Background: Cholecystokinin receptor type 1 (CCK1R) stimulates satiety.
Results: Binding and activity of a CCK1R agonist/CCK2R antagonist are studied at wild-type and chimeric receptors, and ligand-guided model refinement is utilized.
Conclusion: The small molecule agonist docking site is distinct from the antagonist site, with benzodiazepines docked with consistent pose, including approximation with Leu7.39.
Significance: The molecular model and determinants for small molecule agonist action should facilitate drug development.
Keywords: Allosteric Regulation, Calcium Signaling, G Protein-coupled Receptors (GPCR), Receptor Modification, Signaling, Benzodiazepine Agonist, Cholecystokinin Receptor, Binding, Biological Activity
Abstract
Understanding the molecular basis of drug action can facilitate development of more potent and selective drugs. Here, we explore the molecular basis for action of a unique small molecule ligand that is a type 1 cholecystokinin (CCK) receptor agonist and type 2 CCK receptor antagonist, GI181771X. We characterize its binding utilizing structurally related radioiodinated ligands selective for CCK receptor subtypes that utilize the same allosteric ligand-binding pocket, using wild-type receptors and chimeric constructs exchanging the distinct residues lining this pocket. Intracellular calcium assays were performed to determine biological activity. Molecular models for docking small molecule agonists to the type 1 CCK receptor were developed using a ligand-guided refinement approach. The optimal model was distinct from the previous antagonist model for the same receptor and was mechanistically consistent with the current mutagenesis data. This study revealed a key role for Leu7.39 that was predicted to interact with the isopropyl group in the N1 position of the benzodiazepine that acts as a “trigger” for biological activity. The molecular model was predictive of binding of other small molecule agonists, effectively distinguishing these from 1065 approved drug decoys with an area under curve value of 99%. The model also selectively enriched for agonist compounds, with 130 agonists identified by ROC analysis when seeded in 2175 non-agonist ligands of the type 1 CCK receptor (area under curve 78%). Benzodiazepine agonists in this series docked in consistent pose within this pocket, with a key role played by Leu7.39, whereas the role of this residue was less clear for chemically distinct agonists.
Introduction
The type 1 cholecystokinin (CCK)2 receptor is a class A guanine nucleotide-binding protein (G protein)-coupled receptor (GPCR) that is physiologically important for nutritional homeostasis, contributing to meal-stimulated pancreatic exocrine secretion and gallbladder contraction, whereas regulating gastric emptying and enteric transit, and eliciting postcibal satiety (1). It is the latter effect, induction of satiety without the ingestion of a caloric load, which has attracted substantial interest in development of type 1 CCK receptor agonists. The closest related GPCR is the type 2 CCK receptor, sharing many structural features, but having a very different tissue distribution and repertoire of activities (2). Gastrin action at the type 2 CCK receptor on the gastric parietal cell stimulates acid secretion, whereas CCK stimulation of this receptor in the central nervous system has been implicated in anxiety, analgesia, learning, memory, and dopamine-related behaviors.
The natural peptide ligands of the CCK receptors bind predominantly to the extracellular loop and amino-terminal tail regions, whereas both receptors possess intramembranous, interhelical pockets that are able to bind small molecule ligands. For the type 1 CCK receptor, this pocket has been demonstrated to represent a site of action of allosteric ligands. We recently reported the molecular characteristics of these pockets in the two subtypes of CCK receptors in their inactive states, and the key determinants for antagonist ligand docking to each (3). The geometry of these binding pockets and the specific receptor residues shown to interact with the antagonist ligands were unique for the type 1 and type 2 CCK receptors. Residues 6.51, 6.52, and 7.39 were most important for the binding of CCK1R-selective benzodiazepine antagonists, whereas residues 2.61 and 7.39 were most important for the binding of CCK2R-selective benzodiazepine antagonists. These models strongly recapitulated the empirically observed subtype binding selectivity of known CCK antagonists and thus serve as highly predictive tools for evaluation of novel CCK ligands.
In the current work, we have substantially extended these insights by focusing on the characteristics of the same pocket in the type 1 CCK receptor when occupied with a full agonist. A key molecule used in these studies was the 1,5-benzodiazepine developed at GlaxoSmithKline Research Laboratories, GI181771X, which has a unique functional profile, being a type 1 CCK receptor agonist and a type 2 CCK receptor antagonist (4, 5). This provided an ideal ligand to study binding and activation mechanisms of the two closely related CCK receptors. We utilized intracellular calcium mobilization assays and competition binding for small molecule benzodiazepine radioligands, selective for each of the two receptors, in combination with a series of chimeric CCK1R/CCK2R constructs systematically interchanging the distinct residues lining this pocket in these two subtypes of CCK receptors (3), to elucidate the key interactions defining the distinct pharmacologies.
To guide mechanistic interpretation of the data, a ligand-directed approach (6) was used to build molecular models for the intramembranous, interhelical small molecule agonist-binding pocket within the type 1 CCK receptor, starting from the previously described model of the antagonist-occupied receptor (3). The optimal model in this series was distinct from the previously described antagonist pocket and fully compatible with all the experimental data for the CCK receptor constructs. The model effectively differentiated type 1 CCK receptor agonists from approved drugs, as well as from other CCK receptor ligands. Of note, this model provided insights into the molecular basis for agonist action, particularly of benzodiazepine agonists, which uniformly interacted with Leu7.39 in transmembrane segment 7. For GI181771X, the portion of the ligand predicted to interact with this receptor residue was the isopropyl group in the N1 position of the benzodiazepine ring; this position was previously identified in ligand structure-activity series as an “agonist trigger” (4). The molecular models that were generated in this work also provided an explanation for why this compound did not exhibit any biological agonist activity at the structurally related type 2 CCK receptor.
EXPERIMENTAL PROCEDURES
Materials
Ham's F-12 medium, Opti-MEM medium, l-glutamine, LipofectamineTM LTX and PLUSTM reagents were from Invitrogen. Quest Fluo-8-AMTM was from AAT Bioquest Inc. (Sunnyvale, CA). Fetal Clone II tissue culture medium supplement was from Hyclone Laboratories (Logan, UT). MicroscintTM, Unifilter 96-well microplates with bonded GF/B filters were from PerkinElmer Life Sciences. Costar 96-well V bottom assay plates and the black assay plates with clear bottoms were from Corning (Corning, NY). Cholecystokinin octapeptide (CCK-26–33 based on the numbering of CCK-33; also known as CCK-8) was purchased from Peninsula Laboratories (Belmont, CA). The CCK-like radioactive tracer, 125I-d-Tyr-Gly-[(Nle28,31)CCK-26–33], was prepared by oxidative radioiodination of the parental peptide using IODO-BEADs (Pierce) with purification to homogeneity on reversed-phase HPLC, as described previously (7). All other reagents were analytical grade.
Benzodiazepine Ligands
The 1,5-benzodiazepine agonist ligand (GI181771X) was provided by GlaxoSmithKline Research Laboratories (Research Triangle Park, NC). This ligand has unique characteristics, acting as a specific agonist for type 1 CCK receptors (CCK1R) and an antagonist for type 2 CCK receptors (CCK2R) (4, 5, 8). Radioiodinated 1,4-benzodiazepine ligands for the type 1 and type 2 CCK receptors were prepared by oxidative radioiodination using IODO-BEADs, and purifying the product to homogeneity with a specific radioactivity of ∼2000 Ci/mmol on reversed-phase HPLC, as described previously (9).
CCK Receptor Constructs and Cell Lines
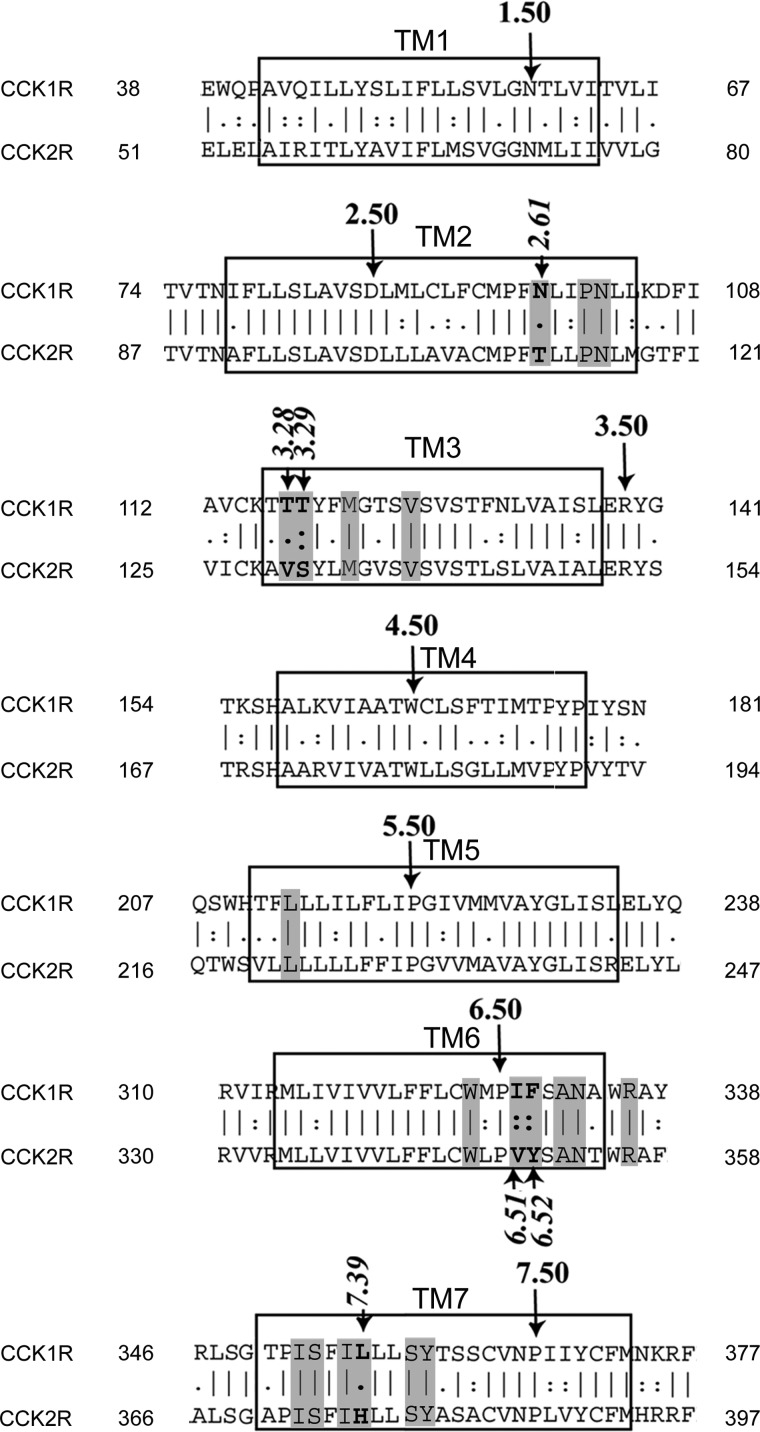
Flp-In Chinese hamster ovary (CHO) cell lines were engineered to stably express a series of CCK receptor constructs, using the approach previously reported (10). These included both wild-type CCK1R and CCK2R receptors, as well as a series of intramembranous chimeric receptor constructs previously studied as transiently expressed on COS cells (3). The design of these chimeric receptor constructs was based on the residues lining the intramembranous, interhelical small molecule binding pocket, exchanging those residues that are different in the two subtypes of CCK receptors. Fig. 1 shows the alignment of the sequences of types 1 and 2 CCK receptors predicted to represent the seven transmembrane segments. Shaded residues are those predicted to line this pocket. Residues involved in the chimeric constructs are identified in italics (2.61, 3.28, 3.29, 6.51, 6.52, and 7.39).
FIGURE 1.
Sequences of transmembrane segments of CCK1R and CCK2R. Shown are the alignments of primary sequences of transmembrane segments of subtypes of CCK receptors with those residues lining the small molecule binding pocket shaded. Those residues that are different in the two receptors were exchanged in the chimeric constructs. These are numbered in italics according to the nomenclature of Ballesteros and Weinstein (30) based on the most highly conserved residue in each segment as number 50.
The receptor constructs were cloned into the FRT/pcDNA5.0 expression vector and sequences were confirmed by DNA sequencing. The parental Flp-In CHO cells not expressing either type of CCK receptor were transfected when at 80% confluence in 6-well plates using a combination of 0.5 μg of receptor cDNA expressed in FRT/pcDNA5 vector and 4.5 μg of pOG44 (Flp recombinase expression vector) using Lipofectamine LTX and PLUS reagent mixture in Opti-MEM medium following the manufacturer's instructions. Stable transfectants were selected using 400 μg/ml of hygromycin to isolate antibiotic-resistant cells. Levels of receptor expression in the cell lines were characterized using radioligand binding assays (described below). These cell lines were grown in tissue culture flasks containing Ham's F-12 medium supplemented with 5% Fetal Clone II in a humidified environment containing 5% carbon dioxide. Cells were passaged approximately two times per week.
Receptor-enriched Cell Membranes
Membrane fractions enriched in plasma membranes were isolated from the receptor-bearing cell lines, as described previously (10). Cells at 80–90% confluence were harvested mechanically using a cell scraper and were suspended in ice-cold phosphate-buffered saline, pH 7.4. The membrane fraction of interest was isolated using sucrose density gradient centrifugation, as described previously (11), and was suspended in Krebs-Ringer/HEPES (KRH) medium (25 mm HEPES, pH 7.4, 104 mm NaCl, 5 mm KCl, 1.5 mm CaCl2, 1.0 mm KH2PO4, 1.2 mm MgSO4, 1.2 mm MgCl2) containing 0.01% soybean trypsin inhibitor and 1 mm phenylmethylsulfonyl fluoride. Membranes were stored at −80 °C until use.
Receptor Binding Assays
Radioligand binding assays were carried out using CCK-like or benzodiazepine radioligands (the BDZ-1 radioligand binds with high affinity to the type 1 CCK receptor and the BDZ-2 radioligand binds with high affinity to the type 2 CCK receptor), as described previously (9). The membrane suspensions (5–15 μg of protein/sample) were incubated in duplicate with radioligand (∼10,000 cpm/well) in KRH medium at room temperature for 60 min in the absence and presence of increasing concentrations of unlabeled CCK or GI181771X. The amounts of radioactivity bound in the presence of 1 μm unlabeled BDZ ligands or 0.1 μm CCK in the respective assays were defined as non-saturable binding. The receptor-bound fraction and the free radioligand were separated by vacuum filtration using Unifilter 96-well microplate-bound membrane GF/B filter mats in a Filtermate Harvester (PerkinElmer Life Sciences). The plates were washed three times with wash buffer (0.9% NaCl and 0.2% bovine serum albumin), air-dried, soaked in 30 μl of MicroscintTM, and radioactivity was quantified using a Top CountNXTTM instrument (Packard, Meriden, CT) that had counting efficiency for 125I of 23%. Data were analyzed using the LIGAND program of Munson and Rodbard (12), and graphed using the nonlinear least-squares curve-fitting routine in Prism 5 (GraphPad, San Diego, CA).
Intracellular Calcium Assays
Ligand-stimulated biological activity was quantified by measuring the intracellular calcium responses to agonists in intact cells (13). The natural peptidyl agonist, CCK, was used as control and the benzodiazepine that is the focus of this work, GI181771X, was used as the experimental agonist. All intracellular calcium responses to GI181771X were expressed as percentages of the control responses to CCK. Cells were seeded in sterile clear-bottom black 96-well tissue culture plates 24 h before the assay, and studied when they reached approximate confluence of 70–80%. They were washed with KRH medium containing 2.5 mm probenecid and 0.2% bovine serum albumin, and incubated with 1.5 μm Quest Fluo 8 (dissolved in anhydrous dimethyl sulfoxide) for 1 h at 37 °C in the dark. The calcium response assay was initiated by addition of agonist ligands. The incubation was terminated by aspirating the medium and washing the cells. The assay was performed in a Flexstation 3.0 plate reader (Molecular Devices, Sunnyvale, CA) using robotic addition of the appropriate agonist ligand (GI181771X or CCK). Intracellular calcium responses were measured at 37 °C by quantifying the fluorescence emission intensity at 525 nm after exciting the samples at 485 nm, with data collection every 4 s over a 120-s period. Data were plotted using Prism 5 (Graphpad), and peak responses were identified.
Molecular Modeling
All molecular modeling was conducted using a stochastic global energy optimization procedure in internal coordinate mechanics (ICM) (14) with the ICM-Pro package version 3.7-2 (MolSoft LLC, San Diego, CA), as we have previously reported (14). This procedure consisted of three iterative steps: (a) random conformational change of a dihedral angle according to the biased-probability Monte Carlo method (15); (b) local minimization of all free dihedral angles; and (c) acceptance or rejection of the new conformation based on the Metropolis criterion at the simulation temperature, usually at 600 K (16).
The ligand-guided homology modeling method (6) that we previously used to construct a model of the type 1 CCK receptor in its inactive conformation (3) was utilized in this work. The inactive structure of this receptor was used as the starting point for the modeling of the active state ligand-binding pocket, and the principal intramembranous pocket was located and defined using ICM PocketFinder (17). A collection of 102 type 1 CCK receptor agonist ligands selected from the ChEMBL database (18) was used to mold the inactive pocket into an active conformation. A distance restraint was used between the ligand and an anchor residue on the receptor to limit the size of the sampling space and to keep the ligand within the pocket during the molecular modeling process. Previously, after attempting to use several different hydrogen bond donor-acceptor pairs, the best models were found to come from use of Asn6.55 as the anchor residue (3). A defined hydrogen bond distance restraint with this residue was used during the simulations, and each ligand was docked to the pocket followed by cycles of stochastic global energy optimization, side chain sampling, backbone minimization, and loop modeling. This resulted in an ensemble of multiple receptor conformations for the agonist-occupied type 1 CCK receptor.
Each pocket conformation in the ensemble was then evaluated using a composite score that included the ability to differentiate structurally diverse positives from decoys by docking scores, the percentage of ligands forming hydrogen bonds with the anchor residue (Asn6.55), and the consistency of docking poses based on clustering of these poses as assessed by Atomic Property Fields (19). The best model was then formally evaluated using plots of receiver operating characteristic (ROC) curves (20) to evaluate the ability to differentiate a set of positive ligands seeded with 1065 decoys, representing approved drugs in the ChEMBL database, as well as the ability to identify 130 known type 1 CCK receptor agonists having activities greater than 1 μm seeded into 2175 non-agonist ligands of the type 1 CCK receptor present in the ChEMBL database. This was evaluated in the most conservative way possible, because not all of the ligands have been tested for agonist activity, with the possibility that some molecules graded as false negatives were actual positives.
Statistical Analysis
Differences in receptor binding parameters between various constructs were analyzed with one-way analysis of variance and Dunnett's post test. Differences were considered to be significant at p < 0.05 (Prism 5, GraphPad).
RESULTS
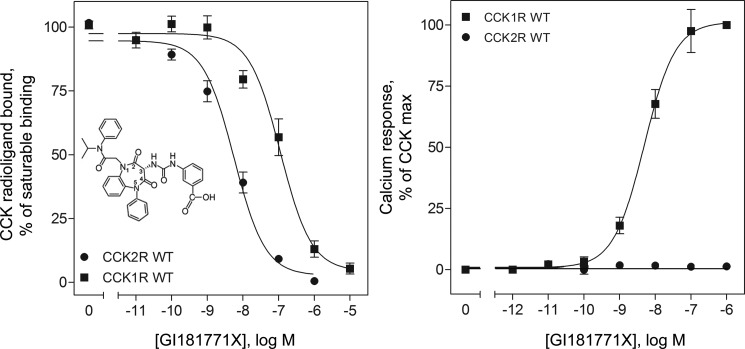
As has been previously described, the 1,5-benzodiazepine, GI181771X, bound specifically to type 1 and type 2 CCK receptors, and was a full agonist at the type 1 CCK receptor, whereas having no agonist activity at the type 2 CCK receptor (Fig. 2) (5). In this type of binding assay, GI181771X completely inhibited the saturable binding of the CCK-like radioligand to the type 1 CCK receptor with an IC50 of 105 ± 13 nm and inhibited its saturable binding to the type 2 CCK receptor with an IC50 of 5.6 ± 0.1 nm. However, because benzodiazepines are allosteric ligands at CCK receptors (21), binding to an intramembranous interhelical region of these receptors (22), binding affinities of GI181771X were also determined by competition for previously described, selective, benzodiazepine radioligands of the CCK receptors, 125I-BDZ-1 and 125I-BDZ-2 (9). The Ki for GI181771X inhibition of binding of the BDZ-1 radioligand to the type 1 CCK receptor was 104.7 ± 19.6 nm, whereas it was 5.1 ± 1.6 nm for inhibition of the BDZ-2 radioligand binding to the type 2 CCK receptor (Fig. 3 and Tables 1 and 2). Despite GI181771X exhibiting 20.5-fold higher affinity for the type 2 CCK receptor than for the type 1 CCK receptor, this compound exhibited agonist activity only at the type 1 CCK receptor, where it elicited a maximal response equivalent to that of the natural agonist, CCK.
FIGURE 2.
Binding and biological activity of GI181771X at wild-type CCK receptors. Shown in the inset is the chemical structure of the 1,5-benzodiazepine ligand, GI181771X, with the positions around the benzodiazepine ring numbered. Also shown are curves representing the abilities of this compound to compete for the saturable binding of the CCK-like radioligand ,125I-d-Tyr-Gly-[(Nle28,31)CCK-26–33], to CHO-CCK1R and CHO-CCK2R cells (left panel), and its abilities to stimulate these cells to increase intracellular calcium responses (right panel). Saturable binding of the CCK-like radioligand was determined by competition with 1 μm CCK, and biological responses were determined relative to the maximal response of the cells to CCK. Data represent mean ± S.E. of values from five to eight independent experiments performed in duplicate.
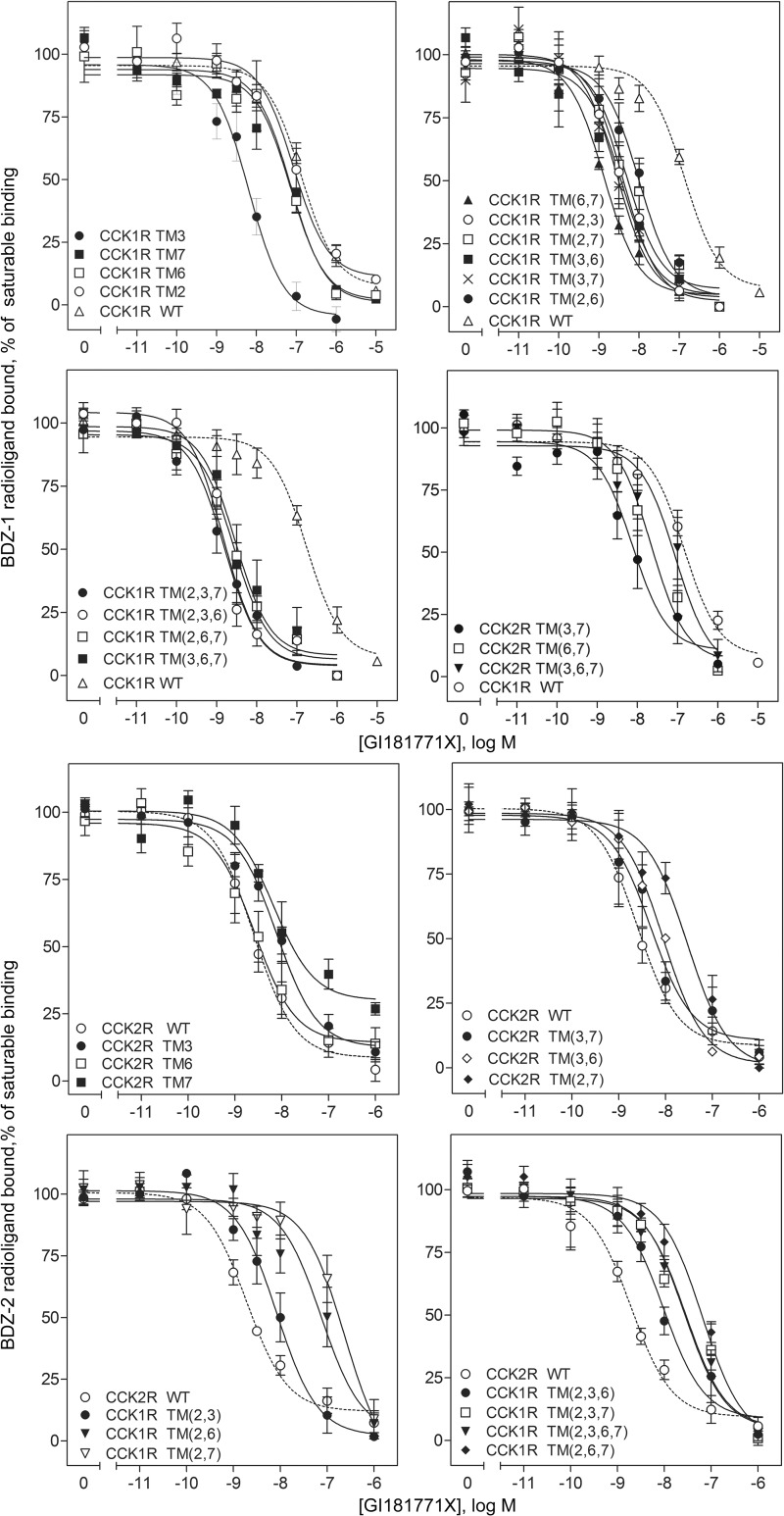
FIGURE 3.
Receptor binding characteristics of chimeric CCK receptor constructs. Shown are curves representing the abilities of GI181771X to compete for saturable binding of the CCK receptor subtype-selective benzodiazepine radioligands, 125I-BDZ-1 and 125I-BDZ-2, to membranes isolated from CHO cell lines expressing wild-type and chimeric CCK receptors. Saturable binding of the benzodiazepine radioligands was determined by competition with 1 μm concentration of the analogous non-radioactive benzodiazepine ligand (BDZ-1 or BDZ-2). Data represent mean ± S.E. of values from three to five independent experiments performed in duplicate.
TABLE 1.
Binding parameters of CCK1 receptor-based chimeric constructs expressed in CHO cells
| CCK1R-based chimeric receptors | Receptor abbreviations | 125I-BDZ-1 vs GI181771X Ki | 125I-BDZ-1 vs GI181771X Bmax | 125I-BDZ-2 vs GI181771X Ki | 125I-BDZ-2 vs GI181771X Bmax |
|---|---|---|---|---|---|
| nm | pmol/mg protein | nm | pmol/mg protein | ||
| CCK1R WT | CCK1R | 104.7 ± 19.6 | 41 ± 7 | NDBa | NDB |
| N2.61T | CCK1R TM2 | 89.0 ± 37.0 | 16 ± 5 | NDB | NDB |
| T3.28V, T3.29S | CCK1R TM3 | 6.3 ± 1.4b | 20 ± 7 | NDB | NDB |
| I6.51V, F6.52Y | CCK1R TM6 | 73.6 ± 7.9 | 80 ± 6b | NDB | NDB |
| L7.39H | CCK1R TM7 | 109.0 ± 43.9 | 36 ± 19 | NDB | NDB |
| N2.61T, T3.28V, T3.29S | CCK1R TM (2,3) | 6.3 ± 3.5b | 14 ± 5b | 11.9 ± 6.1 | 30 ± 6c |
| N2.61T, I6.51V, F6.52Y | CCK1R TM (2,6) | 19.9 ± 4.5b | 23 ± 6 | 111.6 ± 33.7c | 87 ± 44 |
| N2.61T, L7.39H | CCK1R TM (2,7) | 22.2 ± 5.7b | 29 ± 7 | 238.7 ± 28.9c | 87 ± 6c |
| T3.28V, T3.29S, I6.51V, F6.52Y | CCK1R TM (3,6) | 4.6 ± 2.8b | 5 ± 1b | NDB | NDB |
| T3.28V, T3.29S, L7.39H | CCK1R TM (3,7) | 9.4 ± 3.2b | 10 ± 4b | NDB | NDB |
| I6.51V, F6.52Y, L7.39H | CCK1R TM (6,7) | 1.4 ± 0.3b | 6 ± 2b | NDB | NDB |
| N2.61T, T3.28V, T3.29S, I6.51V, F6.52Y | CCK1R TM (2,3,6) | 1.4 ± 0.2b | 11 ± 2b | 45.6 ± 20.3c | 69 ± 18c |
| N2.61T, T3.28V, T3.29S, L7.39H | CCK1R TM (2,3,7) | 1.9 ± 0.5b | 11 ± 3b | 107.7 ± 44.2c | 79 ± 13c |
| N2.61T, I6.51V, F6.52Y, L7.39H | CCK1R TM (2,6,7) | 2.8 ± 0.4b | 12 ± 2b | 109.5 ± 34.2c | 39 ± 9c |
| T3.28V, T3.29S, I6.51V, F6.52Y, L7.39H | CCK1R TM (3,6,7) | 2.3 ± 0.1b | 11 ± 1b | NDB | NDB |
| N2.61T, T3.28V, T3.29S, I6.51V, F6.52Y, L7.39H | CCK1R TM (2,3,6,7) | NDB | NDB | 70.4 ± 16.3c | 83 ± 20c |
| CCK2R WT | CCK2R | 5.1 ± 1.6 | 6 ± 2 |
a NDB, no detectable binding.
b p < 0.05 compared to values for wild-type CCK1R.
c p < 0.05 compared to values for wild-type CCK2R.
TABLE 2.
Binding parameters of CCK2 receptor-based chimeric constructs expressed in CHO cells
| CCK2R-based chimeric receptors | Receptor abbreviations | 125I-BDZ-2 vs GI181771X Ki | 125I-BDZ-2 vs GI181771X Bmax | 125I-BDZ-1 vs GI181771X Ki | 125I-BDZ-1 vs GI181771X Bmax |
|---|---|---|---|---|---|
| nm | pmol/mg protein | nm | pmol/mg protein | ||
| CCK2R WT | CCK2R | 5.1 ± 1.6 | 6 ± 2 | NDBa | NDB |
| T2.61N | CCK2R TM2 | NDB | NDB | NDB | NDB |
| V3.28T, S3.29T | CCK2R TM3 | 15.4 ± 4.3b | 5 ± 1 | NDB | NDB |
| V6.51I, Y6.52F | CCK2R TM6 | 4.6 ± 2.5 | 16 ± 7 | NDB | NDB |
| H7.39L | CCK2R TM7 | 51.5 ± 21.2b | 4 ± 1 | NDB | NDB |
| T2.61N, V3.28T, S3.29T | CCK2R TM (2,3) | NDB | NDB | NDB | NDB |
| T2.61N, V6.51I, Y6.52F | CCK2R TM (2,6) | NDB | NDB | NDB | NDB |
| T2.61N, H7.39L | CCK2R TM (2,7) | 71.4 ± 29.2b | 3 ± 1 | NDB | NDB |
| V3.28T, S3.29T, V6.51I, Y6.52F | CCK2R TM (3,6) | 11.7 ± 3.6 | 5 ± 1 | NDB | NDB |
| V3.28T, S3.29T, H7.39L | CCK2R TM (3,7) | 12.9 ± 6.6 | 4 ± 2 | 8.0 ± 3.3c | 53 ± 13 |
| V6.51I, Y6.52F, H7.39L | CCK2R TM (6,7) | NDB | NDB | 47.4 ± 12.9c | 113 ± 47 |
| T2.61N, V3.28T, S3.29T, V6.51I, Y6.52F | CCK2R TM (2,3,6) | NDB | NDB | NDB | NDB |
| T2.61N, V3.28T, S3.29T, H7.39L | CCK2R TM (2,3,7) | NDB | NDB | NDB | NDB |
| T2.61N, V6.51I, Y6.52F, H7.39L | CCK2R TM (2,6,7) | NDB | NDB | NDB | NDB |
| V3.28T, S3.29T, V6.51I, Y6.52F, H7.39L | CCK2R TM (3,6,7) | NDB | NDB | 150.3 ± 17.8 | 42 ± 10 |
| T2.61N, V3.28T, S3.29T, V6.51I, Y6.52F, H7.39L | CCK2R TM (2,3,6,7) | NDB | NDB | NDB | NDB |
| CCK1R WT | CCK1R | 104.7 ± 19.6 | 41 ± 7 |
a NDB, no detectable binding.
b p < 0.05 compared to values for wild-type CCK2R.
c p < 0.05 compared to values for wild-type CCK1R.
To gain insight into the mode of docking and activation of the type 1 CCK receptor by this compound, we also studied a series of chimeric CCK receptor constructs in which each of the residues lining this allosteric ligand-binding pocket that are distinct in the human type 1 and type 2 CCK receptors were systematically exchanged. This approach is analogous to that recently reported for elucidation of the molecular nature of the antagonist benzodiazepine ligand-binding pockets within these receptors (3). The GI181771X binding characteristics for these constructs are shown in Fig. 3 and Tables 1 and 2.
CCK1 Receptors with Substitution of CCK2 Receptor TM Segments
Radioligand Binding Studies
It is noteworthy that many of the constructs replacing type 1 CCK receptor residues with those of the type 2 CCK receptor, alone or in combination, resulted in higher affinity binding for GI181771X in competition for the BDZ-1 radioligand. Of the single transmembrane segment substitutions, only TM3 of the type 2 CCK receptor (where threonines present in positions 3.28 and 3.29 in the type 1 CCK receptor were replaced with valine and serine, respectively) led to increased affinity (16.6-fold). Combination of TM3 with any of the other TMs alone did not further enhance affinity, whereas combined substitution of TM2 of the type 2 CCK receptor and either TM6 or TM7 segments engendered a modest (∼5-fold) increase in affinity. The combined substitution of TM6 and TM7, however, led to a marked gain in affinity (>50-fold), although further combination of these two TM segments with either TM2 or TM3 of the type 2 CCK receptor produced no further gain (or loss) in affinity. Introducing the combination of either TMs 2, 3, and 6 or TMs 2, 3, and 7 of the type 2 CCK receptor resulted in higher affinity than was seen with pairings of segments with either TM2 or TM3. The substitution of all 4 TM segments led to loss of measurable BDZ-1 radioligand binding, although the GI181771X compound could still bind, as demonstrated by its ability to compete for binding of the BDZ-2 radioligand. As previously described (3), whereas substitution of the TM2 segment of the type 2 CCK receptor alone was insufficient to engender saturable BDZ-2 radioligand binding, only those chimeras containing this segment enabled measurable binding of the BDZ-2 ligand. At these constructs, the apparent affinities of GI181771X in competition for the BDZ-2 radioligand were 2–100-fold lower than those determined from competition with the BDZ-1 radioligand. This is consistent with two (or more), slowly interconverting receptor conformations that each preferentially favors binding of either the BDZ-1 or BDZ-2 radioligand (3).
Functional Studies
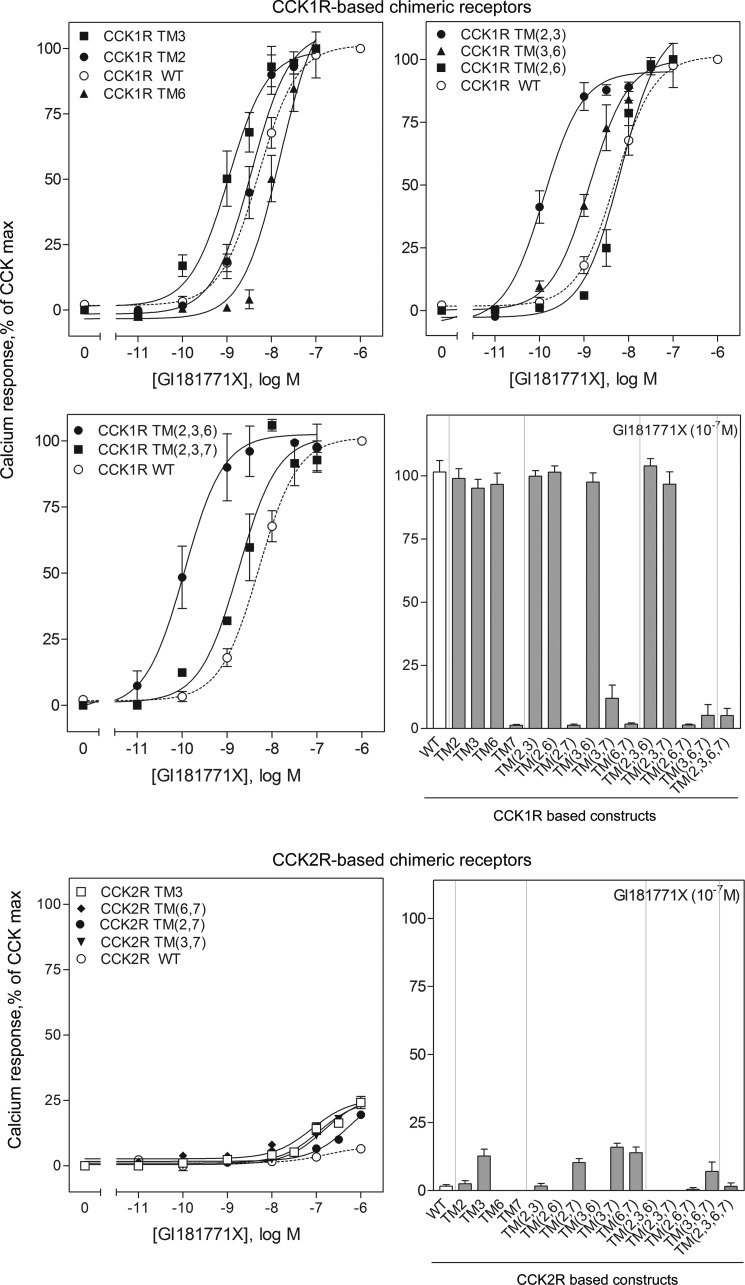
Despite low affinity binding of GI181771X to the type 1 CCK receptor, it is a highly efficacious agonist with potency for stimulating intracellular calcium mobilization shifted nearly 17-fold to the left relative to its affinity (ratio of EC50 to Ki value was 0.06) (Table 3), in contrast to its interaction with the type 2 CCK receptor where it exhibited considerably higher binding affinity but no efficacy to stimulate an intracellular calcium response. Chimeric exchange of individual or combination of TM segments of the type 1 CCK receptor for those of the type 2 CCK receptor revealed key roles for these segments in GI181771X agonism (Fig. 4 and Table 3).
TABLE 3.
Biological activity of CCK1 receptor-based chimeric constructs expressed in CHO cells
| CCK1R-based chimeric receptors | Receptor abbreviations | GI181771X EC50 (nM) | GI181771X ratio EC50/Ki |
|---|---|---|---|
| nm | |||
| CCK1R WT | CCK1R | 6.1 ± 1.4 | 0.06 |
| N2.61T | CCK1R TM2 | 4.5 ± 1.0 | 0.05 |
| T3.28V, T3.29S | CCK1R TM3 | 2.5 ± 1.3 | 0.40 |
| I6.51V, F6.52Y | CCK1R TM6 | 19.6 ± 5.5 | 0.27 |
| L7.39H | CCK1R TM7 | NRa | |
| N2.61T, T3.28V, T3.29S | CCK1R TM (2,3) | 0.2 ± 0.1b | 0.03 |
| N2.61T, I6.51V, F6.52Y | CCK1R TM (2,6) | 5.9 ± 0.7 | 0.30 |
| N2.61T, L7.39H | CCK1R TM (2,7) | NR | |
| T3.28V, T3.29S, I6.51V, F6.52Y | CCK1R TM (3,6) | 1.4 ± 0.3b | 0.30 |
| T3.28V, T3.29S, L7.39H | CCK1R TM (3,7) | NR | |
| I6.51V, F6.52Y, L7.39H | CCK1R TM (6,7) | NR | |
| N2.61T, T3.28V, T3.29S, I6.51V, F6.52Y | CCK1R TM (2,3,6) | 0.1 ± 0.1b | 0.07 |
| N2.61T, T3.28V, T3.29S, L7.39H | CCK1R TM (2,3,7) | 1.9 ± 0.4 | 1.00 |
| N2.61T, I6.51V, F6.52Y, L7.39H | CCK1R TM (2,6,7) | NR | |
| TT3.28V, T3.29S, I6.51V, F6.52Y, L7.39H | CCK1R TM (3,6,7) | NR | |
| N2.61T, T3.28V, T3.29S, I6.51V, F6.52Y, L7.39H | CCK1R TM (2,3,6,7) | NR | |
| CCK2R WT | CCK2R | NR |
a NR, no response.
b p < 0.05 compared to values for wild-type CCK1R.
FIGURE 4.
Biological activity at chimeric CCK receptor constructs. Shown are curves representing the abilities of GI181771X to stimulate intracellular calcium responses in wild-type and chimeric CCK receptor-bearing cells (in which there were significant responses over basal values), with data plotted relative to the maximal responses of each cell line to the natural agonist control, CCK. Also shown are bar graphs of the intracellular calcium responses in cells expressing all of the constructs in each series, with constructs based on the type 1 CCK receptor separated from those based on the type 2 CCK receptor. Data represent mean ± S.E. of values from four to six independent experiments performed in duplicate.
The most notable effect was seen with substitution of TM7 of the type 2 CCK receptor, specifically, the mutation of Leu7.39 to histidine. In almost all cases, the incorporation of histidine in the 7.39 position of the type 1 CCK receptor either alone or in combination with other TM segments from the type 2 CCK receptor, resulted in a marked loss in GI181771X efficacy, even when binding affinity was unchanged or increased. Indeed, with the exception of the TM(2,3,7) chimeric construct, all constructs containing TM7 of the type 2 CCK receptor failed to respond to the ligand (Table 3), illustrating a critical role for this position in the agonist activity of GI181771X. All of these receptor constructs had significant intracellular calcium responses to the CCK control. It was also noteworthy that no other TM segment of the type 2 CCK receptor inserted into the type 1 CCK receptor was alone capable of significant modification of the GI181771X activity. Only three of all of the chimeric constructs resulted in a significant increase in potency of GI181771X activity, those including TM(2,3), TM(3,6) and TM(2,3,6). Each of these constructs also increased binding affinities. Of these constructs, only the CCK1R TM(2,3) construct had a lower ratio of EC50 to Ki than the type 1 wild-type receptor, suggesting that these mutations may lead to relaxation of the barrier to receptor activation. Full efficacy was maintained by the constructs incorporating TM2, TM3, TM6, TM(2,3), TM(2,6), TM(3,6), TM(2,3,6), and TM(2,3,7) from the type 2 CCK receptor. Marked loss of efficacy was observed for all of the constructs including TM7, except for the TM(2,3,7) construct noted above (Fig. 4 and Table 3). Indeed, incorporation of the TM7 segment of the type 1 CCK receptor that included a leucine in the position of the histidine in position 7.39 in several of the type 2 CCK receptor chimeric constructs resulted in partial agonist activity for the GI181771X (TM(6,7), TM(2,7), and TM(3,7)) (Fig. 4). This series of constructs suggested unique importance for residue 7.39 in the functional profile of GI181771X.
CCK2 Receptors with Substitution of CCK1 Receptor TM Segments
Binding and Functional Studies
Consistent with the importance of Thr2.61 present in the type 2 CCK receptor for BDZ-2 binding seen with the type 1 CCK receptor-based receptor chimeras (3), substitution of Thr2.61 for Asn was detrimental to BDZ-2 radioligand binding to the type 2 CCK receptor-based chimeras, with only the CCK1 TM(2,7) chimeric construct displaying measurable BDZ-2 binding. GI181771X had ∼10-fold lower affinity at this construct than at the type 2 wild-type CCK receptor. Of those other chimeric constructs that retained BDZ-2 radioligand binding, only the CCK1 TM6 construct did not significantly alter measured GI181771X affinity. Substitution of TM3 resulted in a 3-fold decrease in GI181771X affinity, whereas the TM7 and TM(2,7) chimeric constructs had 10–15-fold lower affinities. Three type 2 CCK receptor chimeric constructs gained binding of the BDZ-1 radioligand, those incorporating TM(3,7), TM(6,7), and TM(3,6,7) segments of the type 1 CCK receptor. GI181771X affinities for the TM(3,7) and TM(6,7) chimeric constructs, as demonstrated in competition for the BDZ-1 ligand, were higher than observed for the type 1 wild-type CCK receptor. The TM(3,7) chimeric construct was the only type 2 CCK receptor-based construct that bound both radioligands, and GI181771X had similar measured affinities (12.9 versus 8.0 nm for the BDZ-2 and BDZ-1 ligands, respectively, Table 2) in competition binding studies. Nevertheless, the BDZ-1 radioligand labeled ∼10 times greater number of binding sites than the BDZ-2 radioligand, suggesting that the two radioligands labeled two distinct populations of receptors.
Very few of the type 2 CCK receptor-based chimeras supported agonism of the GI181771X ligand, and those that did had low potency and efficacy (≤ 25% of the CCK peptide) in response to the compound. Paired chimeras containing TM7 of the type 1 CCK receptor each displayed low potency agonism (Fig. 4), indicating that Leu7.39 can facilitate receptor activation, but only in the context of specific conformations of the other TM helical residues within the binding pocket. Interestingly, substitution of TM3 of the type 1 CCK receptor alone also engendered agonism, although the mechanistic basis for this is unclear. Of note, the functional EC50 values were lower than the Ki values for binding, measured by competition for the antagonist radioligands (Fig. 4 and Table 3). This may imply that activation of the receptor is via a conformation(s) that does not readily interconvert with the sites labeled by the antagonists, although the functional assays were not performed under equilibrium conditions and this may also contribute to the observed differences.
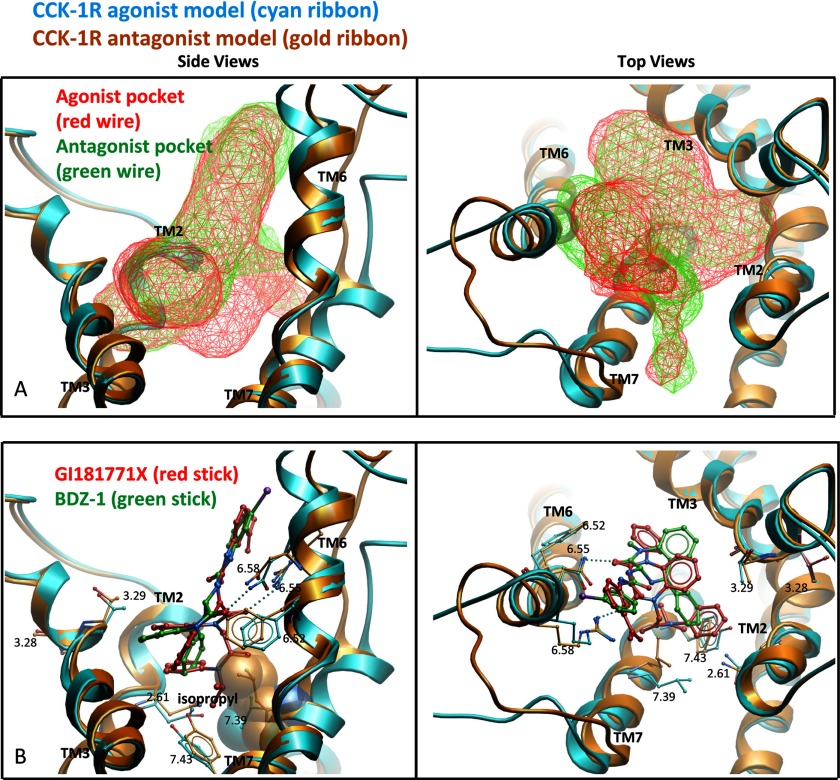
Fig. 5 illustrates the best performing model of GI181771X docking to the type 1 CCK receptor and compares this with the docking of the BDZ-1 antagonist to the same receptor. These structurally related, but biologically distinct molecules appear to dock in similar locations and with similar orientations, but the nature of the docking pockets is quite distinct. The pocket in the type 1 CCK receptor agonist-occupied active model extended further down into the helical bundle compared with the pocket observed in the antagonist-occupied inactive model (Fig. 5A). Analysis using ICM PocketFinder (17, 23) revealed that the predicted agonist pocket had a larger volume between TM5, TM6, and TM7 compared with the antagonist pocket.
FIGURE 5.
Molecular modeling of docking at CCK1R. Shown are the best performing models for docking the agonist, GI181771X (cyan ribbon and stick) and antagonist, BDZ-1 (gold ribbon and stick) ligands to the type 1 CCK receptor. All ligand conformations have been extensively refined in the Merck Molecular Force Field (31). The left viewpoint is from the side of the TM domain looking through TM4 and the right viewpoint is from the extracellular side with the loops cut away for clarity. A, a comparison of the allosteric ligand binding pockets in the agonist (red wire mesh) and antagonist (green wire mesh) models. The agonist pocket was observed to extend deeper into the TM domain, whereas the antagonist pocket was observed to extend more toward the extracellular region. The surface of the ligand binding pockets were calculated using ICM PocketFinder (17, 23). B, a comparison of the binding pose of GI181771X (red stick) in the agonist model compared with the binding pose of BDZ-1 (green stick) in the antagonist model, as previously reported (3). The isopropyl group in GI181771X was observed to cause a shift in the residues at positions 7.39 (CPK spheres, left image) and 7.43 compared with the antagonist model.
The GI181771X ligand was predicted to dock with its N1- and N5-benzodiazepine substituents pointing down into the core of the TM bundle (Fig. 5B). The predicted antagonist pocket extended further into the extracellular region, particularly at the top of TM2, TM6, and TM7 (Fig. 5A). In both the agonist- and antagonist-occupied models (as seen with BDZ-1(3)) the substituents at the C3 position were oriented toward the extracellular region. The N5 phenyl group was predicted to establish π-π interactions with Trp6.48 and the benzo ring was predicted to point toward TM3 (Fig. 5B). In the agonist-occupied model, the C4 carbonyl substituent of GI181771X was predicted to establish a hydrogen bond with Asn6.55, which is similar to the binding pose for the BDZ-1 antagonist in the inactive model. An additional hydrogen bond was also predicted to occur between the C2 carbonyl and Arg6.58, which causes the benzodiazepine ring to shift compared with the pose of BDZ-1 in the inactive model.
Ligand structure-activity relationship data indicate that the N1 substituent confers agonism, with an isopropyl group being optimal for full agonist activity (4, 24). In the type 1 CCK receptor agonist model, this substituent pointed toward a hydrophobic part of the pocket near TM7. The residues interacting with the isopropyl group include Tyr7.43 and Leu7.39, the latter residue, in this study, being critical for type 1 CCK receptor activation. Both of these residues were predicted to move out to accommodate the isopropyl group compared with their positions in the antagonist structure. The volume available for ligand growth in this region appeared to be restrictive, and the model agrees with the tight ligand structure-activity relationship data that have been observed (3).
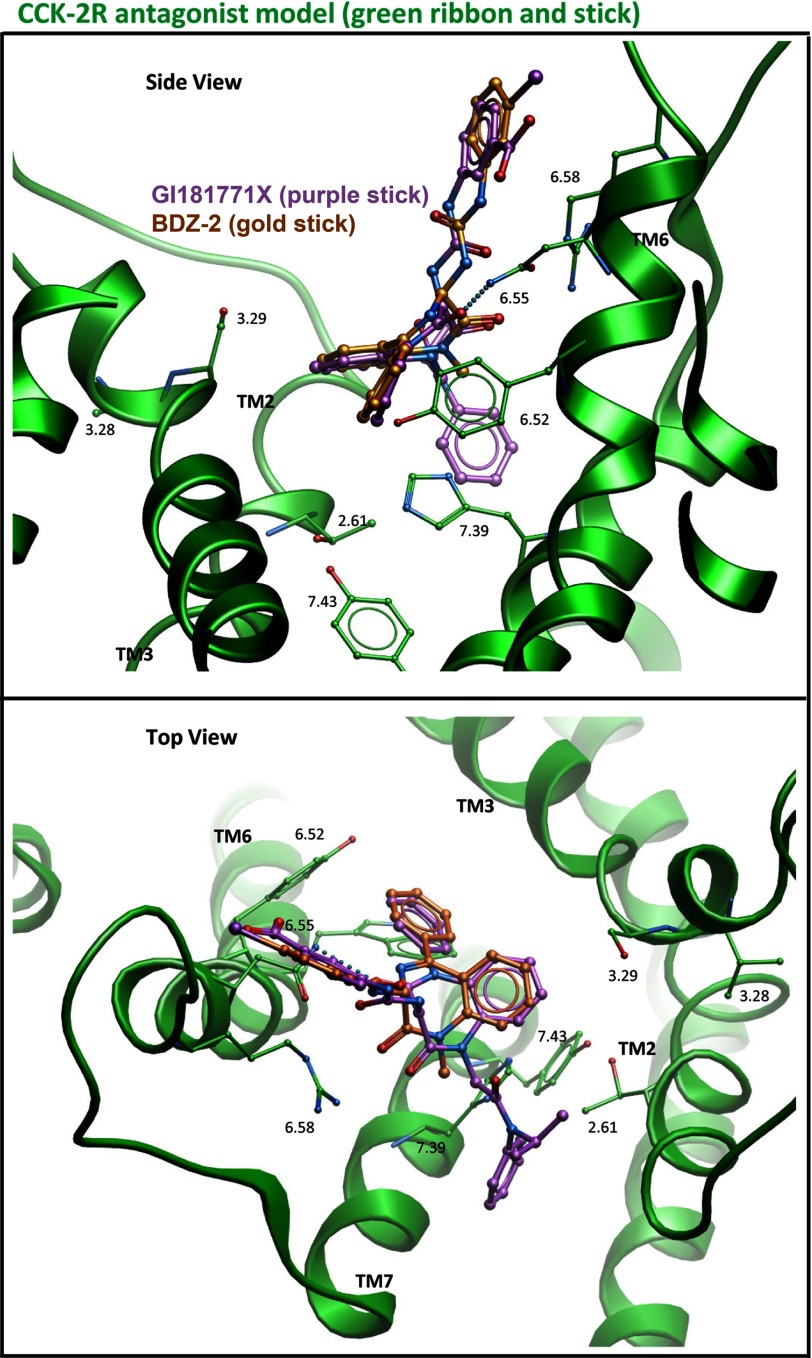
We also docked GI181771X to the type 2 CCK receptor. The best performing model for this complex is shown in Fig. 6; this model required minimal modification of our previously published inactive model of this receptor (3).
FIGURE 6.
Molecular modeling of docking at CCK2R. Shown are the best performing models for docking GI181771X (purple stick) and BDZ-2 (gold stick) to the type 2 CCK receptor in its inactive conformation (green ribbon). The top viewpoint is from the side of the TM domain looking through TM4 and the bottom viewpoint is from the extracellular side with the loops cut away for clarity.
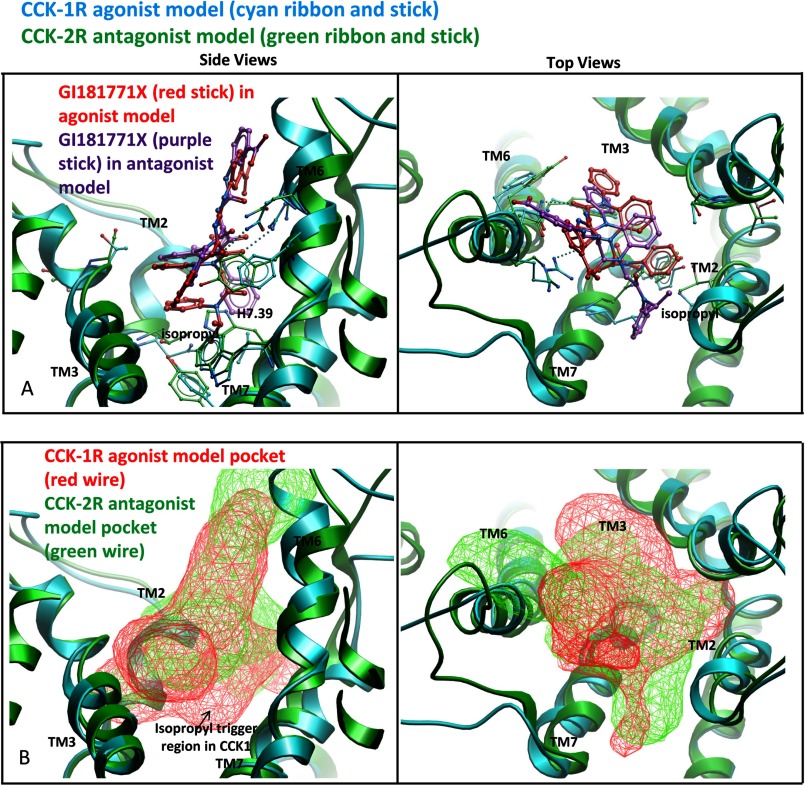
The predicted molecular basis for the receptor subtype-selective activation of the CCK receptors is illustrated in Fig. 7 in which the docking of GI181771X to the type 1 and type 2 CCK receptors is shown. The His7.39 residue in the type 2 CCK receptor is predicted to block the docking of the isopropyl group, whereas this group is accommodated nicely in the type 1 CCK receptor model in which a leucine residue is present in the 7.39 position. This interaction was key in establishing different orientations for this ligand in the two receptors, with the isopropyl group of the ligand oriented toward type 1 CCK receptor residues 7.39 and 7.43, but toward the extracellular region of the type 2 CCK receptor.
FIGURE 7.
Molecular basis for receptor subtype-selective activation of CCK receptors. Shown is a comparison of the predicted docking poses of GI181771X to the type 1 CCK receptor where it is an agonist (receptor in cyan ribbon, ligand in red stick) and to the type 2 CCK receptor where it is an antagonist (receptor in green ribbon, ligand in purple stick). The left viewpoint is from the side of the TM domains looking through TM4 and the right viewpoint is from the extracellular side with the loops cut away for clarity. In the upper pair of panel A, the ligand appears to be unable to make the same interactions in the type 2 CCK receptor that it makes in the type 1 CCK receptor, because His376 (7.39) blocks the binding of the isopropyl group. The isopropyl group was orientated toward residues 7.39 and 7.43 in the type 1 CCK receptor model (left) but toward the extracellular region in the type 2 CCK receptor model (right). We postulate that this steric hindrance prevents the isopropyl group from acting as a trigger in the type 2 CCK receptor. In the lower pair of panel B, the binding pocket for the agonist within the type 1 CCK receptor (red wire mesh) is compared with the binding pocket for the antagonist within the type 2 CCK receptor (green wire mesh). There is a lack of pocket volume in the isopropyl trigger region in the type 2 CCK receptor model.
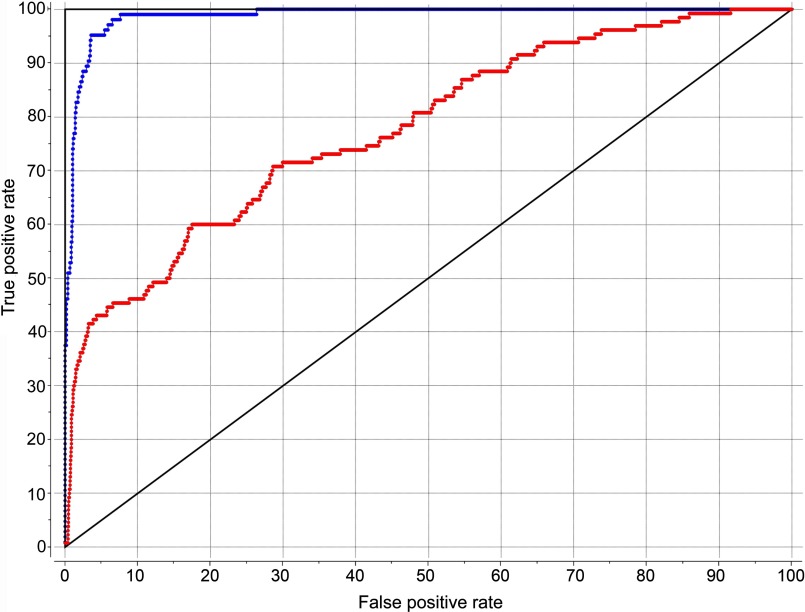
The predictive performance of the agonist model of the type 1 CCK receptor is illustrated in the ROC curves shown in Fig. 8. This model is able to discriminate agonists from decoys representing approved drugs, with an area under curve value of 99%. It is also able to identify 130 known type 1 CCK receptor agonists seeded into a library of 2175 non-agonist ligands of the type 1 CCK receptor present in the latest version of the ChEMBL database. The area under this curve is greater than 78%, significantly different from the 50% line in a random model. It is possible that the performance is actually better than this, because some of the compounds in the decoy list that were found to represent apparent false positives do not have biological activity documented in the database, and could actually represent real positives. This conservative evaluation supports the discriminatory power of this model for identifying agonists from antagonists of the receptor. It is also notable that the poses of docked benzodiazepine agonist ligands in this series were quite similar, with the Leu7.39 playing an analogous and important role in all of these agents.
FIGURE 8.
ROC curves. Shown are ROC curves showing the rate of finding true positives versus the rate of finding false positives. The blue curve reflects the ability to distinguish 102 small molecule agonists of the type 1 CCK receptor from 1065 approved drugs in ChEMBL as decoys. The red curve reflects the ability to distinguish 130 small molecule agonists of the type 1 CCK receptor from 2175 non-agonist ligands of the type 1 CCK receptor present in ChEMBL as decoys.
DISCUSSION
Rational drug design and refinement of drug candidates are dependent on a detailed molecular understanding of the target and the conformational changes in that target associated with the activity of interest. GPCRs are the largest group of plasma membrane receptors that are present on essentially every excitable cell of the body. This protein class is one of the most frequent targets of currently approved drugs, with approximately one-third of current drugs targeting a very small percentage of the members of this superfamily. Thus, the potential for future GPCR drug development is extraordinary.
With the recent solution of high resolution crystal structures of more than a dozen members of the class A family of GPCRs, there is improved understanding into the well conserved structure of the helical bundles of receptors in this family (25). These structures now include not only inactive antagonist-occupied states, but also agonist-occupied active states (26), providing understanding of the conformational changes in this bundle that can be associated with receptor activation. These structural insights have allowed an improved understanding of structure-based drug design for this protein family (27). However, despite the advances in the ability to obtain crystal structures for GPCRs with their ligands bound, the number of proteins in this superfamily present in high-resolution crystal structures is still relatively small. Thus, robust predictive models still play a central role in rational drug design for this protein class, and are key for the broad application of structural insights to drug development.
The structurally closely related types 1 and 2 CCK receptors are class A GPCRs that are both attractive potential drug targets. Type 1 CCK receptor agonists have been clinically evaluated as anti-obesity agents (28), whereas antagonists have been studied as treatments for bowel motility disorders, gastroesophageal reflux disease, and to enhance analgesia (29). Similarly, type 2 CCK receptor antagonists have been investigated for the treatment of anxiety, functional dyspepsia, nociception, and sleep disorders (29). To fully exploit these receptors as drug targets, an improved understanding of the molecular determinants of binding selectivity and functional activity is needed. We recently utilized unique radioligands and a series of chimeric CCK1R/CCK2R constructs in which all of the residues lining the small drug-binding intramembranous, interhelical pocket that are distinct in each receptor were interchanged, separately as well as in groups, to gain insight into the determinants for binding antagonists to each of these receptors (3). In the current work we have continued to utilize these powerful tools to study a novel 1,5-benzodiazepine ligand that has agonist activity at the type 1 CCK receptor and antagonist activity at the type 2 CCK receptor.
Characterization of the impact of each receptor structural change on ligand binding as well as biological activity provided a unique set of data in which the determinants for these functions were directly evaluated. The insights coming from these studies were consistent with the optimized ligand-directed model of the binding pocket within the type 1 CCK receptor. The molecular modeling also provided an explanation for why this compound exhibited no biological agonist activity at the closely related type 2 CCK receptor.
The biological activity studies in the series of chimeric receptor constructs identified a uniquely important role for receptor residue 7.39. Previous structure-activity studies during the development of this agonist ligand pointed to the importance of the isopropyl group in the N1 position as a possible trigger for stimulating biological activity (4). Indeed, in the best model of the ligand-occupied type 1 CCK receptor, this isopropyl group was found to interact with the leucine residue in position 7.39 in transmembrane segment 7. Furthermore, when histidine is in the 7.39 position in the type 2 CCK receptor, the isopropyl group in the ligand was not accommodated and shifted in position, changing the docking pose of the entire ligand. Other groups in close contact with this isopropyl group when the compound is docked at the type 1 CCK receptor are the tryptophan in position 6.48, isoleucine in position 6.51, serine in position 7.42, and tyrosine in position 7.43. Three of these four residues are fully conserved in the type 2 CCK receptor, with only isoleucine in position 6.51 being replaced by a similar amino acid, valine, in the type 2 CCK receptor.
Structure-activity data in the literature provide some insights into how substituents of the N1 acetamide nitrogen in the benzodiazepine ligands affect their functional status at the type 1 CCK receptor. In the series of compounds related to GI181771X described by Aquino et al. (4), the N-H and N-methyl compounds had no demonstrable agonist activity at the type 1 CCK receptor when used at 30 μm. These compounds had demonstrable antagonist activity, shifting the CCK concentration-response curve to the right, thereby supporting the ability of these compounds to bind to this receptor. Increasing the steric bulk of the alkyl substituent on this amide (N-ethyl and N-propyl) resulted in observable, but partial agonist activity, whereas further increasing the bulk of the substituent by branching off of the carbon proximal to the amide nitrogen (isopropyl) increased the agonist activity (efficacy) to that of the natural full agonist, CCK. There was also an interesting relationship between size and shape of the substituents and their agonist activity, because sterically larger and more lipophilic groups than the isopropyl group, such as cyclohexyl and phenyl substituents, resulted in receptor ligands with reduced agonist activity. However, because the direct analysis of binding of these ligands was not studied, it is not clear whether their reduced biological activity reflected predominant impact on their receptor binding affinity or on their modulation of the receptor to move it toward an inactive conformation.
Although the N-H and N-methyl compounds noted above would be expected to dock quite similarly to GI181771X, these smaller groups would be expected to have looser contacts with the key receptor residues in this region of this pocket. This may suggest that agonist activity is influenced by the ability of the isopropyl group to not only contact, but to move one or more of these five residues and to modify the underlying helical bundle. We would expect to see a change in the conformation of the helical bundle that is similar to that observed in recent class A GPCR crystal structures, but the ligand-guided modeling approach, whereas providing significant insights into the ligand pocket, cannot provide insights into more distant conformational changes.
Studying GI181771X binding and agonist activity at the systematic series of receptor constructs also provided some interesting observations in which binding affinity could be enhanced without inducing parallel effects on biological activity. Eleven of the type 1 CCK receptor-based constructs were observed to exhibit increased binding affinities, with only three having increased potency to stimulate biological responses, CCK1R TM(2,3), TM(3,6), and TM(2,3,6). Of note, the CCK1R TM(2,3) and TM(2,3,6) constructs displayed potency that improved by more than 10-fold. Five of the constructs displaying increased binding affinities lost all significant biological responses, including CCK1R TM(2,7), TM(3,7), TM(6,7), TM(2,6,7), and TM(3,6,7). It is noteworthy that all of these constructs included the TM7 segment of the type 2 CCK receptor, thus eliminating the leucine in position 7.39 that seems to be so important for biological activity. Three of the constructs displaying increased binding affinities had no significant change in their potency to stimulate biological activity (CCK1R TM3, TM(2,6), and TM(2,3,7)). Clearly, in developing an agonist drug, the absolute binding affinity of a ligand is less important than the particular determinants of that binding and their impact on inducing a conformational change in the receptor that is capable of resulting in activation, presumably by stabilizing the interaction with a G protein.
These studies provide unique and highly instructive insights into small molecule ligand docking and activation of closely related members of the GPCR superfamily. A very small change in the binding pocket can result in highly significant differences in binding affinities, docking poses, and the ability to activate the receptor. With these new insights into the intramembranous small molecule docking pocket in the active state of the type 1 CCK receptor, rational refinement of the properties of other ligands binding to this pocket may also now be possible.
Acknowledgments
We thank Drs. P. S. Portoghese and E. Akgun from the University of Minnesota for providing the BDZ ligands, and A. M. Ball and M. L. Augustine for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK032878 (to L. J. M.), U01 GM094612 (to R. A.), and U54 GM094618 (to R. A.).
- CCK
- cholecystokinin
- BDZ
- benzodiazepine
- CCK1R
- type 1 cholecystokinin receptor
- CCK2R
- type 2 cholecystokinin receptor
- GPCR
- G protein-coupled receptor
- KRH
- Krebs-Ringer's/HEPES
- ICM
- internal coordinate mechanics
- ROC
- receiver operating characteristic
- TM
- transmembrane.
REFERENCES
- 1. Dockray G. J. (2012) Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 19, 8–12 [DOI] [PubMed] [Google Scholar]
- 2. Dockray G. J. (2004) Clinical endocrinology and metabolism. Gastrin. Best Pract. Res. Clin. Endocrinol. Metab. 18, 555–568 [DOI] [PubMed] [Google Scholar]
- 3. Cawston E. E., Lam P. C., Harikumar K. G., Dong M., Ball A. M., Augustine M. L., Akgün E., Portoghese P. S., Orry A., Abagyan R., Sexton P. M., Miller L. J. (2012) Molecular basis for binding and subtype selectivity of 1,4-benzodiazepine antagonist ligands of the cholecystokinin receptor. J. Biol. Chem. 287, 18618–18635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aquino C. J., Armour D. R., Berman J. M., Birkemo L. S., Carr R. A., Croom D. K., Dezube M., Dougherty R. W., Jr., Ervin G. N., Grizzle M. K., Head J. E., Hirst G. C., James M. K., Johnson M. F., Miller L. J., Queen K. L., Rimele T. J., Smith D. N., Sugg E. E. (1996) Discovery of 1,5-benzodiazepines with peripheral cholecystokinin (CCK-A) receptor agonist activity. 1. Optimization of the agonist “trigger.” J. Med. Chem. 39, 562–569 [DOI] [PubMed] [Google Scholar]
- 5. Hirst G. C., Aquino C., Birkemo L., Croom D. K., Dezube M., Dougherty R. W., Jr., Ervin G. N., Grizzle M. K., Henke B., James M. K., Johnson M. F., Momtahen T., Queen K. L., Sherrill R. G., Szewczyk J., Willson T. M., Sugg E. E. (1996) Discovery of 1,5-benzodiazepines with peripheral cholecystokinin (CCK-A) receptor agonist activity (II). Optimization of the C3 amino substituent. J. Med. Chem. 39, 5236–5245 [DOI] [PubMed] [Google Scholar]
- 6. Katritch V., Rueda M., Lam P. C., Yeager M., Abagyan R. (2010) GPCR 3D homology models for ligand screening. Lessons learned from blind predictions of adenosine A2a receptor complex. Proteins 78, 197–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearson R. K., Miller L. J., Powers S. P., Hadac E. M. (1987) Biochemical characterization of the pancreatic cholecystokinin receptor using monofunctional photoactivatable probes. Pancreas 2, 79–84 [DOI] [PubMed] [Google Scholar]
- 8. Castillo E. J., Delgado-Aros S., Camilleri M., Burton D., Stephens D., O'Connor-Semmes R., Walker A., Shachoy-Clark A., Zinsmeister A. R. (2004) Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G363–G369 [DOI] [PubMed] [Google Scholar]
- 9. Akgün E., Körner M., Gao F., Harikumar K. G., Waser B., Reubi J. C., Portoghese P. S., Miller L. J. (2009) Synthesis and in vitro characterization of radioiodinatable benzodiazepines selective for type 1 and type 2 cholecystokinin receptors. J. Med. Chem. 52, 2138–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadac E. M., Ghanekar D. V., Holicky E. L., Pinon D. I., Dougherty R. W., Miller L. J. (1996) Relationship between native and recombinant cholecystokinin receptors. Role of differential glycosylation. Pancreas 13, 130–139 [DOI] [PubMed] [Google Scholar]
- 11. Harikumar K. G., Pinon D. I., Wessels W. S., Prendergast F. G., Miller L. J. (2002) Environment and mobility of a series of fluorescent reporters at the amino terminus of structurally related peptide agonists and antagonists bound to the cholecystokinin receptor. J. Biol. Chem. 277, 18552–18560 [DOI] [PubMed] [Google Scholar]
- 12. Munson P. J., Rodbard D. (1980) Ligand. A versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107, 220–239 [DOI] [PubMed] [Google Scholar]
- 13. Harikumar K. G., Potter R. M., Patil A., Echeveste V., Miller L. J. (2013) Membrane cholesterol affects stimulus-activity coupling in type 1, but not type 2, CCK receptors. Use of cell lines with elevated cholesterol. Lipids 48, 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abagyan R. A., Totrov M. M., Kuznetsov D. A. (1994) Icm: a new method for protein modeling and design. Applications to docking and structure prediction from the distorted native conformation. J. Comp. Chem. 15, 488–506 [Google Scholar]
- 15. Abagyan R., Totrov M. (1994) Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J. Mol. Biol. 235, 983–1002 [DOI] [PubMed] [Google Scholar]
- 16. Metropolis N. R. A., Rosenbluth M. N., Teller A. H., Teller E. (1953) Equation of state calculations by fast computing machines. J. Chem. Phys. 21, 1087–1092 [Google Scholar]
- 17. An J., Totrov M., Abagyan R. (2005) Pocketome via comprehensive identification and classification of ligand binding envelopes. Mol. Cell Proteomics 4, 752–761 [DOI] [PubMed] [Google Scholar]
- 18. Overington J. (2009) ChEMBL. An interview with John Overington, team leader, chemogenomics at the European Bioinformatics Institute Outstation of the European Molecular Biology Laboratory (EMBL-EBI). Interview by Wendy A. Warr. J. Comput. Aided Mol. Des. 23, 195–198 [DOI] [PubMed] [Google Scholar]
- 19. Totrov M. (2008) Atomic property fields. Generalized three-dimensional pharmacophoric potential for automated ligand superposition, pharmacophore elucidation and three-dimensional QSAR. Chem. Biol. Drug Design 71, 15–27 [DOI] [PubMed] [Google Scholar]
- 20. Truchon J. F., Bayly C. I. (2007) Evaluating virtual screening methods. Good and bad metrics for the “early recognition” problem. J. Chem. Inf. Model 47, 488–508 [DOI] [PubMed] [Google Scholar]
- 21. Gao F., Sexton P. M., Christopoulos A., Miller L. J. (2008) Benzodiazepine ligands can act as allosteric modulators of the type 1 cholecystokinin receptor. Bioorg. Med. Chem. Lett. 18, 4401–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hadac E. M., Dawson E. S., Darrow J. W., Sugg E. E., Lybrand T. P., Miller L. J. (2006) Novel benzodiazepine photoaffinity probe stereoselectively labels a site deep within the membrane-spanning domain of the cholecystokinin receptor. J. Med. Chem. 49, 850–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kufareva I., Ilatovskiy A. V., Abagyan R. (2012) Pocketome. An encyclopedia of small-molecule binding sites in four-dimensional. Nucleic Acids Res. 40, D535–D540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henke B. R., Aquino C. J., Birkemo L. S., Croom D. K., Dougherty R. W., Jr., Ervin G. N., Grizzle M. K., Hirst G. C., James M. K., Johnson M. F., Queen K. L., Sherrill R. G., Sugg E. E., Suh E. M., Szewczyk J. W., Unwalla R. J., Yingling J., Willson T. M. (1997) Optimization of 3-(1H-indazol-3-yl-methyl)-1,5-benzodiazepines as potent, orally active CCK-A agonists. J. Med. Chem. 40, 2706–2725 [DOI] [PubMed] [Google Scholar]
- 25. Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2-adrenergic receptor-Gs protein complex. Nature 477, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoichet B. K., Kobilka B. K. (2012) Structure-based drug screening for G-protein-coupled receptors. Trends Pharmacol. Sci. 33, 268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henke B. R., Willson T. M., Sugg E. E., Croom D. K., Dougherty R. W., Jr., Queen K. L., Birkemo L. S., Ervin G. N., Grizzle M. K., Johnson M. F., James M. K. (1996) 3-(1H-Indazol-3-yl-methyl)-1,5-benzodiazepines. CCK-A agonists that demonstrate oral activity as satiety agents. J. Med. Chem. 39, 2655–2658 [DOI] [PubMed] [Google Scholar]
- 29. Berna M. J., Tapia J. A., Sancho V., Jensen R. T. (2007) Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr. Opin. Pharmacol. 7, 583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ballesteros J. A., Weinstein H. (1992) Analysis and refinement of criteria for predicting the structure and relative orientations of transmembranal helical domains. Biophys. J. 62, 107–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halgren T. A. (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comp. Chem. 17, 490–519 [Google Scholar]