- 22.See Sullivan, Transitions, supra, note 16; Mouly, supra note 17; Mouriceau, supra note 20; see also Thompson Jason V, Sullivan Regina M, Wilson Donald A. Developmental Emergence of Fear Learning Corresponds with Changes in Amygdala Synaptic Plasticity, 1200. Brain Research. 2008;58:58–65. doi: 10.1016/j.brainres.2008.01.057. Shionoya Kiseko, et al. Maternal Attenuation of Hypothalamic Paraventricular Nucleus Norepinephrine Switches Avoidance Learning to Preference Learning in Preweanling Rat Pups, 52. Hormones & Behav. 2007;391:391–400. doi: 10.1016/j.yhbeh.2007.06.004.
- 23.See McEwen, supra note 1 and accompanying text.
- 24.See Hofer, supra note 10 and accompanying text.
- 25.Tang Akaysha C, et al. Maternal Modulation of Novelty Effects on Physical Development, 109. Proc. Nat’l Acad. Sci. 2012;2120:2120–2125. doi: 10.1073/pnas.1121056109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heim Christine, et al. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene, 41. Frontiers Behav. Neurosci. 2009;1:1–10. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.See Bowlby, supra note 15. See generally Roth Tania L, Sweatt J David. Annual Research Review: Epigenetic Mechanisms and Environmental Shaping of the Brain During Sensitive Periods of Development, 52. J. Child Psychol. & Psychiatry. 2011:398. doi: 10.1111/j.1469-7610.2010.02282.x. Weaver Ian CG, et al. Reversal of Maternal Programming of Stress Responses in Adult Offspring Through Methyl Supplementation: Altering Epigenetic Marking Later in Life, 25. J. Neurosci. 2005:11045. doi: 10.1523/JNEUROSCI.3652-05.2005.
- 27.Kaufman Joan. Child Abuse and Psychiatric Illness, 71. Biological Psychiatry. 2012;280:280–281. doi: 10.1016/j.biopsych.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dannlowski Udo, et al. Limbic Scars: Long-Term Consequences of Childhood Maltreatment Revealed By Functional and Structural Magnetic Resonance Imaging, 71. Biological Psychiatry. 2012;286:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]; Claessens Sanne EF, et al. Development of Individual Differences in Stress Responsiveness: An Overview of Factors Mediating the Outcome of Early Life Experiences, 214. Psychopharmacology. 2011;141:141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; McGowan Patrick O, et al. Epigenetic Regulation of the Glucocorticoid Receptor in Human Brain Associates with Childhood Abuse, 12. Nature Neurosci. 2009;342:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.See Thompson et al., supra note 2.