Abstract
Expression of the GABAA π receptor has been described previously in the human endometrium in both luminal epithelium and stroma. Its expression is increased during decidualization in rodents and in the implantation window of human endometrium. Here we localized GABA π subunit receptor protein in human endometrium and identified regulators of gene expression and activation. GABAA π was localized to the cell surface, and expression increased during the window of embryo implantation in human endometrium. The well-differentiated human endometrial adenocarcinoma cell line Ishikawa was treated with progesterone and transfected with pcDNA-HOXA10, HOXA10 siRNA, or respective controls. GABAA π receptor mRNA expression was evaluated by real-time RT-PCR. Protein expression and localization were evaluated using immunofluorescence. GABAA π receptor mRNA expression was increased significantly after either progesterone treatment or HOXA10 transfection. Coadministration of progesterone along with HOXA10 transfection had no additional effect on the expression of GABAA π receptor mRNA over either agent alone. Blocking HOXA10 expression with siRNA prevented progesterone-induced GABAA π receptor mRNA expression. Additionally, either HOXA10 or progesterone independently caused increased translocation of the GABA receptor from the cytoplasm to the cell membrane. Translocation in response to progesterone was blocked with HOXA10 siRNA. Progesterone-induced GABAA π subunit receptor expression is likely mediated indirectly through progesterone's regulation of HOXA10 expression. Modification of subtype composition and translocation of the GABA receptor ion channel likely modulate endometrial receptivity. Whereas HOXA10 typically enhances the expression of progesterone-responsive genes, here HOXA10 expression leads to production of a less progestin-responsive GABA receptor subtype, likely buffering the effects of luteal phase progesterone on GABA receptor activity.
Keywords: homeobox A10, γ-aminobutyric acid
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian brain. Its effects are mediated via interactions with integral membrane proteins, the GABA receptors. These receptors are classified according to their pharmacological properties and mechanism of signal transduction. (7, 38, 46)
GABAA receptors are fast-acting, ligand-gated chloride ion channels that are allosterically regulated by compounds such as anxiolytics, barbiturates, anesthetics, and neurosteroids, including reduced derivatives of progesterone such as allopregnanolone. (7, 19, 25, 44) Several GABAA subunits have been cloned, many with several isoforms (1, 11, 31). They are also present in peripheral tissues outside of the brain, including the female reproductive tract, where the subunit composition of the GABAA receptor fluctuates in the rat uteri throughout pregnancy. (2, 12–14, 30) Isoform selection affects the sensitivity and responsiveness of the GABAA receptor to progesterone metabolites, which may help regulate parturition. (17).
Specifically, the GABAA π receptor is expressed in the brain and in several nonneuronal tissues, including the human endometrium and rat uterus (21). The subunit concentration has been found to vary throughout the cycle and gestation (23, 33). Receptor expression is upregulated during the window of implantation in well-characterized human endometrial biopsies. (23) The receptor's phenotypic localization and timing of expression have been suggested to play an important role in the generation of receptive endometrium.
Homeobox genes are a family of highly evolutionarily conserved transcriptional regulators termed HOX in humans and Hox in mice. (29) Several HOX/Hox genes regulate endometrial growth and development. Specifically, homeobox A10 (HOXA10) is a homeobox gene that is expressed in the human endometrial glands and stroma throughout the menstrual cycle (40). Estrogen and progesterone regulate endometrial expression of HOXA10. HOXA10 expression rises dramatically at the time of implantation, suggesting a role for HOXA10 in this process in humans. It is essential for fertility in mice. Targeted disruption of the Hoxa10 gene renders mice infertile due to defective endometrial receptivity and failed implantation. (4, 37) Hoxa10−/− embryos implant normally upon transfer to the uteri of pseudopregnant wild-type mice, and conversely, embryos from wild-type mice fail to implant in Hoxa10−/− mice (4, 37). Altering Hoxa10 expression in the adult murine endometrium affects the number of implantation sites, demonstrating a necessity for the maternal Hoxa10 (3). Transfection with Hoxa10 antisense yields decreased Hoxa10 expression, number of implantation sites, and litter size in murine uteri. Conversely, overexpression of Hoxa10 increases litter size (3). HOXA10 is likely to mediate a number of sex steroid effects by activation or repression of target genes, potentially including those encoding for the GABAA receptors.
Here we investigated the regulation of GABAA π receptor subunit expression and its functional activation through membrane translocation. Both HOXA10 and GABAA π subunit receptors are expressed in the endometrium at the time of embryonic implantation (21). They are both regulated and/or modulated by the sex steroid progesterone (45). A recent microarray-based study from our laboratory identified the GABAA π subunit as a potential Hoxa10 target gene in the murine endometrium (45). Here we demonstrate that not only was GABAA π subunit gene expression regulated by HOXA10, its membrane translocation was as well.
MATERIALS AND METHODS
Cell culture.
Ishikawa is a well-differentiated endometrial adenocarcinoma cell line that has previously been used to model human endometrial epithelial cells. Ishikawa cells express progesterone receptors and other markers of endometrial function (5, 20, 22, 26–28, 36, 39). HOX gene expression has previously been well characterized in these cells (10, 35, 42, 43). Ishikawa cells were maintained in 25-cm2 flasks, using Dulbecco's minimal essential medium supplemented with 10% (vol/vol) fetal bovine serum and 1% penicillin-streptomycin. Cells were maintained at 37°C in a humidified atmosphere (5% CO2 in air) and allowed to reach confluency. Cells were passaged by standard methods of trypsinization and plated on both glass microscope slides for immunofluorescence studies and on six-well plates for RNA analysis until they reached 75–80% confluence. The cells used for RNA extraction were treated with 10−6 M progesterone for 1 h. The cells used for immunofluorescence were treated with 10−6 M progesterone for 24 h. Each experiment was performed in triplicate and repeated four times.
Transfection.
Preconfluent (75–80%) Ishikawa cells on glass slides were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with 1.6 μg of either pcDNA-HOXA10 3.1 or empty pcDNA3.1. Preconfluent (75–80%) Ishikawa cells in six-well plates were similarly transfected with 4 μg of either pcDNA-HOXA10 3.1 or empty pcDNA3.1, siRNA control, or 20 μM HOXA10 siRNA. pcDNA-HOXA10 3.1 expresses HOXA10 constitutively at high levels, as described previously, whereas pcDNA3.1 served as the respective control (10, 32, 35, 42, 43). HOXA10 siRNA was synthesized by siGENOME duplex (Dharmacon, Lafayette, CO). An unrelated siRNA served as a control. The cells were then washed with cold phosphate-buffered saline after 24 h and treated with serum-free, phenol red-free medium for 24 h. The cells were then treated with either 10−6 M progesterone or vehicle control for either 1 or 24 h for RNA or immunofluorescence studies, respectively.
Real-time RT-PCR.
RNA from cells was isolated using Trizol (Life Technologies, Carlsbad, CA) pursuant to the manufacturer's guidelines. One microgram of total RNA was reverse transcribed in 20 μl of reaction mixture containing l0 mM each of dATP, dCTP, dGTP, and dTTP, 20 pmol oligo(dT), 40 μm/μl ribonuclease inhibitor, 10 μm/μl avian myeloblastosis virus-reverse transcriptase, and 10 μm/μl avian myeloblastosis-reverse transcriptase buffer for 30 min at 61°C. The GABAA π receptor intron-spanning primers were selected, using the primer selection program Primer3 developed by the Whitehead Institute for Biomedical Research. Quantitative real-time RT-PCR was performed using the Light cycle SYBR Green RT-PCR kit from Roche. The Light Cycler monitored the increasing fluorescence of PCR products during amplification. The samples were quantification adjusted to the quantitative expression of β-actin from the same samples. Melting curve analysis was conducted to determine the specificity of the amplified products and to ensure the absence of primer-dimer formation. All products obtained yielded the predicted melting temperature. Construction of a standard curve allowed quantification. At least three independent experiments were conducted. Paired and unpaired t-tests were applied as appropriate. Data are presented as means ± SE. A value of P < 0.05 was considered significant.
Immunocytochemistry.
Tissue was obtained by endometrial biopsy from 10 normal reproductive-age women at the time of tubal ligation under a protocol that was approved by the Yale University School of Medicine Human Investigations Committee. Samples were fixed with 4% paraformaldehyde for 15 min at room temperature and permeabilized by a 50–50% mixture of ethanol-acetone for 10 min followed by 0.05% Triton X-100 in phosphate-buffered saline (PBS) for 2 min at room temperature. They were then washed with PBS with 2% Tween. Nonspecific binding sites were then blocked by incubation with 0.5% bovine serum albumin (BSA) in PBS for 15 min. Samples were then incubated overnight at 4°C with the primary antibodies [GABAA π receptor (sc-21338) from Santa Cruz Biotechnology (Santa Cruz, CA) and cytokeratin 5/6/18 (VP-C418) from Vector Laboratories (Burlingame, CA)]. This step was omitted in the negative control samples. The next day, the cells were incubated with the appropriate fluorescence-conjugated secondary antibodies in PBS-0.5% BSA [goat α-rabbit IgG (FI-1000) and horse α-mouse (TI-2000); Vector Laboratories] for 90 min at room temperature, washed again, and DNA stained with TO-PRO-3 [(1:10,000 for 10 min) and mounted with PBS-90% glycerol-1% p-phenylenediamine] or Dapi Vectashield with 4′,6 diamidino-2-phenylindole (Vector laboratories, Burlingame, CA). Samples were then examined by confocal laser-scanning microscopy.
Confocal laser-scanning microscopy.
Confocal microscopy was performed using a Zeiss LSM 510NLO/Multiphoton microscope. This microscope uses an argon laser and two HeNe lasers for excitation (at 458, 477, 488, 514, 543, and 633 nm) and also has three detection channels. Optical tomography was performed at 0.5-μm intervals using a 40- and 60-fold oil immersion objective. All observations were made on single Ishikawa cells in monolayers. Treated slides were divided into four quadrants. Each quadrant was evaluated by counting the number of cells with peripheral localization of GABAA π receptors, as evident by immunofluorescent staining per high-power field. Statistical significance was measured using Student t-tests.
RESULTS
GABA π subunit expression is increased at the window of embryo implantation in human endometrium.
Immunohistochemistry was used to identify the timing of GABA π subunit expression in human endometrium through the menstrual cycle. The subunit was expressed throughout the menstrual cycle in both epithelial and stromal cells. Expression was increased in the window of embryo implantation in human endometrium, as demonstrated in Fig. 1. The average number of endometrial cells expressing high levels of GABA π subunit protein was 2% in the proliferative phase; the percentage increased to 93% in the midluteal phase (P < 0.006). Increased expression corresponded to the known time of acquisition of endometrial maturation to allow embryo implantation.
Fig. 1.
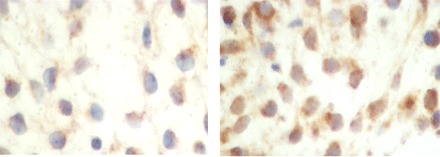
Immunohistochemical analysis of γ-aminobutyric acid (GABA)A π receptor staining in endometrium on days 17 and 24, corresponding to the time of transition into the window of implantation. Day 17 (left) shows minimal stromal staining for GABAA π receptor, and day 24 (right) shows increased positive staining for the receptor.
Progesterone induces π-subunit expression in endometrial cells through a HOXA10-mediated pathway.
A significant increase in π-subunit receptor mRNA levels was seen in endometrial cells transfected with HOXA10 and treated with progesterone or the combination of the two compared with corresponding control vector-transfected or vehicle-treated samples, respectively (Fig. 2). π-Subunit receptor mRNA level was increased approximately threefold after HOXA10 transfection upon normalization with β-actin (P = 0.001). Similarly, subunit mRNA was increased approximately threefold after progesterone treatment (P = 0.022) and threefold after coadministration of both progesterone and HOXA10 (P = 0.014). No additive effect was observed in π-subunit receptor mRNA levels when progesterone was added to the HOXA10-transfected cells (P = 0.294) compared with progesterone-only-treated cells, suggesting a common signal transduction pathway.
Fig. 2.
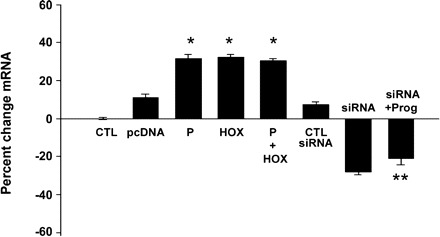
Regulation of GABAA π receptor mRNA. There was a significant increase in mRNA levels of the receptor subunit in cells treated with progesterone (P), transfected with pcDNA-HOXA10 (HOX), or the combination of the two. There was a significant decrease in the receptor mRNA levels in cells transfected with HOXA10 siRNA (siRNA). Simultaneous treatment with P and HOXA10 siRNA (siRNA + Prog) blocked the P effect on subunit expression. *P < 0.05 vs. control (CTL); **P < 0.01 vs. P.
To address the significance of the HOXA10-mediated positive regulation of the GABA receptor, cellular levels of HOXA10 were inhibited by an siRNA knockdown construct in Ishikawa cells. Cells were treated with HOXA10 siRNA for 24 h. This resulted in significant inhibition of mRNA levels of the π-subunit compared with controls. Cells treated with nonsilencing siRNA constructs showed no significant change in the receptor levels. π-Subunit receptor levels were decreased from basal levels when cells were transfected with HOXA10 siRNA constructs (P = 0.009). Progesterone treatment of cells along with transfection of HOXA10 siRNA also resulted in significantly decreased induction of π-subunit expression in Ishikawa cells, indicating that the effect of progesterone requires HOXA10.
Progesterone induction of HOXA10 expression leads to membrane translocation of the GABAA π receptor.
To assess the localization pattern of GABAA π receptors within the cells, immunocytochemical analysis was performed on the Ishikawa cells and assessed by confocal microscopy. A significantly increased number of cells displayed peripheral localization of π-subunit receptor expression when cells were treated with progesterone compared with corresponding vehicle-treated cells (Fig. 3, A and B). Blockade of HOXA10 expression with siRNA resulted in elimination or significant reduction of GABAA π receptor membrane translocation even in the presence of progesterone (Fig. 3C). Cells transfected with HOXA10 also demonstrated enhanced membrane translocation of the receptor in the absence of progesterone (Fig. 4). Quantification of the number of cells showing membrane translocation is displayed in Fig. 5. Cells treated with progesterone (P = 0.002), transfected with HOXA10 (P = 0.001), or treated with the combination of the two (P = 0.008) each showed significant membrane translocation compared with vehicle or vector-transfected cells. Similarly, HOXA10 siRNA blocked the progesterone-mediated membrane translocation.
Fig. 3.
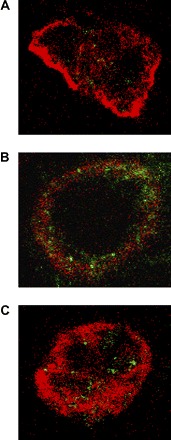
Immunocytochemical visualization of GABAA π receptor localization on confocal laser microscopy and regulation by progesterone. Cell periphery was stained with Texas red against cytokeratin 5/6/18. GABAA π receptors were stained with fluorescein. A: vehicle control-treated cells with nonlocalized receptors. B: treatment with progesterone leads to an increase in localization of the receptor to the cell membrane. C: treatment of the cells with progesterone and HOXA10 siRNA prevents peripheral translocation of the receptors.
Fig. 4.
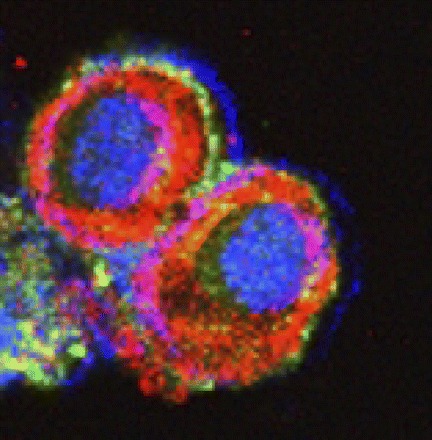
Immunocytochemical visualization of GABAA π receptor localization on confocal laser microscopy. Cell periphery was stained with Texas red, using an antibody against cytokeratin 5/6/18. GABAA π receptors were stained with fluorescein. The nucleus was stained with Topro-3. Cells were transfected with pcDNA-HOXA10, leading to greatly enhanced peripheral localization of the receptor.
Fig. 5.
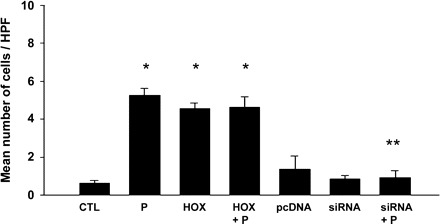
Quantification of GABAA π receptor peripheral localization. Displayed are the mean numbers of cells with peripheralization of the receptor per field. Treatment with P (P = 0.002) or transfection with HOX (P = 0.001) each led to significantly increased peripheralization. The P-induced peripheralization was blocked with HOXA10 siRNA (siRNA), indicating a role for HOXA10 in mediating the effect of P. *P < 0.01 vs. CTL; **P < 0.01 vs. P treatment. HPF, high-power field.
DISCUSSION
In addition to its role as a neurotransmitter, GABA may perform multiple functions in nonneuronal tissues. GABAA receptor activity depends on a number of variables; these include subunit arrangement and isoform selection, concentration, and timing of expression as well as localization to the plasma membrane. The human endometrium expresses the GABAA π receptor (16, 24). The expression of this receptor is known to be modulated by progesterone and its metabolite allopregnanolone (16, 17, 24). The receptor is upregulated during the window of implantation in human endometrium (1, 11, 31) and is thought to play a role in endometrial receptivity. The expression of this receptor, as well as its membrane translocation, is likely to be involved in initiating and maintaining a receptive endometrium.
HOX genes encode highly evolutionarily conserved transcription factors that have evolved to serve an essential role in endometrial receptivity in mammals (8, 15). Endometrial HOXA10 expression fluctuates with the menstrual cycle, which is regulated by estradiol and progesterone levels. This results in an upregulation of the gene in the midluteal phase, a period corresponding to the time of implantation (36, 40). Hoxa10 expression is necessary for endometrial receptivity to embryo implantation in mice (3, 4, 37). Similarly, women with low implantation rates demonstrate diminished endometrial HOXA10 expression (6, 9, 34, 41).
There are no previous reports identifying regulators of GABAA π receptor expression or activation. Here we established that HOXA10 regulates GABAA π receptor mRNA expression and, surprisingly, also regulates its membrane translocation. HOX genes encode transcriptional regulators that typically bind regulatory elements of target genes and influence RNA synthesis. Due to the rapid induction of expression, it is likely that HOXA10 directly regulates the expression of GABA receptor mRNA. The receptor membrane localization occurs only after a delay of several hours; therefore, it is likely that this function of Hoxa10 is indirect. HOXA10 likely induces the expression of proteins involved in translocation to the plasma membrane. Future efforts will focus on delineating target chaperone proteins necessary for protein mobilization.
The function of GABA receptors in the uterus is still incompletely characterized. In the central nervous system, allopregnanolone modulates receptor activity and neuronal inhibition by altering the frequency and duration of GABAA channel opening. The assembly of the π-subunit into the GABA receptor reduces sensitivity to allopregnanolone. Suppression of GABA activity in the uterus is likely beneficial, since enhanced gabagenergic tone is associated with contractions and labor. Therefore, subunit selection may enable the tolerance of the dramatic increase in progesterone and allopregnanolone in the luteal phase. Reduced sensitivity to allopregnanolone may facilitate implantation by attenuating GABA signaling and inducing quiescence at the time of implantation.
Although progesterone action is generally considered to be essential for endometrial differentiation and endometrial receptivity, not all of the myriad effects of progesterone necessarily promote implantation. HOXA10 is known to enhance progesterone action on the expression of many genes. However, here it likely buffers the effects of increased progesterone on this signal transduction pathway. We identify a novel role for HOXA10 as both a positive and negative modulator of progesterone responsiveness in the endometrium.
GRANTS
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Grant HD-0388679 and through cooperative agreement U54-HD-052668 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Andriamampandry C, Taleb O, Kemmel V, Humbert JP, Aunis D, Maitre M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB J 21: 885–895, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Apud JA, Tappaz ML, Celotti F, Negri-Cesi P, Masotto C, Racagni G. Biochemical and immunochemical studies on the GABAergic system in the rat fallopian tube and ovary. J Neurochem 43: 120–125, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Bagot CN, Troy PJ, Taylor HS. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther 7: 1378–1384, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 122: 2687–2696, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Castelbaum AJ, Ying L, Somkuti SG, Sun J, Ilesanmi AO, Lessey BA. Characterization of integrin expression in a well differentiated endometrial adenocarcinoma cell line (Ishikawa). J Clin Endocrinol Metab 82: 136–142, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 88: 238–243, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 21: 1–56, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev 27: 331–355, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Daftary GS, Taylor HS. Hydrosalpinx fluid diminishes endometrial HOXA10 expression: a mechanism for decreased implantation. Fertil Steril 78: 577–580, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol 16: 571–579, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Da Settimo F, Taliani S, Trincavelli ML, Montali M, Martini C. GABA A/Bz receptor subtypes as targets for selective drugs. Curr Med Chem 14: 2680–2701, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Erdö SL, Lapis E. Presence of GABA receptors in rat oviduct. Neurosci Lett 33: 275–279, 1982 [DOI] [PubMed] [Google Scholar]
- 13. Erdö SL, László A, Kiss B, Zsolnai B. Presence of gamma-aminobutyric acid and its specific receptor binding sites in the human term placenta. Gynecol Obstet Invest 20: 199–203, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Erdö SL, László A, Szporny L, Zsolnai B. High density of specific GABA binding sites in the human fallopian tube. Neurosci Lett 42: 155–160, 1983 [DOI] [PubMed] [Google Scholar]
- 15. Eun Kwon H, Taylor HS. The role of HOX genes in human implantation. Ann NY Acad Sci 1034: 1–18, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Follesa P, Concas A, Porcu P, Sanna E, Serra M, Mostallino MC, Purdy RH, Biggio G. Role of allopregnanolone in regulation of GABA(A) receptor plasticity during long-term exposure to and withdrawal from progesterone. Brain Res Brain Res Rev 37: 81–90, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol 57: 1262–1270, 2000 [PubMed] [Google Scholar]
- 18. Fujii E, Mellon SH. Regulation of uterine gamma-aminobutyric acid(A) receptor subunit expression throughout pregnancy. Endocrinology 142: 1770–1777, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Gavish M. Hormonal regulation of peripheral-type benzodiazepine receptors. J Steroid Biochem Mol Biol 53: 57–59, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Hata H, Kuramoto H. Immunocytochemical determination of estrogen and progesterone receptors in human endometrial adenocarcinoma cells (Ishikawa cells). J Steroid Biochem Mol Biol 42: 201–210, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem 272: 15346–15350, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Holinka CF, Gurpide E. Growth-promoting effects of progesterone in a human endometrial cancer cell line (Ishikawa-Var I). J Steroid Biochem Mol Biol 43: 635–640, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Kao LC, Tulac S, Lobo S, Imani B, Yang IP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143: 2119–2138, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kaura V, Ingram CD, Gartside SE, Young AH, Judge SJ. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol 17: 108–115, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lan NC, Chen JS, Belelli D, Pritchett DB, Seeburg PH, Gee KW. A steroid recognition site is functionally coupled to an expressed GABA(A)-benzodiazepine receptor. Eur J Pharmacol 188: 403–406, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Lessey BA, Ilesanmi AO, Castelbaum AJ, Yuan L, Somkuti SG, Chwalisz K, Satyaswaroop PG. Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the alpha1 integrin. J Steroid Biochem Mol Biol 59: 31–39, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Littlefield BA, Gurpide E, Markiewicz L, McKinley B, Hochberg RB. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells: estrogenic action of delta 5 adrenal steroids. Endocrinology 127: 2757–2762, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Markiewicz L, Gurpide E. Estrogenic and progestagenic activities of physiologic and synthetic androgens, as measured by in vitro bioassays. Methods Find Exp Clin Pharmacol 19: 215–222, 1997 [PubMed] [Google Scholar]
- 29. McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell 68: 283–302, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Murashima YL, Kato T. Distribution of gamma-aminobutyric acid and glutamate decarboxylase in the layers of rat oviduct. J Neurochem 46: 166–172, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Neelands TR, Macdonald RL. Incorporation of the pi subunit into functional gamma-aminobutyric Acid(A) receptors. Mol Pharmacol 56: 598–610, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Penna I, Du H, Ferriani R, Taylor HS. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Mol Hum Reprod 14: 613–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quezada M, Henríquez S, Vargas M, Cardenas H, Tapia A, Rios M, Salvatierra AM, Orihuela PA, Croxatto HB, Velasquez L. Proenkephalin A and the gamma-aminobutyric acid A receptor pi subunit: expression, localization, and dynamic changes in human secretory endometrium. Fertil Steril 86: 1750–1757, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakkas D, Lu C, Zulfikaroglu E, Neuber E, Taylor HS. A soluble molecule secreted by human blastocysts modulates regulation of HOXA10 expression in an epithelial endometrial cell line. Fertil Steril 80: 1169–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Sarno JL, Kliman HJ, Taylor HS. HOXA10, Pbx2, and Meis1 protein expression in the human endometrium: formation of multimeric complexes on HOXA10 target genes. J Clin Endocrinol Metab 90: 522–528, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374: 460–463, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Sieghart W. Pharmacology of benzodiazepine receptors: an update. J Psychiatry Neurosci 19: 24–29, 1994 [PMC free article] [PubMed] [Google Scholar]
- 39. Tada A, Sasaki H, Nakamura J, Yoshihama M, Terashima Y. Aromatase activity and the effect of estradiol and testosterone on DNA synthesis in endometrial carcinoma cell lines. J Steroid Biochem Mol Biol 44: 661–666, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 101: 1379–1384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor HS, Bagot C, Kardana A, Olive DL, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14: 1328–1331, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab 84: 1129–1135, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Troy PJ, Daftary GS, Bagot CN, Taylor HS. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol 23: 1–13, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veleiro AS, Burton G. Structure-activity relationships of neuroactive steroids acting on the GABAA receptor. Curr Med Chem 16: 455–472, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Vitiello D, Pinard R, Taylor HS. Gene expression profiling reveals putative HOXA10 downstream targets in the periimplantation mouse uterus. Reprod Sci 15: 529–535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wellendorph P, Bräuner-Osborne H. Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br J Pharmacol 156: 869–884, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


