Abstract
RhoA prenylation may play an important step in the translocation of RhoA in the basal internal anal sphincter (IAS) smooth muscle tone. Statins inhibit downstream posttranslational RhoA prenylation by 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition (HMGCRI). The role of statins in relation to RhoA prenylation in the pathophysiology of the spontaneously tonic smooth muscle has not been investigated. In the present studies, we determined the effect of classical HMGCRI simvastatin on the basal IAS tone and RhoA prenylation and in the levels of RhoA/Rho kinase (ROCK) in the cytosolic vs. membrane fractions of the smooth muscle. Simvastatin produced concentration-dependent decrease in the IAS tone (via direct actions at the smooth muscle cells). The decrease in the IAS tone by simvastatin was associated with the decrease in the prenylation of RhoA, as well as RhoA/ROCK in the membrane fractions of the IAS, in the basal state. The inhibitory effects of the HMGCRI were completely reversible by geranylgeranyltransferase substrate geranylgeranyl pyrophosphate. Relaxation of the IAS smooth muscle via HMGCRI simvastatin is mediated via the downstream decrease in the levels of RhoA prenylation and ROCK activity. Studies support the concept that RhoA prenylation leading to RhoA/ROCK translocation followed by activation is important for the basal tone in the IAS. Data suggest that the role of HMG-CoA reductase may go beyond cholesterol biosynthesis, such as the regulation of the smooth muscle tone. The studies have important implications in the pathophysiological mechanisms and in the novel therapeutic approaches for anorectal motility disorders.
Keywords: internal anal sphincter, mevalonate, geranylgeranyltransferase, Rho kinase
spontaneous tone in the internal anal sphincter (IAS) smooth muscle is considered to be primarily myogenic in nature and plays a crucial role in anorectal continence (20, 27). Hypertensive IAS has been associated with motility disorders like Hirschsprung's disease (22, 27), recurrent anal fissures, and hemorrhoids (7, 19). Hypotensive IAS on the other hand results in rectoanal incontinence (20). Molecular mechanisms for the regulation of IAS tone are not completely understood. Such information is important for the understanding of the pathophysiology of the above abnormalities and their therapeutic managements.
Activation of Ser/Thr kinase Rho kinase (ROCK) by GTP·RhoA is a critical step in RhoA-ROCK-mediated Ca2+ sensitization in the smooth muscle. RhoA cycles between a biologically inactive GDP-bound state and an active GTP-bound state. Thus, in resting state, Rho GDP dissociation inhibitor (Rho GDI) binds to GDP-RhoA and extracts GDP-RhoA from the membrane to the cytosol (36). With the agonist stimulation of G protein-coupled receptors (GPCRs), guanine nucleotide exchange factors convert GDP-RhoA to GTP-RhoA. GTP-RhoA associates with the plasma membrane via its prenylated tail, leading to the activation of ROCK (14).
It follows, therefore, that RhoA prenylation may be an important step for the translocation of RhoA to the cellular membrane. RhoA is posttranslationally modified by the isoprenoid lipid geranylgeranyl (6). In addition, prenyltransferase, geranylgeranyltransferase I (GGTase I), catalyzes the covalent attachment of the geranylgeranyl group from geranylgeranyl pyrophosphate (GGPP) to the carboxyl-terminal cysteine of RhoA (39).
Commonly used lipid-lowering agents or statins work via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in the biosynthesis of cholesterol in the liver. HMG-CoA reductase catalyzes the conversion of HMG-CoA to mevalonic acid. In addition to inhibiting cholesterol synthesis, statins also block the synthesis of isoprenoid intermediates such as farnesyl pyrophosphate and GGPP (13). By inhibiting these isoprenoid intermediates, statins prevent the prenylation of GTPases including RhoA. Because of this prominent effect, actions of statins may go beyond the inhibition of cholesterol biosynthesis. Consequently, HMG-CoA reductase may play an important role not only in the cholesterol biosynthesis but also in the increased smooth muscle contractility (6), neurogenic relaxation of the smooth muscle via G protein coupling (33), neuroinflammation, neurological diseases and stroke (40), and embryonic development (23).
The purpose of the present investigation was to determine the upstream regulation of RhoA prenylation via HMG-CoA reductase in the IAS tone using classical HMG-CoA reductase inhibition (HMGCRI) simvastatin. In this regard, we determined the levels and the cellular distribution of prenylated vs. nonprenylated RhoA ROCK II and ROCK activity in the basal state of the IAS, before and after HMGCRI simvastatin. For direct comparison of this regulation, some studies were performed in the adjoining nontonic smooth muscle of the rectum (RSM).
MATERIALS AND METHODS
Tissue preparation.
The studies were performed in the spontaneously tonic smooth muscle of the IAS and the adjoining nontonic smooth muscle, the RSM. Sprague-Dawley rats (300–350 g) were euthanized by decapitation. The anorectal tissues were then quickly removed and transferred to oxygenated (95% O2-5% CO2) Krebs physiological solution (KPS) of the following composition (in mmol): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose (37°C). Circular smooth muscle strips (∼0.5 × 7 mm) of the IAS and the RSM were prepared as explained previously (29).
Pretreatment of the smooth muscle tissues with simvastatin.
The IAS and RSM strips were cultured in Leibovitz medium (L-15) (25) with 5% penicillin-streptomycin, 50 μg/ml gentamycin, and 2 μg/ml amphotericin B at room temperature in a sterile environment in tissue culture hood. The smooth muscle strips from the IAS and RSM were incubated for 24 h with varying concentrations of simvastatin (0.1, 1.0, and 10.0 μM). Appropriate vehicle was added to the culture medium for control strips for the same duration.
Measurement of isometric tension.
Smooth muscle strips prepared above were transferred to 2-ml muscle baths containing oxygenated KPS at 37°C. Isometric tension was measured via force transducers (model FT03; Grass Instruments, Quincy, MA) using the PowerLab/8SP data-acquisition system and Chart 5 (AD Instruments, Colorado Springs, CO) (10, 31). Decrease in the basal IAS tone was expressed as a percentage of maximal decrease caused by 10 mM EDTA, and increase in the tone was expressed as a percentage of maximal by 300 μM bethanechol, determined at the end of each experiment (5).
The experimental protocol of the study was approved by the institutional Animal Care and Use Committee of Thomas Jefferson University and was in accordance with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Drug responses.
Changes in the basal IAS tone and the cumulative concentration-response curves (CRCs) for U-46619 (1 nM to 10 μM) in the IAS strips were determined before and after pretreatment with simvastatin (0.1 to 10.0 μM). To determine the reversibility by GGTase substrate, some experiments were performed with the simultaneous incubations of the smooth muscles with GGPP (10 μM) plus simvastatin.
Immunoprecipitation of prenylated proteins and Western blot analysis.
Following the incubation with either simvastatin or the vehicle, the smooth muscle tissues were snap frozen in liquid N2 and immediately stored at −80°C. At appropriate time, the frozen tissues were cut into small pieces, and homogenization buffer (50 mM HEPES, pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2 mM NaVO4, 25 mM NaF) was added in a volume equal to five times the weight of the tissues. The mixture was then homogenized on ice. The homogenates were then centrifuged (14,000 revolution/min) for 5 min, and supernatants were collected. Protein concentration in resultant supernatants was determined by the method of Lowry et al. (18) using BSA as a standard (Pierce Biotechnology, Rockford, IL). Prenylated RhoA was immunoprecipitated using Roche Diagnostics immunoprecipitation kit (Protein G) (Fisher, Allentown, PA), following manufacturer's instructions. Briefly, 200 μg of tissue lysate in 250 μl volume was precleared with 25 μl protein G agarose beads. Precleared lysate was incubated with 1 μg of anti-farnesyl rabbit polyclonal antibody (Calbiochem, San Diego, CA) for 1 h (4). Then 25 μl of protein G agarose beads were added and further incubated overnight to immobilize prenylated proteins. Agarose beads were centrifuged for 20 s at 10,000 g, and supernatants were transferred to a fresh tube for a fraction containing unprenylated proteins. Agarose beads were washed repeatedly with wash buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate). Later, 50 μl Lamellae sample buffer (LSB; with final concentrations 62.5 mM Tris, 1% SDS, 15% glycerol and 0.005% bromophenol blue, and 2% β-mercaptoethanol) was added to the beads and placed in a boiling water bath for 5 min. Twenty micrograms of unprenylated fraction was also similarly added with LSB. Protein samples were separated by 15% SDS-polyacrylamide gel.
The proteins separated above were electrophoretically transferred onto a nitrocellulose membrane at 100 V for 1 h at 4°C. To block nonspecific antibody binding, the membranes were soaked overnight at 4°C in Tris-buffered saline with Tween (TBS-T; composed of: 20 mM Tris pH 7.6, 137 mM NaCl, and 0.1% Tween-20) containing 5% nonfat dry milk. The membrane was then incubated with the RhoA primary antibody raised in rabbit (1:1,000 diluted in TBS-T containing 1% milk) for 1 h at room temperature. After being washed with TBS-T three times (10 min each wash), the membranes were incubated with the horseradish peroxidase-conjugated bovine anti-rabbit secondary antibody (1:10,000). The membranes were washed three times with TBS-T, and the corresponding bands were visualized with enhanced chemiluminescence substrate using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) and Hyperfilm MP (Amersham Bioscience, Piscataway, NJ). Bands corresponding to different proteins on X-ray films were scanned with a scanner (model SNAPSCAN 310; Agfa, Ridgefield Park, NJ), and their relative densities were determined by using Image-Pro Plus 4.0 software (Media Cybernetics, Silver Spring, MD).
Cytosolic and particulate fractions.
The IAS and RSM tissues incubated with the vehicle (control) as well as HMG-CoA reductase inhibitor were used for cytosolic and particulate fraction collections. For this, the respective tissues were homogenized in ice-cold homogenization buffer (10 mM Tris·HCl, pH 7.5, 5 mM MgCl2, 2 mM EDTA, 250 mM sucrose, and 1 mM dithiothreitol). The homogenates were centrifuged at 10,000 g for 30 min at 4°C (L8–70M Ultracentrifuge; Beckman, Fullerton, CA). The supernatants were then transferred to a fresh tube and used as the cytosolic fraction. Pellets containing membrane proteins were resuspended in homogenization buffer containing 1% Triton X-100 and homogenized. The pellet extract was centrifuged at 800 g for 10 min, and the supernatant was collected as the particulate fraction (12).
Above prepared protein extracts (20 μg) were mixed with LSB and separated by 15% SDS-polyacrylamide gel. The separated proteins were electrophoretically transferred onto a nitrocellulose membrane, RhoA Western blots were performed as described before (30), and bands were captured on X-ray film. Nitrocellulose membranes were stripped of secondary and primary antibodies by incubating with Restore Western blot stripping buffer (Pierce Biotechnology) for 15 min at room temperature and then reprobed for α-actin as described earlier (10).
ROCK activity assay.
ROCK activity was determined by monitoring the levels of phosphorylation of the endogenous ROCK substrate regulatory subunit of myosin light-chain phosphatase (MYPT1) in total IAS smooth muscle tissue extracts using a rat anti-phospho-Thr696 antibody (17). For this, the IAS tissues were pretreated with different concentrations (0.1 to 10.0 μM) of simvastatin and (as explained above) and 10 μM of selective ROCK inhibitor Y 27632.
Preparation of dispersed IAS smooth muscle cells and measurement of cell lengths.
Smooth muscle cells (SMCs) were isolated from the IAS smooth muscle by sequential enzymatic digestion, filtration, and centrifugation as described previously (10). Briefly, the IAS smooth muscle strips were incubated in KPS containing 0.1% collagenase and 0.01% trypsin inhibitor. The partly digested strips were washed, and SMCs were allowed to disperse spontaneously for 30 min. The SMC were harvested by filtration through 500 μM Nitex mesh and centrifuged twice at 350 g for 10 min. The cells were cultured in 10-cm plates in DMEM containing 10% fetal bovine serum, 5% penicillin-streptomycin, 50 μg/ml gentamycin, and 2 μg/ml amphotericin B at 37°C with 5% CO2. The cells were then incubated with simvastatin (0.1, 1.0, and 10.0 μM) for 24 h. Control cells were incubated with the vehicle solution only. Individual cell length was measured by computerized image microscopy. The average length of cells in the control state or with a test agent was obtained from 50 cells encountered randomly in successive microscopic fields. The experiments were repeated in at least four animals (n = 4).
Data analysis.
Data are presented as means ± SE. Western blot data were expressed as a ratio of relative densities in particulate over cytosolic fractions. One-way ANOVA followed by Bonferroni post hoc test was used (P < 0.05) to calculate statistical significance for comparing more than two groups. CRC curves were analyzed using two-way ANOVA test.
Drugs and chemicals.
Simvastatin sodium was purchased from Calbiochem (San Diego, CA). U-46619 was from Bachem Bioscience (King of Prussia, PA), and GGPP ammonium and bethanechol were from Sigma Chemical (St. Louis, MO). RhoA, pThr696-MYPTI, and α-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA) and Sigma Chemical, respectively. Leibovitz medium (L-15) and DMEM medium were from Fisher (Allentown, PA).
RESULTS
Effect of HMGCRI simvastatin on the basal IAS tone.
Pretreatment of the IAS smooth muscle strips with HMGCRI simvastatin caused concentration-dependent decrease in the basal tone. Simvastatin (10 μM) caused 62.5 ± 6.4% inhibition in the basal IAS tone (*P < 0.05; n = 6; Fig. 1). These results were compared with the non-simvastatin-treated smooth muscle strips (incubated with the vehicle only, considered as controls).
Fig. 1.
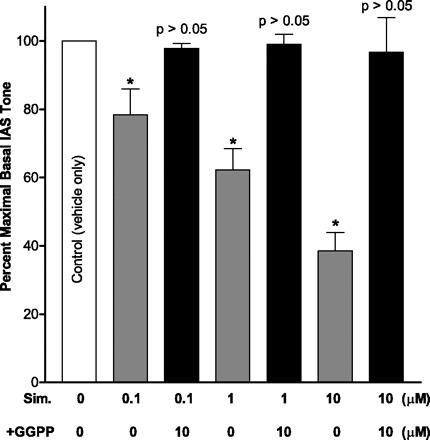
Effect of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (HMGCRI) simvastatin on percent maximal basal tone of the internal anal sphincter (IAS). Simvastatin (Sim.) causes a significant decrease in the basal IAS tone in a concentration-dependent manner (*P < 0.05; n = 6). Geranylgeranyltransferase substrate geranylgeranyl pyrophosphate (GGPP) ameliorates this inhibitory effect of simvastatin.
The inhibitory effect of simvastatin was reversed following GGTase substrate GGPP (10 μM) so that the values obtained following the combined treatment were not significantly different from those of controls (P > 0.05; n = 6; Fig. 1).
Effect of HMGCRI simvastatin on the IAS vs. the RSM SMCs.
To demonstrate the site of action of the HMGCRI on the IAS SMC, we determined the effect of simvastatin on the isolated SMC from the IAS vs. RSM. Data show that simvastatin caused concentration-dependent greater increase in the lengths of the SMC from the IAS compared with those from the RSM (*P < 0.05; Fig. 2). Simvastatin (10 μM) caused maximal relaxation of 28.9 ± 3.8% of the IAS SMCs compared with 6.2 ± 2.3% in the case of the RSM.
Fig. 2.

Simvastatin causes significantly greater and concentration-dependent increase in the lengths (relaxation) of the IAS smooth muscle cells (SMCs) (*P < 0.05; n = 4) compared with the rectal smooth muscle (RSM) (P > 0.05; n = 4). The observed basal lengths of the IAS and RSM SMC in these experiments were 45.2 ± 2.5 and 72. 6 ± 3.7 μm, respectively.
Effect of simvastatin on U-46619-induced increase in the IAS tone.
Thromboxane A2 analog U-46619 caused concentration-dependent increase in IAS tone (the maximal effect, Emax = 59.9 ± 5.1%, with 3 × 10−6 M; EC50 = 2.5 × 10−8 M; n = 6). Pretreatment with simvastatin caused significant attenuation of these responses (Emax = 25.5 ± 5.5%; EC50 = 3.2 × 10−6 M; P < 0.05; n = 4, Fig. 3A).
Fig. 3.
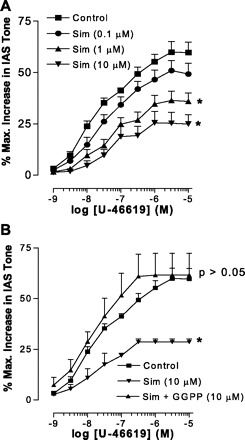
Comparison of concentration-response curve with thromboxane A2 analog U-46619 (1 nM to 10 μM) on percent maximal increase in IAS tone before and after pretreatment with 0.1, 1.0, and 10.0 μM simvastatin (A). As shown, simvastatin causes significant and concentration-dependent attenuation of U-46619-induced increase in the IAS tone (*P < 0.05; n = 4). In a separate series of experiments, 10 μM GGPP reverses the inhibitory effect of simvastatin (10 μM) (B).
The suppressant effect of simvastatin on U-46619-induced increase in the IAS tone was reversed by 10 μM GGPP so that U-46619 CRC examined in the simvastatin + GGPP (both 10 μM) group was not significantly different from control (P > 0.05; Fig. 3B).
Effect of HMGCRI on RhoA prenylation.
Prenylated proteins were immunoprecipitated using anti-farnesyl rabbit antibody (4). The precipitates containing prenylated proteins were then specifically analyzed for the levels of prenylated RhoA via Western blot using RhoA antibody. Similarly, supernatants (containing unprenylated proteins) were analyzed for unprenylated RhoA. Whereas anti-farnesyl antibody recognizes both farnesyltransferase and geranyltransferase-induced isoprenylation, GGTase I may selectively geranylgeranylate rather than farnesylate RhoA (34).
Pretreatment of the IAS smooth muscles with simvastatin significantly reduced the levels of prenylated RhoA but caused increase in the levels of unprenylated RhoA, in a concentration-dependent manner (*P < 0.05; n = 4; Fig. 4). Not shown, these effects of simvastatin were reversed by GGPP.
Fig. 4.
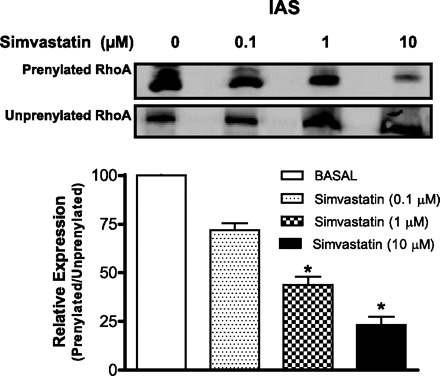
Western blots showing prenylated vs. unprenylated RhoA levels under basal (0) and following 24-h pretreatment with different concentrations of simvastatin. Samples of tissue lysates (200 μg) were incubated with anti-farnesyl antibody. The prenylated proteins were immobilized and captured on protein G agarose beads. The proteins bound on beads were then denatured in sample buffer and separated by SDS-PAGE, followed by Western blotting using RhoA antibody. The supernatants containing unprenylated proteins were also analyzed. Data show a significant decrease in the levels of prenylated RhoA following simvastatin (*P < 0.05; n = 4).
Effect of HMGCRI on the cellular distribution of RhoA in IAS vs. the RSM.
Molecular localization of RhoA in the IAS vs. RMS SMC was determined by Western blot analysis after separation of corresponding cell homogenates in cytosol and membrane fractions, before and after pretreatments with different concentrations of simvastatin. Data revealed higher levels of RhoA in the IAS membrane vs. the cytosolic fraction (Fig. 5, left). Pretreatment with simvastatin reversed the pattern of distribution, a significant decrease in RhoA in the membrane fraction and increased in the cytosolic fraction, in a concentration-dependent manner (*P < 0.05; n = 4; Fig. 5).
Fig. 5.

Western blot analyses for the levels of RhoA in the particulate vs. cytosolic fractions of the IAS smooth muscles before (0) and after different concentrations of simvastatin (left), compared with the RSM (right). Densitometric analysis (particulate:cytosolic ratios in relation to α-actin levels) reveal concentration-dependent and significant decrease (*P < 0.05; n = 4) in the levels of RhoA in the particulate fractions of the IAS but not RSM by simvastatin (P > 0.05; n = 4).
In the phasic tissue of the RSM, in sharp contrast with the tonic IAS, higher levels of RhoA were present in the cytosolic compared with the particulate fractions, and simvastatin or Y 27632 produced no significant reversal of this pattern (P > 0.05; n = 4; Fig. 5, right).
These data in the IAS and RSM have been summarized as the ratios of RhoA in particulate/cytosolic expressions (Fig. 5, bottom).
Effect of HMGCRI on cellular distribution of ROCK II in the IAS vs. the RSM.
To determine the selectivity of action of simvastatin on the distribution of RhoA in the IAS, we determined the effect of HMG-CoA reductase inhibitor on RhoA effector target protein ROCK II.
As shown, in the basal state of the IAS, higher levels of ROCK II were present in the particulate (membranous) compared with the cytosolic fractions. Simvastatin as well as ROCK inhibitor Y 27632 significantly reversed this pattern of cellular distribution of ROCK II (*P < 0.05; n = 4; Fig. 6, left).
Fig. 6.
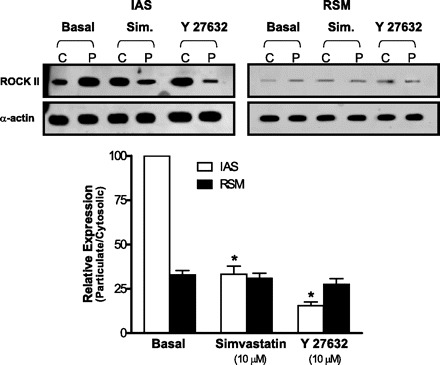
Western blot showing the effect of simvastatin on the levels of Rho kinase (ROCK) II in the cytosolic (C) vs. particulate (P) fractions in the IAS (left) vs. RSM (right). Densitometric analysis (calculated as the particulate:cytosolic ratios) reveals that in the IAS simvastatin and Y 27632 (in comparison with the basal state) cause significant redistribution of ROCK II levels from the particulate to the cytosolic fractions (*P < 0.05; n = 4). However, in the case of RSM, simvastatin or Y 27632 caused no such significant redistributions of ROCK II (P > 0.05; n = 4).
In contrast, however, in the basal state in the RSM, pattern of the cellular distribution of ROCK II was reverse, higher in the cytosolic vs. particulate fractions. In addition, here, neither simvastatin nor Y 27632 had any significant effect on this pattern of ROCK II distribution (P > 0.05; n = 4; Fig. 6, right). These data have been summarized as the ratios of ROCK II in particulate/cytosolic expressions (Fig. 6; bottom).
Effect of HMGCRI on the levels of pThr696-MYPTI.
To determine specifically the effect of HMGCRI on the ROCK activity, we compared the effects of simvastatin and Y 276322 on pThr696-MYPTI in the IAS. Data show that simvastatin causes concentration-dependent decrease in ROCK activity. The residual levels of pThr696-MYPTI following pretreatment with 10 μM simvastatin and Y 27632 were 28.10 ± 2.56% and 6.35 ± 1.26%, respectively (*P < 0.05; n = 4; Fig. 7). Data suggest that the HMGCRI via RhoA prenylation cause downstream inhibition of ROCK activity, leading to decrease in the IAS tone.
Fig. 7.

Western blot data showing the effect simvastatin on the levels of phosphoregulatory subunit of myosin light-chain phosphatase (pThr696-MYPT1) in the IAS smooth muscle in the basal state and following treatment with different concentrations of simvastatin and 10 μM Y 27632. Data show that simvastatin causes significant and concentration-dependent decrease in the levels of pThr696-MYPT1 (*P < 0.05; n = 4). These data suggest that the HMGCRI causes ROCK inactivation via its downstream inhibitory effect on RhoA prenylation.
DISCUSSION
The studies for the first time show that the HMG-CoA reductase provides important regulation of the IAS smooth muscle tone via RhoA trafficking regulated by RhoA prenylation. Inhibition of RhoA geranylageranylation and decrease in the IAS tone with the HMGCRI are similar to that with the GGTase I inhibitor GGTI-297. In addition, HMGCRI also attenuates the agonist-specific increase in the IAS tone.
It is well known that in the basal state RhoA/ROCK are largely present in the cytosol of the SMCs, and during sustained phase of the smooth muscle contraction they translocate to the membrane during the activation of RhoA/ROCK (36). Activated ROCK inhibits myosin light-chain phosphatase (MLCP) either directly or via phosphorylation of PKC-potentiated inhibitor or endogenous inhibitor of myosin light-chain phosphatase (CPI-17). ROCK-mediated MLCP inhibition decreases dephosphorylation of phosphorylated MLC20 (p-MLC20). The resultant increase in p-MLC20 has been suggested to be responsible for the maintenance of the IAS tone in the basal state (24, 28, 30). There is a large body of evidence for this concept for the agonist-induced sustained contraction of different smooth muscles (16, 21).
As depicted in Fig. 8, HMG-CoA reductase not only provides the necessary building blocks for cholesterol biosynthesis, but also for the isoprenoids farnesyl and GGPPs (32). These pyrophosphates play a significant role in the posttranslational modification of Ras and Rho GTPase, respectively. HMG-CoA reductase provides upstream regulation of these events. In agreement with this concept, simvastatin, a typical HMGCRI reduces, not only cholesterol biosynthesis, but also the supply of isoprenoids for Rho prenylation (32). In contrast to the GGTI-297 (which inhibits GGTase I directly), HMGCRI exerts downstream inhibition of RhoA geranylgeranylation via mevalonate/GGPP/GGTase I (2, 37).
Fig. 8.

Simplified schematics of the molecular changes for RhoA prenylation leading to RhoA/ROCK activation responsible for the basal tone in the IAS. HMG-CoA reductase exerts upstream regulation of RhoA prenylation via geranylgeranyltransferase I (GGTase I). The latter catalyzes the covalent attachment of the geranylgeranyl group from GGPP to RhoA (RhoA prenylation). RhoA prenylation plays an important role in the translocation of RhoA to the membrane followed by the activation of RhoA/ROCK. Data show that simvastatin blocks this upstream regulation of RhoA prenylation/RhoA/ROCK pathways, thus causing a decrease in the basal IAS tone, as well as its increase by thromboxane A2 (TXA2) analog U-46619. MLCP, myosin light-chain phosphatase.
Simvastatin, a classical HMGCRI, has been reported to cause vasodilatation of arteries (3). Diminished isoprenoid intermediates are associated with simvastatin-induced decrease in force in vascular smooth muscle from spontaneously hypertensive rats, partially reversible with mevalonate (26). This information is based on the attenuation of agonist-induced smooth muscle contraction, and there are no data in the spontaneously tonic smooth muscle. Our systematic studies in the tonic smooth muscle of the IAS reveal that significant and specific decreases in the basal tone and RhoA/ROCK activity by simvastatin require 24-h incubation.
Longer incubations for the observed changes in the tone and RhoA/ROCK were necessary in the case of, not only HMGCRI, but also RhoA prenylation inhibitor. This is in agreement with the slow turnover of isoprenylation and half life of HMG-CoA reductase (11) lasting hours or days. Following such regimen of pretreatment, simvastatin reduces the basal and agonist-induced increase in the IAS tone. Interestingly, similar results were obtained with the downstream inhibition of geranylgeranylation by GGTI-297 (25). Decrease in IAS tone correlates the decrease in the levels of prenylated RhoA with the corresponding increase in the levels of unprenylated RhoA, in the presence of simvastatin. These findings are of particular interest because similar treatment of the nontonic smooth muscle of the RSM reveals no significant changes either in the force or in the levels of prenylated RhoA.
In agreement with the above concept, we observed significant shifts in the cellular distribution of RhoA/ROCK II following HMGCRI simvastatin, i.e., an increase in the cytosolic and decrease in the membrane RhoA/ROCK II. These data suggest dependence of basal tone on RhoA prenylation, leading to RhoA/ROCK II translocation. This concept draws significant support from the literature as follows. Activation and translocation of RhoA are important processes for the agonist-induced Ca2+ sensitization and force development (14, 36). In this regard, RhoA prenylation appears to be critical (1). Studies by Gong et al. (15) reported that prenylated GTP·RhoAval14 Ca2+ sensitizes smooth muscle mildly permeabilized with β-escin but not with Triton X-100. These observations suggest that intact membrane is important for RhoA translocation. The studies further demonstrated that unprenylated GTP·RhoAval14 fails to produce Ca2+ sensitization-dependent changes in smooth muscle contractility.
The IAS was the major focus of present studies because of its specific importance in the anorectal continence and a number of other anorectal motility disorders including Hirschsprung's disease, recurrent anal fissures, and hemorrhoids (7, 20, 27). The basal IAS tone is considered to be primarily myogenic because of its maintenance in the absence of any extrinsic stimulus. The RSM in contrast is primarily phasic in nature. The IAS has characteristically higher levels of RhoA/ROCK II in the membranous vs. the cytosolic fractions in the IAS, and the pattern is reverse in the RSM. On the basis of the presently available data, we speculate that inhibition of RhoA prenylation by HMGCRI provides upstream regulation of RhoA/ROCK in causing decrease in the IAS tone by the statins. Data show that HMGCRI simvastatin and prenylation inhibitor GGTI-297 cause the redistribution of prenylated RhoA (from the membrane to the cytosol) associated with the decrease in the basal tone in the IAS. These functional and biochemical changes in the IAS are reversible with the GGTase substrate GGPP.
In contrast with the IAS, data from the RSM reveal that neither simvastatin nor GGTI-297 have any significant effect on the cellular distribution of prenylated RhoA and RhoA/ROCK II. This is in agreement with the earlier data showing predominantly higher levels of GDI bound RhoA (the inhibited form of RhoA) in the phasic smooth muscles (35). In addition, the effect of HMGCRI on the basal tone in the IAS and on RhoA/ROCK activation is similar to that of classical ROCK inhibitor Y 27632. This was further evident by the concentration-dependent decrease in ROCK activity following pretreatment of the IAS tissues with simvastatin. These data suggest the significance of sequential events of RhoA prenylation leading to RhoA/ROCK activation.
The inhibitory effect of HMGCRI on the spontaneous and thromboxane A2 analog U-46619-induced increase in the IAS tone may be explained on the basis of the endogenous control of the IAS tone by thromboxane pathway, via GPCR activation (8, 9).
In summary, the present studies identify upstream regulation of RhoA prenylation via HMG-CoA reductase as important step in the gastrointestinal smooth muscle tone. These data may be similar to the restorative actions of HMGCRIs in the cardiovascular hypertensive smooth muscles without the untoward effects in normal individuals (38). Consequently, we speculate that HMGCRI may have significantly more potent inhibitory effects in the hypertensive in contrast with the normotensive IAS. This may explain a lack of adverse gastrointestinal effects of statins in the cardiovascular hypertensive patients on HMGCRI medication. Present findings provide an important mechanism of action of statins in the smooth muscle tone, beyond inhibition of cholesterol biosynthesis.
GRANTS
The work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant DK-35385 and an institutional support from Thomas Jefferson University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
The author appreciates valuable comments and technical assistance of Dr. Jagmohan Singh.
REFERENCES
- 1. Adamson P, Paterson HF, Hall A. Intracellular localization of the P21 rho proteins. J Cell Biol 119: 617–627, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allai C, Favre G, Couderc B, Salicio S, Sixou S, Hamilton AD, Sebti SM, Lajoie-Mazenc I, Pradines A. Rho A prenylation is required for promotion of cell growth and transformation and cytoskeleton organization but not for induction of serum response element transcription. J Biol Chem 275: 31001–31008, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Alvarez de Sotomayor M, Herrera MD, Marhuenda E, Andriantsitohaina R. Characterization of endothelial factors involved in the vasodilatory effect of simvastatin in aorta and small mesenteric artery of the rat. Br J Pharmacol 131: 1179–1187, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baron R, Fourcade E, Lajoie-Mazenc I, Allai C, Couderc B, Barbaras R, Favre G, Faye J, Pradines A. RhoA prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidence in vivo by an anti-farnesyl cysteine antibody. Proc Natl Acad Sci USA 97: 11626–11631, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biancani P, Walsh JH, Behar J. Vasoactive intestinal polypeptide: a neurotransmitter for relaxation of the rabbit internal anal sphincter. Gastroenterology 89: 867–874, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Chiba Y, Sato S, Hanazaki M, Sakai H, Misawa M. Inhibition of geranylgeranyltransferase inhibits bronchial smooth muscle hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol 297: L984–L991, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Collins EE, Lund JN. A review of chronic anal fissure management. Tech Coloproctol 11: 209–223, 2007 [DOI] [PubMed] [Google Scholar]
- 8. De Godoy MAF, Rattan N, Rattan S. Arachidonic acid metabolites follow the preferential course of cyclooxygenase pathway for the basal tone in the internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 296: G727–G734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Godoy MAF, Rattan N, Rattan S. COX1 vs. COX-2 as a determinant of the basal tone in the internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 296: G219–G225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Godoy MAF, Rattan S. Autocrine regulation of internal anal sphincter tone by renin-angiotensin system: comparison with phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 289: G1164–G1175, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Faust JR, Luskey KL, Chin DJ, Goldstein JL, Brown MS. Regulation of synthesis and degradation of 3-hydroxy-3-methlylgluataryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholestrol in UT-1 cells. Proc Natl Acad Sci USA 79: 5205–5209, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujihara H, Walker LA, Gong MC, Lemichez E, Bouquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Mol Biol Cell 8: 2437–2447, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 343: 425–430, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Gong MC, Fujihara H, Somlyo AV, Somlyo AP. Translocation of RhoA associated with Ca2+ sensitization of rabbit smooth muscle. J Biol Chem 272: 10704–10709, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins ras-family or trimeric proteins or both in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci USA 93: 1340–1345, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259: 197–209, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Liu PY, Liao JK. A method for measuring Rho kinase activity in tissues and cells. Methods Enzymol 439: 181–189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 19. Madoff RD, Fleshman JW. American gastroenterological association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 126: 1463–1473, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Mills K, Chess-Williams R. Pharmacology of the internal anal sphincter and its relevance to faecal incontinence. Auton Autacoid Pharmacol 29: 85–95, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 22. O'Kelly TJ, Davies JR, Tam PK, Brading AF, Mortensen NJMC. Abnormalities of nitric-oxide-producing neurons in Hirschsprung's disease: Morphology and implications. J Ped Surg 29: 294–300, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Ohashi K, Osuga J, Tozawa R, Kitamine T, Yagyu H, Dikiya M, Tomita S, Okazaki H, Tamura Y, Yahagi N, Iizuka Y, Harada K, Gotoda T, Shimano H, Yamada N, Ishibashi S. Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J Biol Chem 278: 42936–42941, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Patel CA, Rattan S. RhoA prenylation inhibitor produces relaxation of tonic smooth muscle of internal anal sphincter. J Pharmacol Exp Ther 321: 501–508, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Perez-Guerrero C, Marquez-Martin A, Herrera MD, Marhuenda E, Alvarez de Sotomayor M. Regulation of vascular tone from spontaneously hypertensive rats by the HMG-CoA reductase inhibitor, simvastatin. Pharmacology 74: 209–215, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil 17: 50–59, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Rattan S, Benjamin P, Maxwell IVPJ. RhoA/ROCK-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rattan S, Chakder S. Role of nitric oxide as a mediator of internal anal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 262: G107–G112, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Rattan S, De Godoy MAF, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Rattan S, Regan RF, Patel CA, De Godoy MAF. Nitric oxide not carbon monoxide mediates nonadrenergic noncholinergic relaxation in the murine internal anal sphincter. Gastroenterology 129: 1954–1966, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res 97: 1232–1235, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sari R, Nemeth J, Porszasz R, Horvath P, Blasig IE, Ferdinandy P, Nagy I, Lonovics J, Szilvassy Z. Impairment by lovastatin of neural relaxation of the rabbit sphincter of Oddi. Eur J Pharmacol 432: 91–97, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Solski PA, Helms W, Keely PJ, Su L, Der CJ. RhoA biological activity is dependent on prenylation but independent of specific isoprenoid modification. Cell Growth Differ 13: 363–373, 2002 [PMC free article] [PubMed] [Google Scholar]
- 35. Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Sun J, Qian Y, Hamilton AD, Sebti SM. Both farnesyltransferase and geranylgeranyltransferase I inhibitors are required for inhibition of oncogenic K-Ras prenylation but each alone is sufficient to suppress human tumor growth the nude mouse xenografts. Oncogene 16: 1467–1473, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Terzoli L, Mircoli L, Raco R, Ferrari AU. Lowering of elevated ambulatory blood pressure by HMG-CoA reductase inhibitors. J Cardiovasc Pharmacol 46: 310–315, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Yokoyama K, Gelb MH. Purification of a mammalian protein geranylgeranyltransferase. Formation and catalytic properties of an enzyme-geranylgeranyl pyrophosphate complex. J Biol Chem 268: 4055–4060, 1993 [PubMed] [Google Scholar]
- 40. Zipp F, Waiczies S, Aktas O, Neuhaus O, Hemmer B, Schraven B, Nitsch R, Hartung HP. Impact of HMG-CoA reductase inhibition on brain pathology. Trends Pharmacol Sci 28: 342–349, 2007 [DOI] [PubMed] [Google Scholar]


