Abstract
γ-Hydroxybutyrate (GHB) is a drug of abuse with multiple mechanisms of action. Consistent with its ability to modulate dopaminergic systems, GHB reportedly shares behavioral effects with neuroleptics and interacts with them in a synergistic manner. Here, we examined the ability of GHB and haloperidol to induce catalepsy and to affect operant responding. When given alone, both compounds induced catalepsy and decreased response rate. When given together, however, they produced these effects in an additive manner. This is further evidence that GHB has neuroleptic-like effects, but suggests that GHB interacts additively, not synergistically, with neuroleptics. The mechanisms involved in GHB- and haloperidol-induced catalepsy are different because the N-methyl-D-aspartate (NMDA) receptor antagonist, dizocilpine (MK-801), attenuated the cataleptic effects of haloperidol, but enhanced those of GHB. The latter finding suggests that other NMDA receptor antagonists (e.g., the drugs of abuse—phencyclidine and ketamine) may also interact synergistically with GHB.
Keywords: GHB (γ-hydroxybutyrate), Haloperidol, Dizocilpine, Catalepsy, Synergism
1. Introduction
γ-Hydroxybutyrate (GHB) occurs naturally in the brain, where it is believed to function as a neurotransmitter (for a review, see Maitre et al., 2000). GHB is thought to play a physiological role in modulating dopaminergic neurons (Roth et al., 1980). Consistent with its inhibitory effects on dopamine release (e.g., Howard and Feigenbaum, 1997), GHB and some of its analogs have antidopaminergic effects in vivo, as evidenced by their ability to produce catalepsy in rodents (Snead and Bearden, 1980; Hechler et al., 1993; Navarro et al., 1998; Itzhak and Ali, 2002). GHB reportedly not only shares behavioral effects with neuroleptics, but may interact synergistically with neuroleptics to produce these effects (Navarro et al., 1998).
The present study was aimed at further examining the generality of the neuroleptic-like effects of GHB by investigating its ability, in comparison with haloperidol, to produce catalepsy and to decrease food-reinforced operant responding in rats. Two catalepsy tests were used—one involving the forelimbs and the other the hindlimbs—because neuroleptics can differentially affect forelimb and hindlimb movements (Ellenbroek et al., 1987). The results of combinations of GHB and haloperidol were analyzed with recently introduced methods for testing the statistical significance of drug interactions (Tallarida, 2000).
Catalepsy is not only produced by neuroleptics, but also by opioids, such as morphine (e.g., Kuschinsky and Hornykiewicz, 1972; Costall and Naylor, 1973). The underlying mechanisms, however, are not identical. For example, glutamatergic transmission appears to be involved differently in haloperidol- and morphine-induced catalepsy because competitive and noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonists attenuate the cataleptic effects of haloperidol (Schmidt and Bubser, 1989; Kretschmer et al., 1992), but enhance the cataleptic effects of morphine (Trujillo and Akil, 1991; Tzschentke and Schmidt, 1996). To examine whether glutamatergic transmission is involved in the cataleptic effects of GHB, the present study investigated the effects of the noncompetitive NMDA receptor antagonist, dizocilpine (MK-801), on GHB-induced catalepsy in comparison with its effects on haloperidol-induced catalepsy.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN), weighing 300–400 g, were individually housed in an environmentally controlled room (temperature, 24 °C; relative humidity, 45%), under a 12/12 h light/dark cycle (lights on at 0800 h), with water continuously available. Food (Purina Lab Chow) was freely available for the rats used in the catalepsy tests and was restricted to 12 g/day for the rats used to examine drug effects on food-maintained operant responding. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2. Catalepsy
In the bar test, the forelimbs were placed on a horizontal, cylindrical metal bar (diameter, 1.0 cm; height, 10 cm) and the time until both forelimbs touched the table surface was recorded up to a maximum of 120 s. In the hindlimb test, the right hindlimb was placed on a metal platform (height, 5 cm) and the time until the limb touched the table surface was recorded up to a maximum of 30 s.
2.3. Schedule-controlled responding
Operant chambers (ENV-001; Med Associates, St. Albans, VT), enclosed in sound-attenuating compartments, were controlled by MED-PC IV software (Med Associates). On one side of each chamber was a 5 × 4.2-cm opening allowing access to a tray to which pellets (45 mg, PJAI-0045, Noyes Precision Pellets; Research Diets, New Brunswick, NJ) could be delivered. Two metal response levers on either side of the food tray were located 7.3 cm above the floor, consisting of metal rods (4.8 mm in diameter, spaced 1.6 cm apart). A white cue light, 2.5 cm in diameter, was centered 6 cm above each lever.
Rats were trained to press a lever for food pellets under a fixed ratio (FR) 10 schedule. Daily sessions consisted of six cycles, and each cycle consisted of a 15-min pretreatment period followed by a 5-min response period during which a light above the active lever was illuminated and completion of the FR10 requirement produced a food pellet. The response period ended after 10 pellets were delivered, or after 5 min, whichever occurred first. Response rate was measured as responses per second.
2.4. Drugs
For each drug, given alone or together, cumulative doses were administered at the beginning of each of six 25-min (bar test, hindlimb test) or 20-min (schedule-controlled responding) cycles. When GHB and haloperidol were given together, they were administered as constant fractions of their D50 (i.e., 0.0625:0.0625, 0.125:0.125, 0.25:0.25, 0.5:0.5, 1:1, and 1.5:1.5), in accordance with the design described by Tallarida (2000). For example, the D50 values of GHB and haloperidol in the bar test, calculated as described below, were 485 and 0.16 mg/kg, respectively. Thus, GHB and haloperidol were administered together as 485 and 0.16 mg/kg (quantity of each compound as a fraction of 1.0 of its D50), as 242.5 and 0.08 mg/kg (quantity of each compound as a fraction of 0.5 of its D50), etc.
Dizocilpine (0.32 mg/kg) or vehicle was administered immediately before cumulative doses of GHB or haloperidol that were injected at the beginning of each of six 25-min cycles. Dizocilpine (0.32 mg/kg) and vehicle were administered also before saline was injected at the beginning of each of the six cycles.
GHB and haloperidol were purchased from Sigma-Aldrich (St. Louis, MO), and dizocilpine was from Research Biochemicals International (Natick, MA). GHB and dizocilpine were dissolved in 0.9% NaCl. Haloperidol was dissolved in 0.9% NaCl with a drop of lactic acid. All drugs were administered intraperitoneally (i.p.).
2.5. Data analysis
Catalepsy was measured as the mean time until the animal touched the surface of the table. Response rates were expressed as a percentage of control and were subsequently averaged across animals. Mean values were used to calculate doses needed to produce 50% of the maximal response (D50) by log-linear regression with PharmTools Pro (The McCary Group, Elkins Park, PA). In the procedure described by Tallarida (2000), a combination of two drugs is administered as though it were a new, third compound, and its D50 is compared with the D50 expected from additivity. Here, the D50 values (± S.E.M.) for GHB and haloperidol, when given together, were compared by means of a modified Student’s t test implemented in PharmTools Pro, with the D50 values (± S.E.M.) expected from additivity.
The maximal mean catalepsy value during the six cycles after the administration of dizocilpine alone was compared with the mean value after the corresponding saline injection by means of Student’s t test. Differences between D50 values to produce catalepsy obtained after pretreatment with dizocilpine or vehicle were tested for statistical significance by fitting log-linear regression lines to the data obtained after pretreatment with dizocilpine or vehicle, testing possible slope differences, constraining the regression lines to parallelism, and calculating the 95% confidence interval of the potency ratio (PharmTools Pro); if this interval did not contain 1, the effect of dizocilpine on the D50 value was considered statistically significant.
3. Results
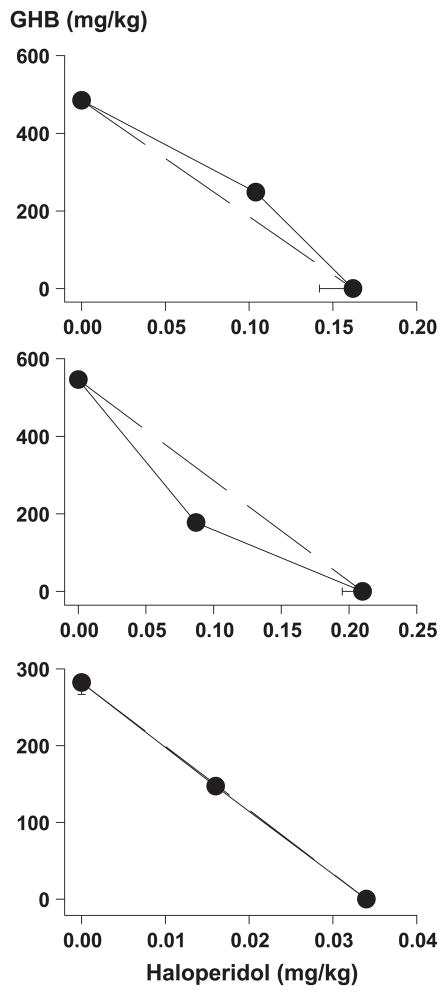
GHB and haloperidol produced catalepsy in the bar test and in the hindlimb test, with GHB being at least 2500-fold less potent (Table 1). The isobolograms in the upper and middle panels of Fig. 1 show the D50 values obtained with each of the drugs alone and with their combination. These latter values were not significantly different from the D50 values expected from additivity (Table 1).
Table 1.
D50 ± S.E.M. values for GHB and haloperidol, given alone and together, to produce catalepsy and to decrease the rate of food-maintained responding in rats (n = 8 per drug or drug combination)
|
D50 (mg/kg), i.p.
|
|||
|---|---|---|---|
| Bar test | Hindlimb test | Schedule-controlled responding | |
| GHB | 484.8 ± 14.2 | 546.3 ± 10.2 | 282.2 ± 15.4 |
| Haloperidol | 0.16 ± 0.02 | 0.21 ± 0.02 | 0.03 ± 0.00 |
| Predicted | 215.0 ± 9.6 | 240.4 ± 6.1 | 147.1 ± 2.8 |
| Observed | 248.2 ± 7.1 | 177.7 ± 8.8 | 147.1 ± 1.2 |
| Predicted/observed | 0.87 (NS) | 1.35 (NS) | 1.00 (NS) |
The D50 values observed with the combination of GHB and haloperidol were compared with the values expected from additivity by means of the modified t test described by Tallarida (2000).
Fig. 1.
Isobolograms showing D50 values of GHB and haloperidol, when given alone and when given together, to produce catalepsy (upper panel: bar test; middle panel: hindlimb test) and to decrease the rate of food-maintained responding (lower panel) in rats (n = 8 per drug or drug combination). The dashed line in each panel represents the line of additivity.
GHB and haloperidol decreased the rate of responding at doses twofold to sevenfold lower than those producing catalepsy (Table 1). The D50 value obtained with the combination of GHB and haloperidol was not significantly different from that expected from additivity (Table 1, lower panel of Fig. 1).
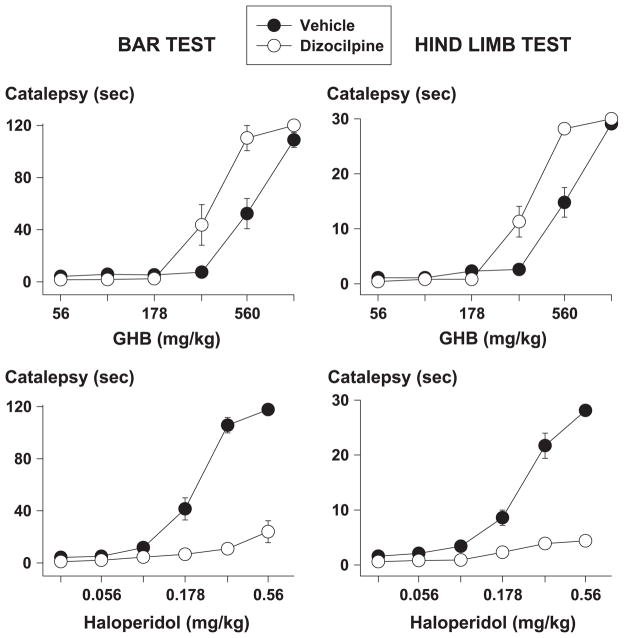
When given alone, dizocilpine did not produce catalepsy [data not shown; maximum mean catalepsy time (± S.E.M.) in the bar test and hindlimb test after 0.32 mg/kg dizocilpine: 2.57 ± 0.47 and 1.24 ± 0.29 s, respectively, which were not significantly different from the corresponding saline values, i.e., 5.70 ± 2.19 and 1.54 ± 0.41 s, respectively]. When given before GHB or haloperidol, dizocilpine enhanced GHB-induced catalepsy and attenuated haloperidol-induced catalepsy (Fig. 2). In both catalepsy tests, dizocilpine significantly decreased the D50 of GHB by almost a factor of two (Table 2). In contrast, dizocilpine increased the D50 of haloperidol in both tests, by more than a factor of two.
Fig. 2.
Cataleptic effects of GHB (upper panels) and haloperidol (lower panels) in the bar test (left panels) and in the hindlimb test (right panels) after pretreatment with 0.32 mg/kg dizocilpine (open symbols) or its vehicle (closed symbols) in rats (n = 9 per condition). Symbols represent mean ± S.E.M.; if not shown, S.E.M. values are contained by the symbol.
Table 2.
D50 ± S.E.M. values for GHB and haloperidol, given after 0.32 mg/kg dizocilpine or vehicle, to produce catalepsy (n = 9 for each pretreatment and treatment combination)
| Pretreatment | Treatment | D50 (mg/kg), i.p.
|
|
|---|---|---|---|
| Bar test | Hindlimb test | ||
| Dizocilpine Vehicle | GHB | 345.1 ± 24.5 | 338.7 ± 23.8 |
| GHB | 589.5 ± 14.9 | 551.8 ± 9.11 | |
| Potency ratio | 1.7 (1.4–2.1) | 1.6 (1.3–2.0) | |
| Dizocilpine Vehicle | Haloperidol | >0.56 | >0.56 |
| Haloperidol | 0.20 ± 0.019 | 0.23 ± 0.029 | |
| Potency ratio | >2.8 | >2.4 | |
The ratios of the D50 values obtained after pretreatment with dizocilpine or vehicle differed significantly from 1 because their 95% confidence limits (indicated between parentheses) did not contain 1.
4. Discussion
GHB, like haloperidol, produced catalepsy, as measured in two different tests involving either the forelimbs or the hindlimbs, and decreased food-maintained responding. The finding that GHB has neuroleptic-like effects confirms and extends previous reports (Snead and Bearden, 1980; Hechler et al., 1993; Navarro et al., 1998; Itzhak and Ali, 2002) and is consistent with inhibitory effects of GHB on dopamine release (Howard and Feigenbaum, 1997).
The present results agree in part with a previous report on cataleptic effects of GHB (Navarro et al., 1998). Like Navarro et al. (1998), we found GHB to produce catalepsy. Unlike Navarro et al. (1998), however, we did not find evidence for a synergistic interaction between GHB and the neuroleptic, haloperidol. In the present study, the interaction between GHB and haloperidol was examined using a recently introduced procedure that, unlike visualization by means of isobolograms, allows a statistical analysis of drug interactions (Tallarida, 2000). Using this procedure, no evidence of synergistic interactions between GHB and haloperidol was obtained. The discrepancy between the present results and those reported previously may be related to the use of different neuroleptics (haloperidol instead of tiapride), different species (rats instead of mice), and different procedures (complete dose–effect curves instead of single doses).
Although it has been suggested that GHB exerts its antidopaminergic effects directly through GHB receptors on dopaminergic terminals (Maitre, 1997), indirect effects mediated by opioid or γ-aminobutyric acid (GABA)ergic systems may be involved as well. Morphine produces catalepsy (e.g., Kuschinsky and Hornykiewicz, 1972; Costall and Naylor, 1973) and dopaminergic effects of GHB have been reported to be mimicked by opioids (Snead and Bearden, 1982) and to be attenuated by naloxone (Snead and Bearden, 1980). A role for GABAergic systems is suggested by the finding that the GABAB receptor agonist, baclofen, shares behavioral effects with GHB (e.g., Carter et al., 2003), including catalepsy (Maitre, 1997), and by the finding that the effects of baclofen on dopaminergic neurons are similar to those of GHB (Da Prada and Keller, 1976). Taken together, these observations suggest that the effects of GHB on dopamine systems may be mediated in part by GABA- and opioid-containing striatal neurons.
Whichever mechanisms underlie the cataleptic effects of GHB, they are clearly different from those involved in the cataleptic effects of the neuroleptic, haloperidol, because decreasing glutamatergic transmission with dizocilpine had opposite effects on GHB- and haloperidol-induced catalepsy. Dizocilpine attenuated the cataleptic effects of haloperidol, consistent with previous findings with noncompetitive and competitive NMDA receptor antagonists (Schmidt and Bubser, 1989; Kretschmer et al., 1992). In contrast, dizocilpine enhanced the cataleptic effects of GHB. This latter finding, together with reports that NMDA receptor antagonists enhance the cataleptic effects of morphine (Trujillo and Akil, 1991; Tzschentke and Schmidt, 1996), is further evidence of commonalities between GHB and opioids and suggests that glutamatergic transmission is similarly involved in GHB- and morphine-induced catalepsy.
GHB is a drug of abuse that is often used together with alcohol. This combination is described in the popular press as producing synergistic effects. However, a recent study of the effects of GHB and alcohol on operant responding in rats showed that when GHB was combined with alcohol, the effects of the two drugs were less than additive (Lamb et al., 2003). The authors of that study concluded that the steepness of the dose–response curves of both GHB and alcohol may account for the serious effects seen clinically when seemingly moderate doses of the two drugs are combined, rather than synergistic actions of the drug combination. Although alcohol may not interact synergistically with GHB, the present study might be the first to identify a compound that interacts in a synergistic manner with GHB. Because dizocilpine is an NMDA receptor antagonist, other NMDA receptor antagonists are likely to interact synergistically with GHB. Some of these other antagonists are, like GHB, drugs of abuse [e.g., phencyclidine (PCP), ketamine]. PCP and ketamine, like GHB, are “club drugs” that are used recreationally in social settings. The present study suggests a potentially important synergistic interaction with PCP and ketamine in the recreational use of GHB.
In summary, the present results provide further evidence for the importance of dopaminergic systems in the behavioral effects of GHB. The results suggest that GHB interacts additively, not synergistically, with neuroleptics. However, the mechanisms involved in GHB- and haloperidol-induced catalepsy are different because dizocilpine attenuated the cataleptic effects of haloperidol, but enhanced those of GHB. This latter finding suggests that other NMDA receptor antagonists (e.g., the drugs of abuse, PCP, and ketamine) may also interact synergistically with GHB.
Acknowledgments
This work was supported by USPHS grant DA14986 and RCA grant DA00211 to CPF.
References
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABAB receptors in the discriminative stimulus effects of {gamma}-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Neuroleptic and non-neuroleptic catalepsy. Arzneimittelforschung. 1973;23:674–683. [PubMed] [Google Scholar]
- Da Prada M, Keller HH. Baclofen and gamma-hydroxybuty-rate: similar effects on cerebral dopamine neurones. Life Sci. 1976;19:1253–1263. doi: 10.1016/0024-3205(76)90261-7. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Peeters BW, Honig WM, Cools AR. The paw test: a behavioural paradigm for differentiating between classical and atypical neuroleptic drugs. Psychopharmacology (Berlin) 1987;93:343–348. doi: 10.1007/BF00187254. [DOI] [PubMed] [Google Scholar]
- Hechler V, Peter P, Gobaille S, Bourguignon JJ, Schmitt M, Ehrhardt JD, Mark J, Maitre M. Gamma-hydroxybutyrate ligands possess antidopaminergic and neuroleptic-like activities. J Pharmacol Exp Ther. 1993;264:1406–1414. [PubMed] [Google Scholar]
- Howard SG, Feigenbaum JJ. Effect of gamma-hydroxybutyrate on central dopamine release in vivo. A microdialysis study in awake and anesthetized animals. Biochem Pharmacol. 1997;53:103–110. doi: 10.1016/s0006-2952(96)00664-8. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Repeated administration of gamma-hydroxybutyric acid (GHB) to mice: assessment of the sedative and rewarding effects of GHB. Ann NY Acad Sci. 2002;965:451–460. doi: 10.1111/j.1749-6632.2002.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD, Zadow B, Volz TL, Volz L, Schmidt WJ. The contribution of the different binding sites of the N-methyl-D-aspartate (NMDA) receptor to the expression of behavior. J Neural Transm Gen Sect. 1992;87:23–35. doi: 10.1007/BF01253108. [DOI] [PubMed] [Google Scholar]
- Kuschinsky K, Hornykiewicz O. Morphine catalepsy in the rat: relation to striatal dopamine metabolism. Eur J Pharmacol. 1972;19:119–122. doi: 10.1016/0014-2999(72)90086-6. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Munn J, Duiker NJ, Coop A, Wu H, Koek W, France CP. Interactions of γ-hydroxy butyrate with ethanol and NCS 382. Eur J Pharmacol. 2003;470:157–162. doi: 10.1016/s0014-2999(03)01791-6. [DOI] [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Maitre M, Andriamampandry C, Kemmel V, Schmidt C, Hode Y, Hechler V, Gobaille S. Gamma-hydroxybutyric acid as a signaling molecule in brain. Alcohol. 2000;20:277–283. doi: 10.1016/s0741-8329(99)00092-0. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Pedraza C, Martin M, Manzaneque JM, Davila G, Maldonado E. Tiapride-induced catalepsy is potentiated by gamma-hydroxybutyric acid administration. Prog Neuro-Psychopharmacol Biol Psychiatry. 1998;22:835–844. doi: 10.1016/s0278-5846(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Roth RH, Doherty JD, Walters JR. Gamma-hydroxybutyrate: a role in the regulation of central dopaminergic neurons? Brain Res. 1980;189:556–560. doi: 10.1016/0006-8993(80)90368-6. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Bubser M. Anticataleptic effects of the N-methyl-D-aspartate antagonist MK-801 in rats. Pharmacol Biochem Behav. 1989;32:621–623. doi: 10.1016/0091-3057(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Snead OC, III, Bearden LJ. Naloxone overcomes the dopaminergic EEG, and behavioral effects of gamma-hydroxybutyrate. Neurology. 1980;30:832–838. doi: 10.1212/wnl.30.8.832. [DOI] [PubMed] [Google Scholar]
- Snead OC, III, Bearden LJ. The epileptogenic spectrum of opiate agonists. Neuropharmacology. 1982;21:1137–1144. doi: 10.1016/0028-3908(82)90171-x. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose – Effect Data Analysis. Chapman and Hall/CRC Press; Boca Raton: 2000. [Google Scholar]
- Trujillo KA, Akil H. The NMDA receptor antagonist MK-801 increases morphine catalepsy and lethality. Pharmacol Biochem Behav. 1991;38:673–675. doi: 10.1016/0091-3057(91)90032-w. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Morphine-induced catalepsy is augmented by NMDA receptor antagonists, but is partially attenuated by an AMPA receptor antagonist. Eur J Pharmacol. 1996;295:137–146. doi: 10.1016/0014-2999(95)00667-2. [DOI] [PubMed] [Google Scholar]