Abstract
Gastroesophageal reflux (GER) frequently triggers or worsens cardiac pain or symptoms in patients with coronary heart disease. This study aimed to determine whether GER enhances the activity of upper thoracic spinal neurons receiving noxious cardiac input. Gastric fundus and pyloric ligations as well as a longitudinal myelotomy at the gastroesophageal junction induced acute GER in pentobarbital-anesthetized, paralyzed, and ventilated male Sprague-Dawley rats. Manual manipulations of the stomach and lower esophagus were used as surgical controls in another group. At 4–9 h after GER surgery, extracellular potentials of single neurons were recorded from the T3 spinal segment. Intrapericardial bradykinin (IB) (10 μg/ml, 0.2 ml, 1 min) injections were used to activate cardiac nociceptors, and esophageal distensions were used to activate esophageal afferent fibers. Significantly more spinal neurons in the GER group responded to IB compared with the control group (69.1 vs. 38%, P < 0.01). The proportion of IB-responsive neurons in the superficial laminae of GER animals was significantly different from those in deeper layers (1/8 vs. 46/60, P < 0.01); no difference was found in control animals (7/25 vs. 20/46, P > 0.05). Excitatory responses of spinal neurons to IB in the GER group were greater than in the control group [32.4 ± 3.5 impulses (imp)/s vs. 13.3 ± 2.3 imp/s, P < 0.01]. Forty-five of 47 (95.7%) neurons responded to cardiac input and ED, which was higher than the control group (61.5%, P < 0.01). These results indicate that acute GER enhanced the excitatory responses of thoracic spinal neurons in deeper laminae of the dorsal horn to noxious cardiac stimulus.
Keywords: visceral pain, hypersensitivity, angina pectoris, esophagitis, spinal cord
gastroesophageal reflux disease (GERD), a common esophageal disorder, affects ∼19 million people in the U.S. (4, 11, 18, 27, 42). GERD typically presents as heartburn and/or acid regurgitation; however, extraesophageal presentations such as noncardiac chest pain are increasingly recognized (16, 25, 39). These extraesophageal manifestations are indistinguishable from true cardiac angina described as substernal squeezing, burning, tightness, heaviness, radiating to the arm, neck, back, or jaw (17). Besides coronary artery disease (CAD) resulting from myocardial ischemia, GERD is the second most frequent cause of chest pain (5, 9). Interestingly, it has long been noted that GERD is associated with CAD. For example, the concomitant prevalence of CAD and esophageal dysfunction has been reported in 10–70% of patients (12, 22, 26, 47, 49). Pathophysiologically, esophageal insults or acid can significantly reduce coronary blood flow velocity and volume, generate cardiac arrhythmias, elicit ischemic ECG changes, reduce the exertional angina threshold, and produce typical anginal pain in patients with and without CAD (3, 6, 7, 10, 31, 41, 50, 51). It is also known that GERD patients who do not have ischemic heart disease often have chest pain that mimics angina (28); 20–50% of patients with nonischemic chest pain have esophageal abnormalities (7); 46% of patients with normal coronary angiograms but with chest pain are found to have GERD as detected by 24-h esophageal pH monitoring (45). Furthermore, it has been suggested that antireflux therapy as well as acid-reducing drugs may benefit patients with known cardiac arrhythmias and ischemia (12, 26, 47). For example, 75% of CAD patients with optimal antianginal therapy showed improvement in chest pain following treatment with acid-suppressing drugs (47). The coexistence of esophageal and cardiac diseases suggests a strong pathophysiological communication between the heart and esophagus in producing and worsening of symptoms. However, the mechanisms underlying cross-organ communication between the esophagus and heart have not been fully elucidated.
The esophagus and heart share a common innervation of vagal and sympathetic neural systems, which originate from the medulla and spinal cord, respectively. Infusion of acid into the esophagus attenuates coronary blood flow in patients with and without CAD. A similar response is not seen in heart transplant patients, suggesting a neurally mediated esophagocardiac reflex (7). Therefore, a common viscerovisceral cross-talk pathway in the central nervous system may contribute to the clinical associations of esophageal and cardiac diseases. Spinal visceral afferents are well known to convey nociceptive information from visceral organs to neurons in the thoracic spinal cord. The responses are modulated by descending circuits. In addition, vagal parasympathetic afferents may also contribute to esophageal and cardiac nociception (17, 46). Human studies have shown that esophageal acid alters sensory perception of the resulting chest pain, which has been attributed to visceroreceptive sensitization of the central nervous system (13, 43, 44). It has been proposed from a human study that esophageal hyperalgesia may be caused by an increase of spinal visceroreceptive neuronal excitability (spinal sensitization) of spinal dorsal horn neurons, but animal studies are not available to support their argument (23, 43, 44). We and others have shown the afferent pathways and spinal processing of esophageal mechanical stimulation in animal models (14, 34). Furthermore, esophageal HCl and inflammatory mediators sensitized the responses of upper thoracic spinal neurons to esophageal distension (36). In addition, single thoracic spinal neurons with afferent input from the esophagus have been also shown to receive spinal afferent input from the heart (20, 34). Esophagocardiac convergence onto thoracic spinal neurons apparently provides a neural substrate to mediate viscerovisceral nociception and reflexes. Increased responsiveness to cardiac noxious stimulation could occur if esophageal irritation sensitizes spinal neurons. This possibility is based on evidence from our previous study showing that inflammation of the colon sensitizes lumbosacral spinal neurons with urinary bladder input (35). In that study, activities of lumbosacral neurons to distension of a “normal” urinary bladder were significantly increased after colitis compared with animals with a normal colon. Therefore, the aim of the present study was to compare the excitability and responsiveness of upper thoracic spinal neurons that receive noxious cardiac input in gastroesophageal reflux (GER) and control rats. A preliminary report of this work has been published in an abstract form (37).
METHODS
Male Sprague-Dawley rats (Charles River) weighing between 230 and 380 g were used for this study. Animals were housed in the animal facility under a 12-h light:12-h dark cycle with free access to food and water. After arrival, rats were acclimated to the animal facility for at least 1 wk before surgery. Rats were anesthetized with pentobarbital sodium (60 mg/kg ip), and a tracheotomy was performed for artificial ventilation with use of a constant-volume pump (55–60 strokes/min, 3.0–5.0 ml stroke volume). Catheters were inserted into the right carotid artery to monitor blood pressure and into the left jugular vein to infuse pentobarbital sodium (15–25 mg·kg−1·h−1) for maintenance of anesthesia. Animals remained anesthetized for surgical procedures, during the development of reflux, and during the measurement period to be described below. Animals were paralyzed with an initial injection of pancuronium bromide (0.4 mg/kg ip) followed by 0.2 mg/kg dose (ip) each hour during the experiment. A thermostatically controlled heating pad and overhead infrared lamps were used to keep rectal temperature between 37 and 38°C. At the end of the experiment, the animals were euthanized with an overdose of intravenous pentobarbital sodium (200 mg/kg). The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center.
Induction of gastroesophageal reflux.
Animals were randomly divided into GER (n = 8) and surgical control rats (n = 8). A laparotomy was performed in anesthetized animals to expose the stomach. In GER animals, the pylorus of the stomach and the junction between the forestomach and corpus was ligated with 2-0 silk thread. A longitudinal myelotomy (1.0–1.5 cm) was made across the gastroesophageal junction to facilitate reflux from the stomach into the esophagus. In the surgical control animal, after laparotomy, light stretching and squeezing were applied to the pylorus of the stomach, the gastroesophageal junction, and the forestomach.
To evaluate the presence or absence of esophageal inflammation, the thoracic esophagus was examined histologically in separate animals placed in four groups: 4-h GER (n = 4), 4-h GER surgical control (n = 4), 8-h GER (n = 4), and 8-h GER surgical control (n = 4). For this, 4 or 8 h after surgery, the rats were perfused intracardially with phosphate-buffered saline (PB, pH 7.4), followed by 4% paraformaldehyde in 0.1 M PB. The experimental and surgical controls were treated as matched pairs for each GER duration (e.g., 4 and 8 h). The esophagi from each pair of animals were removed, postfixed overnight and then dehydrated and infiltrated with paraffin. The specimens were embedded in paraffin and cross sectioned (8 μm thick) beginning at the caudal end. The sections were stained with hematoxylin-eosin and examined and digitized under a light microscope (Olympus, Spot camera). The images were saved in tiff format at a resolution of 300 dpi.
Spinal neuronal recordings.
Between 4 and 9 h after GER surgery, extracellular potentials of single neurons were recorded from the upper thoracic (T3) spinal cord to examine response characteristics to visceral and somatic stimuli. Rats were placed in a stereotaxic unit and a pair of clamps were attached to the T2 and T5–6 vertebral processes to stabilize the spinal cord. Laminectomies were made to expose the T3 segments. The dura mater of the spinal cord was carefully removed, and the T3 segment was filled with agar (3–4% in saline) to protect the cord from drying. Carbon filament glass microelectrodes were advanced with an electronic microstepper. Exploration coordinates were 0–1.2 mm from the dorsal surface and 0.5–2 mm from midline of the dorsal surface of the spinal cord. Electrodes were inserted at angles 70–80° to the thoracic cord (rostrocaudal direction) to avoid dimpling the cord. An average of 9 (range 7–11) spinal neurons were obtained and examined for visceral and somatic stimuli from each animal. Extracellular action potentials of single neurons in superficial and deeper laminae were conventionally amplified, displayed on an oscilloscope, and isolated in the window of a discriminator to ensure that activity was recorded from only one unit. Raw neuronal activity and discriminator output were stored on a computer using a CED data-acquisition system with Spike 3 software (Cambridge, UK).
Visceral and somatic stimuli.
A noxious cardiac stimulus was induced by an intrapericardial injection of bradykinin as described previously (34). A high midline thoracotomy was made to expose the pericardial sac by opening the thymus gland. Silicone tubing (0.020 ID, 0.037 OD, 14–16 cm in length) with five to eight small holes in the distal 2 cm was inserted into the pericardial sac over the left ventricle. A solution of bradykinin (10 μg, 0.2 ml) was injected into the pericardial sac for chemical activation of cardiac afferent nerve endings. The protocol for administering bradykinin was to inject warm saline (0.2 ml) into the pericardial sac and withdraw after 60 s to determine volume effects, to inject 0.2 ml of the solution of bradykinin and withdraw after 60 s, and finally to rinse the chemicals within the pericardial sac with two to three saline flushes (0.2 ml each). At least 20 min elapsed between each injection of bradykinin to avoid tachyphylaxis (38). Bradykinin was used because it is released by the ischemic myocardium and is involved in the generation of ischemic pain (17). Esophageal distension (ED) was produced by infusing warm water into a small latex balloon (1.0 cm long) at the end of PE-240 tubing (34). The balloon was inserted perorally 6–8 cm from the front incisors into the middle thoracic region of the esophagus. An injection of 0.4 ml warm water for ED was performed at a rate of 0.05–0.1 ml/s and held for 20 s. This volume has been employed as natural noxious stimuli in behavioral and neuronal processing studies of esophageal reflexes in rats (14, 34).
Different mechanical somatic stimuli were applied to determine the receptive fields of recorded spinal neurons, including innocuous brushing with a camel-hair brush, pressure with a blunt stick, and noxious pinching of skin with a blunt forceps. Neurons were classified as follows: low-threshold (LT) neurons were excited by hair movement and/or light pressure; high-threshold (HT) neurons responded only to noxious pinching of the somatic field; wide-dynamic-range (WDR) neurons were excited by innocuous stimuli and also had greater responses to noxious pinch of somatic fields. Outlines and descriptions of receptive fields were recorded manually for all neurons examined. Areas of the somatic fields were measured and assigned to the following categories: small (ipsilateral, long axis <4 cm); medium (ipsilateral, long axis >4 cm), and large (bilateral fields).
Examination of recording sites.
After the examining protocol for a spinal neuron with cardiac input was completed, an electrolytic lesion (50 μA DC) was made at some recording sites. At the end of the experiment, the upper thoracic spinal cord was removed and placed in 10% buffered formalin solution. Frozen sections (55–60 μm) were viewed to find lesion sites in the gray matter of the spinal cord. Locations were drawn on cross sections traced from the cytoarchitectonic scheme of spinal cord (Fig. 1A).
Fig. 1.
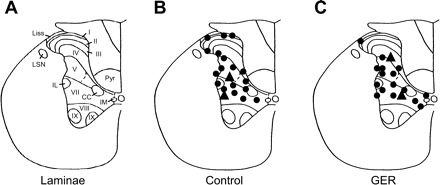
Lesion sites of neurons in representative T3 segment of the rat spinal cord. A: lamina of a T3 spinal segment. I–X, laminae; CC, column of Clarke; IL, intermediolateral nucleus; IM, intermedial nucleus; Liss, Lissauer's tract; LSN, lateral spinal nucleus; Pyr, pyramidal tract. B: neurons with responses to intrapericardial bradykinin (IB) in control animals. ●, Neurons excited by IB; ▲, neurons inhibited by IB. C: neurons with response to IB in gastroesophageal reflux (GER) animals.
Data analysis.
The neuronal discharges were stored in a computer using the Spike 3 data capture system and evaluated by using rate histograms (bin width 1 s). Spontaneous activity was determined by counting activity for 10 s and then dividing by 10 to obtain impulses per second (imp/s). An excitatory response to intrapericardial bradykinin (IB, imp/s) was calculated by subtracting the mean of 10 s of spontaneous activity from the mean of 10 s of the maximal activity during IB. An inhibitory response to IB was calculated by subtracting the mean of 10 s of minimal activity during IB from background activity. For each neuron, a given stimulus was considered effective if the change in activity was ≥20% of control activity. Duration of responses was measured from the onset of IB-evoked responses to the time at which activity returned to control level. Latency of responses was measured from the onset of IB to the starting point of responses to IB. Time to peak represented a period of time from starting point of response to maximal responses to IB. Statistical comparisons between GER and control animals were made by Student's paired or unpaired t-test and Fisher's exact tests. Descriptive data are reported as means ± SE. Comparisons of data were considered statistically different if P < 0.05.
RESULTS
Neuronal general characteristics.
In total, 139 spinal neurons recorded from upper thoracic (T3) segments of the spinal cord were examined for responses to cardiac and somatic stimulation. IB changed the activity of 47/68 (69.1%) of the spinal neurons recorded in GER rats, which is significantly higher than those recorded in control animals (27/71, 38%, P < 0.01). Of the responsive neurons, IB excited 39 neurons and inhibited 8 neurons in the GER group; in the control group, IB excited 23 neurons and inhibited 4 neurons. Figure 2 shows examples of excitatory and inhibitory responses to IB in GER and control groups. On the basis of the spontaneous activity of IB-responsive neurons, spinal neurons excited by IB were divided into two groups: active neurons with high spontaneous activity (>0.5 imp/s) and silent neurons with low spontaneous activity (≤0.5 imp/s). The ratio of active and silent neurons in GER rats (39/7) was similar to those in control animals (23/5), and the mean spontaneous activity (12.9 ± 2.3 imp/s, n = 39) of neurons excited by IB in the GER group was not significantly higher than that in the control group (8.3 ± 1.7 imp/s, n = 23, P > 0.05). Furthermore, the mean excitatory responses to IB (32.4 ± 3.5 imp/s, n = 39) of spinal neurons recorded from GER rats were significantly greater than those recorded from control rats (13.3 ± 2.3 imp/s, n = 23, P < 0.01). A comparison of the characteristics of spontaneous activity and IB responses of spinal neurons from GER and control animals are presented in Table 1.
Fig. 2.
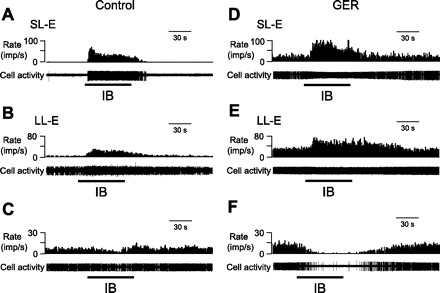
Response patterns of thoracic spinal neurons to IB in control and GER animals. A–C: examples of neurons with short-lasting excitatory (SL-E, A), long-lasting excitatory (LL-E, B), and inhibitory (C) responses to IB in a control animal. D–F: examples of SL-E (D), LL-E (E), and inhibitory (F) responses to IB in a GER animal. imp, Impulses. In all panels, top trace is the rate histogram and bottom trace is raw cell activity.
Table 1.
Comparison of response characteristics of thoracic spinal neurons receiving noxious cardiac input in GER and control animals
| Groups and Neuron Classes | n | Spontaneous Activity, imp/s | Latency, s | Excitatory Response, imp/s | Inhibitory Response, imp/s | Duration of Responses, s |
|---|---|---|---|---|---|---|
| GER | ||||||
| E | 39 | 12.9 ± 2.3 | 5.9 ± 0.6 | 32.4 ± 3.5* | N/A | 105.5 ± 6.6 |
| I | 8 | 9.9 ± 3.0 | 5.1 ± 0.7 | N/A | 5.8 ± 1.4 | 78.6 ± 10.3 |
| Control | ||||||
| E | 23 | 8.3 ± 1.7 | 7.0 ± 0.8 | 13.3 ± 2.3 | N/A | 98.4 ± 9.0 |
| I | 4 | 14.1 ± 6.9 | 7.8 ± 1.6 | N/A | 5.6 ± 1.1 | 56.8 ± 6.1 |
Values are means ± SE. GER, gastroesophageal reflux; E, excitatory responses; I, inhibitory responses.
P < 0.01 compared to corresponding activity of control group.
Short- and long-lasting responses.
On the basis of the recovery time of neuronal activity to the control level after removing bradykinin from the pericardial sac, neurons excited by IB were further subdivided into two subgroups (38): neurons with a recovery time of ≤50 s were classified as short-lasting excitatory (SL-E, Fig. 1, A and B), and neurons with a recovery time of >50 s were classified as long-lasting excitatory (LL-E, Fig. 1, D and E). Quantitative analyses of spontaneous activity, excitatory responses to IB, duration of response, and latency to responses in SL-E and LL-E neurons are shown in Table 2. The mean excitatory responses (34.6 ± 5.2 imp/s, n = 20) of SL-E neurons recorded from GER animals were significantly greater than those in control animals (11.0 ± 3.4 imp/s, n = 12, P < 0.01). Comparison of excitatory responses of LL-E neurons also showed a significant difference between GER and control groups (30.0 ± 4.5 vs. 15.9 ± 2.8 imp/s, P < 0.01). In addition, spontaneous activity (15.9 ± 3.9 imp/s, n = 20) of SL-E neurons recorded from GER animals was significantly higher than those in control group (6.5 ± 2.3 imp/s, n = 12, P < 0.05).
Table 2.
Comparison of short- and long-lasting excitatory responses of thoracic spinal neuron receiving noxious cardiac input in GER and control animals
| Groups and Neuron Classes | n | Spontaneous Activity, imp/s | Latency, s | Excitatory Response, imp/s | Duration of Responses, s |
|---|---|---|---|---|---|
| GER | |||||
| SL-E | 20 | 15.9 ± 3.9* | 5.1 ± 0.7 | 34.6 ± 5.2† | 75.9 ± 3.8 |
| LL-E | 19 | 9.7 ± 2.0 | 6.7 ± 0.8 | 30.0 ± 4.5† | 136.5 ± 8.0 |
| Control | |||||
| SL-E | 12 | 6.5 ± 2.5 | 6.5 ± 1.4 | 11.0 ± 3.4 | 63.9 ± 7.1 |
| LL-E | 11 | 10.3 ± 2.3 | 7.7 ± 0.9 | 15.9 ± 2.8 | 136.1 ± 6.3 |
SL-E, neuron with short-lasting excitatory response. LL-E, neuron with long-lasting excitatory response.
P < 0.05,
P < 0.01 compared to corresponding activity of control group.
Superficial and deeper neurons.
Spinal neurons recorded at depth of ≤300 μm from the dorsal surface of spinal cord were classified as superficial neurons, whereas neurons below 300 μm (to 1,200 μm) were classified as deeper neurons (38). The average depth of the recorded neurons in control animals (range from 12 to 1,180 μm, 589.8 ± 28.2 μm, n = 71) was similar to 610.3 ± 23.4 μm (range from 58 to 1,200 μm, n = 68) in GER groups of animals. Lesions made at the recording sites were histologically identified for 18 neurons in GER group and 20 neurons in control group (Fig. 1, B and C), respectively. Of neurons examined for noxious cardiac stimulus in control groups, IB altered the activity of 7/25 (28%) neurons in the superficial laminae and 20/46 (43.5%) neurons in the deeper spinal gray matter in GER rats. The proportion of IB-responsive neurons in the superficial laminae in GER animals was significantly different from those in deeper neurons (1/8 vs. 46/60, P < 0.01), whereas this difference was not found in control animals (7/25 vs. 20/46, P > 0.05). Furthermore, all of the superficial responsive neurons were excited by IB, whereas all of the neurons with inhibitory responses to IB were located in deeper laminae in both GER and control groups. A comparison of the IB-responsive characteristics of superficial and deeper spinal neurons in GER and control groups of animals is presented in Table 3. The mean excitatory responses of deeper spinal neurons to cardiac noxious stimulus were significantly greater than those in control group (32.6 ± 3.5 vs. 10.9 ± 1.9 imp/s, P < 0.05, Table 3).
Table 3.
Comparison of excitatory responses of superficial and deeper thoracic spinal neurons receiving noxious cardiac input in GER and control animals
| Groups and Neuron Classes | n | Spontaneous Activity, imp/s | Latency, s | Excitatory Response, imp/s | Duration of Responses, s |
|---|---|---|---|---|---|
| GER | |||||
| Superficial | 1 | 0 | 5.1 | 23.5 | 74.1 |
| Deeper | 38 | 13.3 ± 2.3 | 5.9 ± 0.6 | 32.6 ± 3.5* | 106.3 ± 6.6 |
| Control | |||||
| Superficial | 7 | 8.4 ± 3.8 | 8.0 ± 2.3 | 17.3 ± 5.3 | 97.3 ± 10.4 |
| Deeper | 16 | 8.2 ± 1.9 | 6.6 ± 0.7 | 10.9 ± 1.9 | 98.9 ± 12.4 |
P < 0.01 compared to corresponding activity of control group.
Cardioesophageal convergence.
Of the 47 spinal neurons that responded to IB in rats with GER, 45 (95.7%) also responded to noxious ED (0.4 ml, 20 s). This rate was significantly higher than that of the control group (16/26, 61.5%, P < 0.01). Multiple patterns of excitatory (E) and inhibitory (I) responses to IB and ED in same neurons were observed: 37 E-E, 1 E-I, 4 I-I, and 3 neurons responding to IB and ED were identified in GER group; 12 E-E, 2 E-I, 1 I-I, and 1 I-E neurons responding to IB and ED were classified in control group. Thus 84.3% (37/46) and 75% (12/16) neurons were excited by both visceral stimuli in GER and control groups, respectively. An example of a neuron with excitatory responses to both IB and noxious ED in GER group is shown in Fig. 3. Spontaneous activity and excitatory responses to IB of cardioesophageal convergent and nonconvergent neurons are quantitatively compared in Table 4. Inflammation of the esophagus significantly enhanced excitatory responses to IB in cardioesophageal convergent neurons compared with convergent and nonconvergent neurons in control groups.
Fig. 3.
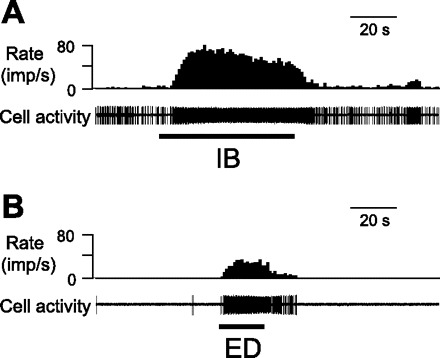
Example of the cardioesophageal convergent neurons excited by IB (A) and noxious esophageal distension (ED, 0.4 ml, 20 s) (B) in a GER rat.
Table 4.
Comparison of excitatory responses of cardioesophageal convergent and nonconvergent thoracic spinal neurons to IB in GER and control animals
| Groups and Neuron Classes | n | Spontaneous Activity, imp/s | Latency, s | Excitatory Response, imp/s | Duration of Responses, s |
|---|---|---|---|---|---|
| GER | |||||
| Convergent | 38 | 13.2 ± 2.3 | 5.8 ± 0.6 | 32.9 ± 3.5* | 104.7 ± 6.6 |
| Nonconvergent | 1 | 3.0 | 8.8 | 12.6 | 136.0 |
| Control | |||||
| Convergent | 14 | 9.1 ± 2.0 | 7.0 ± 0.8 | 14.0 ± 2.5 | 102.4 ± 13.1 |
| Nonconvergent | 9 | 7.0 ± 3.2 | 7.2 ± 1.8 | 12.2 ± 4.5 | 92.2 ± 11.3 |
P < 0.01 compared to excitatory responses of convergent and nonconvergent neurons in control group.
Somatic inputs.
Of the neurons responding to IB, 41/47 (87.2%) neurons in GER animals and 25/27 (92.6%) neurons in the control group received convergent inputs from cutaneous receptive fields. Cutaneous receptive fields were generally on the arms, shoulder, chest, and upper back areas. Figure 4 shows examples of neurons that received inputs from somatic fields. In neurons with noxious cardiac inputs in GER animals, 1 LT, 17 WDR, and 23 HT neurons were classified and the somatic fields of 6 neurons were not found. In the control groups of neurons responding to IB, 2 LT, 9 WDR, and 14 HT neurons were classified and another 2 neurons were not found for somatic field. Of neurons receiving somatic inputs only, 16 LT, 18 WDR, and 9 HT neurons were classified in GER groups, similar to those in control animals (8 LT, 7 WDR, and 2 HT). Thus no statistical difference of somatic field properties of viscerosomatic convergent neurons was found in GER and control groups. For somatic field sizes of viscerosomatic convergent neurons in the GER group, 9 small, 22 medium, and 10 large-size somatic fields were classified. This was not significantly different from those in control group of neurons (with 9 small, 12 medium, and 4 large-size somatic fields). However, for neurons with somatic input only, 5 small, 9 medium, and 3 large somatic fields were identified in the GER group, whereas 34 small, 8 medium, and 1 large somatic field were found in the control group. Therefore, the somatoreceptive neurons with large or medium fields were more frequently were observed in the GER group than in the control group (P < 0.01).
Fig. 4.
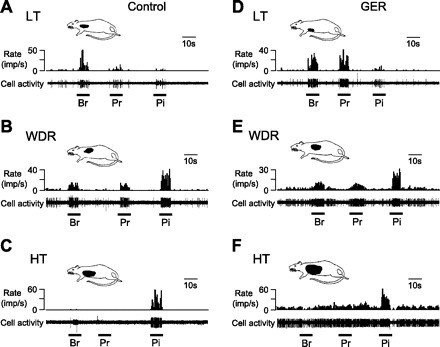
Characteristics of thoracic spinal neurons receiving somatic inputs. A–C: examples of neurons with low-threshold (LT, A), wide-dynamic-range (WDR, B), and high-threshold (HT, C) responses to mechanical stimulation of somatic field in control animals. D–E: examples of neurons with LT (D), WDR (E), and HT (F) responses to mechanical stimulation of somatic field in GER animals. In each panel, top drawing shows location of somatic field and bottom trace shows cell activity. Br, brush; Pr, light pressure; Pi, pinch.
Histological confirmation of esophageal inflammation.
We examined the thoracic esophagi collected from experimental and surgical control animals for histological signs of inflammation. In the 4-h GER surgical control, the esophagus had a normal appearance as evidenced by a smooth and even epithelia and lamina propria (Fig. 5A). In contrast, in the 4-h paired experimental animal, the epithelium was thicker and the lamina propria was invaginated (Fig. 5B). In another pair of animals in which the esophagi were collected 8 h after surgery, we observed further thickening of the lamina propria (arrows in Fig. 5D) in the experimental animal compared with the control (Fig. 5C). Similar differences were observed between all of the experimental and surgical controls. These pathologies, e.g., basal cell hyperplasia and thickening and invagination of the lamina propria, are consistent with those of esophageal inflammation. Similar histological observations have been reported in previous studies in which gastric acid reflux esophagitis developed within 4 h after surgery (2, 32, 33, 48).
Fig. 5.
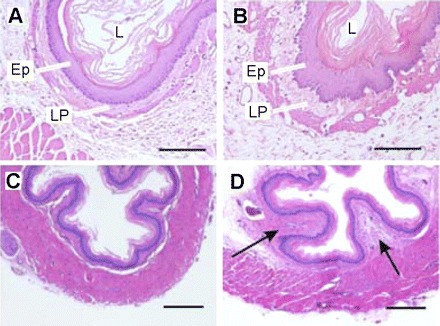
Histological examination of esophagitis in rats with the acute GER and control surgery. Digital photomicrographs of cross sections through the thoracic esophagus at 4 (B) and 8 (D) h after GER surgery. B: early basal cell hyperplasia in the epithelium (EP) and thickening and invagination of the lamina propria (LP) compared with control surgery (A). In D, arrows indicate further thickening of the LP at 8 h compared with control surgery (C). Scale bars in A and B = 50 μm; in C and D = 300 μm. Hematoxylin-eosin staining.
DISCUSSION
The present study shows that upper thoracic spinal neurons responding to IB in rats with acute GER inflammation had significantly greater excitatory responses compared with those in control animals. The increased neuronal responsiveness to IB occurred not only in neurons with short-lasting excitatory responses, but also in neurons with long-lasting responses. Furthermore, GER primarily enhanced the neuronal responses to IB in the deeper laminae of spinal gray matter rather than superficial dorsal horn. These data suggested that visceroreceptive hyperresponsiveness of spinal neurons might be the intraspinal neuronal mechanisms underlying cross-organ sensitization responsible for hypersensitivity of the cardiac perception in rats with esophagitis. The cross-organ sensitization of spinal neuronal processing observed in this study could mediate pathophysiological viscerovisceral communication in patients with cardiac and noncardiac chest pains.
Spontaneous activity.
The spontaneous activity or background discharges of spinal neurons represent to some degree excitability and responsiveness to various afferent inputs and are also modulated by impulses from spinal segmental, intersegmental, and supraspinal sites. In this study, the ratio of active to silent spinal neurons excited by IB in GER animals was not different from those in the control group. Furthermore, although spontaneous activity of SL-E neurons in the GER group was significantly higher than those in the control group, the overall spontaneous activity of all neurons excited by IB in the GER group was not significantly different from those in the control group. This finding is different from a previous observation using a central cross-organ sensitization model, in which lumbosacral spinal neuronal responses to urinary bladder distension (UBD) in rats with colitis were examined (35). The ratio of active to silent neurons with excitatory responses to UBD was significantly higher in rats with colitis than in the control group (P < 0.05). The mean spontaneous activity of neurons excited by UBD in rats with colitis is significantly greater than that in the control group (P < 0.05). This could be due to a chronic and severe colorectal inflammation with bleeding and diarrhea induced by dextran sulfate sodium and that is present for 7–12 days. In the present study, acute esophageal inflammation (4–6 h) was induced by the reflux of gastric contents after GER surgery. First, therefore, the short term of inflammation in the esophagus may have been insufficient to drive and maintain the tonic afferent inputs to spinal cardioreceptive neurons necessary for elevating spontaneous or background activity. Second, the rapid onset of esophageal nociception might quickly trigger and activate descending inhibitory systems originating from supraspinal sites; descending inhibition might suppress background activity of visceroreceptive neurons. It has been presumed that descending facilitatory modulation may not be developed during a short term of noxious esophageal event (40).
Responses to IB.
The incidence of upper thoracic spinal neurons responding to intrapericardial application of an algogenic chemical mixture or bradykinin has been reported in normal rats (34, 38). In the present study, IB changed the activity of 38% T3 spinal neurons in control animals, which is similar to previous observations. In contrast, IB altered the activity of 69.1% spinal neurons recorded in GER animals, which is significantly higher than in control groups. This is unlike a previous study that focuses on cross-organ sensitization of spinal viscerovisceral processing, in which noxious UBD changes the activity of 38 and 36% lumbosacral spinal neurons recorded from rats with colitis or normal colon, respectively (35). The different animal preparations, including tested spinal segments, visceral stimuli, and inflammation, could be responsible. In the present study, interestingly, of cardioreceptive neurons, IB excited 83 and 85% of the neurons with noxious cardiac input in GER and control groups, respectively. The proportions of lumbosacral spinal neurons receiving urinary bladder input are, respectively, 82.8 and 78.2% in rats with and without colitis (35). This suggests that cross-organ sensitization, either in lumbosacral and thoracic spinal neurons, mainly occurs in visceroreceptive spinal neurons with excitatory responses to peripheral visceral stimuli. Furthermore, on the basis of recovery time of neuronal responses to IB, cardioreceptive neurons in the thoracic spinal cord are classified as SL and LL responses (38). The neurons with SL responses have been suggested to mainly receive inputs from mechanosensitive and myelinated A-δ afferent fibers, whereas the LL responses might be more closely correlated with activation of chemosensitive and unmyelinated C afferent fibers. In the present study, both mean SL-E and LL-E responses to IB were significantly greater in rats with GER than in control animals. This suggests that inflammation of the esophagus sensitized and enhanced the activity of both small myelinated and unmyelinated spinal visceral afferents innervating the heart. Since a larger number of spinal neurons in the GER group of animals responded to intrapericardial bradykinin compared with the control group, it was reasonable to suggest that GER might recruit more peripheral visceral afferents activated by esophageal inflammation (36, 46).
Superficial and deep neurons.
Electrophysiologically, quantitative differences of activity characteristics between superficial and deeper viscerosomatic neurons within spinal dorsal horn have been reported in the thoracic spinal segments of rats when intrapericardial bradykinin or other algesic chemicals were used as the noxious cardiac stimulus (15, 34, 38). In general, noxious stimulation of cardiac afferents excites all superficial neurons, whereas deeper neurons exhibit multiple response patterns that are either excitatory or inhibitory, or combinations of the two. The response patterns with IB from the present study are consistent with previous observations (15, 34, 38); however, GER predominantly enhanced neuronal activity in deeper rather than superficial laminae of the dorsal horn. The mean excitatory responses of deeper spinal neurons to cardiac noxious stimulus in the GER group were significantly greater than those in the control group. Thus deeper spinal neurons primarily meditated the cross-organ sensitization between esophagus and heart observed in the present study. Comparing the present results with immunohistochemical techniques, occlusion of the left anterior descending coronary artery and stimulation of cardiac afferent fibers by an intrapericardial infusion of bradykinin or inflammatory solution increase Fos expressions in the dorsal horns of laminae I–IV, VII, and X in upper thoracic spinal segments in rats (1, 24). It should be noted that IB increases c-fos expression in the superficial dorsal laminae (I–IV) in the thoracic spinal cord at the upper thoracic segments, particularly in the medial parts of layers II and III. In contrast, the intermediolateral cells and the dorsal spinal nucleus, located near the spinal canal, only contain a few Fos-positive cells (1). Thus neurons expressing c-fos after noxious cardiac stimulation are mainly located in superficial laminae of the spinal dorsal horn, whereas neurons with electrophysiological responses are mostly found in deeper laminae. Although the increased expression of c-fos is often used as a marker of neuronal activity, such expression does not appear to follow either increases or decreases in neuronal discharges as obtained electrophysiologically in the present study. Therefore, differences in the laminar distribution of c-fos-expressive and visceroreceptive neurons to noxious cardiac stimulus in spinal cord are difficult to interpret.
Viscerovisceral convergence.
Spinal viscerovisceral sensory neurons with convergent inputs from different visceral domains have been reported in the thoracic and lumbosacral spinal cord in several species of animals. For example, single thoracic spinal neurons with mechanical afferent inputs from the esophagus also respond to stimulation of cardiac spinal visceral afferents in cats (20) as well as intrapericardial algogenic chemicals in rats (34). It is believed that esophagocardiac convergence on thoracic spinal neurons provides a neural substrate that mediates esophageal and cardiac interactions in nociception and reflexes. In the present study, 95.7% of neurons in the GER group had esophagocardiac convergence, which was significantly higher than that (61.5%) in the control group (P < 0.01). On the other hand, a previous study of cross-organ sensitization shows that 64 and 58% viscerovisceral convergent neurons receiving noxious inputs from both colon and urinary bladder were identified in lumbosacral spinal cord (L6–S2) of rats with colitis and normal colon, respectively (35). The differences might be due to the different gastrointestinal inflammations, visceral organs, and spinal segments examined. Thus increased afferent inputs from an inflamed esophagus onto viscerovisceral convergent neurons could facilitate the excitability and responsiveness of individual neurons and elevate the visceroreceptive sensitivity to noxious cardiac input. Another possible source of convergence to spinal cord is that afferent fibers have branches from both the esophagus and heart. It has been showed that ∼15% of the neurons in the dorsal root ganglia at thoracolumber and lumbosacral spinal levels are double labeled by dyes injected in both the colon and urinary bladder in rats (8, 29, 30). These DRG neurons receive afferent inputs from both colon and urinary bladder and are believed to play a role in prespinal or peripheral pathway for cross-organ sensitization (30). However, to date, there is no study to examine whether visceral afferent fibers innervate both esophagus and heart. The higher incidence of convergent neurons after GER in the present study (Table 4) is consistent with sensitization of neurons in the dorsal horn; however, we do not have the data to distinguish between enhanced recruitment of afferents and spinal sensitization.
Somatic inputs.
Clinically, pain of visceral origin is referred to overlying somatic structures and often results in somatic hyperalgesia, also termed viscerosomatic referred pain. It is usually explained by the spinal convergence-projection theory based on the interaction of visceral and somatic afferent inputs onto viscerosomatic convergent neurons in the spinal dorsal horn (17). In the present study, 87.2% of neurons responding to IB in GER animals and 92.6% of neurons in the control group also received somatic input. Unexpectedly, no significant difference was found in somatic field location, properties, and sizes between GER and control groups, although the somatoreceptive (somatic-only) neurons with large or medium sizes field were more frequently observed in GER groups than in control groups. Apparently, acute esophageal inflammation did not induce a generalized sensitization of spinal esophagocardiac convergent neurons for both visceral and somatic afferent inputs. In contrast, in another study of cross-sensitization for spinal colorectal and urinary bladder inputs, colitis increased the proportion of lumbosacral viscerovisceral convergent and nonconvergent neurons with WDR properties and/or large somatic fields compared with the control group. In that model, sensitivity of spinal neurons to somatic input is increased (35). Once again, duration of inflammation as well as spinal segments activated could account for this difference.
Clinical implication.
It is well known that pain of visceral origin is frequently referred to overlying somatic structures and often results in somatic hyperalgesia. This phenomenon is also termed viscerosomatic referred pain. Primary visceral pain that originates in one injured internal organ is also referred secondarily to adjacent visceral domains, which is called viscerovisceral hyperalgesia or viscerovisceral cross-organ sensitization (21, 34, 35). For example, viscerovisceral pathological interactions and reflexes between the bowel and urinary bladder are suggested to be mediated by a peripheral afferent-afferent interaction and by central mechanism that process viscerovisceral convergent inputs from different visceral organs (30, 34, 35). Viscerovisceral cross talk not only occurs in pelvic and abdominal cavities but is also commonly manifested in the thoracic visceral organs. Physiologically, the esophagus and heart are linked by a variety of viscerovisceral reflexes, which are initiated with the activation of esophageal receptors and nerve endings in the cardiac coronary vessels and specific conducting system. In pathological conditions, these reflexes became more sensitive and more intense than normal so as to produce cardiac symptoms and disorders such as bradycardia, tachycardia, dysrhythmias, coronary angiospasm, and angina-like chest pain (3, 6, 7, 10, 31, 41, 50, 51). Therefore, there is a clinically significant interaction between cardiac disease and esophageal disorders. For example, GERD and CAD frequently coexist and may further interact to produce and/or worsen chest pain and other symptoms (12, 22, 26, 48, 49). The major finding of this study is that acute esophageal inflammation induced by gastric reflux in rats enhanced the visceroreceptive sensitivity of upper thoracic spinal neurons with afferent inputs from the heart. In other words, the central hypersensitivity resulting from acute esophagitis occurred in spinal neurons that responded to cardiac noxious stimulus. The cross-organ afferent-afferent interaction in single viscerovisceral convergent spinal neurons observed in the present study provided a cross-talk mechanism between the esophagus and heart. This suggests that there is an intraspinal neuronal link to chest pain perceived secondary to gastroesophageal reflux. In patients with noncardiac chest pain, increased central processing of sensory input after esophageal inflammatory stimulation may be in part responsible for patients with both GERD and myocardial ischemia. This theory may be applied to diagnostic and therapeutic approach to patients with GERD and CAD.
GRANTS
This study was supported by National Institutes of Health grants (NS-35471, HL-075524).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank for Dr. K. M. Garrett for helpful comments and G. M. Wienecke and D. Holston for excellent technical assistance.
REFERENCES
- 1. Albutaihi IA, DeJongste MJ, Ter Horst GJ. An integrated study of heart pain and behavior in freely moving rats (using fos as a marker for neuronal activation). Neurosignals 13: 207–226, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil 19: 681–691, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bennett JR, Atkinson M. The differentiation between oesophageal and cardiac pain. Lancet 2: 1123–1127, 1966 [DOI] [PubMed] [Google Scholar]
- 4. Brook RA, Wahlqvist P, Kleinman NL, Wallander MA, Campbell SM, Smeeding JE. Cost of gastro-oesophageal reflux disease to the employer: a perspective from the United States. Aliment Pharmacol Ther 26: 889–898, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Castell DO. Chest pain of undetermined origin: overview of pathophysiology. Am J Med 92: 2S–4S, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Chauhan A, Petch MC, Schofield PM. Effect of oesophageal acid instillation on coronary blood flow. Lancet 341: 1309–1310, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Chauhan A, Mullins PA, Taylor G, Petch MC, Schofield PM. Cardioesophageal reflex: a mechanism for “linked angina” in patients with angiographically proven coronary artery disease. J Am Coll Cardiol 27: 1621–1628, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder, and colon sensory innervation occurs at the primary afferent level. Pain 128: 235–243, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooke RA, Anggiansah A, Smeeton NC, Owen WJ, Chambers JB. Gastroesophageal reflux in patients with angiographically normal coronary arteries: an uncommon cause of exertional chest pain. Br Heart J 72: 231–236, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies HA, Page Z, Rush EM, Brown AL, Lewis MJ, Petch MC. Oesophageal stimulation lowers exertional angina threshold. Lancet 1: 1011–1014, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Dean BB, Crawley JA, Schmitt CM, Wong J, Ofman JJ. The burden of illness of gastro-oesophageal reflux disease: impact on work productivity. Aliment Pharmacol Ther 17: 1309–1317, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Dobrzycki S, Baniukiewicz A, Korecki J, Bachorzewska-Gajewska H, Prokopczuk P, Musial WJ, Kaminski KA, Dabrowski A. Does gastro-esophageal reflux provoke the myocardial ischemia in patients with CAD? Int J Cardiol 104: 67–72, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Drewes AM, Schipper KP, Dimcevski G, Petersen P, Andersen OK, Gregersen H, Arendt-Nielsen L. Multi-modal induction and assessment of allodynia and hyperalgesia in the human oesophagus. Eur J Pain 7: 539–549, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Euchner-Wamser I, Sengupta JN, Gebhart GF, Meller ST. Characterization of responses of T2-T4 spinal cord neurons to esophageal distension in the rat. J Neurophysiol 69: 868–883, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Euchner-Wamser I, Meller ST, Gebhart GF. A model of cardiac nociception in chronically instrumented rats: behavioral and electrophysiological effects of pericardial administration of algogenic substances. Pain 58: 117–1128, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Farrokhi F, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux. Oral Dis 13: 349–359, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 61: 143–167, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Frank L, Kleinman L, Ganoczy D, McQuaid K, Sloan S, Eggleston A, Tougas G, Farup C. Upper gastrointestinal symptoms in North America: prevalence and relationship to healthcare utilization and quality of life. Dig Dis Sci 45: 809–818, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Frye JW, Vaezi MF. Extraesophageal GERD. Gastroenterol Clin North Am 37: 845–858, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Garrison DW, Chandler MJ, Foreman RD. Viscerosomatic convergence onto feline spinal neurons from esophagus, heart and somatic fields: effects of inflammation. Pain 49: 373–382, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Giamberardino MA. Recent and forgotten aspects of visceral pain. Eur J Pain 3: 77–92, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Hewson EG, Dalton CB, Hackshaw BT, Wu WC, Richter JE. The prevalence of abnormal esophageal test results in patients with cardiovascular disease and unexplained chest pain. Arch Intern Med 150: 965–969, 1990 [PubMed] [Google Scholar]
- 23. Hobson AR, Aziz Q. Brain processing of esophageal sensation in health and disease. Gastroenterol Clin North Am 33: 69–91, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Hua F, Harrison T, Qin C, Reifsteck A, Ricketts B, Carnel C, Williams CA. c-Fos expression in rat brain stem and spinal cord in response to activation of cardiac ischemia-sensitive afferent neurons and electrostimulatory modulation. Am J Physiol Heart Circ Physiol 287: H2728–H2738, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Jaspersen D. Extra-esophageal disorders in gastroesophageal reflux disease. Dig Dis Sci 22: 115–119, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Liuzzo JP, Ambrose JA. Chest pain from gastroesophageal reflux disease in patients with coronary artery disease. Cardiol Rev 13: 167–173, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Locke GR. The epidemiology of gastroesophageal reflux disease. Gastroenterology 112: 1448–1456, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Malfertheiner P, Hallerback B. Clinical manifestations and complications of gastroesophageal reflux disease (GERD). Int J Clin Pract 59: 346–355, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon, and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18: 936–948, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience 149: 660–672, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mellow MH, Simpson AG, Watt L, Schoolmeester L, Haye OL. Esophageal acid perfusion in coronary artery disease. Induction of myocardial ischemia. Gastroenterology 85: 306–312, 1983 [PubMed] [Google Scholar]
- 32. Nagahama K, Yamato M, Kato S, Takeuchi K. Protective effect of lafutidine, a novel H2-receptor antagonist, on reflux esophagitis in rats through capsaicin-sensitive afferent neurons. J Pharm Sci 93: 55–61, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Nagahama K, Yamato M, Nishio H, Takeuchi K. Essential role of pepsin in pathogenesis of acid reflux esophagitis in rats. Dig Dis Sci 51: 303–309, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Qin C, Chandler MJ, Foreman RD. Esophagocardiac convergence onto thoracic spinal neurons: comparison of cervical and thoracic esophagus. Brain Res 1008: 193–197, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 129: 1967–1978, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Qin C, Farber JP, Foreman RD. Intraesophageal chemicals enhance responsiveness of upper thoracic spinal neurons to mechanical stimulation of esophagus in rats. Am J Physiol Gastrointest Liver Physiol 294: G708–G716, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Qin C, Malykhina AP, Thompson AM, Farber JP, Foreman RD. Cross-organ sensitization of thoracic spinal neurons receiving noxious cardiac input in rats with acute gastroesophageal reflux. Program No. 173.12/MM28. In: 2008 Neuroscience Meeting Planner (Online). Washington, DC: Society for Neuroscience, 2008. http://www.sfn.org/am2008/ [Google Scholar]
- 38. Qin C, Du JQ, Tang JS, Foreman RD. Bradykinin is involved in the mediation of cardiac nociception during ischemia through upper thoracic spinal neurons. Curr Neurovasc Res 6: 89–94, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Richter JE. Beyond heartburn: extraesophageal manifestations of gastroesophageal reflux disease. Am J Manag Care 7, Suppl 1: S6–S9, 2001 [PubMed] [Google Scholar]
- 40. Robinson DA, Calejesan AA, Wei F, Gebhart GF, Zhuo M. Endogenous facilitation: from molecular mechanisms to persistent pain. Curr Neurovasc Res 1: 11–20, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Roesler H. Esophageal reflex origin of myocardial infarction. Am J Med Sci 240: 159–162, 1960 [PubMed] [Google Scholar]
- 42. Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology 122: 1500–1511, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Sarkar S, Hobson AR, Furlong PL, Woolf CJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 281: G1196–G1202, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Sarkar S, Woolf CJ, Hobson AR, Thompson DG, Aziz Q. Perceptual wind-up in the human oesophagus is enhanced by central sensitisation. Gut 55: 920–925, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schofield PM, Whorwell PJ, Brooks NH, Bennett DH, Jones PE. Oesophageal function in patients with angina pectoris: a comparison of patients with normal coronary angiograms and patients with coronary artery disease. Digestion 42: 70–78, 1989 [DOI] [PubMed] [Google Scholar]
- 46. Sengupta JN. Esophageal sensory physiology. GI Motility (Online). Nature Publishing Group, 2006. http://www.nature.com/gimo/contents/pt1/full/gimo16.html
- 47. Singh S, Richter JE, Hewson EG, Sinclair JW, Hackshaw BT. The contribution of gastroesophageal reflux to chest pain in patients with coronary artery disease. Ann Intern Med 117: 824–830, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Shin YK, Sohn UD, Choi MS, Kum C, Sim SS, Lee MY. Effects of rutin and harmaline on rat reflux oesophagitis. Auton Autocoid Pharmacol 22: 47–55, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Svensson O, Stenport G, Tibbling L, Wranne B. Oesophageal function and coronary angiogram in patients with disabling chest pain. Acta Med Scand 204: 173–178, 1978 [DOI] [PubMed] [Google Scholar]
- 50. Wright RA, Miller SA, Corsello BF. Acid-induced esophagobronchial-cardiac reflexes in humans. Gastroenterology 99: 71–73, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Wright RA, McClave SA, Petruska J. Does physiologic gastroesophageal reflux affect heart rate or rhythm? Scand J Gastroenterol 28: 1021–1024, 1993 [DOI] [PubMed] [Google Scholar]


