Abstract
Clinical studies have demonstrated a strong relationship between visceral fat content and metabolic diseases, such as type 2 diabetes and liver steatosis. Obese mouse models are an excellent tool to study metabolic diseases; however, there are limited methods for the noninvasive measurement of fat distribution in mice. Although micromagnetic resonance imaging and microcomputed tomography are the “gold standards” in the measurement of fat distribution, more economical and accessible methods are required. Dual energy X-ray absorptiometry (DEXA) is an effective method in characterizing fat content; however, it cannot discriminate between visceral and subcutaneous fat depots. We demonstrate that an evaluation of abdominal fat content measured by DEXA through the selection of one localized abdominal area strongly correlates with visceral fat content in C57BL/6J mice. We found that DEXA is able to measure fat pad volume ex vivo with high accuracy; however, the measurement of visceral fat in vivo shows an overestimation caused by subcutaneous tissue interference. The overestimation is almost constant for a wide range of values, and thus it is possible to correct the data for a more accurate estimation of visceral fat content. We demonstrate the utility of this technique in characterizing phenotypes of several obese mouse models (ob/ob, db/db, MC4R-KO, and DIO) and evaluating the effect of treatments on visceral fat content in longitudinal studies. Additionally, we also establish abdominal obesity as a potential biomarker for metabolic abnormalities (liver fat accumulation, insulin resistance/diabetes) in mice, similar to that described in humans.
Keywords: dual-energy X-ray absorptiometry scan, metabolic diseases, glucose tolerance test, fat distribution
adipose tissue is not distributed uniformly in the body; instead, it accumulates in specific compartments as either visceral adipose tissue (VAT) or subcutaneous adipose tissue (SAT). Anatomically distinct body fat depots have unique metabolic properties (30). VAT has proatherogenic and prothrombic characteristics (35). VAT also has relatively more capillaries and efferent sympathetic axons per unit of volume than SAT (39). Visceral adipocytes exhibit increased production of inflammatory mediators such as tumor necrosis factor-α, interleukins, and other cytokines but secrete less leptin and adiponectin than SAT (36). It has also been shown that insulin effect (antilipolytic) is lower and catecholamine effect (lipolytic) higher in VAT, enabling visceral fat to release free fatty acids more extensively. More importantly, due to its anatomic position, secretions from visceral adipocytes have easy access to the liver through the portal system (2).
Body composition measurement and fat distribution are important to study and understand the mechanisms involved in metabolic regulation. In humans, several techniques are available for in vivo measurement of body composition. Magnetic resonance imaging (MRI) and computed tomography (CT) are considered “gold standards” for the measurement of body composition and fat distribution (24, 28). These noninvasive techniques allow work in large longitudinal studies.
However, the use of both techniques in small animals requires specialized equipment, i.e., μMRI and μCT scan. Although proven to be successful in phenotyping mouse models of obesity (4, 23), this equipment is less readily available to most researchers due to high cost. Additionally, both techniques have the disadvantage of long scan times, and CT is considered a moderate to high radiation technique, which limits its use in longitudinal studies. Dual-energy X-ray absorptiometry (DEXA) scan, which was developed originally to examine bone mineral density, has been used to effectively characterize lean and fat volume in humans and rodents. Over the last decade an increasing number of researchers have used DEXA as an alternative technique, instead of direct chemical carcass analysis, to measure body composition. DEXA has the advantages of low cost, low radiation, and short scan times, making it a quick and practical method for assessing body composition in large longitudinal studies. However, this technique is restricted in terms of providing detailed spatial information on fat distribution (23). Nevertheless, development and validation of indirect DEXA scan techniques that are able to measure visceral adispose tissue have been achieved in rats (12, 25) and humans (24).
Abdominal obesity is strongly associated with metabolic disorders in humans (1). More recently, it has become apparent that it is the visceral component of measured abdominal fat that is the most intimately associated with metabolic diseases and adverse outcomes such as type 2 diabetes, hyperlipidemia, and cardiovascular disease (9). In addition, the quantification of both VAT and SAT accumulation is relevant since it has been suggested that the proliferation and differentiation of subcutaneous adipocytes can protect against the ectopic fat storage in muscle and liver (16, 19, 29, 34). Importantly, reductions of equal amounts of visceral and subcutaneous fat do not have the same effect on glucose homeostasis and cardiovascular disease (13, 38).
Few studies have been developed that show to what extent a specific regional accumulation of fat is directly related to metabolic diseases. Considering that the mechanisms linking fat localization and pathogenicity remain poorly understood, it is important to evaluate the fat distribution in obese models associated with metabolic diseases. The diet-induced obese (DIO) mouse model caused by feeding a high-fat diet (HFD) recapitulates the endocrine and metabolic changes found in most obese humans (15). It develops a syndrome of obesity, hyperinsulinemia, hyperglycemia, hyperleptinemia, and hypertension (8, 27, 33). However, the onset of all of these metabolic alterations varies with the duration of the diet; therefore, it is very important to consider the timing of the study. Several monogenetic models of obesity also exist, such as ob/ob mice (spontaneous mutant unable to produce leptin), db/db mice (unable to respond to leptin due to lack of leptin receptors), and MC4R-knockout (KO) mice (lacking the melanocortin 4 receptor). A female model of obesity can be generated either by feeding on a HFD or by ovariectomy, whereby a combination of the two further enhances obesity.
Here, we aim to validate a DEXA scan technique to specifically determine regional visceral adipose tissue distribution in male and female C57BL/6J (most widely used inbred strain in biomedical research) and B6D2 mice (extensively used as a genetic background for the generation of transgenic/KO mice). Using this new approach, we describe the phenotypical fat distribution of different obese mouse models and the effect of treatments (estradiol and diet intervention) on fat distribution. In addition, we also establish abdominal obesity as a potential biomarker for metabolic abnormalities in mice, similar to that described in humans.
MATERIALS AND METHODS
Animals.
A total of 170 mice were used in this study. At 4–5 wk of age, 57 male and 28 female C57BL/6J mice and 58 B6D2 female mice (Monash Animal Services) were fed a regular rodent diet (Specialty Feeds, Glen Forrest, Australia) or a HFD (SF04–001; Specialty Feeds) for 28 (C57BL/6J) or 12 wk (B6D2). Regular diet provided 3.3 kcal/g of energy (59.8% carbohydrate, 20.0% protein, and 5% fat). HFD provided 4.75 kcal/g of energy (41% carbohydrate, 22.6% protein, and 23.5% fat). Five male ob/ob, 11 male db/db, and seven male and four female MC4R-KO mice were fed a regular diet. Mice were housed (5/cage) in a controlled environment. Food and water were available ad libitum. All procedures were performed in accordance with the guidelines and with the approval of the Monash University Animal Ethics Committee.
Ovariectomy and estradiol treatment.
Bilateral ovariectomy was performed in a subset of 24 B6D2 mice at 4 wk of age under isoflurance anaesthesia, as described by Geary and Asarian (11). One week after ovariectomy, mice were fed a HFD for the next 10 wk. After that, eight mice were administered with 17β-estradiol-3 benzoate through subcutaneous injection (2 μg/animal, dissolved in peanut oil, 100 μl) every 4 days for 1 mo (a total of 8 injections). At the same time, 16 mice received vehicle (peanut oil, 100 μl). Food intake and body weight were monitored every 4 days.
Diet restoration from HFD to regular diet.
A subset of eight C57BL/6J DIO mice that had been on a HFD for 20 wk was then switched to a regular diet for 3 wk. DEXA scan was performed before and after diet restoration. Visceral fat was dissected and weighed directly (see measurement of visceral fat by dissection section). As a control, another subset of eight C57BL/6J DIO mice maintained on a HFD throughout the entire experiment was euthanized to measure visceral fat content. Food and water were available ad libitum, and body weight and food intake were recorded weekly.
Measurement of visceral fat by dissection.
A subset of 69 C57BL/6J mice (28 females and 41 males) was euthanized by decapitation under isofluorine anesthesia. Epididymal white adipose depot located around both testes was carefully separated from the epididymis. The perirenal white adipose depot, which is attached dorsally to both kidneys, was then dissected. Finally, the intra-abdominal mesenteric adipose depot was dissected. In females, visceral fat mass was assessed by weighing the perirenal, mesenteric, and periuterine adipose tissue. Fat mass was determined by an analytical balance (Mettler Toledo).
Measurement of body composition by DEXA scan.
Body composition was assessed in all mice using DEXA scan (PIXImus2; Lunar, Madison, WI). Each mouse was anesthetized for the duration of the procedure (5 min) by exposure to 2–3% isoflurane-oxygen gas via nose cone. Each mouse was placed on the scanner bed in the prone position, with the limbs and tail stretched away from the body.
The PIXImus employs a cone beam X-ray source generating energies at 35 and 80 keV with a current of 0.5 mA for both energy levels. The detector is flat (100 × 80 mm) and comprised of individual pixels of 0.18 × 0.18 mm. Based on the attenuation of two energy levels, the system provides quantitative data on the fat tissue content, the lean tissue content, and the total tissue mass within the region of interest (ROI). One scan per mouse was performed and analyzed with PIXImus software (2.10; GE/Lunar). The head was excluded from calculation using a manual ROI. The PIXImus was calibrated with an aluminium/lucite phantom (corresponding to bone mineral density = 0.0592 g/cm2 and 12.5% fat) on each day of testing according to the manufacturer's instructions.
Measurement of abdominal fat by DEXA scan.
To evaluate abdominal fat and to select the more accurate method, three different ROI were defined from the whole body scan. They consist of rectangular boxes extending from one vertebral space to another, with the lateral borders extending to the edge of the abdominal tissue. ROI 1 was defined from S3/S4 vertebral space to L5/L6 vertebral space, ROI 2 was defined from S3/S4 vertebral space to L3/L4 vertebral space, and ROI 3 extends from L1/L2 to L4/L5 vertebral space.
Calculation of abdominal fat to nonabdominal fat ratio.
We calculated the ratio of abdominal fat to nonabdominal fat as an index of visceral fat/subcutaneous fat distribution using the following equation: abdominal fat (DEXA)/[total fat (DEXA) − abdominal fat (DEXA)].
Evaluation of the interference of subcutaneous fat on the measurement of visceral fat by DEXA scan.
A subset of 13 C57BL/6J mice with body weight ranging from 31 to 62 g was euthanized, and the DEXA scan was performed. After that, the skin of the mouse was carefully dissected away from the remaining carcass, and the subcutaneous tissue around the abdominal region (the inguinal white adipose tissue fat pads and any other subcutaneous fat, specifically on the dorsum of the mouse) was removed. The remaining carcass then contained all of the internal fat pads except for the subcutaneous fat pad. The carcass was then wrapped tightly so that the skin imitated the original structure, and DEXA scan was performed. The visceral fat was then dissected and weighed as described previously.
Measurement of liver triglycerides.
Liver samples (∼20 mg) from a subset of 17 C57BL/6J mice on a regular diet and 22 C57BL/6J DIO mice were homogenized and extracted in chloroform-ethanol (2:1, vol/vol). The samples were maintained at room temperature overnight and spun down for 15 min. The chloroform layer was taken and evaporated under nitrogen at 40°C. The resulting pellet was reconstituted in 250 μl of ethanol, and the triglyceride content was measured using a standard enzymatic method (Tg GPO-PAP assay; Roche Diagnostics, Indianapolis, IN).
Glucose tolerance test.
Studies were performed as described previously (8). Briefly, after a 4-h fast, samples were obtained in the morning from saphenous vein bleeds from 28 mice on regular diet and 28 DIO. Blood glucose was measured using a glucometer (Accu-chek; Roche Diagnostics) at 0, 15, 30, 60, and 120 min after an intraperitoneal injection of glucose (1 mg/g).
Statistical analyses.
All values are expressed as means ± SD. Data were analyzed by two-way analysis of variance (ANOVA) for changes in abdominal fat and the ratio of abdominal fat/nonabdominal fat over time. Changes in the ratio of abdominal fat/nonabdominal fat over time in females were analyzed using nonlinear regression (sigmoidal dose respone best-fit curve). To compare abdominal fat and the ratio of abdominal/nonabdominal fat at the age of 10 wk, we used one-way ANOVA, followed by Bonferroni's multiple comparisons. Glucose tolerance tests (GTT) were analyzed by two-way ANOVA, and the area under the curve (AUC) was calculated by trapezoid analysis. Data were checked for normality using the D'Agostino and Pearson tests. The relationship between the weight of dissected visceral fat and DEXA abdominal fat, as well as the relationships between abdominal fat and the ratio of abdominal fat/nonabdominal fat with the AUC of GTT and with total liver triglycerides, was analyzed with a Spearman rank test. Probability values <0.05 were considered statistically significant. The two techniques (dissection/weighing and DEXA) were also compared using Bland-Altman test to quantify the agreement between them in measuring visceral fat mass. Coefficient of variation percentage was calculated to evaluate the precision of the method. Analyses were performed with statistical software (GraphPad Prism 4.0; GraphPad Software, San Diego, CA).
RESULTS
Accuracy and precision of DEXA to measure fat tissue.
The accuracy of DEXA to measure fat content was evaluated by comparing the weight of dissected visceral fat pad measured by DEXA scan and the weight of dissected visceral fat measured by direct weighing. The coefficient of correlation and the bias calculated by Bland-Altman test show a strong relationship and agreement between the two measurements (DEXA and direct weighing). DEXA precision was evaluated by taking eight repeated measurements in each animal. Between DEXA readings, the mice were repositioned slightly. The low coefficient of variation indicates that DEXA is a precise method to measure fat mass (Table 1).
Table 1.
Accuracy and precision of DEXA absorptiometry scan
| Correlation | P Value | Bias ± SD | n | |
|---|---|---|---|---|
| Accuracy | r2 = 0.995* | <0.0001 | −0.12 ± 0.11† | 12 |
| Precision | %CV = 3.7§ | 8 |
DEXA, dual-energy X-ray absorptiometry; %CV, %coefficient of variation.
r2 was calculated by linear regression between the weight of dissected visceral fat pad measured by DEXA scan and by direct weighing.
Bias + SD was calculated by Bland-Altman test.
%CV showing the precision to determine total fat mass with 8 repeated measurements in a single animal.
Validation of visceral fat measurement by DEXA scan.
To evaluate the relationship between the abdominal adipose tissue measured by DEXA scan with the visceral fat content, three ROI extracted from the whole body scan were evaluated (Fig. 1A). Although all ROI show a significant correlation with visceral fat content, we chose ROI 3 because it displayed the lowest bias according to Bland-Altman test (Table 2).
Fig. 1.
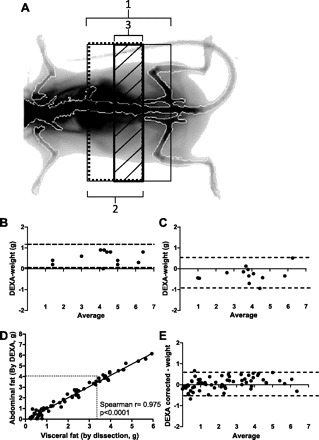
Relationship between visceral fat measured by dual-energy X-ray absorptiometry (DEXA) and visceral fat measured by dissection. A: graph of regions of interest (ROI) used to calculated visceral fat by DEXA scan. ROI 1 extends from S3/S4 vertebral space to L5/L6 vertebral space (thin solid line). ROI 2 extends from S3/S4 vertebral space to L3/L4 vertebral space (dotted line). ROI3 extends from L1/L2 to L4/L5 vertebral space (bold line, diagonally striped bar). B: Bland-Altman plot of the difference between DEXA scan (ROI 3) and dissection of visceral fat against the average of the 2 methods (DEXA + dissection/2). DEXA scan was performed before the removal of SAT (n = 13). C: Bland-Altman plot of the difference between DEXA scan (ROI 3) and dissection of visceral fat against the average of the 2 methods (DEXA + dissection/2). DEXA scan was performed after the removal of SAT (n = 13). D: linear regression between abdominal fat measured by DEXA (corrected by subtraction of bias factor, 0.65) and visceral fat measured by dissection. The solid line represents the regression from which the correlation coefficient was calculated, Spearman r = 0.975, P < 0.0001; n = 69. E: Bland-Altman plot of the difference between DEXA scan (ROI 3) and dissection of visceral fat against the average of the 2 methods (DEXA + dissection/2). DEXA scan was corrected by subtraction of bias factor (n = 69).
Table 2.
Comparison between the 3 ROI
| Test | ROI 1 | ROI 2 | ROI 3 |
|---|---|---|---|
| Spearman | |||
| r | 0.961 | 0.94 | 0.975 |
| 95% CI | 0.93–0.97 | 0.90–0.96 | 0.95–0.98 |
| P | <0.0001 | <0.0001 | <0.0001 |
| Bland-Altman | |||
| Bias | 3.27 | 2.07 | 0.65 |
| SD | 1.75 | 1.04 | 0.28 |
ROI, regions of interest; 95% CI, 95% confidence interval (n = 69).
Three ROI were used to calculate visceral fat by DEXA scan. ROI 1 extends from S3/S4 vertebral space to L5/L6 vertebral space. ROI 2 extends from S3/S4 vertebral space to L3/L4 vertebral space. ROI 3 extends from L1/L2 to L4/L5 vertebral space. Correlation coefficient (r) and bias were calculated for each area using Spearman's rank and Bland-Altman test, respectively, to evaluate the relationship between visceral fat obtained by DEXA scan and dissected visceral fat pad measured by direct weighing. ROI 3 shows the strongest correlation and the lowest bias.
To evaluate the interference of subcutaneous fat in the measurement of visceral adipose tissue by DEXA, subcutaneous fat was removed, and DEXA was performed before and after dissection. Using ROI 3, we found that the correlation was similar before and after removal of subcutaneous fat (r = 0.964, P < 0.0001, and r = 0.921, P < 0.0001, respectively). Furthermore, after removal of subcutaneous fat the bias was decreased, and the values obtained by DEXA were close to excised visceral fat mass (Fig. 1, B and C).
Because the overestimation seems to be constant over a wide range of values, it is possible to improve the selectivity of DEXA to measure visceral fat by subtraction of a bias factor. We obtained the bias factor of 0.65 from Bland-Altman test (Table 2) by analyzing a large number of mice. The resulting calculation (Fig. 1, D and E) shows a stronger agreement between the visceral fat measured by DEXA and the visceral fat measured by dissection and weighing.
This bias factor was then subtracted from DEXA abdominal fat measurements in all subsequent studies. We refer to this corrected data as DEXA visceral fat. Subsequent results using this corrected data were similar to results before correction.
In the same way, we recalculated the ratio of abdominal fat to nonabdominal fat, and it became (DEXA abdominal fat − 0.65)/(DEXA total fat − DEXA abdominal fat − 0.65). Subsequent results using the recalculated ratios were similar to results before correction (data not shown). However, when we characterized the phenotypes of different obese models (Fig. 2C), we found a significant difference between the ratios of monogenetic obese mouse models and lean C57BL/6J mice, which was not seen before the data were corrected.
Fig. 2.
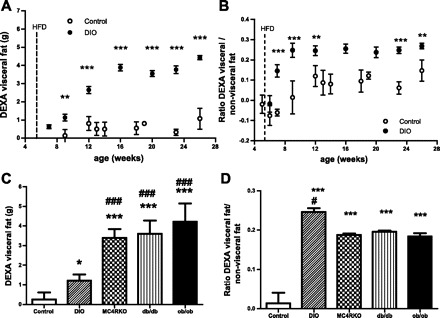
Visceral fat and ratio of visceral fat to nonvisceral fat measured by DEXA scan in different obese mouse phenotypes. A: DEXA visceral fat accumulation in male C57BL/6J mice on regular diet (○) and high-fat diet (HFD) [diet-induced obese (DIO) mice; ●]. B: ratio of DEXA visceral fat to nonvisceral fat in male C57BL/6J mice on regular diet (○) and DIO mice (●). C: DEXA visceral fat accumulation of C57BL/6J mice on regular diet (open bar; n = 9), DIO mice (diagonally striped bar; n = 6), melanocortin 4 receptor-knockout (MC4R-KO) mice (checkered bar; n = 7), db/db mice (vertically striped bar; n = 11), and ob/ob mice (black bar; n = 5) at 10 wk. D: ratio of DEXA visceral fat to nonvisceral fat of C57BL/6J mice on regular diet, DIO mice, MC4R-KO mice, db/db mice, and ob/ob mice at 10 wk. Results are means ± SD. ***P < 0.001, **P < 0.01, and *P < 0.05 vs. control; ###P < 0.001 and #P < 0.05 vs. DIO.
Usefulness of DEXA visceral fat and the ratio of DEXA visceral fat to nonvisceral fat to define obese mice phenotypes.
Using the localized area (ROI 3) by DEXA scan, we analyzed visceral fat accumulation in male C57BL/6J mice (control) and DIO mice. We found that DIO mice accumulate significantly more DEXA visceral fat in the first week of HFD than control mice. DEXA visceral fat continues to increase markedly for 8 wk more and then remains constant up to the last measurement (Fig. 2A).
The ratio of DEXA visceral fat to nonvisceral fat was almost constant throughout the study in C57BL/6J control mice. On the contrary, DIO mice showed a marked and rapid increase in the ratio of DEXA visceral fat to nonvisceral fat, with a remarkable visceral fat accumulation. This pattern of fat distribution was maintained during the study (Fig. 2B).
The monogenetic obese models (ob/ob, db/db, and MC4R-KO) had higher amounts of DEXA visceral fat than control and DIO mice (Fig. 2C). Interestingly, DIO mice exhibited a higher ratio of DEXA visceral to nonvisceral fat than the monogenetic obese mice (Fig. 2D) despite DIO mice having lower DEXA visceral fat content.
Female C57BL/6J mice on a regular diet slowly but constantly gained more visceral fat related to total fat, exhibiting at the age of 16–28 wk a 50% increase in the ratio of DEXA visceral/total fat (Fig. 3, A and B). To illustrate the importance of the timing of study, we compared the findings in C57BL/6J mice with those of female MC4R-KO (Fig. 3, A and B). Although MC4R-KO females show a significant and statistically higher DEXA visceral fat accumulation than C57BL/6J female mice at a young age, this difference disappears beyond 24 wk of age, when both strains of mice display a similar ratio of DEXA visceral fat to nonvisceral fat.
Fig. 3.
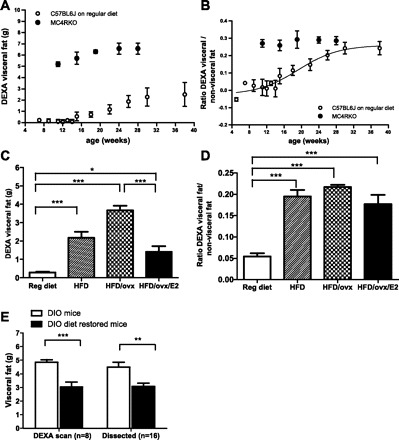
Usefulness of the DEXA visceral fat measurement and ratio of DEXA visceral fat to total fat to define a female obese mouse phenotype. A: DEXA visceral fat accumulation in female C57BL/6J mice on regular diet (○) and female MC4R-KO mice on regular diet (●). B: ratio of DEXA visceral fat to nonvisceral fat in female C57BL/6J mice on regular diet (○) and female MC4R-KO mice (●). C: DEXA visceral fat accumulation of B6D2 mice on regular diet (open bar; n = 26), on HFD (striped bar; n = 8), on HFD plus a previous ovariectomy at 4 wk of age (checkered bar; n = 16), on HFD plus a previous ovariectomy at 4 wk of age, and on estradiol replacement therapy for 1 mo (black bar; n = 8). D: ratio of DEXA visceral fat to nonvisceral fat of B6D2 mice on regular diet at 10 wk of age, on HFD, on HFD plus a previous ovariectomy at 4 wk of age, on HFD plus a previous ovariectomy at 4 wk of age, and on estradiol replacement therapy for 1 mo (black bar; n = 8). E: visceral fat of DIO mice (open bar) and DIO diet-restored mice (black bar) measured by DEXA scan (n = 8) and direct dissection and weighing (n = 16); both methods showed a similar decrease in visceral fat after diet intervention (37.4 vs. 31.4%, respectively). Results are means ± SD. ***P < 0.001; **P < 0.01; *P < 0.05.
We compared B6D2 mice of the same age (10 wk) fed different diets (regular diet and HFD) and found that B6D2 mice fed a HFD exhibited an increase in DEXA visceral fat accumulation, which worsened when these mice had previously been ovariectomized. However, estradiol replacement therapy significantly reduced DEXA visceral fat in these mice (Fig. 3C). The ratio of DEXA visceral to nonvisceral fat is higher in animals on HFD and remains constant despite ovariectomy or estradiol replacement therapy (Fig. 3D).
To strengthen the utility of in vivo localized DEXA scan to perform longitudinal analyses, we evaluated the effect of diet restoration from HFD to regular diet on visceral fat accumulation. We found that the decrease in visceral fat after diet intervention measured by DEXA scan (37.4%) is similar to the decrease in visceral fat measured by dissection (31.4%) (Fig. 3E).
DEXA visceral fat is associated with liver fat accumulation and nonalcoholic liver disease in obese mice.
DIO mice accumulated significantly more triglycerides in the liver than animals on a regular diet (P < 0.001; Fig. 4A). To evaluate whether DEXA visceral fat content or the ratio of DEXA visceral to nonvisceral fat is associated with liver fat accumulation, we analyzed the relationship between these parameters and the triglyceride content of the liver in male control and DIO mice. We found a positive and significant correlation between both parameters (DEXA visceral fat and the ratio of DEXA visceral fat to nonvisceral fat) and triglyceride accumulation. This correlation was found when the data of both groups (control and DIO mice) were analyzed together (Fig. 4, B and D) or when only the DIO mice were considered (Fig. 4, C and E). No such association was found between both parameters in control mice (data not shown).
Fig. 4.
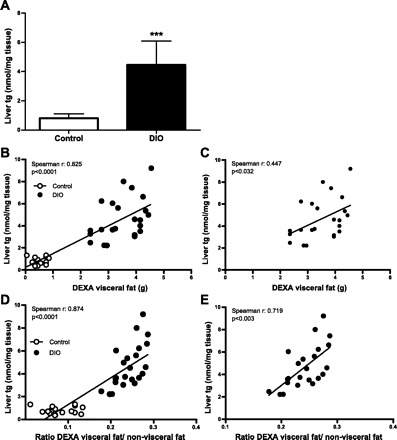
DEXA visceral fat correlates with liver fat accumulation in DIO mice. A: liver triglyceride content in C57BL/6J mice on regular diet (open bar; n = 17) and DIO mice (filled bar; n = 22). B: correlation between visceral fat measured by DEXA scan and liver content in C57BL/6J mice on regular diet (○; n = 17) and DIO mice (●; n = 22). C: correlation between visceral fat measured by DEXA scan and liver content in DIO mice (●; n = 22). D: correlation between the ratio of DEXA visceral fat to nonvisceral fat and liver content in C57BL/6J mice on regular diet (○; n = 17) and DIO mice (●; n = 22). E: correlation between the ratio of DEXA visceral fat to nonvisceral fat and liver content in DIO mice (●; n = 22). Results are means ± SD. ***P < 0.001.
DEXA visceral fat is associated with GTT response in obese mice.
The majority (70%) of DIO mice became insulin resistant and glucose intolerant compared with animals on a regular diet (8). The response to GTT was significantly higher in DIO mice than in control mice (Fig. 5A). To evaluate whether DEXA visceral fat content or the ratio of DEXA visceral fat to nonvisceral fat is associated with glucose intolerance, we analyzed its relationship with the AUC obtained from GTT in mice. We found a positive strong relationship between the visceral fat measured by DEXA scan and the AUC of glucose when the data of both groups (control and DIO mice) were analyzed together (Fig. 5B). This relationship was also found when the DIO mice were considered individually (Fig. 5C). There was also a significant albeit weaker association between the ratio of DEXA visceral fat to nonvisceral fat and the AUC for glucose in both groups analyzed together (Fig. 5D). This association was lost when either the DIO or the control mice were considered individually (Fig. 5E).
Fig. 5.
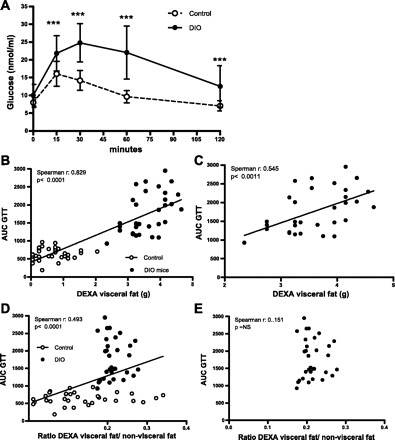
DEXA visceral fat correlates with area under the curve of glucose tolerance test (AUC GTT) in DIO mice. A: GTT of C57BL/6J mice on regular diet (○; n = 28) and DIO mice (●; n = 28). B: correlation between visceral fat measured by DEXA scan and AUC GTT in C57BL/6J mice on regular diet (○; n = 28) and DIO mice (●; n = 28). C: correlation between visceral fat measured by DEXA scan and AUC GTT in DIO mice (●; n = 28). D: correlation between the ratio of DEXA visceral fat to nonvisceral fat and AUC GTT in C57BL/6J mice on regular diet (○; n = 28) and DIO mice (●; n = 28). E: correlation between the ratio of DEXA visceral fat to nonvisceral fat and AUC GTT in DIO mice (●; n = 28). Results are means ± SD. ***P < 0.001.
DISCUSSION
Here, we demonstrate that visceral fat content measured in vivo by DEXA scan and evaluated by the selection of one localized abdominal area has an excellent correlation with visceral fat content measured by excised fat in C57BL/6J lean and obese mice. We analyzed three areas of the abdominal region extracted from the whole DEXA body scan, and we selected the area that showed the strongest correlation with visceral fat pad weights over a large range of data and the lowest bias. This localized DEXA scan was designed specifically to measure body fat in mice and provides a means to assess intra-abdominal visceral fat noninvasively. Recently, Gerbaix et al. (12), using a DEXA scan technique designed specifically for human whole body scans, validated this technique as a potential biomarker of visceral fat tissue in lean and obese male rats. There are some controversies regarding the accuracy of measuring fat mass between DEXA scan and chemical extraction (carcass analysis). Johnston et al. (17) found that DEXA scan underestimated fatty mass for obese animals and overestimated fatty mass for lean animals. However, other authors found that DEXA scan overestimated fat mass content in lean and obese mice (3, 26). These authors suggest these measurements can be corrected by using simple algorithms specific for the older and the newer Piximus software versions. To date, Piximus2 has replaced the older version of Piximus.
We evaluated the accuracy of DEXA to measure fat mass by comparing the weight of dissected visceral fat measured by DEXA scan ex vivo and measured by direct weighing. We found a strong relationship and agreement between the two measurements. Our result was similar to Leung et al. (20), demonstrating that DEXA scan is an excellent method to measure fat tissue.
To validate the measurement of visceral fat in vivo by DEXA scan, we analyzed a specific area of interest (ROI 3) and found an overestimation of visceral fat measured by DEXA scan compared with the results obtained directly by weighing fat mass. The overestimation we found can be explained by the fact that subcutaneous fat was included in the abdominal measurement. We performed DEXA after the removal of subcutaneous fat and found that the measurement of visceral fat was close to the exact values of excised fat.
Selectivity is the capacity of the method to detect a particular compound (visceral fat) in a complex mixture (whole body) without interference from other components. To improve the selectivity of DEXA to measure visceral fat in vivo, we studied a large number of mice, and the results were analyzed by Bland-Altman test. We found a bias factor that seems to be constant over a wide range of values. Therefore, we were able to subtract this bias factor (0.65) from each value obtained from DEXA scan and found a stronger agreement between the visceral fat measured by DEXA and the visceral fat measured by dissection and weighing. This correction allows for a more accurate estimation of visceral fat content in vivo, and therefore, we used this correction in all of our studies. However, because this correction was established only in C57BL/6J mice, using it in other strains of mice gives an estimate of visceral fat only in these mice. Therefore, further research is required to establish true correction factors for different strains of mice.
Clinical studies, including subjects with abdominal obesity and normal BMI, have demonstrated a strong relationship between abdominal (visceral) fat accumulation and metabolic alterations (1, 5, 9, 29). It has been postulated that the detrimental effects of visceral adipocytes on metabolism are due to the fact that they are more lipolitically active, which results in a large influx of free fatty acids into the portal circulation and the liver (lipotoxicity theory) (37). More recently, a complementary theory postulated that visceral adipocytes and their macrophage infiltration produce more proinflamatory cytokines that then influence liver metabolism and increase metabolic risk factors (36, 39). Recent studies have also suggested a possible protective role for subcutaneous fat. In humans, increased subcutaneous fat mass is associated with lower glucose and lipid levels independent of abdominal fat (29, 31). Loss of subcutaneous fat from differents areas of the body, characteristic of lipodystrophy, increases the risk of diabetes and dyslipidemia (10). Moreover, transplantation of subcutaneous fat into visceral compartments in mice improves glucose metabolism (34). The different distribution of fat depots could explain the reduced risk for metabolic disease of the well-known human obese subgroup termed metabolic healthy but obese individuals (18).
We measured DEXA visceral fat and the ratio of DEXA visceral fat to nonvisceral fat as an index of fat distribution. Our results show that the monogenetic models of obesity studied (ob/ob, db/db, and MC4R-KO mice) have a significantly higher visceral fat tissue content than DIO mice but a lower ratio of DEXA visceral fat to nonvisceral fat. When we compared C57BL/6J and B6D2 mice on a regular diet with C57BL/6J and B6D2 mice fed an HFD (DIO), we found that DIO mice accumulate a disproportionate volume of visceral fat, demonstrating a strong influence of diet in fat deposition.
Adipose tissue accumulation is sexually dimorphic, and females have a higher percentage of body fat than males (14, 22). Interestingly, we found that female mice on regular diets have a different pattern of visceral fat accumulation than males, showing an increased visceral fat depot faster than subcutaneous fat from the age of 14 wk. This difference is important to consider when the mice are used as a control of some genetic models, such as MC4R-KO mice. For example, we found that young female MC4R-KO mice exhibit increased DEXA visceral fat accumulation compared with control mice; however, this difference disappears after 24 wk, where the proportion of DEXA visceral fat to total fat is similar. These results demonstrate the utility of in vivo localized DEXA scan to characterize phenotype at different ages.
Because of its noninvasive nature, our localized DEXA scan technique also serves as an efficient tool for testing the efficacy of treatments on fat distribution in mice. We evaluated visceral fat accumulation in obese ovariectomized B6D2 mice before and after estradiol replacement therapy. We found that obese ovariectomized B6D2 mice have a higher visceral fat accumulation than obese B6D2 mice with intact ovaries. Estradiol replacement therapy for 1 mo with a physiological dose of estradiol induced a significant decrease in visceral fat. However, the ratio of DEXA visceral to nonvisceral fat remained unchanged. These results are unexpected, because in humans menopause onset is associated with decreased estradiol and increased total body fat and visceral fat (21). Also, some reports suggest that the reduction in total fat induced by estradiol treatment in mice is predominantly in visceral fat (6, 32). However, these results were obtained through cross-sectional analysis in which different mice were evaluated before and after treatment. In the present study, a noninvasive DEXA scan technique enabled the evaluation of fat depots before and after treatment in the same animal.
An additional advantage of using in vivo localized DEXA scan is it does not involve euthanization of the mice, thus reducing the number of mice required. To demonstrate the effect of diet restoration (from HFD to regular diet) on decreasing visceral fat, we performed direct measurement of visceral fat by dissection on 16 mice. On the contrary, the measurement by DEXA scan required half the number of mice to reach a similar result.
Liver fat accumulation (steatosis) is associated with hepatic insulin resistance (7). If the ability of insulin to suppress the hepatic output of glucose and VLDL is decreased, then it contributes to (postprandial) hyperglycemia and hyperlipidemia, intrinsic features of the metabolic syndrome. We evaluated whether the accumulation of visceral fat, measured by DEXA scan, can serve as a potential biomarker of metabolic abnormalities. We found a significant correlation between visceral fat or the ratio of DEXA visceral to nonvisceral fat with liver triglyceride accumulation in mice on regular diet and HFD when the data of both groups (control and DIO mice) were analyzed together, but more importantly, we found a significant correlation when just the DIO mice data were considered.
Additionally, we found a significant correlation between visceral fat and the AUC of GTT when the data of both groups were analyzed together or when just the DIO mice were considered. The association between the ratio of visceral to subcutaneous fat and the AUC of GTT was weaker when both groups were included in the analysis. This association was lost when the DIO mice data were considered individually. These results show that abdominal fat measured by DEXA scan is a potential biomarker of the response to a glucose challenge in obese mice that seems to be independent of the subcutaneous fat depot.
Ever since the validation of DEXA for measuring the body composition of mice, it has became an established method for conducting longitudinal studies of body composition and bone mineral density in small rodents (3, 26). Here, we demonstrate that, by using the same system and applying a selection of a predetermined localized area of the abdominal region, it is possible to estimate accurately and reproducibly the visceral fat depot in vivo. This new and feasible approach will permit comparisons of visceral adipose tissue and phenotypic characterization across experimental models of obesity in mice.
GRANTS
This work was supported by grants from the National Health and Medical Research Council of Australia, 606662, Pfizer Australia, Monash University, National Heart Foundation of Australia Grant 09M 4306, and National Institutes of Health Grants RR-0163 and DK-62202.
DISCLOSURES
The authors declare not to have any conflict of interest, financial or otherwise, with respect to this article.
AUTHOR CONTRIBUTIONS
W.C., J.L.W., and M.K. performed the experiments; W.C., J.L.W., M.K., M.A.C., and P.J.E. analyzed the data; W.C., J.L.W., and P.J.E. edited and revised the manuscript; W.C., J.L.W., M.K., M.A.C., and P.J.E. approved the final version of the manuscript; M.A.C. and P.J.E. interpreted the results of the experiments; P.J.E. did the conception and design of the research; P.J.E. prepared the figures; P.J.E. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Garcia Rudaz for helpful discussions.
REFERENCES
- 1.Balkau B, Deanfield JE, Despres JP, Bassand JP, Fox KA, Smith SC, Jr, Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C, Haffner SM International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 116: 1942–1951, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 10: 493–496, 1990 [PubMed] [Google Scholar]
- 3.Brommage R. Validation and calibration of DEXA body composition in mice. Am J Physiol Endocrinol Metab 285: E454–E459, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Calderan L, Marzola P, Nicolato E, Fabene PF, Milanese C, Bernardi P, Giordano A, Cinti S, Sbarbati A. In vivo phenotyping of the ob/ob mouse by magnetic resonance imaging and 1H-magnetic resonance spectroscopy. Obesity (Silver Spring) 14: 405–414, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Casanueva FF, Moreno B, Rodríguez-Azeredo R, Massien C, Conthe P, Formiguera X, Barrios V, Balkau B. Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in Spain. Clin Endocrinol (Oxf) 73: 35–40, 2010 [DOI] [PubMed] [Google Scholar]
- 6.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983–35991, 2005 [DOI] [PubMed] [Google Scholar]
- 7.den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol 24: 644–649, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc Med 20: 90–95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg A. Acquired and inherited lipodystrophies. N Engl J Med 350: 1220–1234, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav 67: 141–147, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Gerbaix M, Metz L, Ringot E, Courteix D. Visceral fat mass determination in rodent: validation of dual-energy X-ray absorptiometry and anthropometric techniques in fat and lean rats. Lipids Health Dis 9: 140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48: 839–847, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 34: 989–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens 14: 103S–115S, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 28, Suppl 4: S12–S21, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Johnston SL, Peacock WL, Bell LM, Lonchampt M, Speakman JR. PIXImus DEXA with different software needs individual calibration to accurately predict fat mass. Obes Res 13: 1558–1565, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Karelis AD. Obesity: To be obese—does it matter if you are metabolically healthy? Nat Rev Endocrinol 7: 699–700, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung FW, Murray S, Murray E, Go VL. Determination of body fat distribution by dual-energy X-ray absorptiometry and attenuation of visceral fat vasoconstriction by enalapril. Dig Dis Sci 53: 1084–1087, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32: 949–958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 10: 154–167, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Luu YK, Lublinsky S, Ozcivici E, Capilla E, Pessin JE, Rubin CT, Judex S. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med Eng Phys 31: 34–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy x-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 20: 1109–1114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller CN, Kauffman TG, Cooney PT, Ramseur KR, Brown LM. Comparison of DEXA and QMR for assessing fat and lean body mass in adult rats. Physiol Behav 103: 117–121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8: 392–398, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism 53: 454–457, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Poll L, Wittsack HJ, Willers R, Modder U, Heinemann L, Kapitza C, Rave K. Correlation between anthropometric parameters and abdominal fat volumes assessed by a magnetic resonance imaging method in patients with diabetes. Diabetes Technol Ther 6: 844–849, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 32: 1068–1075, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav 97: 199–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, Seidell JC; Health ABC Study Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 48: 301–308, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. In press [DOI] [PubMed] [Google Scholar]
- 33.Surwit RS, Collins S. Revisiting lessons from the C57BL/6J mouse. Am J Physiol Endocrinol Metab 280: E825–E826, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trayhurn P. Adipose tissue in obesity—an inflammatory issue. Endocrinology 146: 1003–1005, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle - adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem 117: 47–56, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44: 863–870, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Uusitupa M, Lindi V, Louheranta A, Salopuro T, Lindström J, Tuomilehto J; Finnish Diabetes Prevention Study Group Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes 52: 2532–2538, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000 [DOI] [PubMed] [Google Scholar]


