Background: Active STAT5 promotes angiogenesis.
Results: In human brain endothelial cells, active STAT5 promotes the secretion of prolactin, which stimulates endothelial cell migration and tube formation. Prolactin also induces the secretion of VEGF and activates STAT5.
Conclusion: Prolactin and STAT5 are engaged in a positive feedback loop that stimulates angiogenesis.
Significance: Prolactin may be important in pathologic angiogenesis.
Keywords: Angiogenesis, Cell Migration, Cell Signaling, Prolactin, STAT Transcription Factor, STAT5, Autocrine Factors
Abstract
We have shown previously that the murine prolactin/growth hormone family member proliferin plays a pivotal role in angiogenesis induced by the FGF2/STAT5 signaling cascade. To delineate the signaling pathway downstream of STAT5 in the human system, where proliferin does not exist, we expressed constitutively active (CA) or dominant-negative (DN) mutant STAT5A in hCMEC/D3 human brain endothelial cells. We found that conditioned medium from CA-STAT5A- but not from DN-STAT5A-overexpressing endothelial cells (EC) is sufficient to induce EC migration and tube formation but not proliferation, indicating that STAT5A regulates the secretion of autocrine proangiogenic factors. We identified prolactin (PRL) as a candidate autocrine factor. CA-STAT5A expression stimulates PRL production at the RNA and protein level, and STAT5A binds to the PRL promoter region, suggesting direct transcriptional regulation. Medium conditioned by CA-STAT5A-overexpressing EC induces phosphorylation of the PRL receptor and activates MAPK. Knockdown of PRL expression by shRNA or blocking of PRL activity with neutralizing antibodies removed the CA-STAT5A-dependent proangiogenic activity from the conditioned medium of EC. The addition of recombinant PRL restores this activity. STAT5A-induced PRL in the conditioned medium can activate STAT5, STAT1, and to a lesser extent STAT3 in hCMEC/D3 cells, suggesting the existence of a positive feedback loop between STAT5 and PRL that promotes angiogenesis. Furthermore, we find that VEGF, a potent proangiogenic factor, is induced by activation of STAT5A, and VEGF induction depends on PRL expression. These observations demonstrate a STAT5/PRL/VEGF signaling cascade in human brain EC and implicate PRL and VEGF as autocrine regulators of EC migration, invasion, and tube formation.
Introduction
Angiogenesis, the formation of a new microvasculature, is a critical process during normal organ development as well as tumor initiation, progression, and metastasis (1–4). Although potent angiogenesis inducers (VEGF, basic FGF, angiopoietins, thrombospondin) and a variety of downstream effector molecules (integrins, matrix metalloproteases) have been identified, the paracrine and autocrine signaling pathways that regulate angiogenesis remain incompletely understood.
Prolactin (PRL),2 growth hormone, and placental lactogen (PL) are members of a family of polypeptide hormones, which share structural similarities and biological activities. Work by a number of investigators indicates that these hormones along with proliferin (PLF) and proliferin-related protein (PRP), the murine members of this family, can regulate angiogenesis (5–7). The classical textbook model suggests that PRL is secreted by the pituitary gland into the circulation and exerts its effects systemically primarily on the mammary gland. More recently, it has become evident that PRL is also secreted locally in many tissues and acts as a paracrine/autocrine factor (8). One of the proposed local activities of PRL is the regulation of angiogenesis (5). The intact human PRL molecule, with a molecular mass of 23 kDa, has proangiogenic activity and reportedly stimulates endothelial cell (EC) proliferation (9), migration, and tube formation (6). Conversely, proteolytically generated fragments of PRL, typically displaying a molecular mass of 16 kDa, inhibit angiogenesis and block EC proliferation (7, 10–14). PRL is produced by some but not all ECs, suggesting a role of the hormone in the autocrine regulation of angiogenesis (15–17).
The pleiotropic actions of PRL are mediated through the PRL receptor (PRLR), a member of the type I cytokine receptor family (18–20). Several signaling cascades are activated upon ligand binding and receptor dimerization, including the Jak2/STAT5, Ras/Raf/MAPK, Tek/Vav/Rac1, and PI3K/Akt pathways (21). These PRL-triggered signals, especially the Jak2/STAT5 pathway, activate the expression of numerous downstream genes, some of which have roles in angiogenesis.
We have shown previously that PLF plays a pivotal role in angiogenesis induced by FGF/STAT5 in mouse brain microvascular endothelial cells (22). Because the PLF gene does not exist in humans and PRL appears to carry out many PLF functions, we sought to explore whether PRL or its family members carry out roles similar to PLF in human cells. We report here that STAT5-induced human brain EC migration, invasion, and tube formation requires the secretion of an autocrine factor and identify this factor as PRL. We further show that PRL itself activates STAT5 in ECs, thus establishing a positive signal feedback loop. We also show that VEGF, a potent angiogenesis stimulator, is also induced in a PRL-dependent manner during STAT5-mediated angiogenesis. Thus, we describe a novel role for STAT5 in a STAT5-PRL-VEGF signaling cascade that facilitates growth factor-induced migration, invasion, and tube formation of human brain EC.
EXPERIMENTAL PROCEDURES
Cell Culture
Immortalized human microvascular brain endothelial cell line hCMEC/D3, a gift from Dr. B. Weksler, Weill Medical College of Cornell University, New York, NY (23, 24), which has been extensively characterized and shown to retain their EC characteristics, was maintained in EBM-2 basal medium (Lonza, Allendale, NJ; #CC-3156) supplemented with 2% FBS and EGM-2 MV SingleQuots kit supplements and growth factors (Lonza, #CC-4147). Dishes were coated with collagen gel (R&D System, #354236) diluted at 1:500. HUVEC were purchased from Lonza.
Antibodies and Reagents
Stock solutions (reconstituted at 100 μg/ml in sterile 4 mm HCl containing 1 mg/ml BSA) of recombinant human PRL (R&D system, #682-PL) and recombinant human VEGF165 (500 nm, R&D system, #293-VE) were diluted in serum-free DMEM medium containing 0.2% BSA to their final concentration and added to cultures for different time periods with occasional mixing. Sunitinib (LC Laboratories, Woburn, MA) was dissolved in DMSO to make a 1 mm stock solution. All monoclonal antibodies and antisera were obtained from commercial sources. Antibodies to STAT5 (C-17), STAT3 (H-190), STAT1αp91 (M-23), VEGF (A-20), p-Tyr (PY20), p-Tyr (PY99), PRLR (H-300), von Willebrand factor (H-300), and PRL (A-7 and H-105) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Biotinylated anti-human PRL (BAF682) was purchased from R&D Systems (Minneapolis, MN). Streptavidin-HRP was obtained from GE Healthcare. Antibodies against FLAG (F-3165) and β-actin (AC-74) were from Sigma. Antibodies to p-44/42 and Erk1/2 were from Cell Signaling Technology. Monoclonal anti-BrdU antibody (clone Bu20a) was from Dako. Puromycin was from Santa Cruz (sc-108071). Collagen Type I (#354236), Matrigel basement membrane matrix high concentration (#354248), and Matrigel Invasion Chambers (#354483) were purchased from BD Biosciences.
Adenoviral Plasmids
Constitutively active STAT5A mutant (CA-STAT5A) harboring a COOH terminus FLAG tag (pRKmSTAT5AHSFLAG; generated from pRKmSTAT5AcFLAG using site-directed mutagenesis by substituting His-299 and Ser-711 with arginine and phenylalanine, respectively) and a dominant-negative STAT5A mutant (DN-STAT5A) truncated from amino acid 713 to 793 (pRKmSTAT5A713FLAG) were kindly provided by S. J. Collins (Fred Hutchinson Cancer Center, Seattle, WA) (25). These were cloned into an adenoviral expression vector in our laboratory as described previously (26). For adenovirus infection of target cells, hCMEC/D3 cells (5 × 105 cells/well) were seeded into 10-cm Petri dishes 1 day before infection. A fixed volume of a viral stock (50 pfu/cell) was used to infect the target cells for 24 h. The infected hCMEC/D3 cells were then starved in serum-free DMEM medium for an additional 24 h, and conditioned medium was collected for the subsequent experiments as needed.
Lentivirus-mediated PRL-shRNA Expression
PRL expression in hCMEC/D3 cells was silenced with commercially obtained shRNA-containing lentiviral particles (Santa Cruz Biotechnology). Control shRNA (sc-10808) and shRNA targeting PRL (sc-37214-v) consist of a pool of three to five expression constructs each encoding target-specific 19–25-nucleotide (plus hairpin) shRNA. The constructs were introduced into the hCMEC/D3 cells by lentiviral transduction, supplementing the medium with 8 μg/ml Polybrene (Sigma). After selection with puromycin (8 μg/ml; Sigma) for about 2 weeks, cells were pooled and expanded.
Real-time PCR
Total RNA from treated hCMEC/D3 was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed in a reaction volume of 20 μl containing 2 μl of 10× reverse transcription buffer, 4 μl of 25 mm MgCl2, 1 μl of 10 mm dNTP, 1 μl of 50 μm Random primer, and 1 μl of 40 units of RNaseOUT (Invitrogen). Synthesis of cDNA was carried out for 50 min at 50 °C. PCR amplifications were carried out in a 25-μl reaction volume at a 60 °C annealing temperature. The primer sequences used to measure PRL gene expression were: L1, CATGAACATCAAAGGATCGC (forward), and R1, AACAGGTCTCGAAGGGTCAC (reverse); L2, GTGACCCTTCGAGACCTGTT (forward), and R2, GATGGCCTTGGTAATGAACC (reverse). The primers used for chromatin immunoprecipitation (ChIP) assays were purchased from SABiosciences: EpiTec ChIP quantitative PCR assay human PRL, NW_000948.3 (+)01 kb and EpiTec ChIP quantitative PCR Assay human PRL, NW_000948.3 (−)04 kb. The quantitative PCR was performed according to the EpiTec ChIP quantitative PCR Handbook.
ChIP Assay
Chromatin immunoprecipitation was performed using a kit (Upstate Biotechnology, Lake Placid, NY) following the manufacturer's instructions. Briefly, 2.5 × 106 hCMEC/D3 cells (infected with Ad-Con, Ad-CA-STAT5A or Ad-DN-STAT5A at 50 pfu/cells) were grown in 100-mm dishes and cross-linked by adding formaldehyde to a final concentration of 1% and incubated at room temperature for 10 min. Cells were then washed and lysed in 2 ml of ChIP lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris-HCl, pH 8.0). Cells were sonicated to shear DNA to lengths between 200 and 1000 base pairs after incubation on ice for 10 min. Then 60 μl of protein A-agarose beads (Sigma) were added to the sonicated lysates and rotated at 4 °C for 1 h. The agarose beads were pelleted by brief centrifugation. The supernatant fraction was collected (20-μl lysate aliquots were taken out as input control) and immunoprecipitated with an anti-STAT5 antibody (C17, Santa Cruz). For a negative control, the supernatant fraction was incubated with 60 μl of Salmon Sperm DNA/Protein A-agarose, omitting the primary antibody. After the appropriate washing steps, the complex was eluted from the antibody by adding 250 μl of elution buffer (1% SDS, 0.1 m NaHCO3) to the pelleted protein A-agarose/antibody/STAT5 complex, and the supernatant fraction was carefully transferred to another tube before repeating the elution. The combined eluates (total volume ∼500 μl) were mixed with 20 μl of 5 m NaCl, and the STAT5-DNA cross-links were reversed by heating to 65 °C for 4 h. After a brief digestion with Proteinase K, the DNA was recovered by phenol/chloroform extraction and ethanol precipitation. The pellets were washed with 70% ethanol and air-dried. The pellets were resuspended in an appropriate buffer for PCR.
Assessment of Mitogenesis
To measure DNA synthesis, 3000 hCMEC/D3 cells in 100 μl were seeded into 96-well plates and cultured for 24 h at 37 °C in a CO2 incubator. Then the cells were washed twice with warmed PBS, and the culture medium was replaced with conditioned medium for an additional 48 h. DNA synthesis was analyzed with the bromodeoxyuridine (BrdU) incorporation assay using a chemiluminescence-based BrdU ELISA according to Hawker with modifications (27). BrdU (10 μm final concentration; Sigma) was added for the last 6 h of the experiment. At the conclusion of labeling, cultures were fixed with 70% EtOH (20 min, room temperature), acid-denatured with 2 m HCl (20 min, 37 °C), neutralized with 0.1 m borate buffer (pH 9.0, 5 min, room temperature), and treated with blocking buffer (2% goat serum in PBS with 0.1% Triton X-100). Primary monoclonal anti-BrdU antibody was used at 1 μg/ml (60 min, 37 °C; Dako, clone Bu20a). After washing to remove unbound antibody, the cells were incubated with sheep anti-mouse IgG HRP-conjugated secondary antibody (1:2000 dilution; Amersham Biosciences) for 60 min at 37 °C. After another round of washes and a final rinse with dH2O, 100 μl of substrate (SureBlueTM, PKL) were added to each well and developed for 15–30 min until a sufficient blue color developed. Wells were subsequently quenched with 0.5 m H2SO4 (50 μl/well), and the absorbance (optical density) was measured at 450 nm on a Spectramax Microplate Spectrophotometer (Molecular Devices).
For the MTT proliferation assay, 3000 cells in a volume of 100 μl were seeded into 96-well plates, and at the end of the treatments, 50 μl of CellTiter 96Aqueous One Solution Reagent (Promega) were added. The cultures were incubated for 1–4 h at 37 °C, 5% CO2, and absorbance was measured at 490 nm using a 96-well plate reader (SpectraMax, Molecular Devices).
Immunoblot
Cells were collected and washed 3 times with cold PBS and lysed with 150 μl of radioimmune precipitation assay buffer supplemented with protease and phosphatase inhibitors on ice for 20 min. Cell lysates were cleared by centrifugation at 13,000 rpm for 20 min at 4 °C. Protein concentration was measured by BCA assay, and the lysates were mixed with a 5× sample buffer. After boiling for 5 min, 50 μg of total protein from each sample were separated on a 7.5–12% SDS-PAGE gel. After transfer, the nitrocellulose membranes were blocked with Tris-buffered saline containing 5% nonfat dry milk for 1 h at room temperature and incubated with primary antibodies against the proteins of interest in Tris-buffered saline containing 5% nonfat dry milk plus 0.5% Tween 20, overnight at 4 °C. After washing and incubation with a horseradish peroxidase-conjugated secondary antibody, the proteins were revealed using an enhanced chemiluminescent detection kit (Pierce).
Immunoprecipitation
Cells (P100 mm dishes) were lysed in 1 ml of ice-cold lysis buffer (50 mm Tris, pH 7.5, 1 mm EDTA, 1 mm EGTA, 0.5 mm vanadate, 0.1% 2-mercaptoethanol, 1% Triton X-100, 50 mm NaF, 5 mm sodium pyrophosphate, 10 mm sodium glycerophosphate, 0.1 mm PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml antipain). Cell lysates were collected by scraping and clearance at 13,000 rpm for 20 min at 4 °C. Protein concentrations were determined by BCA assay, and 500 μg of cellular proteins were immunoprecipitated with preconjugated anti-PY20/PY99-PAA beads for 6–12 h at 4 °C. Antibody complexes were washed 3 times with lysis buffer and mixed with 2× sample buffer and boiled for 10 min. Then 40 μl of the samples were loaded to assay for p-PRLR, p-STAT5, p-STAT1, p-STAT3, and p-ERK1/2. Another lysate aliquot was probed for PRLR, STAT5, STAT3, STAT1, ERK1/2, and β-actin.
TCA Precipitation of Samples for SDS-PAGE
The volume of serum-free conditioned medium samples was adjusted up to 1 ml, and protein was precipitated with 100 μl of 100% TCA (28.57 g TCA in 20 ml of Milli Q water). The tubes were placed on ice for at least 2 h. Samples were centrifuged at 15,000 × g for 20 min at 4 °C, and the precipitates were washed twice with cold acetone (−20 °C). After brief air-drying, samples were mixed with 1× sample buffer and boiled for 5 min.
Matrigel EC Tube Formation Assay
High Concentration Matrigel Basement Membrane Matrix (#354248) was adjusted to 10 μg/ml with DMEM, and 100 μl/well Matrigel was added to prechilled 96-well plates. The plate was then incubated at 37 °C for 1 h to allow the material to solidify. Starved hCMEC/D3 cells (15,000 cells/well) were seeded on the surface of the Matrigel in 150 μl of conditioned media. After 6–8 h, images of the tube structures were captured under a phase contrast microscope using a SPOT RT Slider digital camera (Diagnostic Instruments) and analyzed using ImageJ (rsb.info.nih.gov). Tube length was assessed by drawing a line along each tubule and measuring the length of the line in pixels. Tube lengths were measured for each sample in five non-overlapping fields at 200× original magnification.
Monolayer Wound Healing Assay
ECs were seeded onto 6-well plates at 5 × 105 cells/well and grown to confluence before a 24-h starvation period in serum-free DMEM. A single scratch wound was introduced in the monolayer using a micropipette tip, and the medium was replaced with conditioned medium from differently treated hCMEC/D3 cells. Wound closure was monitored for 48 h.
EC Invasion Assay
EC invasion was assayed using modified invasion chambers with polycarbonate PVP-free Nucleopore filters (8 μm pore size) coated with 25 μg/filter Matrigel (BD Bioscience). Starved EC cells (2 × 105) were added to the upper chamber in serum-free medium. Conditioned media were applied as a chemoattractant to the lower compartment of the chamber. At the end of a 48 h-incubation period, the cells on the upper surface of the filter were removed with a cotton swab, and cells on the lower surface of the filter were stained with Hoechst 33342 (1 μg/ml). Cells on the lower surface were counted, and each assay was performed in triplicate.
RESULTS
STAT5 Activation in ECs Induces the Secretion of an Autocrine Pro-angiogenic Factor
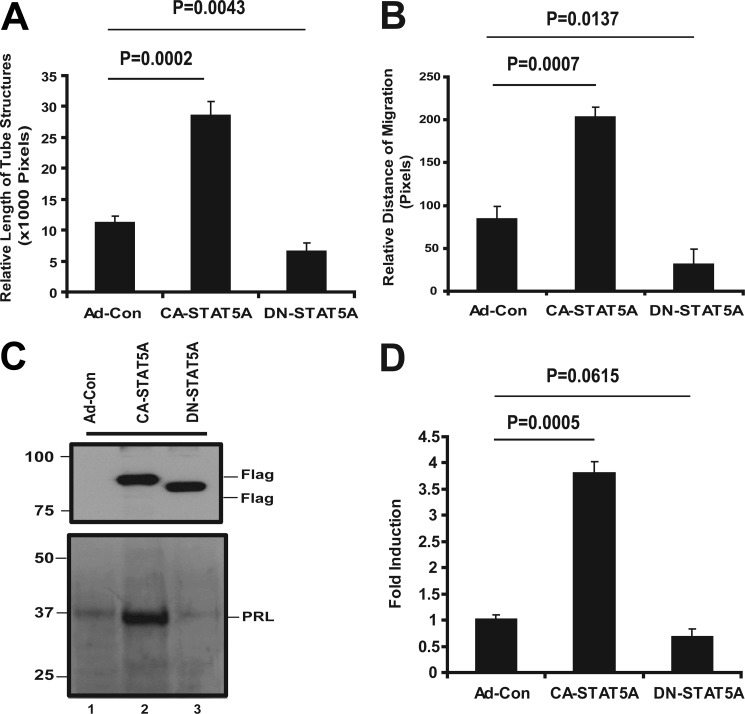
We have recently shown that FGF-induced activation of STAT5 in mouse microvascular ECs results in the secretion of the growth hormone/prolactin family member PLF, which stimulates EC migration, invasion, and tube formation (22, 26). Because the PLF gene does not exist in humans (21, 28–30), we explored whether active STAT5 promotes the secretion of a different autocrine, proangiogenic activity in human ECs. For this purpose, we expressed CA-STAT5A, DN-STAT5A, or a control construct in hCMEC/D3 human brain ECs by adenoviral transduction, collected conditioned media, and tested the ability of the conditioned media to induce angiogenic effects in vitro in native hCMEC/D3 cells. We chose brain endothelial cells because of the importance of angiogenesis in glioma progression and because of our longstanding interest in this tumor type. Consistent with our previously reported observations in mouse ECs (22, 26), conditioned medium from CA-STAT5A-transduced cells compared with conditioned media from cells treated with empty virus or DN-STAT5A-transduced cells stimulates hCMEC/D3 tube formation (Fig. 1A) and migration (Fig. 1B), two processes essential for angiogenesis. Similar effects are also observed after adding conditioned medium from CA-STAT5A-transduced cells to HUVEC cells, suggesting that the secreted activity affects ECs universally (supplemental Fig. 1, A and B). These observations indicate that STAT5 activation in EC leads to the secretion of a soluble factor (or soluble factors) that specifically promotes tube formation and migration via an autocrine loop.
FIGURE 1.
STAT5 activation induces autocrine angiogenic activities associated with prolactin expression. hCMEC/D3 cells at 70% confluence were transduced with Ad-Con, Ad-CA-STAT5A, and Ad-DN-STAT5A at 50 pfu/cell. Cells were incubated for 48 h, and the media were then replaced with fresh serum-free DMEM for an additional 24 h. Conditioned media and cells were collected for subsequent experiments. A, effect of conditioned media on EC tube formation. Starved hCMEC/D3 cells were tested for tube formation in Matrigel as detailed under “Experimental Procedures.” EC tube formation was measured at 6–8 h. Photographs were taken with a phase-contrast microscope, and relative tube length was measured with ImageJ and expressed as the mean ± S.D. for three photographs. Shown is one of three independent experiments. B, effect of conditioned media on EC migration. hCMEC/D3 cell monolayers at ∼95% confluence were wounded with a pipette tip after a 24-h starvation period as described under “Experimental Procedures.” The media were then replaced with conditioned media from Ad-Con, Ad-CA-STAT5A, and Ad-DN-STAT5A. The cells were incubated for two additional days. Photographs were taken with a phase-contrast microscope, and relative migration distance during the gap closure was measured with ImageJ and expressed as the mean ± S.D. for three photographs. Shown is one of two independent experiments. C, CA-STAT5A-induced PRL protein secretion into conditioned medium is shown. Conditioned media from Ad-Con-, Ad-CA-STAT5A-, and Ad-DN-STAT5A-transduced hCMEC/D3 were TCA-precipitated and re-solubilized in sample buffer as described under “Experimental Procedures.” Equal amounts of protein were fractionated on SDS-PAGE gels, and the membranes were probed with antibodies to human PRL. Cell lysates were also analyzed for exogenous expression of FLAG-tagged STAT5, and β-actin served as loading control. D, induction of PRL mRNA by active STAT5A. PRL mRNA was analyzed by qRT-PCR using specific PRL primers in the cells transduction with adenoviral Ad-Con, Ad-CA-STAT5A, and Ad-DN-STAT5A as described above.
STAT5 Activation Results in the Release of PRL, Which Mediates the Phosphorylation of PRLR and ERK1/2 MAPK
Because PLF, the prolactin superfamily member responsible for STAT5-dependent proangiogenic activity in mice, is not present in human cells, we sought to explore whether other prolactin family members might be involved. Indeed, after STAT5 activation in human brain ECs (hCMEC/D3), PRL protein secretion into the conditioned medium was detected (Fig. 1C, supplemental Fig. S1C), and PRL mRNA levels were elevated in the cells (Fig. 1D, supplemental Fig. S1D). Immunolabeling of human gliomas revealed coexpression of PRL and STAT5 in endothelial cells in some but not all glioma tissues (supplemental Fig 2). STAT5 is found primarily in the EC nucleus (supplemental Fig 2A), suggesting activation of this transcription factor in this cell type. Interestingly, PRL also localizes to the nucleus (supplemental Fig. 2B). Although this subcellular location is somewhat unexpected for a secreted hormone, it is consistent with prior reports by Clevenger et al. (31–33) and others, who identified PRL in the nucleus of lymphocytes and breast carcinoma cells and ascribed functional significance to this subcellular localization. STAT5 was also seen in scattered glioma cells (supplemental Fig. 2A), whereas PRL is expressed in the majority of glioma cells (supplemental Fig. 2B).
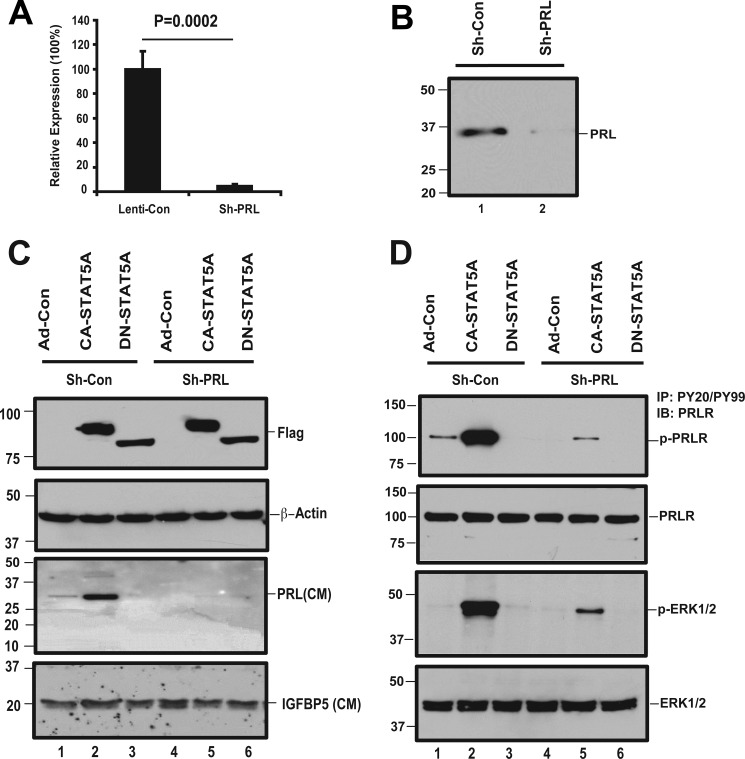
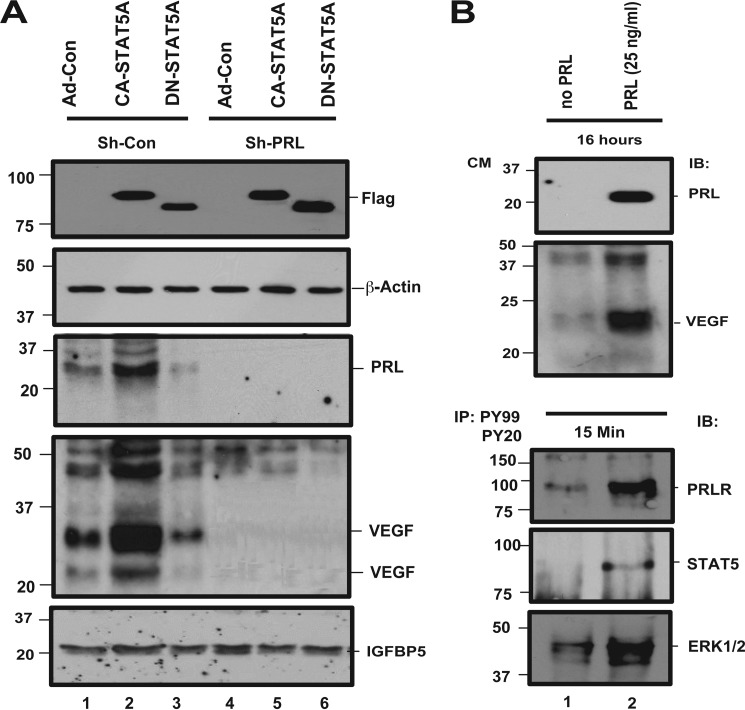
To study the function of PRL secretion, expression of this protein was disrupted with RNAi. shRNA constructs targeting PRL were stably expressed in hCMEC/D3 cells by lentiviral transduction followed by selection in puromycin. qRT-PCR demonstrates a 95–99% reduction of base-line PRL expression (Fig. 2A). The PRL protein level present in the conditioned medium is also significantly decreased (Fig. 2B). Next, we examined whether the shRNA construct can suppress PRL induction by active STAT5. shRNA-expressing clones and controls were transduced with CA-STAT5A, DN-STAT5A, or control virus. CA-STAT5A-induced PRL protein secretion is dramatically suppressed by PRL shRNA (Fig. 2C). The cells expressing mutant STAT5A and/or PRL shRNA were also lysed and processed for Western blotting. Ectopically expressed STAT5A was detected with an antibody to the FLAG epitope (Fig. 2C, upper panel).
FIGURE 2.
Conditioned medium from STAT5A-overexpressing ECs activates PRLR and ERK1/2, dependent on PRL production. A, PRL expression is suppressed by PRL-shRNA. To examine whether the expression of PRL has been knocked down by PRL shRNA constructs, the pooled clones that stably express shRNA or shControl lentivirus were analyzed for PRL mRNA levels by qRT PCR using PRL specific primers. B, PRL shRNA reduces CA-STAT5A-induced PRL secretion into the medium. Equivalent volumes of conditioned media from the pooled clones that stably express shRNA or shControl lentivirus were subjected to TCA protein precipitation. The re-solubilized proteins were analyzed with a 12% acrylamide gel, and the membranes were probed for PRL. C, knockdown of PRL prevents CA-STAT5 mediated induction of PRL. Pooled shRNA expressing clones targeting PRL and controls were transduced with Ad-CA-STAT5A, Ad-DN-STAT5A, or Ad-control virus as described under “Experimental Procedures.” Conditioned media were TCA-precipitated and re-solubilized in sample buffer. Equal amounts of protein were fractionated on SDS-PAGE gels, and the membranes were probed with antibodies to human PRL. The membrane was stripped and reprobed with IGFBP5 as loading control. The cells were lysed and tested for expression of transduced exogenous STAT5 by Western blot. The membranes were probed with an anti-FLAG antibody to detect FLAG-tagged exogenous mutant STAT5A or β-actin as the loading control. D, activation of PRLR and MAPK by CA-STAT5A-conditioned medium is shown. Pooled shRNA expressing clones targeting PRL and controls were transduced with Ad-CA-STAT5A, Ad-DN-STAT5A, or Ad-control to produce conditioned media as detailed under “Experimental Procedures.” hCMEC/D3 cells at 95–100% confluence were starved for 24 h, and the cultures were incubated for 15 min with the conditioned media mentioned above. The treated cells were lysed, and 250 μg of total protein was immunoprecipitated (IP) with antibodies to PY20/PY99 and probed with antibodies to PRLR and ERK1/2. Total cell lysates were also analyzed for expression of endogenous PRLR and ERK1/2 to serve as loading control. IB, immunoblot.
To examine the biologic activity of STAT5-induced PRL, we treated starved hCMEC/D3 cells with conditioned media collected from STAT5 overexpressing cells and assayed PRLR phosphorylation. Conditioned medium from CA-STAT5A but not from DN-STAT5A-expressing cells induces phosphorylation of the PRLR and of ERK1/2 (Fig. 2D). Conditioned medium collected from cells expressing active STAT5 in which PRL expression had been silenced, failed to induce PRLR or ERK1/2 phosphorylation, demonstrating that PRL is responsible for activation of the PRLR signaling pathway. Residual phosphorylated ERK1/2 after PRL expression silencing suggests that STAT5 can also activate ERK1/2 via alternative, PRL-independent pathways (Fig. 2D, lane 5).
STAT5 Binds to the PRL Promoter
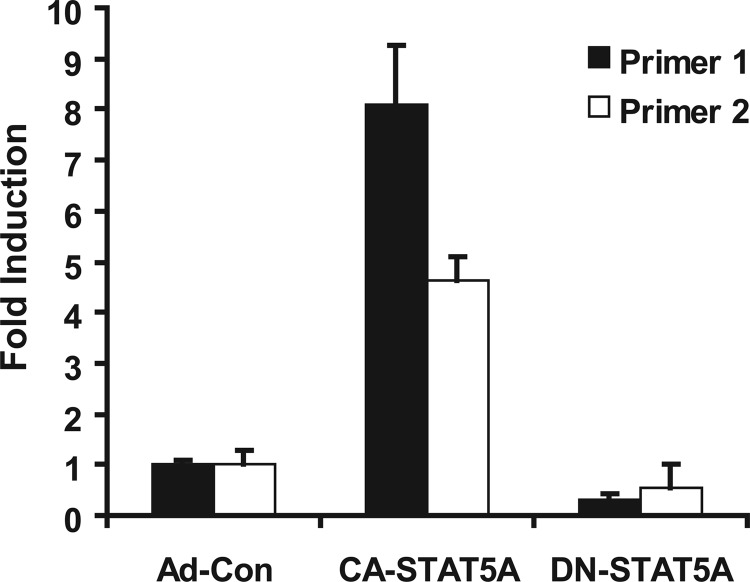
The induction of PRL production by CA-STAT5A could be the result of direct transcriptional regulation or it could be mediated by a yet to be defined indirect regulatory pathway. An NCBI Entrez Gene database analysis revealed multiple potential STAT5 binding sites within the PRL gene promoter region. This prompted us to examine whether STAT5 can bind to the PRL promoter using ChIP. Transduction of hCMEC/D3 with CA-STAT5A led to a significant increase in STAT5 binding to the PRL promoter region, as detected by qRT-PCR using two different primer pairs (Fig. 3). This observation indicates that STAT5 binds to the PRL gene in an activation-dependent manner and suggests direct transcriptional regulation of PRL.
FIGURE 3.
Active STAT5A binds to the PRL promoter. Chromatin immunoprecipitation with a STAT5-specific antibody was performed as described under “Experimental Procedures.” DNA fragments in the precipitates were analyzed by qRT-PCR using two primer pairs designed to amplify the PRL promoter region. Results are normalized to input DNA and expressed relative to control transduction. When a control antibody (rabbit IgG) was used, no signal was detected by qRT-PCR. CA-STAT5A overexpression significantly increases STAT5 binding to the PRL promoter region (p < 0.00001 for both primer pair 1 and primer pair 2). Results shown are representative for three independent experiments in triplicate.
Positive Feedback Loop between STAT5 and PRL
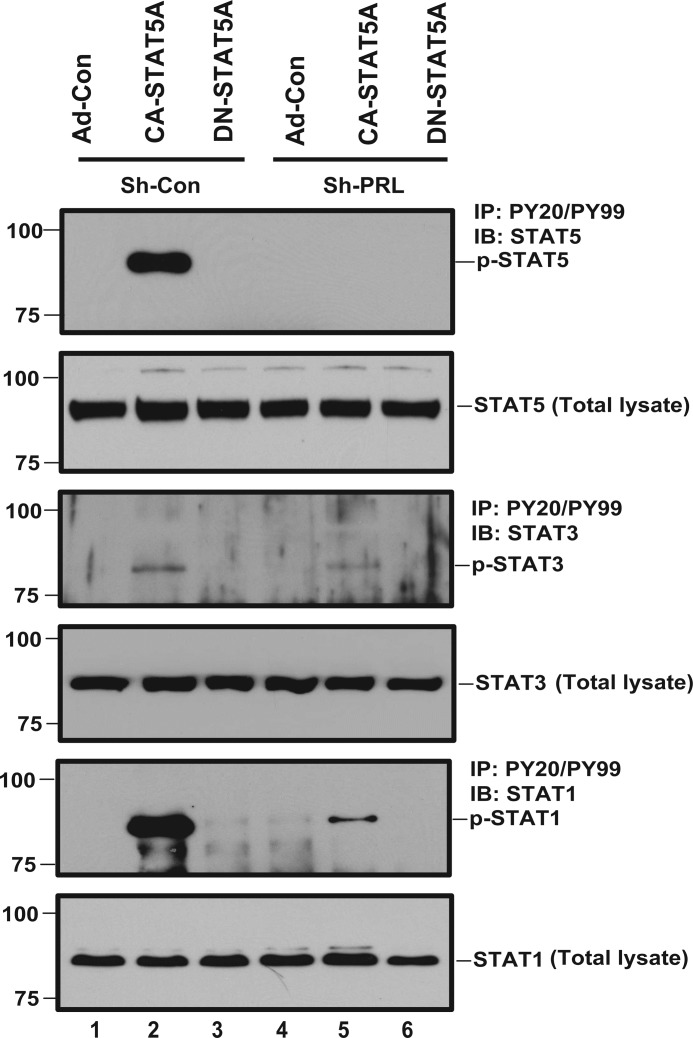
Experimental evidence generated by us and others points toward STAT5 as a transcription factor that integrates signals originating from cytokines and growth factors. Binding of PRL to PRLR has been shown to activate JAK2 and subsequently STAT5 (34). To determine whether this signaling pathway is operative in human ECs, hCMEC/D3 cells were starved and treated with conditioned media collected from hCMEC/D3 cells stably transfected with shPRL or shCon and transduced with Ad-Con, Ad-CA-STAT5A or Ad-DN-STAT5A, respectively. Conditioned medium from Ad-CA-STAT5A-transduced cells stimulates the phosphorylation of STAT5, STAT1, and to a much lesser extent, STAT3 (Fig. 4). Importantly, knockdown of PRL expression in the ECs producing the conditioned medium largely abolishes STAT activation in the responding ECs. Because active STAT5 induces expression and secretion of PRL (Figs. 1C and 2C), these observations point toward a positive feedback loop that would drive enhanced and sustained STAT5 activation via autocrine PRL.
FIGURE 4.
Positive feedback loop between PRL and STAT5. hCMEC/D3 cells at 95–100% confluence were starved for 24 h. The media were then replaced with conditioned media collected from shCon or shPRL-expressing hCMEC/D3 cells that had been transduced with Ad-Con, Ad-CA-STAT5A, or Ad-DN-STAT5A. The cells were treated with the conditioned media for 15 min. The conditioned media-treated cells were lysed, and 250 μg of total protein was immunoprecipitated (IP) with anti-PY20/PY99 antibody. The membranes were probed with antibodies to STAT5, STAT3, and STAT1, respectively. Total cell lysates were also analyzed for endogenous expression of STAT5, STAT3, and STAT1 protein, respectively, to serve as loading control. IB, immunoblot.
STAT5-induced PRL Activity Does Not Promote Cell Proliferation
We previously demonstrated that PLF, secreted by mouse ECs expressing CA-STAT5A, induces EC migration, invasion, and tube formation but fails to stimulate proliferation. To determine whether PRL has a similar activity in human ECs, we studied EC mitogenesis using the BrdU incorporation assay. Conditioned medium from ECs transduced with CA-STAT5A had no effect on hCMEC/D3 cell mitogenesis. Neither silencing of PRL expression by shRNA nor neutralization of PRL activity with a blocking antibody had any effect on EC mitogenesis (supplemental Fig. 3, A and B). Similarly, recombinant PRL used at a concentration of up to 1 μg/ml failed to induce a mitogenic effect (supplemental Fig. 3C). This result is intriguing considering that PRL secretion leads to robust ERK1/2 activation (Fig. 2D).
STAT5-induced PRL Activity Is Required for Endothelial Cell Migration, Invasion, and Tube Formation
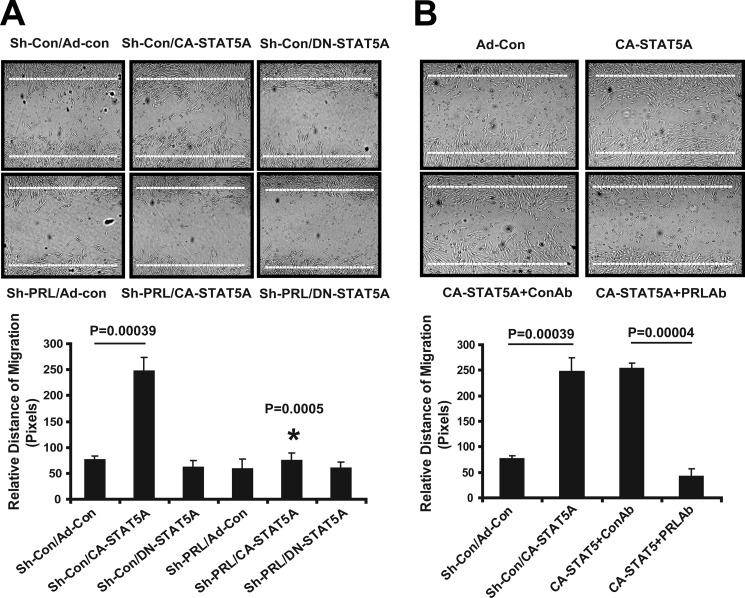
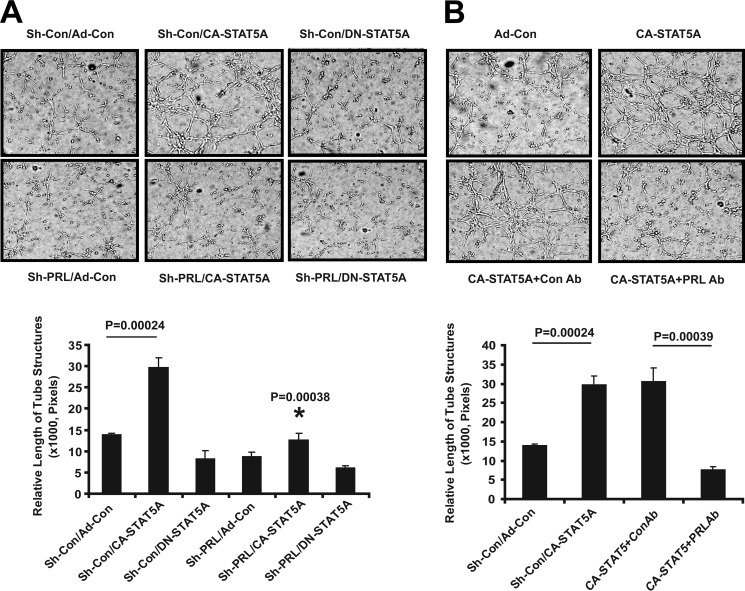
Endothelial cell migration, tube formation, and invasion in Matrigel are considered relevant in vitro surrogate assays for angiogenesis (35). Compared with control, conditioned medium from CA-STAT5A-transduced ECs induces EC migration 2–3-fold (Fig. 5A). shRNA silencing of PRL expression in the producer ECs (Fig. 5A) or neutralization of PRL activity in the conditioned medium (Fig. 5B) abolishes CA-STAT5A-induced EC migration, implicating PRL as the active constituent. Conditioned medium from CA-STAT5A-transduced ECs induces the formation of capillary tube-like structures in EC cultures in matrigel gels by ∼2–2.5-fold. This activity is significantly reduced by disrupting PRL expression with shRNA or blocking PRL with a neutralizing antibody. These findings indicate that PRL is required for STAT5-induced capillary morphogenesis (Fig. 6, A and B).
FIGURE 5.
PRL is necessary for EC migration. A, dependence of hCMEC/D3 migration on PRL is shown. hCMEC/D3 cell monolayers at ∼95% confluence were wounded with a pipette tip after a 24-h starvation period. The medium was then replaced with conditioned medium as indicated, and the cells were incubated for 2 additional days. Photographs were taken with a phase-contrast microscope, and relative migration distance during the gap closure was measured with ImageJ and expressed as the mean ± S.D. for three photographs. Shown is one of two independent experiments. Silencing of PRL expression in CA-STAT5-transduced cells resulted in decreased cell migration (*, p = 0.0005). B, neutralizing anti-PRL antibody decreases cell migration. Dialyzed polyclonal rabbit anti human PRL at a final concentration of 5 μg/ml was used to neutralize PRL in the conditioned medium. An identical concentration of rabbit serum was used as control antibody. Photographs were taken with a phase-contrast microscope, and relative length of migration was measured with ImageJ and expressed as the mean ± S.D. after analyzing three photographs. Neutralization of PRL resulted in decreased cell migration (p = 0.00004). Shown is one of two independent experiments.
FIGURE 6.
PRL is required for STAT5A-induced EC tube formation. A, hCMEC/D3 stably expressing shPRL or shCon were transduced with Ad-CA-STAT5A or empty virus, and then conditioned media were collected and tested for their ability to induce EC tube formation in Matrigel as detailed under “Experimental Procedures.” EC tube formation was measured at 6 h. Photographs were taken with a phase-contrast microscope, and relative tube length was measured with ImageJ. Results are expressed as the mean ± S.D. for three photographs. CA-STAT5A-stimulated tube formation was diminished by shPRL, indicating that PRL is required for this activity (*, p = 0.00038). Shown is one of three independent experiments. B, neutralizing antibody to PRL abolishes EC tube formation stimulated by conditioned medium from CA-STAT5A-expressing hCMEC/D3 cells. Neutralizing antibody directed against human PRL or rabbit serum control were added to conditioned medium produced by CA-STAT5A expressing hCMEC/D3 cells at a final concentration of 5 μg/ml. The antibody-treated conditioned medium was added to starved hCMEC/D3 cells, and tube formation was measured after 6 h. Photographs were taken with a phase-contrast microscope, and relative tube length was measured with ImageJ and expressed as the mean ± S.D. analyzing three photographs (chart). Data represent one of three independent experiments in triplicate. The addition of anti-PRL antibody significantly inhibited tube formation (p = 0.00039).
To examine whether the pro-angiogenic effect of PRL is limited to hCMEC/D3 cells or applies more universally to other ECs, we repeated the experiments in similar form using HUVEC, a well characterized human endothelial cell model. The results in HUVEC closely mirror our findings in hCMEC/D3 cells. HUVEC secrete PRL, and expression is silenced by shRNA (supplemental Fig. 4A). CA-STAT5A expression stimulates PRL secretion (supplemental Figs. 1C and 4B), which is reduced by lentiviral delivery of a PRL shRNA construct (supplemental Fig. 4B). Conditioned medium produced by CA-STAT5A-expressing HUVEC induces migration (supplemental Fig. 5), tube formation (supplemental Fig. 6), and invasion (supplemental Fig. 7) in a PRL-dependent manner. As in hCMEC/D3 cells, conditioned medium from CA-STAT5A-expressing HUVEC induces PRLR and ERK1/2 phosphorylation in native HUVEC, which is abolished by silencing of PRL expression in the producer cells (supplemental Fig. 8).
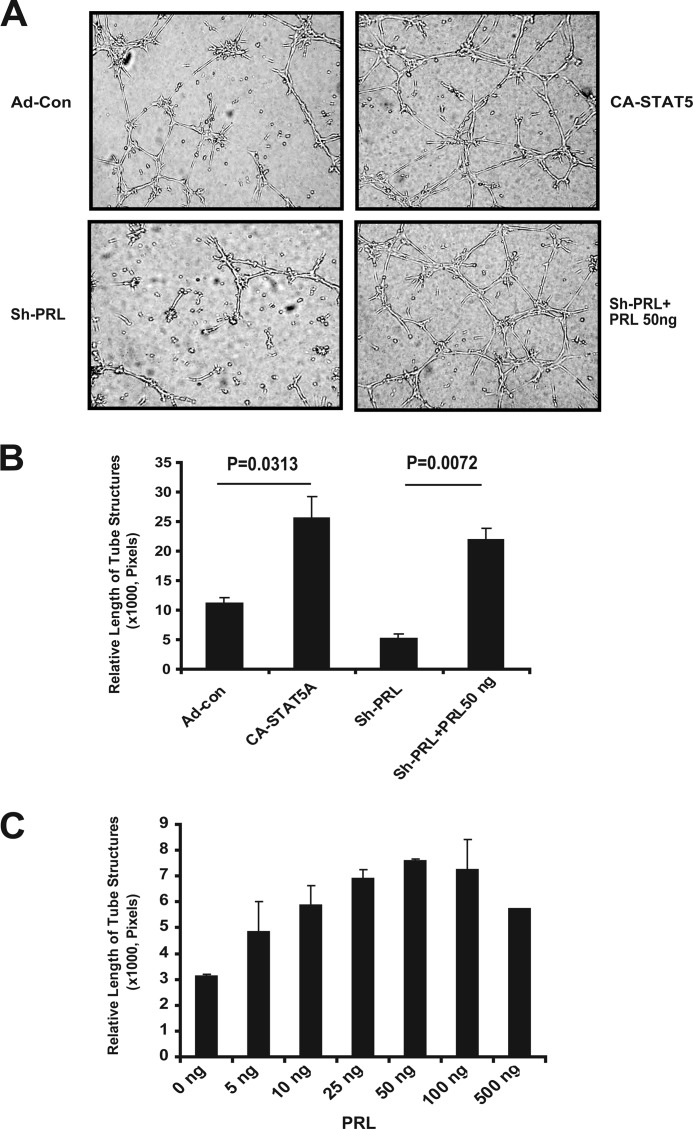
Recombinant PRL Restores Activity after shRNA Expression Silencing
To further confirm a critical role for PRL in capillary morphogenesis and to control for potential off-target effects of the shRNA treatment, we examined whether recombinant PRL can rescue EC tube formation in conditioned medium produced by cells in which PRL expression had been knocked down. Indeed, recombinant PRL at a concentration of 50 ng/ml completely restores EC tube formation, indicating that PRL is necessary and sufficient for the induction of capillary tube formation (Fig. 7).
FIGURE 7.
Recombinant PRL restores EC tube formation in CA-STAT5-expressing cells after PRL knockdown with shPRL RNA. A, conditioned medium from EC with and without CA-STAT5 and with and without shPRLRNA was added to ECs to test tube formation in starved EC. The addition of recombinant PRL (50 ng/ml) restored tube formation of conditioned medium produced by EC in which PRL production had been silenced by shRNA. EC tube formation was measured at 6–8 h. Photographs were taken with a phase-contrast microscope, and relative tube length was measured with ImageJ and expressed as mean ± S.D. for three photographs. Shown is one of three independent experiments. B, quantitation of tube formation is shown. Data represent one of three independent experiments in triplicate. C, recombinant human PRL stimulates tube formation in hCMEC/D3. Starved hCMEC/D3 cells were tested for tube formation in serum-free medium containing different concentrations of human recombinant PRL. EC tube formation was measured at 6–8 h. Photographs were taken with a phase-contrast microscope, and relative tube length was measured with ImageJ and expressed as the mean ± S.D. for three photographs.
STAT5 Mediates PRL-dependent VEGF Induction
VEGF is a potent proangiogenic factor that is transcriptionally regulated by STAT3 in certain models (36). To determine whether VEGF plays a role in STAT5-mediated angiogenesis in our system, we first examined whether VEGF secretion is induced by CA-STAT5 overexpression. hCMEC/D3 cells stably transfected with shCon or shPRL were transduced with CA-STAT5A, DN-STAT5A, or control adenovirus. CA-STAT5A expression results in a robust induction of VEGF secretion (Fig. 8A). Surprisingly, STAT5-dependent VEGF secretion is abolished by shRNA-mediated PRL expression silencing, suggesting that PRL signaling is required upstream of VEGF production. Consistent with this model, recombinant PRL stimulates VEGF secretion (Fig. 8B, upper panel), indicating that PRL signaling is sufficient for VEGF induction. Recombinant PRL is active in vitro, because as expected it induces PRLR and ERK1/2 phosphorylation (Fig. 8B, lower panel). These observations are consistent with a model in which active STAT5 mediates VEGF expression through a PRL-dependent pathway. In a feedback loop, PRL further activates STAT5, which enhances expression of PRL and subsequently VEGF.
FIGURE 8.
PRL-dependent induction of VEGF. A, effect of PRL on VEGF induction. hCMEC/D3 stably expressing shPRL or shCon were transduced with Ad-CA-STAT5A or empty virus as detailed under “Experimental Procedures.” Then conditioned media were collected and tested for expression of PRL and VEGF. The treated cells were lysed, and lysates were also analyzed for expression of exogenous STAT5A and β-actin, which served as the loading control. B, effect of recombinant PRL. Starved hCMEC/D3 cells were treated with recombinant PRL at 25 ng/ml for 16 h (upper panel) or 15 min (lower panel) in serum-free medium condition. The conditioned media (CM) from cells treated for 16 h were analyzed for expression of VEGF and PRL, and the cells treated for 15 min were analyzed for the activation of PRLR, STAT5, and ERK1/2 as described for Fig. 2. IP, immunoprecipitation; IB, immunoblot.
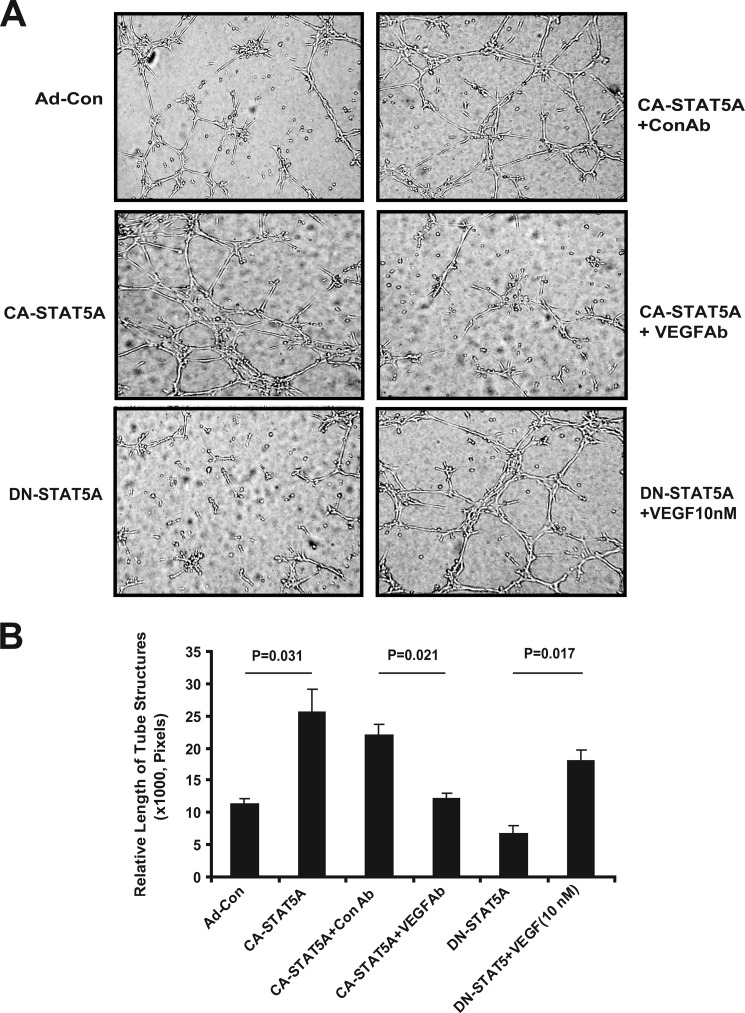
VEGF Signaling via VEGFR2 Participates in STAT5-dependent Autocrine Stimulation of EC Tube Formation
To study whether VEGF secretion contributes to the proangiogenic effect of the conditioned medium from CA-STAT5A overexpressing cells, we blocked VEGF activity and analyzed the ability of the medium to induce endothelial cell tube formation. Neutralization of VEGF significantly reduces EC tube formation, indicating that VEGF participates in the signaling pathway (Fig. 9). The addition of recombinant VEGF to medium conditioned by ECs expressing DN-STAT5A partially restores EC tube formation. To further investigate the role of STAT5-dependent PRL and VEGF secretion in the regulation of angiogenic events, we analyzed whether the VEGFR2 is involved in EC tube formation stimulated by conditioned medium from ECs expressing active STAT5. The inhibition of VEGFR2 by Sunitinib (1.0 μg/ml) led to a slight but significant reduction in EC tube formation after conditioned medium from CA-STAT5A-overexpressing ECs had been added, indicating that VEGFR2 signaling does play a role in CA-STAT5A-induced angiogenic activity (supplemental Fig. 9A). Although EC tube formation induced by recombinant VEGF is significantly diminished by the inhibition of VEGFR2 by Sunitinib (supplemental Fig. 10), EC tube formation stimulated by the addition of PRL alone is not affected by inhibition of the VEGFR2 (supplemental Fig. 9B). In aggregate, these data suggest that secretion of PRL and VEGF both contribute to STAT5-induced angiogenesis.
FIGURE 9.
VEGF participates in the proangiogenic activity of conditioned medium produced by CA-STAT5A-expressing hCMEC/D3 cells. A, a tube formation assay. Neutralizing polyclonal rabbit anti-VEGF (final 5 μg/ml) antibody was used to block VEGF, and recombinant VEGF (10 nm) was used for rescue. Rabbit serum was used as the control. Photographs were taken with a phase-contrast microscope, and relative tube length was measured with ImageJ and expressed as the mean ± S.D. analyzing three photographs. Photos represent one of three independent experiments in triplicate. B, quantitation of tube formation assay. Data represent one of three independent experiments in triplicate.
DISCUSSION
We show here that STAT5 activation in ECs induces the transcription and secretion of PRL. PRL, in turn, activates the PRLR and induces EC migration, invasion, and tube formation but not EC proliferation. Binding of PRL to PRLR activates STAT5, which suggests an autocrine positive feedback loop that would be expected to amplify and sustain angiogenic signaling. PRL signaling also induces the secretion of the powerful pro-angiogenic factor VEGF. Neutralization of VEGF activity abolishes most of the CA-STAT5A effect on EC tube formation (Fig. 9), whereas inhibition of the VEGFR2 by Sunitinib has a more limited effect (supplemental Fig. 10A).
Roles of PRL in Angiogenesis
The classical view of PRL as a pituitary gland-derived hormone with roles in mammary gland development and lactation had to be revised when PRL production in extrapituitary cell types such as fibroblasts and neurons was shown (37, 38). PRL is also secreted by various ECs (15–17), a cell type that also expresses PRLR (6, 15, 39, 40) and thus might be subject to autocrine stimulation by this hormone. Some reports describe an effect of PRL on EC proliferation (9), whereas we and others have failed to observe this activity (6). This discrepancy may be due to different EC types used in the experiments or different functional states of the target cells. Reuwer et al. (6) observed enhanced EC migration and tube formation in response to PRL, but not proliferation, which is consistent with our findings. The relevance of PRL signaling for angiogenesis induction in vivo is also beginning to emerge. Ko et al. (41) observed testicular angiogenesis in response to systemic PRL expression and full-length PRL increases in vascular density in the chicken chorioallantoic membrane assay (5–7). The proangiogenic role of PRL family members appears to be conserved across species, with PLF fulfilling this role in mice (22, 42).
Some of the earlier work on PRL and angiogenesis focused on shorter 16-kDa fragments of PRL family members, so-called vasoinhibins, which potently inhibit angiogenesis (7, 10, 12, 43). The 16-kDa fragments are generated by proteolytic cleavage of full-length PRL, growth hormone, and PL, catalyzed by several enzymes including cathepsin D, matrix metalloproteases and bone morphogenic protein (43–45). Although full-length PRL utilizes the PRLR, its proteolytic fragments appear to signal via a different receptor (39) or an alternative signaling mechanism that relies on the molecule's physical properties as “tilted” peptides (13). The existence of distinct signaling pathways of full-length and 16-kDa forms of PRL is also supported by in vivo studies. 16-kDa PRL is active during the early, proliferative phase of the chorioallantoic membrane assay, when full-length PRL is inactive, whereas the reverse effect is seen during the late “differentiation” phase of the chorioallantoic membrane assay (5–7). The dual function of PRL as autocrine stimulator and inhibitor of angiogenesis dependent on protein processing constitutes an efficient and unique regulatory mechanism (7, 10).
PRL and STAT5 Participate in a Positive Feedback Loop
STAT5 is well characterized as a critical transcription factor downstream of the PRLR that mediates many PRL activities, particularly in mammary gland development and breast cancer (21, 46). Our observation that active STAT5 induces the production of PRL in ECs is novel and expands our understanding of the interaction between these two signaling molecules. STAT5-induced PRL is bioactive as it induces the phosphorylation of PRLR and of ERK1/2 and stimulates the phosphorylation of STAT5 (and to a lesser degree STAT1 and STAT3). This sets the stage for a positive feedback loop, which could amplify or sustain angiogenesis independent of the original paracrine pro-angiogenic signal. This loop could be switched off by proteases such as cathepsin D or morphogenic protein, which process full-length PRL to the antiangiogenic 16-kDa peptide.
PRL-dependent VEGF Expression and Angiogenesis
VEGF is an important paracrine and autocrine stimulator of angiogenesis (47–49). Multiple factors have been implicated in the regulation of VEGF expression in different tissues, including hypoxia-induced pathways, cytokines, and steroid hormones. VEGF secretion in estrogen-induced PRL-secreting rat pituitary tumors as well as the PRL-secreting rat pituitary tumor cell line GH3 is elevated compared with normal pituitary glands (50). Furthermore, patients with pituitary tumors have elevated serum VEGF levels (51). This finding also coincides with the observation that the production of active VEGF isoforms by mammary epithelial cells is increased during lactation when PRL levels are elevated (52). These observations point to a role of PRL in the regulation of VEGF expression.
Regulation of VEGF gene transcription occurs as the result of several transcription factors acting on the VEGF promoter including estrogen receptor (53), Sp1/Sp3 (54), and STAT3 (55). PRL-induced VEGF secretion has been described by others (56, 57) and involves both SP1 and Egr1 transcription factors (56). Whether STAT5 activation plays a direct role in PRL-induced VEGF production in our system remains an open question.
Clinical Relevance and Role of PRL in Tumor Angiogenesis
Autocrine VEGF secretion is required for maintaining the integrity of the vascular system (48) although no vascular abnormalities have been described in either PRL- (58) or PRLR-deficient mice (46). Therefore, it is unlikely that autocrine PRL signaling is necessary for vascular development or vessel maintenance in adults. A potential role for PRL in tumor angiogenesis is more compelling. Paracrine and autocrine PRL signaling has been linked to prostate (59) and breast carcinoma (21) development and progression, and PRLR expression has been identified in tumor vessel EC of breast carcinomas (6). PRL is found in benign CNS tumors (60) and is secreted by malignant glioma cells.3 Tumor or stromal cell-derived PRL in addition to PRL secreted by EC may participate in a complex signaling network that drives and sustains tumor angiogenesis. The autocrine feedback loop involving PRL and STAT5, which appears to occupy a central role in this network, offers an attractive target for therapeutic intervention. PRLR antagonists have recently been described (61, 62) that could be used alone or in combination with STAT5 blockers (63, 64) to disrupt the positive feedback loop. Similarly, 16-kDa PRL or related peptides may be administered to counteract full-length PRL. The viability of such strategies for the antiangiogenic therapy of tumors remains to be tested in preclinical models. In summary, we describe a novel proangiogenic, autocrine feedback loop that centers around STAT5 and PRL signaling and involves VEGF as a downstream effector molecule.
Acknowledgments
We thank Dr. Charles Clevenger, Northwestern University, for immunohistochemical labeling of the glioma samples and Dr. Babette Weksler, Weill Cornell Medical College, for kindly providing hCMEC/D3 cells. Korise Rasmusson helped with manuscript preparation. We thank the University of Wisconsin Translational Research Initiatives in Pathology laboratory, in part supported by the UW Department of Pathology and Laboratory Medicine and UW Carbone Cancer Center Grant P30 CA014520 for use of its facilities and services.
This work was supported by Award I01BX000137 from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development.

This article contains supplemental Figs. S1–S10.
X. Yang and A. Friedl, unpublished observations.
- PRL
- prolactin
- PRLR
- PRL receptor
- PL
- placental lactogen
- EC
- endothelial cell
- CA
- constitutively active
- PLF
- proliferin
- HUVEC
- human umbilical vein endothelial cell(s)
- qRT
- quantitative real-time.
REFERENCES
- 1. Carmeliet P. (2005) Angiogenesis in life, disease, and medicine. Nature 438, 932–936 [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P., Jain R. K. (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Folkman J., Watson K., Ingber D., Hanahan D. (1989) Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339, 58–61 [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer. The next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 5. Corbacho A. M., Martínez De La Escalera G., Clapp C. (2002) Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J. Endocrinol. 173, 219–238 [DOI] [PubMed] [Google Scholar]
- 6. Reuwer A. Q., Nowak-Sliwinska P., Mans L. A., van der Loos C. M., von der Thüsen J. H., Twickler M. T., Spek C. A., Goffin V., Griffioen A. W., Borensztajn K. S. (2012) Functional consequences of prolactin signalling in endothelial cells. A potential link with angiogenesis in pathophysiology? J. Cell. Mol. Med. 16, 2035–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Struman I., Bentzien F., Lee H., Mainfroid V., D'Angelo G., Goffin V., Weiner R. I., Martial J. A. (1999) Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis. An efficient mechanism for the regulation of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 96, 1246–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernichtein S., Touraine P., Goffin V. (2010) New concepts in prolactin biology. J. Endocrinol. 206, 1–11 [DOI] [PubMed] [Google Scholar]
- 9. Castilla A., García C., Cruz-Soto M., Martínez de la Escalera G., Thebault S., Clapp C. (2010) Prolactin in ovarian follicular fluid stimulates endothelial cell proliferation. J. Vasc. Res. 47, 45–53 [DOI] [PubMed] [Google Scholar]
- 10. Clapp C., Aranda J., González C., Jeziorski M. C., Martínez de la Escalera G. (2006) Vasoinhibins. Endogenous regulators of angiogenesis and vascular function. Trends Endocrinol. Metab. 17, 301–307 [DOI] [PubMed] [Google Scholar]
- 11. Dueñas Z., Torner L., Corbacho A. M., Ochoa A., Gutiérrez-Ospina G., López-Barrera F., Barrios F. A., Berger P., Martínez de la Escalera G., Clapp C. (1999) Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest. Ophthalmol. Vis. Sci. 40, 2498–2505 . [PubMed] [Google Scholar]
- 12. Ferrara N., Clapp C., Weiner R. (1991) The 16K fragment of prolactin specifically inhibits basal or fibroblast growth factor stimulated growth of capillary endothelial cells. Endocrinology 129, 896–900 [DOI] [PubMed] [Google Scholar]
- 13. Nguyen N. Q., Tabruyn S. P., Lins L., Lion M., Cornet A. M., Lair F., Rentier-Delrue F., Brasseur R., Martial J. A., Struman I. (2006) Prolactin/growth hormone-derived antiangiogenic peptides highlight a potential role of tilted peptides in angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 103, 14319–14324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabruyn S. P., Nguyen N. Q., Cornet A. M., Martial J. A., Struman I. (2005) The antiangiogenic factor, 16-kDa human prolactin, induces endothelial cell cycle arrest by acting at both the G0-G1 and the G2-M phases. Mol. Endocrinol. 19, 1932–1942 [DOI] [PubMed] [Google Scholar]
- 15. Clapp C., López-Gómez F. J., Nava G., Corbacho A., Torner L., Macotela Y., Dueñas Z., Ochoa A., Noris G., Acosta E., Garay E., Martínez de la Escalera G. (1998) Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells. evidence for autocrine effects. J. Endocrinol. 158, 137–144 [DOI] [PubMed] [Google Scholar]
- 16. Corbacho A. M., Macotela Y., Nava G., Torner L., Dueñas Z., Noris G., Morales M. A., Martínez De La Escalera G., Clapp C. (2000) Human umbilical vein endothelial cells express multiple prolactin isoforms. J. Endocrinol. 166, 53–62 [DOI] [PubMed] [Google Scholar]
- 17. Ochoa A., Montes de Oca P., Rivera J. C., Dueñas Z., Nava G., de La Escalera G. M., Clapp C. (2001) Expression of prolactin gene and secretion of prolactin by rat retinal capillary endothelial cells. Invest. Ophthalmol. Vis. Sci. 42, 1639–1645 [PubMed] [Google Scholar]
- 18. Brooks C. L. (2012) Molecular mechanisms of prolactin and its receptor. Endocr. Rev. 33, 504–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris J., Stanford P. M., Oakes S. R., Ormandy C. J. (2004) Prolactin and the prolactin receptor. new targets of an old hormone. Ann. Med. 36, 414–425 [DOI] [PubMed] [Google Scholar]
- 20. Kelly P. A., Boutin J. M., Jolicoeur C., Okamura H., Shirota M., Edery M., Dusanter-Fourt I., Djiane J. (1989) Purification, cloning, and expression of the prolactin receptor. Biol. Reprod. 40, 27–32 [DOI] [PubMed] [Google Scholar]
- 21. Clevenger C. V., Furth P. A., Hankinson S. E., Schuler L. A. (2003) The role of prolactin in mammary carcinoma. Endocr. Rev. 24, 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X., Qiao D., Meyer K., Pier T., Keles S., Friedl A. (2012) Angiogenesis induced by signal transducer and activator of transcription 5A (STAT5A) is dependent on autocrine activity of proliferin. J. Biol. Chem. 287, 6490–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poller B., Gutmann H., Krähenbühl S., Weksler B., Romero I., Couraud P. O., Tuffin G., Drewe J., Huwyler J. (2008) The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J. Neurochem. 107, 1358–1368 [DOI] [PubMed] [Google Scholar]
- 24. Weksler B. B., Subileau E. A., Perrière N., Charneau P., Holloway K., Leveque M., Tricoire-Leignel H., Nicotra A., Bourdoulous S., Turowski P., Male D. K., Roux F., Greenwood J., Romero I. A., Couraud P. O. (2005) Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 [DOI] [PubMed] [Google Scholar]
- 25. Si J., Collins S. J. (2002) IL-3-induced enhancement of retinoic acid receptor activity is mediated through Stat5, which physically associates with retinoic acid receptors in an IL-3-dependent manner. Blood 100, 4401–4409 [DOI] [PubMed] [Google Scholar]
- 26. Yang X., Qiao D., Meyer K., Friedl A. (2009) Signal transducers and activators of transcription mediate fibroblast growth factor-induced vascular endothelial morphogenesis. Cancer research 69, 1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hawker J. R., Jr. (2003) Chemiluminescence-based BrdU ELISA to measure DNA synthesis. J. Immunol. Methods 274, 77–82 [DOI] [PubMed] [Google Scholar]
- 28. Jackson-Grusby L. L., Pravtcheva D., Ruddle F. H., Linzer D. I. (1988) Chromosomal mapping of the prolactin/growth hormone gene family in the mouse. Endocrinology 122, 2462–2466 [DOI] [PubMed] [Google Scholar]
- 29. Wiemers D. O., Shao L. J., Ain R., Dai G., Soares M. J. (2003) The mouse prolactin gene family locus. Endocrinology 144, 313–325 [DOI] [PubMed] [Google Scholar]
- 30. Wilder E. L., Linzer D. I. (1986) Expression of multiple proliferin genes in mouse cells. Mol. Cell. Biol. 6, 3283–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clevenger C. V., Altmann S. W., Prystowsky M. B. (1991) Requirement of nuclear prolactin for interleukin-2-stimulated proliferation of T lymphocytes. Science 253, 77–79 [DOI] [PubMed] [Google Scholar]
- 32. Reynolds C., Montone K. T., Powell C. M., Tomaszewski J. E., Clevenger C. V. (1997) Expression of prolactin and its receptor in human breast carcinoma. Endocrinology 138, 5555–5560 [DOI] [PubMed] [Google Scholar]
- 33. Rycyzyn M. A., Clevenger C. V. (2002) The intranuclear prolactin/cyclophilin B complex as a transcriptional inducer. Proc. Natl. Acad. Sci. U.S.A. 99, 6790–6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brockman J. L., Schroeder M. D., Schuler L. A. (2002) PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol. Endocrinol. 16, 774–784 [DOI] [PubMed] [Google Scholar]
- 35. Auerbach R., Lewis R., Shinners B., Kubai L., Akhtar N. (2003) Angiogenesis assays. A critical overview. Clin. Chem. 49, 32–40 [DOI] [PubMed] [Google Scholar]
- 36. Kujawski M., Kortylewski M., Lee H., Herrmann A., Kay H., Yu H. (2008) Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Invest. 118, 3367–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richards R. G., Hartman S. M. (1996) Human dermal fibroblast cells express prolactin in vitro. J. Invest. Dermatol. 106, 1250–1255 [DOI] [PubMed] [Google Scholar]
- 38. Shivers B. D., Harlan R. E., Pfaff D. W. (1989) A subset of neurons containing immunoreactive prolactin is a target for estrogen regulation of gene expression in rat hypothalamus. Neuroendocrinology 49, 23–27 [DOI] [PubMed] [Google Scholar]
- 39. Clapp C., Weiner R. I. (1992) A specific, high affinity, saturable binding site for the 16-kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology 130, 1380–1386 [DOI] [PubMed] [Google Scholar]
- 40. Merkle C. J., Schuler L. A., Schaeffer R. C., Jr., Gribbon J. M., Montgomery D. W. (2000) Structural and functional effects of high prolactin levels on injured endothelial cells. Evidence for an endothelial prolactin receptor. Endocrine 13, 37–46 [DOI] [PubMed] [Google Scholar]
- 41. Ko J. Y., Ahn Y. L., Cho B. N. (2003) Angiogenesis and white blood cell proliferation induced in mice by injection of a prolactin-expressing plasmid into muscle. Mol. Cells 15, 262–270 [PubMed] [Google Scholar]
- 42. Jackson D., Volpert O. V., Bouck N., Linzer D. I. (1994) Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science 266, 1581–1584 [DOI] [PubMed] [Google Scholar]
- 43. Clapp C., Martial J. A., Guzman R. C., Rentier-Delure F., Weiner R. I. (1993) The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology 133, 1292–1299 [DOI] [PubMed] [Google Scholar]
- 44. Ge G., Fernández C. A., Moses M. A., Greenspan D. S. (2007) Bone morphogenetic protein 1 processes prolactin to a 17-kDa antiangiogenic factor. Proc. Natl. Acad. Sci. U.S.A. 104, 10010–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piwnica D., Touraine P., Struman I., Tabruyn S., Bolbach G., Clapp C., Martial J. A., Kelly P. A., Goffin V. (2004) Cathepsin D processes human prolactin into multiple 16K-like N-terminal fragments. Study of their antiangiogenic properties and physiological relevance. Mol. Endocrinol. 18, 2522–2542 [DOI] [PubMed] [Google Scholar]
- 46. Ormandy C. J., Camus A., Barra J., Damotte D., Lucas B., Buteau H., Edery M., Brousse N., Babinet C., Binart N., Kelly P. A. (1997) Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11, 167–178 [DOI] [PubMed] [Google Scholar]
- 47. Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362, 841–844 [DOI] [PubMed] [Google Scholar]
- 48. Lee S., Chen T. T., Barber C. L., Jordan M. C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K. P., Iruela-Arispe M. L. (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 [DOI] [PubMed] [Google Scholar]
- 50. Banerjee S. K., Zoubine M. N., Tran T. M., Weston A. P., Campbell D. R. (2000) Overexpression of vascular endothelial growth factor164 and its co-receptor neuropilin-1 in estrogen-induced rat pituitary tumors and GH3 rat pituitary tumor cells. Int. J. Oncol. 16, 253–260 [DOI] [PubMed] [Google Scholar]
- 51. Komorowski J., Jankewicz J., Stepień H. (2000) Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and soluble interleukin-2 receptor (sIL-2R) concentrations in peripheral blood as markers of pituitary tumours. Cytobios 101, 151–159 [PubMed] [Google Scholar]
- 52. Hovey R. C., Goldhar A. S., Baffi J., Vonderhaar B. K. (2001) Transcriptional regulation of vascular endothelial growth factor expression in epithelial and stromal cells during mouse mammary gland development. Mol. Endocrinol. 15, 819–831 [DOI] [PubMed] [Google Scholar]
- 53. Mueller M. D., Vigne J. L., Minchenko A., Lebovic D. I., Leitman D. C., Taylor R. N. (2000) Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc. Natl. Acad. Sci. U.S.A. 97, 10972–10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maeno T., Tanaka T., Sando Y., Suga T., Maeno Y., Nakagawa J., Hosono T., Sato M., Akiyama H., Kishi S., Nagai R., Kurabayashi M. (2002) Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioloalveolar carcinoma cells. Am. J. Respir. Cell Mol. Biol. 26, 246–253 [DOI] [PubMed] [Google Scholar]
- 55. Jung J. E., Lee H. G., Cho I. H., Chung D. H., Yoon S. H., Yang Y. M., Lee J. W., Choi S., Park J. W., Ye S. K., Chung M. H. (2005) STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 19, 1296–1298 [DOI] [PubMed] [Google Scholar]
- 56. Goldhar A. S., Vonderhaar B. K., Trott J. F., Hovey R. C. (2005) Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol. Cell. Endocrinol. 232, 9–19 [DOI] [PubMed] [Google Scholar]
- 57. Malaguarnera L., Imbesi R. M., Scuto A., D'Amico F., Licata F., Messina A., Sanfilippo S. (2004) Prolactin increases HO-1 expression and induces VEGF production in human macrophages. J. Cell. Biochem. 93, 197–206 [DOI] [PubMed] [Google Scholar]
- 58. Horseman N. D., Zhao W., Montecino-Rodriguez E., Tanaka M., Nakashima K., Engle S. J., Smith F., Markoff E., Dorshkind K. (1997) Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16, 6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rouet V., Bogorad R. L., Kayser C., Kessal K., Genestie C., Bardier A., Grattan D. R., Kelder B., Kopchick J. J., Kelly P. A., Goffin V. (2010) Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc. Natl. Acad. Sci. U.S.A. 107, 15199–15204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ciccarelli E., Razzore P., Gaia D., Todaro C., Longo A., Forni M., Ghè C., Camanni F., Muccioli G., Faccani G., Lanotte M. M. (2001) Hyperprolactinaemia and prolactin binding in benign intracranial tumours. J. Neurosurg. Sci. 45, 70–74 [PubMed] [Google Scholar]
- 61. Bernichtein S., Kayser C., Dillner K., Moulin S., Kopchick J. J., Martial J. A., Norstedt G., Isaksson O., Kelly P. A., Goffin V. (2003) Development of pure prolactin receptor antagonists. J. Biol. Chem. 278, 35988–35999 [DOI] [PubMed] [Google Scholar]
- 62. Goffin V., Bernichtein S., Touraine P., Kelly P. A. (2005) Development and potential clinical uses of human prolactin receptor antagonists. Endocr. Rev. 26, 400–422 [DOI] [PubMed] [Google Scholar]
- 63. Bar-Natan M., Nelson E. A., Walker S. R., Kuang Y., Distel R. J., Frank D. A. (2012) Dual inhibition of Jak2 and STAT5 enhances killing of myeloproliferative neoplasia cells. Leukemia 26, 1407–1410 [DOI] [PubMed] [Google Scholar]
- 64. Nelson E. A., Walker S. R., Weisberg E., Bar-Natan M., Barrett R., Gashin L. B., Terrell S., Klitgaard J. L., Santo L., Addorio M. R., Ebert B. L., Griffin J. D., Frank D. A. (2011) The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood 117, 3421–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]