Abstract
Neuronal ceroid lipofuscinosis (NCL), first clinically described in 1826 and pathologically defined in the 1960s, refers to a group of disorders mostly diagnosed in the childhood years that involve the accumulation of lysosomal storage material with characteristic ultrastructure and prominent neurodegenerative features including vision loss, seizures, motor and cognitive function deterioration, and oftentimes, psychiatric disturbances. All NCL disorders evidence early morbidity, and treatment options are limited to symptomatic and palliative care. While distinct genetic forms of NCL have long been recognized, recent genetic advances are considerably widening the NCL genotypic and phenotypic spectrum, highlighting significant overlap with other neurodegenerative diseases. This review will discuss these recent advances and the expanded potential for increased awareness and new research that will ultimately lead to effective treatments for NCL and related disorders.
Keywords: neuronal ceroid lipofuscinosis, NCL, Batten disease, Kufs disease, Parkinson's disease, parkinsonism, frontotemporal lobar degeneration, FTLD, endosome, lysosome, lysosomal disease, subunit c, saposin, progranulin, DNAJC5, CSPα, CLN6, cathepsin F, KCTD7, ATP13A2
Introduction
The NCL disorders, sometimes referred to as Batten disease, are pan-ethnic neurodegenerative disorders that affect all age groups and are mostly inherited in an autosomal recessive fashion, although autosomal dominant forms are also recognized (for a detailed reference see [1]). While currently reliable worldwide incidence estimates are not available, country-specific estimates typically range from 1 to 2.5 in 100,000 live births, with enrichment in some geographic regions, such as within the Scandinavian Peninsula (Appendix 1 in [1]).
Infantile and childhood forms of NCL are the most common and are characterized by visual failure that progresses to blindness, behavioral problems, onset of seizures, loss of cognitive and motor function (developmental regression) and ultimately, premature death. The less common early-adult forms tend to present with early dementia, myoclonic seizures, movement abnormalities (ataxia, extrapyramidal), and sometimes, psychiatric disturbance, and lack visual involvement [1]. Onset and progression of the clinical manifestations of NCL can be very heterogeneous, even within families [2-4]. A hallmark of NCL is the histopathologic finding of lysosomal autofluorescent lipopigments in brain and other tissue types, which display a distinctive ultrastructural pattern by electron microscopy (EM) described as curvilinear (CV), rectilinear (RL), fingerprint (FP) or granular osmiophilic deposit (GROD) profiles, and predominantly contain the lipophilic proteins subunit c of the mitochondrial ATP synthase and/or the sphingolipid activator proteins, saposins A and D (SAPs) (Table 1) [1, 5].
Table 1.
Genotype-phenotype correlations of the NCL disorder.
| Disease | Prior designation | OMIM # | MI | Gene | Age of onset | Clinical phenotype | Early Symptoms and Signs | Usual survival | Pathology |
|---|---|---|---|---|---|---|---|---|---|
| CLN1 | INCL | 256730 | AR | PPT1 | 6 -24mos | classic infantile | developmental failure, seizures, myoclonus, visual failure | 2 - 6 yrs | GROD |
| 2 - 4yrs | late-infantile | dev regression, seizures, visual failure | 5 - 12+ yrs | ||||||
| 4 - 6yrs | juvenile | visual failure, seizures, behavioral, dementia | 10 -20yrs | ||||||
| CLN2 | vLINCL | 204500 | AR | TPP1 | 2 - 4yrs | classic late infantile, [juvenile] | malignant seizures, myoclonus, developmental & visual failure | 6 - 12+ yrs | CL |
| CLN3 | JNCL (Batten) | 204200 | AR | CLN3 | 3 - 8yrs | classic juvenile | visual failure, dementia, behavioral and motor difficulties, late seizures | 20 -40yrs | Vacuolated lymphocytes, FP, CL, RL |
| CLN4 | ANCL (Parry) | 162350 | AD | DNAJC5 | teenage - 30+ | juvenile to adult | dementia, seizures, myoclonus, without visual loss | 5 - 20yrs | GROD |
| CLN5 | vLINCL 1 | 256731 | AR | CLN5 | 3 - 7yrs | Variant late infantile, [juvenile, adult] | behavior abnl, cognitive decline, seizures, visual faliure, myoclonus, motor difficulties | teenage+ | CL, FP, RL |
| CLN6 | vLINCL 2 | 601780 | AR | CLN6 | 1.5 -8yrs | Variant late infantile | visual failure, cognitive decline, seizures, myoclonus | teenage+ | FP, CL |
| ANCL Kufs, type A | 204300 | 16 - 51 yrs | adult-onset | progressive myoclonic epilepsy, no retinal changes | FP, GROD | ||||
| CLN7 | vLINCL 3 | 610951 | AR | MFSD8 | 1.5 -6yrs | Variant late infantile, [juvenile, adult] | visual failure, dementia, seizures, myoclonus | teenage+ | RL, FP |
| CLN8 | vLINCL | 600143 | AR | CLN8 | 1.5 -7yrs | variant late infantile | myoclonic seizures, visual failure, ataxia, cognitive decline | teenage+ | GROD, CL [FP] |
| EPMR | 610003 | 5 -10yrs | Northern epilepsy | seizures, slow dementia without visual loss | 40+ | GROD, CL | |||
| CLN10 | CNCL | 610127 | AR | CTSD | neonatal | congenital, [late infantile, adult] | Epileptic encephalopathy, microcephaly | < 1 yr | GROD |
| CLN11 | 614706 | AR | GRN | early 20s | adult | visual failure, myoclonic seizures, ataxia, dementia | 30+ | FP | |
| FTLD-GRN | 607485 | AD | 35 -65yrs | adult | myoclonic seizures, ataxia, cognitive decline | 50+ | TDP-43 | ||
| CLN12 | Kufor-Rakeb syndrome | 606693 | AR | ATP13A2 | 8 - 12 yrs | juvenile | parkinsonism, ataxia, dementia | 30+ | FP, vacuolated lymphocytes, NBIA |
| CLN13 | Kufs, type B | AR | CTSF | 20 -30yrs | adult | movement abnl (ataxia, tremor) and dementia, rare seizures | FP [brain] | ||
| CLN14 | EPM3 | 611726 | AR | KCTD7 | <2 years | infantile | seizures, motor & speech regression, visual failure | teenage | FP. GROD |
Variant initially described in a Finnish population and referred to as the Finnish variant.
Formerly described as Lake-Cavanagh early-juvenile NCL variant and Indian-variant late-infantile NCL.
Formerly also called Turkish variant.
OMIM: Online Mendelian Inheritance in Man (www.omim.org)
MI: Mode of inheritance
AR: autosomal recessive
AD: autosomal dominant
INCL: infantile NCL
LINCL: late-infantile NCL; vLINCL: variant LINCL
ANCL: adult NCL
CNCL: congenital NCL
EPMR: epilepsy, progressive, with mental retardation
EPM3: epilepsy, progressive myoclonic 3, with or without intracellular inclusions; mos: months
yrs: years
dev: developmental
abnl: abnormal
GROD: granular osmiophilic deposit
CL: curvilinear
FP: fingerprint
RL: rectilinear
NBIA: Neurodegeneration with brain iron accumulation
Solving the molecular basis of the NCL disorders began in 1995 with the discovery in the same year of palmitoyl protein thioesterase 1 (PPT1), a soluble lysosomal enzyme encoded by the PPT1 gene (sometimes referred to as CLN1) [6], and the CLN3 gene, encoding a putative lysosomal membrane protein [7]. In the following years, mutations in 6 more genes were shown to cause NCL: TPP1 (sometimes referred to as CLN2) in 1997 [8], CLN5 in 1998 [9], CLN8 in 1999 [10], CLN6 in 2002 [11, 12], CLN10/CTSD, encoding cathepsin D, in 2006 [13] and CLN7/MSFD8 in 2007 [14] (Table 1). Nearly two decades of research have uncovered important roles for the NCL proteins in lysosomal catabolism and recycling of proteins and lipids, though the precise functions of most NCL proteins remain unsolved. Kousi and colleagues have recently reviewed the spectrum of mutations in these NCL genes [15], and Kollmann and colleagues have recently reviewed the current understanding of the NCL proteins [16]. Of note is the discovery that distinct sets of CLN6 mutations cause two forms of NCL. A severe form, thought to result from complete loss of function mutations, displays lysosomal storage primarily of the FP and RL types, and clinical symptom onset in the late infantile years presenting with seizures, followed by severe vision loss, ataxia, mental regression and death in the mid twenties [11, 12]. A milder, early-adult onset form, thought to result from mutations that cause reduced protein activity but not a complete loss of function, displays lysosomal storage primarily of the FP and GROD types, presents with progressive myoclonic epilepsy (PME) alone, and does not involve visual failure [17].
Here we will review the most recent discoveries in NCL genetics, which underscore the need for further studies aimed at elaborating the functions of the NCL proteins and genotype-phenotype relationships. Once attained, these insights will have great potential to impact our understanding, recognition and treatment of NCL and related disorders.
Recent genetic advances broaden understanding of the molecular basis of the NCL disorders
While more than a decade of genetic research into the molecular causes of recessive, infantile and childhood-onset NCL yielded the eight key NCL genes mentioned above, most cases of adult-onset NCL (sometimes referred to as Kufs disease), and some infantile/childhood-onset cases, have remained unsolved until recently. Technological advances and dropping costs of next generation sequencing have led to the discovery of five additional NCL genes in the past year (Table 1).
CLN4
Multiple groups identified a major cause of autosomal dominant adult-onset NCL, using a combination of classic linkage approaches and whole exome sequencing (WES). Noskova and colleagues initially reported the identification of two different mutations in DNAJC5 in independent families with NCL-like clinical features and GROD-type storage material [18]. The same mutations were confirmed in three subsequent WES reports [19-21]. DNAJC5 encodes cysteine-string protein alpha (CSPα), a chaperone protein highly expressed in brain and known to be heavily palmitoylated in a manner that influences synaptic vesicle membrane association (reviewed in [22]). Though its precise function is not fully understood, CSPα appears to serve an important role in neuronal synaptic activity through regulation of SNARE protein levels, and CSPα is required for activity dependent neuronal cell survival [23, 24]. The NCL-associated CSPα mutations, p.Leu116del and p.Leu115Arg, which have arisen independently multiple times [18, 21], impact the palmitoylated cysteine-string domain. Evidence suggests these mutations likely lead to higher order CSPα self-aggregation, possibly also incorporating wildtype CSPα protein into the aggregates, causing a toxic gain-of-function [25]. In light of this discovery, it is intriguing that PPT1 catabolizes the palmitoyl linkages on protein substrates [26] and that CLN3 has been proposed to be involved in regulating saturation of palmitoylated side chains, which may regulate protein trafficking [27, 28]. Like CSPα, both PPT1 and CLN3 have been localized at or near the synapse, in vesicles and in the plasma membrane [29-32]. Specific PPT1 and CLN3 palmitoylated substrates have not yet been identified, though several (including CSPα) have been proposed [33, 28]. Whether palmitoylated CSPα is a substrate for PPT1 or CLN3-mediated activities requires further experimental testing.
CLN11
Smith and colleagues recently reported on a pair of siblings with a recessive form of NCL, with vision loss and seizure onset in their late 20s and skin biopsy evidence of NCL-like storage material of the FP type [34]. WES identified a homozygous progranulin (GRN) mutation that was identical to that previously seen with an autosomal dominant inheritance pattern in frontotemporal lobar degeneration with ubiquitinated TDP-43 inclusions (FTLD-TDP) [34]. Progranulin, most recently reviewed by Cenik et al [35] and Jian et al [36], is a secreted protein that is localized within the endoplasmic reticulum (ER) and endosomal-lysosomal system and undergoes proteolytic processing into the low molecular weight granulins. The functions of progranulin and the cleaved granulins are unknown, but their expression is particularly high in neurons and activated immune cells [37-39], suggesting they are likely to play an important role in neuronal function and inflammation [35, 36]. Intriguingly, progranulin has also been demonstrated to bind sortilin, which is thought to direct its trafficking into the endosomal-lysosomal system [40]. It is noteworthy that interactions between other NCL-related proteins and sortilin and/or its retrograde trafficking receptor, retromer, have also been demonstrated [41-43, 28], highlighting further commonalities among at least several forms of NCL (Figure 1). The potential overlap in disease pathophysiology between GRN-associated NCL and FTLD raised by these new genetic data will be further discussed in the next section of this review.
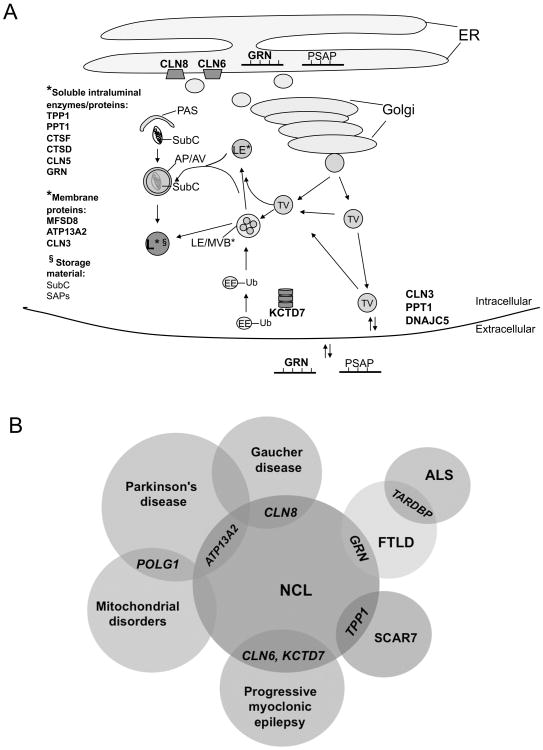
Figure 1. The broadening NCL biological pathway and genotypic and phenotypic spectrum.
A. A hypothetical model of the expanding NCL biological pathway(s) is depicted. The genes mutated in NCL patients are indicated by boldface type and their putative protein cellular localizations are shown (*). The NCL proteins may function in common or intersecting pathways. Alternatively, one or more NCL proteins may function in parallel pathways that, when dysfunctional, lead to a shared pathology. Progranulin (GRN) and prosaposin (PSAP) similarly exist as both precursor polypeptides in the endoplasmic reticulum (ER) and in the secretory pathway (Golgi and transport vesicles [TV]) and extracellular space, and are cleaved into lower molecular weight peptides in the endosomal-lysosomal system. Lines on GRN and PSAP depict cleavage sites. GRN, PSAP, and cathepsin D (CTSD) are sorted within the secretory pathway and endosomal system via sortilin. CLN5 also binds sortilin/retromer complex. Ubiquitination (Ub) is an increasingly recognized mechanism for regulating trafficking in the endosomal-lysosomal system. Subunit c (SubC) and the saposins (SAPs), cleaved from PSAP, are the major proteins accumulating in NCL storage material within autophagic vacuoles (AV) and lysosomes (L). Subunit c is the pore-forming subunit of the mitochondrial ATP synthase complex (Complex V), and the SAPs are lipid carrying accessory proteins in the endosomal-lysosomal system. *indicates the protein localization; §indicates localization of the listed storage material. TV=transport vesicle; EE=early endosome; Ub=ubiquitin; LE=late endosome; MVB=multivesicular body; PAS=preautophagosomal structure; AP/AV=amphisome/autophagic vacuole; L=lysosome. B. Venn diagram showing that new NCL genes (GRN, ATP13A2, and KCTD7) as well as previously defined NCL genes (TPP1, CLN6, and CLN8) genes intersect with other neurodegenerative disorders. As described in the text, polymorphisms in the CLN8 locus that influence its expression appear to be genetic modifiers of Gaucher disease. Other genes such as POLG and TARDBP, although not specifically identified as causative genes in NCL, may offer additional insight into NCL biology given their association with diseases showing clinicopathological overlap with NCL, including Parkinson's disease, mitochondrial disease, and frontotemporal lobar dementia (FTLD). TARDBP (TAR DNA-binding protein 43) encodes TDP-43, the protein that loses its nuclear localization and forms ubiquitinated, hyperphosphorylated inclusions in various forms of FTLD and amyotrophic lateral sclerosis (ALS). SCAR7, autosomal recessive spinocerebellar ataxia type 7.
CLN12
In 2011, a canine form of NCL was described by Wohlke and colleagues, which was due to a one base pair deletion in ATP13A2 [44]. Subsequent to that report in 2012, Bras and colleagues reported their finding in another WES study that a homozygous missense mutation (c.T2429G/p.Met810Arg) in the same gene segregated with the disease in an NCL family with FP-type storage material [45]. ATP13A2, also called PARK9, was previously associated with Kufor-Rakeb syndrome, a rare monogenic parkinsonism disorder [46, 47]. Like a number of the other NCL genes (Table 1), ATP13A2 encodes a transmembrane protein and is suspected of being a P-type transporter, possibly of cations or glycolipids [45, 46, 48], and overexpression studies localized the protein to the lysosomal membrane [46, 49]. The potential overlap in disease pathophysiology between ATP13A2-associated NCL and monogenic parkinsonism raised by this recent discovery will be further discussed in the next section of this review.
CLN13
It was previously known that mutations in the cathepsin D (CTSD) gene, which encodes an aspartyl protease within the endosomal-lysosomal system, lead to the most severe form of NCL, congenital NCL, and less frequently to a form of early childhood NCL [13, 50]. Perhaps not surprisingly then, a second member of the cathepsin family of proteases was also recently demonstrated to be linked to NCL. As reported by Smith and colleagues, WES of samples from a family with a recessive NCL disorder with FP-type storage material led to the identification of a homozygous missense CTSF mutation, encoding cathepsin F, a lysosomal cysteine protease whose in vivo substrates and intracellular pathway are unknown [51]. Further sequencing of the CTSF gene in additional NCL patient samples for which no other known gene defect was present, identified a second NCL family with compound heterozygous CTSF mutations. Further investigation of a Ctsf knock-out mouse model strongly supported these findings, as these mice likewise displayed classical NCL-like storage material and phenotypes [51].
CLN14
WES of a sib-pair with infantile-onset NCL and a mixture of FP and GROD-type lysosomal storage, uncovered a homozygous mutation in KCTD7, encoding potassium channel tetramerization domain containing protein 7 (KCTD7) [52]. Different mutations in the KCTD7 gene have also been identified in PME patients, but without lysosomal storage [53, 54]. Given the limited number of patients with KCTD7 mutations and for which skin biopsy lysosomal storage analysis has been performed, it remains unclear whether these observations represent a variant genotype-phenotype relationship or whether KCTD7 mutations are more broadly associated with this key NCL feature. KCTD7 is not yet well studied, but suspected functions include regulation of ion channels and function in the ubiquitin-proteasomal system [55]. KCTD7 is known to interact with cullin-3 (CUL3), an E3 ubiquitin ligase [55]. The missense mutation reported in this NCL family disrupted the KCTD7-CUL3 interaction as shown by co-immunoprecipitation studies using an overexpression system, supporting the hypothesis that the disease pathobiology involves regulation of CUL3 function [52]. CUL3 has also been recently recognized as playing an important role in late endosomal maturation [56], and it is known that ubiquitin modification is an important mechanism regulating protein trafficking and signaling pathways within the endosomal system [57]. Future research studies into the possible role of ubiquitin-mediated processes in NCL disease pathogenesis are warranted.
The primary neurological symptoms in the NCL disorders, and the enrichment of NCL proteins at or near the neuronal synapse, support the importance of the NCL proteins in neuronal function. A hypothetical model of NCL protein and clinical syndrome overlap is depicted in Figure 1. The NCL proteins may function in a single common pathway, or in intersecting or parallel pathways, leading to highly similar end-stage features involving lysosomal storage and neurological dysfunction. NCL gene expression data and studies in genetic models [16] also predict significant consequences of NCL gene mutations in other cell types that, like neurons, heavily rely on coordinated trafficking to and from the cell surface and that have high energy demands, including immune cells, secretory cells, and cardiomyocytes. Further targeted studies are important to understand the full impact of the NCL gene mutations and protein disruption on non-neuronal cells and organ function. Indeed, cardiac pathology in later stages of late-infantile and juvenile NCL (CLN2 and CLN3, respectively), and an abnormal autoimmune response in juvenile NCL (CLN3) have been documented [58-60]. As a result of the latter findings, suppression of the immune system using mycophenolate mofetil (CellCept®) in CLN3 patients is currently being tested in a Phase II clinical trial (www.clinicaltrials.gov).
Overlap between NCL and other neurological diseases
Increased understanding of the molecular basis of the NCL disorders has established compelling evidence that altered NCL gene function may be implicated in other neurological diseases (Figure 1). These discoveries augment a growing body of evidence that endosomal-lysosomal system dysfunction plays a significant role in neurodegeneration.
FTLD
After Alzheimer's disease, FTLD is the second most common form of presenile dementia, with a usual age of onset between 45 and 65 years. FTLD is broadly characterized by neurodegeneration of the frontal and temporal cortices leading to behavioral and language disturbances with usual sparing of memory and visuospatial functions. This disorder is further categorized pathologically into sub-types based on differing histologic features (see recent review [61]). FTLD-TDP is distinguished by intraneuronal cytoplasmic deposits, which are tau-negative and positive for ubiquitin and TDP-43 (an RNA-binding protein and splicing regulator encoded by TARDBP). The surprising finding that identical mutations in GRN underlie NCL and FTLD-TDP in autosomal recessive and autosomal dominant fashion, respectively, raises intriguing new questions regarding whether there are also overlapping pathophysiologies underlying these two forms of neurodegeneration [34, 35]. Notably, in addition to the core features of FTLD, FTLD-TDP associated with GRN mutations may also affect the basal ganglia and parietal cortex, resulting in parkinsonism, corticobasal syndrome, and memory impairment [62].
The majority of FTLD-associated GRN mutations lead to reduced progranulin levels and are suspected to cause disease by haploinsufficiency, rather than toxic gain-of-function mechanisms [35, 63]. Two independent Grn-deficient mouse lines show recessively inherited intraneuronal ubiquitin-positive, autofluorescent storage consistent with lipofuscin [38, 64], and one of these analyzed ultrastructurally displayed the characteristic appearance of NCL storage material [38, 34]. Other studies describe some behavioral abnormalities in homozygous mice, including social interaction deficits and enhanced aggression, that may be seen in clinical FTLD [65-67], but interestingly, heterozygous mice, which might be expected to model human FTLD-TDP given the suspected haploinsufficiency mechanism, have been reported to lack overt behavioral or histopathologic abnormalities [65-67]. Further studies are certain to shed more light on the intriguing relationship between progranulin and neurodegeneration.
Parkinsonism
The potential for shared disease pathophysiology between NCL and parkinsonism is also particularly intriguing. Clinical features of classical Parkinson's disease (PD) as well as monogenic forms of parkinsonism, including bradykinesia, rigidity, and to a lesser extent, resting tremor, are well-documented in later stages of juvenile NCL caused by mutations in CLN3 [3]. Functional evidence for nigrostriatal pathology in these patients includes decreased striatal uptake of [18F]-fluorodopa [68] and decreased striatal dopamine transporter density revealed by SPECT imaging using the labeled cocaine analog [131I]β-CIT [69]. In addition, the extrapyramidal features of juvenile NCL respond to antiparkinsonian therapy, albeit variably [70]. Parkinsonian features have also been reported in a late-infantile NCL patient with TPP1 mutations who responded to Levodopa [71], and in a large Dutch family with NCL caused by DNAJC5 mutation [72, 18]. Neuropathology of an unrelated DNAJC5 patient showed considerable degeneration of the substantia nigra, consistent with parkinsonian features late in the clinical course [73, 18].
The discovery of mutations in ATP13A2 in NCL patients provides the first genetic link between parkinsonism and NCL. While ATP13A2/PARK9 mutations had previously been linked to Kufor-Rakeb syndrome, without neuropathology available for these patients, it is difficult to know whether these in fact represent the same or distinct diseases. Of note, however, mice homozygous for an Atp13a2 knock-out allele showed pathologic features of both NCL (lipofuscin deposition in the cerebellum, cortex, and hippocampus) and PD (increased insoluble α-synuclein, however, without apparent dopaminergic neuron loss or decreased striatal dopamine) [74]. Moreover, ATP13A2 has been shown to significantly influence α-synuclein and manganese toxicity in yeast and mammalian PD models, potentially linking environmental and genetic factors influencing parkinsonism [75].
The relationship between PD and lysosomal dysfunction was previously recognized by the finding that Gaucher disease gene mutations (GBA1, encoding glucocerebrosidase) are also risk variants for PD (reviewed in [76]). Polymorphisms in linkage disequilibrium with the CLN8 locus, and associated with altered CLN8 gene expression in cultured fibroblasts, were recently implicated as possible Gaucher disease genetic modifiers [77], further supporting a hypothesis of common or intersecting biological pathways.
PME
NCL is part of the differential diagnosis of isolated PMEs, which are clinically and genetically heterogeneous epilepsy syndromes of childhood to early adulthood. The PME disorders typically have in common myoclonus (often prominent in the face and distal extremities and inducible with photic stimulation), multiple seizure types, psychomotor regression, and cerebellar signs [78]. As discussed previously, mutations in CLN6 cause a subset of adult-onset NCL in which PME is frequently a presenting feature and visual failure does not develop [17]. Moreover, CLN6 mutations are documented in PME patients with no evidence of lysosomal storage [79]. Similarly, patients with mutations in KCTD7 commonly present with PME, which may or may not involve lysosomal storage. Further genotype-phenotype studies are therefore needed to better understand the relationship between lysosomal dysfunction and the KCTD7 and CLN6 genes.
SCAR7
A recent study has also uncovered a surprisingly expanded phenotypic spectrum for mutations in TPP1, the gene typically mutated in classic late-infantile NCL (CLN2) [80]. Affected individuals in a previously described Dutch family [81] as a well as an unrelated sporadic SCAR7 case were found to be compound heterozygous for the common TPP1 splice site mutation c.509-1G>C and the missense mutation p.Val466Gly [80]. Affected individuals presented in childhood or adolescence with cerebellar ataxia and pyramidal signs but at the time of the report had not yet manifested seizures or ophthalmologic abnormalities. Skin biopsies were performed on 2 patients; one showed GROD and FP profiles, but not the CV profiles characteristic of classic late-infantile NCL (Table 1); the other biopsy did not show storage material. A TPP1 enzyme activity assay showed 10-15% of normal levels in peripheral blood and 5% of normal levels in cultured fibroblasts (compared to 9% and 0.4%, respectively, in the reference set of classic late-infantile NCL cases). These findings suggest that higher residual TPP1 activity produces a more protracted atypical NCL clinical course [80].
Mitochondrial disorders (primary or secondary)
The core clinical features of NCL, including pigmentary retinopathy, seizures, movement abnormalities, and regression, are also observed in a broad range of mitochondrial disorders. In some cases the major distinguishing feature identified was the diagnostic inclusions of NCL [82]. It is therefore not altogether surprising that certain subtypes of NCL show alterations in mitochondrial morphology, subcellular distribution, respiratory chain complex activity, or basal ATP synthesis [83-86]. The role of impaired mitochondrial homeostasis has been recognized in other neurodegenerative diseases, most notably PD. Mutations in DNA polymerase gamma 1 (POLG1), which encodes the catalytic subunit of the mitochondrial DNA polymerase, have been linked to early-onset forms of parkinsonism [87, 88]. Turnover of damaged mitochondria by a regulated form of autophagy, known as mitophagy, is impaired by mutations in PARK2, which encodes the ubiquitin ligase parkin [89]. Kufor-Rakeb associated mutations in ATP13A2 have also been linked to defective mitochondrial quality control mediated by mitophagy [90], suggesting that forms of NCL caused by ATP13A2 mutations may reveal similar pathology. Further highlighting the potential intersection between NCL and mitochondrial disease is a case report suggesting that a variant in POLG1 (c.1550G>T, p.Gly517Val) may modify the clinical phenotype of CLN5 [91].
Conclusions
Twenty years after the identification of the first NCL genes, the pathophysiological pathways remain to be fully elucidated. The development of rational therapies for most forms of NCL is in the very early stages, and includes Phase I safety trials using gene therapy and stem cells to treat enzymatic forms (CLN1 and CLN2), and a Phase II trial of mycophenolate mofetil (Cellcept®) as an anti-inflammatory agent in the juvenile form/CLN3 (www.clinicaltrials.gov). Progress in identifying therapeutic targets has been hampered by the genotypic and phenotypic heterogeneity seen in the NCL disorders, as well as the lack of clear understanding of the functions of the NCL proteins. These proteins tend to be ubiquitously expressed, are possibly multifunctional proteins, and are enzymes whose substrates are mostly unknown or are difficult to study membrane proteins [16]. Nevertheless, the molecular discoveries in the recent years widen the opportunities for new research efforts aimed at detailed high-throughput biological profiling approaches (e.g. proteomics, lipidomics, and metabolomics) to advance knowledge of the NCL protein network and the pathways that are central to the disease process in the NCL disorders and related neurodegenerative diseases [92, 93]. Translation of these efforts will be greatly facilitated by more patient-oriented research efforts, such as improved access to patient samples (DNA, cell lines, and autopsy tissue) linked to clinical phenotype and genotype information (e.g. The NCL Mutation and Patient Database, www.ucl.ac.uk/ncl/mutation.shtml), biomarker development and natural history studies, as well as investigation of disease pathogenesis in human cell-based systems, such as those offered by cellular reprogramming to produce genotype-specific induced pluripotent stem cells (iPS cells). Development of these human cell based systems will also greatly improve drug-screening efforts.
The recent discoveries in NCL genetics also underscore the notion that lysosomal storage analysis on tissue biopsy is an imperfect marker for NCL. If NCL-type storage material is found in multiple cell types in a patient with clinical symptoms of the disorder, a positive clinical NCL diagnosis can be made [1], but absence of storage material in rectal or skin punch biopsy or in peripheral blood lymphocytes cannot rule out the disease. In all cases, further definitive molecular diagnostic approaches should be undertaken, which may include measurement of enzymatic activity for PPT1 (CLN1) and TPP1 (CLN2), and sequencing and deletion/duplication analysis of NCL genes using traditional molecular methodologies. Next generation sequencing panels are also now available on a clinical basis and will target a variable number of well-known NCL mutations. Although bioinformatic analysis and interpretation is still challenging, clinical whole exome and whole genome sequencing are also now options for molecular diagnosis of simplex cases, or small clinically homogenous cohorts that remain molecularly unresolved. Results of these larger sequencing efforts will likely continue to extend the NCL genotypic and phenotypic spectrum.
Footnotes
Conflict of Interest: Susan L. Cotman declares no potential conflicts of interest relevant to this article. Amel Karaa declares no potential conflicts of interest relevant to this article. John F. Staropoli declares no potential conflicts of interest relevant to this article. Katherine B. Sims declares no potential conflicts of interest relevant to this article.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Susan L. Cotman, Email: cotman@helix.mgh.harvard.edu, Center for Human Genetic Research, Department of Neurology, Massachusetts General Hospital (MGH) 185 Cambridge Street Boston, MA 02114 PH: 617.726.9180.
Amel Karaa, Email: akaraa@partners.org, Center for Human Genetic Research, MGH, 185 Cambridge Street, Boston, MA 02114; Harvard Medical School, Clinical Genetics Program, 77 Louis Pasteur Avenue, NRB suite 252, Boston, MA 02115, PH: 617.726.5732.
John F. Staropoli, Email: jstaropoli@partners.org, Center for Human Genetic Research, Department of Pathology, MGH, 185 Cambridge Street, Boston, MA 02114, PH: 917.658.6066.
Katherine B. Sims, Email: sims@helix.mgh.harvard.edu, Center for Human Genetic Research, Department of Neurology, MGH, 185 Cambridge Street, Boston, MA 02114, PH: 617.726.5718.
Bibiography & References Cited
- 1.Mole SE, Williams RE, Goebel HH. Contemporary Neurology Series. Second. Oxford: Oxford University Press; 2011. The Neuronal Ceroid Lipofuscinoses (Batten Disease) [Google Scholar]
- 2.Santorelli FM, Garavaglia B, Cardona F, Nardocci N, Bernardina BD, Sartori S, et al. Molecular epidemiology of childhood neuronal ceroid-lipofuscinosis in Italy. Orphanet J Rare Dis. 2013;8:19. doi: 10.1186/1750-1172-8-19. 1750-1172-8-19 [pii] 10.1186/1750-1172-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvela I, Autti T, Lamminranta S, Aberg L, Raininko R, Santavuori P. Clinical and magnetic resonance imaging findings in Batten disease: analysis of the major mutation (1.02-kb deletion) Ann Neurol. 1997;42(5):799–802. doi: 10.1002/ana.410420517. [DOI] [PubMed] [Google Scholar]
- 4.Munroe PB, Mitchison HM, O'Rawe AM, Anderson JW, Boustany RM, Lerner TJ, et al. Spectrum of mutations in the Batten disease gene, CLN3. Am J Hum Genet. 1997;61(2):310–6. doi: 10.1086/514846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6(3):107–26. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- 6.Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, et al. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376(6541):584–7. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 7.International Batten Disease Consortium. Isolation of a novel gene underlying Batten disease, CLN3. The International Batten Disease Consortium. Cell. 1995;82(6):949–57. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 8.Sleat DE, Donnelly RJ, Lackland H, Liu CG, Sohar I, Pullarkat RK, et al. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science. 1997;277(5333):1802–5. doi: 10.1126/science.277.5333.1802. [DOI] [PubMed] [Google Scholar]
- 9.Savukoski M, Klockars T, Holmberg V, Santavuori P, Lander ES, Peltonen L. CLN5, a novel gene encoding a putative transmembrane protein mutated in Finnish variant late infantile neuronal ceroid lipofuscinosis. Nat Genet. 1998;19(3):286–8. doi: 10.1038/975. [DOI] [PubMed] [Google Scholar]
- 10.Ranta S, Zhang Y, Ross B, Lonka L, Takkunen E, Messer A, et al. The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat Genet. 1999;23(2):233–6. doi: 10.1038/13868. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler RB, Sharp JD, Schultz RA, Joslin JM, Williams RE, Mole SE. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am J Hum Genet. 2002;70(2):537–42. doi: 10.1086/338708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao H, Boustany RM, Espinola JA, Cotman SL, Srinidhi L, Antonellis KA, et al. Mutations in a novel CLN6-encoded transmembrane protein cause variant neuronal ceroid lipofuscinosis in man and mouse. Am J Hum Genet. 2002;70(2):324–35. doi: 10.1086/338190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, et al. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129(Pt 6):1438–45. doi: 10.1093/brain/awl107. [DOI] [PubMed] [Google Scholar]
- 14.Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, et al. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Hum Genet. 2007;81(1):136–46. doi: 10.1086/518902. S0002-9297(07)62823-7 [pii] 10.1086/518902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2012;33(1):42–63. doi: 10.1002/humu.21624. 10.1002/humu.21624. Comprehensive review of the spectrum of mutations in the NCL genes discovered prior to the recent discoveries in the past year, described in the present review. [DOI] [PubMed] [Google Scholar]
- 16.Kollmann K, Uusi-Rauva K, Scifo E, Tyynela J, Jalanko A, Braulke T. Cell biology and function of neuronal ceroid lipofuscinosis-related proteins. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.01.019. S0925-4439(13)00033-1 [pii] 10.1016/j.bbadis.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 17•.Arsov T, Smith KR, Damiano J, Franceschetti S, Canafoglia L, Bromhead CJ, et al. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet. 2011;88(5):566–73. doi: 10.1016/j.ajhg.2011.04.004. S0002-9297(11)00145-5 [pii] 10.1016/j.ajhg.2011.04.004. Identified a distinct subset of CLN6 mutations in adult-onset NCL (Kufs disease) patients. CLN6 mutations had only previously been recognized in late infantile-onset NCL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Noskova L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, et al. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet. 2011;89(2):241–52. doi: 10.1016/j.ajhg.2011.07.003. S0002-9297(11)00297-7 [pii] 0.1016/j.ajhg.2011.07.003. Elegantly used a combination of classic linkage analysis and next-generation sequencing to identify the first gene associated with a dominant form of NCL. Only recessive forms of NCL were genetically identified prior to this report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benitez BA, Alvarado D, Cai Y, Mayo K, Chakraverty S, Norton J, et al. Exome-sequencing confirms DNAJC5 mutations as cause of adult neuronal ceroid-lipofuscinosis. PLoS One. 2011;6(11):e26741. doi: 10.1371/journal.pone.0026741. 10.1371/journal.pone.0026741 PONE-D-11-16499 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velinov M, Dolzhanskaya N, Gonzalez M, Powell E, Konidari I, Hulme W, et al. Mutations in the gene DNAJC5 cause autosomal dominant Kufs disease in a proportion of cases: study of the Parry family and 8 other families. PLoS One. 2012;7(1):e29729. doi: 10.1371/journal.pone.0029729. 10.1371/journal.pone.0029729 PONE-D-11-16743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadieux-Dion M, Andermann E, Lachance-Touchette P, Ansorge O, Meloche C, Barnabe A, et al. Recurrent mutations in DNAJC5 cause autosomal dominant Kufs disease. Clin Genet. 2012 doi: 10.1111/cge.12020. 10.1111/cge.12020. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JN, Ahrendt E, Braun JE. CSPalpha: the neuroprotective J protein. Biochem Cell Biol. 2010;88(2):157–65. doi: 10.1139/o09-124. o09-124 [pii] 10.1139/o09-124. [DOI] [PubMed] [Google Scholar]
- 23.Sharma M, Burre J, Sudhof TC. CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol. 2011;13(1):30–9. doi: 10.1038/ncb2131. ncb2131 [pii] 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, et al. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron. 2004;42(2):237–51. doi: 10.1016/s0896-6273(04)00190-4. S0896627304001904 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Greaves J, Lemonidis K, Gorleku OA, Cruchaga C, Grefen C, Chamberlain LH. Palmitoylation-induced aggregation of cysteine-string protein mutants that cause neuronal ceroid lipofuscinosis. J Biol Chem. 2012;287(44):37330–9. doi: 10.1074/jbc.M112.389098. M112.389098 [pii] 10.1074/jbc.M112.389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993;268(30):22566–74. [PubMed] [Google Scholar]
- 27.Narayan SB, Rakheja D, Tan L, Pastor JV, Bennett MJ. CLN3P, the Batten's disease protein, is a novel palmitoyl-protein Delta-9 desaturase. Ann Neurol. 2006;60(5):570–7. doi: 10.1002/ana.20975. [DOI] [PubMed] [Google Scholar]
- 28.Kama R, Kanneganti V, Ungermann C, Gerst J. The yeast Batten disease ortholog, Btn1, controls endosome-Golgi retrograde transport via SNARE assembly. J Cell Biol. 2011;195(2):203–15. doi: 10.1083/jcb.201102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehtovirta M, Kyttala A, Eskelinen EL, Hess M, Heinonen O, Jalanko A. Palmitoyl protein thioesterase (PPT) localizes into synaptosomes and synaptic vesicles in neurons: implications for infantile neuronal ceroid lipofuscinosis (INCL) Hum Mol Genet. 2001;10(1):69–75. doi: 10.1093/hmg/10.1.69. [DOI] [PubMed] [Google Scholar]
- 30.Suopanki J, Lintunen M, Lahtinen H, Haltia M, Panula P, Baumann M, et al. Status epilepticus induces changes in the expression and localization of endogenous palmitoyl-protein thioesterase 1. Neurobiol Dis. 2002;10(3):247–57. doi: 10.1006/nbdi.2002.0503. S0969996102905036 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Zhang Z, Sarkar C, Tsai PC, Lee YC, Dye L, et al. Palmitoyl protein thioesterase-1 deficiency impairs synaptic vesicle recycling at nerve terminals, contributing to neuropathology in humans and mice. J Clin Invest. 2008;118(9):3075–86. doi: 10.1172/JCI33482. 10.1172/JCI33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luiro K, Kopra O, Lehtovirta M, Jalanko A. CLN3 protein is targeted to neuronal synapses but excluded from synaptic vesicles: new clues to Batten disease. Hum Mol Genet. 2001;10(19):2123–31. doi: 10.1093/hmg/10.19.2123. [DOI] [PubMed] [Google Scholar]
- 33.Saja S, Buff H, Smith AC, Williams TS, Korey CA. Identifying cellular pathways modulated by Drosophila palmitoyl-protein thioesterase 1 function. Neurobiol Dis. 2010;40(1):135–45. doi: 10.1016/j.nbd.2010.02.010. S0969-9961(10)00057-4 [pii] 10.1016/j.nbd.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90(6):1102–7. doi: 10.1016/j.ajhg.2012.04.021. S0002-9297(12)00254-6 [pii] 10.1016/j.ajhg.2012.04.021. This study established the first genetic link between NCL and FTLD, demonstrating a novel effect of GRN mutation allele dosage on phenotypic presentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G. Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J Biol Chem. 2012;287(39):32298–306. doi: 10.1074/jbc.R112.399170. R112.399170 [pii] 10.1074/jbc.R112.399170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol. 2013;93(2):199–208. doi: 10.1189/jlb.0812429. jlb.0812429 [pii] 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan CL, Baranowski DC, Chitramuthu BP, Malik S, Li Z, Cao M, et al. Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci. 2009;10:130. doi: 10.1186/1471-2202-10-130. 1471-2202-10-130 [pii] 10.1186/1471-2202-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, et al. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177(1):311–24. doi: 10.2353/ajpath.2010.090915. S0002-9440(10)60087-9 [pii] 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petkau TL, Neal SJ, Orban PC, MacDonald JL, Hill AM, Lu G, et al. Progranulin expression in the developing and adult murine brain. J Comp Neurol. 2010;518(19):3931–47. doi: 10.1002/cne.22430. 10.1002/cne.22430. [DOI] [PubMed] [Google Scholar]
- 40.Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68(4):654–67. doi: 10.1016/j.neuron.2010.09.034. S0896-6273(10)00776-2 [pii] 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamo A, Jules F, Dumaresq-Doiron K, Costantino S, Lefrancois S. The role of ceroid lipofuscinosis neuronal protein 5 (CLN5) in endosomal sorting. Mol Cell Biol. 2012;32(10):1855–66. doi: 10.1128/MCB.06726-11. MCB.06726-11 [pii] 10.1128/MCB.06726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003;22(24):6430–7. doi: 10.1093/emboj/cdg629. 10.1093/emboj/cdg629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canuel M, Korkidakis A, Konnyu K, Morales CR. Sortilin mediates the lysosomal targeting of cathepsins D and H. Biochem Biophys Res Commun. 2008;373(2):292–7. doi: 10.1016/j.bbrc.2008.06.021. S0006-291X(08)01159-5 [pii] 10.1016/j.bbrc.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Wohlke A, Philipp U, Bock P, Beineke A, Lichtner P, Meitinger T, et al. A one base pair deletion in the canine ATP13A2 gene causes exon skipping and late-onset neuronal ceroid lipofuscinosis in the Tibetan terrier. PLoS Genet. 2011;7(10):e1002304. doi: 10.1371/journal.pgen.1002304. 10.1371/journal.pgen.1002304 PGENETICS-D-10-00617 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet. 2012;21(12):2646–50. doi: 10.1093/hmg/dds089. dds089 [pii] 0.1093/hmg/dds089. The first genetic link in humans between NCL and monogenic parkinsonism, predicted by an earlier finding of a homozygous ATP13A2 mutation in a canine model of NCL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type. ATPase Nat Genet. 2006;38(10):1184–91. doi: 10.1038/ng1884. ng1884 [pii] 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 47.Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68(19):1557–62. doi: 10.1212/01.wnl.0000260963.08711.08. 68/19/1557 [pii] 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt K, Wolfe DM, Stiller B, Pearce DA. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem Biophys Res Commun. 2009;383(2):198–202. doi: 10.1016/j.bbrc.2009.03.151. S0006-291X(09)00652-4 [pii] 10.1016/j.bbrc.2009.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JS, Mehta P, Cooper AA, Veivers D, Heimbach A, Stiller B, et al. Pathogenic effects of novel mutations in the P-type ATPase ATP13A2 (PARK9) causing Kufor-Rakeb syndrome, a form of early-onset parkinsonism. Hum Mutat. 2011;32(8):956–64. doi: 10.1002/humu.21527. 10.1002/humu.21527. [DOI] [PubMed] [Google Scholar]
- 50.Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W, et al. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet. 2006;78(6):988–98. doi: 10.1086/504159. S0002-9297(07)63920-2 [pii] 10.1086/504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith KR, Dahl HH, Canafoglia L, Andermann E, Damiano J, Morbin M, et al. Cathepsin F mutations cause Type B Kufs disease, an adult-onset neuronal ceroid lipofuscinosis. Hum Mol Genet. 2013;22(7):1417–23. doi: 10.1093/hmg/dds558. dds558 [pii] 10.1093/hmg/dds558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staropoli JF, Karaa A, Lim ET, Kirby A, Elbalalesy N, Romansky SG, et al. A homozygous mutation in KCTD7 links neuronal ceroid lipofuscinosis to the ubiquitin-proteasome system. Am J Hum Genet. 2012;91(1):202–8. doi: 10.1016/j.ajhg.2012.05.023. S0002-9297(12)00308-4 [pii] 10.1016/j.ajhg.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Bogaert P, Azizieh R, Desir J, Aeby A, De Meirleir L, Laes JF, et al. Mutation of a potassium channel-related gene in progressive myoclonic epilepsy. Ann Neurol. 2007;61(6):579–86. doi: 10.1002/ana.21121. 10.1002/ana.21121. [DOI] [PubMed] [Google Scholar]
- 54.Kousi M, Anttila V, Schulz A, Calafato S, Jakkula E, Riesch E, et al. Novel mutations consolidate KCTD7 as a progressive myoclonus epilepsy gene. J Med Genet. 2012;49(6):391–9. doi: 10.1136/jmedgenet-2012-100859. jmedgenet-2012-100859 [pii] 10.1136/jmedgenet-2012-100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azizieh R, Orduz D, Van Bogaert P, Bouschet T, Rodriguez W, Schiffmann SN, et al. Progressive myoclonic epilepsy-associated gene KCTD7 is a regulator of potassium conductance in neurons. Mol Neurobiol. 2011;44(1):111–21. doi: 10.1007/s12035-011-8194-0. 10.1007/s12035-011-8194-0. [DOI] [PubMed] [Google Scholar]
- 56.Huotari J, Meyer-Schaller N, Hubner M, Stauffer S, Katheder N, Horvath P, et al. Cullin-3 regulates late endosome maturation. Proc Natl Acad Sci U S A. 2012;109(3):823–8. doi: 10.1073/pnas.1118744109. 1118744109 [pii] 10.1073/pnas.1118744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–47. doi: 10.1146/annurev.cellbio.23.090506.123319. 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostergaard JR, Rasmussen TB, Molgaard H. Cardiac involvement in juvenile neuronal ceroid lipofuscinosis (Batten disease) Neurology. 2011;76(14):1245–51. doi: 10.1212/WNL.0b013e31821435bd. 76/14/1245 [pii] 10.1212/WNL.0b013e31821435bd. [DOI] [PubMed] [Google Scholar]
- 59.Fukumura S, Saito Y, Saito T, Komaki H, Nakagawa E, Sugai K, et al. Progressive conduction defects and cardiac death in late infantile neuronal ceroid lipofuscinosis. Dev Med Child Neurol. 2012;54(7):663–6. doi: 10.1111/j.1469-8749.2011.04170.x. 10.1111/j.1469-8749.2011.04170.x. [DOI] [PubMed] [Google Scholar]
- 60.Chattopadhyay S, Ito M, Cooper JD, Brooks AI, Curran TM, Powers JM, et al. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Hum Mol Genet. 2002;11(12):1421–31. doi: 10.1093/hmg/11.12.1421. [DOI] [PubMed] [Google Scholar]
- 61.Bigio EH. Making the diagnosis of frontotemporal lobar degeneration. Arch Pathol Lab Med. 2013;137(3):314–25. doi: 10.5858/arpa.2012-0075-RA. 10.5858/arpa.2012-0075-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohn-Hokke PE, Elting MW, Pijnenburg YA, van Swieten JC. Genetics of dementia: update and guidelines for the clinician. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(6):628–43. doi: 10.1002/ajmg.b.32080. 10.1002/ajmg.b.32080. [DOI] [PubMed] [Google Scholar]
- 63.Sun L, Eriksen JL. Recent insights into the involvement of progranulin in frontotemporal dementia. Curr Neuropharmacol. 2011;9(4):632–42. doi: 10.2174/157015911798376361. 10.2174/157015911798376361 CN-9-632 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wils H, Kleinberger G, Pereson S, Janssens J, Capell A, Van Dam D, et al. Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol. 2012;228(1):67–76. doi: 10.1002/path.4043. 10.1002/path.4043. [DOI] [PubMed] [Google Scholar]
- 65.Yin F, Dumont M, Banerjee R, Ma Y, Li H, Lin MT, et al. Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J. 2010;24(12):4639–47. doi: 10.1096/fj.10-161471. fj.10-161471 [pii] 10.1096/fj.10-161471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis. 2012;45(2):711–22. doi: 10.1016/j.nbd.2011.10.016. S0969-9961(11)00348-2 [pii] 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, et al. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185(2):110–8. doi: 10.1016/j.bbr.2007.07.020. S0166-4328(07)00369-5 [pii] 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 68.Ruottinen HM, Rinne JO, Haaparanta M, Solin O, Bergman J, Oikonen VJ, et al. [18F]fluorodopa PET shows striatal dopaminergic dysfunction in juvenile neuronal ceroid lipofuscinosis. J Neurol Neurosurg Psychiatry. 1997;62(6):622–5. doi: 10.1136/jnnp.62.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aberg L, Liewendahl K, Nikkinen P, Autti T, Rinne JO, Santavuori P. Decreased striatal dopamine transporter density in JNCL patients with parkinsonian symptoms. Neurology. 2000;54(5):1069–74. doi: 10.1212/wnl.54.5.1069. [DOI] [PubMed] [Google Scholar]
- 70.Aberg LE, Rinne JO, Rajantie I, Santavuori P. A favorable response to antiparkinsonian treatment in juvenile neuronal ceroid lipofuscinosis. Neurology. 2001;56(9):1236–9. doi: 10.1212/wnl.56.9.1236. [DOI] [PubMed] [Google Scholar]
- 71.Le NM, Parikh S. Late infantile neuronal ceroid lipofuscinosis and dopamine deficiency. J Child Neurol. 2012;27(2):234–7. doi: 10.1177/0883073811419261. 0883073811419261 [pii] 10.1177/0883073811419261. [DOI] [PubMed] [Google Scholar]
- 72.Nijssen PC, Brusse E, Leyten AC, Martin JJ, Teepen JL, Roos RA. Autosomal dominant adult neuronal ceroid lipofuscinosis: parkinsonism due to both striatal and nigral dysfunction. Mov Disord. 2002;17(3):482–7. doi: 10.1002/mds.10104. 10.1002/mds.10104. [DOI] [PubMed] [Google Scholar]
- 73.Josephson SA, Schmidt RE, Millsap P, McManus DQ, Morris JC. Autosomal dominant Kufs' disease: a cause of early onset dementia. Journal of the Neurological Sciences. 2001;188(1-2):51–60. doi: 10.1016/s0022-510x(01)00546-9. [DOI] [PubMed] [Google Scholar]
- 74.Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, Giasson B, et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited alpha-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt057. ddt057 [pii] 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41(3):308–15. doi: 10.1038/ng.300. ng.300 [pii] 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol. 2012;124(3):325–38. doi: 10.1007/s00401-012-1013-5. 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang CK, Stein PB, Liu J, Wang Z, Yang R, Cho JH, et al. Genome-wide association study of N370S homozygous Gaucher disease reveals the candidacy of CLN8 gene as a genetic modifier contributing to extreme phenotypic variation. Am J Hematol. 2012;87(4):377–83. doi: 10.1002/ajh.23118. 10.1002/ajh.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Siqueira LF. Progressive myoclonic epilepsies: review of clinical, molecular and therapeutic aspects. J Neurol. 2010;257(10):1612–9. doi: 10.1007/s00415-010-5641-1. 10.1007/s00415-010-5641-1. [DOI] [PubMed] [Google Scholar]
- 79.Andrade DM, Paton T, Turnbull J, Marshall CR, Scherer SW, Minassian BA. Mutation of the CLN6 gene in teenage-onset progressive myoclonus epilepsy. Pediatr Neurol. 2012;47(3):205–8. doi: 10.1016/j.pediatrneurol.2012.05.004. S0887-8994(12)00217-2 [pii] 10.1016/j.pediatrneurol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 80•.Sun Y, Almomani R, Breedveld GJ, Santen GW, Aten E, Lefeber DJ, et al. Autosomal Recessive Spinocerebellar Ataxia 7 (SCAR7) is Caused by Variants in TPP1, The Gene Involved in Classic Late-Infantile Neuronal Ceroid Lipofuscinosis 2 Disease (CLN2 Disease) Hum Mutat. 2013 doi: 10.1002/humu.22292. 10.1002/humu.22292. Demonstrates the power of enzyme screening to pick up novel and unexpected phenotypic presentations of lysosomal storage diseases. [DOI] [PubMed] [Google Scholar]
- 81.Breedveld GJ, van Wetten B, te Raa GD, Brusse E, van Swieten JC, Oostra BA, et al. A new locus for a childhood onset, slowly progressive autosomal recessive spinocerebellar ataxia maps to chromosome 11p15. J Med Genet. 2004;41(11):858–66. doi: 10.1136/jmg.2004.019232. 41/11/858 [pii] 10.1136/jmg.2004.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thorburn DR, Rahman S. Mitochondrial DNA-Associated Leigh Syndrome and NARP. 1993 NBK1173 [bookaccession] [Google Scholar]
- 83.Jolly RD, Brown S, Das AM, Walkley SU. Mitochondrial dysfunction in the neuronal ceroid-lipofuscinoses (Batten disease) Neurochemistry International. 2002;40(6):565–71. doi: 10.1016/s0197-0186(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 84.Fossale E, Wolf P, Espinola JA, Lubicz-Nawrocka T, Teed AM, Gao H, et al. Membrane trafficking and mitochondrial abnormalities precede subunit c deposition in a cerebellar cell model of juvenile neuronal ceroid lipofuscinosis. BMC Neuroscience. 2004;5(57) doi: 10.1186/1471-2202-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luiro K, Kopra O, Blom T, Gentile M, Mitchison HM, Hovatta I, et al. Batten disease (JNCL) is linked to disturbances in mitochondrial, cytoskeletal, and synaptic compartments. J Neurosci Res. 2006;84(5):1124–38. doi: 10.1002/jnr.21015. [DOI] [PubMed] [Google Scholar]
- 86.Pezzini F, Gismondi F, Tessa A, Tonin P, Carrozzo R, Mole SE, et al. Involvement of the mitochondrial compartment in human NCL fibroblasts. Biochem Biophys Res Commun. 2011;416(1-2):159–64. doi: 10.1016/j.bbrc.2011.11.016. S0006-291X(11)02015-8 [pii] 10.1016/j.bbrc.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 87.Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, et al. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol. 2006;59(5):859–62. doi: 10.1002/ana.20831. 10.1002/ana.20831. [DOI] [PubMed] [Google Scholar]
- 88.Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, et al. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64(4):553–7. doi: 10.1001/archneur.64.4.553. 64/4/553 [pii] 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- 89.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. jcb.200809125 [pii] 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gusdon AM, Zhu J, Van Houten B, Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 2012;45(3):962–72. doi: 10.1016/j.nbd.2011.12.015. S0969-9961(11)00390-1 [pii] 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Staropoli JF, Xin W, Barone R, Cotman SL, Sims KB. An atypical case of neuronal ceroid lipofuscinosis with co-inheritance of a variably penetrant POLG1 mutation. BMC Med Genet. 2012;13:50. doi: 10.1186/1471-2350-13-50. 1471-2350-13-50 [pii] 10.1186/1471-2350-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jalanko A, Tyynela J, Peltonen L. From genes to systems: new global strategies for the characterization of NCL biology. Biochim Biophys Acta. 2006;1762(10):934–44. doi: 10.1016/j.bbadis.2006.09.001. S0925-4439(06)00174-8 [pii] 10.1016/j.bbadis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Kmoch S, Stranecky V, Emes RD, Mitchison HM. Bioinformatic perspectives in the neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbadis.2012.12.010. S0925-4439(12)00301-8 [pii] 10.1016/j.bbadis.2012.12.010. [DOI] [PubMed] [Google Scholar]