Abstract
Objectives
Psychological stress may play a role in metabolic syndrome. A consequence of metabolic syndrome is endothelial dysfunction, which is also influenced by psychological stress. We sought to compare the effect of consciously resting meditation (CRM), a sound (mantra)-based meditation, with a control intervention of health education (HE) on endothelial function in the setting of metabolic syndrome.
Methods
Sixty-eight black Americans with metabolic system risk factors (age 30 to 65 years) were randomized to either CRM (N=33), or to HE (N=35); interventions were matched for frequency and duration of sessions and lasted 12 months. Endothelial function was assessed by brachial artery flow-mediated dilation (FMD%) at baseline, 6 and 12 months. Arterial elasticity, metabolic risk factors, psychosocial and behavioral variables were secondary endpoints.
Results
Although FMD % improved in the CRM group over 12 months, this increase was not significantly higher than in the HE group (p=0.51 for the interaction between group and time). Non-endothelium dependent dilation and arterial elasticity did not change in either group. Most metabolic syndrome risk factors showed beneficial trends in the CRM group only. A risk factor score counting the number of metabolic syndrome components decreased in the CRM group but not in the control HE group (p=0.049 for the interaction between treatment group and time).
Conclusions
Among black Americans with metabolic syndrome risk factors, CRM, a sound-based meditation, did not improve endothelial function significantly more than a control intervention of health education. CRM resulted in favorable trends in metabolic syndrome risk factors which were examined as secondary outcomes.
Keywords: Endothelium, stress, metabolic syndrome, obesity
Introduction
Cardiovascular disease (CVD) and its complications carry a significantly higher morbidity and mortality in black Americans compared with white Americans. One factor likely to play a role is obesity, which is more prevalent in black Americans and is associated with insulin resistance and other cardiometabolic risk factors including elevated blood pressure (BP), elevated fasting glucose and dyslipidemia.(1,2) It is anticipated that the explosive epidemic of obesity, which is particularly a problem in the Southeast region of the nation, will trigger an increase in CVD, as already observed for hypertension (2) and type 2 diabetes.(3) These trends herald a further widening of the gap in black-white differences in CVD morbidity and mortality.
Although the etiology of insulin resistance is complex, psychological stress may play a role through increasing inflammation and other metabolic and hormonal abnormalities.(4) A major consequence of insulin resistance is endothelial dysfunction,(5) an early marker of CVD which is also influenced by psychological stress.(6-8) Insulin resistance is thought to affect endothelial function through impaired endothelial nitric oxide synthase activity caused by excess superoxide anion formation in vascular endothelial cells.(9,10)
Previous controlled clinical studies have suggested that mind-body interventions for stress reduction using a variety of meditation techniques may reduce psychological stress and improve CVD risk factors in blacks and whites, although results are heterogeneous.(11) Of various meditation techniques, transcendental meditation (TM)(12-15) and breathing awareness meditation (16-18) have been widely used in studies of black Americans and show promise in reducing BP in this population. In addition to elevated BP, it is possible that meditation approaches are beneficial in reducing other adverse consequences of obesity and metabolic syndrome, including endothelial dysfunction, inflammation and insulin resistance. These effects, however, have never been evaluated in community-dwelling blacks. We therefore conducted a randomized controlled trial among black Americans with metabolic syndrome risk factors to test the hypothesis that consciously resting meditation (CRM), a novel sound (mantra)-based meditation, improves vascular function and metabolic risk profile in participants with metabolic syndrome risk factors compared with a control arm of health education (HE) matched for frequency and duration of sessions. The primary outcome was endothelial function measured by flow-mediated dilation (FMD) of the brachial artery using ultrasound. Secondary outcomes included measures of arterial elasticity, metabolic syndrome risk factors, psychosocial and behavioral measures (symptoms of anxiety, depression, perceived stress, anger, and hostility, and physical activity), and inflammatory and metabolic biomarkers.
Methods
Study Design
This trial was part of the “Emory-Morehouse Partnership to Reduce CV Disparities” (U01HL079214), also known as META-Health. The overall goal of this research program was to characterize potential racial/ethnic differences in obesity-related cardiovascular risk. The present study was a single-site, parallel-group randomized controlled trial. The trial was technically single-blinded given that patients were aware of which intervention they were assigned to (CRM or HE). However, participants were masked to the research hypothesis and both interventions were presented to participants as health promoting; this design should minimize expectation bias.(11) Investigators and the staff collecting the data were blinded to the treatment status of the participants. The two treatment providers were not involved with participant recruitment, data collection, analysis or interpretation. The study was approved by the Emory University and Morehouse University institutional review committees. Informed consent was obtained from all participants.
Selection of Participants
Participants were recruited from the community in metropolitan Atlanta through flyers and at health fairs, churches, university campuses and other community locations, as well as through direct referrals. They were included in the trial if they were between the ages of 30 and 65, self-identified as blacks, and met specific criteria for metabolic syndrome. According to current ATP III guidelines,(19) metabolic syndrome is defined as having at least 3 out of 5 of the following criteria: abdominal obesity (waist circumference >102 cm in men and >88 cm in women); triglycerides ≥150 mg/dL; HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women; BP≥130/≥85 mm Hg; and fasting glucose ≥100 mg/dL. However, these criteria have not been extensively validated in the black population. A paradoxical observation is that black Americans meet the metabolic syndrome criteria less often than whites despite a higher prevalence of obesity, hypertension and insulin resistance,(20) partly because of a lower triglycerides/HDL ratio.(21,22) Thus, there is concern that current metabolic syndrome definitions may underestimate risk in black Americans.(23,24) Based on this, in addition to the standard definition above, participants were eligible if they met a modified definition which did not include the lipid criteria, but they met 2 out of the following 3 criteria: abdominal obesity (waist circumference >102 cm in men and >88 cm in women); BP≥130/≥85 mm Hg; and fasting glucose ≥100 mg/dL. Subjects were excluded if they had known CVD or renovascular disease; if they had uncontrolled hypertension (systolic BP>160 mm Hg on two or more occasions or diastolic BP>105 mm Hg); if they were current smokers or had been taking over the counter vitamins such as vitamin A, C or E within three days of study initiation (since these can affect endothelial function assessments); if they were pregnant; and if they had documented history of alcohol or drug abuse or other psychiatric or medical diagnoses that would interfere with ability to attend training sessions and clinic visits for one year.
Study Protocol
After pre-screening for eligibility over the phone, potential participants attended a screening visit (Visit 1). After signing the informed consent, research staff obtained fasting blood, BP measurements and anthropometric data in order to verify the inclusion criteria. Qualifying participants were then invited to a baseline visit (Visit 2) within 2 weeks, where a number of measures were obtained, including vascular function testing, BP, anthropometry and study questionnaires. After completion of baseline assessments, participants were randomized to one of two treatment groups by blocked randomization with stratification on gender. The random allocation sequence was provided in sealed envelopes by the study statistician (who had no contact with participants) to a staff member not involved in data collection. Patients were randomized in subsequent cohorts of participants, with a minimum of 10 and a maximum of 20 in each randomization cohort (yielding no more than 10 participants per group who would start the program at any one time), for a total of 5 cohorts. All outcome data were collected in a blinded fashion with respect to the participants' treatment status. Follow-up visits for outcome assessments were performed at 6 months (Visit 3) and 12 months (Visit 4) after baseline. Three BP measurements were obtained at each visit.(25) Anthropometric measures, including height, weight, and waist circumference were measured and fasting blood was also drawn for lab assessments. Visit 1 through 4 took place in the Cardiovascular Research Unit at the Emory University Hospital. Participant recruitment started on June 7, 2007, and the follow-up of the last randomized cohort ended on January 11, 2010.
Interventions
Both interventions were administered in the National Center for Primary Care at Morehouse School of Medicine, by providers not involved with participant recruitment, data collection, analysis or interpretation, CRM is a sound or mantra based meditation. This meditation approach was chosen because of its standardized protocol and its similarity to the TM program, which has been previously successfully implemented in black American samples. Both TM and CRM use sounds that have no meaning, but their quieting effects have been known for thousands of years. These sounds, when used properly, settle the mind and body down to a state of restful alertness. As with many TM studies, the CRM program included 21 sessions over a one year intervention period where participants learned the technique of consciously resting their mind and body. The core instruction involves a four-step course over four consecutive days (sessions 1-4) and a follow-up program over 12 months (sessions 5-21). Most sessions last 1 to 1.5 hours, and the general format is group meditation plus a lecture/discussion or videotape. In contrast to TM, CRM does not include a private Sanskrit ceremony, which has been a major objection for inner-city meditation projects. Additionally, in contrast to TM, CRM can be taught in groups, is less time consuming for participants, and is potentially more affordable, making it more accessible and easier to disseminate among minority groups. All sessions were taught by the same experienced teacher (KK). Subjects were instructed to practice CRM twice a day for 20 minutes. Participants were also given the same health education reading materials given to the control group.
Subjects randomized to HE attended the same number, size and frequency of group meetings lead by a professional health educator (LB). The program included information on prevention of CVD through lifestyle modification and was modeled on educational material disseminated by the American Heart Association (www.americanheart.org). Topics included the value of a healthy diet, exercise, and weight management. The impact of stress was discussed as it relates to weight management and physical exercise. However, to avoid contamination in the experimental design, the HE sessions did not include instructions on stress reduction or relaxation techniques. To match the 20-minute twice-a-day CRM practice, participants were instructed to undertake a 20-minute twice-a-day home practice session applying the recommendations given in this course—diet, exercise, or other lifestyle habits.
Primary Outcome
Endothelium-Dependent Flow-Mediated Vasodilation (FMD) of the brachial artery was the primary outcome of the trial. It was measured according to established methodology (26) by using an Acuson 10 mHz linear array transducer and an Acuson Aspen ultrasound system. We imaged the participants after they had rested in supine position for at least 10 minutes in a quiet setting. Optimal brachial artery images were obtained 2-10 cm above the antecubital crease. This location was marked, and all subsequent images were obtained at the same location. After baseline measurements, a BP cuff was inflated to 200 mm Hg over the proximal portion of the right arm for 5 minutes. Endothelium-dependent function was determined during the first two minutes of release of the cuff. The flow dependent response was then allowed to return to baseline over a period of five minutes.
Four triggered events (defined as the end of the T wave on the ECG) were recorded. Each triggered event consisted of three sequential frames, for a total of 12 images. These were subsequently downloaded to an analysis system that allows automatic edge detection of the M-line that defines the intima-media interface for both the near wall and the far wall of the artery using customized software (Medical Imaging Applications Inc, Iowa). Measurements from the 12 frames were averaged. In our laboratory, the mean difference and standard deviation (SD) in FMD between assessments performed in 11 participants on consecutive days was 1.26% (SD 0.76%), with a correlation coefficient of 0.75. The mean difference and SD in the FMD between 2 readings of the same11 measurements was 0.82% (SD 0.48%) (r=0.97).
Secondary Outcomes
Endothelium-independent nitroglycerin-mediated vasodilation (NMD) and arterial stiffness were secondary vascular outcomes. NMD was evaluated as a control condition for FMD 3 minutes after administration of 0.4 mg of nitroglycerin sublingually. Data acquisition was done in a similar manner. The end point of measurement was the percent change in diameter in response to endothelium-independent nitroglycerin-mediated vasodilation (NMD%).
Arterial stiffness was assessed by means of pulse wave velocity and radial pulse wave analysis using the SphygmoCor® Pulse Wave Velocity system (PWV Medical, NSW, Australia). Peripheral pressure waveforms were recorded from the radial artery at the wrist using applanation tonometry with a high-fidelity micromanometer. After 20 sequential waveforms were acquired, a validated generalized transfer function was used to generate the corresponding central aortic pressure waveform.(27) Pulse wave velocity (PWV) was calculated as the velocity of the BP waveform between carotid and femoral arteries. Transcutaneous Doppler flow velocity recordings were carried out simultaneously at the base of the neck over the common carotid artery and at the femoral artery in the groin, and the time delay (t) as well as the distance (d) traveled by the pulse wave were measured between the two recording sites using established methods.(27) PWV was calculated as PWV=d/t. Additionally, we obtained the augmentation index (AI) from pulse wave analysis. The merging point of the incident and the reflected wave (the inflection point) was identified on the aortic pressure waveform. The AI is defined as the augmented pressure (the maximum systolic pressure minus the pressure at the inflection point) divided by pulse pressure and expressed as a percentage. Larger values of AI indicate increased wave reflection from the periphery or earlier return of the reflected wave as a result of increased pulse wave velocity, an indication of increased arterial stiffness. Reproducibility studies in our laboratory on consecutive days have demonstrated a coefficient of variation of 20.3% and 3.8% for AI and PWV, respectively.
Psychosocial and behavioral factors were measured as secondary outcomes and included psychosocial stress, measured by means of the Cohen's Perceived Stress Scale,(28) hostility, by means of the Cook-Medley Hostility Inventory,(29) anger, using the Spielberger's Anger Expression Inventory,(30) anxiety, using the Spielberger's State-Trait Anxiety Inventory,(31) depressive symptoms using the Beck II Depression Inventory (BDI), (32) including two subscales of the BDI meant to capture two distinct dimensions of depression, the cognitive/affective subscale and the somatic subscale.(33) Physical activity was measured using the CAPS Typical Week Physical Activity Survey (CAPS-TWPAS), a 28-item questionnaire that has been validated in diverse ethnic populations.(34) This instrument provides total time spent in light physical activity (< 3 METs), moderate physical activity (3-6 METs), and vigorous physical activity (> 6 METs) in a typical week.(34) In our analysis, we focused on moderate and vigorous physical activity; because these were highly skewed, log transformation was performed prior to analysis. Compliance with the interventions was measured by class attendance as indicated by signed attendance sheets.
Metabolic profile
As additional secondary outcomes, we assessed changes in levels of metabolic syndrome risk factors, including abdominal obesity, fasting plasma lipids (triglycerides and HDL-cholesterol), BP, and fasting plasma glucose. We also computed a score counting the number of metabolic syndrome risk factors meeting the criteria for metabolic syndrome definition at baseline and at each of the follow-up visits. Additionally, we assessed changes in metabolic and inflammatory biomarkers, including leptin, adiponectin, C-reactive protein (CRP), interleukin 6 (IL-6), tumor necrosis factor 1 (TNF-α) and plasminogen activator inhibitor 1 (PAI-1). Blood was drawn after an overnight fast. We measured CRP levels were quantified using the Dade-Behring Nephelometry System – BNII. All the other biomarkers were measured using the Fluorokine® MultiAnalyte Profiling Human Obesity Base Kit Kits from R&D Systems (Minneapolis, USA) on a Luminex 200 Bio-Plex platform (Bio-Rad, CA USA). The biomarker assays were done in the Division of Hereditary Blood Disorders at the Centers for Disease Control and Prevention.
Statistical Analysis
Data were analyzed following the intention-to-treat principle, i.e. all available data were analyzed on randomized participants (n=68), irrespective of whether they received the intervention, or completed the study.(35) All outcomes (FMD and secondary outcome measures) were analyzed as continuous variables using repeated measures mixed effects models (36) with treatment group, time point, and other study factors treated as fixed effects. The model-based means are unbiased with unbalanced and missing data, so long as the missing data are non-informative (missing at random).(37) Physical activity scores and biomarker data (CRP, IL-6, adiponectin and PAI-1) were log transformed for analysis when distribution deviated significantly from normality. Because of the lack of preliminary data on the effect of meditation on FMD at the time the study was conceived, we based our sample size on an estimated effect of 3% absolute increase in flow due to CRM, compared with HE, a standard deviation for FMD in middle-aged individuals of 3.7%, an alpha of 0.05 (2-tailed), a beta of 0.80, and an attrition rate of 20%. On the basis of these estimates, 30 participants per group were needed to show a significant difference in FMD between the two groups.
Results
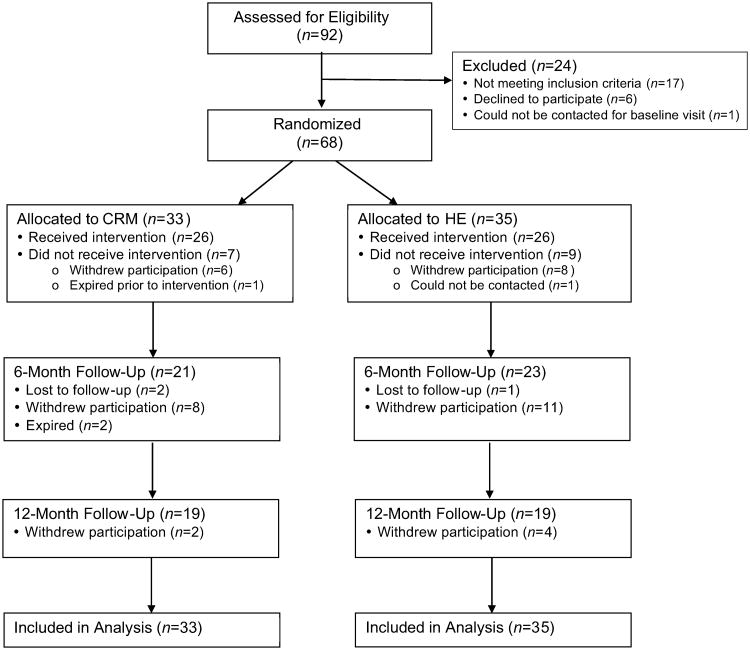
Of 105 potentially eligible participants as determined through phone prescreening, 92 attended the screening visit to assess eligibility. As shown in Figure 1, of these 92 patients 68 were randomized to either CRM (n=33) or HE (n=35). Of the randomized patients, 21 and 23participants in the CRM and HE groups, respectively, attended the 6-month visit and 19 in each group attended the 12-month visit. There was no significant difference in drop-out rate between the two randomized groups. There were also no significant differences in any baseline measures comparing participants who dropped out with those who did not drop out. Compliance, assessed by class attendance averaged throughout the 12 months (21 sessions) was also similar: 50% in the CRM group and 51% in the HE group. No adverse events due to the intervention were reported.
Figure 1.
CONSORT flow diagram for enrollment and follow-up of study participants.
Most participants (82%) were obese (BMI ≥30). Table 1 shows baseline characteristics of the participants by randomization group. The sample was middle-aged and predominantly female. Subjects in the CRM group had higher triglyceride levels (138 ± 81 mg/dLvs. 103 ± 50 mg/dL). There were no significant differences in other baseline factors.
Table 1.
Baseline demographics and risk factors by intervention group.
| Measure | CRM (n=33) | HE (n=35) |
|---|---|---|
| Demographic Factors | ||
| Age (years), mean (SD) | 51.5 (7.5) | 52.1 (7.6) |
| Male, % | 25.0 | 17.1 |
| Married, % | 37.5 | 34.3 |
| More than high school education, % | 76.7 | 78.1 |
| Metabolic Risk Factors and Medical History | ||
| Body mass index (kg/m2), mean (SD) | 38.2 (11.2) | 36.7 (5.6) |
| Systolic blood pressure (mm Hg), mean (SD) | 138.5 (22.2) | 137.5 (21.4) |
| Diastolic blood pressure (mm Hg), mean (SD) | 83.2 (13.1) | 83.5 (12.2) |
| Heart rate (beat/min), mean (SD) | 73.7 (11.8) | 70.9 (13.7) |
| LDL-cholesterol (mg/dL), mean (SD) | 117.3 (32.8) | 121.0 (44.0) |
| HDL-cholesterol (mg/dL), mean (SD) | 54.1 (15.3) | 55.0 (11.1) |
| Triglycerides (mg/dL), mean (SD) | 137.9 (80.8) | 102.7 (50.2) |
| Creatinine (mg/dL), mean (SD) | 0.96 (0.31) | 0.84 (0.25) |
| Glucose (mg/dL) mean (SD) | 116.8 (57.4) | 100.3 (38.3) |
| Insulin (μIU/mL), mean (SD) | 18.3 (31.1) | 14.1 (12.9) |
| History of diabetes, % | 45.5 | 41.2 |
| HOMA index | 1.04 | 0.92 |
| Family history of heart disease before age 55, % | 37.9 | 39.4 |
| Moderate physical activity, minutes/day (SD) | 167 (228) | 230 (242) |
| Vigorous physical activity, minutes/day (SD) | 10.5 (23.8) | 38.3 (75.9) |
| Medications | ||
| On aspirin, % | 21.2 | 14.3 |
| On statins, % | 45.5 | 25.7 |
| On beta blockers, % | 18.2 | 20.0 |
| On ACE Inhibitors, % | 27.3 | 28.6 |
| Psychosocial Risk Factors | ||
| Spielberger's Anger-in, mean (SD) | 14.4 (3.9) | 14.0 (4.0) |
| Spielberger's Anger-out, mean (SD) | 12.9 (3.4) | 13.0 (2.5) |
| Cook-Medley Hostility Inventory, mean (SD) | 17.2 (8.1) | 18.0 (7.4) |
| Cohen's Perceived Stress Scale, mean (SD) | 14.8 (8.4) | 17.4 (8.6) |
| Spielberger's State Anxiety, mean (SD) | 36.8 (13.2) | 40.0 (14.1) |
| Spielberger's Trait Anxiety, mean (SD) | 38.2 (11.9) | 39.5 (12.4) |
| Beck Depression Inventory II, mean (SD) | 10.9 (7.7) | 11.0 (9.7) |
| Beck Depression Somatic Subscore, mean (SD) | 0.64 (0.44) | 0.59 (0.48) |
| Beck Depression Cognitive Subscore, mean (SD) | 2.6 (3.1) | 3.3 (3.9) |
Based on log-transformed values.
Flow-Mediated Vasodilation and Other Vascular Outcomes
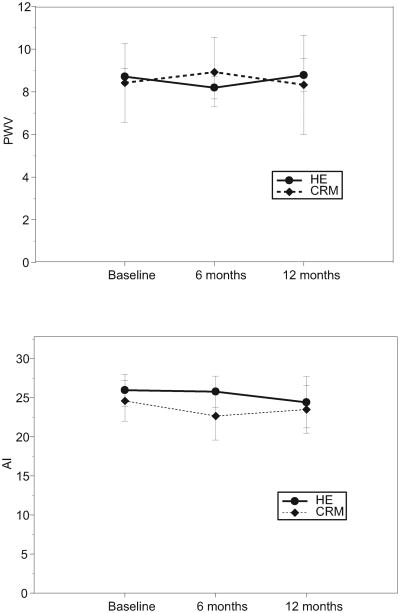
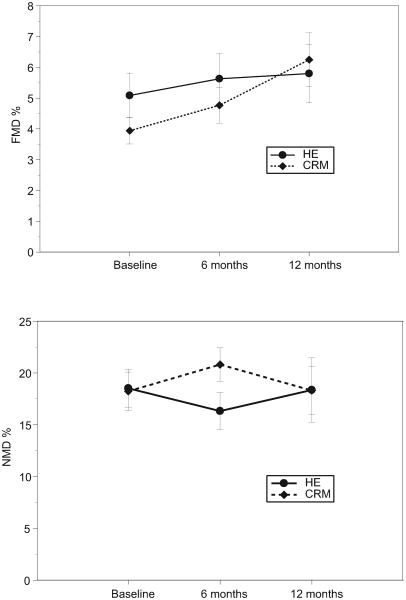
At baseline, there was no significant difference in mean FMD % between the CRM and the HE group (3.9%, SD 2.4%, vs. 5.1%,SD 4.2%, p=0.18). FMD % significantly improved in the CRM group over the 12-month intervention (mean change 2.1%, 95% confidence interval (CI), 0.5 to 3.7%, p=0.009), but less so in the HE group (mean change 1.4%, 95% CI, -0.2 to 2.9%, p=0.094) (Table 2 and Figure 2). The interaction between group and time, however, was not significant (p=0.51), denoting no significant difference in FMD change over time between the two groups. Non-endothelium dependent dilation (% NMD) did not change in either group and arterial elasticity measures (PWV and AI) were similarly unaffected (Figure 3). Compliance assessed as class attendance was not correlated with change in FMD, both in the CRM group (Pearson r=-0.08, p=0.79) and in the HE group (Pearson's r=-0.21, p=0.49).
Table 2.
Changes in flow-mediated vasodilation (FMD) and metabolic risk factors by study group.
| Study Visit Means ± SD | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Study Group | Baseline | 6 Months | 12 Months | Change, Baseline to 12 Months (95% CI)* | P Value for Trend Within Group | P Value for group difference in trend |
| FMD % | CRM | 3.9 (2.4) | 4.8 (2.7) | 6.3 (3.3) | 2.10 (0.53, 3.67) | 0.009 | 0.51 |
| HE | 5.1 (4.2) | 5.6 (4.4) | 5.8 (3.4) | 1.36 (-0.24, 2.95) | 0.094 | ||
| SBP, mm Hg | CRM | 138.2 (22.3) | 135.9 (20.5) | 129.6 (11.1) | -3.84 (-13.07, 5.38) | 0.41 | 0.78 |
| HE | 137.5 (21.5) | 132.7 (25.2) | 133.4 (20.1) | -2.08 (-11.02, 6.86) | 0.64 | ||
| DBP, mm Hg | CRM | 83.1 (13.0) | 83.1 (15.8) | 74.1 (11.1) | -6.24 (-11.71, -0.77) | 0.026 | 0.17 |
| HE | 83.5 (12.2) | 79.6 (14.0) | 82.3 (15.3) | -0.97 (-6.27, 4.33) | 0.72 | ||
| Weight, Kg | CRM | 104.8 (32.7) | 101.8 (30.9) | 100.6 (23.2) | -2.52 (-0.20, -4.83) | 0.033 | 0.26 |
| HE | 101.1 (15.1) | 102.0 (15.5) | 101.0 (16.8) | -0.69 (-1.51, 2.89) | 0.54 | ||
| BMI | CRM | 37.7 (11.2) | 36.8 (10.6) | 37.5 (9.8) | -0.99 (0.13, 1.86) | 0.025 | 0.34 |
| HE | 36.7 (5.6) | 37.0 (5.0) | 36.3 (4.9) | -0.43 (-0.38, 1.23) | 0.29 | ||
| Glucose, mg/dL | CRM | 117.5 (58.2) | 138.5 (68.8) | 111.2 (34.7) | -6.04 (-28.02, 15.94) | 0.59 | 0.095 |
| HE | 100.3 (38.3) | 112.4 (52.6) | 128.1 (58.9) | 20.86 (-1.90, 43.63) | 0.072 | ||
| Insulin, mU/L | CRM | 18.2 (31.6) | 18.4 (21.3) | 14.2 (9.6) | -1.13 (-9.12, 6.85) | 0.78 | 0.68 |
| HE | 14.1 (12.9) | 16.4 (14.8) | 16.9 (24.7) | 1.22 (-6.90, 9.34) | 0.77 | ||
| Triglycerides, mg/dL | CRM | 136.9 (81.9) | 128.9 (57.8) | 109.6 (36.6) | -14.35 (-31.75, 3.06) | 0.10 | 0.012 |
| HE | 102.7 (50.2) | 109.4 (58.1) | 123.8 (90.1) | 17.72 (-0.19, 35.63) | 0.052 | ||
| HDL-C, mg/dL | CRM | 54.2 (15.5) | 55.7 (17.1) | 57.1 (17.5) | 1.47 (-2.04, 4.97) | 0.41 | 0.71 |
| HE | 55.0 (11.1) | 54.8 (9.7) | 56.3 (12.2) | 0.51 (-3.14, 4.16) | 0.70 | ||
| Metabolic syndrome score | CRM | 2.8 (1.1) | 2.8 (1.1) | 2.3 (1.2) | -0.41 (-0.89, 0.06) | 0.089 | 0.049 |
| HE | 2.5 (0.8) | 2.3 (1.0) | 2.7 (1.2) | 0.25 (-0.21, 0.72) | 0.28 | ||
Model estimates, adjusted for individual variance, obtained using repeated measures mixed effects models. Mean estimates for triglyceride are adjusted for baseline triglyceride values.
FMD: flow-mediated vasodilation; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Figure 2.
Brachial artery reactivity testing results by intervention group, including flow-mediated vasodilation (FMD %) and endothelium-independent nitroglycerin-mediated vasodilation (NMD%).
Figure 3.
Arterial stiffness results by intervention group, including pulse wave velocity (PWV) and augmentation index (AI).
Metabolic Syndrome Risk Factors
Virtually all the metabolic syndrome risk factors showed beneficial trends in the CRM group, while they showed less benefit or worsening in the HE group (Table 2). In the CRM arm, changes between baseline and 12 months were significant for diastolic BP (-6.2 mm Hg, 95% CI, -11.7 to -0.8 mm Hg, p=0.026), and weight (-2.5 kg, 95% CI, -0.2 to -4.8 kg, p=0.033); additionally, the interaction between treatment arm and time was significant for triglyceride levels, after adjusting for baseline levels (p=0.012) and marginally significant for fasting glucose (p=0.095). The summation score counting the number of metabolic syndrome risk factors tended to decrease in the CRM group and to worsen in the HE group (p=0.049 for the interaction between treatment group and time, Table 2).
Psychosocial and Behavioral Factors
Both interventions significantly improved depressive symptoms and STAI-State anxiety scores. STAI-Trait anxiety and Perceived Stress Scale scores were reduced slightly more in the HE group. No significant changes were observed in anger and hostility measures for both interventions (Table 3). Neither CRM nor HE was associated with significant changes in moderate physical activity. Vigorous physical activity, however, which was log transformed because of its skewed distribution, increased in the CRM group, from a geometric mean of 3.8 ± 4.2 min/day to 9.1 ±3.9 min/day (p=0.023), while it tended to decrease in the HE group (from 10.5± 5.7 min/day to 6.3± 4.3 min/day, p=0.43), p=0.032 for the interaction between group and time. The increase in physical activity, however, did not substantially explain the effect of CRM on FMD. The mean change in FMD in the CRM group decreased only slightly, from 2.1% (95% CI, 0.5 to 3.7%, p=0.009), to 1.9% (95% CI, 0.3 to 3.6%, p=0.022) once vigorous physical activity was added to the model. Changes in BMI also did not contribute to the findings, as there was no significant correlation between changes in BMI and changes in FMD (Pearson's r=0.04, p=0.63).
Table 3.
Changes in psychosocial scale scores by study group.
| Study Visit Means ± SD | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Study Group | Baseline | 6 Months | 12 Months | Change, Baseline to 12 Months (95% CI)* | P Value for Trend Within Group | P Value for group difference in trend |
| Anger-in | CRM | 14.4 (3.9) | 14.1 (3.8) | 13.7 (3.4) | -0.35 (-1.69, 0.99) | 0.60 | 0.71 |
| HE | 14.0 (4.0) | 13.4 (3.5) | 13.3 (3.6) | 0.0002 (-1.33, 1.33) | 1.00 | ||
| Anger-out | CRM | 12.9 (3.4) | 12.2 (3.2) | 13.4 (3.7) | 0.34 (-0.76, 1.45) | 0.54 | 0.52 |
| HE | 13.0 (2.5) | 13.5 (3.2) | 12.4 (2.5) | -0.17 (-1.27, 0.93) | 0.76 | ||
| Hostility | CRM | 17.2 (8.1) | 17.6 (8.0) | 17.7 (8.0) | 1.69 (-0.46, 3.84) | 0.12 | 0.10 |
| HE | 18.0 (7.4) | 16.7 (8.4) | 15.4 (6.7) | -0.84 (-2.98, 1.30) | 0.44 | ||
| PSS | CRM | 14.8 (8.4) | 14.0 (6.9) | 12.1 (6.2) | -1.66 (-4.42, 1.11) | 0.24 | 0.022 |
| HE | 17.4 (8.6) | 14.0 (7.7) | 9.4 (6.4) | -6.50 (-9.55, -3.45) | <0.001 | ||
| STAI-State | CRM | 36.8 (13.2) | 32.4 (10.1) | 28.4 (7.7) | -6.63 (-10.73, -2.53) | 0.002 | 0.65 |
| HE | 40.0 (14.1) | 35.8 (11.0) | 31.7 (7.6) | -8.01 (-12.40, -3.62) | 0.001 | ||
| STAI-Trait | CRM | 38.2 (11.9) | 34.4 (11.1) | 33.4 (8.7) | -2.43 (-5.22, 0.37) | 0.088 | 0.48 |
| HE | 39.5 (12.4) | 37.7 (13.5) | 33.2 (8.6) | -3.88 (-6.87, -0.88) | 0.012 | ||
| BDI-Somatic | CRM | 0.6 (0.4) | 0.5 (0.3) | 0.3 (0.2) | -0.19 (-0.34, -0.05) | 0.011 | 0.72 |
| HE | 0.6 (0.5) | 0.5 (0.4) | 0.4 (0.4) | -0.16 (-0.31, -0.01) | 0.040 | ||
| BDI-Cognitive | CRM | 2.6 (3.1) | 1.7 (2.1) | 0.6 (0.8) | -1.34 (-2.48, -0.21) | 0.021 | 0.87 |
| HE | 3.3 (3.9) | 2.6 (3.5) | 1.2 (1.6) | -1.47 (-2.61, -0.34) | 0.011 | ||
| BDI-Total | CRM | 10.9 (7.7) | 7.8 (5.4) | 5.1 (3.3) | -3.76 (-6.38, -1.15) | 0.005 | 0.86 |
| HE | 11.0 (9.7) | 9.0 (7.8) | 6.1 (6.0) | -3.44 (-6.04, -0.84) | 0.010 | ||
Model estimates, adjusted for individual variance, obtained using repeated measures mixed effects models.
PSS: Perceived Stress Scale; STAI: State-Trait Anxiety Inventory; BDI: Beck Depression Inventory.
Metabolic/Inflammatory Biomarkers
Biomarker data were available for 27 participants randomized to HE (82%) and 29 participants randomized to CRM (83%). Adiponectin was slightly higher in the HE than in the CRM group at baseline; there were no differences in the baseline levels of leptin or any of the inflammatory biomarkers (CRP, IL-6, TNF-α and PAI-1) between the two groups. Biomarker data did not change significantly in either group during the follow-up (Table 4).
Table 4.
Changes in biomarker levels by study group.
| Study Visit Means ± SD | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Study Group | Baseline | 6 Months | 12 Months | Change, Baseline to 12 Months (95% CI)* | P Value for Trend Within Group | P Value for group difference in trend |
| Leptin (ng/mL) | CRM | 71.1 (40.6) | 77.2 (40.4) | 72.5 (46.9) | -3.77 (-16.54, 9.00) | 0.56 | 0.65 |
| HE | 70.4 (37.4) | 71.5 (39.9) | 67.5 (37.2) | 0.36 (-12.53, 13.24) | 0.96 | ||
| LgAdiponectin (ng/mL) | CRM | 9.0 (0.5) | 9.0 (0.5) | 9.0 (0.4) | 0.05 (-0.13, 0.23) | 0.58 | 0.40 |
| HE | 9.2 (0.4) | 9.0 (0.4) | 9.2 (0.5) | -0.06 (-0.24, 0.12) | 0.52 | ||
| LgCRP (mg/L) | CRM | 1.2 (1.2) | 1.2 (0.8) | 1.1 (1.1) | -0.04 (-0.41, 0.32) | 0.81 | 0.24 |
| HE | 1.4 (1.1) | 1.4 (1.0) | 1.6 (1.0) | 0.27 (-0.11, 0.64) | 0.16 | ||
| LgIL-6 (pg/mL) | CRM | 0.1 (1.0) | 0.3 (1.0) | 0.3 (0.6) | 0.16 (-0.29, 0.62) | 0.47 | 0.57 |
| HE | 0.1 (1.2) | 0.3 (1.5) | 0.6 (1.0) | 0.35 (-0.10, 0.79) | 0.13 | ||
| TNF-α (pg/mL) | CRM | 2.3 (1.3) | 2.8 (1.2) | 2.4 (1.6) | -0.13 (-0.69, 0.44) | 0.66 | 0.24 |
| HE | 2.4 (1.8) | 3.4 (1.5) | 3.4 (1.4) | 0.35 (-0.22, 0.92) | 0.23 | ||
| LgPAI-1 (pg/mL) | CRM | 10.3 (1.0) | 10.2 (0.5) | 10.0 (0.7) | -0.03 (-0.41, 0.36) | 0.89 | 0.95 |
| HE | 10.4 (1.1) | 9.9 (0.8) | 9.9 (0.7) | -0.01 (-0.40, 0.38) | 0.96 | ||
Model estimates, adjusted for individual variance, obtained using repeated measures mixed effects models.
CRP: C-reactive protein; IL-6: interleukin 6; TNF-α: tumor necrosis factor α. PAI-1: plasminogen activator inhibitor 1.
Discussion
Among black Americans with components of the metabolic syndrome, CRM, a sound-based meditation intervention, did not improve endothelial function significantly more than a control intervention of health education, which was matched to the CRM intervention for frequency of meetings and time with the instructor. In the CRM group, several metabolic risk factors tended to improve at 12 months, while they did not improve or tended to worsen in the HE group, as did a cumulative metabolic risk score that included BP, weight, glucose, HDL-cholesterol and triglyceride levels. These effects occurred despite no substantial difference in the impact of the interventions on perceived stress and other psychosocial risk factors.
An innovative aspect of our study is the inclusion of an urban black population, a group that remains understudied. While studies have linked chronic psychosocial and environmental stressors, such as social and economic disadvantage and discrimination, to CVD and its risk factors,(38-41) these associations tend to be more pronounced among blacks.(42,43) These differences may in part be mediated by race differences in autonomic nervous system responses and behavioral and psychosocial factors such as depression and anger expression.(39,44,45) For example, community-dwelling blacks have higher rates of discrimination ratings and depressive symptoms than whites,(42,46-48) and these factors tend to be more predictive of hypertension and CVD in blacks than in whites.(42,48-50)
Our results are in agreement with previous clinical trials of stress reduction interventions using meditation that have shown beneficial effects on CVD risk factors.(13,51) Recently, the effects of TM on insulin resistance were examined in 103 patients with clinically-confirmed CVD who were randomized to either TM or a HE control group for 16 weeks; 84 completed the protocol.(52) Consistent with our trial, this study found a significant improvement in BP and other metabolic risk factors in the patients randomized to TM compared to HE. FMD was examined as secondary outcome. There was a non-significant improvement in FMD (0.11%) in the TM group and a non-significant decline in the HE group (-0.81) (p=0.24 for difference between groups). Our results are in agreement with this previous report in terms of failing to find a significant between-group difference in FMD, although we did find a somewhat larger (2.1%) improvement in FMD within the meditation group. It should be noted that this earlier trial studied a different population, i.e., patients with established coronary artery disease, many of whom may not have had metabolic syndrome and most of whom had already advanced vascular disease with a limited potential for improvement in endothelial function. In contrast, we excluded persons with previous coronary artery disease and selected participants with metabolic syndrome risk factors.
Several, if not all, of the metabolic syndrome risk factors,(53-55) as well as visceral fat and insulin resistance,(56,57) have been linked to adrenocortical and autonomic disturbances. Recently, in the Chicago site of the Study of Women's Health Across the Nation, both depressive symptoms and experiences of discrimination were associated with visceral fat, an important substrate for metabolic syndrome, but not with subcutaneous fat, both assessed by computed tomography; these relationships were observed both in black and white women.(47,58) Possible effects of stress-reduction on lifestyle behaviors may also play a role. In fact, we found that participants randomized to CRM increased their level of vigorous physical activity more than participants randomized to HE. One might speculate that this reflects an increase in energy level in the CRM group relative to the control group, or an increased focus on personal physical activity goals. Nonetheless, our physical activity assessment was somewhat imprecise and the increase in physical activity did not substantially explain the effects of CRM on FMD.
Limitations of our trial include its small size that limited the power to detect a significant difference in the change in outcome measures between the two randomized groups. The study power was also affected by a smaller than anticipated effect on FMD, and a higher attrition rate. The relatively high drop-out rate, however, is not surprising for a year-long intervention in an at-risk population, and there was no difference in retention rate between the two randomized groups. Furthermore, we used a statistical approach for unbalanced data that allowed all available data to be included in the analysis. Nonetheless, we recognize that generalizability may be affected. Although participants were masked to the research hypothesis, we were not able to evaluate whether this masking was successful because data on participants' expectations were not collected. It is also possible that baseline differences in metabolic and vascular factors may have influenced the results. However, with exception for triglycerides, the two groups were balanced for baseline factors, and we adjusted for baseline triglyceride levels in the model. Finally, we studied a novel meditation approach, CRM, that has not been evaluated before in a controlled fashion. However, CRM follows closely the TM standardized protocol with few but significant modifications, and has the possible advantage of being more acceptable to community-dwelling blacks. TM has failed to disseminate in the black community, possibly because it requires substantial investment in cost and time. CRM could be more successfully disseminated at the community level. Another advantage of our study is the use of a control intervention that rigorously controlled for frequency and duration of the training sessions, as well as practice time at home. Indeed, a limitation of many previous trials is lack of an adequate control intervention.(11)
In conclusion, among black Americans with metabolic syndrome risk factors, CRM, a sound-based meditation intervention, did not improve endothelial function significantly more than a control intervention of health education. Favorable trends in metabolic risk factors were observed at the end of the 1-year intervention period denoting improvement in the CRM group and no change or worsening in the HE group.
Acknowledgments
Funding Sources: This study was supported by funding from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute U01 HL079156 and U01 HL79214; NIH, National Center for Research Resources (NCRR) Grant M01-RR00039 for the Emory General Clinical Research Center; NIH/NCRR 5P20RR11104 for the Morehouse Clinical Research Center; and NIH K24HL077506.
Abbreviations
- BP
Blood Pressure
- CRM
Consciously Resting Meditation
- CRP
C Reactive Protein
- CVD
Cardiovascular Disease
- FMD
Flow-Mediated Dilation
- HE
Health Education
- PWV
Pulse-Wave Velocity
- NMD
Nitroglycerin-Mediated Vasodilation
- TM
Transcendental Meditation
Footnotes
Disclosures: Kofi A. Kondwani, Ph.D., is the founder and CEO of Consciously Resting Meditation (CRM), Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. Jama. 2003;290:199–206. doi: 10.1001/jama.290.2.199. see comment. [DOI] [PubMed] [Google Scholar]
- 3.Fagot-Campagna A, Pettitt DJ, Engelgau MM, Burrows NR, Geiss LS, Valdez R, Beckles GL, Saaddine J, Gregg EW, Williamson DF, Narayan KM. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–72. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 4.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 5.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 6.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental Stress Induces Transient Endothelial Dysfunction in Humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood A, Johnson K, Blumenthal JA, Hinderliter AL. Endothelial function and hemodynamic responses during mental stress. Psychosom. Med. 1999;61:365–370. doi: 10.1097/00006842-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 9.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 10.Shinozaki K, Ayajiki K, Nishio Y, Sugaya T, Kashiwagi A, Okamura T. Evidence for a causal role of the renin-angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension. 2004;43:255–262. doi: 10.1161/01.HYP.0000111136.86976.26. [DOI] [PubMed] [Google Scholar]
- 11.Ospina MB, Bond TK, Karkhaneh M, Tjosvold L, Vandermeer B, Liang Y, Bialy L, Hooton N, Buscemi N, Dryden DM, Klassen TP. Evidence Report/Technology Assessment No 155 AHRQ Publication No 07-E010. Rockville, MD: Quality AfHRa; Jun, 2007. Meditation Practices for Health: State of the Research. [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider RH, Nidich SI, Salerno JW. The Transcendental Meditation program: reducing the risk of heart disease and mortality and improving quality of life in African Americans. Ethn Dis. 2001;11:159–60. [PubMed] [Google Scholar]
- 13.Schneider RH, Alexander CN, Staggers F, Orme-Johnson DW, Rainforth M, Salerno JW, Sheppard W, Castillo-Richmond A, Barnes VA, Nidich SI. A randomized controlled trial of stress reduction in African Americans treated for hypertension for over one year. Am J Hypertens. 2005;18:88–98. doi: 10.1016/j.amjhyper.2004.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes VA, Treiber FA, Davis H. Impact of Transcendental Meditation on cardiovascular function at rest and during acute stress in adolescents with high normal blood pressure. J Psychosom Res. 2001;51:597–605. doi: 10.1016/s0022-3999(01)00261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes VA, Treiber FA, Johnson MH. Impact of transcendental meditation on ambulatory blood pressure in African-American adolescents. Am J Hypertens. 2004;17:366–9. doi: 10.1016/j.amjhyper.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes VA, Pendergrast RA, Harshfield GA, Treiber FA. Impact of breathing awareness meditation on ambulatory blood pressure and sodium handling in prehypertensive African American adolescents. Ethn Dis. 2008;18:1–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Gregoski MJ, Barnes VA, Tingen MS, Harshfield GA, Treiber FA. Breathing awareness meditation and LifeSkills Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2010;48:59–64. doi: 10.1016/j.jadohealth.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes VA, Davis HC, Murzynowski JB, Treiber FA. Impact of meditation on resting and ambulatory blood pressure and heart rate in youth. Psychosom Med. 2004;66:909–14. doi: 10.1097/01.psy.0000145902.91749.35. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, for the Conference Participants Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Arterioscler Thromb Vac Biol. 2004;24:13e–18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 20.Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the atherosclerosis risk in communities study: 1987-1998. Diabetes Care. 2002;25:1358–64. doi: 10.2337/diacare.25.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoratti R. A review on ethnic differences in plasma triglycerides and high-density-lipoprotein cholesterol: is the lipid pattern the key factor for the low coronary heart disease rate in people of African origin? Eur J Epidemiol. 1998;14:9–21. doi: 10.1023/a:1007492202045. [DOI] [PubMed] [Google Scholar]
- 22.Lin SX, Carnethon M, Szklo M, Bertoni A. Racial/Ethnic Differences in the Association of Triglycerides with Other Metabolic Syndrome Components: The Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2010 doi: 10.1089/met.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155:S7–e7-11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumner AE, Harman JL, Buxbaum SG, Miller BV, 3rd, Tambay AV, Wyatt SB, Taylor HA, Rotimi CN, Sarpong DF. The triglyceride/high-density lipoprotein cholesterol ratio fails to predict insulin resistance in African-American women: an analysis of Jackson Heart Study. Metab Syndr Relat Disord. 2010;8:511–4. doi: 10.1089/met.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anonymous. The Sixth Report of the Joint National Commitee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 26.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 27.Pauca AL, O'Rourke MF, Kon ND. Prospective Evaluation of a Method for Estimating Ascending Aortic Pressure From the Radial Artery Pressure Waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health & Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 29.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RBJ. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Spielberger CD. Professional manual for the State-Trait Anger Expression Inventory Research Ed. Tampa, Fla: University of South Florida; 1988. [Google Scholar]
- 31.Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety (STAI) manual. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. BDI-II Beck Depression Inventory. Second. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 33.Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol. 1999;55:117–28. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Whitt MC, Levin S, Ainsworth BE, Dubose KD. Evaluation of a two-part survey item to assess moderate physical activity: the Cross-Cultural Activity Participation Study. J Womens Health (Larchmt) 2003;12:203–12. doi: 10.1089/154099903321667537. [DOI] [PubMed] [Google Scholar]
- 35.Shao J, Zhong B. Last observation carry-forward and last observation analysis. Stat Med. 2003;22:2429–41. doi: 10.1002/sim.1519. [DOI] [PubMed] [Google Scholar]
- 36.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 37.Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clin Trials. 2004;1:368–76. doi: 10.1191/1740774504cn032oa. [DOI] [PubMed] [Google Scholar]
- 38.Williams DR, Neighbors H. Racism, discrimination and hypertension: evidence and needed research. Ethn Dis. 2001;11:800–16. [PubMed] [Google Scholar]
- 39.Guyll M, Matthews KA, Bromberger JT. Discrimination and unfair treatment: relationship to cardiovascular reactivity among African American and European American women. Health Psychol. 2001;20:315–25. doi: 10.1037//0278-6133.20.5.315. [DOI] [PubMed] [Google Scholar]
- 40.Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68:362–8. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- 41.Brondolo E, Libby DJ, Denton EG, Thompson S, Beatty DL, Schwartz J, Sweeney M, Tobin JN, Cassells A, Pickering TG, Gerin W. Racism and ambulatory blood pressure in a community sample. Psychosom Med. 2008;70:49–56. doi: 10.1097/PSY.0b013e31815ff3bd. [DOI] [PubMed] [Google Scholar]
- 42.Lewis TT, Barnes LL, Bienias JL, Lackland DT, Evans DA, Mendes de Leon CF. Perceived discrimination and blood pressure in older African American and white adults. J Gerontol A Biol Sci Med Sci. 2009;64:1002–8. doi: 10.1093/gerona/glp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 2003;22:300–9. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- 44.Anderson NB, McNeilly M, Myers H. Autonomic reactivity and hypertension in blacks: a review and proposed model. Ethn Dis. 1991;1:154–70. [PubMed] [Google Scholar]
- 45.Gentry WD, Chesney AP, Gary HE, Jr, Hall RP, Harburg E. Habitual anger-coping styles: I. Effect on mean blood pressure and risk for essential hypertension. Psychosom Med. 1982;44:195–202. doi: 10.1097/00006842-198205000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Amyre Morris A, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, Gibbons G, Din-Dzietham R, Quyyumi A, Vaccarino V. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosom Med. 2011;73:462–8. doi: 10.1097/PSY.0b013e318222379c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis TT, Kravitz HM, Janssen I, Powell LH. Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. Am J Epidemiol. 2011;173:1223–31. doi: 10.1093/aje/kwq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis TT, Guo H, Lunos S, Mendes de Leon CF, Skarupski KA, Evans DA, Everson-Rose SA. Depressive symptoms and cardiovascular mortality in older black and white adults: evidence for a differential association by race. Circulation Cardiovascular quality and outcomes. 2011;4:293–9. doi: 10.1161/CIRCOUTCOMES.110.957548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch Intern Med. 2000;160:1495–500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- 50.Lewis TT, Everson-Rose SA, Colvin A, Matthews K, Bromberger JT, Sutton-Tyrrell K. Interactive effects of race and depressive symptoms on calcification in African American and white women. Psychosom Med. 2009;71:163–70. doi: 10.1097/PSY.0b013e31819080e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider RH, Staggers F, Alexander C, Sheppard W, Rainforth M, Kondwani K, Smith S, King CG. A randomized controlled trial of stress reduction for hypertension in older African Americans. Hypertension. 1995;26:820–827. doi: 10.1161/01.hyp.26.5.820. [DOI] [PubMed] [Google Scholar]
- 52.Paul-Labrador M, Polk D, Dwyer JH, Velasquez I, Nidich S, Rainforth M, Schneider R, Merz CN. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–24. doi: 10.1001/archinte.166.11.1218. [DOI] [PubMed] [Google Scholar]
- 53.Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med. 1999;16:373–383. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 54.Liao D, Sloan R, Cascio W, Folsom A, Liese A, Evans G, Cai J, Sharrett A. Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care. 1998;21:2116–2122. doi: 10.2337/diacare.21.12.2116. [DOI] [PubMed] [Google Scholar]
- 55.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GDO, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: Nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 56.Epel EE, Moyer AE, Martin CD, Macary S, Cummings N, Rodin J, Rebuffe-Scrive M. Stress-induced cortisol, mood, and fat distribution in men. Obes Res. 1999;7:9–15. doi: 10.1002/j.1550-8528.1999.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 57.Raikkonen K, Matthews KA, Kuller LH. Anthropometric and psychosocial determinants of visceral obesity in healthy postmenopausal women. Int J Obes Relat Metab Disord. 1999;23:775–782. doi: 10.1038/sj.ijo.0800917. [DOI] [PubMed] [Google Scholar]
- 58.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med. 2009;71:410–6. doi: 10.1097/PSY.0b013e3181a20c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]