Abstract
Folate plays an essential role in one-carbon metabolism, and a relationship exists between methyl group metabolism and pancreatic exocrine function. Little, however, is known about the mechanism(s) and regulation of folate uptake by pancreatic acinar cells and the effect of chronic alcohol use on the process. We addressed these issues using the rat-derived pancreatic acinar cell line AR42J and freshly isolated primary rat pancreatic acinar cells as models. We found [3H]folic acid uptake to be 1) temperature and pH dependent with a higher uptake at acidic than at neutral/alkaline pH; 2) saturable as a function of substrate concentration at both buffer pH 7.4 and 6.0; 3) inhibited by folate structural analogs and by anion transport inhibitors at both buffer pH 7.4 and 6.0; 4) trans-stimulated by unlabeled folate; 5) adaptively regulated by the prevailing extracellular folate level, and 6) inhibited by modulators of the cAMP/PKA-mediated pathway. Both the reduced folate carrier (RFC) and the proton-coupled folate transporter (PCFT) were found to be expressed in AR42J and in primary pancreatic acinar cells, as well as in native human pancreas with expression of RFC being higher than PCFT. Chronic alcohol feeding of rats (4 wk; 36% of calories from ethanol) led to a significant decrease in folate uptake by freshly isolated primary pancreatic acinar cells compared with cells from pair-fed controls; this effect was associated with a parallel decrease in the level of expression of RFC and PCFT. These studies reveal that folate uptake by pancreatic acinar cells is via a regulated carrier-mediated process which may involve RFC and PCFT. In addition, chronic alcohol feeding leads to a marked inhibition in folate uptake by pancreatic acinar cells, an effect that is associated with reduction in level of expression of RFC and PCFT.
Keywords: RFC, PCFT, transporter, pancreas, chronic alcohol use
folate, a water-soluble vitamin, is essential for maintaining normal cellular function, growth, and development. The vitamin acts as a cofactor in a number of critical metabolic reactions that include synthesis of precursors of DNA and RNA, metabolism of certain amino acids (e.g., homocysteine), and other methylation reactions. Thus it is not surprising that folate deficiency leads to a variety of clinical abnormalities that range from megaloblastic anemia to neural tube defects.
All mammalian cells, including those of the pancreatic acini, are unable to synthesize folate and thus must obtain the vitamin from the extracellular environment via transport across the plasma membrane. The pancreas maintains the second highest level of folate after the liver (3, 30), and folate is essential for its normal exocrine function and health. A reduction in amylase secretion, appearance of immature secretory granules in pancreatic cells, and disappearance of secreted materials in the pancreatic duct as well as disturbances in methyl metabolism (with a significant reduction in S-adenosylmethionine-to-S-adenosylhomocysteine ratio) have all been observed in rats rendered folate deficient (3–5). Other studies have suggested that disturbances in the folate-dependent methyl group metabolism in the pancreas contribute to the pathogenesis of several pancreatic disorders (reviewed in Ref. 15) and that an inverse relationship exists between serum folate level and the risk of human pancreatic cancer (25). Despite the importance of folate for the normal physiology and health of the exocrine pancreas, there is little known about the mechanism(s) of folate uptake by pancreatic acinar cells and its regulation.
Chronic alcohol use is often associated with folate deficiency and is a major cause of acute and chronic pancreatitis (7). The mechanism(s) by which chronic alcohol consumption negatively affect pancreatic physiology and health is not fully understood but appears to be multifactorial and may include an increase in the predisposition of the pancreas to stress conditions and injurious agents that leads to alteration in the resting state of the pancreas and reduction in its defense mechanisms (7, 10, 16, 19, 20). A negative effect(s) of chronic alcohol consumption on pancreatic folate physiology (and homeostasis) may be such a contributing factor, but little is known about the effect of chronic alcohol consumption on folate uptake by pancreatic acinar cells.
Our aims in this investigation were to delineate the mechanism(s) involved in folate uptake by pancreatic acinar cells and its regulation, as well as to examine the effect of chronic alcohol feeding on the uptake process. We used both cultured rat-derived pancreatic acinar AR42J cells and freshly isolated primary rat acinar cells as models in our investigations. The results showed the involvement of carrier-mediated processes for folate uptake by pancreatic acinar cells. In addition, both of the major folate uptake systems [reduced folate carrier (RFC) and proton-coupled folate transporter (PCFT)] were found to be expressed in pancreatic acinar cells as well as in native human pancreas with expression of the former being higher than the latter. Furthermore, the pancreatic acinar folate uptake process was adaptively regulated by the prevailing extracellular substrate level and appeared to be under the regulation of an intracellular cAMP/PKA-mediated pathway. Moreover, chronic alcohol feeding resulted in a significant inhibition in folate uptake by pancreatic acinar cells, an effect that was associated with a marked reduction in the level of expression of RFC and PCFT.
MATERIALS AND METHODS
[3H]Folic acid (specific activity 10 pmol GBq/mmol; radiochemical purity >98%) was obtained from Moravek Biochemicals (Brea, CA). TRIzol reagent was purchased from Life Technologies (Rockville, MD). DNA oligonucleotide primers were from Sigma Genosys (The Woodlands, TX). All chemicals, routine reagents, and kits were of the analytical or molecular biology grade.
Cell culture and uptake studies.
Rat-derived pancreatic acinar AR42J cells were obtained from American Type Tissue Collection, ATCC (Rockville, MD) and cultured in Kaighn's F12-K growth medium containing 20% FBS. Cells were used between passages of 20 to 35. Prior to use for uptake studies, AR42J cells were cultured in a growth medium containing 100 nM dexamethasone for 48 h in order achieve maximum differentiation of cells (14). Uptake was measured at 37°C (unless otherwise stated) in cells suspended in Krebs-Ringer buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES; pH adjusted as indicated). Labeled (and unlabeled) folic acid was added to the incubation medium at the onset of incubation. At the end of reaction, cell suspension was placed on nitrocellulose filters under negative pressure, washed with 5 ml of ice-cold buffer, and dissolved in scintillation fluid, and radioactivity was counted in a liquid scintillation counter. Protein content of cell digests were measured with a Bio-Rad Dc protein assay kit.
Isolation of rat primary pancreatic acinar cells.
Pancreatic acinar cells were isolated from Sprague-Dawley rats (100–150 g) by a collagenase digestion method as described previously (6, 8) with minor modifications that included reducing the exposure period of the pancreas to collagenase (type IV) digestion. Isolated acinar cells were suspended in Krebs-Ringer buffer and uptake was performed within 1 h of isolation. Trypan blue exclusion was used to test viability of the isolated acinar cells from control and alcohol fed rats with viability estimated at greater than 90% in both cases.
Chronic alcohol feeding of rats.
Adult male rats (100–150 g) were housed at the Animal Core of the Research Center for Alcohol Liver and Pancreatic Diseases at the University of Southern California, Los Angeles, CA. Animal use committee of both the University of Southern California and the Long Beach VA Medical Center approved the experimental protocols. Rats were fed for 4 wk the Lieber-DeCarli alcohol liquid diet (ethanol provided 36% of total ingested calories; 5 g of ethanol/dl diet; Bio Serv, French Town, NJ) (12). Control rats were pair fed the same liquid diet but without alcohol (maltose-dextrin isocalorically replaced ethanol). Each rat was euthanized at the time of study and the pancreas was removed and processed immediately for isolation of pancreatic acinar cells.
Real-time and semiquantitative RT-PCR analysis.
Five micrograms of total RNA isolated from AR42J cells and primary rat pancreatic acinar cells, as well as human pancreatic RNA obtained from Clontech (Palo Alto, CA) were used for the first-strand cDNA synthesis via an Invitrogen Superscript synthesis system (Invitrogen, CA). Gene specific primers used in real-time and semiquantitative PCR analysis are shown in Table 1. The conditions for real-time and semiquantitative PCR were followed as described previously (13, 22). For real-time quantitative PCR, a SYBR green PCR kit (Qiagen, Valencia, CA) was utilized. Data was normalized relative to 18S rRNA or β-actin and calculated by a relative relationship method supplied by iCycler (Bio-Rad, Hercules, CA). The semiquantitative PCR products were analyzed on 2% agarose gels, the images were captured using GelDoc system (Bio-Rad), and bands were quantified by using UN-SCAN-IT software (Silk Scientific, Orem, UT). The specific bands for RFC and PCFT were normalized relative to their respective 18S rRNA.
Table 1.
Primers used for semiquantitative reverse transcription-polymerase chain reaction analysis of rat and human RFC, PCFT, 18S rRNA, and β-actin
| Gene | Primer Sequence (5′–3′) | Amplicon Size, bp |
|---|---|---|
| Rat | ||
| RFC | Forward ATCCGCTGGGCTCTGTGGTCAAA | 162 |
| Reverse AAGCGCAGTGGCAAGGAAAGTGTT | ||
| PCFT | Forward CACAGGGTACGGATTACTCTTC | 116 |
| Reverse CATGGCCAAGCTATTCACACAG | ||
| FR | Forward CTCAATGTCTGCATGGATGC | 108 |
| Reverse CGGTACAGGTAGGAAATGTC | ||
| 18S rRNA | Forward GGGAGGTAGTGACGAAAAATAACAAT | 97 |
| Reverse TTGCCCTCCAATGGATCCTC | ||
| Human | ||
| RFC | Forward CACCGACTACCTGCGCTACA | 135 |
| Reverse GCCATGGTGACGCTGTAGAA | ||
| PCFT | Forward ATGCAGCTTTCTGCTTTGGT | 100 |
| Reverse GGAGCCACATAGAGCTGGAC | ||
| β-actin | Forward CATCCTGCGTCTGGACCT | 116 |
| Reverse TAATGTCACGCACGATTTCC |
RFC, reduced folate carrier; PCFT, proton-coupled folate transporter; FR, folate receptor.
Western blot analysis.
Western blot analysis was performed with membranous fractions isolated from pancreatic acinar AR42J or primary cells as described before (2, 26). Fifteen micrograms of membranous protein was treated with Laemmli buffer, resolved on a 4–12% Tris-Bis gel (Invitrogen), and electroblotted onto polyvinylidene difluoride membrane. Membranes were blocked with 5% dried milk in PBS-Tween 20 and incubated with either anti-rat RFC-specific polyclonal antibody (1:2,) or anti-rat PCFT-specific polyclonal antibody (1:2,) (Thermo Fisher Scientific, Huntsville, AL). Blots were washed twice with PBS-Tween 20 buffer (Sigma), and immunodetection was performed with anti-rabbit IgG conjugated with horseradish peroxidase (1:3,) (Santa Cruz Biotechnology, Santa Cruz, CA) by use of an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL). The respective blots were stripped by use of reblot solution (Chemicon, Temecula, CA) and reprobed with anti-GAPDH antibody as described above.
Data presentation and statistical analysis.
Uptake data are means ± SE of multiple individual determinations and are expressed in picomoles or femtomoles per milligram protein per unit time. The Student's t-test and ANOVA were used for statistical analysis. P < 0.05 was considered statistically significant. Uptake by the saturable component of folic acid was determined by subtracting uptake by simple diffusion from the total uptake at each folic acid concentration examined. Uptake by simple diffusion was calculated from the slope of the line between uptake at high pharmacological concentration (1 mM) and the point of origin. Kinetic parameters of the saturable folic acid uptake process [i.e., maximal velocity (Vmax) and the apparent Km] were calculated as described by Wilkinson (29). All semiquantitative PCR determinations were performed on at least three separate occasions.
RESULTS
General characteristics of folic acid uptake by rat pancreatic acinar AR42J and freshly isolated primary cells.
Figure 1 shows uptake of folic acid (10 nM) by pancreatic acinar AR42J cells as a function of time at buffer pH 7.4 and 6.0. Uptake was linear for 5 min of incubation and uptake was higher at buffer pH 6.0 than 7.4 and occurred at a rate of 9.48 and 6.04 fmol/mg protein, respectively. A 5-min incubation period was used as the standard incubation time in all subsequent experiments. A more detailed pH profile of the initial rate of folic acid (10 nM) uptake by AR42J cells is shown in Fig. 2. Effect of buffer pH on uptake of folic acid (10 nM) by freshly isolated rat primary pancreatic acinar cells was also examined and again found to be significantly (P < 0.01) higher at buffer pH 6.0 than 7.4 (46.77 ± 1.98 and 26.83 ± 3.9 fmol·mg protein−1·5 min−1, respectively).
Fig. 1.
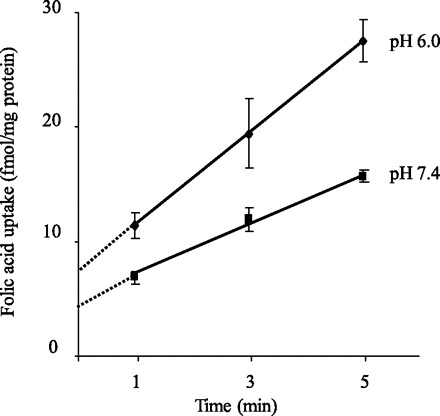
Uptake of folic acid by pancreatic acinar AR42J cells as a function of time. AR42J cells were incubated in Krebs-Ringer buffer pH 7.4 and 6.0 at 37°C for different periods of time in the presence of 10 nM [3H]folic acid. Each data point represents mean ± SE of 3 separate uptake determinations.
Fig. 2.
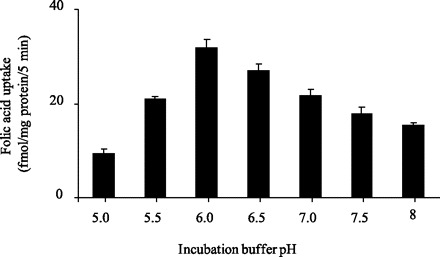
Effect of incubation buffer pH on initial rate of folic acid uptake by pancreatic acinar AR42J cells. AR42J cells were incubated for 5 min in Krebs-Ringer buffer of varying pH (5 to 8.5) at 37°C in the presence of 10 nM [3H]folic acid. Values are means ± SE of 3 separate uptake determinations.
Initial rate of uptake of folic acid (10 nM) by AR42J cells was also examined as a function of incubation temperature at both buffer pH 7.4 and 6.0. At both pH values, uptake was markedly decreased as a function of lowering the incubation temperature from 37 to 22 and 4°C (Fig. 3).
Fig. 3.
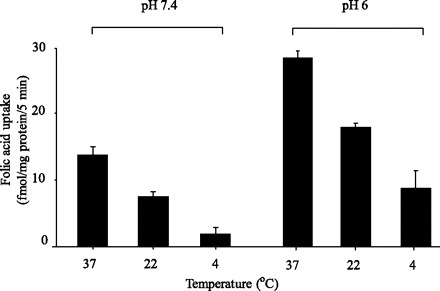
Effect of incubation temperature on initial rate of folic acid uptake by pancreatic acinar AR42J cells as a function of temperature. AR42J cells were incubated for 5 min in Krebs-Ringer buffer, pH 7.4 and 6.0, at different temperatures (4, 22, and 37°C) in the presence of 10 nM [3H]folic acid. Values are means ± SE of 3 separate uptake determinations.
We also investigated the role of Na+ in the incubation medium on the initial rate of folic acid (10 nM) uptake by AR42J cells. The results showed similar folic acid uptake in the presence and absence of Na+ and at both pH 7.4 and 6.0 (Li+ isoosmotically replaced Na+ in the incubation medium) (uptake at buffer pH 7.4 was 11.60 ± 0.63 and 10.50 ± 0.49 fmol·mg protein−1·5 min−1; uptake at buffer pH 6.0 was 20.96 ± 0.71 and 20.40 ± 1.02 fmol·mg protein−1·5 min−1 in the presence and absence of Na+, respectively).
Functional evidence for existence of carrier-mediated mechanism(s) for folic acid uptake by rat pancreatic acinar AR42J and primary cells.
We examined the initial rate of folic acid uptake by AR42J cells as a function of concentration (0.1–10 μM) in the incubation medium at both buffer pH 7.4 and 6.0. The results showed saturation in the substrate uptake at both pH values (Fig. 4). The apparent Km and Vmax of the saturable folic acid uptake component at pH 7.4 were 2.71 ± 0.23 μM and 11.21 ± 0.15 pmol·mg protein−1·5 min−1; whereas at buffer pH 6.0 were 4.55 ± 1.23 μM and 26.21 ± 3.23 pmol·mg protein−1·5 min−1, respectively. These findings provide functional evidence for existence of carrier-mediated processes for folic acid uptake at pH 7.4 and 6.0.
Fig. 4.
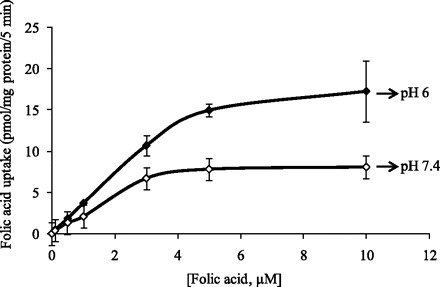
Uptake of folic acid by pancreatic acinar AR42J cells as a function of concentration. AR42J cells were incubated for 5 min (initial rate) at 37°C in Krebs-Ringer buffer pH 7.4 and 6.0 in the presence of different concentrations of folic acid. Values are means ± SE of at least 3 separate uptake determinations. When not shown, error bars are smaller than the symbol.
We also examined the effect of the folic acid structural analogs methotrexate (MTX) and 5-methyltetrahydrofolate (5-MTHF) (both at 100 μM) on the initial rate of [3H]folic acid (10 nM) uptake by pancreatic acinar AR42J cells, at both pH 7.4 and 6.0. The results (Fig. 5) showed significant (P < 0.01 for all) inhibition of [3H]folic acid by both analogs at both pH values. In another study, we examined the ability of unlabeled folic acid to induce efflux of [3H]folic acid from preloaded AR42J cells. Cells were preloaded with [3H]folic acid (by incubation for 10 min in the presence of 30 nM [3H]folic acid) followed by incubation for 10 min in Krebs-Ringer buffer pH 7.4 in the presence and absence of 100 μM unlabeled folic acid. The results showed a significantly (P < 0.01) lower [3H]folic acid content in cells incubated in the presence of unlabeled folic acid in the incubation buffer compared with those incubated in its absence (4.18 ± 0.05 and 8.93 ± 0.3 fmol/mg protein, respectively), demonstrating a trans-stimulation in the vitamin movement across pancreatic acinar cell membrane. These observations provide further functional evidence for the involvement of carrier-mediated processes in folic acid uptake at acidic and at neutral/alkaline buffer pH.
Fig. 5.
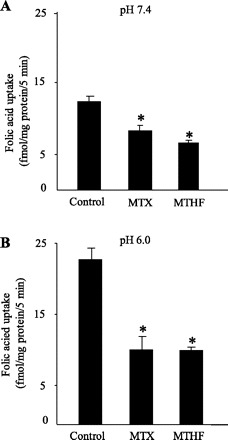
Effect of structural analogs on [3H]folic acid uptake by pancreatic acinar AR42J cells. AR42J cells were incubated for 5 min at 37°C in Krebs-Ringer buffer pH 7.4 (A) and 6.0 (B) in the presence of [3H]folic acid (10 nM) and 100 μM methotrexate (MTX) or 100 μM 5-methyltetrahydrofolate (5-MTHF) 5-MTHF. Values are means ± SE of at least 3 separate uptake determinations. *P < 0.01.
In a related study we examined the effect of unlabeled folic acid (1 mM) on [3H]folic acid (10 nM) uptake by freshly isolated rat primary pancreatic acinar cells and found a significant (P < 0.01) inhibition at buffer pH 7.4 and 6.0 (uptake at pH 7.4 was 31.5 ± 1.6 and 4.4 ± 2.65 fmol·mg protein−1·5 min−1; uptake at buffer pH 6.0 was 46.45 ± 3.2 and 2.67 ± 1.6 fmol·mg protein−1·5 min−1 for control and in the presence of unlabeled folic acid, respectively). Similarly, the folate structural analog MTX (100 μM) caused a significant (P < 0.01) inhibition in folic acid (10 nM) uptake by primary rat pancreatic acinar cells at both pH 7.4 and 6.0 (uptake at pH 7.4 was 34.02 ± 1.65 and 23.68 ± 0.85 fmol·mg protein−1·5 min−1; uptake at pH 6.0 was 46.47 ± 3.2 and 23.75 ± 5.5 fmol·mg protein−1·5 min−1 in control and presence of MTX, respectively). These findings provide functional confirmation for the data obtained with the AR42 J cell line and point to the existence of carrier-mediated mechanisms for folic acid uptake by native pancreatic acinar cells.
Finally, we examined the effect of the membrane transport inhibitors DIDS and SITS (both at 0.5 mM) on the initial rate of folic acid (10 nM) uptake by AR42J cells. The study was performed at both buffer pH 7.4 and 6.0. Both DIDS and SITS resulted in a significant (P < 0.01) inhibition in folic acid uptake at both buffer pH values (uptake at pH 7.4 was 14.0 ± 3.01, 5.04 ± 1.3, and 8.32 ± 1.1 fmol·mg protein−1·5 min−1; uptake at buffer pH 6.0 was 27.52 ± 1.5, 11.7 ± 3.3, and 12.54 ± 1.95 fmol·mg protein−1·5 min−1, respectively, for control and in cells pretreated with DIDS and SITS, respectively).
Molecular aspects of folic acid uptake process in rat pancreatic acinar AR42J and primary cells as well as in native human pancreas.
Three main transport mechanisms have been identified for folate uptake by mammalian cells: the transmembrane RFC, which operates optimally at neutral/alkaline pH (24, 28), the transmembrane PCFT, which operates optimally at acidic pH (9, 18, 21), and the membrane anchored (via a glycosyl phosphatidyl-inositol linkage) folate receptor (FR) (1, 23). In this study, we examined mRNA expression of these folate transport systems in rat pancreatic acinar AR42J and primary cells. Expression was examined by mean of real-time PCR using total RNA and gene-specific primers corresponding to sequences in the open-reading frame of rat RFC, PCFT, and FR (materials and methods; Table 1). The results showed that both RFC and PCFT are expressed in rat AR42J and primary pancreatic acinar cells (Fig. 6, A and B), with expression of the former being significantly (P < 0.01 for both) higher than that of the latter. However, no expression for the FR was detected in both of these cell types, confirming previous immunological observations (27).
Fig. 6.
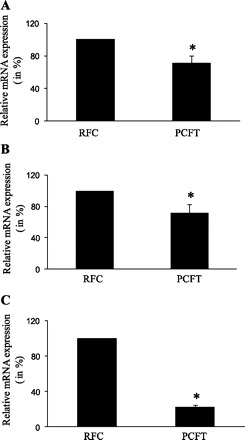
Expression of reduced folate carrier (RFC) and proton-coupled folate transporter (PCFT) at the mRNA level in cultured rat-derived pancreatic acinar AR42J cells (A), primary rat pancreatic acinar cells (B), and native human pancreas (C). Real-time PCR was performed using gene specific primers for rat and human RFC and PCFT (see Table 1) as described in materials and methods. Data were normalized relative to β-actin. Experiments were run on 3 different occasions. Data are presented as percentage relative to RFC expression. *P < 0.01.
We also examined mRNA expression of RFC and PCFT in human pancreas RNA. We assumed that mRNA prepared from whole human pancreas is a representative of the overall level of acinar cell transcripts since this cell type makes up the majority (80–85%) of cells in this tissue. The results (Fig. 6C) showed expression of both RFC and PCFT with expression of the former again being significantly (P < 0.01) higher than that of the latter (Fig. 6C).
Regulation of the folic acid uptake process of pancreatic acinar cells: adaptive regulation by substrate level.
The effect of maintaining the pancreatic acinar AR42J cells (for 6–8 days) in folate deficient (0.03 μM) compared with control (9 μM) growth media on initial rate of folic acid uptake was examined. The results showed a significant (P < 0.01) induction in [3H]folic acid (10 nM) uptake in cells maintained in a folate-deficient medium compared with those maintained in control growth medium (Fig. 7). This effect of extracellular folate level on folate uptake by AR42J cells was seen whether the substrate uptake was measured at buffer pH 7.4 or 6.0 (Fig. 7, A and B). We also examined the effect of changing the extracellular folate level on expression of RFC and PCFT at the mRNA and protein levels. Results of the quantitative PCR showed a significant (P < 0.05 for both) increase in RFC and PCFT mRNA levels in cells maintained under folate-deficient condition compared with controls (Fig. 8A). Results of Western blot analysis showed a marked induction in the level of RFC and PCFT proteins in cells maintained in folate-deficient growth medium compared with those maintained in control growth medium (Fig. 8B). Maintaining AR42J in a growth medium oversupplemented with folate (100 μM) caused a marked inhibition in initial rate of [3H]folic acid (10 nM) uptake as well as in the level of expression of RFC and PCFT compared with cells maintained in control growth medium (data not shown).
Fig. 7.
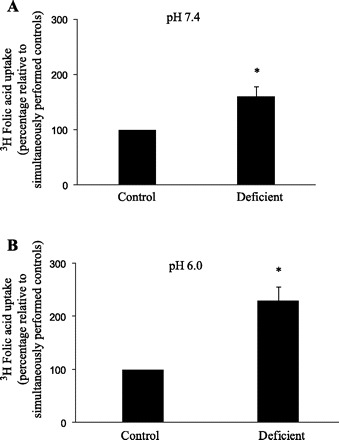
Effect of maintaining pancreatic acinar AR42J cells in a culture medium with different concentrations of folic acid on initial rate of [3H]folic acid uptake. Cells were maintained for 6 to 8 days in folate-deficient (contains 0.03 μM folate) or control (contains 9 μM folate) growth medium. Initial rate of 10 nM [3H]folic acid uptake was then examined at buffer pH 7.4 (A) and pH 6.0 (B). Data are mean ± SE of at least 3 separate uptake determinations and are expressed as percentage relative to simultaneously performed controls. *P < 0.01.
Fig. 8.
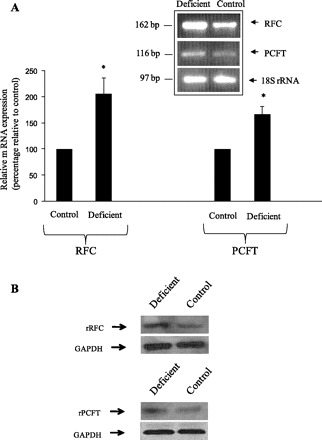
Effect of maintaining pancreatic acinar AR42J cells in a culture medium with different concentrations of folic acid on level of expression of RFC and PCFT mRNA (A) and protein (B). A: semiquantitative PCR was performed using RNA samples from cells maintained in folate-deficient (contains 0.03 μM folate) or control (contains 9 μM folate) growth medium and gene-specific primers for rat (r)RFC, PCFT, and 18S rRNA. The products were resolved on a 2% agarose gel. Level of mRNA expression for RFC and PCFT were normalized relative to 18S rRNA and expressed as percentage relative to simultaneously performed control. Inset is from a representative gel of 3 separate runs. *P < 0.05; **P < 0.01. B: Western blotting was performed by using specific anti-rRFC and anti-rPCFT polyclonal antibodies and membranous fractions (30 μg) of AR42J cells maintained in folate-deficient or control growth medium as describe in materials and methods. Western blotting were run on 2 separate occasions by use of different membranous preparations, and a representative set is shown.
Role of intracellular regulatory pathways.
Involvement of specific intracellular regulatory pathways [protein tyrosine kinase (PTK) and protein kinase A (PKA)] in the regulation of folate uptake by pancreatic acinar AR42J cells was investigated by examining the effect of pretreating (for 1 h) these cells with different concentrations of specific modulators of the respective pathways on initial rate of folic acid (10 nM) uptake. The results showed that whereas modulators of the PTK-mediated pathway did not affect folic acid uptake (data not shown), modulators of the cAMP/PKA-mediated pathway caused a significant (P < 0.01 for both) inhibition in folic acid uptake at both buffer pH 7.4 and 6.0 (uptake at pH 7.4 was 13.76 ± 2.42, 2.52 ± 1.4, and 7.15 ± 1.5 fmol·mg protein−1·5 min−1, respectively; uptake at pH 6.0 was 24.8 ± 0.91, 5.2 ± 2.1, and 8.9 ± 3.0 fmol·mg protein−1·5 min−1, for control and in the presence of 5 mM of dibutyryl cAMP and 8-bromo-cyclic AMP, respectively).
Effect of chronic alcohol feeding of rats on folate uptake by freshly isolated primary pancreatic acinar cells.
An important aim of this study was to examine the effect of chronic alcohol feeding on folate uptake by pancreatic acinar cells. Thus, following the characterization of the folate uptake process by these cells, we examined the effect of chronic alcohol feeding of rats (for 4 wk; see materials and methods) on folic acid (10 nM) uptake by freshly isolated primary pancreatic acinar cells at buffer pH 7.4 and 6.0. Data were compared with folic acid uptake by pancreatic acinar cells isolated simultaneously from pair-fed control rats. The results showed a significantly (P < 0.01 for both) lower carrier-mediated folic acid uptake by pancreatic acinar cells isolated from chronic alcohol fed rats compared with those isolated from pair-fed controls (Fig. 9).
Fig. 9.
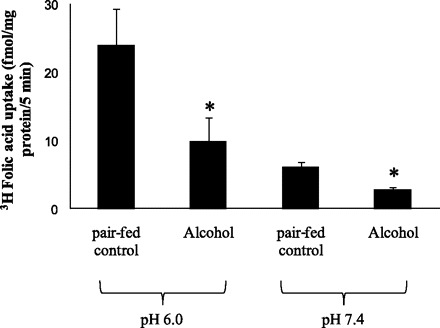
Effect of chronic alcohol feeding on folic acid uptake by rat primary pancreatic acinar cells. Rats were fed ethanol liquid diet (ethanol provides 36% of total calories) for 4 wk. Control rats were pair fed the same liquid diet but without alcohol (maltose-dextrin replaced ethanol isocalorically). Freshly isolated primary pancreatic acinar cells were incubated for 5 min at 37°C in Krebs-Ringer buffer pH 7.4 and 6.0 in the presence of 10 nM [3H]folic acid. Each data point represents mean ± SE of 3 separate uptake determinations. *P < 0.01.
We also examined the effect of chronic alcohol feeding on level of expression of RFC and PCFT mRNA in pancreatic acinar cells. Results of our semiquantitative PCR (normalized to the house keeping gene 18S rRNA) showed a significantly (P < 0.01 for both) lower level of expression of RFC and PCFT mRNA in acinar cells isolated from chronic alcohol fed rats compared with those isolated from pair-fed controls (Fig. 10).
Fig. 10.
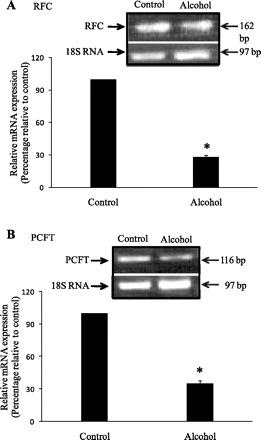
Effect of chronic alcohol feeding of rats on level of expression of RFC (A) and PCFT (B) mRNA in primary pancreatic acinar cells. Semiquantitative PCR and data normalization and presentation are as described in Fig. 8A. *P < 0.01.
DISCUSSION
Folate is important for the normal function of the exocrine pancreas and a relationship exists between methyl group metabolism (a folate-dependent event) and this function of the pancreas (3–6, 15). Indeed folate deficiency leads to disturbance in methyl metabolism and a reduction in pancreatic exocrine secretions (3–5). Despite the importance of folate for the exocrine pancreas, little was known about the mechanism(s) involved in folate uptake by pancreatic acinar cells and its regulation. Little is also known about the effect of chronic alcohol consumption on folate uptake by these cells. Addressing the latter issue is important since chronic alcohol consumption is a major cause of pancreatitis and since a negative effect of chronic alcohol use on pancreatic folate uptake may lead to impairment in pancreatic acini folate homeostasis and disturbance of normal methyl metabolism (3, 15, 25). We addressed these issues using the rat-derived pancreatic acinar cells AR42J and freshly isolated rat primary acinar cells as models.
Our results showed that whereas folic acid uptake by pancreatic acinar cells at buffer pH 7.4 is considerable, a significant increase in this uptake occurs at pH 6.0. This increase in folic acid uptake at acidic compared with neutral/alkaline pH, however, was less dramatic than that seen in other epithelial cells of the digestive system, such as the pancreatic duct, where folate uptake at buffer pH 7.4 was less than 5% of the uptake at the more acidic pH 6.0 (17). Uptake of folic acid by pancreatic acinar cells was also found to be highly temperature dependent at both buffer pH 7.4 and 6.0, an observation that argues against the involvement of simple diffusion as a mechanism for uptake. This suggestion was supported by findings of saturation in the initial rate of folic acid uptake as a function of concentration at both buffer pH 7.4 and 6.0, findings that support the involvement of carrier- mediated mechanisms at both pH conditions. The ability of unlabeled folic acid and its structural analogs MTX and 5-MTHF, as well as the ability of the anion transport inhibitors DIDS and SITS to significantly inhibit [3H]folic acid uptake at both buffer pH 7.4 and 6.0, provide further functional evidence for the involvement of carrier-mediated mechanisms in the vitamin uptake by pancreatic acinar cells at acidic as well as alkaline and neutral pH conditions.
Based on the above functional data, we set out to determine the expression of the three main known folate uptake systems, i.e., RFC, PCFT, and FR, in pancreatic acinar cells. The results showed expression of both of the main transmembrane transporters (RFC and PCFT), whereas the membrane-anchored FR was not detected. Furthermore, RFC was expressed at a significantly higher level than PCFT. Similarly, human pancreas was found to express RFC and PCFT with expression of the former being higher than that of the latter. Since RFC is known to function optimally at pH 7.4 (with much less activity at acidic pH values; Refs. 24, 28), whereas PCFT is known to function optimally at acidic pH values (with much less activity at neutral/alkaline pH values; Refs. 9, 18, 21), it may be reasonable to assume that folate uptake by pancreatic acinar cells at pH 7.4 is mediated mainly by RFC and that at pH 6.0 it is mediated mainly by the PCFT. Further studies are needed to confirm this assumption and to establish which of these system(s) is mainly utilized under normal physiological conditions in vivo.
Following the delineation of mechanism of folic acid uptake by pancreatic acinar cells, we examined possible adaptive regulation of the uptake process by extracellular folate level. Our results showed that maintaining pancreatic acinar AR42J cells in a folate-deficient medium leads to a significant upregulation in the initial rate of [3H]folic acid uptake. This adaptive response in the pancreatic acinar cells folate uptake process was observed at both buffer pH 7.4 and 6.0, and was associated with parallel changes in the level of expression of RFC and PCFT at the protein and mRNA levels. The observed change in message level of the folate transporters in response to the change in extracellular folate level raises the possibility of involvement of a transcriptional mechanism(s), as has been observed in our laboratory with other cell types (2, 26). Further studies, however, are needed to investigate this possibility.
We also examined possible regulation of the folate uptake process of pancreatic acinar cells by specific intracellular regulatory mechanisms. We focused on possible role of the cAMP/PKA- and the PTK-mediated pathways since these two pathways were reported to play a role in regulating folate uptake by other cells (11, 17). The results showed that whereas modulators of cAMP/PKA-mediated pathway cause significant inhibition in folate uptake (and at both buffer pH 7.4 and 6.0), modulators of the PTK-mediated pathway failed to affect the uptake process. Since the cAMP/PKA-mediated regulatory pathway affects folate uptake in other cell types (11, 17), it may be reasonable to assume that this pathway is a common regulatory pathway used by mammalian cells to regulate folate uptake. On the other hand, the inability of specific modulators of the PTK-mediated pathway to affect folate uptake by pancreatic acinar cells, although they inhibit folate uptake by other cell types (11), suggests that this pathway may be used in a cell-specific manner to regulate folate uptake.
We also examined the effect of chronic alcohol feeding on folate uptake by freshly isolated rat pancreatic acinar cells. A pair-feeding regimen with alcohol and control liquid diets (for 4 wk) was followed. Our results showed a significantly lower folic acid uptake by pancreatic acinar cells isolated from alcohol-fed rats compared with those isolated from pair-fed controls. This decrease in folic acid uptake was associated with a marked reduction in the level of expression of both RFC and PCFT. The reduction in the level of expression of RFC in pancreatic acinar cells of alcohol fed rats is in line with previously published microarray data (10). In the microarray study (10), chronic alcohol consumption was also shown to stimulate the expression of many genes including those responsible for transport of other substrates. These findings demonstrate the diverse effects that chronic alcohol feeding has on mammalian gene expression. The observed reduction in mRNA levels of RFC and PCFT in pancreatic acinar cells of alcohol fed rats raises the possibility that the effect may be (at least in part) exerted at the transcriptional level and/or mediated via alteration of mRNA stability of the affected transporters; Further studies, however, are needed to confirm this suggestion. The observed inhibition in folate uptake by pancreatic acinar cells in chronic alcohol feeding may lead to a disturbance in folate homeostasis in pancreatic acinar cells, which in turn may lead to a disturbance in normal methyl metabolism. Disturbance in this metabolic pathway is believed to play a role in the pathogenesis of several pancreatic diseases including pancreatitis (15).
In summary, our findings show the involvement of carrier-mediated mechanisms in folate uptake by pancreatic acinar cells, which may involve the folate transporters RFC and PCFT. In addition, the uptake process appears to be adaptively regulated by extracellular substrate level and by an intracellular cAMP/PKA-mediated pathway. Furthermore, chronic alcohol feeding leads to a significant inhibition in folic acid uptake by pancreatic acinar cells, an effect that is associated with marked decrease in level of expression of both RFC and PCFT.
GRANTS
This study was supported by grants from the National Institutes of Health (AA018071 and DK56061 to H. M. Said; and the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases P50-AA11999) and the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Antony AC. Folate receptors. Annu Rev Nutr 16: 501–521, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86: 159–166, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Balaghi M, Horne DW, Woodward SC, Wagner C. Pancreatic one-carbon metabolism in early folate deficiency in rats. Am J Clin Nutr 58: 198–203, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Balaghi M, Wagner C. Folate deficiency inhibits pancreatic amylase secretion in rats. Am J Clin Nutr 61: 90–96, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Balaghi M, Wagner C. Methyl group metabolism in the pancreas of folate-deficient rats. J Nutr 122: 1391–1396, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Capdevila A, Decha-Umphai W, Song KH, Borchardt RT, Wagner C. Pancreatic exocrine secretion is blocked by inhibitors of methylation. Arch Biochem Biophys 345: 47–55, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Greensberger NJ, Toskes PP. Acute and chronic pancreatitis. In: Harrison's Principles of Internal Medicine, edited by Kasper DL. New York: McGraw-Hill, 2005 [Google Scholar]
- 8. Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest 100: 1853–1862, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue K, Nakai Y, Ueda S, Kamigaso S, Ohta KY, Hatakeyama M, Hayashi Y, Otagiri M, Yuasa H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am J Physiol Gastrointest Liver Physiol 294: G660–G668, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek DE, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Kumar CK, Moyer MP, Dudeja PK, Said HM. A protein-tyrosine kinase-regulated, pH-dependent, carrier-mediated uptake system for folate in human normal colonic epithelial cell line NCM460. J Biol Chem 272: 6226–6231, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol 100: 1200–1208, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Longnecker DS. Abnormal methyl metabolism in pancreatic toxicity and diabetes. J Nutr 132: 2373S–2376S, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 282: G501–G507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nabokina SM, Ma TY, Said HM. Mechanism and regulation of folate uptake by human pancreatic epithelial MIA PaCa-2 cells. Am J Physiol Cell Physiol 287: C142–C148, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, Otagiri M, Hayashi Y, Yuasa H. Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther 322: 469–476, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, Solomon TE, Gukovskaya AS, Tsukamoto H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 117: 706–716, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Ponnappa BC, Marciniak R, Schneider T, Hoek JB, Rubin E. Ethanol consumption and susceptibility of the pancreas to cerulein-induced pancreatitis. Pancreas 14: 150–157, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Selhub J. Folate binding proteins. In: Nutrient Regulation During Pregnancy, Lactation, and Infant Growth, edited by Allen L, King J, Lonnerdal B. New York: Plenum: 141–149, 1994 [Google Scholar]
- 24. Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr 19: 91–122, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Stolzenberg-Solomon RZ, Albanes D, Nieto FJ, Hartman TJ, Tangrea JA, Rautalahti M, Sehlub J, Virtamo J, Taylor PR. Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. J Natl Cancer Inst 91: 535–541, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Subramanian VS, Chatterjee N, Said HM. Folate uptake in the human intestine: promoter activity and effect of folate deficiency. J Cell Physiol 196: 403–408, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Weitman SD, Weinberg AG, Coney LR, Zurawski VR, Jennings DS, Kamen BA. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res 52: 6708–6711, 1992 [PubMed] [Google Scholar]
- 28. Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter. Biochem J 367: 629–640, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilkinson GN. Statistical estimations in enzyme kinetics. Biochem J 80: 324–332, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeo EJ, Wagner C. Tissue distribution of glycine N-methyltransferase, a major folate-binding protein of liver. Proc Natl Acad Sci USA 91: 210–214, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]


