Abstract
The primary metabolite of the herbicide atrazine (ATRA), diaminochlorotriazine (DACT), has been suggested to cause disruption in the hypothalamic-pituitary-gonadal axis leading to inhibition of luteinizing hormone (LH) release. DACT is a reactive electrophile known to form covalent protein adducts both in vitro and in vivo following ATRA exposure and maybe targeting proteins involved in GnRH-induced calcium signaling and subsequent LH release. To test this hypothesis, LβT2 pituitary cells were exposed to 300 μM DACT for 24 hrs and examined by fluorescence microscopy for GnRH-induced changes in intracellular calcium and LH release. LβT2 cells exposed to DACT had markedly diminished GnRH-induced intracellular calcium transients and a significant decreased LH release in response to GnRH. DACT appeared to cause a selective decrease in caffeine-sensitive ryanodine receptor-operated calcium stores in LβT2 cells, rather than in thapsigargin-sensitive ER calcium stores. This sensitivity correlated with the formation of covalent protein adducts by DACT, as determined by mass spectrometry. ERp57 was identified by mass spectrometry as a target of DACT adduction in the ER that could potentially mediate the effects of DACT on inhibition of GnRH-induced calcium signaling and inhibition of LH release. Intracellular calcium responses to GnRH and release of LH were restored in DACT-treated cells with the addition of a calcium ionophore (A23187). These data suggest that DACT forms adducts on proteins involved in calcium handling within the ER and that dysfunction in this critical signaling system is associated with loss of normal sensitivity to GnRH and subsequent decreased release of LH.
Keywords: atrazine, chlorotriazines, DACT, protein adducts, brain
Introduction
Atrazine (2-chloro-4-ethylamino-6-isopropyl- amino-s-triazine, (ATRA) a chlorotriazine (Cl-TRI) herbicide is one of the most heavily used herbicides in the United States, an estimated 76.4 million pounds are applied to crops each year. ATRA and other Cl-TRIs have been reported to disrupt the estrous cycle in various laboratory rat strains.1 In other animal studies, lifetime exposure to high levels of ATRA and other CL-TRIs cause premature reproductive senescence. Normal reproductive aging results from a decrease in the hypothalamic regulation of the luteinizing hormone (LH) secretion, the inability to produce enough LH to initiate ovulation causes persistent estrus.2 It has been shown in previous studies that ATRA inhibits pulsatile LH release,3 which is attributed to premature reproductive senescence. Additionally, ATRA has been shown to delay the onset of puberty in both sexes4,5 and cause a reduction in basal testosterone levels.6,7,8 These effects may be related to the atrazine induced suppression of LH release because it has been demonstrated that the onset of puberty is associated with an increased nocturnal release of LH9,10,11 and testosterone release from the testes is associated with an hourly release of LH.12,13
In the body, ATRA is extensively metabolized by P450 isozymes to mono-dealkylated chlorinated metabolites 2-chloro-4-ethyl- amino-6-amino-1,3,5-triazine (ETHYL) and 2-chloro- 4-amino-6-isopropylamino-1,3,5-triazine (ISO). Subsequent metabolism of these dealkylated intermediates produces the di-dealkylated metabolite, diaminochlorotriazine (DACT). Our group has recently shown that DACT is the major metabolite in the plasma after dosing with ATRA, accounting for over 95% of the total chlorotriazine plasma area under the curve (AUC). Thus DACT would be the likely metabolite to cause a disruption in the hypothalamic-pituitary-gonadal axis.
We have previously reported that DACT is capable of forming covalent adducts with numerous proteins in the pituitary of ATRA treated rats and in DACT treated LBT2 anterior pituitary cells.14 Although the mechanisms responsible for the suppression of the LH surge by ATRA are unknown, it is possible the LH signaling cascade is being disrupted by the covalent modifications of key proteins by DACT. We postulated in the present studies that DACT might inhibit GnRH-induced calcium signaling and thereby blunt the LH surge from pituitary gonadotrophs, possibly through covalent modification of critical calcium regulatory proteins.
Intracellular calcium (Ca2+) plays a critical role in the exocytosis of hormones and release from the endoplasmic reticulum (ER) is an essential step in the signal transduction cascade necessary for LH secretion from anterior pituitary gonadotrophs. The binding of GnRH to its G-protein coupled receptor on anterior pituitary gonadotrophs begins the signaling cascade, which causes a rapid release of calcium from ER stores that results in secretion of LH.15 Therefore, alterations in intracellular calcium release by modification of proteins within this signaling pathway could be responsible for suppression of LH release. ERp57 is a protein that has been identified as a target of DACT adduction,14 which also plays a role in calcium signaling. ERp57 modulates the redox state of SERCA 2b thiols, which could provide dynamic control of ER Ca2+ homeostasis.16
The data presented here demonstrate that DACT effectively suppresses GnRH-induced intracellular calcium transients in LβT2 pituitary cells and that direct restoration of intracellular calcium levels with a calcium ionophore is sufficient to restore LH release even in DACT treated cells. ERp57 was identified by mass spectroscopy as a target of DACT adduction in the ER that potentially could be mediating the effects of DACT on inhibition of GnRH-induced calcium signaling.
Materials and Methods
Chemicals and Materials
Diaminochlorotriazine (97.1% purity) was a gift from Syngenta Crop Science Inc. (Greensboro, NC). The DACT adduct specific antibody was generated by Strategic Biosolutions (Newark, DE), and the horseradish peroxidase-conjugated anti-rabbit antibody was purchased from Santa Cruz Biotechnology, Inc, (Santa Cruz, CA). Methanol and acetic acid were purchased from Mallinckrodt Baker Inc. (Paris, KY). Acetonitrile, bromophenol blue, Tween-80, and ammonium bicarbonate were acquired from Fisher Chemical Company (Fair Lawn, NJ). Proteomic-grade porcine trypsin, iodoacetamide (IAA), Tris-HCl, glycerol, CHAPS, urea, triflouroacetic acid, sodium citrate, hydrogen peroxie, trtiton-X-100 agarose, and Pefabloc SC were purchased from Sigma Chemical Co. (St. Louis, MO). Sypro Ruby, acrylamide, sodium dodecylsulfate (SDS), KCl, MgCl2, dithiothreitol (DTT), Tris-base, mineral oil, and glycine were purchased from Bio-Rad Laboratories (Hercules, CA).
LβT2 Cell Culture DACT Exposures
LβT2 immortalized murine anterior pituitary cells were cultured in a T-150 flask with complete Dulbelco's modified essential medium (DMEM) containing 4.5 g/l of glucose, supplemented with 10% fetal bovine serum and 1% Pen/Strep (Media Tech, Herndon VA). Cultures were grown in a humidified environment with 95% air and 5% CO2 at 37 °C to 80% confluence and then split into 4 treatment groups (N=5), which were grown on 60 mm culture dishes. The four treatment groups were exposed to DMSO (DACT vehicle control), 300 μM DACT, 300 μM DACT/25 μM A23187, and 300 μM DACT/0.25% DMSO (A23187 vehicle control). All cells were primed with 10 nM GnRH in the media for 15 mins, 4 times a day for 3 days. 12 h following the final priming with GnRH, three of the treatment groups were then exposed to 300 μM DACT in DMEM and the 4th to DMSO in DMEM (control) for 24 h.17 The cells were then rinsed with PBS and covered with 1.8ml of Earl's buffered salt solution containing 1.75 mM CaCl2 (EBSS-Ca+2). 10nM GnRH alone or in combination with 25 μM A23187 or DMSO was spiked into the EBSS-Ca+2 media for 3 mins and then the media was removed and frozen in liquid nitrogen for radioimmunoassy (RIA) analysis. LH in the EBSS-Ca+2 media were analyzed by RIA at the Colorado State University Endocrinology Laboratory as previously described.18 The remaining cells were rinsed with DMEM followed by PBS and then scraped from the flask into 15-ml conical tube. Tubes were centrifuged for 10 min at 14,000 rpm (16,000g) to form a tight cell pellet, snap frozen with liquid nitrogen, and stored at -80° C until analysis.
Cell Microsomal Fractionation
LβT2 cell pellets were fractionated into cytoplasmic, microsomal, nuclear, and cytoskeleton fractions with a Calbiochem ProteoExtract® Subcellular Proteome Extraction Kit (San Diego, CA) following manufactures protocols. Proteins from the microsomal fraction were precipitated with the Bio-Rad 2-D Clean-up Kit (Hercules, CA) following the manufacturer's protocols and dissolved by sonication in 400 μl of rehydration buffer (8M urea, 0.3% w/v DTT, 2% w/v CHAPS, pH 3-10 buffer, bromophenol blue).
Identification of DACT Adducted ERp57 in Microsomal Fractions
DACT adducted ERp57 was identified in the microsomal fraction by 2DE/Western blotting followed by mass spectral analysis of targeted protein spots. For the first dimension (isoelectric focusing) of the 2DE, the microsomal fraction (200 μl) was pipetted into a rehydrating tray, a 7 cm immobilized pH gradient (IPG) strip, pH 5-8 (Bio-Rad, Hercules, CA), was placed on the sample solution, and the strip was allowed to rehydrate passively for at least 12 h. Using a Protean IEF Cell (Bio-Rad, Hercules, CA), the protein mixture was focused using the voltage program of an increase from 0 to 500 V over 1 min, 500 to 3500 over 5 h, and steady exposure to 3500 V for 17.5 h. For the second dimension (SDS-PAGE) separation, the IPG strip was removed from the focusing tray, reduced in 2% w/v DTT in equilibration buffer (6 M urea, 30% v/v glycerol, 2% w/v SDS, 24 mM Tris-HCl) for 15 min, followed by alkylation in 2.5% w/v IAA in equilibration buffer for 5 min. The IPG strip was placed on a slab gel (12% polyacrylamide) and proteins separated for 1.5 h at 200 V in a Mini-Protean III cell (Bio-Rad, Hercules, CA). 2-D gels were either stained with Sypro Ruby for protein detection or subjected to Western blotting for immunodetection of DACT-adducted proteins as previously described.14 For protein detection, the gel was stained in 100 ml of Sypro Ruby stain for 2 hours and then destained with 10% methanol/7% acetic acid. Stained proteins were visualized with 320 nm UV light using a BioChemi Bioimaging system (UVP, Upland, CA) and the image saved for comparison with Western blotting results. Protein spots from the Western blot were matched to spots on Sypro-stained gels using the Delta 2D 3.6 (Decodon, Greifswald, Germany) gel image analysis program. Spot patterns were matched between the gel and Western blot first by automation creating a fusion image, followed by manual warping to align spots. Matched protein spots thought to be ERp57 based on mass and isoelectric point were digested with trypsin and analyzed with MALDI TOF/TOF mass spectrometry as previously described.14 The combined MS and MS/MS spectra for each spot were searched using the Mascot (version 2.2) database search engine against the Rattus (Taxonomy ID 101144) entries in Swiss-Prot (version 51.6) database containing 5769 sequence entries. Parameters used in the database search were: peptide mass tolerance of 0.15 Da, fragment ion mass tolerance of 0.8 Da, trypsin peptides allowing for 1 missed cleavage, variable modifications of cysteine carbamidomethylation and methionine oxidation.
LBT2 Cell Culture for Calcium Imagining
Immortalized LβT2 anterior gonadotrophs (Courtesy of Dr. Colin Clay, Colorado State University) were cultured in DMEM media with L-glutamine, supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 units/mL penicillin, 50 ng/mL streptomycin, and 100 ng/mL neomycin (PSN) at 37 °C, 5% CO2 in a humidified atmosphere. Cells were grown in 60 mm culture dishes for protein expression studies or subcultured onto FBS-coated 2-well borosilicate chambered coverglass slides (Thermo-Fischer, Rochester, NY). Cells were allowed to adhere for 48 hr prior to any treatments and then phenotypically primed with 10 nM GnRH for 15 min every 1.5 hr, 4 times a day for 3 days. Approximately 400,000 LβT2 cells/well were plated onto chambered coverglass slides for imaging studies.
Intracellular Calcium Imaging
To detect changes in intracellular calcium, LβT2 cells were incubated with 2 μM Fluo-4-acetylmethylesther (AM) (Invitrogen, Carlsbad, CA) for 15 min at 37 ºC in culture medium under a humidified atmosphere at 5% CO2. Cells were then transferred to imaging medium (Earl's buffered salt solution, EBSS) containing 1.75 mM CaCl2 (EBSS+Ca2+). For experiments employing calcium-free media, EBSS was supplemented with 2 mM EGTA instead of CaCl2 (EBSS-Ca2+). Cells were mounted on the stage of a Zeiss 200M inverted fluorescence equipped with a DG-4 xenon excitation source (Sutter Instruments, Novato, CA) and ORCA-ER cooled interline charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan). Images were acquired every 10 sec for 5 min and various pharmacologic agonists were added at the 30 sec time point (10 nM GnRH, 2 μM thapsigargin or 10 mM caffeine). Images of Fluo-4 fluorescence were detected at 490 nm excitation/515 nm emission through a 20× NeoFluor air objective. Data were analyzed as the mean background-subtracted fluorescence intensity of each cell normalized to the intensity of the first image (F/F0). Both data acquisition and analysis were performed using Slidebook software (Version 4.0, Intelligent Imaging Innovations, Denver, CO). Cells utilized for the DACT portion of the experiments were treated with 300 μM DACT (96.8% pure, Syngenta Corporation) for 24 hr following the final GnRH priming on day 3. Minimums of 500 – 800 cells were imaged per treatment group over at least 3 independent experiments.
Radioimmunoassay for Luteinizing Hormone (LH)
LβT2 cells were cultured in 60 mm tissue culture dishes to 80-85% confluency and primed with GnRH as described above. After priming, cells were exposed to 300 μM DACT or vehicle control (DMSO) for 24 hr. The cells were then rinsed gently with PBS and 1.8 mL of EBSS-Ca2+ was added. 10 nM GnRH was then added to the cells for 3 min, followed by removal of the EBSS-Ca2+ which was then frozen at -80° C for LH RIA. RIA was performed by the Colorado State University Endocrinology Laboratory as previously described.18
Statistical Analysis
Multiple groups were analyzed by one-way ANOVA and Tukey's post-hoc test. Two-group comparisons were analyzed the Students t-test. Differences were considered significant at p<0.05. Data were analyzed using Prism software (v4.0a, Graphpad Software, Inc., San Diego, CA). Differing letters (a, b, c, etc) in graphs indicate significant differences between groups. Significance was set at p<0.05.
Results
Identification of DACT Adducted ERp57 in Microsomal Fractions
LβT2 cells exposed to 300 μM DACT showed a representative 2DE spot pattern seen in Figure 1A. Western Blots of these gels using a DACT adduct specific antibody showed several DACT modified proteins in the 57kDa/5.8 IP range (Figure 1B), while blots from DMSO exposed control cells showed no spots (Figure 1C). MS analysis of these spots identified two as ERp57, again with slightly different isoelectic points suggesting differential post-translational modifications. Proteins spots identified as DACT adducted ERp57 in LβT2 cells exposed to DACT are listed in Table 1, along with Mascot protein ion scores and cutoffs, and sequence coverage for each identified protein.
Figure 1. DACT bind to ERp57 localized in the ER of LβT2 cells.

(A) 2DE of Sypro Ruby-stained water-soluble proteins from LβT2 cells exposed to 300 μM DACT within the 50-75 kDa and 5-6 isoelectric point range. (B) 2DE Western blots for DACT adducted proteins in LβT2 cells exposed to 300 μM DACT and (C) 2DE western blots showing no adducted proteins in unexposed LβT2 cells. Numbering corresponding to protein identifications in Table 1.
Table 1.
Protein spots identified with MALDI TOF/TOF MS as DACT adducted ERp57 in the L®T2 cells exposed to 300 ⌠M DACT (spots 1 and 2).
| Spot No. | Protein Designation (Accession) | Mascot proteinion score* (cutoff score) | Sequence coverage (matched peaks) | Peptide sequences identified by MS/MS spectra | Mascot MS/MS score* (cutoff score) |
|---|---|---|---|---|---|
| 1 | ERp57 (P11598) | 388 (50) | 31% (17) | K.FVMQEEFSR.D R.ELNDFISYLQR.E K.FISDKDASVVGFFR.D K.EYDDNGEGITIFRPLHLANK.F | 184 (23) |
| 2 | ERp57 (P11598) | 333 (50) | 48% (24) | K.FVMQEEFSR.D R.ELNDFISYLQR.E K.EYDDNGEGITIFRPLHLANK.F | 118 (23) |
Protein ion scores greater than cutoff score are significant (p<0.05)
GnRH Dependent Intracellular Calcium Imaging
The effect of DACT on GnRH-induced intracellular Ca2+ levels (Ca2+i) in LβT2 cells was examined in Figure 2. Following pretreatment with 300 μM DACT for 24 hrs, LβT2 cells were stimulated with 10 nM GnRH and relative changes in Ca2+i detected by wide-field fluorescence microscopy. Addition of GnRH produced a rapid rise in Ca2+i in control LβT2 cells that was strongly attenuated by pretreatment with DACT. The amplitude of the initial Ca2+i peak was decreased but the sustained or ‘plateau’ phase of the transient remained unchanged (Figure 2A). Quantification of fluorescence intensity (Figure 2B) demonstrated a significant decrease in the average maximum amplitude of GnRH-induced Ca2+i transients in DACT treated LβT2 cells, relative to vehicle treated control cells.
Figure 2. Pretreatment with DACT blunts GnRH-induced intracellular calcium transients in LβT2 cells.
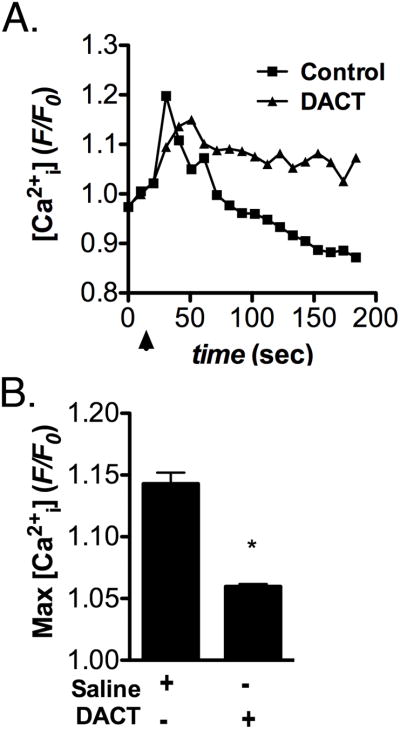
LβT2 cells were phenotypically primed by exposure to GnRH as described in Materials and Methods and exposed to vehicle control (DMSO) or DACT (300 μM) for 24 hrs prior to calcium imaging. Intracellular calcium (Ca2+i) was detected using Fluo-4-AM and fluorescence images were collected at 10 Hz. Following baseline acquisition for 30 sec, GnRH was added to induce Ca2+i transients. (A) Representative traces showing the time course of GnRH-induced Ca2+i transients in in control and DACT-treated LβT2 cells. (B) Quantification of the maximal amplitude of GnRH-induced Ca2+i transients indicates that DACT pretreatment potently suppresses Ca2+i transients in LβT2 cells. Data were combined from 3 independent experiments, averaging 700 - 800 cells total per experimental group. (*p<0.05)
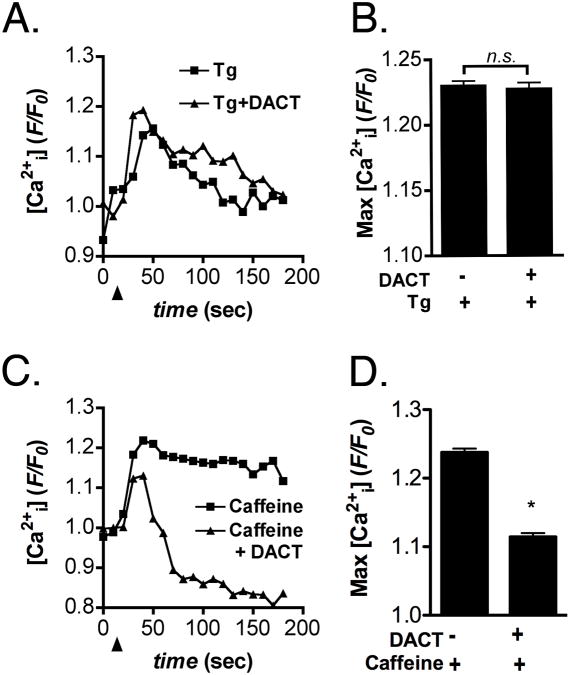
To examine potential causes of DACT-induced inhibition of GnRH-dependent Ca2+ signaling, releasable Ca2+ from intracellular stores was evaluated in Figure 3. LβT2 cells primed by GnRH exposure were exposed for 24 hrs to 300 μM DACT and examined for changes in Ca2+i following stimulation with either 10 μM thapsigargin (Tg) or 5 mM caffeine to induce release of Ca2+ release from Tg- or ryanodine receptor-dependent ER stores, respectively. There was no difference in Tg-induced release of ER Ca2+ between control and DACT treated cells (Figure 3A,B), whereas caffeine-induced Ca2+ release from ryanodine receptor-sensitive ER stores was significantly decreased (Figure 3C,D).
Figure 3. DACT selectively depletes ER calcium stores in LβT2 cells.
LβT2 cells primed by GnRH exposure were exposed for 24 hrs to 300 μM DACT or vehicle control (DMSO) and examined for changes in intracellular Ca2+ following stimulation with either 10 μM thapsigargin (Tg) or 5 mM caffeine. (A,B) Tg-induced release of Ca2+ from ER stores was unaffected in LβT2 cells treated with DACT, relative to vehicle-treated control cells. (C,D) Caffeine-induced release of Ca2+ from ryanodine receptor-sensitive stores was reduced following treatment with 300 μM DACT. Data were combined from 3 independent experiments, averaging 500 - 600 cells total per experimental group. (*p<0.05)
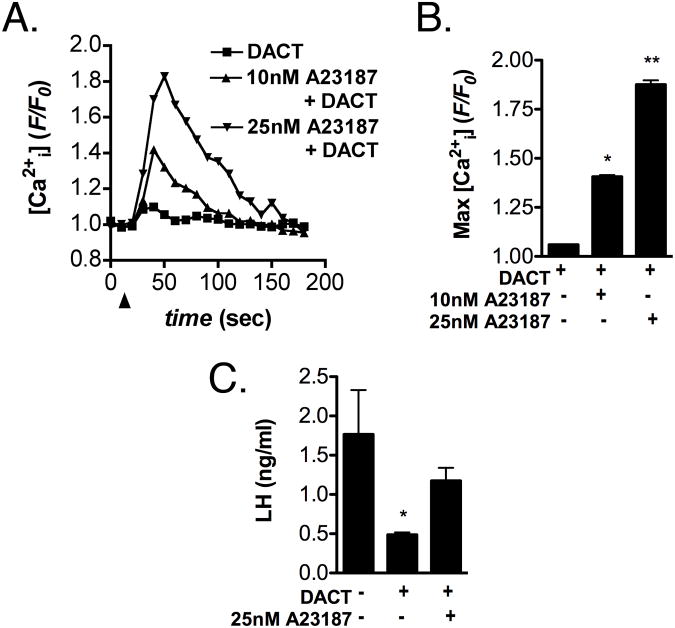
To determine whether augmenting Ca2+i could reverse the effects of DACT on GnRH-induced LH release, DACT-treated LβT2 cells were examined for changes in Ca2+i induced by increasing concentrations of the Ca2+ ionophore, A23187 (Figure 4). Although DACT-pretreatment abolished the effects of GnRH on Ca2+i transients, stimulation with 10 and 25 nM A23187 still resulted in a rapid increase in Ca2+i in DACT-pretreated LTβ2 cells (Figure 4A). Quantification of the maximum amplitude of A23187-induced Ca2+i transients (Figure 4B) revealed a dose-dependent increase in the peak amplitude of Ca2+i transients similar to that induced by GnRH in control LTβ2 cells. GnRH-induced release of LH was also examined in control and DACT-pretreated LTβ2 cells by radioimmunoassay (Figure 4C). GnRH-induced release of LH was inhibited in LβT2 cells exposed to 300 μM DACT for 24 hrs and this effect was reversed by increasing Ca2+i through stimulation with 25 nM A23187.
Figure 4. Restoration of intracellular calcium levels reverses the effects of DACT on GnRH-induced release of LH.
(A) LβT2 cells were exposed to DACT or vehicle control (DMSO) for 24 hrs and examined for changes in Ca2+i transients following stimulation with the Ca2+ ionophore, A23187 (0, 10, and 25 nM). (B) Quantification of the average maximum amplitude of A23187-induced Ca2+i transients indicates a dose-dependent increase in Ca2+i in DACT-treated cells. (C) LH levels were determined by radioimmunoassay (RIA) in cell culture supernatants following stimulation with GnRH. GnRH-induced release of LH was inhibited in LβT2 cells were exposed to DACT but was restored upon stimulation with 25 A23187. Data were combined from 3 independent experiments, averaging 500 - 600 cells total per experimental group (A,B) or from 5 - 7 independent cell cultures. (*Different from control, p<0.05. **Different from control and 10 nM A23187, p<0.05)
Discussion
DACT is the major metabolite in the plasma shortly after dosing with ATRA19 and could cause a disruption in the hypothalamic-pituitary-gonadal axis leading to the numerous abnomalites observed following ATRA exposure in the rodent model. DACT is reactive towards exposed cysteine residues in vitro forming stable covalent adducts through a non-enzymatic nucleophilic substitution reaction at the chlorine moiety.20 Numerous DACT-adducted proteins have been identified in the pituitary and hypothalamus in previous studies with ATRA exposed rodents suggesting widespread protein modification.14,21 Nearly all of the identified modified proteins share the common characteristic of exposed cysteine residues. ERp57 was one of these proteins that was found to be modified by DACT in both the pituitary and hypothalamus following in vivo and in vitro ATRA exposures.
ERp57 is an oxidoreductase found in the endoplasmic reticulum (ER) of cells, where its exact function is not completely understood. It has been shown to be involved in formation of disulphide bonds and folding glycoproteins when associated with calnexin and careticulin,22,23 as well as in the redox modulation of SERCA 2b (calcium ATPase) providing dynamic control of ER calcium homeostasis.16 ERp57 contains two redox active Cys-Gly-His-Cys motifs (Cys-57, 60, 406, 409) that forms mixed disulfide bonds with substrate protiens. SERCA 2b contains two conserved cysteine residues exposed to the lumen of the ER (Cys 875 and Cy887). Formation of a disulphide bond between ERp57 and these lumen cysteine residues of SERCA 2b has been shown to disrupt calcium cycling consistent with inhibition of the pump.16 These Cys residues are potential targets for DACT and it is possible that alkylation by DACT could prevent the ERp57/SERCA 2b interaction and thereby reduce dynamic control of calcium homeostasis.
Using cellular fractionation procedures, we were able to isolate proteins from the microsomal fraction of LβT2 cells exposed to DACT at concentrations shown to disrupt LH secretion. From this isolated microsomal fractions, we were able to identify ERp57 as a target of DACT through immunoreactivity and identification with mass spectrometry (Table 1). This identification of DACT adducted ERp57 in the lumen of the ER indicates that the ERp57 interaction and possible control of SERCA 2b could be compromised with DACT exposure. The resulting disruption of calcium homeostatisis could be of significant detrimental consequence and explain the suppression of LH release from murine LβT2 cells by suppressing GnRH-induced intracellular calcium transients.
The data in Figure 2 indicate that the capacity of GnRH to induce Ca2+ transients in LβT2 cells is dramatically blunted by DACT. This inhibition closely correlates with decreased release of LH, suggesting that the mechanism by which DACT inhibits LH release in this cell type involves a decreased capacity to mobilize calcium from intracellular stores. This is supported by the data in Figure 2A demonstrating that only the peak Ca2+ levels were suppressed by DACT, whereas steady state levels remained similar to control cells minutes after the initial high amplitude transient subsided. It is therefore unlikely that capacitive calcium entry from extracellular sources is compromised by DACT but rather that mobilization of calcium from intracellular stores is the likely site of inhibition of the GnRH-induced Ca2+i transient.
To test this hypothesis, we used selective pharmacologic stimulants for the two major pools of ER calcium in control and DACT-treated cells (Figure 3). Releasable Ca2+ from thapsigargin-sensitive ER stores was not inhibited by DACT, whereas DACT caused a significant decrease in ryanodine receptor-releasable ER calcium stores following stimulation with 5 mM caffeine. These data identify ryanodine receptor-linked ER calcium stores as a direct target of DACT in LβT2 cells and suggest that loss of mobilization from this intracellular pool of calcium may underlie the capacity of DACT to inhibit release of LH in response to GnRH signaling. GnRH binding to metabotropic receptors elicits activation of phospholipase C and rapid mobilization of Ca2+ through IP3 receptors on the ER, which are tightly linked to regulatory proteins such as ERp44, ERp57 and Ero1alpha that mediate rapid and reversible flux of calcium through the ER.24 It is therefore plausible that ERp57 or other proteins in the pathway regulating ryanodine receptor-mediated storage or uptake of calcium are inhibited by DACT.
The functional consequence of restoring intracellular levels in DACT treated cells was examined using the calcium ionophore A23187. The data in Figure 4 indicate that exposure to low nanomolar doses of A23187 was sufficient to cause rapid, dose-dependent increases in Ca2+ in LβT2 cells that were equivalent in amplitude to native transients induced by stimulation with GnRH, even after pretreatment with DACT. Moreover, A23187 stimulation resulted in dose-dependent increases in LH in DACT treated cells similar to untreated control cells (Figure 4C). These data strongly support that impaired intracellular Ca2+ release is a fundamental mechanism underlying the capacity of DACT to inhibit GnRH-induced LH release in LβT2 cells. Moreover, because A23187-stimulated cells release similar levels of LH as untreated control cells, it is unlikely that reduced production of LH by pituitary gonadotrophs is the cause of the blunted LH surge in response to exposure to ATRA in experimental animals. The capacity of DACT-treated LβT2 cells to release LH upon stimulation with A23187 also suggests that calcium-dependent vesicular release of LH is not impaired, further implicating ryanodine receptor-linked ER calcium stores as a critical target of DACT in this cell type.
In summary, the results of in vitro experiments presented herein argue that in part, the mechanism of action for previously observed reduction of LH levels in experimental animals could involve disruption of pituitary calcium signaling associated with protein adduction (ERp57) by the reactive ATRA metabolite DACT. Since ERp57 has previously been found to be modified by DACT in both the pituitary and hypothalamus following in vivo ATRA exposures,14,21 we propose that adduction of the ERp57 protein interferes with regulatory networks associated with receptor-mediated storage and/or calcium uptake, which in turn results in LH suppression. Moreover, preliminary unpublished studies conducted in ATRA treated female SD rats have initially confirmed that ATRA is capable of blunting LH release from perifused hemi-pituitary fragments challenged with 100 nM GnRH and 200 mM KCL further confirming that there is a direct effect of ATRA (via DACT) on the pituitary. However, further animal studies will be needed to confirm our in vitro findings using LβT2 cells as well as the above described initial unpublished observations.
Supplementary Material
Acknowledgments
This project was supported by the Center for Environmental Medicine, The College of Veterinary Medicine and Biomedical Sciences (College Research Council) and the High Plains Intermountain Center for Agricultural Health and Safety pilot grant program (CDC/NIOSH grant U50 OH0 08085).
References
- 1.Cooper R, Stoker T, Tyrey L, Goldman J, McElroy W. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- 2.Cooper R, Stoker T, Goldman J, Parrish M, Tyrey L. Effect of atrazine on ovarian function in the rat. Reprod Toxicol. 1996;10:257–264. doi: 10.1016/0890-6238(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 3.Foradori C, Hinds L, Hanneman W, Legare M, Clay C, Handa R. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin-releasing hormone receptor agonist in female Wistar rats. Biol Reprod. 2009;81:40–45. doi: 10.1095/biolreprod.108.075713. [DOI] [PubMed] [Google Scholar]
- 4.Stoker T, Guidici D, Laws S, Cooper R. The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol Sci. 2002;67:198–206. doi: 10.1093/toxsci/67.2.198. [DOI] [PubMed] [Google Scholar]
- 5.Laws S, Ferrell J, Stoker T, Cooper R. Pubertal development in female Wistar rats following exposure to propazine and atrazine biotransformation by-products, diamino-S-chlorotriazine and hydroxyatrazine. Toxicol Sci. 2003;76:190–200. doi: 10.1093/toxsci/kfg223. [DOI] [PubMed] [Google Scholar]
- 6.Stoker T, Laws S, Guidici D, Cooper R. The effect of atrazine on puberty in male Wister rats: and evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci. 2000;58:52–59. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- 7.Trentacoste S, Friedmann A, Youker R, Breckenridge C, Zirkin B. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Andology. 2001;22:142–148. [PubMed] [Google Scholar]
- 8.Friedmann A. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 9.Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972(287):582–586. doi: 10.1056/NEJM197209212871203. [DOI] [PubMed] [Google Scholar]
- 10.Parker D, Judd H, Rossman L, Yen S. Pubertal sleep-wake patterns of episodic LH, FSH and testosterone release in twin boys. J Clin Endocrinol Metab. 1975;40:1099–1109. doi: 10.1210/jcem-40-6-1099. [DOI] [PubMed] [Google Scholar]
- 11.Kulin H, Moore R, Santner S. Circadian rhythms in gonadotropin excretion in prepubertal and pubertal children. J Clin Endocrinol Metab. 1976;42:770–773. doi: 10.1210/jcem-42-4-770. [DOI] [PubMed] [Google Scholar]
- 12.Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243:257–263. doi: 10.1152/ajpendo.1982.243.3.E257. [DOI] [PubMed] [Google Scholar]
- 13.Dufau M, Veldhuis J, Fraioli F, Johnson M, Beitins I. Mode of secretion of bioactive luteinizing hormone in man. J Clin Endocrinol Metab. 1983;57:993–1000. doi: 10.1210/jcem-57-5-993. [DOI] [PubMed] [Google Scholar]
- 14.Dooley G, Reardon K, Prenni J, Tjalkens R, Legare M, Foradori C, Tessari J, Hanneman W. Proteomic analysis of diaminochlorotriazine adducts in wistar rat pituitary glands and LbetaT2 rat pituitary cells. Chem Res Toxicol. 2008;21:844–851. doi: 10.1021/tx700386f. [DOI] [PubMed] [Google Scholar]
- 15.Tse A, Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992;255:462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Camacho P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol. 2004;164:35–46. doi: 10.1083/jcb.200307010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas P, Mellon PL, Turgeon J, Waring DW. The L beta T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology. 1996;137:2979–89. doi: 10.1210/endo.137.7.8770922. [DOI] [PubMed] [Google Scholar]
- 18.Niswender G, Reichert L, Jr, Midgley A, Nalbandov A. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- 19.McMullin T, Brzezicki J, Cranmer B, Tessari J, Andersen M. Pharmacokinetic modeling of disposition and time-course studies with C14-atrazine. J Toxicol Env Health Pt A. 2003;66:941–964. doi: 10.1080/15287390306454. [DOI] [PubMed] [Google Scholar]
- 20.Dooley G, Prenni J, Prentiss P, Cranmer B, Andersen M, Tessari J. Identification of a novel hemoglobin adduct in Sprague Dawley rats exposed to atrazine. Chem Res Toxicol. 2006;19:692–700. doi: 10.1021/tx060023c. [DOI] [PubMed] [Google Scholar]
- 21.Dooley G, Ashley A, Legare M, Handa R, Hanneman W. Proteomic analysis of diaminochlorotriazine (DACT) adducts in three brain regions of Wistar rats. Toxicol Lett. 2010;199:17–21. doi: 10.1016/j.toxlet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Zapun A, Darby N, Tessier D, Michalak M, Bergeron J, Thomas D. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J Biol Chem. 1998;273:6009–6012. doi: 10.1074/jbc.273.11.6009. [DOI] [PubMed] [Google Scholar]
- 23.High S, Lecomte F, Russell S, Abell B, Oliver J. Glycoprotein folding in the endoplasmic reticulum: a tale of three chaperones? FEBS Lett. 2000;476:38–41. doi: 10.1016/s0014-5793(00)01666-5. [DOI] [PubMed] [Google Scholar]
- 24.Simmen T, Lynes E, Gesson K, Thomas G. (2010) Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM) Biochim Biophys Acta. 2010;1798:1465–1473. doi: 10.1016/j.bbamem.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.