Abstract
Radiation therapy is an essential modality in the treatment of colorectal cancers. Radiation exerts an antiangiogenic effect on tumors, inhibiting endothelial proliferation and survival in the tumor microvasculature. However, damage from low levels of irradiation can induce a paradoxical effect, stimulating survival in endothelial cells. We used human intestinal microvascular endothelial cells (HIMEC) to define effects of radiation on these gut-specific endothelial cells. Low-level irradiation (1–5 Gy) activates NF-κB and the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is involved in cell cycle reentry and cell survival in HIMEC. A downstream target of PI3K/Akt is mammalian target of rapamycin (mTOR), which contributes to endothelial proliferation and angiogenesis. The aim of this study was to investigate the signaling molecules involved in the radiosensitizing effects of curcumin on HIMEC subjected to low levels of irradiation. We have demonstrated that exposure of HIMEC to low levels of irradiation induced Akt and mTOR phosphorylation, which was attenuated by curcumin, rapamycin, LY294002, and mTOR small interference RNA (siRNA). Activation of NF-κB by low levels of irradiation was inhibited by curcumin, SN-50, and mTOR siRNA. Curcumin also induced apoptosis by induction of caspase-3 cleavage in irradiated HIMEC. In conclusion, curcumin significantly inhibited NF-κB and attenuated the effect of irradiation-induced prosurvival signaling through the PI3K/Akt/mTOR and NF-κB pathways in these gut-specific endothelial cells. Curcumin may be a potential radiosensitizing agent for enhanced antiangiogenic effect in colorectal cancer radiation therapy.
Keywords: mammalian target of rapamycin, radiation, human intestinal microvascular endothelial cells
colorectal cancer is the second leading cause of cancer death, as over 150,000 individuals are diagnosed annually in the U.S. each year and 60,000 individuals die from the disease. It has been shown that less than 20% of patients respond to chemotherapy completely, because they are resistant to radiation therapy (36). Reasons for the resistance and lack of response to radiation are not known. Recent advances in cancer biology have defined a critical role for the microvascular endothelium and angiogenesis in tumor progression and metastasis (12, 25, 48, 62). Signaling pathways such as NF-κB, STAT3, growth factors, reactive oxygen species, Bcl-2, phosphatidylinositol 3-kinase (PI3K)/Akt, multidrug resistance proteins, and cyclooxygenase-2 (COX-2) have been linked to the progression, aggressiveness, and tumor resistance to chemotherapy and radiotherapy (22, 28, 38, 45, 53). Specific signaling cascades in gut endothelium associated with tumor angiogenesis have not been defined.
Mechanisms of gut-specific microvascular endothelial cell survival and proliferation are presently undergoing characterization. A critical downstream target of Akt, which controls the regulation of endothelial cell growth and proliferation is mTOR (mammalian target of rapamycin) (46). Inhibition of endothelial proliferation, increased apoptosis, and induction of autophagy by rapamycin indicate that mTOR plays a key role in cell survival (43, 64). It has been shown that treatment of solid tumors with mTOR inhibitor RAD001 (Everolimus) increases the tumor radiosensitivity via effects on vascular cells in addition to effects on tumor itself (41).
Curcuma longa Linn, Zingiberaceae (curcumin) has been used for centuries in Ayurvedic traditional medicine as well as in food preparation (60). Curcumin has been shown to regulate cell growth and proliferation of various cancers through effects on many different signaling pathways (54, 56). Curcumin suppresses and downregulates NF-κB, AP-1, STAT3 and STAT5, Egr-1, PPARγ, β-catenin, Bcl-2, Bcl-XL, COX-2, MMP9, and cyclin D1 and it has an antineoplastic potential, inhibiting tumors of the oral cavity, skin, forestomach, duodenum, and colon in rodents (1, 55). The effect of curcumin on gastrointestinal microvascular biology in the context of cancer and radiation therapy has not been defined.
The effect of curcumin in radiation biology has demonstrated divergent responses in various cell populations. Pretreatment with curcumin demonstrated both radiosensitizing as well as radioprotective effects, whereas postradiation treatment demonstrated consistent ameliorative effect. Curcumin (5 μM) has been shown to act as a radiosensitizer in the PC3 prostate cancer cell line with enhanced radiation-induced apoptosis, which correlated with inhibition of NF-κB and PI3K, and decreased expression of COX-2 (2, 13). An opposite, radioprotective, effect has been demonstrated by Inano and Onoda (34), who showed that mammary and pituitary tumors in rats induced by radiation could be prevented by curcumin pretreatment (1% of dietary weight/day). The inhibitory effect on tumor development was seen with both pretreatment as well as posttreatment after radiation exposure. These authors further demonstrated that a combination of curcumin pretreatment and posttreatment prevented death in rats exposed to lethal dosages of whole body irradiation (9.6 Gy). The ameliorative effect of curcumin (200 mg·kg−1·day−1) in the treatment of radiation-induced injury has demonstrated a significant decrease in mucositis in rats following exposure to 10–30 Gy radiation (51).
Nuclear factor-κB (NF-κB) plays an important role in both radioresistance and induced radiosensitivity (19). Curcumin has been shown to downregulate NF-κB activation, which is mediated through inhibition of IκB kinase and subsequent IκBα phosphorylation (3, 4, 7, 57), thereby suppressing proliferation and inducing apoptosis (3, 4, 7, 42). Thus cells that are resistant to irradiation become susceptible to apoptosis when treated in conjunction with curcumin. Moreover, it has been shown that curcumin sensitizes human colorectal cancer xenografts in nude mice to radiation by targeting NF-κB-regulated gene products, resulting in inhibition of angiogenesis (37).
Previous studies from our group have shown that curcumin blocked VEGF-induced angiogenesis in human intestinal microvascular endothelial cells (HIMEC) (8). In this study we characterized the radiosensitizing effect of curcumin in HIMEC following low-dose (1–5 Gy) external beam irradiation. Our findings demonstrate that HIMECs pretreated with curcumin are more sensitive to radiation than non-curcumin-treated cells. Curcumin sensitization of HIMEC occurred through inhibition of NF-κB, Akt/mTOR activity, and induction of cell death. Finally, we demonstrate that silencing of the mTOR gene with small interference RNA (siRNA) mimicked the effect of curcumin in irradiated HIMEC.
MATERIALS AND METHODS
Reagents.
Antibodies against phosphorylated and nonphosphorylated Akt (Ser473), mTOR (Ser2448), p65 NF-κB subunit, total and cleaved caspase 3, and FOXO antibodies sampler kit were obtained from Cell Signaling Technology (Danvers, MA). MDM2 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). LY294002 (PI3K/Akt), SN-50 (NF-κB), and rapamycin (mTOR) inhibitors were obtained from Biomol (Plymouth Meeting, PA). All electrophoresis reagents were from Bio-Rad (Hercules, CA). Nuclear protein extraction kit and nonradioactive TransAM DNA-binding ELISA kit for detection of NF-κB activity were obtained from Active Motif (Carlsbad, CA). Oligonucleotide and primers were purchased from IDT (Integrated DNA Technologies, Coralville, IA). RNA Cell Protect reagent and RNeasy Plus Mini Kits were obtained from Qiagen (Valencia, CA). mTOR siRNA (target siRNA), GAPDH siRNA (positive control), and nontargeting siRNA (negative control) were obtained from Dharmacon (Chicago, IL). iScript cDNA synthesis kit, SYBR Green Master Mix, iQ5 software, and all other electrophoresis reagents were obtained from Bio-Rad. Mayer's hematoxylin and eosin solutions and unless otherwise indicated, all other chemicals used in this study were purchased from Sigma Chemical (St. Louis, MO).
HIMEC isolation and culture.
HIMECs were isolated and cultured as previously described (9). All experiments were approved by the Institutional Review Board of the Medical College of Wisconsin. Experiments were performed on three independent HIMEC lines unless otherwise specified. All images displayed were a representative result of one of three independent experiments.
Animal studies.
All experiments were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (MCW). Male Sprague-Dawley rats (35 days old; 200 g; WAG/RijMCW strain) were divided into four groups (n = 3 in each group) that were evaluated over a 7-day time period: 1) a nonradiated control group; 2) an irradiated (6 Gy) group; 3) a nonradiated curcumin-fed (2% of daily diet) control group; and 4) a curcumin-fed and 6 Gy-irradiated group. Animals received a single dose of total body irradiation (6 Gy) by dosimetry in the Radiation Countermeasure Center facilities at MCW. Head and partial limb were shielded to prevent the need for hematological bone marrow transplantation. Curcumin was incorporated into the animals' diets from a commercial vendor (Harlan Teklad, Madison, WI). This dosage of curcumin (2% of daily diet) was based on prior published data in rats and mice, in which curcumin exhibited no toxicity in the treatment of chemically induced gut inflammation (i.e., TNBS colitis) (59). Clinical parameters including animal weights as well as the presence of diarrhea and animal mortality were examined. Overall assessments of the animals' status were made by veterinarians (i.e., hunched posture, cowering, etc.). After 7 days animals were euthanized, and gastrointestinal tissues were harvested and prepared for standard histology, RT-PCR, and Western blotting.
HIMEC IRRADIATION.
Irradiation was performed in a Mark I Cesium-137 irradiator (J. L. Shepherd and Associates, San Fernando, CA) at the dose of 1, 2, and 5 Gy/min at room temperature.
Pharmacological modulation of HIMEC.
Curcumin (10 μM), rapamycin (100 nM), LY294002 (10 μM), and SN-50 (18 μM) were used to determine signaling pathways underlying HIMEC survival following irradiation. HIMECs were pretreated with inhibitors for 1 h and then exposed to 1–5 Gy of radiation. The 2 Gy irradiation was selected for most experiments, because it demonstrated the modulatory effect of curcumin. Of note, we have previously performed dose-response studies for these inhibitors and chose the most potent but nontoxic doses for our experimental analysis. These inhibitors demonstrated no toxicity at the dosages used in this study.
Cell survival and cell death assays.
HIMECs were grown to subconfluence and either were pretreated with curcumin or various inhibitors for 60 min before irradiation or were left untreated. The cells were then incubated at 37°C for 10 days. Following staining with Trypan blue, five random high-power fields in the HIMEC monolayers were counted by use of an ocular grid, as previously described (49). For the cell death assay, HIMEC were treated and grown as above and the cells were washed and then fixed in 1% paraformaldehyde. Using a TdT-mediated dUTP nick-end labeling (TUNEL) assay kit according to manufacturer's instruction (In Situ Cell Death Detection Kit, Roche Diagnostics, Indianapolis, IN), we evaluated the percentage of apoptotic cells.
Matrigel in vitro tube formation assay. endothelial.
Tube formation was performed by using Matrigel, a solubilized extracellular basement membrane matrix extracted from the Engelbreth-Holm-Swarm mouse sarcoma, as described previously (8, 49). Twenty-four-well dishes were coated with 250 μl of complete medium containing 5 mg/ml Matrigel, and HIMEC resuspended in complete growth medium were seeded at a density of 1 × 104. Then HIMEC tube formation with or without inhibitors followed by irradiation was assessed. Separate wells received rapamycin (100 nM), LY294002 (10 μM), or curcumin (10 μM) followed by irradiation. Control cells remained free of irradiation and inhibitors. HIMEC were stimulated with 50 ng/ml of VEGF, which served as a positive control. Cells were cultured on Matrigel for 16 h and endothelial tube formation was enumerated by an observer blinded to treatment regimen by inverted phase-contrast microscopy using previously established protocols (49, 8). Five high-power fields per condition were examined, and experiments were repeated in three independent HIMEC cultures.
Western blot analysis.
Confluent HIMEC monolayers in 35-mm culture dishes (one dish per condition) were pretreated with various inhibitors for 60 min or left untreated before irradiation (1–5 Gy) and then incubated for indicated times mentioned in figure legends. SDS-PAGE and Western blot analysis were performed using specific antibodies for NF-κB p65 subunit, Akt, and mTOR (phosphorylated and nonphosphorylated), MDM2 and FOXOs (phosphorylated and nonphosphorylated) as described previously (8, 49).
Assay of transcription factor NF-κB activation.
Nuclear protein from irradiated HIMEC with or without various inhibitors was extracted by using the Nuclear Protein Extraction kit according to the manufacturer's protocol. The samples were analyzed in a 96-well plate on which oligonucleotide containing the NF-κB consensus site (5′-GGG ACT TTCC-3′) had been immobilized. The activated form of NF-κB in nuclear extract from HIMEC bound to this oligonucleotide. Using an antibody against NF-κB p65 subunit and a horseradish peroxidase-conjugated secondary antibody resulted in a colorimetric readout, which was quantified at 450 nm by using a Beckman DU-650 spectrophotometer. Data from triplicate wells were expressed as means ± SD.
Immunofluorescence staining.
HIMEC monolayers were grown on coverslips to 80% confluence. Following treatment with various inhibitors and irradiation, using NF-κB p65 subunit antibody and a FITC-conjugated secondary antibody, immunofluorescence staining was performed as described previously (47). 4,6-Diamidino-2-phenylindole (DAPI) staining was performed to ensure the nuclear localization of p65 subunit in response to irradiation. TNF-α/LPS-activated HIMEC served as a positive control. Coverslips were mounted on Superfrost slides (Fisher Scientific) with Prolong Antifade mounting media (Invitrogen, Carlsbad, CA) and visualized via a fluorescence microscope (Olympus BX-40) and a Leica DFC 300FX camera.
mTOR siRNA transfection.
Transfection was done by using Amaxa's primary endothelial transfection kit and Nucleofector device according to the manufacturer's instructions (Amaxa, Cologne, Germany). HIMEC were transfected with 100 nM of either mTOR siRNA (target gene), GAPDH siRNA (positive control), or nontargeting siRNA (negative control). Pooled siRNAs for each gene were obtained from Dharmacon. At 48 h after transfection, cells were either exposed to 2 Gy of irradiation or were remained unirradiated as per protocol.
Real-time PCR.
RNA was then isolated by using Qiagen's RNeasy Plus Mini Kit according to manufacturer's instructions. Reverse transcription was done with 1 μg of RNA by using either Bio-Rad's iScript cDNA synthesis kit or Invitrogen SuperScript III First-Strand Synthesis System for RT-PCR. mTOR gene knockdown was analyzed by real-time PCR using Bio-Rad's SYBR Green Master Mix, 2 μl of cDNA, and 250 nM primers in 25 μl reactions. Cycling parameters were 95°C for 3 min, then 45 cycles of 95°C for 10 s and 60°C for 30 s. Generation of a single product was confirmed with a melt cycle. Real-time data were analyzed by use of Bio-Rad's iQ5 software. Primer sequences were as follows: mTOR forward 5′-CCT CCA AAA GGC CTG GGG CG-3′ and reverse 5′-GCG CAG GGA GGG CGA TGA TG-3′ (123-bp product); GAPDH forward 5′-TGC ACC ACC AAC TGC TTA GC-3′ and reverse 5′-GGC ATG GAC TGT GGT CAT GAG-3′; FOXO1 forward 5′-GGC GGG CTG GAA GAA TTC AA-3′ and reverse 5′-AGA TTT CCC GCT CTT GCC AC-3′ (130-bp product).
RESULTS
Effect of irradiation on HIMEC cell survival and cell death.
We determined the effect of curcumin, rapamycin, SN-50, and LY294002 on cell survival in irradiated HIMEC. Cell survival was assessed after 10 days by enumeration of adherent and viable cells with Trypan blue exclusion. The increased surviving fraction of HIMEC exposed to 2 Gy irradiation was twofold greater compared with control cells (Fig. 1A). Pretreatment of HIMEC with curcumin (10 μM), rapamycin (100 nM), and LY294002 (10 μM) followed by 2 Gy of irradiation significantly decreased cell survival. There were no detectable changes beyond the baseline from the inhibitors alone.
Fig. 1.
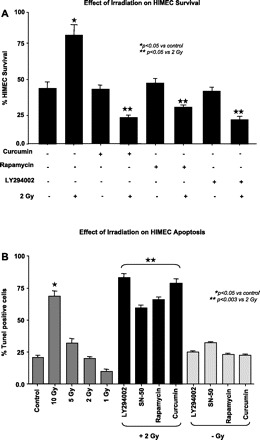
Effect of 2 Gy irradiation on human intestinal microvascular endothelial cells (HIMEC) survival and death. A: the increased surviving fraction of HIMEC between those exposed to 2 Gy irradiation was greater than that of controls after 10 days. Pretreatment of HIMEC with curcumin (10 μM), rapamycin (100 nM), and LY294002 (10 μM) significantly suppressed cell survival when subsequently exposed to radiation. B: terminal transferase-mediated dUTP-digoxigenin nick-end labeling (TUNEL) staining was performed to determine the induction of apoptosis by curcumin (10 μM), rapamycin (100 nM), SN-50 (18 μM), and LY294002 (10 μM) pretreatment of HIMEC followed by 2 Gy of irradiation. There was no detectable effect by the inhibitors alone beyond the baseline. Data are representative of 3 independent experiments.
For the cell death assay, HIMEC were treated and grown as above and fixed in 1% paraformaldehyde. Using a TUNEL we evaluated the percentage of apoptotic cells. Figure 1B shows that 10 Gy of irradiation significantly increased the HIMEC apoptosis; however, the number of apoptotic cells in response to 2 Gy was similar to control nonirradiated HIMEC. Pretreatment of HIMEC with curcumin (10 μM), rapamycin (100 nM), and LY294002 (10 μM) followed by 2 Gy of irradiation significantly increased the number of apoptotic cells. There were no detectable changes in apoptosis beyond the baseline from the inhibitors alone. These results indicate that low doses of irradiation increase cell survival and inhibition of PI3K/Akt/mTOR with curcumin prior to irradiation increases HIMEC apoptosis, making the endothelial cells more sensitive to low doses of irradiation.
Effect of irradiation on caspase 3 cleavage in HIMEC.
In the next series of experiments, HIMEC were either pretreated with rapamycin (100 nM), curcumin (10 μM), LY294002 (10 μM), and SN-50 (18 μM) for 60 min or left untreated then exposed to 2 Gy irradiation and incubated for another 5 h. Using antibodies against total and cleaved caspase 3, we found that these inhibitors significantly increased caspase 3 cleavage (17-kDa and 19-kDa cleaved bands) compared with irradiated HIMEC alone without changing the level of total caspase 3 (Fig. 2). These results indicate that inhibition of PI3K/Akt/mTOR and NF-κB with curcumin resulted in HIMEC apoptosis and made the endothelial cells more sensitive to low doses of irradiation.
Fig. 2.
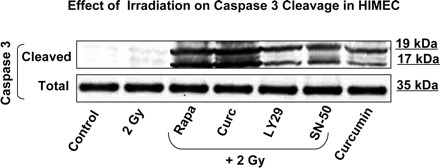
Effect of 2 Gy irradiation on caspase 3 in HIMEC. Western blot analysis for the cleavage of caspase 3 induction demonstrates that pretreatment of HIMEC with curcumin (10 μM), rapamycin (100 nM), LY294002 (10 μM), and SN-50 (18 μM) significantly increased caspase 3 cleavage (17 and 19 kDa) compared with irradiated HIMEC alone (upper blot). The levels of total caspase 3 remained unchanged (lower blot). Blot is representative of 3 independent experiments.
Effect of irradiation on HIMEC in vitro tube formation.
Next, we examined endothelial in vitro tube formation using the Matrigel assay. HIMEC in complete growth medium were seeded onto a three dimensional extracellular matrix preparation (Matrigel) and incubated for 16 h at 37°C. Where indicated, HIMEC monolayers were irradiated with or without pretreatment with inhibitors of mTOR (rapamycin, 100 nM), PI3K/Akt (LY294002, 10 μM) and curcumin (10 μM). Naive HIMEC seeded onto Matrigel in complete growth medium or in the presence of VEGF display formation of robust tubelike structures after 16 h (Fig. 3, A and B). Exposure of HIMEC to 2 Gy irradiation increased the number of endothelial tubes formed in Matrigel (Fig. 3, C). Inhibitors of mTOR, PI3K/Akt and curcumin at doses specific for their signaling targets exhibited a marked inhibitory effect on the formation of tubelike structures by HIMEC, visible by the disruption of tubelike structures and cells remaining coherent in spherical clusters (Fig. 3, D–F). Moreover, anti-VEGFR2 antibody pretreatment of HIMEC prior to 2 Gy irradiation inhibited tube formation (not shown). Silencing the mTOR gene by siRNA exerted an inhibitory effect on tube formation similar to curcumin (Fig. 3). These results indicate that activation of PI3K is required for in vitro tube formation in HIMEC, defining the crucial role of Akt/mTOR signaling pathways in functional angiogenesis of HIMEC.
Fig. 3.
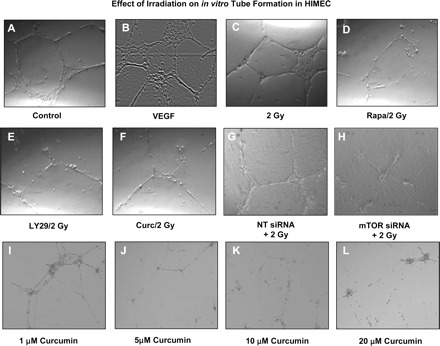
Effect of 2 Gy irradiation on HIMEC in vitro tube formation. HIMEC were seeded onto Matrigel-coated plates, and tube formation was assessed by phase-contrast microscopy (original magnification ×200). HIMEC initially form tubelike sprouts within 4 h with further maturation after 16 h (A). VEGF stimulation of HIMEC increased the number of tubes (B). The number of tube formed by 2 Gy of irradiation was same as VEGF (C). Endothelial tube formation of HIMEC was inhibited by incubation with rapamycin (Rapa; 100 nM), LY294002 (LY29; 10 μM), and curcumin (Curc; 10 μM) prior to irradiation (D–F). Similarly, inhibition of mammalian target of rapamycin (mTOR) by small interference RNA (siRNA) prior to irradiation inhibited tube formation in HIMEC (G and H). NT, nontargeting. Treatment of HIMEC with various doses of curcumin (1–20 μM) resulted in a dose-dependent inhibition of tube formation (I–L). Low doses of curcumin (1–5 μM) did not completely inhibit the tube formation in HIMEC, but the tubes were thinner than untreated controls. Phase-contrast microscopy data are representative of 3 independent experiments.
Effect of irradiation on PI3K/Akt in HIMEC.
Given the important role of the PI3K/Akt pathway in endothelial cell survival and death, we assessed the effect of irradiation on Akt activation in HIMEC. Western blot analysis using phosphorylated Akt (pAkt) antibody revealed that 2 Gy of irradiation induced increased Akt phosphorylation in HIMEC which was time dependent, enhanced by 15 min, lasted 30 min, and declined by 60 min (Fig. 4A). VEGF (50 ng/ml) stimulated HIMEC was used as a positive control. Akt phosphorylation was significantly inhibited by pretreatment of HIMEC with curcumin and LY294002, a specific PI3K/Akt inhibitor (Fig. 4B). These findings suggest that PI3K/Akt may play a role in radiation-induced cell survival in this endothelial cell population and inhibition of PI3K/Akt by LY294002 and curcumin enhanced the HIMEC radiosensitivity.
Fig. 4.
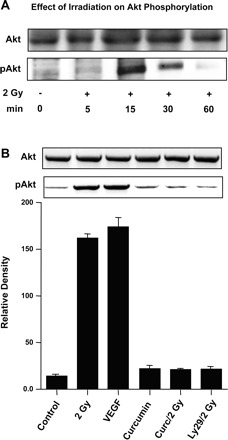
Effect of 2 Gy irradiation on phosphatidylinositol 3-kinase (PI3K)/Akt in HIMEC. A: phosphorylation of Akt (pAkt) in control and irradiated HIMEC was assessed by Western blot analysis. Total cell lysates from HIMEC monolyers exposed to 2 Gy of irradiation with or with inhibitors were analyzed by SDS-PAGE and immunoblotted with Akt antibodies (phosphorylated and unphosphorylated). In a time-dependent fashion, Akt phosphorylation in HIMEC was enhanced by 15 min, lasted for 30 min, and declined by 60 min. B: Akt phosphorylation was significantly inhibited by pretreatment of HIMEC with 10 μM of curcumin and 10 μM of LY294002, a specific PI3K/Akt inhibitor. Lysate from VEGF-stimulated HIMEC was used as a positive control for Akt phosphorylation. Level of total unphosphorylated Akt remained unchanged. The representative figure shown is 2 of 3 independent experiments.
Effect of irradiation on mTOR induction in HIMEC.
Next the effect of irradiation on mTOR phosphorylation was determined. As shown in Fig. 5A, exposure of HIMEC to irradiation dose dependently enhanced mTOR phosphorylation as demonstrated by Western blot analysis using phospho-specific mTOR antibodies. Irradiation-induced mTOR phosphorylation was also time dependent, increased at 30 min, then decreased by 60 min and was abolished by 90 min (Fig. 5B). Treatment of HIMEC with rapamycin (100 nM), curcumin (10 μM), and LY294002 (10 μM) alone did not affect mTOR phosphorylation beyond the control basal levels (Fig. 5C). Pretreatment of HIMEC with rapamycin (100 nM), curcumin (10 μM), and LY294002 (10 μM) completely blocked mTOR phosphorylation, which was induced by 2 Gy of irradiation (Fig. 5D). Similarly, silencing of mTOR gene by siRNA inhibited the effect of 2 Gy of irradiation on mTOR phosphorylation (not shown).
Fig. 5.
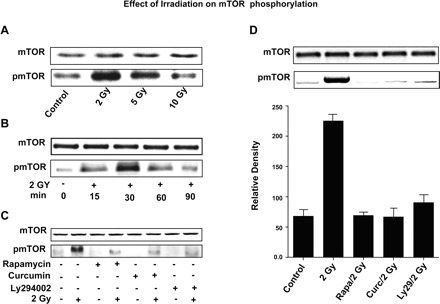
Effect of 2 Gy irradiation on mTOR in HIMEC. A: Western blot analysis of HIMEC lysates demonstrates that exposure of HIMEC to irradiation dose dependently enhanced mTOR phosphorylation using phospho-specific mTOR (pmTOR) antibody. Note the strong increased mTOR phosphorylation by 2 Gy, compared with control. B: enhanced mTOR phosphorylation induced by 2 Gy of irradiation was also time dependent, increased at 30 min, then decreased by 60 min and abolished by 90 min. C: treatment of HIMEC with rapamycin (100 nM), curcumin (10 μM), and LY294002 (10 μM) alone without irradiation did not exert any noticeable changes on mTOR expression. D: pretreatment of HIMEC with rapamycin (100 nM), curcumin (10 μM), and LY294002 (10 μM) completely blocked the effect of 2 Gy irradiation on mTOR phosphorylation. The representative figure shown is 1 of 3 independent experiments.
Gene silencing of mTOR in HIMEC.
To confirm whether the effect of irradiation on HIMEC is linked to mTOR, we performed gene-silencing experiments using transfection with siRNA specific for mTOR. Using appropriate controls (GAPDH siRNA as a positive control and nontargeted siRNA as a negative control), we demonstrated specific knockdown of mTOR by mTOR siRNA by real-time PCR (Fig. 6). After confirming significant silencing of mTOR expression, the effect of irradiation on tube formation and NF-κB p65 subunit nuclear translocation in these mTOR-silenced cells were determined (Figs. 3 and 7B).
Fig. 6.
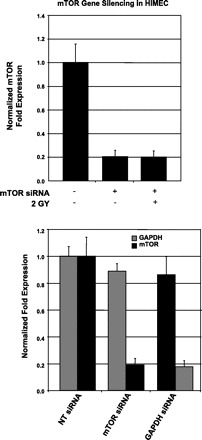
mTOR gene silencing in HIMEC. Real-time PCR demonstrates the specific knockdown of mTOR gene by mTOR siRNA using GAPDH siRNA as a positive and nontargeted siRNA as the negative controls.
Fig. 7.
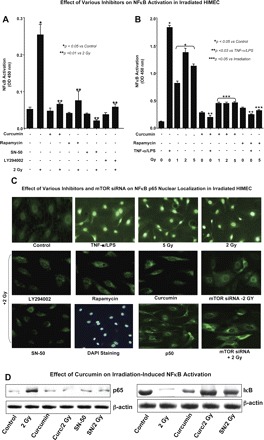
Effect of 2 Gy irradiation on NF-κB activation in HIMEC. A: using a DNA-binding ELISA based assay, we determined NF-κB activation in HIMEC in response to radiation exposure. NF-κB-DNA binding activity was completely inhibited by SN-50 (18 μM), rapamycin (100 nM), LY294002 (10 μM), and curcumin (10 μM) pretreatment of HIMEC prior to irradiation. Inhibitors alone did not alter the NF-κB activity. B: exposure of HIMEC to 1, 2, and 5 Gy produce similar results, TNF/LPS was used as a positive control for the NF-κB activity. C: immunofluorescence staining of HIMEC using NF-κB p65 demonstrates that in resting there is no nuclear staining of NF-κB p65 subunit. In contrast, in HIMECs that were exposed to 2 and 5 Gy of irradiation NF-κB p65 subunit but not NF-κB p50 subunit was translocated to nucleus. TNF-α/LPS-activated HIMEC served as a positive control. NF-κB p65 subunit translocation in was inhibited by LY294002, rapamycin, curcumin, SN-50, and mTOR siRNA. 4,6-Diamidino-2-phenylindole (DAPI) staining confirmed that the nuclear staining in the HIMEC was exposed to 2 Gy of irradiation. Moreover, NF-κB p50 subunit did not translocate to the nucleus. D: Western blot analysis shows the NF-κB p65 subunit and IκB immunoreactivity in nuclear and cytoplasmic protein fractions of irradiated HIMEC. Both SN-50 (SN) and curcumin exerted an inhibitory effect on NF-κB p65 subunit immunoreactivity. The representative figure shown is 1 of 3 independent experiments.
Effect of irradiation on NF-κB activation in HIMEC.
We then investigated whether NF-κB activation also plays a role in HIMEC radiosensitivity. Using a DNA-binding ELISA-based assay (Active Motif), we determined the NF-κB activation in HIMEC after exposure to radiation (Fig. 7A). NF-κB-DNA binding activity was completely inhibited by SN-50 (18 μM), rapamycin (100 nM), LY294002 (10 μM), and curcumin (10 μM) pretreatment of HIMEC prior to 2 Gy irradiation. These inhibitors did not alter NF-κB activity in HIMEC when administered alone. Figure 7B demonstrates that 1, 2, and 5 Gy irradiation all activate the NF-κB in HIMEC and that curcumin was a potent inhibitor of NF-κB activity. TNF-α/LPS was used as a positive control in these experiments. Next, we used immunofluorescence staining to demonstrate that exposure of HIMEC to low-dose irradiation (2 and 5 Gy) results in translocation of the NF-κB p65 subunit into the nucleus (Fig. 7C). TNF-α/LPS activated HIMEC served as a positive control. Pretreatment of HIMEC with LY294002 (10 μM), rapamycin (100 nM), curcumin (10 μM), SN-50 (18 μM), and mTOR siRNA resulted in inhibition of NF-κB p65 subunit nuclear translocation with no detectable nuclear staining of NF-κB p50 subunit. DAPI staining confirmed the p65 subunit indeed is localized in the nucleus after 2 Gy irradiation (Fig. 7C). Furthermore, Western blot analysis from nuclear protein fractions of irradiated HIMEC revealed the NF-κB p65 subunit immunoreactivity, which was inhibited by pretreatment of HIMEC with SN-50 and curcumin (Fig. 7D). IκB was not detected in irradiated cells alone but was seen in cells exposed to SN-50 or curcumin prior to irradiation. These findings demonstrate that low doses of irradiation activate NF-κB, which may result in radioresistance in HIMEC, and inhibition of NF-κB activity by curcumin would sensitize HIMEC exposed to subsequent irradiation. Together these data suggest that NF-κB, PI3K/Akt, and mTOR are the key pathways induced by low-dose radiation in HIMEC, which lead to increased cell survival.
Effect of irradiation on FOXO and MDM2 induction in HIMEC.
Next, we examined the effect of irradiation on the induction of both FOXOs and MDM2 in HIMEC. Our preliminary data indicate that 2 and 5 Gy irradiation did not affect the induction of FOXO1 at the gene or protein levels, and no increase in activation beyond the basal levels compared with control HIMEC was seen (Fig. 8, A–C, E). Neither curcumin nor mTOR siRNA exerted any effect on the expression of FOXOs in HIMEC. Interestingly, the level of phosphorylated FOXOs in control cells were slightly, but not significantly higher than either irradiated or curcumin pretreated HIMEC (Fig. 8D). Inhibition of mTOR by siRNA did not affect the FOXOs protein expression and phosphorylation (data not shown). Similarly, neither radiation nor curcumin affect the MDM2 protein levels in HIMEC beyond the basal levels demonstrated in resting cells (Fig. 8F).
Fig. 8.
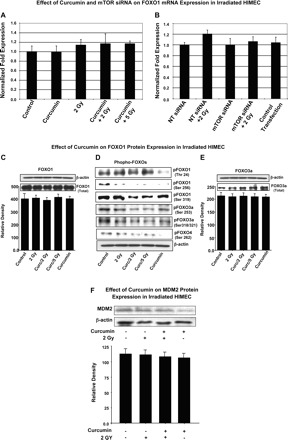
Effect of irradiation on FOXO and MDM2 induction in HIMEC. A: real-time PCR demonstrates that neither irradiation nor curcumin alters the FOXO1 mRNA expression in HIMEC compared with control cells. B: real-time PCR from HIMEC transfected with mTOR siRNA also showed no effect in FOXO1 mRNA expression. These results demonstrate that neither irradiation nor curcumin alter the FOXO1 mRNA expression in HIMEC compared with control cells. C and E: irradiation did not affect the induction of FOXO1 and FOXO3a total protein levels beyond the base level compared with control HIMEC. D: levels of phosphorylated FOXOs (1, 3a, and 4) were slightly but not significantly higher in control nonirradiated HIMEC than in either irradiated or curcumin-pretreated HIMEC. Inhibition of mTOR by siRNA did not affect the FOXOs protein expression and phosphorylation (not shown). F: neither irradiation nor curcumin affects the MDM2 protein level in HIMEC beyond the basal level of resting cells.
Effect of curcumin on rats exposed to whole body irradiation.
Preliminary experiments used histopathological assessment of small and large bowel tissues from rats fed a 2% curcumin diet, exposed to 6 Gy whole body irradiation, and evaluated over a 7-day time period. Rats with curcumin pretreatment prior to radiation demonstrated decreased villous number and height compared with control animals. Curcumin-fed and irradiated animals also showed and increased apoptotic bodies and ballooning of cells, vascular space with mild endothelial cell edema, but no significant vasculitis or thrombosis (short radiation exposure), all features of radiation injury, in the epithelium from both the small and large bowel (Fig. 9, A and B).
Fig. 9.
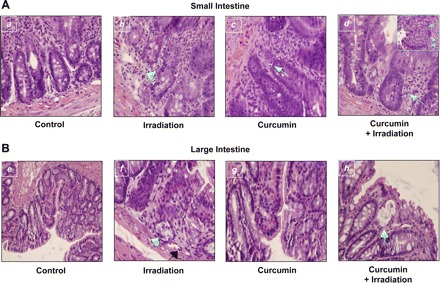
Histological analysis. A: hematoxylin and eosin (H&E) staining of cross section from rat's small intestine (n = 3). a: Normal appearance of mucosa in control tissue. b: 6 Gy irradiated rat small intestine; the arrow indicates an apoptotic body present in the crypt of the small intestine, the number of apoptotic bodies were within normal ranges. c: Curcumin-fed section shows moderate levels of damage indicated by the apoptotic bodies in the crypt. d: Curcumin-fed and irradiated tissue demonstrates more extensive apoptosis in the crypts, with crypt abscess. Inset in d shows ballooning of the crypt, apoptosis, and much more extensive damage to the tissue. The damage was more widespread and severe in the rats that were fed curcumin diet and received radiation (d) than in the rats that received radiation alone (b). B: H&E staining of cross section from rats large intestine (n = 3). e: Control rat large intestine displays the normal morphology of the crypts, villi, and the presence of goblet cells. f: Irradiated rat large intestine; the arrow indicates a crypt abscess in the mucosa of the large intestine. This is a region of apoptotic cells that is severe enough to lead to the collapse of a crypt. Black arrow shows vascular space with mild endothelial cell edema. g: Curcumin-fed sample shows the normal morphology similar to control samples. h: Curcumin-fed/irradiated sample demonstrates the loss of goblet cells with mucus lakes and flattening of the mucosa.
Effect of curcumin on caspase 3 induction in irradiated rat intestine.
In the next series of experiments we isolate RNA and protein from tissue homogenates of all four groups of rats. Using rat specific caspase 3 primers and real-time PCR, we show that the level of caspase 3 RNA was increased in animals that were fed curcumin and irradiated compare to irradiated alone in both small and large intestine (Fig. 10, A and B).
Fig. 10.
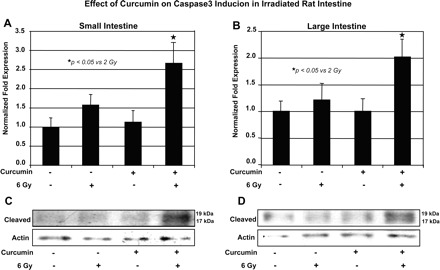
Effect of curcumin on caspase 3 induction in irradiated rat intestine. A and B: real-time PCR, using rat- specific caspase 3 primers, shows that the caspase 3 mRNA level was increased in animals that were fed curcumin and irradiated compare to irradiated alone in both small and large intestine. C and D: Western blot analysis of small and large intestine homogenates using antibody to cleaved caspase 3.
DISCUSSION
In the present study, we have shown that low-level irradiation induces survival pathways in HIMEC by activating NF-κB, PI3K/Akt, and mTOR signaling pathways. Moreover, curcumin and inhibitors of NF-κB (SN-50), PI3K/Akt (LY294002), mTOR (rapamycin) pretreatment, and mTOR siRNA sensitized HIMEC to irradiation. These data suggest that microvascular sensitivity to radiation therapy can be modulated by pretreatment with agents that exert an effect on the gut-specific endothelial cell population.
PI3K/Akt is the most targeted pathway in human cancers, since its activation leads to cell proliferation and cell survival via mechanisms that are presently not well defined (53). Akt is an important molecular junction in intracellular cell signaling, because multiple growth factor-driven signaling pathways converge through this molecule (45). Among possible downstream targets of Akt signaling, mTOR is of particular interest since its activity is induced by radiation and it is involved in cancer initiation and progression (28).
The involvement of Forkhead family of transcription factors (FOXO1, FOXO4, and FOXO3a) in tumorigenesis has been reported (10, 21, 44). The role of FOXOs in TGF-β-mediated upregulation of p21, which is negatively regulated by PI3K, has been shown (6). Phosphorylation of FOXOs by Akt (Thr24, Ser256, and Ser319) results in their nuclear translocation and inhibition of their transcriptional activity (63). Moreover, it has been reported that curcumin dephosphorylates and inactivates the constitutively active Akt, FOXO, and GSK3 in acute T-cell leukemia (32). In this study, we demonstrate that neither irradiation nor curcumin alters the FOXO expression in HIMEC.
Moreover, PI3K/Akt has been also implicated in the induction of MDM2 transcription through mTOR/ETS2, suggesting that these proteins may promote cell proliferation and inactivate p53 (38). Inhibitory effect of curcumin on MDM2 via PI3K/mTOR/ETS2 in several cancer cell lines has been demonstrated (38). Our preliminary studies demonstrate that neither irradiation nor curcumin affect the MDM2 level in HIMEC beyond the basal level of resting cells; more detail experiments are needed to determine the role of FOXOs and MDM2 in these primary endothelial cells.
Targeting mTOR for cancer therapy is an attractive approach because rapamycin and its derivative drugs exhibit significant anticancer activity in various tumor cell lines (11, 31). Activated mTOR phosphorylates eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and 70-kDa S6 kinase 1 (S6K1) (50). Phosphorylated S6K1 is a biomarker for mTOR activation (20). Association between rapamycin resistance and decreased levels of 4E-BP1 has been shown (18). Sensitization of U87 glioma xenografts to radiotherapy by rapamycin has been reported (20).
In the present study we addressed the effect of curcumin on HIMEC radiosensitivity. Curcumin, a dietary component of the spice turmeric, has been show to inhibit the formation of carcinogen-induced cancers of the colon (35), oral cavity (39), forestomach (58), esophagus (61), stomach (33), lung (29), liver (15), and skin (40) in rodents. In a phase I clinical trial, curcumin has demonstrated beneficial effects on patients with high-risk or premalignant lesions (14). It has been shown that curcumin modulates several oncogenes and tumor suppressor genes; however, the main cellular target(s) of curcumin in targeting cancer remain unknown (1, 52).
Our results demonstrate that HIMEC monolayers pretreated with curcumin were more sensitive to irradiation as measured by cell survival and apoptosis. These findings were similar to the effects of rapamycin, LY294002, and SN-50, which all enhanced HIMEC radiosensitivity. We have demonstrated that low-level irradiation resulted in phosphorylation of both Akt and mTOR, the survival molecules in HIMEC. Moreover, irradiation enhanced HIMEC in vitro tube formation (a key component of angiogenesis) and cell survival. The radiosensitizing effect of curcumin on HIMEC monolayers was shown by inhibition of angiogenesis (tube formation) and cell survival assays. Inhibition of angiogenic activity in HIMEC is possibly mediated through inhibition of VEGF expression as blocking the VEGF receptor by anti-VEGFR2 antibody prior to irradiation inhibited the endothelial tube formation. It has been shown that VEGF inhibition has a radiosensitizing effect on tumor vasculature and reduced VEGF expression exerted antiangiogenic effects in an S6K1-dependent fashion (23, 64). Thus, in addition to its direct effects on tumor cells, rapamycin also possess antiangiogenic effects (26).
The intestine is an important dose-limiting organ during radiation therapy. Intestinal radiation toxicity is classified as early or delayed, relative to the time of radiation exposure. Early radiation toxicity is the result of intestinal crypt cell death, disruption of the epithelial barrier, and mucosal inflammation, whereas delayed radiation toxicity is characterized by progressive intestinal wall fibrosis and vascular sclerosis, which may develop after several years. The severity of intestinal radiation toxicity depends on intestinal crypt cell death and radiation-induced cellular and functional changes secondary to cell death. Using hematoxylin and eosin staining, we demonstrate a variable presence of apoptotic cells in response to radiation and/or curcumin. Rats that were fed curcumin (2%) in their diet prior to radiation treatment had more apoptotic cells and showed vascular space with mild endothelial cell edema and no significant vasculitis or thrombosis (short radiation exposure) with an increased level of caspase 3 mRNA. Radiation injury scores in our rat intestinal experimental model could not be adequately addressed because of the short radiation time (7 days).
Results from the HIMEC in vitro tube formation experiments suggest that mTOR inhibition enhanced the effects of radiation on the migratory ability of these gut-specific microvascular endothelial cells. Curcumin demonstrated significant inhibitory effects on endothelial tube formation, which was similar to rapamycin's antiangiogenic effects. Thus it appears that mTOR inhibitors are an important radiosensitizer of gut microvascular endothelial cells. Significant reduction in vascularity and blood flow in tumors treated with mTOR inhibitors alone has been shown (26). How cells respond to mTOR inhibition is dependent on different mutations and protein expression (30). It has been reported that malignant cell lines may respond to rapamycin with a remarkable variance in sensitivity and radioresistance (17). It is possible that downstream targets of mTOR (e.g., S6K1, 4E-BP1) may also be mutated or decreased in expression, resulting in rapamycin resistance (16, 18, 24). Differences in these proteins may also explain the different responses seen in different cell lines. These differences also provide a rationale for focusing on the tissue specific microvascular endothelial cells as a target for radiosensitization due to less heterogeneity among this cell population compared with the heterogeneity associated with cancer cells.
Our present study also demonstrate that curcumin increased HIMEC radiosensitivity by inhibiting more than one intracellular signaling pathway and transcription factor. Curcumin inhibited NF-κB activation and nuclear translocation of the p65 subunit. A common mechanism underlying upregulation of angiogenesis and endothelial proliferation occurs via activation of NF-κB and the biological effects of curcumin are at least partly mediated through inhibition of this transcription factor. As shown in results, radiation exposure of HIMEC resulted in NF-κB activation similar to the effect of TNF-α/LPS in HIMEC. This endothelial activation of NF-κB inhibits the apoptotic response to radiation and provides a mechanism for tumor microvasculature to evade the potential cytotoxicity and antiangiogenic effect of radiation therapy (5, 27). Thus inhibition of the NF-κB pathway by curcumin could potentially overcome this radioresistance and enhance the efficacy of radiation therapy in patients with colorectal cancers.
In summary, we have demonstrated that curcumin can inhibit human intestinal endothelial cell survival and sensitize these cells to irradiation. The sensitization most likely occurs by regulating apoptosis, as confirmed by the effect of curcumin on cleavage of caspase 3 inductions as well as inhibition of Akt/mTOR and NF-κB activity. These finding provide a scientific foundation for the investigation of curcumin in clinical trials as a potential radiosensitizing agent for the microvascular endothelium.
GRANTS
This work was supported by National Institutes of Health Grant 5U19-AI067734 and support from the Cancer Center of the Medical College of Wisconsin.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23: 363–398, 2003 [PubMed] [Google Scholar]
- 2. Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res 11: 7490–7498, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol 69: 195–206, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer 111: 679–692, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Cell. [PMC free article] [PubMed] [Google Scholar]
- 6. Arden KC. FoxO: linking new signaling pathways. Mol Cell 14: 416–418, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 101: 1053–1062, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57: 1509–1517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 112: 1895–1907, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Borkhardt A, Repp R, Haas OA, Leis T, Harbott J, Kreuder J, Hammermann J, Henn T, Lampert F. Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11) (q13;q23). Oncogene 14: 195–202, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O'Reilly T, Stolz B, Marti A, Thomas G, Lane HA. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res 64: 252–261, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, Xing X, Durduran T, Yodh AG, Evans SM, Koch CJ, Hahn SM, Quon H, Sehgal CM, Lee WM, Maity A. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One 4: e6539, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 23: 1599–1607, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21: 2895–2900, 2001 [PubMed] [Google Scholar]
- 15. Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 21: 331–335, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol 16: 6242–6251, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dilling MB, Dias P, Shapiro DN, Germain GS, Johnson RK, Houghton PJ. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res 54: 903–907, 1994 [PubMed] [Google Scholar]
- 18. Dilling MB, Germain GS, Dudkin L, Jayaraman AL, Zhang X, Harwood FC, Houghton PJ. 4E-binding proteins, the suppressors of eukaryotic initiation factor 4E, are down-regulated in cells with acquired or intrinsic resistance to rapamycin. J Biol Chem 277: 13907–13917, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ding GR, Honda N, Nakahara T, Tian F, Yoshida M, Hirose H, Miyakoshi J. Radiosensitization by inhibition of IkappaB-alpha phosphorylation in human glioma cells. Radiat Res 160: 232–237, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res 62: 7291–7297, 2002 [PubMed] [Google Scholar]
- 21. Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, 3rd, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet 5: 230–235, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal 7: 1630–1647, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Geng L, Donnelly E, McMahon G, Lin PC, Sierra-Rivera E, Oshinka H, Hallahan DE. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res 61: 2413–2419, 2001 [PubMed] [Google Scholar]
- 24. Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15: 2852–2864, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grimm D, Bauer J, Schoenberger J. Blockade of neoangiogenesis, a new and promising technique to control the growth of malignant tumors and their metastases. Curr Vasc Pharmacol 7: 347–357, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 8: 128–135, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Gururaj AE, Belakavadi M, Venkatesh DA, Marme D, Salimath BP. Molecular mechanisms of antiangiogenic effect of curcumin. Biochem Biophys Res Commun 297: 934–942, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8: 179–183, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett 137: 123–130, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist Updat 4: 378–391, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol 3: 371–377, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Hussain AR, Al-Rasheed M, Manogaran PS, Al-Hussein KA, Platanias LC, Al Kuraya K, Uddin S. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in acute T cell leukemias. Apoptosis 11: 245–254, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Ikezaki S, Nishikawa A, Furukawa F, Kudo K, Nakamura H, Tamura K, Mori H. Chemopreventive effects of curcumin on glandular stomach carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine and sodium chloride in rats. Anticancer Res 21: 3407–3411, 2001 [PubMed] [Google Scholar]
- 34. Inano H, Onoda M. Radioprotective action of curcumin extracted from Curcuma longa LINN: inhibitory effect on formation of urinary 8-hydroxy-2′-deoxyguanosine, tumorigenesis, but not mortality, induced by gamma-ray irradiation. Int J Radiat Oncol Biol Phys 53: 735–743, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N, Tanaka Y, Osawa T, Uraji T, Murakoshi M, Nishino H, Tsuda H. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 19: 81–85, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Krishnan S, Janjan NA, Skibber JM, Rodriguez-Bigas MA, Wolff RA, Das P, Delclos ME, Chang GJ, Hoff PM, Eng C, Brown TD, Crane CH, Feig BW, Morris J, Vadhan-Raj S, Hamilton SR, Lin EH. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 66: 762–771, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res 14: 2128–2136, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res 67: 1988–1996, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Li N, Chen X, Liao J, Yang G, Wang S, Josephson Y, Han C, Chen J, Huang MT, Yang CS. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23: 1307–1313, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett 116: 197–203, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Manegold PC, Paringer C, Kulka U, Krimmel K, Eichhorn ME, Wilkowski R, Jauch KW, Guba M, Bruns CJ. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (Everolimus) increases radiosensitivity in solid cancer. Clin Cancer Res 14: 892–900, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene 21: 8852–8861, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Munafo DB, Colombo MI. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci 114: 3619–3629, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem 274: 15982–15985, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Nakamura JL, Karlsson A, Arvold ND, Gottschalk AR, Pieper RO, Stokoe D, Haas-Kogan DA. PKB/Akt mediates radiosensitization by the signaling inhibitor LY294002 in human malignant gliomas. J Neurooncol 71: 215–222, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344: 427–431, 1999 [PMC free article] [PubMed] [Google Scholar]
- 47. Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol 288: C272–C281, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Qiu M, Yi C, Hou M. [Combined low-dose chemotherapy inhibiting angiogenesis and growth of Lewis lung cancinoma xenografts in mice]. Sichuan Da Xue Xue Bao Yi Xue Ban 37: 534–537, 2006 [PubMed] [Google Scholar]
- 49. Rafiee P, Heidemann J, Ogawa H, Johnson NA, Fisher PJ, Li MS, Otterson MF, Johnson CP, Binion DG. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun Signal 2: 3, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA 98: 7037–7044, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rezvani M, Ross GA. Modification of radiation-induced acute oral mucositis in the rat. Int J Radiat Biol 80: 177–182, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 41: 1955–1968, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441: 424–430, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer 31: 243–305, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann NY Acad Sci 1056: 206–217, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol 595: 127–148, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Singh N, Aggarwal S. The effect of active oxygen generated by xanthine/xanthine oxidase on genes and signal transduction in mouse epidermal JB6 cells. Int J Cancer 62: 107–114, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Singh SV, Hu X, Srivastava SK, Singh M, Xia H, Orchard JL, Zaren HA. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis 19: 1357–1360, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 123: 1912–1922, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428: 305–327, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Ushida J, Sugie S, Kawabata K, Pham QV, Tanaka T, Fujii K, Takeuchi H, Ito Y, Mori H. Chemopreventive effect of curcumin on N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Jpn J Cancer Res 91: 893–898, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol 29: 1172–1178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 24: 1021–1032, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yu Y, Sato JD. MAP kinases, phosphatidylinositol 3-kinase, and p70 S6 kinase mediate the mitogenic response of human endothelial cells to vascular endothelial growth factor. J Cell Physiol 178: 235–246, 1999 [DOI] [PubMed] [Google Scholar]


