Abstract
The prevalence of obesity is increasing globally, and obesity is a major risk factor for metabolic diseases such as type 2 diabetes. Previously, we reported that oral administration of homobrassinolide (HB) to healthy rats triggered a selective anabolic response that was associated with lower blood glucose. Therefore, the aim of this study was to evaluate the effects of HB administration on glucose metabolism, insulin sensitivity, body composition, and gluconeogenic gene expression profiles in liver of C57BL/6J high-fat diet-induced obese mice. Acute oral administration of 50–300 mg/kg HB to obese mice resulted in a dose-dependent decrease in fasting blood glucose within 3 h of treatment. Daily chronic administration of HB (50 mg/kg for 8 wk) ameliorated hyperglycemia and improved oral glucose tolerance associated with obesity without significantly affecting body weight or body composition. These changes were accompanied by lower expression of two key gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase), and increased phosphorylation of AMP-activated protein kinase in the liver and muscle tissue. In vitro, HB treatment (1–15 μM) inhibited cyclic AMP-stimulated but not dexamethasone-stimulated upregulation of PEPCK and G-6-Pase mRNA levels in H4IIE rat hepatoma cells. Among a series of brassinosteroid analogs related to HB, only homocastasterone decreased glucose production in cell culture significantly. These results indicate the antidiabetic effects of brassinosteroids and begin to elucidate their putative cellular targets both in vitro and in vivo.
Keywords: homobrassinolide, glucose, insulin resistance, diabetes, phosphoenolpyruvate carboxykinase
brassinosteroids are plant-specific polyhydroxylated derivatives of 5α-cholestane, structurally similar to cholesterol-derived animal steroid hormones and ecdysteroids from insects. They are found at low levels in pollen, seeds, leaves, and young vegetative tissues throughout the plant kingdom (2). The first biologically active plant brassinosteroid was isolated from the pollen of rapeseed Brassica napus in 1979 (16). The natural occurrence of more than 50 compounds of this group has been reported following the initial discovery (13). Similar to animal steroid hormones (24), brassinosteroids regulate the expression of specific plant genes and complex physiological responses involved in growth (9) partly via interactions with other hormones setting the frame for brassinosteroid responses (28). Brassinosteroid signaling in plants resembles the Wnt pathway and is mediated by GSK-3-like kinase (18). Moreover, application of brassinosteroids increased sugar and starch content in plants (35), whereas a brassinosteroid-deficient Arabidopsis mutant had an impaired carbohydrate metabolism (32).
Very little is known about effects of brassinosteroids in animals. Natural brassinosteroids inhibited growth of several human cancer cell lines without affecting the growth of normal cells (25). A synthetic brassinosteroid analog prevented HSV-1 multiplication and viral spreading in a human conjunctival cell line with no cytotoxicity and reduced the incidence of herpetic stromal keratitis in mice when administered topically (27), possibly by the modulation of the response of epithelial and immune cells to HSV-1 infection (26). 24-Epibrassinolide, the most widely used brassinosteroid in agriculture, has a favorable safety profile. The median lethal dose (LD50) of this compound is >1,000 mg/kg in mice and >2,000 mg/kg in rats when applied orally or subcutaneously (22).
In our previous study, we observed that oral administration of homobrassinolide (HB; Fig. 1A) to healthy rats triggered a selective anabolic response that was associated with lower blood glucose (11). Since another plant ecdysteroid, 20-hydroxyecdysone (20HE), was shown to decrease weight and hypeglycemia in obese mice (21), we sought to explore the effects of HB on glucose metabolism and insulin resistance in the high-fat diet-induced obese C57BL/6J mouse model. These mice were selectively bred for divergent body fat mass and thus model complex polygenic human obesity (7).
Fig. 1.

Chemical structure of 28-homobrassinolide (HB; A) compared with other synthetic and natural brassinosteroid analogs (B).
MATERIALS AND METHODS
Reagents.
HB [(22S,23S,24S)-2a,3a,22,23-tetrahydroxy-24 ethyl-b-homo-7-oxo-5a-cholestane-6-one; Fig. 1A] was purchased from Waterstone Technology (Carmel, IN), and its structure was confirmed by electrospray ionization/liquid chromatography-mass spectrometry and NMR. Brassinosteroid analogs 2–9 (Fig. 1B), including homocastasterone (22S,23S,24S)-2a,3a,22,23-tetrahydroxy-24-ethyl-5a-cholestan-6-one (2), (22S,23S,24R)-3a-fluoro-22,23-dihydroxy-24-ethyl-b-homo-7-oxa-5a-cholestan-6-one (3), (22S,23S,24S)-3a-fluoro-22,23-dihydroxy-24-ethyl-5α-cholestan-6-one (4), (22S,23S,24S)-2α,3α,22,23-tetrahydroxy-24-ethyl-b-homo-7-aza-5α-cholestan-6-one (5), (22S,23S,24S)-2α,3α,22,23-tetrahydroxy-24-ethyl-b-homo-6-aza-5α-cholestan-7-one (6), (22R,23R,24S)-2α,3α,22,23-tetrahydroxy-b-homo-7-oxa-5α-cholestan-6-one (7),(22S,23S,24R)-2α,3α,22,23-tetrahydroxy-24-methyl-b-homo-7-oxa-5α-cholestan-6-one (8), and (22R,23R,24R)-2α,3α,22,23-tetrahydroxy-24-methyl-b-homo-7-oxa-5α-cholestan-6-one (9), were synthesized or purchased previously (12) and are shown in Fig. 1B. All other chemicals and cell culture media were obtained from Sigma (St. Louis, MO) or Invitrogen (Carlsbad, CA) unless specified otherwise.
Animal studies.
All animal experiments were performed according to procedures approved by the Rutgers Institutional Animal Care and Use Committee in an American Association for Accreditation of Laboratory Animal Care-accredited animal care facility. Six-week-old male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained on a high-fat diet (HFD) containing 60% fat-derived calories (D12492; Research Diets) with 12:12-h light-dark cycles for additional 4 wk. Then animals were further randomized into two groups and fed HFD for an additional 6 wk. At this time, the control group (n = 8) was gavaged daily with a vehicle solution alone (5% DMSO in corn oil), whereas a treatment group (n = 8) received 50 mg/kg body wt of HB for another 8 wk. The body weight of each animal and the total amount of food consumed (accounted for spillage) were recorded every week for the duration of the brassinosteroid supplementation.
Fasted plasma glucose concentrations were measured immediately prior to gavage and 3 h postgavage at weeks 1 and 8 of the brassinosteroid supplementation in submandibular vein blood samples using a glucometer (Lifescan; Johnson & Johnson). Blood samples were collected in EDTA-coated tubes and centrifuged for 20 min at 1,500 g, and separated plasma was stored at −80°C until analysis. Plasma concentrations of insulin were determined by rat/mouse insulin ELISA kit (Millipore, Billerica, MA). Plasma and tissue triglycerides and total cholesterol were measured by enzymatic colorimetric assays (Wako Diagnostics, Richmond, VA).
To perform the glucose tolerance test at week 8 of the brassinosteroid supplementation, mice were fasted overnight (16 h) and gavaged orally with 2 g/kg glucose solution. Plasma glucose levels were measured immediately before and 30, 60, and 120 min after the glucose challenge.
At the end of experiment, blood was collected by heart puncture after CO2 inhalation, and animal body composition was assessed prior to necropsy using dual-energy X-ray absorptiometry (DEXA) analysis on PIXImus equipment (Lunar, Madison, WI). At necropsy, tissue weights were recorded, and then tissue samples were collected by snap-freezing in the liquid nitrogen and stored at −80°C for further studies.
Western blot analysis.
Whole cell extracts were prepared from liver or gastrocnemius muscle samples in ice-cold RIPA buffer supplemented with 10 mM sodium floride, 2 mM sodium orthovanadate, 1 mM PMSF, and protease inhibitor cocktail (Sigma) and centrifuged for 20 min at 12,000 g at 4°C. Equal amounts of protein (50 μg) from the supernatants were separated on 10% SDS polyacrylamide gels and blotted onto the nitrocellulose membrane. Western blot detection was performed with monoclonal antibodies for phosphorylated (phospho) AMP-activated protein kinase (AMPK) and AMPK (Cell Signaling Technology, Danvers, MA), phospho-insulin receptor substrate-1 (IRS-1) (Ser636/Ser639) and IRS-1, phosphatidylinositol 3-kinase (PI3K), phospho-Akt1 and (Ser473) and Akt1 (EMD Millipore, Bedford, MA), phospho-Akt2 (Ser474) and Akt2 (Bioworlde Technologies, St. Paul, MN), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) as a loading control according to the manufacturer's instructions. After being washed, the blots were incubated with an anti-rabbit peroxidase-labeled secondary antibody and visualized using ECL Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ).
Cell culture and qPCR.
The H4IIE hepatoma cells were cultured in 24-well tissue culture plates (Greiner Bio One, Monroe, NC) and grown to near confluence in Dulbecco's modified Eagle's medium containing 2.5% (vol/vol) fetal bovine serum and 2.5% (vol/vol) horse serum. When appropriate, cells were treated with 500 nM dexamethasone and/or 0.1 mM 8-CTP-cAMP (Dex-cAMP) for 8 h to induce phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) gene expression together with different concentrations of brassinosteroid or 10 nM insulin as a positive control. Three wells were allocated for each treatment, including the negative control (vehicle alone). Total RNA extraction, cDNA synthesis, and quantitative PCR were performed essentially as described earlier (15).
Glucose production assay.
H4IIE rat hepatoma cells were serum starved overnight in the glucose production buffer (glucose-free Dulbecco's modified essential medium, pH 7.4, containing 20 mM sodium lactate and 2 mM sodium pyruvate without phenol red) and treated for 8 h with Dex-cAMP in the presence or absence of 10 nM insulin or different concentrations of brassinosteroid for 8 h. At the end of the incubation, 0.5 ml of medium was taken to measure the glucose concentration in the culture medium using the Amplex Red glucose assay kit (Invitrogen). Corrections for cell number were made on the basis of the protein concentration measured using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
Statistics.
Statistical analyses were performed using Prism 4.0 (GraphPad Software, San Diego, CA) and expressed as means ± SE. Unless otherwise noted, two-tailed t-test or one-way ANOVA (as appropriate) was applied, and P < 0.05 was considered significant. Post hoc analyses of differences between individual experimental groups were made using the Dunnett's multiple comparison test. Body weight gain and glucose tolerance were analyzed by two-factor repeated-measures ANOVA, with time and treatment as independent variables.
RESULTS
HB decreases fasting blood glucose.
Obesity was induced by feeding a HFD to C57BL/6J mice for 4 wk before the animals were randomized into the control and treatment groups. Mice were maintained on HFD for an additional 6 wk prior to brassinosteroid supplementation. At the beginning of the treatment (after 10 wk on HFD), all animals had similar weight (33.2 ± 0.9 g) and developed hyperglycemia (204 ± 11 mg/dl fasting blood glucose). HB showed moderate efficacy at lowering blood glucose levels in C57BL/6J mice following the acute single-dose treatment (Fig. 2). The effect was dose dependent in the range of 50 to 300 mg/kg body wt and reached one-half of that observed for metformin (a standard reference drug used at the highest dose of 300 mg/kg) 3 h after oral administration.
Fig. 2.
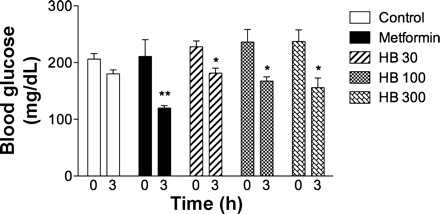
Acute lowering effect of HB on plasma glucose in C57BL/6J mice 3 h after administration of 50, 100, or 300 mg/kg HB (n = 8). Metformin at 300 mg/kg was used as a positive control. Values are means ± SE. *P < 0.05 and **P < 0.01 when compared with control by 1-way ANOVA followed by Dunnett's post hoc test.
HB improves insulin sensitivity without affecting body weight.
To test the chronic effect of HB in the in vivo diabetes model, C57BL/6J mice were kept on the HFD for another 8 wk. During this time, the treatment group received an oral gavage of 50 mg/kg HB daily, whereas control animals were gavaged with vehicle alone. The final body weights did not differ between two groups (Table 1); no changes in food intake (not shown) or body weight gain have also been noted (Fig. 3A). DEXA analysis confirmed these findings by showing no significant changes in either body composition (lean or fat mass) or bone mineral content. The baseline blood glucose levels continued to rise for the duration of the treatment and were not significantly different between either group at the end of the treatment (229 ± 17 mg/dl). However, animals administered with 50 mg/kg HB exhibited lower blood glucose levels following an acute treatment with HB several days before the end of the study (Fig. 3B). Moreover, an oral glucose tolerance test performed at the end of the study revealed significantly increased insulin sensitivity in HB-treated animals. Whereas blood glucose levels peaked similarly in both groups 30 min following an oral glucose challenge, they were significantly lower at 60 and 120 min after administration of HB to treated animals relative to the control group (Fig. 3C). The total area under the curve decreased by an average of 18% by treating mice with HB.
Table 1.
Body composition and metabolic biochemistry profiles of mice treated with HB
| Control | HB | |
|---|---|---|
| Body weight, g | 39.4 ± 1.5 | 38.4 ± 3.4 |
| Lean mass, g | 20.6 ± 0.4 | 20.1 ± 0.7 |
| Fat mass, g | 18.8 ± 1.3 | 19.3 ± 3.5 |
| Bone mineral content, g | 0.439 ± 0.02 | 0.430 ± 0.02 |
| Liver triglycerides, mg/g | 15.4 ± 2.6 | 13.9 ± 2.1 |
| Muscle triglycerides, mg/g | 2.5 ± 0.2 | 2.1 ± 0.2 |
Results are expressed as means ± SE. HB, homobrassinolide. Mice were fed high-fat diet and gavaged daily with 50 mg/kg body wt HB for 8 wk (n = 8). Body composition was measured by dual-energy X-ray absorptiometry.
Fig. 3.
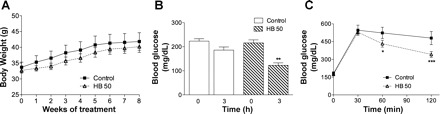
Chronic insulin-sensitizing effect of HB on body weight gain (A), fasting blood glucose (B), and oral glucose tolerance test (C) in the C57BL/6J mice. Six-week-old male mice were fed a high-fat diet for 6 wk and kept on the same diet for additional 8 wk combined with daily gavage with vehicle or 50 mg/kg HB. B: fasting blood glucose levels in animals fed high-fat diet (n = 8) or high-fat diet treated with 50 mg/kg HB (n = 8) at 6 wk. C: oral glucose tolerance test curves of groups fed high-fat diet or high-fat diet animals receiving the HB treatment. Values are means ± SE. *P < 0.05 and ***P < 0.001 when compared with control by 1-way ANOVA followed by Dunnett's post hoc test. **P > 0.01.
HB inhibits liver gluconeogenesis in obese mice.
Glucose homeostasis requires a precise balance between glucose production and utilization. Both acute and chronic effects of HB administration on blood glucose levels could be explained partially by reduced gluconeogenesis in liver tissue of these animals. To test this hypothesis, we evaluated mRNA expression levels for two key gluconeogenic enzymes, PEPCK and G-6-Pase, which are regulated on the transcriptional level. PEPCK is highly expressed in the liver, where it is adaptively regulated by a variety of different hormones and other agents in a manner that parallels gluconeogenic flux (17). G-6-Pase is expressed mainly in the liver and in the kidney cortex, most particularly in the starved and diabetic states (30). In animals treated with HB, the mRNA levels were reduced significantly for PEPCK and G-6-Pase two- and threefold, respectively (Fig. 4A). Additionally, Western blot analysis of liver tissue of these animals revealed an increased phosphorylation of AMPK (Fig. 4B), a key regulator of cellular energy homeostasis and suppressor of gluconeogenesis (5). A smaller increase in AMPK phosphorylation was also evident in the muscle tissue of the HB animals (Fig. 4C).
Fig. 4.

Hepatic expression of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) (A) and activation of liver AMP-acticated protein kinase phosphorylation (AMPK-P; B) in C57BL/6J mice treated with 50 mg/kg HB. PEPCK and G-6-Pase mRNAs were normalized to β-actin mRNA. Liver tissue lysates were analyzed by immunoblotting with phospho- and non-phospho-specific AMPK antibodies. Values are means ± SE. *P < 0.05, 2-tailed t-test vs. control.
Next, we analyzed the status of the insulin-signaling pathways in liver and muscle tissues of control and HB animals. We observed a significant increase in Akt1 and Akt2 phosphorylation in the liver and a significant increase in IRS-1 and Akt1 phosphorylation in the muscle as well as an increase in total PI3K in muscle tissue. Taken together, these data suggest that HB supplementation resulted in activation of the insulin-signaling pathway in liver and muscle tissues of diet-induced obese mice (Fig. 5).
Fig. 5.

Effect of HB administration on insulin signaling in liver (A) and skeletal muscle (B). Tissue lysates were analyzed by immunoblotting with phospho- and non-phospho-specific antibodies for insulin receptor substrate-1 (IRS-1), phosphatidylinositol 3-kinase (PI3K), Akt1, Akt2, and β-actin as loading controls. Four animals were tested from each group, and a representative Western blot is shown. Ctr, control.
HB inhibits cAMP- but not dexamethasone-induced upregulation of PEPCK and G-6-Pase.
Starvation and diabetes cause a two- to threefold increase in gluconeogenic enzyme activity in the liver that is associated with a two- to fourfold increase in PEPCK and G-6-Pase mRNA (1). Glucocorticoids and specifically dexamethasone cause a larger (≤10-fold) increase in G-6-Pase activity and in the level of its mRNA in cultured hepatoma cells (33). Changes in cAMP concentration are also directly involved in transcriptional regulation of these genes through several cis-acting sequences present in their promoters (8). To confirm our observation that HB treatment decreased PEPCK and G-6-Pase mRNA levels in liver tissue of obese animals, a quantitative analysis of the mRNA expression patterns of PEPCK and G-6-Pase in cAMP-stimulated and Dex-cAMP-induced H4IIE cells was performed to determine whether the effect of HB on glucose production is related to its effect on expression of these genes. Untreated cells were used to measure the basal level of PEPCK and G-6-Pase expression, whereas the β-actin gene was chosen as an internal standard since the level of β-actin mRNA remained unaffected by the treatments. HB treatment (1 or 10 μM for 8 h) achieved weak suppression of glucose production (20%) from Dex-cAMP-induced H4IIE cells in vitro (Fig. 6A). A similar weak decrease in PEPCK and G-6-Pase mRNA levels (0–10%) was observed following an induction with a dexamethasone-cAMP mixture (Fig. 6B); however, a very strong dose-dependent decrease in target gene expression (≤70% for PEPCK and 85% for G-6-Pase) was observed in cells induced by cAMP alone (Fig. 6C). Under the same conditions, insulin at 10 nM decreased glucose production by 50% and PEPCK mRNA expression by 80% and totally suppressed the G-6-Pase mRNA transcript.
Fig. 6.

A: production of glucose in the H4IIE rat hepatoma cell culture in response to HB treatment following inducement with the dexamethasone (Dex)-cAMP mixture. B and C: hepatic expression of the gluconeogenic enzymes PEPCK and G-6-Pase in cell culture induced with the Dex-cAMP mixture (B) or cAMP alone (C). PEPCK and G-6-Pase mRNAs were normalized to β-actin mRNA. Values are means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with control by 1-way ANOVA followed by Dunnett's post hoc test.
Effect of brassinosteroid analogs on glucose production in vitro.
Next, we compared bioactivity of HB to that of its natural and synthetic analogs (12). Among eight compounds tested, only homocastasterone (2) was able to suppress glucose production from Dex-cAMP-induced cells, similar to HB (Fig. 7). Removal of the 2a-hydroxyl group and fluorination at C-3 in the A ring (compounds 3 and 4) led to an 80% decrease in bioactivity. Replacement of 7-oxalactone group with amine in the B ring of compound 5 reduced biological activity by 90%, whereas a similar replacement of the 6-carbonyl group with amine in 6 resulted in a complete loss of suppression of glucose production. Modifications in the side chain (compounds 7–9) also abolished the activity.
Fig. 7.

Production of glucose in the H4IIE rat hepatoma cell culture in response to treatment of HB (1), homocastasterone (2), and other synthetic and natural brassinosteroid analogs (3–9). Cells were incubated with 10 μM brassinosteroids for 8 h at 37°C. Values are means ± SE. *P < 0.05 and **P < 0.01 when compared with control by 1-way ANOVA followed by Dunnett's post hoc test.
DISCUSSION
Brassinosteroids are present in small quantities in foods and plants (2). They are similar in many respects to animal steroids (Fig. 1) but appear to function very differently at the cellular level. Whereas animal steroid hormones act through the nuclear receptor family of transcription factors, plant brassinosteroids signal through a cell surface receptor kinase-mediated signal transduction pathway (29, 34). At the same time, brassinosteroids share some similarities with ecdysteroids that have a wide array of physiological and pharmacological effects in animals and insects (4), including modulation of protein synthesis (14) and carbohydrate metabolism (21).
Oral administration of HB to obese mice with hyperglycemia lowered blood glucose levels after the acute single-dose treatment (Fig. 2). This hypoglycemic activity was approximately one-half that of the known antidiabetic drug metformin. The principal function of metformin is to reduce hepatic glucose production and to improve peripheral insulin sensitivity, thus ameliorating hyperglycemia (20). Metformin has been shown to activate AMPK and to inhibit the expression of the hepatic gluconeogenic genes PEPCK and G-6-Pase similarly to insulin (19). The activation of AMPK improves insulin sensitivity by stimulating glucose uptake and lowering blood glucose, whereas the activity of AMPK is suppressed in disorders associated with insulin resistance (31). At molecular levels, a complex relationship between the AMPK and insulin-signaling pathways exists. It has been reported that AMPK regulates IRS-1 and Akt/PKB (23), whereas insulin and Akt have negative impacts on AMPK activation in adipocytes (6).
To test the effect of HB in the in vivo diabetes model, male C57BL/6J mice were fed a HFD and treated daily by oral administration of 50 mg/kg HB for 8 wk following a 10-wk HFD feeding to develop hyperglycemia and insulin resistance (Fig. 3). At the end of the brassinosteroid supplementation, the body weight of the HB group was not significantly different from that of the controls. Oral gavage technique may induce stress response in mice and thus influence weight gain in chronic feeding studies. This is possibly reflected in the low weight gain in mice in this study (Fig. 3A), and an alternative method for low-stress drug delivery, i.e., peanut butter pills (10), may be considered for future chronic studies for obesity or type 2 diabetes in rodents.
No differences in animal feeding behavior or lean/fat body mass composition were noted by DEXA (Table 1). However, at the end of the treatment HB animals had slightly lower blood glucose and significantly improved glucose tolerance, suggesting that liver or muscle tissue is the primary target for brassinosteroid bioactivity. In the present study, both control and treatment groups of mice were obese and showed clear signs of hyperglycemia and insulin resistance. Liver and skeletal muscle triglyceride levels decreased with HB treatment but did not reach significance (Table 1). Therefore, it is possible that healthy animals with physiological activity of the insulin-signaling pathway responded stronger to chronic HB administration (11). An alternative explanation for this phenomenon would be the innate difference in control of glucose utilization between rodents since the original observation was reported in rats. However, the reduction of blood glucose associated with HB administration to healthy rats was only a trend that did not reach significance, and mean fasting blood glucose values decreased from 5.0 to 4.5–4.8 mM in response to HB treatment (11).
Previously, we have observed that 20HE, a plant ecdysteroid structurally similar to brassinosteroids, lowered blood glucose in obese mice, whereas it caused a significant decrease in body weight gain and adipose mass. Similarly to HB, 20HE had no effect on food consumption in this model (21). Taken together, these data suggest that plant brassinosteroids and ecdysteroids have similar effects on glucose metabolism and insulin sensitivity in animals; however, the underlying molecular mechanisms and targets may be different. For example, similarly to 20HE, HB decreased PEPCK and G-6-Pase mRNA levels and induced AMPK phosphorylation in liver and, to a lesser degree, muscle tissues of the treated animals (Fig. 4). Normally, PEPCK and G-6-Pase gene expression is induced by glucagon (through cAMP), glucocorticoids, and cathecholamines during periods of fasting and in response to stress but is dominantly inhibited by glucose-induced increases in insulin secretion upon feeding. The impaired insulin response in the liver caused by insulin resistance secondary to type 2 diabetes results in a continuous elevated expression of PEPCK and G-6-Pase due to the unopposed action of glucagon. This permits continuous hepatic glucose output, thereby contributing significantly to basal and fasting hyperglycemia and the complications associated with diabetes. Both 20HE (21) and HB decreased glucose production in H4IIE rat hepatoma cell culture; however, HB treatment suppressed only gluconeogenic gene expression induced by cAMP and not the dexamethasone-cAMP combination (Fig. 6). In this instance, HB acted similar to metformin in regard to inability to repress hormone-induced PEPCK expression (36).
Previously, we have also described the anabolic effect of HB in muscle tissue of healthy rats that was mediated by Akt activation (11) and synthesis of several brassinosteroid analogs with various ability to induce Akt signaling in L6 rat skeletal muscle cells (12). In this study, we observed positive effects of HB administration on some components of the insulin-signaling pathway, including an increase in Akt1 and Akt2 phosphorylation in the liver and a significant increase in IRS-1 and Akt1 phosphorylation in the muscle as well as an increase in total PI3K in muscle tissue. Therefore, there is a possibility that HB modulates glucose metabolism by a combined effect on both Akt and AMPK signaling pathways in liver and muscle tissues; however, this hypothesis has not been tested in the present study and needs further clarification. However, it is clear that several structural similarities are required for plant sterols to modulate carbohydrate metabolism in mammals. These include 2a- and 3a-hydroxyl groups at the C-3 position in the A ring, 6-keto group in B ring, and 22a, 23a-hydroxyls in the side chain of the molecule (Fig. 7). These requirements are very similar to those reported from plants, where (2a, 3a)- and (22a, 23a)-vicinal diol moieties are required for optimum bioactivity (3). Another interesting observation is that the 6-keto group shared by 20HE, HB, and homocastasterone is preferable for retaining glucose metabolism-modulating activity rather than a 6-keto-7-lactone group present in classical brassinosteroids.
In conclusion, we hypothesize that HB may exert its glucose-lowering effect by repressing glucose production and activating AMPK and Akt signaling in liver and skeletal muscle. Stimulatory effect of HB on glucose metabolism subsequently translates into a whole body insulin-sensitizing effect such as improved oral glucose tolerance.
GRANTS
This work was supported in part by Rutgers University and by the 5P50AT002776-05 grant from the National Center for Complementary and Alternative Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.E., S.K., and I.R. did the conception and design of the research; D.E., P.K., and S.K. performed the experiments; D.E. and S.K. analyzed the data; D.E., P.K., and S.K. interpreted the results of the experiments; D.E. and S.K. prepared the figures; D.E. and S.K. drafted the manuscript; D.E., S.K., and I.R. edited and revised the manuscript; D.E., P.K., S.K., and I.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Reneta Pouleva and Ruth Dorn for excellent technical assistance and Dr. Sue Shapses for reviewing and commenting on the manuscript.
REFERENCES
- 1. Argaud D, Zhang Q, Pan W, Maitra S, Pilkis SJ, Lange AJ. Regulation of rat liver glucose-6-phosphatase gene expression in different nutritional and hormonal states: gene structure and 5′-flanking sequence. Diabetes 45: 1563– 1571, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Bajguz A, Tretyn A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62: 1027– 1046, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Baron DL, Luo W, Janzen L, Pharis RP, Back TG. Structure-activity studies of brassinolide b-ring analogues. Phytochemistry 49: 1849– 1858, 1998 [Google Scholar]
- 4. Bathori M, Toth N, Hunyadi A, Marki A, Zador E. Phytoecdysteroids and anabolic-androgenic steroids—structure and effects on humans. Curr Med Chem 15: 75– 91, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Berasi SP, Huard C, Li D, Shih HH, Sun Y, Zhong W, Paulsen JE, Brown EL, Gimeno RE, Martinez RV. Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J Biol Chem 281: 27167– 27177, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Berggreen C, Gormand A, Omar B, Degerman E, Goransson O. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am J Physiol Endocrinol Metab 296: E635– E646, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Bunger L, Hill WG. Inbred lines of mice derived from long-term divergent selection on fat content and body weight. Mamm Genome 10: 645– 648, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Chatelain F, Pegorier JP, Minassian C, Bruni N, Tarpin S, Girard J, Mithieux G. Development and regulation of glucose-6-phosphatase gene expression in rat liver, intestine, and kidney: in vivo and in vitro studies in cultured fetal hepatocytes. Diabetes 47: 882– 889, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Clouse SD. Brassinosteroids. Plant counterparts to animal steroid hormones? Vitam Horm 65: 195– 223, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes 29: 607– 614, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Esposito D, Komarnytsky S, Shapses S, Raskin I. Anabolic effect of plant brassinosteroid. FASEB J 25: 3708– 3719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esposito D, Rathinasabapathy T, Poulev A, Komarnytsky S, Raskin I. Akt-dependent anabolic activity of natural and synthetic brassinosteroids in rat skeletal muscle cells. J Med Chem 54: 4057– 4066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujioka S, Yokota T. Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54: 137– 164, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Gorelick-Feldman J, Maclean D, Ilic N, Poulev A, Lila MA, Cheng D, Raskin I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem 56: 3532– 3537, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab 293: E1503– E1510, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Steffens GL, Flippenanderson JL, Cook JC. Brassinolide, a plant growth-promoting steroid isolated from Brassica-napus pollen. Nature 281: 216– 217, 1979 [Google Scholar]
- 17. Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66: 581– 611, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang JX, Burlingame AL, Wang ZY. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11: 1254– 1260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57: 306– 314, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 137: 25– 33, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kizelsztein P, Govorko D, Komarnytsky S, Evans A, Wang Z, Cefalu WT, Raskin I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab 296: E433– E439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuzmitsky BB, Mizulo NA. Study of Acute Toxicity of Epibrassinolide and its Preparative Forms: Technical Report. Minsk, Belarus: Academy of Sciences of Belarus, 1991. (1–44). [Google Scholar]
- 23. Longnus SL, Segalen C, Giudicelli J, Sajan MP, Farese RV, Van Obberghen E. Insulin signalling downstream of protein kinase B is potentiated by 5′AMP-activated protein kinase in rat hearts in vivo. Diabetologia 48: 2591– 2601, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4: 46– 56, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Malikova J, Swaczynova J, Kolar Z, Strnad M. Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry 69: 418– 426, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Michelini FM, Berra A, Alche LE. The in vitro immunomodulatory activity of a synthetic brassinosteroid analogue would account for the improvement of herpetic stromal keratitis in mice. J Steroid Biochem Mol Biol 108: 164– 170, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Michelini FM, Ramirez JA, Berra A, Galagovsky LR, Alche LE. In vitro and in vivo antiherpetic activity of three new synthetic brassinosteroid analogues. Steroids 69: 713– 720, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Müssig C. Brassinosteroid-promoted growth. Plant Biol (Stuttg) 7: 110– 117, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203– 212, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 117: 132– 139, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Ruderman NB, Saha AK. Metabolic syndrome: adenosine monophosphate-activated protein kinase and malonyl coenzyme A. Obesity 14, Suppl 1: 25S–33S, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Schluter U, Kopke D, Altmann T, Mussig C. Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroids deficient cbb1 mutant. Plant Cell Environ 25: 783– 791, 2002 [Google Scholar]
- 33. Schmoll D, Allan BB, Burchell A. Cloning and sequencing of the 5′ region of the human glucose-6-phosphatase gene: transcriptional regulation by cAMP, insulin and glucocorticoids in H4IIE hepatoma cells. FEBS Lett 383: 63– 66, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380– 383, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55: 1135– 1143, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Yuan L, Ziegler R, Hamann A. Inhibition of phosphoenolpyruvate carboxykinase gene expression by metformin in cultured hepatocytes. Chin Med J (Engl) 115: 1843– 1848, 2002 [PubMed] [Google Scholar]


