Background: Defective folding and trafficking of β-cell ATP-sensitive potassium (KATP) channels causes congenital hyperinsulinism.
Results: Carbamazepine improves the processing and surface expression of trafficking-impaired KATP channels harboring a subset of sulfonylurea receptor 1 mutations.
Conclusion: Carbamazepine is a novel corrector of KATP channels.
Significance: Carbamazepine may be used to treat congenital hyperinsulinism caused by defective KATP channel trafficking.
Keywords: ABC Transporter, CFTR, Intracellular Trafficking, Molecular Chaperone, Potassium Channels, KATP Channel, β-Cells, Carbamazepine, Hyperinsulinism, Sulfonylurea Receptor 1
Abstract
ATP-sensitive potassium (KATP) channels consisting of sulfonylurea receptor 1 (SUR1) and the potassium channel Kir6.2 play a key role in insulin secretion by coupling metabolic signals to β-cell membrane potential. Mutations in SUR1 and Kir6.2 that impair channel trafficking to the cell surface lead to loss of channel function and congenital hyperinsulinism. We report that carbamazepine, an anticonvulsant, corrects the trafficking defects of mutant KATP channels previously identified in congenital hyperinsulinism. Strikingly, of the 19 SUR1 mutations examined, only those located in the first transmembrane domain of SUR1 responded to the drug. We show that unlike that reported for several other protein misfolding diseases, carbamazepine did not correct KATP channel trafficking defects by activating autophagy; rather, it directly improved the biogenesis efficiency of mutant channels along the secretory pathway. In addition to its effect on channel trafficking, carbamazepine also inhibited KATP channel activity. Upon subsequent removal of carbamazepine, however, the function of rescued channels was recovered. Importantly, combination of the KATP channel opener diazoxide and carbamazepine led to enhanced mutant channel function without carbamazepine washout. The corrector effect of carbamazepine on mutant KATP channels was also demonstrated in rat and human β-cells with an accompanying increase in channel activity. Our findings identify carbamazepine as a novel small molecule corrector that may be used to restore KATP channel expression and function in a subset of congenital hyperinsulinism patients.
Introduction
Congenital hyperinsulinism is an infant/childhood disease characterized by persistent insulin secretion despite life-threatening hypoglycemia (1). The most common causes of the disease are loss-of-function mutations in ABCC8 and KCNJ11, which encode the sulfonylurea receptor 1 (SUR1)2 and inwardly rectifying potassium channel Kir6.2 subunits of ATP-sensitive potassium (KATP) channels, respectively (2–4). In pancreatic β-cells, KATP channels serve as molecular sensors of intracellular ATP/ADP ratios commensurate with glucose metabolism and translate this metabolic signal into changes in β-cell membrane potential to control insulin secretion (5–7). When glucose concentrations are low, the ATP/ADP ratio falls, and KATP channels open to keep the β-cell membrane potential hyperpolarized, thus preventing insulin secretion. Failure to open KATP channels due to defects in channel gating and/or expression uncouples β-cell membrane potential from glucose concentrations, resulting in inappropriate insulin secretion in the face of severe hypoglycemia (8). Although some KATP hyperinsulinism cases are successfully treated with the channel opener diazoxide, in the severe, diffuse form of the disease associated with recessive mutations that prevent channel expression at the cell surface, total or subtotal pancreatectomy is often required (1).
Many genetic diseases arise as a result of protein folding and trafficking defects (9). Mutant proteins are recognized by cellular quality control systems, retained in the ER, and targeted for degradation (10–13). Significant progress has been made in recent years to develop strategies that will correct protein folding and trafficking defects. One clinically promising approach is to identify small molecules that can trigger a cellular response that enhances protein folding capacity or act as specific pharmacological chaperones for the misfolded proteins (for reviews, see Refs. 14 and 15). In a number of cases, rescued mutant proteins have been shown to be fully or partially functional and thus would be expected to alleviate the disease phenotype. The cystic fibrosis transmembrane conductance regulator (CFTR) folding mutation ΔF508, the most prevalent mutation underlying cystic fibrosis, is a prominent example (16–18). Screening of chemical libraries has led to the identification of compounds that enhance function of this mutant chloride channel: those that boost processing and surface expression (referred to as correctors) and those that improve channel gating (referred to as potentiators) (19–23). An emerging concept is that optimal functional restoration may be achieved through a combination of correctors and potentiators (24, 25).
A large number of congenital hyperinsulinism-causing KATP channel mutations, especially in the regulatory subunit SUR1, have been shown to impair channel expression at the cell membrane (8, 26, 27) (collectively referred to as trafficking mutations hereinafter). In most cases, mutant proteins are retained in the ER and rapidly degraded possibly due to folding or assembly defects (28–31). Sulfonylureas are drugs commonly used to treat type II diabetic patients by binding to the KATP complex and inhibiting channel activity. We have shown previously that these drugs are efficient pharmacological chaperones that correct trafficking defects of a subset of SUR1 mutants (29, 31). Some of the rescued mutants exhibit normal sensitivity to intracellular ATP and MgADP (29), indicating that they would be able to sense metabolic changes if their surface expression were restored in the patients. Translation of this finding to disease treatment, however, has been hampered by high affinity binding of the most effective chaperone, glibenclamide, to KATP channels and the resulting irreversible inhibitory effect of glibenclamide on channel function. Although a reversible sulfonylurea, tolbutamide, is also effective in correcting KATP channel trafficking defects and can be washed out to recover channel function (29), this drug has fallen out of favor following the release of the University Group Diabetes Program study, which implicated tolbutamide in increased mortality secondary to cardiovascular events (32, 33). Therefore, there is a need to identify additional compounds that can correct KATP channel trafficking defects.
SUR1 and CFTR both belong to the ATP-binding cassette (ABC) transporter protein superfamily and share structural similarities in the ABC core domain (see Fig. 1A) (34). Furthermore, a number of CFTR proteostasis regulators such as Hsp90 and derlin-1 also play a role in KATP channel regulation (35–38). We therefore postulate that small molecules effective in rescuing CFTRΔF508 may also correct folding/trafficking defects caused by mutations in SUR1 (39). In this study, we focused on a previously identified CFTRΔF508 corrector, carbamazepine (19), because it is approved by the Food and Drug Administration for treatment of epilepsy and bipolar disorder and has an established biosafety profile. In addition, carbamazepine has recently been shown to alleviate liver disease in mice expressing the folding mutant α1-antitrypsin Z (40) as well as pathology caused by protein misfolding in several neurodegenerative disease models (41) by activating the autophagic pathway. Herein we report that carbamazepine improves the processing and surface expression of a subset of 19 previously identified SUR1 trafficking mutants. Strikingly, of the mutations that were responsive to carbamazepine, all are located in the N-terminal transmembrane domain of SUR1, a domain involved in physical and functional coupling to the pore-forming subunit Kir6.2 (42, 43). Carbamazepine corrected surface expression of mutant channels by enhancing the processing efficiency of newly synthesized SUR1 independently of autophagy. In addition to its corrector effect, carbamazepine inhibited KATP channel activity in isolated membrane patches, suggesting that the drug may interact with the channel complex. This channel inhibition was reversible, and functional recovery of mutant channels rescued to the cell surface could be achieved upon washout of carbamazepine and enhanced by the KATP channel opener diazoxide. In rat insulinoma cells and human β-cells, carbamazepine similarly corrected mutant channel trafficking defects and restored KATP currents. Our findings identify KATP channels as a novel target for carbamazepine and have significant therapeutic implications for a subset of congenital hyperinsulinism patients.
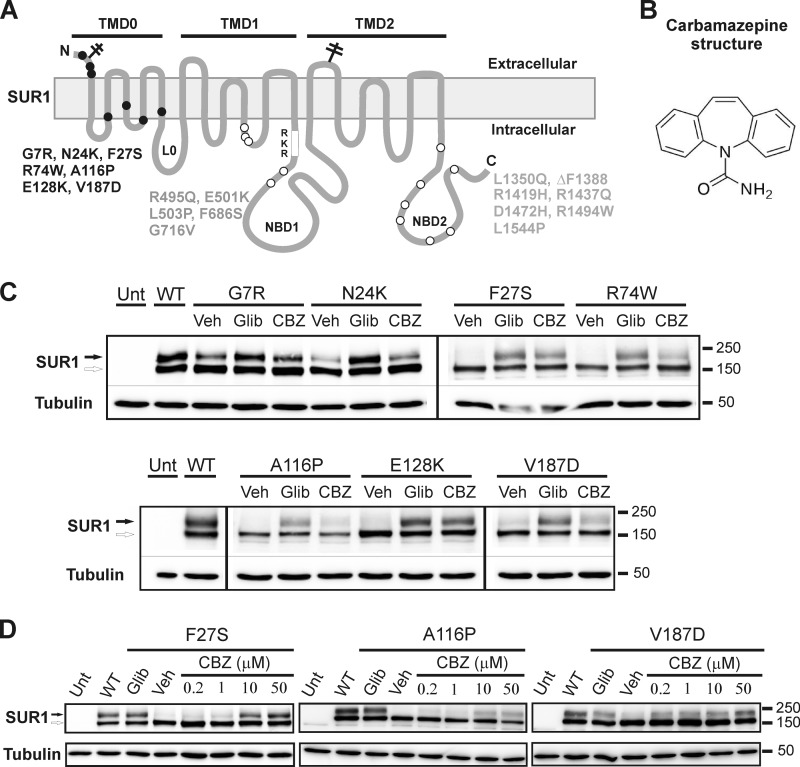
FIGURE 1.
Carbamazepine corrects SUR1 processing defects caused by a subset of mutations previously identified in congenital hyperinsulinism. A, location of SUR1 mutations included in this study. SUR1 topology is based on Conti et al. (70). B, chemical structure of carbamazepine. C, Western blots of SUR1 from COSm6 cells co-transfected with WT Kir6.2 and WT or mutant SUR1 cDNAs and treated with 0.1% DMSO (Veh), 5 μm glibenclamide (Glib), or 10 μm carbamazepine (CBZ) for 16 h. Untransfected cells (Unt) and cells expressing WT channels were included for comparison. Only mutations that exhibit a significant response to carbamazepine are shown (all in TMD0). The thin lines separate different parts of the same blot, and thick vertical lines separate different blots. The empty arrow points to the core-glycosylated immature SUR1, and the solid arrow points to the complex-glycosylated mature SUR1. Molecular mass markers shown on the right side of the blots are in kDa in this and all subsequent figures. D, dose response of SUR1 mutants to the rescue effect of carbamazepine.
MATERIALS AND METHODS
Expression Constructs
SUR1 was tagged with a FLAG epitope (DYKDDDDK) at the extracellular N terminus (referred to as f-SUR1), and the cDNA was cloned into the pECE vector. We have shown in previous studies that these epitope tags do not affect the trafficking or function of the channels (44, 45). Kir6.2 cDNA was cloned into pcDNA1 as described previously (44). Construction of adenoviruses carrying KATP subunit cDNA was as described previously (46). The f-SUR1 recombinant adenovirus was made using a modified pShuttle plasmid (AdEasy kit, Stratagene) containing a tetracycline-inducible promoter and requires co-infection of a virus carrying the cDNA encoding a tetracycline-inhibited transactivator (tTA) for expression. Recombinant viruses were amplified in HEK293 cells and purified according to the manufacturer's instructions.
Transduction of INS-1 Cells or Human Islets with Recombinant Adenoviruses
INS-1 cell clone 832/13 (kindly provided by Dr. Christopher Newgard) was plated in 10-cm plates and cultured for 24 h in RPMI 1640 medium with 11.1 mm d-glucose (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mm HEPES, 2 mm glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol (47). Cells at ∼70% confluence were washed once with phosphate-buffered saline (PBS) and then incubated for 1.5 h at 37 °C in Opti-MEM containing low FBS and a mixture of viruses including the tTA virus, the tTA-regulated f-SUR1 virus, and the Kir6.2 virus as described previously (45, 46). The multiplicity of infection (m.o.i.) for each virus was determined empirically. After 90 min, 2× growth medium was added, and the cells were incubated at 37 °C until they reached the appropriate density for the various experiments.
Human islets with 80–95% purity and 80–95% viability were obtained through the Integrated Islets Distribution Program. Islets received were maintained overnight in INS-1 growth medium as described above minus penicillin and streptomycin. m.o.i. calculations assumed 2000 cells per islet equivalent (as defined by the Integrated Islets Distribution Program) (48). Groups of islets (100 islet equivalents) in each well of a 12-well plate were infected with Ad-tTA (m.o.i., 500), Ad-Kir6.2 (m.o.i., 2000), and either Ad-f-SUR1 (m.o.i., 1000) or mutant Ad-A116P f-SUR1 (m.o.i., 1000) or Ad-F27S f-SUR1 (m.o.i., 500) for 16 h in 0.5 ml of Opti-MEM (Invitrogen) at 37 °C. The islets were then incubated for an additional 24 h in RPMI 1640 medium with 10% FBS containing either DMSO, glibenclamide, or carbamazepine before being harvested for immunoblotting. For electrophysiological experiments, human islets were dissociated into single or groups of cells by trituration in a solution containing 116 mm NaCl, 5.5 mm d-glucose, 3 mm EGTA, and 0.1% bovine serum albumin (BSA), pH 7.4. Dissociated cells were spun down and plated on gelatin (0.4%)-coated coverslips at least 3–4 h prior to infection with adenoviruses as described above for INS-1 cells.
Immunoblotting
COSm6 cells (transfected with SUR1 and Kir6.2 cDNAs using FuGENE® 6 according to the manufacturer's instructions), INS-1 cells, or human islets (control or transduced with recombinant adenoviruses) were lysed in lysis buffer (50 mm Tris·HCl, pH 7.0, 150 mm NaCl, and 1% Triton X-100 with CompleteTM protease inhibitors) on ice for 30 min. Cell lysate was centrifuged at 16,000 × g for 5 min at 4 °C, and an aliquot of the supernatant was run on SDS-PAGE and transferred to nitrocellulose membrane. The membrane was probed with a mouse anti-FLAG (Sigma) monoclonal antibody or a rabbit anti-SUR1 serum raised against a C-terminal peptide of SUR1 (KDSVFASFVRADK) followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) and visualized by enhanced chemiluminescence (Super Signal West Femto, Pierce). Tubulin was also probed and served as a loading control.
Surface Protein Biotinylation
Transfected COSm6 cells were placed on ice and washed twice with cold PBS. Cells were then incubated with 1 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (sulfosuccinimidyl-2-[biotinamido]ethyl-1,3-dithiopropionate; Pierce) in PBS for 30 min on ice for biotinylation of surface proteins. The reaction was terminated by incubating cells with 20 mm glycine in PBS for 10 min on ice followed by three washes with cold PBS. Cells were then lysed immediately in 1% Triton X-100 lysis buffer. Cell lysate was cleared by centrifugation at 14,000 rpm for 20 min at 4 °C. Biotinylated proteins from equal amounts of total protein of each sample were pulled down by incubation with NeutrAvidin-agarose beads (Pierce) overnight at 4 °C. The beads were washed three times with lysis buffer, and proteins were eluted by incubation with SDS sample buffer. Eluted proteins were separated by SDS-PAGE, and SUR1 was detected with an anti-SUR1 antibody as described previously (49).
Immunofluorescence Staining
COSm6 cells transfected with WT Kir6.2 and WT or F27S f-SUR1were plated on coverslips 1 day before the experiment and treated overnight with DMSO, tolbutamide, or carbamazepine. To label channels expressed at the cell surface, cells were washed once with ice-cold PBS and then incubated in M2 anti-FLAG antibody (10 μg/ml in Opti-MEM plus 0.1% BSA) for 1 h at 4 °C. The cells were washed once with cold PBS, fixed with ice-cold methanol for 10 min on ice, washed three times with cold PBS, and subsequently incubated in blocking buffer (PBS + 2% BSA + 1% normal goat serum) for 1 h followed by incubation with Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (Invitrogen; 1:300 dilution in blocking buffer) for 1 h at room temperature. The cells were then washed twice with PBS, and the coverslips were mounted on microscope slides using VECTASHIELD Mounting Medium for Fluorescence with DAPI to counterstain the nuclei. Cells were viewed using a Zeiss LSM780 confocal microscope.
Chemiluminescence Assay
COSm6 cells in 35-mm dishes were fixed with 2% paraformaldehyde for 20 min at room temperature 48 h post-transfection. Fixed cells were preblocked in PBS + 0.1% BSA for 1 h, incubated in M2 anti-FLAG antibody (10 μg/ml) for 1 h, washed 4 × 30 min in PBS + 0.1% BSA, incubated in horseradish peroxidase-conjugated anti-mouse secondary antibodies (Amersham Biosciences; 1:1,000 dilution) for 20 min, and washed again 4 × 30 min in PBS + 0.1% BSA and 2 × 5 min in PBS. The chemiluminescence signal was read in a TD-20/20 luminometer (Turner Designs) after 10-s incubation in Power Signal ELISA luminol solution (Pierce). Results of each experiment are the average of two dishes. Signals observed in untransfected cells were subtracted as background (∼10% of that observed in cells expressing WT channels). Data shown in figures are the average of at least three independent experiments.
Metabolic Labeling and Immunoprecipitation
COSm6 cells were plated on 35-mm dishes and transfected with WT Kir6.2 and F27S f-SUR1. At 40–48 h post-transfection, cells were washed twice with methionine/cysteine-free DMEM, starved in methionine/cysteine-free DMEM supplemented with 5% dialyzed FBS for 30 min, and then metabolically labeled with l-[35S]methionine/cysteine (Tran35S-Label, 120–200 μCi, MP Biomedicals, Solon, OH) for 30 min in the same medium. Labeled cultures were washed once with PBS, twice with regular medium (DMEM supplemented with 10% FBS), and chased for various times in regular medium at 37 °C. At the end of each chase, cells were washed twice with ice-cold PBS and lysed in 200 μl of 1% Triton X-100 lysis buffer described above on ice for 30 min. For immunoprecipitation, each sample was incubated with 50 μl of FLAG antibody-agarose beads overnight at 4 °C. The precipitate was washed three times with lysis buffer and once with Tris-buffered saline, and the proteins were eluted with FLAG peptide. The eluted proteins were separated by SDS-PAGE, and the dried gel was visualized by autoradiography using a Quantity One phosphorimaging system (Bio-Rad).
Patch Clamp Recording
COSm6 cells were transfected with f-SUR1 and Kir6.2 cDNAs along with cDNA encoding the green fluorescent protein to help identify transfected cells. Cells were replated onto coverslips 24 h post-transfection and used for inside-out patch voltage clamp recording the following day. Micropipettes were pulled from non-heparinized Kimble glass (Fisher) on a horizontal puller (Sutter Instrument, Novato, CA) with a resistance of typically ∼1–2 megaohms. The bath (intracellular) and pipette (extracellular) solutions were K-INT (140 mm KCl, 10 mm K-HEPES, and 1 mm K-EGTA, pH 7.3). ATP was added as the potassium salt. Recording was performed at room temperature, currents were measured at a membrane potential of −50 mV, and inward currents are shown as upward deflections.
For whole cell current recordings, INS-1 and human islets β-cells were held at −70 mV, and the KATP current was recorded at two voltage steps (−50 and −90 mV) applied every 2 s. Pipettes had typical resistance of 3–5 megaohms and were filled with K-INT solution without ATP. Outside Tyrode's solution contained 137 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 0.5 mm MgCl2, 5 mm Na-HEPES, 3 mm NHCO3, and 0.16 mm NaH2PO4, pH 7.2. To identify human β-cells, dissociated human islet cells plated on coverslips were placed into the recording chamber and stained briefly with 0.01% dithizone solution (in PBS) followed by a few minutes of washout with normal Tyrode's solution. Only dithizone-positive cells were recorded.
86Rb+ Efflux Assay
Transfected COSm6 cells in 6-well plates were incubated overnight in medium containing 86RbCl (0.1 μCi/ml) with or without carbamazepine. In experiments shown in Fig. 6B, carbamazepine was removed for 15, 30, or 60min before incubating cells with metabolic inhibitors (2.5 μg/ml oligomycin and 1 mm 2-deoxy-d-glucose) in Krebs-Ringer solution for 30 min in the presence of 86Rb+. In experiments shown in Fig. 6C, there was no preremoval of carbamazepine prior to incubation with metabolic inhibitors, and efflux was measured in Ringer's solution containing metabolic inhibitors with or without 200 μm diazoxide. Efflux was measured over a 40-min incubation period. At the end of the incubation, the efflux solution was collected, and cells were lysed. 86Rb+ in the solution and the cell lysate was counted. The percentage of efflux was calculated as the radioactivity in the efflux solution divided by the total activity from the solution and cell lysate.
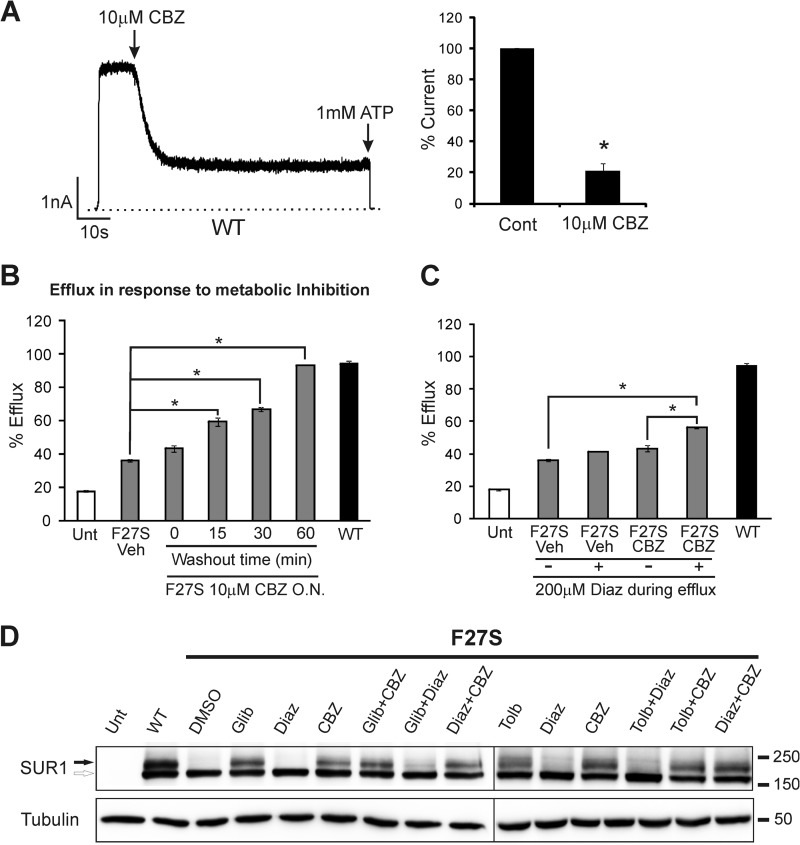
FIGURE 6.
Effects of carbamazepine on KATP channel function. A, carbamazepine inhibits WT channel activity in isolated membrane patches. Carbamazepine (CBZ) (10 μm) was applied acutely to the cytoplasmic face of isolated membrane patches containing WT channels. A representative current trace is shown on the left, and the averaged data (mean ± S.E.) are shown on the right (n = 3). Cont, control. *, p < 0.001 by Student's t test. B, KATP channel activity in intact cells in response to metabolic inhibition as assessed by the 86Rb+ efflux assay. Cells were treated with metabolic inhibitors for 30 min prior to efflux measurements. Efflux over a 40-min period is expressed as percentage of total counts. F27S cells were treated overnight (O.N.) with 10 μm carbamazepine, and carbamazepine was removed 15, 30, or 60 min (washout) prior to incubation with metabolic inhibitors. Untreated untransfected cells (Unt) and cells transfected with WT channels are included as controls. C, same as B except that carbamazepine was not washed out prior to incubation with metabolic inhibitors, and the efflux was measured with or without the addition of 200 μm diazoxide (Diaz). In B and C, each bar represents the mean ± S.E. (n = 3–10). *, p < 0.001 comparing F27S vehicle (Veh) with various treatment groups by one-way analysis of variance with Bonferroni post hoc test. Error bars represent S.E. D, Western blots show the effects of different drug combinations on the processing efficiency of F27S SUR1 co-expressed with WT Kir6.2 in COSm6 cells. The concentrations of the drugs used in the overnight (16-h) combination treatment are 5 μm glibenclamide (Glib), 300 μm tolbutamide (Tolb), 10 μm carbamazepine, and 200 μm diazoxide. Note that although diazoxide nearly abolished the rescue effect of glibenclamide or tolbutamide it had no effect on the ability of carbamazepine to correct the processing defect of F27S SUR1. The lines separate different blots.
Statistics
Data are shown as means ± S.E. Statistical analysis was performed using one-way analysis of variance and Bonferroni post hoc test when comparing three or more groups. When only two groups were compared, Student's t test was used.
RESULTS
Carbamazepine Improves the Processing of Trafficking-impaired SUR1 Mutants with Domain Specificity
SUR1 belongs to the ABC transporter protein family with a core structure consisting of two transmembrane domains designated TMD1 and TMD2, each followed by nucleotide binding domains NBD1 and NBD2, respectively. In addition, it contains an N-terminal transmembrane domain designated TMD0, which is linked to the core structure via a cytoplasmic loop called L0 (50) (Fig. 1A). SUR1 has two N-linked glycosylation sites that undergo core glycosylation in the ER. Co-assembly with Kir6.2 conceals -RKR- ER retention/retrieval motifs in the SUR1/Kir6.2 channel and allows the complex to traffic to the Golgi where N-linked glycosylation of SUR1 undergoes further processing to give rise to the higher molecular weight complex-glycosylated form (51). To test whether carbamazepine can correct processing defects of KATP channels caused by SUR1 mutations, we screened a total of 19 mutations (Fig. 1A), which have been shown previously to impair channel expression at the cell surface (29, 31, 44, 52). COSm6 cells were co-transfected with mutant SUR1 and WT Kir6.2 cDNAs and treated overnight (∼16 h) with 10 μm carbamazepine (structure shown in Fig. 1B) or 0.1% DMSO as vehicle control. Western blotting of whole-cell lysate was used to assess the processing efficiency of the mutants. WT channels served as a positive control, and cells treated with 5 μm glibenclamide were included for comparison as mutations in TMD0 of SUR1 are effectively rescued by this sulfonylurea drug (29, 31). Carbamazepine at 10 μm improved the processing of several mutants as judged by an increase in the mature, complex-glycosylated upper band (Fig. 1C). The identity of this band as the complex-glycosylated SUR1 was confirmed by its sensitivity to the peptide-N-glycosidase F (data not shown and Ref. 53). To our surprise, all mutations responsive to carbamazepine are located in TMD0 and have been shown previously to be rescued by sulfonylureas. By contrast, all mutations located outside of TMD0 in the ABC core structure shared by CFTR showed little or no increase in the upper band signal (data not shown).
The TMD0 mutants responded to carbamazepine to variable extents. At 10 μm, the F27S and E128K mutations exhibited the greatest improvement to nearly the level seen with 5 μm glibenclamide; R74W, A116P, and V187D showed moderate responses; whereas G7R and N24K, which have less severe processing defects (31), had weak responses (Fig. 1C). Dose-response relationships were further determined for F27S, A116P, and V187D. These mutants have been shown previously to exhibit normal gating properties when rescued to the plasma membrane and are thus suitable for subsequent functional rescue studies (29, 31). Fig. 1D shows that carbamazepine in the range of 0.2–50 μm improved the intensity of the upper band of all three SUR1 mutants in a dose-dependent manner.
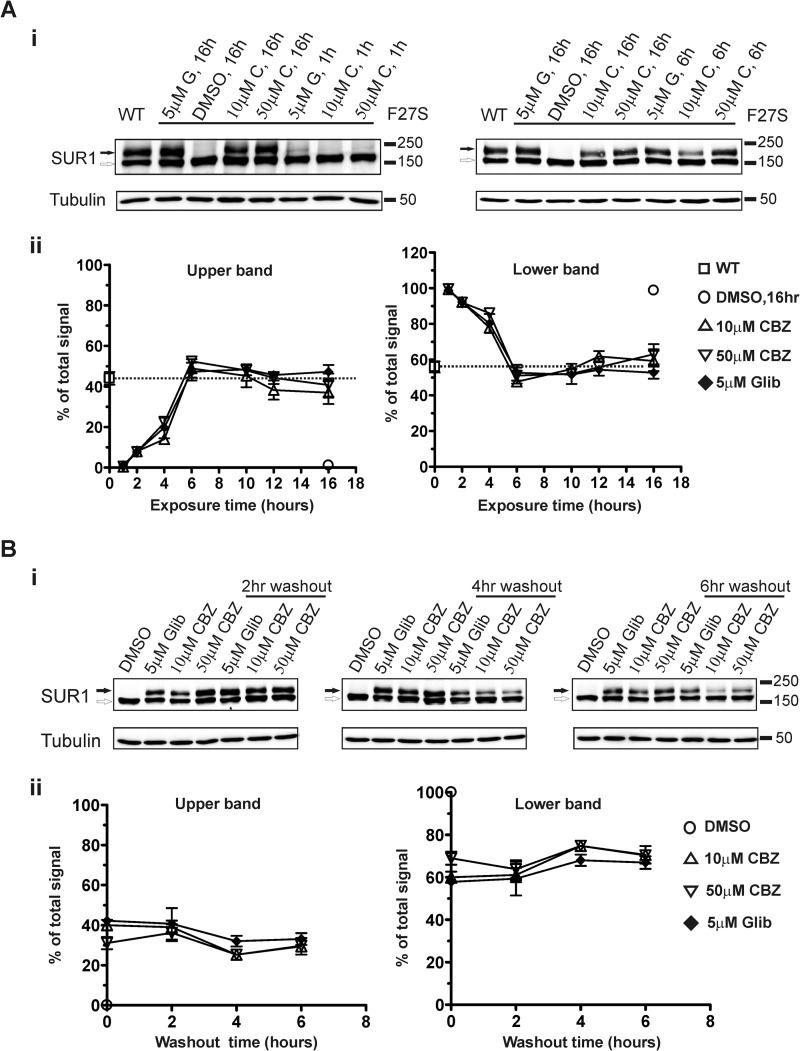
Time Course and Duration of the Carbamazepine Rescue Effect
To characterize the carbamazepine effect further, we determined the time course and duration of the rescue effect using the F27S mutation as an example. The effect of carbamazepine at 50 μm could be detected as early as 1 h and peaked at ∼6 h after treatment; 10 μm carbamazepine followed a similar time course (Fig. 2A). To determine the duration of the rescue effect, carbamazepine was removed after a 16-h treatment for various times, and the glycosylation states of mutant SUR1 were determined. Our results show that rescue persisted for up to 6 h after drug removal (Fig. 2B). For comparison, glibenclamide treatment was included in both series of experiments. Interestingly, the time course of action of carbamazepine as in mutation specificity was very similar to that of glibenclamide (Fig. 2, A and B).
FIGURE 2.
Time course and duration of the rescue effect of carbamazepine. A, COSm6 cells were transiently transfected with WT Kir6.2 and WT or F27S mutant SUR1 cDNAs and treated with 10 or 50 μm carbamazepine (C) or 5 μm glibenclamide (G) for 0, 1, 2, 4, 6, 10, 12, or 16 h. Panel i, blots showing cells treated with glibenclamide or carbamazepine for 1 h (left) when an effect on the upper band signal was first detected and for 6 h (right) when the effect began to plateau. WT channels and mutant receiving various treatments for 16 h are included for comparison. Panel ii, time course of the effect of glibenclamide and carbamazepine. Upper and lower band signals (left and right plots, respectively) from blots similar to those shown in panel i were quantified by densitometry and expressed as percentage of the total signal (upper + lower) of each sample. Each data point represents the mean ± S.E. of three to five independent experiments. WT without any treatment and F27S mutant treated with DMSO (0.1%) for 16 h are shown for comparison. B, COSm6 cells transfected with WT Kir6.2 and F27S mutant SUR1 cDNAs were treated with 0.1% DMSO, 10 or 50 μm carbamazepine (CBZ), or 5 μm glibenclamide (Glib) for 16 h. The drugs were then removed from the culture medium, and cells were cultured for an additional 2, 4, or 6 h in fresh medium. Panel i, blots showing that the rescue effect of carbamazepine remains for at least 6 h after drug removal. Panel ii, quantification of blots shown in panel i by densitometry. Upper and lower band signals (left and right plots, respectively) were expressed as percentage of the total signal of each sample. F27S treated with DMSO for 16 h is shown for comparison. Each data point represents the mean ± S.E. of three independent experiments. No statistical difference was found between the 0 time point and 2, 4, or 6 h of washout in all three treatment groups (p > 0.05 by Student's t test). Error bars represent S.E.
Carbamazepine Restores Surface Expression of Trafficking-impaired KATP Channels
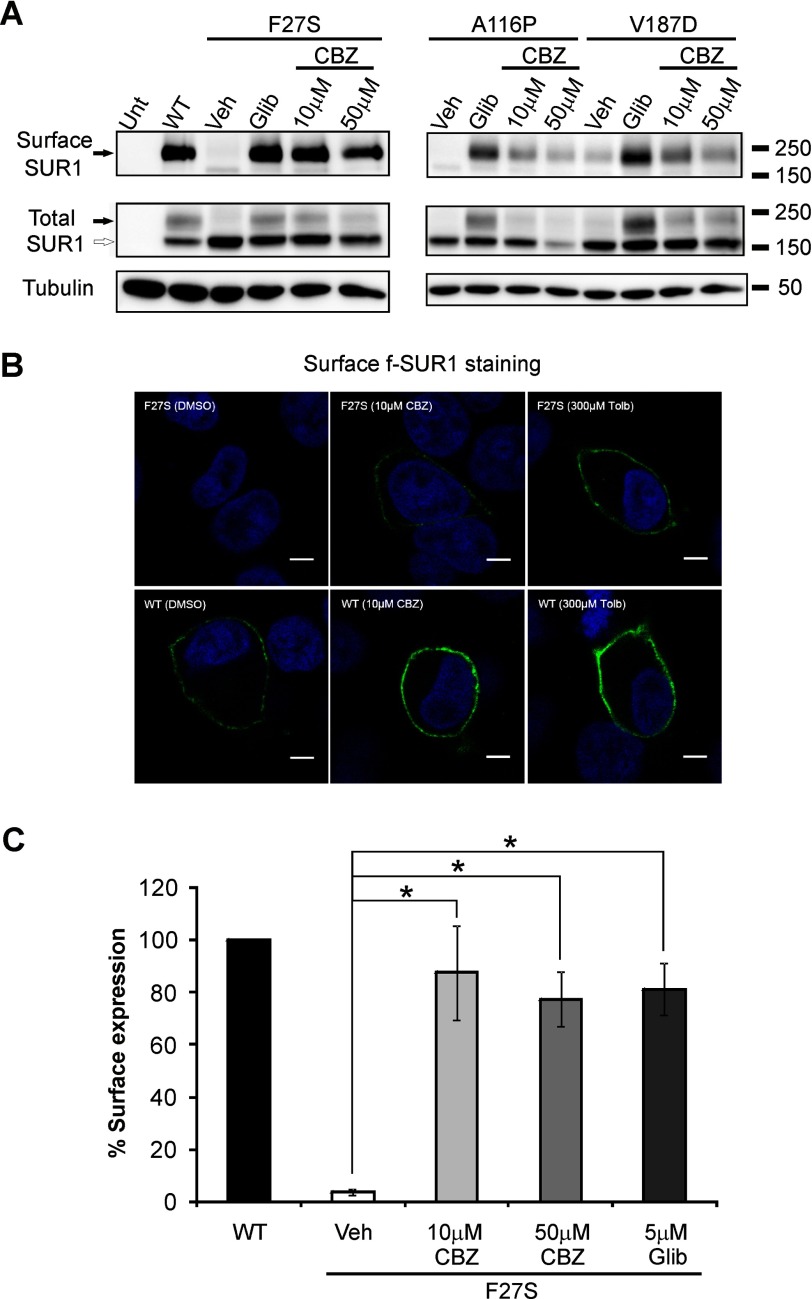
To directly test whether increased processing of mutant SUR1 upon carbamazepine treatment leads to increased surface expression of mutant channels, we monitored mutant channel expression in the plasma membrane using three different approaches. In surface protein biotinylation experiments, there was a significant increase in biotinylated F27S, A116P, or V187D SUR1 in cells treated with carbamazepine or glibenclamide as compared with cells treated with vehicle alone (Fig. 3A). The F27S mutant was further analyzed by surface staining and chemiluminescence assays. Surface staining of FLAG-tagged (N terminus) SUR1 showed a clear increase in surface expression of the F27S mutant upon carbamazepine treatment, resembling that seen in cells treated with the sulfonylurea drug tolbutamide (Fig. 3B). In addition, we quantified the amount of SUR1 surface expression using a chemiluminescence assay as described under “Materials and Methods” and observed a pronounced increase in surface expression of F27S mutant channels in response to 10 or 50 μm carbamazepine treatment (Fig. 3C). These results demonstrate the utility of carbamazepine in restoring surface expression of the trafficking-impaired TMD0 mutants.
FIGURE 3.
Carbamazepine restores surface expression of trafficking-impaired SUR1 mutants. A, surface SUR1 detected using surface protein biotinylation followed by immunoprecipitation and immunoblotting. COSm6 cells were transiently transfected with WT Kir6.2 and WT or mutant SUR1 cDNAs; treated with 0.1% DMSO (Veh), 10 or 50 μm carbamazepine (CBZ), or 5 μm glibenclamide (Glib) for 12 h; and then subjected to surface biotinylation. Only the upper complex-glycosylated band was pulled down by the NeutrAvidin beads (top). Total SUR1 detected in whole-cell lysate in the corresponding samples is also shown with tubulin as a loading control (middle and lower). B, surface expression of mutant SUR1 upon rescue by carbamazepine and tolbutamide (Tolb) as detected by immunostaining of the extracellular FLAG epitope tag of f-SUR1 in non-permeabilized cells. Scale bar, 5 μm. C, quantification of surface expression of KATP channels by chemiluminescence assays. Each bar represents mean ± S.E. of three to four experiments. *, p < 0.001 comparing F27S vehicle with various treatment groups by one-way analysis of variance with Bonferroni post hoc test. Error bars represent S.E. Unt, untransfected cells.
Carbamazepine Improves Mutant Channel Biogenesis Efficiency to Increase Channel Surface Expression Independently of Its Effect on Autophagy
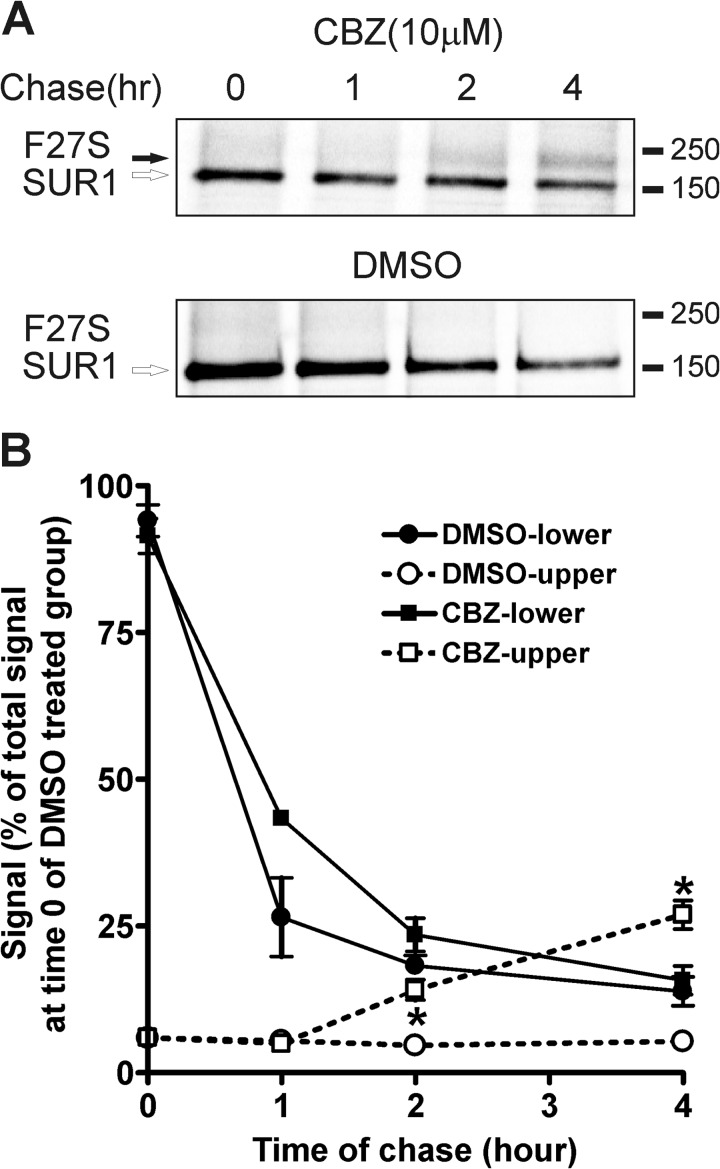
Carbamazepine could increase the abundance of the SUR1 upper band by enhancing mutant channel processing efficiency directly akin to the mechanism we have reported for sulfonylureas (54). To test this, we conducted metabolic pulse-chase experiments to monitor the kinetics of F27S mutant SUR1 maturation from the core-glycosylated to the complex-glycosylated band in cells treated with carbamazepine or the vehicle DMSO. As shown in Fig. 4, in cells receiving carbamazepine treatment during the chase, mutant SUR1 acquired the complex-glycosylated form within 2 h, similar to that reported previously for WT SUR1 (30). By contrast, mutant SUR1 remained as the core-glycosylated form even after 4 h of chase in cells treated with DMSO. Note that the low maturation efficiency of SUR1 from the core-glycosylated to the complex-glycosylated form observed in the carbamazepine-treated F27S mutant (∼20% of pulse-labeled SUR1) is similar to the value obtained previously for WT SUR1 in experiments where pulse-labeled SUR1 was chased for up to 24 h (29, 30, 36).
FIGURE 4.
Carbamazepine increases F27S mutant channel surface expression by improving processing and maturation of the channel complex during biogenesis. A, COSm6 cells transfected with F27S SUR1 and WT Kir6.2 were pulse-labeled with Tran35S-Label for an hour and chased for 0–4 h in regular medium. SUR1 was immunoprecipitated using anti-FLAG-agarose beads and analyzed by phosphorimaging. Shown are representative gels from cells treated with 10 μm carbamazepine (CBZ) or DMSO vehicle control during the chase. B, the upper and lower band signals from gels shown in A were quantified using a Bio-Rad phosphorimaging system and expressed as percentage of total signal at time 0 of DMSO-treated group. Data points represent the mean ± S.E. of three to four experiments. The upper band signals in the carbamazepine-treated group are significantly higher than those in the DMSO-treated group at 2 and 4 h of chase. *, p < 0.001 by Student's t test. Error bars represent S.E.
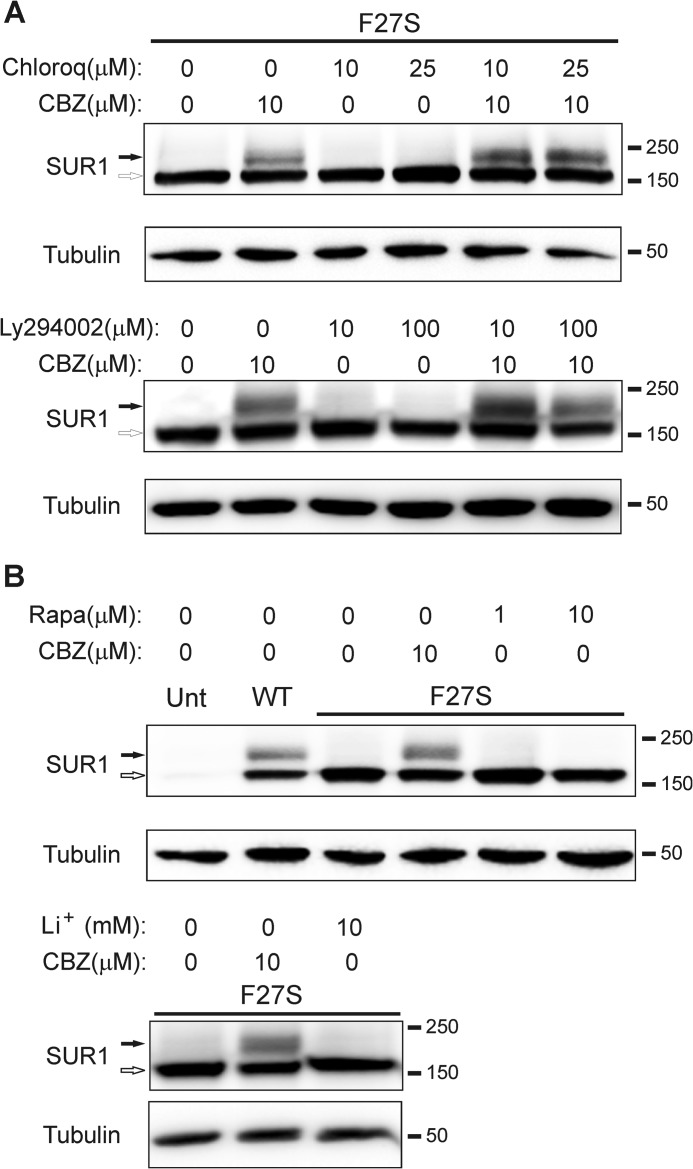
A recent study has shown that carbamazepine stimulates autophagy to accelerate clearance of misfolded α1-antitrypsin (40), raising the possibility that the drug may improve the overall functional capacity of the secretory pathway by preventing pathological accumulation of misfolded proteins. To determine whether carbamazepine improves mutant channel processing efficiency by activating the autophagy-lysosome pathway, we incubated cells with carbamazepine in the presence or absence of chloroquine, which inhibits autolysosome function (55). Chloroquine at either 10 or 25 μm did not diminish the effect of carbamazepine; in fact, chloroquine appeared to further increase the intensity of the mutant SUR1 upper band (Fig. 5A; see Discussion). Another compound, Ly294002, which inhibits PI3K to inhibit autophagy (56, 57), also failed to block the rescue effect of carbamazepine (Fig. 5A). In addition, we tested whether rapamycin, which is known to induce cell autophagy by inhibiting mammalian target of rapamycin (58), as well as Li+, which stimulates autophagy by inhibiting phosphoinositol phosphatases (59), would mimic the rescue effect of carbamazepine. Unlike carbamazepine, rapamycin at both 1 and 10 μm and lithium at 10 mm all failed to improve the processing efficiency of mutant SUR1 (Fig. 5B). Collectively, our results provide strong evidence that carbamazepine rescues mutant channels by improving the efficiency of their biogenesis independently of the autophagy pathway.
FIGURE 5.
The rescue effect of carbamazepine is not due to activation of autophagy. A, Western blot of SUR1 from COSm6 cells transfected with F27S SUR1 and WT Kir6.2 and treated with carbamazepine (CBZ), chloroquine (Chloroq), or both for 16 h (upper panel) or carbamazepine, Ly294002, or both for 16 h (lower panel) as indicated. WT SUR1 is shown as a control. B, Western blot of SUR1 from COSm6 cells transfected with F27S SUR1 and WT Kir6.2 and treated with carbamazepine or rapamycin (Rapa) or Li+, both of which are autophagy inducers, for 16 h as indicated.
Functional Effects of Carbamazepine on KATP Channels
Carbamazepine has been studied widely with documented actions on voltage-gated sodium channels (60), Ca2+ channels (61), and GABAA receptors (62). However, effects of carbamazepine on KATP channel function have not been reported. Because pharmacological correctors could inhibit the function of target proteins (14), we sought to determine whether carbamazepine affects the activity of rescued KATP channels. In inside-out patch clamp recordings, acute bath application of carbamazepine caused a rapid decline of WT channel currents (Fig. 6A), indicating that carbamazepine inhibits KATP channel activity. To test whether this inhibitory effect compromises the ability of rescued mutant channels to respond to metabolic inhibition, we performed 86Rb+ efflux assays, which report channel activity in intact cells. We chose the F27S mutant for this analysis because it exhibited the greatest response to rescue by carbamazepine and has been shown previously to have WT-like gating properties (31). In F27S-transfected cells treated overnight with 10 μm carbamazepine, metabolic inhibition induced only a low level of efflux similar to that seen in vehicle-treated cells. This is in marked contrast to cells expressing WT channels (Fig. 6B). This result indicates that carbamazepine does prevent rescued F27S channels from being activated by metabolic inhibition. To test whether the inhibitory effect of carbamazepine could be reversed, the drug was removed from the medium for 15, 30, or 60 min prior to incubation with metabolic inhibitors, and 86Rb+ efflux was measured as described under “Materials and Methods.” A progressive increase in efflux through rescued F27S channels over a 40-min period was observed with longer washout; the efflux level was nearly comparable with that seen in WT channels with 60-min washout (Fig. 6B). These results demonstrate that carbamazepine modulates KATP channels in two ways: it promotes channel surface expression but also inhibits channel function. The inhibitory effect of carbamazepine on KATP channel function is reversible; upon extensive washout (∼1 h), physiological function of rescued surface F27S channels was recovered nearly completely.
An emerging concept in overcoming protein misfolding/mistrafficking diseases is that compounds that correct protein trafficking defects and those that potentiate the function of the protein may be combined to achieve greater functional recovery of misfolded proteins (24, 63). We tested this idea by examining the effects of carbamazepine and the KATP channel opener diazoxide on F27S channels using the 86Rb+ assay. In cells treated overnight with 10 μm carbamazepine without washout, metabolic inhibition alone failed to induce a statistically significant increase in efflux over a 40-min period compared with vehicle-treated cells (Fig. 6C). However, combination of metabolic inhibitors and 200 μm diazoxide resulted in a significantly higher efflux through the F27S channels in cells treated overnight with 10 μm carbamazepine compared with cells treated with vehicle alone without washout (Fig. 6C). These findings suggest that by combining the corrector effect of carbamazepine and the potentiator effect of diazoxide partial surface expression and functional recovery of mutant channels can be achieved without the need to remove carbamazepine. To ensure that diazoxide and carbamazepine can be co-administered without compromising the rescue effect of carbamazepine, we compared the processing efficiency of F27S SUR1 in cells treated overnight with diazoxide, carbamazepine, or both together. As a control, we also included cells treated with sulfonylureas or diazoxide alone or both sulfonylureas and diazoxide. Our results show that although diazoxide abolished the chaperoning effect of either glibenclamide or tolbutamide it did not interfere with the ability of carbamazepine to correct the processing defect of F27S SUR1 (Fig. 6D). These results support the feasibility of the carbamazepine-diazoxide combination therapy.
Carbamazepine Restores Surface Expression and Activity of Mutant KATP Channels in Rat Insulinoma Cells and Human Islet β-Cells
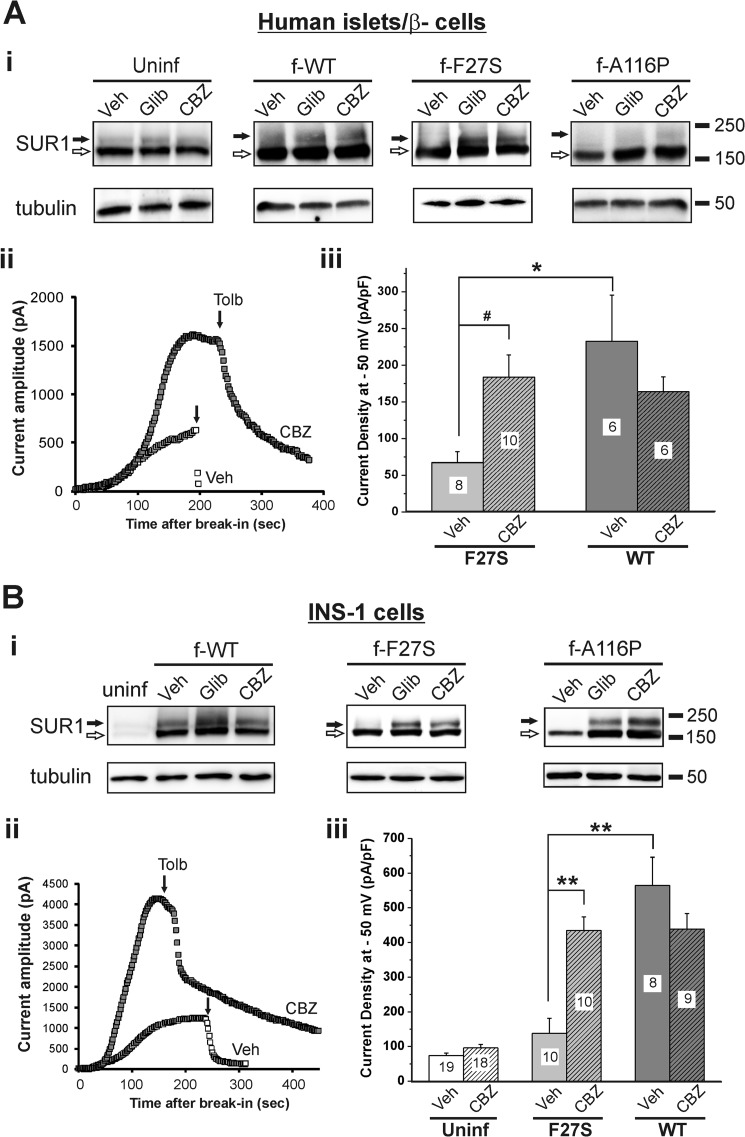
Having demonstrated the corrector effect of carbamazepine on mutant KATP channels expressed in COSm6 cells, we next asked whether the effect could be reproduced in pancreatic β-cells. This is important if carbamazepine is to be considered for clinical use in congenital hyperinsulinism patients harboring KATP channel trafficking mutations. We used human islets and β-cells as well as rat insulinoma INS-1 cells for these experiments. Human islets obtained through the Integrated Islet Distribution Program were co-infected overnight with adenoviruses carrying Kir6.2 and WT, F27S, or A116P f-SUR1 cDNAs. The FLAG epitope added to the N terminus of SUR1 allows distinction between exogenously expressed mutant and endogenously expressed WT KATP channels. Infected islets were then treated overnight with 0.1% DMSO, 5 μm glibenclamide, or 10 μm carbamazepine and harvested for Western blots. As was observed in COSm6 cells, overnight glibenclamide and carbamazepine treatments led to a marked increase in the upper SUR1 band in the F27S mutant and an obvious albeit weaker increase in the upper band in the A116P mutant in whole islet lysates (Fig. 7A, panel i). An increase in endogenous (detected by anti-SUR1 antibody in uninfected islets) and exogenous (detected by anti-FLAG antibody) WT upper SUR1 band was also observed. To measure surface channel density, we performed whole-cell patch clamp recordings (Fig. 7A, panel ii) in dissociated islet cells that were identified as β-cells based on positive staining with the Zn2+-binding dye dithizone as described under “Materials and Methods.” Results show that cells from islets treated overnight with carbamazepine followed by washout had significantly higher current density (184 ± 30 pA/picofarad in carbamazepine-treated versus 67 ± 15 pA/picofarad in DMSO-treated group, n = 10–11, p < 0.05), which was close to the value observed in cells from islets infected with WT channel viruses and treated with or without carbamazepine (Fig. 7A, panel iii). Because we cannot exclude the possibility that some dissociated human islet cells analyzed were misidentified as β-cells, the same experiments were repeated using the rat insulinoma INS-1 cells. These experiments yielded similar results (Fig. 7B). Together, these results demonstrate that carbamazepine effectively improved the processing and surface expression of the F27S and A116P SUR1 trafficking-impaired mutant KATP channels in pancreatic β-cells.
FIGURE 7.
Carbamazepine restores surface expression and function of trafficking-impaired SUR1 mutants in β-cells. A, panel i, representative SUR1 blots from uninfected human islets (probed with anti-SUR1 antibody) and human islets infected with adenoviruses carrying WT Kir6.2 and WT or F27S or A116P mutant f-SUR1 cDNAs (probed with anti-FLAG antibody) and treated with DMSO, 5 μm glibenclamide (Glib), or 10 μm carbamazepine (CBZ) for 16 h. Panel ii, representative whole-cell patch clamp recordings measuring KATP current density in control and drug-treated human β-cells infected with the F27S mutant viruses (recordings are from two cells with similar membrane capacitance of ∼10 picofarads (pF)). Dissociated human islet β-cells were identified by staining with 0.01% dithizone briefly followed by washout before recording. KATP currents were activated upon perfusion with Tyrode's solution containing 200 μm diazoxide. Tolbutamide (Tolb) (300 μm) was used to verify that the currents observed were from KATP channels. Panel iii, the averaged current density measured by whole-cell recordings. Each bar represents the mean ± S.E. of the number of patches shown in the bar. B, same as in A except INS-1 cells were used for the experiments. #, p < 0.05; *, p < 0.01; **, p < 0.001 by one-way analysis of variance with Bonferroni post hoc test. Error bars represent S.E. uninf, uninfected; Veh, vehicle.
DISCUSSION
Mutations in KATP channels that prevent channels from reaching the cell surface are major causes underlying congenital hyperinsulinism. Patients with these mutations usually present with a severe disease phenotype unresponsive to diazoxide treatment, necessitating partial or total pancreatectomy to prevent life-threatening hypoglycemia. Identification of small molecules that correct the folding and trafficking defects of such mutant channels would provide an alternative treatment option for these patients. In this study, we show that carbamazepine, a clinically used anticonvulsant, corrects the surface expression defects caused by a subset of SUR1 mutations identified in congenital hyperinsulinism. In addition, we report that carbamazepine inhibits KATP channel activity. The function of mutant channels rescued to the cell surface, however, can be recovered after carbamazepine is washed out. These findings reveal KATP channels as a new target for carbamazepine with dual effects on trafficking and gating.
Mechanism by Which Carbamazepine Corrects KATP Channel Processing Defects
Discovered in the 1950s, carbamazepine is used primarily to treat seizure disorders and neuropathic pain but also other mental illnesses such as bipolar disorder. Its predominant mechanism of action is thought to be the stabilization of the inactivated state of the voltage-gated sodium channels, although effects on Ca2+ channels and GABAA receptors have also been reported (60). More recently, carbamazepine has been shown to induce cell autophagy, thus facilitating clearance of misfolded protein aggregates by autolysosomes and alleviating pathology caused by protein misfolding in a number of experimental models of neurodegenerative diseases and liver disease (40, 64). Interestingly, stimulation of autophagy has also been proposed as a strategy to treat cystic fibrosis caused by the misfolding CFTR mutant ΔF508 by promoting mutant trafficking to the cell surface (65). Carbamazepine has emerged as a CFTR corrector from a chemical library screen (19), although whether it corrects the CFTRΔF508 trafficking defect by stimulating autophagy is yet to be determined. These recent studies raise the possibility that carbamazepine may improve mutant KATP channel processing by stimulating autophagy. However, our data suggest that this is not the case. First, inhibition of autophagy by chloroquine (55) or Ly294002 (57) did not abolish the ability of carbamazepine to rescue mutant KATP channels. In fact, we observed an even greater increase in the intensity of the SUR1 upper band when chloroquine was co-administered with carbamazepine. A likely explanation is that chloroquine inhibits lysosomal degradation of endocytosed surface channels rescued by carbamazepine. Second, treating cells with rapamycin or Li+, both of which are known to stimulate autophagy by discrete mechanisms (58, 59), did not improve mutant KATP channel processing. Instead, our findings that carbamazepine directly inhibits KATP channel activity in isolated membrane patches and improves the processing efficiency of SUR1 in metabolic pulse-chase experiments suggest that carbamazepine may act as a small molecule chaperone, like sulfonylureas, to overcome the folding/processing defects of mutant KATP channels.
To our surprise, all mutations that respond to carbamazepine reside in the SUR1 TMD0 domain, a domain absent in CFTR. The TMD0 domain is known to play a key role in the physical and functional coupling of SUR1 and Kir6.2 (42, 43). A reasonable hypothesis is that the TMD0 mutations render channels unable to reach a mature conformation by disrupting interactions with Kir6.2, and small molecules such as sulfonylureas and carbamazepine stabilize TMD0-Kir6.2 association to allow channel maturation. Consistent with this idea, the effects of sulfonylureas and carbamazepine are only seen when SUR1 mutants carrying TMD0 mutations are co-expressed with Kir6.2. SUR1 proteins with TMD0 trafficking mutations and in which the ER retention signal RKR has been mutated to AAA to allow mutant protein to escape the ER quality control surveillance in the absence of the assembly partner Kir6.2 do not respond to sulfonylureas or carbamazepine in the absence of Kir6.2 co-expression (54).3 Future work will determine whether carbamazepine binds directly to the channel complex and if so where the binding site is located. Finally, another potential mechanism that needs to be considered is whether carbamazepine affects channel biogenesis efficiency indirectly by targeting other proteostasis regulators of KATP channels such as cellular chaperones and ER-associated degradation machinery.
Therapeutic Implications
Many pharmacological chaperones that stabilize misfolded target proteins act as specific inhibitors for those proteins (66). As such, only reversible inhibitors are suitable for clinical use. We have shown previously that glibenclamide, which rescues a number of KATP trafficking mutants with high efficiency, remains bound to rescued channels due to its high binding affinity even after the drug has been removed (29). Consequently, mutant channels rescued to the cell surface remain non-functional. By contrast, the inhibitory effects of the low affinity sulfonylurea tolbutamide and the new KATP corrector carbamazepine identified here are reversible upon extensive washout to recover channel function. Interestingly, combining the KATP channel opener diazoxide with metabolic inhibition significantly increased the activity of carbamazepine-rescued mutant channels even without washout of carbamazepine (Fig. 6C). This suggests that diazoxide may antagonize the inhibitory effect of carbamazepine to potentiate the function of rescued F27S channels. These findings are of clinical relevance. In a recent study in which a large number of diazoxide-responsive versus diazoxide-unresponsive dominant congenital hyperinsulinism gating mutations were analyzed, a correlation between the severity of the MgADP/diazoxide gating defects and clinical responsiveness to diazoxide treatment was observed (67). This study reveals a threshold-like phenomenon in which residual activity of mutant channels above a certain level, even if still much lower than WT channels, is strongly correlated with positive clinical outcome. Thus, partial functional recovery of trafficking-impaired channels in response to a combined carbamazepine and diazoxide treatment may be sufficient to alleviate disease symptoms to avoid pancreatectomy. This would provide an example of how correctors and potentiators may be used together to restore the function of trafficking-impaired proteins. Feasibility of the carbamazepine and diazoxide combination therapy strategy is further supported by our finding that the two drugs can be co-applied to cells without compromising the ability of carbamazepine to rescue the trafficking defects of the F27S mutant (Fig. 6D). This is in stark contrast to the rescue effect of sulfonylureas that was abolished when diazoxide was co-administered. An interesting question to address in the future is what is the structural basis that underlies this compatibility difference.
Importantly, our results demonstrate that the effect of carbamazepine is observed not only in COSm6 cells but also in two additional physiologically relevant experimental systems, namely the rat β-cell line INS-1 and freshly isolated primary human β-cells. The effective concentrations of carbamazepine observed to rescue KATP mutants in our study are similar to those used to demonstrate effects on Na+ channels (60), suggesting that the clinically approved dosage (circulating concentration is ∼4–12 mg/liter, which is ∼17–51 μm) is likely to have an effect on KATP channels. Although our study focused mostly on one mutation (F27S) that responded well to carbamazepine, there are many additional congenital hyperinsulinism-associated SUR1 TMD0 mutations waiting to be tested (4). Thus, the mutations that can be targeted for carbamazepine-based therapy will undoubtedly expand. Aside from implications in treating congenital hyperinsulinism caused by trafficking-impaired KATP channels with SUR1 TMD0 mutations, the finding that carbamazepine has additional targets than previously appreciated has implications for its clinical use for the treatment of approved diseases. It is worth noting that aside from their well established role in regulating hormone secretion KATP channels are also widely expressed in the brain and have been implicated in epilepsy (68, 69). Whether the clinical effect of carbamazepine as an anticonvulsant is related in part to an effect on KATP channels in the central nervous system is an interesting issue to address in the future. Regardless, by understanding the full pharmacological profile of carbamazepine, it might be possible to better manage side effects that arise from unintended targets.
In summary, we demonstrate in this study that carbamazepine is a novel corrector for KATP channels with mutations in the TMD0 domain of SUR1. Our finding raises many future questions. It remains to be determined whether carbamazepine binds directly to the KATP channel complex or whether it targets other proteins to indirectly affect channel biogenesis and/or gating. If carbamazepine binds to KATP channels directly, delineation of the binding site(s) may shed light on how it overcomes the folding/processing defects caused by SUR1 TMD0 mutations and on the mechanisms governing the folding and assembly of the heteromeric KATP channel complex. Lastly, a direct demonstration of the beneficial effect of carbamazepine on restoring surface expression and function of mutant KATP channels in β-cells derived from congenital hyperinsulinism patients carrying SUR1 TMD0 trafficking mutations will help shepherd translation of our finding into disease therapy.
Acknowledgments
The INS-1 cell line clone 832/13 was kindly provided by Dr. Christopher B. Newgard. We thank Dr. Leslie Satin for advice on preparation of single cells from human islets for electrophysiological recording. We are grateful to Dr. William Skach and Dr. Prasanna Devaraneni for helpful comments on the manuscript. Human islets were provided by the Integrated Islets Distribution Program through the Pilot Program for Human Islet Research.
This work was supported, in whole or in part, by National Institutes of Health Grants T32 DK007680 (to Richard H. Goodman; in support of P.-C. C.) and DK57699 (to S.-L. S.). This work was also supported by March of Dimes Foundation Research Grant 1-2001-707 (to S.-L. S.).
E. M. Olson and S.-L. Shyng, unpublished data.
- SUR1
- sulfonylurea receptor 1
- CFTR
- cystic fibrosis transmembrane conductance regulator
- KATP
- ATP-sensitive potassium
- Kir6.2
- inwardly rectifying potassium channel 6.2
- NBD
- nucleotide binding domain
- TMD
- transmembrane domain
- ER
- endoplasmic reticulum
- ABC
- ATP-binding cassette
- tTA
- tetracycline-inhibited transactivator
- m.o.i.
- multiplicity of infection.
REFERENCES
- 1. Stanley C. A. (2002) Advances in diagnosis and treatment of hyperinsulinism in infants and children. J. Clin. Endocrinol. Metab. 87, 4857–4859 [DOI] [PubMed] [Google Scholar]
- 2. Dunne M. J., Cosgrove K. E., Shepherd R. M., Aynsley-Green A., Lindley K. J. (2004) Hyperinsulinism in infancy: from basic science to clinical disease. Physiol. Rev. 84, 239–275 [DOI] [PubMed] [Google Scholar]
- 3. Glaser B., Thornton P. S., Herold K., Stanley C. A. (1998) Clinical and molecular heterogeneity of familial hyperinsulinism. J. Pediatr. 133, 801–802 [DOI] [PubMed] [Google Scholar]
- 4. Gloyn A. L., Siddiqui J., Ellard S. (2006) Mutations in the genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 27, 220–231 [DOI] [PubMed] [Google Scholar]
- 5. Aguilar-Bryan L., Bryan J. (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20, 101–135 [DOI] [PubMed] [Google Scholar]
- 6. Ashcroft F. M. (2005) ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Investig. 115, 2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nichols C. G. (2006) KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476 [DOI] [PubMed] [Google Scholar]
- 8. Huopio H., Shyng S. L., Otonkoski T., Nichols C. G. (2002) KATP channels and insulin secretion disorders. Am. J. Physiol. Endocrinol. Metab. 283, E207–E216 [DOI] [PubMed] [Google Scholar]
- 9. Welch W. J. (2004) Role of quality control pathways in human diseases involving protein misfolding. Sem. Cell Dev. Biol. 15, 31–38 [DOI] [PubMed] [Google Scholar]
- 10. Määttänen P., Gehring K., Bergeron J. J., Thomas D. Y. (2010) Protein quality control in the ER: the recognition of misfolded proteins. Semin. Cell Dev. Biol. 21, 500–511 [DOI] [PubMed] [Google Scholar]
- 11. Aridor M., Hannan L. A. (2000) Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1, 836–851 [DOI] [PubMed] [Google Scholar]
- 12. Aridor M., Hannan L. A. (2002) Traffic jams II: an update of diseases of intracellular transport. Traffic 3, 781–790 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y., Bellamy W. P., Seabra M. C., Field M. C., Ali B. R. (2005) ER-associated protein degradation is a common mechanism underpinning numerous monogenic diseases including Robinow syndrome. Hum. Mol. Genet. 14, 2559–2569 [DOI] [PubMed] [Google Scholar]
- 14. Perlmutter D. H. (2002) Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr. Res. 52, 832–836 [DOI] [PubMed] [Google Scholar]
- 15. Powers E. T., Morimoto R. I., Dillin A., Kelly J. W., Balch W. E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 [DOI] [PubMed] [Google Scholar]
- 16. Anderson M. P., Welsh M. J. (1992) Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science 257, 1701–1704; Correction (1992) Science258, 1719 [DOI] [PubMed] [Google Scholar]
- 17. Qu B. H., Strickland E., Thomas P. J. (1997) Cystic fibrosis: a disease of altered protein folding. J. Bioenerg. Biomembr. 29, 483–490 [DOI] [PubMed] [Google Scholar]
- 18. Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834 [DOI] [PubMed] [Google Scholar]
- 19. Carlile G. W., Robert R., Zhang D., Teske K. A., Luo Y., Hanrahan J. W., Thomas D. Y. (2007) Correctors of protein trafficking defects identified by a novel high-throughput screening assay. Chembiochem 8, 1012–1020 [DOI] [PubMed] [Google Scholar]
- 20. Robert R., Carlile G. W., Liao J., Balghi H., Lesimple P., Liu N., Kus B., Rotin D., Wilke M., de Jonge H. R., Scholte B. J., Thomas D. Y., Hanrahan J. W. (2010) Correction of the Delta phe508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol. Pharmacol. 77, 922–930 [DOI] [PubMed] [Google Scholar]
- 21. Robert R., Carlile G. W., Pavel C., Liu N., Anjos S. M., Liao J., Luo Y., Zhang D., Thomas D. Y., Hanrahan J. W. (2008) Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Mol. Pharmacol. 73, 478–489 [DOI] [PubMed] [Google Scholar]
- 22. Sampson H. M., Robert R., Liao J., Matthes E., Carlile G. W., Hanrahan J. W., Thomas D. Y. (2011) Identification of a NBD1-binding pharmacological chaperone that corrects the trafficking defect of F508del-CFTR. Chem. Biol. 18, 231–242 [DOI] [PubMed] [Google Scholar]
- 23. Verkman A. S., Lukacs G. L., Galietta L. J. (2006) CFTR chloride channel drug discovery—inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr. Pharm. Des. 12, 2235–2247 [DOI] [PubMed] [Google Scholar]
- 24. Lukacs G. L., Verkman A. S. (2012) CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol. Med. 18, 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molinski S., Eckford P. D., Pasyk S., Ahmadi S., Chin S., Bear C. E. (2012) Functional rescue of F508del-CFTR using small molecule correctors. Front. Pharmacol. 3, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snider K. E., Becker S., Boyajian L., Shyng S. L., MacMullen C., Hughes N., Ganapathy K., Bhatti T., Stanley C. A., Ganguly A. (2013) Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J. Clin. Endocrinol. Metab. 98, E355–E363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Otonkoski T., Ammälä C., Huopio H., Cote G. J., Chapman J., Cosgrove K., Ashfield R., Huang E., Komulainen J., Ashcroft F. M., Dunne M. J., Kere J., Thomas P. M. (1999) A point mutation inactivating the sulfonylurea receptor causes the severe form of persistent hyperinsulinemic hypoglycemia of infancy in Finland. Diabetes 48, 408–415 [DOI] [PubMed] [Google Scholar]
- 28. Crane A., Aguilar-Bryan L. (2004) Assembly, maturation, and turnover of KATP channel subunits. J. Biol. Chem. 279, 9080–9090 [DOI] [PubMed] [Google Scholar]
- 29. Yan F., Lin C. W., Weisiger E., Cartier E. A., Taschenberger G., Shyng S. L. (2004) Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J. Biol. Chem. 279, 11096–11105 [DOI] [PubMed] [Google Scholar]
- 30. Yan F. F., Lin C. W., Cartier E. A., Shyng S. L. (2005) Role of ubiquitin-proteasome degradation pathway in biogenesis efficiency of β-cell ATP-sensitive potassium channels. Am. J. Physiol. Cell Physiol. 289, C1351–C1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan F. F., Lin Y. W., MacMullen C., Ganguly A., Stanley C. A., Shyng S. L. (2007) Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes 56, 2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwartz T. B., Meinert C. L. (2004) The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect. Biol. Med. 47, 564–574 [DOI] [PubMed] [Google Scholar]
- 33. Luna B., Feinglos M. N. (2001) Oral agents in the management of type 2 diabetes mellitus. Am. Fam. Physician 63, 1747–1756 [PubMed] [Google Scholar]
- 34. Higgins C. F., Linton K. J. (2001) Structural biology. The xyz of ABC transporters. Science 293, 1782–1784 [DOI] [PubMed] [Google Scholar]
- 35. Wang F., Olson E. M., Shyng S. L. (2012) Role of Derlin-1 in proteostasis regulation of ATP-sensitive potassium channels. J. Biol. Chem. 287, 10482–10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan F. F., Pratt E. B., Chen P. C., Wang F., Skach W. R., David L. L., Shyng S. L. (2010) Role of Hsp90 in biogenesis of the β-cell ATP-sensitive potassium channel complex. Mol. Biol. Cell 21, 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loo M. A., Jensen T. J., Cui L., Hou Y., Chang X. B., Riordan J. R. (1998) Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 17, 6879–6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun F., Zhang R., Gong X., Geng X., Drain P. F., Frizzell R. A. (2006) Derlin-1 promotes the efficient degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR folding mutants. J. Biol. Chem. 281, 36856–36863 [DOI] [PubMed] [Google Scholar]
- 39. Sampson H. M., Lam H., Chen P. C., Zhang D., Mottillo C., Mirza M., Qasim K., Shrier A., Shyng S. L., Hanrahan J. W., Thomas D. Y. (2013) Compounds that correct F508del-CFTR trafficking can also correct other protein trafficking diseases: an in vitro study using cell lines. Orphanet J. Rare Dis. 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hidvegi T., Ewing M., Hale P., Dippold C., Beckett C., Kemp C., Maurice N., Mukherjee A., Goldbach C., Watkins S., Michalopoulos G., Perlmutter D. H. (2010) An autophagy-enhancing drug promotes degradation of mutant α1-antitrypsin Z and reduces hepatic fibrosis. Science 329, 229–232 [DOI] [PubMed] [Google Scholar]
- 41. Renna M., Jimenez-Sanchez M., Sarkar S., Rubinsztein D. C. (2010) Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J. Biol. Chem. 285, 11061–11067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Babenko A. P., Bryan J. (2003) SUR domains that associate with and gate KATP pores define a novel gatekeeper. J. Biol. Chem. 278, 41577–41580 [DOI] [PubMed] [Google Scholar]
- 43. Chan K. W., Zhang H., Logothetis D. E. (2003) N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 22, 3833–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cartier E. A., Conti L. R., Vandenberg C. A., Shyng S. L. (2001) Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc. Natl. Acad. Sci. U.S.A. 98, 2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin C. W., Yan F., Shimamura S., Barg S., Shyng S. L. (2005) Membrane phosphoinositides control insulin secretion through their effects on ATP-sensitive K+ channel activity. Diabetes 54, 2852–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin Y. W., Bushman J. D., Yan F. F., Haidar S., MacMullen C., Ganguly A., Stanley C. A., Shyng S. L. (2008) Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism. J. Biol. Chem. 283, 9146–9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hohmeier H. E., BeltrandelRio H., Clark S. A., Henkel-Rieger R., Normington K., Newgard C. B. (1997) Regulation of insulin secretion from novel engineered insulinoma cell lines. Diabetes 46, 968–977 [DOI] [PubMed] [Google Scholar]
- 48. Kapturczak M., Zolotukhin S., Cross J., Pileggi A., Molano R. D., Jorgensen M., Byrne B., Flotte T. R., Ellis T., Inverardi L., Ricordi C., Nick H., Atkinson M., Agarwal A. (2002) Transduction of human and mouse pancreatic islet cells using a bicistronic recombinant adeno-associated viral vector. Mol. Ther. 5, 154–160 [DOI] [PubMed] [Google Scholar]
- 49. Chen P. C., Bruederle C. E., Gaisano H. Y., Shyng S. L. (2011) Syntaxin 1A regulates surface expression of β-cell ATP-sensitive potassium channels. Am. J. Physiol. Cell Physiol. 300, C506–C516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. (1995) Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268, 423–426 [DOI] [PubMed] [Google Scholar]
- 51. Zerangue N., Schwappach B., Jan Y. N., Jan L. Y. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22, 537–548 [DOI] [PubMed] [Google Scholar]
- 52. Taschenberger G., Mougey A., Shen S., Lester L. B., LaFranchi S., Shyng S. L. (2002) Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J. Biol. Chem. 277, 17139–17146 [DOI] [PubMed] [Google Scholar]
- 53. Raab-Graham K. F., Cirilo L. J., Boettcher A. A., Radeke C. M., Vandenberg C. A. (1999) Membrane topology of the amino-terminal region of the sulfonylurea receptor. J. Biol. Chem. 274, 29122–29129 [DOI] [PubMed] [Google Scholar]
- 54. Yan F. F., Casey J., Shyng S. L. (2006) Sulfonylureas correct trafficking defects of disease-causing ATP-sensitive potassium channels by binding to the channel complex. J. Biol. Chem. 281, 33403–33413 [DOI] [PubMed] [Google Scholar]
- 55. Amaravadi R. K., Yu D., Lum J. J., Bui T., Christophorou M. A., Evan G. I., Thomas-Tikhonenko A., Thompson C. B. (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Investig. 117, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mousavi S. A., Brech A., Berg T., Kjeken R. (2003) Phosphoinositide 3-kinase regulates maturation of lysosomes in rat hepatocytes. Biochem. J. 372, 861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Petiot A., Ogier-Denis E., Blommaart E. F., Meijer A. J., Codogno P. (2000) Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998 [DOI] [PubMed] [Google Scholar]
- 58. Ravikumar B., Vacher C., Berger Z., Davies J. E., Luo S., Oroz L. G., Scaravilli F., Easton D. F., Duden R., O'Kane C. J., Rubinsztein D. C. (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36, 585–595 [DOI] [PubMed] [Google Scholar]
- 59. Sarkar S., Floto R. A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L. J., Rubinsztein D. C. (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ambrósio A. F., Soares-Da-Silva P., Carvalho C. M., Carvalho A. P. (2002) Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 27, 121–130 [DOI] [PubMed] [Google Scholar]
- 61. Ambrósio A. F., Silva A. P., Malva J. O., Soares-da-Silva P., Carvalho A. P., Carvalho C. M. (1999) Carbamazepine inhibits L-type Ca2+ channels in cultured rat hippocampal neurons stimulated with glutamate receptor agonists. Neuropharmacology 38, 1349–1359 [DOI] [PubMed] [Google Scholar]
- 62. Granger P., Biton B., Faure C., Vige X., Depoortere H., Graham D., Langer S. Z., Scatton B., Avenet P. (1995) Modulation of the γ-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol. Pharmacol. 47, 1189–1196 [PubMed] [Google Scholar]
- 63. Skach W. R. (2007) Pharmacological chaperoning: two 'hits' are better than one. Biochem. J. 406, e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sarkar S., Perlstein E. O., Imarisio S., Pineau S., Cordenier A., Maglathlin R. L., Webster J. A., Lewis T. A., O'Kane C. J., Schreiber S. L., Rubinsztein D. C. (2007) Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat. Chem. Biol. 3, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luciani A., Villella V. R., Esposito S., Brunetti-Pierri N., Medina D., Settembre C., Gavina M., Pulze L., Giardino I., Pettoello-Mantovani M., D'Apolito M., Guido S., Masliah E., Spencer B., Quaratino S., Raia V., Ballabio A., Maiuri L. (2010) Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 12, 863–875 [DOI] [PubMed] [Google Scholar]
- 66. Ringe D., Petsko G. A. (2009) What are pharmacological chaperones and why are they interesting? J. Biol. 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Macmullen C. M., Zhou Q., Snider K. E., Tewson P. H., Becker S. A., Aziz A. R., Ganguly A., Shyng S. L., Stanley C. A. (2011) Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1. Diabetes 60, 1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giménez-Cassina A., Martínez-François J. R., Fisher J. K., Szlyk B., Polak K., Wiwczar J., Tanner G. R., Lutas A., Yellen G., Danial N. N. (2012) BAD-dependent regulation of fuel metabolism and KATP channel activity confers resistance to epileptic seizures. Neuron 74, 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hattersley A. T., Ashcroft F. M. (2005) Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 54, 2503–2513 [DOI] [PubMed] [Google Scholar]
- 70. Conti L. R., Radeke C. M., Shyng S. L., Vandenberg C. A. (2001) Transmembrane topology of the sulfonylurea receptor SUR1. J. Biol. Chem. 276, 41270–41278 [DOI] [PubMed] [Google Scholar]