Abstract
Genomewide association studies have linked a polymorphism in the zinc transporter 8 (Znt8) gene to higher risk of developing type 2 diabetes. Znt8 is highly expressed in pancreatic β-cells where it is involved in the regulation of zinc transport into granules. However, Znt8 is also expressed in other tissues including α-cells, where its function is as yet unknown. Previous work demonstrated that mice lacking Znt8 globally were more susceptible to diet-induced obesity (Lemaire et al., Proc Natl Acad Sci USA 106: 14872–14877, 2009; Nicolson et al., Diabetes 58: 2070–2083, 2009). Therefore, the main goal of this study was to examine the physiological impact of β-cell-specific Znt8 deficiency in mice during high-fat high-calorie (HFHC) diet feeding. For these studies, we used β-cell-specific Znt8 knockout (Ins2Cre:Znt8loxP/loxP) and whole body Znt8 knockout (Cre-:Znt8−/−) mice placed on a HFHC diet for 16 wk. Ins2Cre:Znt8loxP/loxP mice on HFHC diet had similar body weights throughout the study but displayed impaired insulin biosynthesis and secretion and were glucose intolerant compared with littermate control Ins2Cre mice. In contrast, Cre-:Znt8−/− mice became remarkably obese, hyperglycemic, hyperinsulinemic, insulin resistant, and glucose intolerant compared with littermate control Cre- mice. These data show that β-cell Znt8 alone does not considerably aggravate weight gain and glucose intolerance during metabolic stress imposed by an HFHC diet. However, global loss of Znt8 is involved in exacerbating diet-induced obesity and resulting insulin resistance, and this may be due to the loss of Znt8 activity in a tissue other than the β-cell. Thus, our data suggest that Znt8 contributes to the risk of developing type 2 diabetes through β-cell- and non-β-cell-specific effects.
Keywords: zinc transporter 8, obesity, slc30a8
zinc and zinc transporters have been found in a wide variety of tissues. In pancreatic β-cells, insulin is crystalized with zinc, forming dense core granules (36). Studies have shown high expression of zinc transporter 8 (Znt8) on insulin granules (10, 63) and further suggest that Znt8 is the primary transporter of zinc into these structures (39). Interestingly, studies have correlated a Znt8 deficiency-dependent defect in zinc transport into insulin granules with abnormal crystalization of insulin (34, 39, 63). In humans, genome-wide association studies have demonstrated a link between a nonsynonymous polymorphism in the gene coding for Znt8 (SLC30A8) and type 2 diabetes (48, 49, 52, 54, 65). In addition, autoantibodies to Znt8 have been found in type 1 diabetic patients (61). Together, these studies outline a role for Znt8 in the pathophysiology of both type 1 and type 2 diabetes.
Two mutant mouse models were used to characterize the function of Znt8 in β-cells (34, 39, 45, 63). Whole body Znt8 knockout (Cre-:Znt8−/−) mice have elevated fasting blood glucose and impaired glucose tolerance, which was correlated with lower insulin secretion in vivo (39). β-Cells from Cre-:Znt8−/− mice have reduced granular zinc content and fewer dense core insulin granules. However, two other studies have shown that global deletion of Znt8 does not impair glucose tolerance in male transgenic mice (34, 45). Since Znt8 mRNA was shown to be expressed in human adipose tissue (53) and blood lymphocytes (43) as well as at the protein level in thyroid follicles (37), the adrenal cortex, and α- (63) and pancreatic polypeptide islet cells (56), a β-cell-specific knockout mouse model (Ins2Cre:Znt8loxP/loxP) was generated to more precisely define the role of Znt8 in β-cells (63). Specific deletion of Znt8 in β-cells was associated with glucose intolerance, reduced first-phase glucose-stimulated insulin secretion in vitro, and reduced insulin processing enzyme transcripts in islets (63).
It has been shown that obesity is tightly linked to type 2 diabetes, as it can be causative of or exacerbatory to the disease process. During obesity, adipose tissue increases its release of cytokines such as TNF-α or IL-6 (51, 60), nonesterified fatty acids (7, 46), and hormones, which overall contribute to insulin resistance (22). β-Cells compensate for this insulin resistance by increasing insulin secretion, thus creating metabolic stress. Data on the role of Znt8 in conditions of metabolic stress are scarce. Genome-wide association studies conducted in Asian and Caucasian populations detected an association between the risk allele rs13266634 carried by the Znt8 gene and body mass index (9, 64). Our initial studies as reported in Nicolson et al. (39) showed that Cre-:Znt8−/− mice fed with a high-fat high-calorie (HFHC) diet display greater body weight gain, higher fasting insulin level, and blood glucose, as well as glucose intolerance and insulin resistance. Another study also revealed that Cre-:Znt8−/− mice fed an HFHC diet were at greater risk of being glucose intolerant and diabetic (34). Nevertheless, whether deficiency of Znt8 specifically in β-cells confers a greater risk for developing diabetes during HFHC dietary intake remained unknown.
Therefore, in the present study we aimed to identify the role of Znt8 in β-cells during metabolic stress induced by HFHC feeding. In conjunction with the Cre-:Znt8−/− mice, we fed Ins2Cre:Znt8loxP/loxP mice an HFHC diet and performed in vivo and in vitro characterization of their phenotypes.
MATERIALS AND METHODS
Animal care and generation of Znt8 knockout mice.
The Animal Care Committee at the University of Toronto approved all experiments. Animals were handled according to the guidelines of the Canadian Council of Animal Care. The Cre-:Znt8−/− mouse model was generated by Genoway (France) using the following strategy: a Znt8 loxP/loxP mouse line (hybrid C57BL/6J/129sv backcrossed 6 times onto C57BL/6J mice) was bred to a CMV promoter-driven Cre recombinase mouse line (C57BL/6J) to excise the LoxP flanked genomic region (exon 1 of Znt8) in all cells of the animals. Then, whole body Znt8KO mice (Cre+/+Znt8−/−) were backcrossed onto C57BL/6J mice three times. Over the course of the backcrossing, offspring showing lack of Cre expression and heterozygous Znt8 (Znt8+/−) were kept. We interbred these heterozygous mice. Cre-Znt8+/+ offspring were designated as Cre- controls. From the same litter, Cre- and homozygous Znt8−/− mice were used as whole body Znt8KO mice and termed Cre-:Znt8−/−. Therefore, Cre- mice and Cre-:Znt8−/− mice were littermates and had the same genetic background (39). Our β-cell-specific Znt8KO mouse model was generated by crossing TgN(Ins2-Cre)25Mgn mice (maintained on an hybrid C57BL/6/129J background) to Znt8loxP/loxP mice (hybrid C57BL/6J/129sv backcrossed six times onto C57BL/6J mice). Then, Ins2Cre+/− and Znt8+/loxP were interbred for two generations. Offspring expressing the Cre transgene alone were used as control mice (Ins2Cre). From the same litters, offspring expressing the Cre transgene and Znt8loxP/loxP were used as β-cell-specific Znt8KO mice and termed Ins2Cre:Znt8loxP/loxP. Therefore, control Ins2Cre mice and Ins2Cre:Znt8loxP/loxP mice were littermates and had the same mixed genetic background. To generate our inducible β-cell-specific Znt8KO mouse model, Znt8loxP/loxP mice were crossed to Pdx1CreER mice, which express a tamoxifen-inducible Cre recombinase driven by the insulin gene transcription factor pancreatic and duodenal homeobox 1 (Pdx1) (16, 18). Heterozygous offspring were interbred to generate homozygous-inducible β-cell-specific Znt8KO mice and termed Pdx1CreER:Znt8loxP/loxP. Pdx1CreER:Znt8loxP/loxP mice were crossed for three generations. Mice were genotyped using tail DNA and standard multiplex PCR using flox and cre primers (Fig. 1B). To induce Znt8 gene ablation in Pdx1CreER:Znt8loxP/loxP mice (Fig. 1A), at 5 wk of age, tamoxifen (125 mg/kg) or vehicle (5% ethanol-corn oil, Sigma) was injected intraperitoneally three times every other day (16). The tamoxifen stock solution was prepared by initially soaking the tamoxifen in alcohol. Then 50 mg/ml stock solution of tamoxifen was prepared in 5% ethanol-corn oil at 60°C in a water bath. The solution was shaken vigorously until complete dissolution and filtered with a 0.2-μm syringe filter. Before each injection, tamoxifen stock solution aliquots were thawed at 60°C. All the mice used in our study were age matched and male.
Fig. 1.
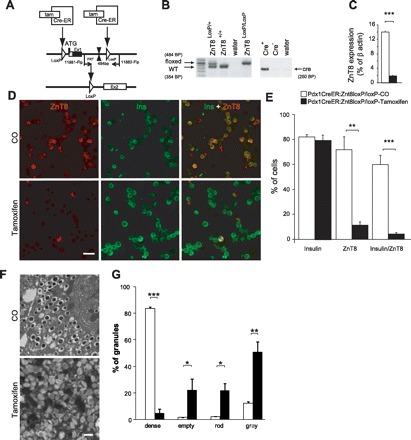
Acute zinc transporter 8 (Znt8) gene deletion in Pdx1Cre:ERZnt8LoxP/LoxP mice. A: tamoxifen (125 mg/kg, 3 injections every other day) was used to trigger deletion of Znt8 through the CreER system driven by Pdx1 promoter. Tamoxifen activates CreER, promoting translocation of CreER into the nucleus. Then, Cre induces recombination between LoxP sites flanking exon 1 of SLC30A8, leading to excision of the start codon and inhibiting the expression of Znt8. B: genotyping strategy used to identify Pdx1Cre:ERLoxP/LoxP mice. C: qPCR analysis of the expression of Znt8 in isolated islets of corn oil (CO) and tamoxifen-injected mice (***P < 0.001, n = 3). D: immunohistochemistry experiments were performed on dispersed islet cells obtained from CO and tamoxifen-injected mice labeled for insulin and Znt8. E: % of cells labeled were calculated and summarized (n = 3). Scale bar, 25 μm. F and G: granule morphological analysis of β-cells in CO- and tamoxifen-injected Pdx1CreERLoxP/LoxP mice (n = 3). F: representative electron micrographs (scale bar, 500 nm). G: quantification of different types of β-cell granules (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001.
High-fat diet feeding.
From 6 to 22 wk of age, mice were fed an HFHC diet (kcal%, protein 20, carbohydrate 35, fat 45; D12451 Research Diets). Weekly body weights were monitored. Food consumption of Pdx1CreER:Znt8loxP/loxP mice was calculated weekly by weighing total food amount available for each cage. After 16 wk on the diet, all mice were euthanized for in vitro experiments.
Transmission electron microscopy.
Freshly isolated islets were prepared, and images were acquired as previously described (39). Samples were observed under a Philips CM100 electron microscope operating at 75 kV. Dense core, empty, light core (gray), and atypical (rod-shaped) granules were manually counted and quantified (63).
Immunostaining and confocal microscopy.
Islets were isolated and dispersed as previously described (19, 20). Dispersed islet cells were immunostained for Znt8 and insulin, and images were acquired on a Zeiss confocal microscope using the LSM510 software package as previously described (63). Pancreas and fat were sectioned and labeled with an anti-rat Znt8 polyclonal antibody (1:1,000 dilution) as previously described (63).
Quantitative real-time PCR.
Quantitative real-time PCR (qPCR) analysis was performed as previously described (19). Primers were designed using Primer Express version 2.0 software (Applied Biosystems) and are listed in Table 1. Data were normalized to mouse β-actin mRNA.
Table 1.
PCR primer sequences used for mouse genotyping and qPCR
| Gene | Forward (5′->3′) | Reverse (5′->3′) | Accession Number |
|---|---|---|---|
| Znt1 | ATTCTTCTACAAACTGTCCCTAAGCAA | ACTTCCTCAACGCCGTCAAC | NM_009579 |
| Znt2 | ACAGAGCCTGGCCATCATG | GAGGGCGAAGAGGCTAATGAG | NM_001039677 |
| Znt3 | TCACTGGCATCCTCCTGTACCT | GGCACCCGCCTCAATG | NM_011773 |
| Znt4 | AATGGACCCCTGTGACAACTG | CGATGGTCAGCCTGGTCTTC | NM_011774 |
| Znt5 | GCATATGTCCGGCCTGAGTAC | TTGCAGTATTTCAGGGATTCCA | NM_022885 |
| Znt6 | AGCCCGGTTATTCTTCTGAACA | GTATGGTGTGTGTCCGCGATT | NM_144798 |
| Znt7 | CCTCTTTAATGGTGCTCTAGATCACA | CGTGCTTGGCTTCGTGACT | NM_023214 |
| Znt8 | AAGCCTGACTACAAAATTGCTGATC | GACGGTGCTGGCCAAAAC | NM_172816 |
| Znt9 | GATCCGAGTACAAATGTTATTCTATTGG | GGAGGTAAGGCCCATGCA | NM_178651 |
| Znt10 | GGAGCCAACATCTTGTTAGACAAG | GTGACCGTGCACCACTTCTG | NM_001033286 |
| Kcnj11 | GACATCCCCATGGAGAATGG | TCGATGACGTGGTAGATGATGAG | NM_010602 |
| β-Actin | CTGAATGGCCCAGGTCTGA | CCCTGGCTGCCTCAACAC | NM_007393 |
| Flox | AGTTATTGACTGAACACACCTATCTTATGTCCTGC | GCTATATACTCTTCCACTCAGCTACATCGCTACC | NT_039621.7 |
| Cre | GGCAGTAAAAACTATCCAGCAA | GTTATAAGCAATCCCCAGAAATG | AB542060 |
| Human Znt8 | TGACTGGCGTGCTAGTGTACCT | ACAGTCGCCTGGATCTGGTAAT | NM_173851.2 |
| Human β-actin | TGAGCTGCGTGTGGCTCCC | AGGGATAGCACAGCCTGGATAGCA | NM_001101.3 |
Znt, zinc transporter.
Oral glucose tolerance test (OGTT).
Following a 6-h fast, glucose (1.5 g/kg body wt) was given by oral gavage, and blood glucose was measured at 0, 10, 20, 30, 60, and 120 min from tail vein blood with a glucometer. Blood was centrifuged at 8,000 rpm for 10 min at 4°C. Supernatant (plasma) was used to measure insulin. Area under the curve was calculated using GraphPad Prism software.
Insulin tolerance test (ITT).
Following a 4-h fast, insulin (0.75 IU/kg body wt) was injected intraperitoneally, and plasma glucose was measured at 0, 15, 30, 60, and 120 min from tail vein blood with a glucometer.
Glucagon, insulin, and proinsulin measurements.
Blood was collected from fed or 16-h-fasted mice from their tail vein. Plasma insulin and plasma proinsulin were measured using ELISA kits from ALPCO diagnostics and Mercodia (Sweden), respectively. Islet insulin content and plasma glucagon were measured by radioimmunoassay (RIA) kits from Millipore, Canada. HOMA-IR (homeostasis model assessment of insulin resistance) was calculated using the formula: HOMA-IR (mmol/l × μU/ml) = fasting glucose (mmol/l) × fasting insulin (μU/ml)/22.5.
Islet morphological analysis.
Pancreatic islet morphology was analyzed as described in previous reports (32). Results were normalized to whole slice area (μm2). Islet number per slice was manually counted and normalized to whole slice area (μm2).
Zinc content measurement.
Cell zinc content was measured as previously described (39). Dispersed islet cells were seeded on coverslips and loaded with 2 μM Zinquin (Mellitech) for 50 min at 37°C in Kreb's ringer buffer containing (in mM) 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 NaHCO3, 1 glucose, and 10 HEPES, pH 7.4. Cells were washed and imaged using an Olympus BX51W1 fluorescent microscope controlled with Image Master3 software (PTI) with a high-speed monochromator (PTI, Lawrenceville, USA). Zinquin emission was monitored using 365-nm excitation, 375-nm beam splitter, and 385-nm long-pass filter.
Insulin and glucagon secretion.
Islets were isolated as previously described (19, 20). Fresh isolated mouse islets (20/vial) were preincubated for 60 min in a glucose-free Krebs-Ringer-HEPES buffer [125 mM NaCl, 5.9 mM KCl, 1.28 mM CaCl2, 5.0 mM NaCO3, 25 mM HEPES, 1.2 mM MgSO4, 1.2 mM KH2PO4 and 0.1% (wt/vol) bovine serum albumin]. The islets were then incubated for 90 min in 0, 11.1, and 20 mM glucose. Supernatant was collected, and the cell pellets were lysed with acid-ethanol [75% ethanol containing 1.5% (vol/vol) HCl]. DNA was dried with a centriVap centrifugal vacuum concentrator connected to a cold trap and a pump (Labconco), redissolved in 50 μl of ultrapure water, and quantified with a BioPhotometer Plus spectrophotometer (Eppendorf, Hamburg. Germany). Insulin was measured in the supernatant using a Linco Research RIA kit and normalized to DNA content. Area under the curve was calculated using GraphPad Prism software.
Human islets.
Human islets were generously provided by Dr. Tatsuya Kin (Clinical Islet Laboratory, University of Alberta, Edmonton, AB, Canada) under the Transplant Program (26).
In vitro free fatty acid exposure.
Oleate and palmitate were complexed to fatty acid-free bovine serum albumin in Krebs-Ringer-HEPES buffer following protocols used in previous studies (28, 42). Then, fatty acid Krebs-Ringer solutions were added to 6 mM glucose-RPMI 1640 culture medium (0.5% wt/vol final fatty acid-free bovine serum albumin). Freshly isolated mouse islets or human islets (150 islets/condition) were incubated for 48 h in oleate (0.4 mM) or palmitate (0.4 mM) or control medium. Control culture medium contained all the ingredients without fatty acids.
Statistical analysis.
Data are expressed as means ± SE. Significance was determined using Student's t-test or two-way repeated-measures ANOVA with Tukey-Kramer or Bonnferroni's post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Cre-:Znt8−/− but not Ins2Cre:Znt8loxP/loxP mice on HFHC diet were more obese.
Cre-:Znt8−/− mice were significantly more obese than their control mice (Cre-), as seen with their body weight curve and area under the body weight curve from 7 to 16 wk on the diet (P < 0.05 to P < 0.001, n = 11; Fig. 2, A and B). In contrast, after 7 wk on the HFHC diet, Ins2Cre:Znt8loxP/loxP mice gained less weight than their control Ins2Cre mice, as seen from the area under the body weight curve (P < 0.05, n = 15; Fig. 2, C and D). To demonstrate that the HFHC diet did have an effect on body weight in our Ins2Cre:Znt8loxP/loxP mice, we compared the weight gain of our mice to previous studies examining control mice fed a normal chow diet. A previous study showed that 19-wk-old C57Bl/6J mice fed a chow diet had gained on average 21% of their original 6-wk-old body weight (27). By comparison, our 19-wk-old Ins2Cre and Ins2Cre:Znt8loxP/loxP mice fed the HFHC diet gained 84 and 73% of their 6-wk-old body weight, respectively. In addition, three other studies using Ins2Cre mice fed a normal chow diet showed body weight gains below what we report here (11, 47, 58). Therefore, HFHC diet feeding resulted in an appreciable increase in body weight gain compared with chow-fed mice.
Fig. 2.
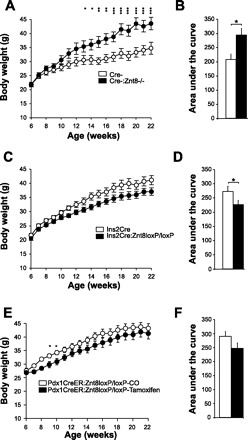
Weekly body weight and area under the body weight curve in Cre-:Znt8−/− (A and B, n = 11) Ins2Cre:Znt8loxP/loxP (C and D, n = 15), Pdx1CreER:Znt8loxP/loxP (E and F, n = 9) and their respective control mice fed a high-fat high-calorie (HFHC) diet. *P < 0.05, **P < 0.01, ***P < 0.001.
Cre-:Znt8−/−, but not Ins2Cre:Znt8loxP/loxP, mice on HFHC were insulin resistant.
Similar to mice fed a chow diet (39), Cre-:Znt8−/− mice fed an HFHC diet for 12 wk were significantly more hyperglycemic (P < 0.05, n = 11; Fig. 3A) and hyperinsulinemic (P < 0.01, n = 11; Fig. 3B) compared with control Cre- mice. HOMA-IR showed that Cre-:Znt8−/− mice were more insulin resistant than control Cre- mice (P < 0.01, n = 11; Fig. 3C), which was confirmed by an ITT performed after 14 wk on the diet (P < 0.05, n = 5; Fig. 4, C and D). Ins2Cre:Znt8loxP/loxP mice were normoglycemic and secreted significantly less insulin than control Ins2Cre mice (Fig. 3, E and F, n = 13). HOMA-IR was also significantly lower in Ins2Cre:Znt8loxP/loxP (P < 0.05, n = 13; Fig. 3G). Fasting plasma glucagon was unchanged in both groups of Znt8-deleted mice compared with their respective controls (Fig. 3, D and H, n = 9).
Fig. 3.
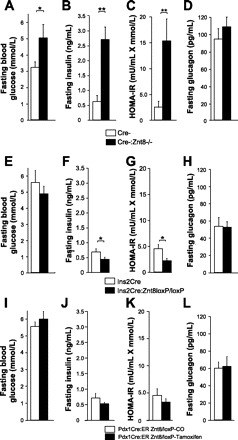
Fasting blood glucose (A, E, I), plasma insulin (B, F, J), HOMA-IR index (C, G, K), and plasma glucagon (D, H, L) in Cre-:Znt8−/− (A–D, n = 11), Ins2Cre:Znt8loxP/loxP (E–H, n = 11), and Pdx1CreER:Znt8loxP/loxP (I–L, n = 9) and their respective control mice fed an HFHC diet for 12 wk and following a 16-h fast. *P < 0.05, **P < 0.01.
Fig. 4.
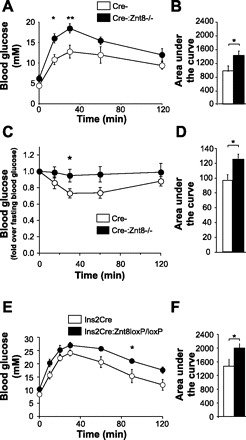
Oral glucose tolerance test in 6-h-fasted Cre-:Znt8−/− (A, n = 5) Ins2Cre:Znt8loxP/loxP (E, n = 5), and their respective control mice after 15 wk on an HFHC diet. Area under the glucose curve for the entire test was calculated (B and F). Insulin tolerance tests in 4-h-fasted Cre-:Znt8−/− mice after 14 wk on HFHC diet (C, n = 9). D: area under the glucose curve for the entire test was calculated. *P < 0.05, **P < 0.01.
At week 15 on the HFHC diet, glucose tolerance was measured using an OGTT. Cre-:Znt8−/− mice showed glucose intolerance during OGTT compared with their control Cre- mice (P < 0.05, P < 0.01, n = 5; Fig. 4, A and B). Ins2Cre:Znt8loxP/loxP mice showed mild glucose intolerance as they displayed a higher blood glucose excursion curve, and the corresponding area under the OGTT curve was increased compared with their control Ins2Cre mice (P < 0.05, n = 5; Fig. 4, E and F). Insulin secretion was measured during the OGTT and showed consistently lower levels of insulin in the Ins2Cre:Znt8loxP/loxP mice (not significant; Table 2, n = 5).
Table 2.
Plasma insulin levels during OGTTs performed in Ins2Cre and Ins2Cre:Znt8loxP/loxP mice
| 0 min | 15 min | 30 min | 60 min | |
|---|---|---|---|---|
| Ins2Cre | 4.52 ± 0.58 | 6.25 ± 2.18 | 4.4 ± 1.46 | 2.84 ± 0.68 |
| Ins2Cre:Znt8loxP/loxP | 3.34 ± 1.28 | 3.22 ± 0.78 | 3.32 ± 0.87 | 2.79 ± 0.92 |
Values are means ± SE in ng/ml; n = 5.
OGTT, oral glucose tolerance test.
Cre-:Znt8−/− but not Ins2Cre:Znt8loxP/loxP mice on HFHC diet displayed β-cell compensation.
Cre-:Znt8−/− mice fed an HFHC diet for 16 wk had a significantly increased insulin-positive area, islet number, and islet size (P < 0.05, P < 0.01, n = 3; Fig. 5, A, B, D) compared with control Cre- mice. In Ins2Cre:Znt8loxP/loxP mice, insulin-positive area was significantly reduced, islet number was unchanged, and islet size was significantly smaller compared with Ins2Cre mice (P < 0.01, n = 3; Fig. 5, E, F, H). Interestingly, insulin-positive area was approximately two times bigger in Cre- mice (Fig. 5A) than in Ins2Cre mice (Fig. 5E) and may reflect differences in their respective genetic backgrounds. Ins2Cre:Znt8loxP/loxP and Cre-:Znt8−/− mice did not show any differences in their glucagon-positive area compared with their respective controls (Fig. 5, C and G, n = 3).
Fig. 5.
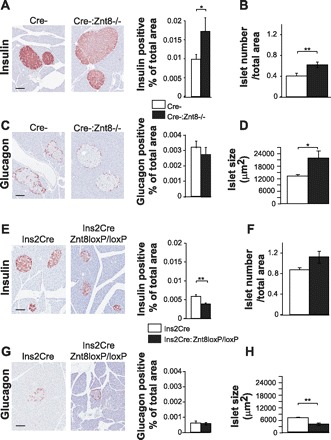
Islet morphological analysis in Cre-:Znt8−/− (A–D, n = 3), Ins2Cre:Znt8loxP/loxP (E–H, n = 3), and their respective control mice fed an HFHC diet for 16 wk. Histological sections were used to calculate islet number per pancreatic slice area (B and F) and islet size (D and H). β-Cell area (A and E) and α-cell area (C and G) calculated from images of insulin and glucagon staining. *P < 0.05, **P < 0.01; scale bar, 100 μm.
Ins2Cre:Znt8loxP/loxP and Cre-:Znt8−/− mice on HFHC diet have lower granule zinc content and abnormal insulin crystalization.
Granular zinc content was estimated using Zinquin after 16 wk on the HFHC diet. As previously reported, both Cre-:Znt8−/− and Ins2Cre:Znt8loxP/loxP pancreatic islet cells had significantly reduced zinc content than their respective control cells (Cre-:Znt8−/−: P < 0.001, Fig. 6, A–C, n = 3; Ins2Cre:Znt8loxP/loxP: P < 0.001, Fig. 6, F–H, n = 3). Similarly, both groups of knockout mice showed altered granular morphology with reduced dense core granules and increased abnormal (rod-shaped) granules compared with their respective controls (Cre-:Znt8−/−: P < 0.001, Fig. 6, D and E, n = 3; Ins2Cre:Znt8loxP/loxP: P < 0.001, Fig. 6, I and J, n = 3).
Fig. 6.
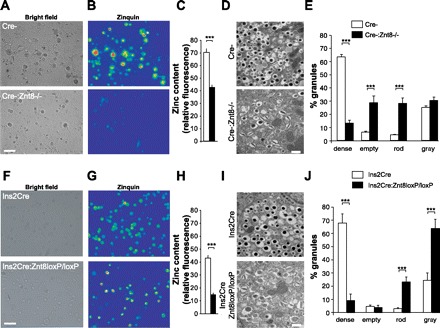
Intracellular zinc content in primary pancreatic islet cells obtained from Cre-:Znt8−/− (A–C, n = 3), Ins2Cre:Znt8loxP/loxP (F–H, n = 3), and their respective control mice after 16 wk on HFHC diet. Representative bright field images of primary pancreatic cells (A and F) loaded with zinquin (B and G). Intracellular zinc content was estimated by measuring zinquin fluorescence and is represented on bar graphs (C and H, n = 3, ***P < 0.001; scale bar, 10 μm). Granule morphological analysis in Cre-:Znt8−/− (D and E, n = 3), Ins2Cre:Znt8loxP/loxP (I and J, n = 3), and their respective control mice after 16 wk on HFHC diet. Representative electron micrographs (D and I) and quantification of different types of β-cell granules (E and J, n = 3, ***P < 0.001; scale bar, 500 nm).
Cre-:Znt8−/− and Ins2Cre:Znt8loxP/loxp mice on HFHC diet have impaired insulin processing.
After 16 wk on the HFHC diet, the average fed plasma insulin levels were similar between Cre-:Znt8−/− and control mice; however, proinsulin levels were significantly higher in Cre-:Znt8−/− mice and therefore so was the ratio of proinsulin to insulin (P < 0.05, P < 0.01, Fig. 7, A–C, n = 5). The average fed plasma insulin levels were similar between Ins2Cre:Znt8loxP/loxP and Ins2Cre mice, and again, proinsulin levels were significantly higher in Ins2Cre:Znt8loxP/loxP mice without modulating significantly the ratio of proinsulin to insulin (P < 0.05, Fig. 7, D–F, n = 10).
Fig. 7.
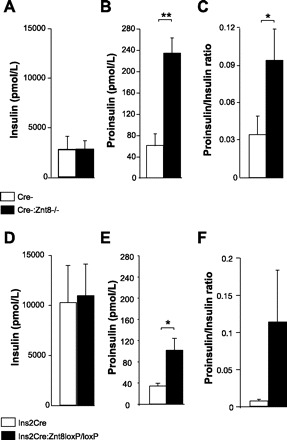
Fed plasma insulin, proinsulin, and proinsulin-to-insulin ratio in Cre-:Znt8−/− (A–C, n = 5), Ins2Cre:Znt8loxP/loxP (n = 10, D–F) and their respective controls fed HFHC diet for 16 wk. *P < 0.05, **P < 0.01.
Ins2Cre:Znt8loxP/loxp islets have decreased, whereas Cre-:Znt8−/− islets have increased, insulin secretion.
In vitro insulin secretion was assessed after 16 wk on the HFHC diet. In Cre-:Znt8−/− islets, we observed significantly increased insulin secretion in the presence of 20 mM glucose (P < 0.05, Fig. 8A, n = 6) but reduced glucagon secretion at 11.1 and 20 mM glucose compared with Cre-islets (P < 0.05, Fig. 8C, n = 6). Total insulin content from freshly isolated islets was similar between Cre-:Znt8−/− and Cre- islets (Fig. 8B, n = 10). We observed a significant decrease in insulin secretion from Ins2Cre:Znt8loxP/loxP islets in the presence of 11 mM glucose (P < 0.05, Fig. 8D, n = 6), with unchanged glucagon secretion or total insulin content from freshly isolated islets compared with control Ins2Cre islets (Fig. 8, E and F, n = 6–7).
Fig. 8.
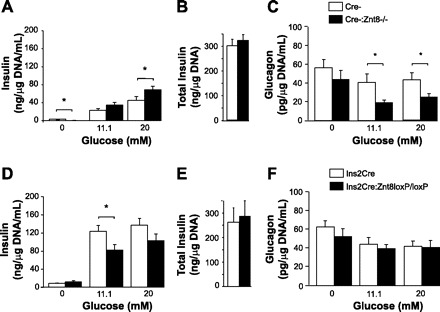
Glucose-stimulated insulin secretion from Cre-:Znt8−/− (A, n = 6), Ins2Cre:Znt8loxP/loxP (D, n = 6), and their respective control islets measured at 0, 11.1, and 20 mmol/l glucose. Total insulin content of isolated islets from Cre-:Znt8−/− (B, n = 10), Ins2Cre:Znt8loxP/loxP (E, n = 7), and controls. Glucagon secretion from Cre-:Znt8−/− (C, n = 6), Ins2Cre:Znt8loxP/loxP (F, n = 6), and control islets measured at 0, 11.1, and 20 mmol/l glucose. Islets were collected after 16 wk of HFHC diet. *P < 0.05.
Tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice maintain similar body weights.
Znt8 mRNA expression was evaluated by qPCR, and we showed an 87% decrease in Znt8 expression in pancreatic islets of tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice (P < 0.001, Fig. 1C, n = 3). Immunohistochemical analysis of primary islet cells from tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice showed a significant decrease in the number of cells colabeled for Znt8 and insulin. Some insulin-negative cells remained labeled for Znt8 and are likely α- or pancreatic polypeptide cells (P < 0.01, P < 0.001, n = 3; Fig. 1, D and E). β-Cells of tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice were analyzed under an electron microscope and showed altered granular morphology with reduced dense core granules and increased abnormal (rod-shaped, gray, and empty) granules compared with their respective corn oil-injected Pdx1CreER:Znt8loxP/loxP mice (P < 0.05, P < 0.001, Fig. 1, F and G, n = 3).
Over the course of the HFHC diet study, tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice gained less weight than the corn oil-injected mice, as seen from the area under the body weight curve (Fig. 2, E and F, n = 9). Food consumption was measured weekly in these mice, and there was no change in the feeding rate (data not shown). To evaluate a potential nonspecific effect on body weight and food consumption due to the injections or tamoxifen itself, a C57BL/6J control group was injected with corn oil and tamoxifen and fed the same HFHC diet. Body weight and food consumption was measured weekly over a 16-wk period and did not reveal a detectable difference between the corn oil- and tamoxifen-injected groups (n = 10, data not shown). Tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice were normoglycemic and had a tendency to secrete less insulin than control mice (Fig. 3, I and J, n = 7). HOMA-IR was unchanged (Fig. 3K, n = 7), and fasting plasma glucagon was similar between corn oil- and tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice (Fig. 3L, n = 9).
Islet Znt8 expression is reduced by high-fat feeding.
Znt1–10 expression was measured in islets from chow-fed and HFHC diet-fed Ins2Cre mice. Interestingly, we observed a decrease in the expression of Znt8 in HFHC diet-fed mice (P < 0.05, Fig. 9A, n = 3). Expression of most other zinc transporters, especially Znt5, was elevated in HFHC diet-fed mice (P < 0.05, Fig. 9A, n = 9). Similarly, incubation of 8-wk-old Ins2Cre mouse islets with 0.4 mM palmitate for 48 h significantly reduced the expression of Znt8, whereas 0.4 mM oleate treatment had no significant effect (P < 0.05, Fig. 9B, n = 3). Interestingly, both oleate and palmitate treatment significantly decreased Znt8 expression in human islets isolated in the Clinical Islet Laboratory (22) compared with those incubated in control conditions (P < 0.05, Fig. 9C, n = 3). To demonstrate the effect of high-fat feeding on ZnT8 expression in non-β-cell tissues, we performed qPCR. Very low levels of Znt8 expression was detected in the hypothalamus, adrenal gland, inguinal fat, and skeletal muscle of Cre- mice fed a chow diet (Fig. 9D, n = 4–6). Immunohistochemistry was also performed on abdominal, inguinal, and subcutaneous fat and did not reveal any specific expression of Znt8 (data not shown).
Fig. 9.
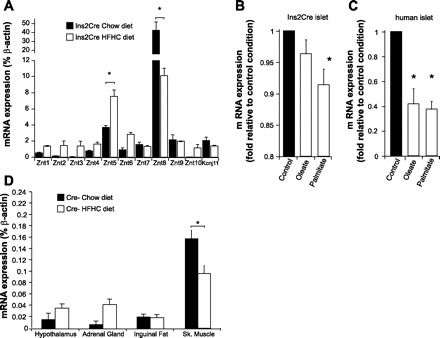
Znt8 and other Znt transcript expression in islets of Ins2Cre mice on chow or HFHC fed for 16 wk (A, n = 3). Znt8 transcript expression in Ins2Cre mouse islets (B, n = 3) and in human islets (C, n = 3) after 48-h incubation in culture medium only (control) or supplemented with oleate (0.4 mM) or palmitate (0.4 mM). Znt8 transcript expression in hypothalamus, adrenal gland, inguinal fat, and skeletal muscle (Sk. Muscle) in Cre- mice on chow or HFHC diet fed for 16 wk (D, n = 4–6). *P < 0.05.
DISCUSSION
We and others (34, 39, 63) have shown that the loss of Znt8 in mouse β-cells results in lower granular zinc content and abnormal granules. These defects are now considered hallmarks of β-cell Znt8 deficiency and were observed in Cre-:Znt8−/−, Ins2Cre:Znt8loxP/loxP, and tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice in the present study.
Recent studies have shown that Znt8 polymorphisms are a potential risk factor for type 2 diabetes (48, 50, 52, 54, 55, 57, 65). Some studies in humans have shown no association between adiposity/dietary fat intake and the SLC30A8 risk allele (38, 41, 44, 59), while two recent genome-wide association studies have shown that the SLC30A8 risk allele confers higher risk of developing type 2 diabetes in a lower-BMI population (64) or nonobese subjects (9). We observed in our Ins2Cre:Znt8loxP/loxP mice that the the β-cell-specific deletion of Znt8 seemed to have a protective effect against diet-induced obesity compared with controls. HFHC diet-fed Ins2Cre:Znt8loxP/loxP mice showed some glucose intolerance and reduced insulin secretion, consistent with the same mice on a chow diet (63), without a vast impairment in glucose homeostasis or onset of obesity. The reduced insulin secretion in these mice may have resulted from the small decrease in the β-cell area and/or an impairment in insulin processing and secretion and was likely responsible for the glucose intolerance observed during the OGTT. Reduced insulin secretion combined with lower body weight confirms the improved insulin sensitivity in these mice, which may have developed as compensation for the lower insulin levels. Interestingly, obesity and plasma insulin are tightly linked, as many studies have shown that hyperinsulinemia in both mice and humans aggravates diet-induced body weight gain and insulin resistance (1–4, 35, 40). An insulin secretion defect and/or reduced insulin due to lower β-cell mass in Ins2Cre:Znt8loxP/loxP mice may have prevented the development of hyperinsulinemia during the HFHC diet, and this may have protected against uncontrolled body weight gain and obesity. The origin of the decrease in β-cell area in the Ins2Cre:Znt8loxP/loxP mice is unclear. It is possible that increased insulin sensitivity may have created a reduced demand for insulin, thus decreasing β-cell mass over time. Alternately, previous studies indicate that zinc deficiency may lead to reduced β-cell viability (15, 25). Thus, we could also speculate that the reduced zinc levels in β-cells of Ins2Cre:Znt8loxP/loxP mice may have resulted in more β-cell death, leading to reduced β-cell area independently of insulin sensitivity. In addition, zinc deficiency in β-cells is known to modulate expression of the insulin genes, genes involved in glucose sensing and insulin biosynthesis in 8-wk-old mice (63). It is therefore possible that a similar gene modulation occurred in our Ins2Cre:Znt8loxP/loxP mice fed over the course of the 16-wk HFHC diet period and induced a decrease in β-cell area. Consequently, these data suggest that β-cell Znt8 deficiency does not necessarily exacerbate diet-induced glucose intolerance and may be providing a protective effect against diet-induced obesity.
However, control Ins2Cre mice are reportedly already glucose intolerant with defects in insulin secretion compared with wild-type mice (31). In our study, we cannot confirm that our Ins2Cre mice are glucose intolerant, as we did not compare them with age matched wild-type mice. To address the possibility of some glucose intolerance and potential developmental complications or compensatory mechanisms in the Ins2Cre:Znt8loxP/loxP mouse model, we created the Pdx1CreER:Znt8loxP/loxP mouse. Tamoxifen injection of Pdx1CreER:Znt8loxP/loxP mice induced a significant deletion of the Znt8 gene in islets as shown by qPCR. Furthermore, costaining with insulin confirmed the effectiveness of tamoxifen to specifically delete Znt8 in β-cells. When fed our HFHC diet, the body weight curve and area under the body weight curve of corn oil-injected control and tamoxifen-injected Pdx1CreER:Znt8loxP/loxP mice were similar to those of Ins2Cre:Znt8loxP/loxP and their controls, thus confirming that constitutive or temporal β-cell-specific deletion of Znt8 does not significantly impact HFHC diet-induced obesity.
Endogenous expression of the Ins2 promoter in the mouse brain has been demonstrated over the course of the embryo development (13, 14) and at the adult stage in the hypothalamus (12, 17, 29, 62). Pdx1 promoter activity was reported in the hypothalamus as well (62). We found Znt8 transcripts at negligible levels in the hypothalamus of Cre- mice. Therefore, it is unlikely that the phenotype observed in Ins2Cre:Znt8loxP/loxP mice originated from deletion of Znt8 in the hypothalamus. Nevertheless, Ins2 promoter activity was reported in many other extrahypothalamic brain areas. The choroid plexus (30), cortex, caudate putamen, ventral pallidum, substantia nigra, pons (17), and mid- and ventral regions of the brain were all shown to have Ins2 promoter activity (62). Importantly, Znt8 expression was reported in the cortex, as seen in the Allen Mouse Brain Atlas (http://www.brain-map.org) (33). Thus, Znt8 deletion in this extrahypothalamic brain region may have played a role in the mild phenotype observed in our β-cell-specific knockout mice. Future studies should be geared to address these concepts.
As stated above, HFHC diet led to a drastic deterioration of insulin sensitivity and development of obesity in global Znt8 knockout mice in contrast to our β-cell-specific deletion models. Some discrepancies between the Ins2Cre:Znt8loxP/loxP and global knockout models may arise simply due to differences in their genetic backgrounds; however, deletion of Znt8 from non-β-cell tissues is most likely responsible. Although our results thus far suggest a lack of appreciable expression of Znt8 in hypothalamus, adrenal glands, fat, and skeletal muscle, it is probable that Znt8 deficiency in some other subset of cells could lead to the insulin resistance or obesity in the global knockout mice. As we only observed changes in glucagon secretion in vitro, likely as a result of elevated insulin secretion, coupled with the unaltered fasting plasma glucagon levels, it suggests that Znt8 deficiency in α-cells may not play a role in defining the insulin resistance phenotype in these mice. Interestingly, pancreatic polypeptide was previously shown to decrease food intake and increase energy expenditure (5). As Znt8 was reportedly found in pancreatic polypeptide cells, this may be an important point of regulation of energy balance that also needs to be further examined (56).
Cre-:Znt8−/−mice were hyperglycemic, hyperinsulinemic, and glucose intolerant. Interestingly, a previous study also showed that HFHC-fed Cre-:Znt8−/− mice had a higher risk of becoming diabetic, whereas all control mice remained nondiabetic (34). Regardless of whether this oversecretion of insulin is a consequence or a prerequisite of the insulin resistance, the resulting hyperinsulinema may lead to insulin resistance of the hypothalamus, resulting in overfeeding and obesity. Increased fat mass is a trigger in itself to propagate further insulin resistance due to increased release of cytokines and nonesterified fatty acids (7, 46, 51, 60). The β-cells may have attempted to compensate for this insulin resistance by increasing their mass, which subsequently increased islet size and insulin-secretory capacity. On a similar note, enhancement of glucose-stimulated insulin secretion was also apparent in islets isolated from chow-fed Cre-:Znt8−/− mice (39). Thus, the hyperinsulinemia in our Cre-:Znt8−/− mice may have made them more prone to obesity and worsened the phenotype compared with the control mice.
Interestingly, we show that an HFHC diet significantly reduces β-cell Znt8 gene expression in our mice. A similar decrease in Znt8 was found previously in the pancreata of obese ob/ob and db/db mice (24, 56), as well as following oleate and palmitate treatment in rat insulinoma cells (21). In the present study, we have shown once again that in vitro treatment with oleate and palmitate causes a significant decrease in Znt8 transcript levels in human and mouse islets. However, the reduction in Znt8 expression observed in pancreata from the Akita mouse, which is diabetic due to endoplasmic reticulum stress without an obese phenotype, suggests that factors other than circulating fat that stress the islet can decrease Znt8 expression (56). To this end, Znt8 gene expression was significantly reduced in islets upon in vitro cytokine treatment. However, not all stressors decrease Znt8 expression, as a recent report failed to see any change in Znt8 gene expression upon incubation in high glucose concentrations (6). The decrease in Znt8 expression that we observed in control mice fed an HFHC diet did not translate into any apparent change in β-cell morphology or zinc content, which may be the result of compensation by other Znt transporters, such as Znt5, which has been shown to be expressed in the Golgi and on β-cell insulin granules (23). However, such compensation was not previously observed in Cre-:Znt8−/− or Ins2Cre:Znt8loxP/loxP mice (39, 63).
Interestingly, high glucose is a stimulator of zinc influx transporters 6 and 7 (ZIP6 and ZIP7) expression, which could lead to an increase in the cytoplasmic zinc concentration in β-cells (6). Another recent article demonstrated the toxic effect of zinc in cells (8). Therefore, it is conceivable that the phenotype of mice stressed with an HFHC diet could originate as well from the long-term effect glucose has on ZIP6 and ZIP7 in β-cells, which would result in toxic levels of cytoplasmic zinc.
In summary, we show here that, during HFHC diet-induced metabolic stress, global Cre-:Znt8−/− mice develop severe insulin resistance and obesity, whereas mice with specific deletion of Znt8 in β-cells do not. We suggest that the obesity in Cre-:Znt8−/− mice is likely dictated by the presence of hyperinsulinemia, not seen in Ins2Cre:Znt8loxP/loxP mice. Consequently, our study suggests that β-cell-specific Znt8 may not confer a higher risk of developing diabetes associated with obesity.
GRANTS
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR; MOP-102588) to M. B. Wheeler and the National Institutes of Health (NIH; BADERC P30 DK-057521 PF) to D. Kong. A doctoral award from CIHR supported N. Wijesekara.
DISCLOSURES
Dr. Fabrice Chimienti is employed by Mellitech.
AUTHOR CONTRIBUTIONS
Author contributions: A.H., N.W., K.J.P., and M.W. conception and design of research; A.H., N.W., I.G., K.J.P., A.B., and D.K. performed experiments; A.H., N.W., and K.J.P. analyzed data; A.H., N.W., K.J.P., and M.W. interpreted results of experiments; A.H. prepared figures; A.H. drafted manuscript; A.H., N.W., I.G., K.J.P., A.B., D.K., F.C., and M.W. edited and revised manuscript; A.H., N.W., I.G., K.J.P., A.B., D.K., F.C., and M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Minna Woo (Ontario Cancer Institute/Princess Margaret Hospital, Toronto, ON, Canada) for generously providing the TgN(Ins2-Cre)25Mgn mice. Pdx1CreER mice were generated by Dr. Douglas Melton (Howard Hughes Medical Institute, Harvard University, Boston, MA) and provided by Dr. Minna Woo.
REFERENCES
- 1. Alemzadeh R, Holshouser S, Massey P, Koontz J. Chronic suppression of insulin by diazoxide alters the activities of key enzymes regulating hepatic gluconeogenesis in Zucker rats. Eur J Endocrinol 146: 871–879, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Alemzadeh R, Jacobs W, Pitukcheewanont P. Antiobesity effect of diazoxide in obese Zucker rats. Metabolism 45: 334–341, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Alemzadeh R, Langley G, Upchurch L, Smith P, Slonim AE. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab 83: 1911–1915, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Alemzadeh R, Slonim AE, Zdanowicz MM, Maturo J. Modification of insulin resistance by diazoxide in obese Zucker rats. Endocrinology 133: 705–712, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM, Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 124: 1325–1336, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bellomo EA, Meur G, Rutter GA. Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP) and metallothionein gene expression in primary pancreatic islet (beta)-cells. J Biol Chem 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10, 1997 [PubMed] [Google Scholar]
- 8. Bozym RA, Chimienti F, Giblin LJ, Gross GW, Korichneva I, Li Y, Libert S, Maret W, Parviz M, Frederickson CJ, Thompson RB. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp Biol Med (Maywood) 235: 741–750, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cauchi S, Nead KT, Choquet H, Horber F, Potoczna N, Balkau B, Marre M, Charpentier G, Froguel P, Meyre D. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med Genet 9: 45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119: 4199–4206, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Choi D, Cai EP, Schroer SA, Wang L, Woo M. Vhl is required for normal pancreatic β-cell function and the maintenance of beta cell mass with age in mice. Lab Invest 91: 527–538, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C. Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24: 258–269, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deltour L, Leduque P, Blume N, Madsen O, Dubois P, Jami J, Bucchini D. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci USA 90: 527–531, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deltour L, Montagutelli X, Guenet JL, Jami J, Paldi A. Tissue- and developmental stage-specific imprinting of the mouse proinsulin gene, Ins2. Dev Biol 168: 686–688, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol 146: 443–459, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab 10: 285–295, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26: 139–142, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Gyulkhandanyan AV, Lee SC, Bikopoulos G, Dai F, Wheeler MB. The Zn2+-transporting pathways in pancreatic beta-cells: a role for the L-type voltage-gated Ca2+ channel. J Biol Chem 281: 9361–9372, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hardy AB, Fox JE, Giglou PR, Wijesekara N, Bhattacharjee A, Sultan S, Gyulkhandanyan AV, Gaisano HY, MacDonald PE, Wheeler MB. Characterization of Erg K+ channels in alpha- and beta-cells of mouse and human islets. J Biol Chem 284: 30441–30452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hardy OT, Hohmeier HE, Becker TC, Manduchi E, Doliba NM, Gupta RK, White P, Stoeckert CJ, Jr, Matschinsky FM, Newgard CB, Kaestner KH. Functional genomics of the beta-cell: short-chain 3-hydroxyacyl-coenzyme A dehydrogenase regulates insulin secretion independent of K+ currents. Mol Endocrinol 21: 765–773, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem 277: 19049–19055, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18: 706–716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK, Kim KW, Lee MS. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes 49: 367–372, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Kin T, O'Gorman D, Schroeder A, Onderka C, Richer B, Rosichuk S, Zhai X, Shapiro AM. Human islet distribution program for basic research at a single center. Transplant Proc 43: 3195–3197, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Kizelsztein P, Govorko D, Komarnytsky S, Evans A, Wang Z, Cefalu WT, Raskin I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab 296: E433–E439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koshkin V, Dai FF, Robson-Doucette CA, Chan CB, Wheeler MB. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic beta-cells. J Biol Chem 283: 7936–7948, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest 114: 917–927, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamotte L, Jackerott M, Bucchini D, Jami J, Joshi RL, Deltour L. Knock-in of diphteria toxin A chain gene at Ins2 locus: effects on islet development and localization of Ins2 expression in the brain. Transgenic Res 13: 463–473, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem 281: 2649–2653, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol 203: 33–43, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, Gilon P, in't Veld PA, Schuit FC. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA 106: 14872–14877, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lustig RH, Greenway F, Velasquez-Mieyer P, Heimburger D, Schumacher D, Smith D, Smith W, Soler N, Warsi G, Berg W, Maloney J, Benedetto J, Zhu W, Hohneker J. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes (Lond) 30: 331–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michael J, Carroll R, Swift HH, Steiner DF. Studies on the molecular organization of rat insulin secretory granules. J Biol Chem 262: 16531–16535, 1987 [PubMed] [Google Scholar]
- 37. Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis 19: 431–439, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Ng MC, Park KS, Oh B, Tam CH, Cho YM, Shin HD, Lam VK, Ma RC, So WY, Cho YS, Kim HL, Lee HK, Chan JC, Cho NH. Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes 57: 2226–2233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58: 2070–2083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Odeleye OE, de Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes 46: 1341–1345, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Omori S, Tanaka Y, Takahashi A, Hirose H, Kashiwagi A, Kaku K, Kawamori R, Nakamura Y, Maeda S. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes 57: 791–795, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes 56: 2927–2937, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 83: 368–380, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Pecioska S, Zillikens MC, Henneman P, Snijders PJ, Oostra BA, van Duijn CM, Aulchenko YS. Association between type 2 diabetes loci and measures of fatness. PLoS One 5: e8541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O'Brien RM. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J 421: 371–376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37: 1020–1024, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Robson-Doucette CA, Sultan S, Allister EM, Wikstrom JD, Koshkin V, Bhatacharjee A, Prentice KJ, Sereda SB, Shirihai OS, Wheeler MB. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes 60: 2710–2719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316: 1331–1336, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Scott DA, Fisher AM. The insulin and the zinc content of normal and diabetic pancreas. J Clin Invest 17: 725–728, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316: 1341–1345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881–885, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Smidt K, Pedersen SB, Brock B, Schmitz O, Fisker S, Bendix J, Wogensen L, Rungby J. Zinc-transporter genes in human visceral and subcutaneous adipocytes: lean versus obese. Mol Cell Endocrinol 264: 68–73, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39: 770–775, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Takeuchi F, Serizawa M, Yamamoto K, Fujisawa T, Nakashima E, Ohnaka K, Ikegami H, Sugiyama T, Katsuya T, Miyagishi M, Nakashima N, Nawata H, Nakamura J, Kono S, Takayanagi R, Kato N. Confirmation of multiple risk Loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes 58: 1690–1699, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tamaki M, Fujitani Y, Uchida T, Hirose T, Kawamori R, Watada H. Downregulation of ZnT8 expression in pancreatic b-cells of diabetic mice. Islets 1: 124–128, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Timpson NJ, Lindgren CM, Weedon MN, Randall J, Ouwehand WH, Strachan DP, Rayner NW, Walker M, Hitman GA, Doney AS, Palmer CN, Morris AD, Hattersley AT, Zeggini E, Frayling TM, McCarthy MI. Adiposity-related heterogeneity in patterns of type 2 diabetes susceptibility observed in genome-wide association data. Diabetes 58: 505–510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang L, Liu Y, Yan Lu S, Nguyen KT, Schroer SA, Suzuki A, Mak TW, Gaisano H, Woo M. Deletion of Pten in pancreatic ss-cells protects against deficient ss-cell mass and function in mouse models of type 2 diabetes. Diabetes 59: 3117–3126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Webster RJ, Warrington NM, Beilby JP, Frayling TM, Palmer LJ. The longitudinal association of common susceptibility variants for type 2 diabetes and obesity with fasting glucose level and BMI. BMC Med Genet 11: 140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wellen KE, Hotamisligil GS. Inflammation, stress, diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 104: 17040–17045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, Bernal-Mizrachi E, Elghazi L, Roe MW, Labosky PA, Myers MM, Jr, Gannon M, Powers AC, Dempsey PJ. Conditional gene targeting in mouse pancreatic (beta)-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59: 3090–3098 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53: 1656–1668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu Y, Li H, Loos RJ, Yu Z, Ye X, Chen L, Pan A, Hu FB, Lin X. Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes 57: 2834–2842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316: 1336–1341, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


