Abstract
Specialized forms of physiologic cell death lacking certain characteristic morphologic features of apoptosis occur in terminally differentiating tissues, such as in the outer cell layers of epidermis. In these cell layers, NF-κB translocates from the cytoplasm to the nucleus and induces target gene expression. In light of its potent role in regulating apoptotic cell death in other tissues, NF-κB activation in these cells suggests that this transcription factor regulates cell death during terminal differentiation. Here, we show that NF-κB protects normal epithelial cells from apoptosis induced by both TNFα and Fas, whereas NF-κB blockade enhances susceptibility to death via both pathways. Expression of IκBαM under control of keratin promoter in transgenic mice caused a blockade of NF-κB function in the epidermis and provoked premature spontaneous cell death with apoptotic features. In normal tissue, expression of the known NF-κB–regulated antiapoptotic factors, TRAF1, TRAF2, c-IAP1, and c-IAP2, is most pronounced in outer epidermis. In transgenic mice, NF-κB blockade suppressed this expression, whereas NF-κB activation augmented it, consistent with regulation of cell death by these NF-κB effector proteins. These data identify a new role for NF-κB in preventing premature apoptosis in cells committed to undergoing physiologic cell death and indicate that, in stratified epithelium, such cell death normally proceeds via a distinct pathway that is resistant to NF-κB and its antiapoptotic target effector genes.J. Clin. Invest. 105:253–260 (2000).
Introduction
Specialized forms of programmed cell death occur in terminally differentiating tissues with a capacity for continuous self-renewal, including the outer layers of stratified epithelium (1, 2). Tissue homeostasis in this setting is dependent on a balance between proliferation and programmed cell death. Apoptosis in many settings involves distinctive ultrastructural and molecular alterations including chromatin condensation, DNA fragmentation, membrane blebbing, cellular shrinkage and collapse (3). Some of these features are lacking in terminal differentiation–associated cell death in stratified epithelium. In the case of epidermis, cells at the junction of the granular and cornified layers change abruptly from metabolically active cells to the nonviable cells lacking nuclei or cellular organelles that form the stratum corneum (4). Cells undergoing this physiologic programmed cell death lack the pyknotic nuclei, cell shrinkage and collapse seen in epidermal apoptosis after certain stimuli, such as ultraviolet radiation (4, 5). The mechanisms regulating terminal differentiation–associated epidermal cell death are not well understood.
The NF-κB family of gene regulatory proteins can profoundly influence induction of cell death (6). NF-κB opposes death in number of settings (6–10), through induction of a number of antiapoptotic factors, including TRAF1, TRAF2, c-IAP1, c-IAP2 (11), IEX-1L (12), and XIAP (13, 14). Its role, however, may differ in different cell types and cell commitment steps, as in such settings as lymphoid development (15), reactive oxygen species-triggered apoptosis in T cells (16), and in cerebral ischemia (17), where NF-κB promotes cell death. In addition, NF-κB has been implicated as an important component in malignant progression characterized by a resistance to apoptosis (18, 19), and NF-κB inhibition is considered a promising potential tumor therapy (20). Thus, NF-κB can play a decisive role in cell fate by either opposing or promoting cell death in different settings; however, the role of NF-κB in epithelial tissues is not well established.
Recently, NF-κB subunits have been shown to translocate from the cytoplasm to the nucleus in cells within the differentiating suprabasal layers of stratified epithelium (21), raising the possibility that NF-κB may either positively or negatively regulate terminal differentiation–associated cell death. In support of this possibility, disruption of normal components involved in NF-κB activation by targeted mutation of the IκB kinase 1 (IKK1) gene (22–24) is associated with evidence of abnormally distributed epidermal cell death in one study at embryonic day 19 (23). To further study this possibility, we studied the impact of altering NF-κB function on epithelial cell death in vitro and in vivo. Here we show that NF-κB strongly inhibits epithelial cell death and that premature apoptotic cell death occurs in the absence of normal NF-κB function in stratified epithelium. These data identify a role for NF-κB in the prevention of premature apoptosis in cells committed to terminal epithelial differentiation and indicate that physiologic cell death in stratified epithelium occurs via a pathway resistant to NF-κB and its effectors TRAF1, TRAF2, c-IAP1, and c-IAP2.
Methods
Cell death analysis.
For terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling (TUNEL) assay, cells were grown on glass slides, treated with TNFα (30 ng/mL) or CH-11. After different time points and cytospinning, cells were subjected to TUNEL assay (Apoptag; Oncor Inc., Gaithersburg, Maryland, USA) following the manufacturer’s protocol. Tissue cryosections were prepared and also subjected to TUNEL assay. Briefly, slides were fixed in 4% formalin for 10 minutes at room temperature, treated with 2% hydrogen peroxide in PBS for 5 minutes, and washed with PBS. Digoxigenin-labeled nucleotides were incubated with terminal deoxyribonucleotidyl transferase for 1 hour at 37°C, viewed by using a peroxidase–anti-digoxigenin antibody, counterstained with methyl green, and mounted with xylene. For analysis of nuclear morphology, propidium iodide staining was performed. After cytospin, cells were fixed with acetone, washed in PBS, and stained with propidium iodide (50 ng/mL) for 15 seconds, followed by a 30-minute wash in PBS, and mounted with VECTASHIELD (Vector Laboratories, Burlingame, California, USA).
Cell culture, vectors, and gene transfer.
Human keratinocytes were isolated from normal human skin biopsies and grown in a 1:1 mixture of SFM (GIBCO-BRL, Grand Island, New York, USA) and Medium 154 (Cascade Biologics, Portland, Oregon, USA) as described (25). The cDNA sequences for the coding regions of human p50 (amino acids 1–502, XbaI truncation) (26), p65 (27), and the dominant-negative mutants IκBαM (10) and ΔSP (28) were expressed through the retroviral expression vector LZRS (21, 29). Vector production and keratinocyte gene transfer was performed as described previously (30, 31). For the cell death analysis above, 3 independent transductions were analyzed at each time point with data presented as SD between these 3 independent experiments; P values were calculated using the Student’s t test.
Production of transgenic epithelial tissues.
Mice transgenic for sequences encoding the IκBαM mutant and constitutively active p50 and p65 expressed through the 2075-bp human keratin 14 promoter were generated as described previously (21).
Analysis of gene expression.
At 6, 12, and 18 hours after retroviral transduction with vectors expressing p50/p65, IκBαM, and lacZ control, primary human skin epithelial cells were harvested and mRNA was prepared. RNase protection was performed for a panel of antiapoptotic genes (hAPO5 apoptosis reagent panel; PharMingen, San Diego, California, USA) and confirmatory Northern blotting was performed using probes for c-IAP1, c-IAP2, XIAP, TRAF1, TRAF2, IEX-1L, survivin, as well as β-actin control. Immunohistochemical analysis was performed as described previously (21) on skin cryosections from IκBαM[+] transgenic and control mice using antibodies to c-IAP1, c-IAP2, TRAF1, and TRAF2 (Santa Cruz Biotechnology, Santa Cruz, California, USA).
Results
NF-κB subunits protects epithelial cells against Fas and TNFα-induced death in vitro.
It has been shown previously in lymphoid cells that NF-κB can oppose cell death triggered by Fas and TNFα (32). To study the function of NF-κB subunits in epithelial cell death, normal human keratinocytes were transduced with retroviral expression vectors for active NF-κB subunits p50 and p65 as well as the trans-dominant IκBαM super-repressor that blocks function of all 5 mammalian NF-κB subunits (10). Transductions were performed using high-titer vector preparations in an approach shown to consistently yield a transduction efficiency of more than 95% (30). Whereas control cells display only moderate levels of diffuse expression, NF-κB subunit-expressing cells show marked expression of nuclear localized protein; as anticipated, the IκBαM super-repressor retains NF-κB subunits predominantly in the cytoplasm (Figure 1 and data not shown). NF-κB subunit–expressing cells appear larger and differ morphologically from controls. This is a consistent finding that develops 48 hours after gene transfer and is dependent on intact NF-κB subunit transcription activation domains in that these changes are not observed with transcriptionally inactive mutants; the basis for this effect is unclear. This approach, however, has been shown previously to produce the predicted respective induction or blockade of NF-κB–driven gene expression in epithelial cells, both in the case of p50 and p65 alone as well as both subunits together (21) and provides a basis for determining the effects altering NF-κB function on apoptosis in this setting.
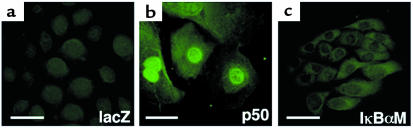
Figure 1.
Generation of epithelial cells with altered NF-κB function. Normal keratinocytes were transduced with retroviral expression vector for (a) lacZ normal control, (b) constitutively active p50, and (c) the trans-dominant IκBαM super-repressor, then subjected to immunofluorescence staining with antibody to p50 (bars = 5 μm). Note marked nuclear expression in p50-transduced cells and blockade of nuclear-localized p50 in IκBαM.
Fas is expressed in epithelial cells and is implicated as a potentially important mediator of epithelial cell death in settings of inflammation (33–35) and ultraviolet injury (5, 36). Cotransduction with vectors altering NF-κB function and a retroviral vector for human Fas/CD95 was used to study NF-κB effects on Fas-triggered apoptosis in epithelial cells. Fas activation in normal control and in IκBαM[+] cells leads to rapid cellular rounding, shrinkage, and detachment in the majority of cells; however, these changes are not seen in NF-κB subunit–expressing cells (Figure 2, a–c and f). These morphologic changes are consistent with apoptosis, a possibility supported by detection of DNA strand breakage using TUNEL assay and characteristic nuclear morphologic condensation and collapse in these cells (Figure 2, d and e).
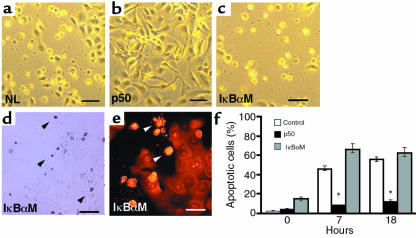
Figure 2.
NF-κB subunit protects epithelial cells against Fas-induced apoptosis in vitro. Normal human keratinocytes were cotransduced with a retroviral vector of the wild-type human Fas protein along with vectors for p50, IκBαM, and lacZ normal control (NL). One day after double transduction, Fas signal transduction was triggered by the anti–Fas antibody CH-11 (1 μg/mL) and cellular morphology was analyzed by phase-contrast microscopy (bars = 10 μm). (a–c) Morphology of (a) normal control, (b) p50[+], and (c) IκBαM[+] cells 7 hours after Fas activation. Note the rounded, shrunken, and detached morphology of IκBαM[+] and normal controls and the lack of such changes in p50[+] cells. Representative (d) TUNEL assay stains and (e) propidium iodide nuclear stains demonstrate apoptotic changes in cells with altered morphology; note positive nuclei in TUNEL staining as well as nuclear condensation and collapse evident in propidium iodide stained cells (arrows). (f) Quantitation of NF-κB effects on Fas-induced epithelial apoptosis in vitro. The percentage of apoptotic cells was determined by cell morphology, TUNEL, and nuclear stains. Time points analyzed were 0, 7, and 18 hours after Fas cross-linking with CH-11. Three independent transductions were analyzed at each time point; data shown are based on morphologic changes and are presented as ± SD between these 3 independent experiments. *P < 0.01 difference of p50 from IκBαM and normal controls.
TNFα is another known trigger of apoptosis in many tissues that also impacts NF-κB function. TNFα activation of NF-κB through the TNF receptor TNFR1 opposes TNFα-induced apoptosis in a number of cell types in a process dependent on TRAF2 (8, 37). Whereas TNFα failed to alter normal keratinocytes or those expressing active NF-κB subunits, NF-κB blockade renders these cells very susceptible to TNFα-induced apoptosis (Figure 3, a–d), suggesting an analogous role for NF-κB in preventing apoptosis in epithelial cells.
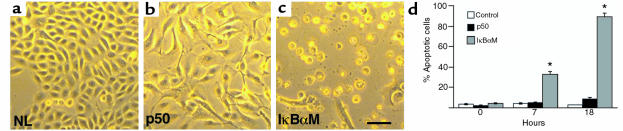
Figure 3.
NF-κB blockade renders normal epithelial cells susceptible to TNFα-triggered apoptosis. Normal keratinocytes were transduced with retroviral expression vectors for p50, IκBαM, and lacZ normal control (NL), then treated 24 hours later with TNFα. (a) Normal control and (b) p50[+] cells; note failure to demonstrate cellular rounding, shrinkage, and detachment characteristic of apoptosis in contrast to (c) IκBαM[+] cells (bar = 10 μm). (d) Quantitation of NF-κB effects on TNFα-triggered epithelial apoptosis in vitro. The percentage of apoptotic cells was determined by cell morphology, TUNEL, and nuclear stains. Time points analyzed were 0, 7, and 18 hours after addition of TNFα. Three independent transductions were analyzed at each time point; data shown are based on morphologic changes and are presented as ± SD between these 3 independent experiments. *P < 0.01 difference of IκBαM from p50 and controls.
Blockade of NF-κB function leads to premature epidermal cell apoptosis in vivo.
Normal stratified epithelium maintains a balance between cellular proliferation and a specialized form of programmed cell death confined to the outer differentiated cell layer at the stratum granulosum–stratum corneum interface (4). This terminal differentiation–associated cell death is not accompanied by classic cell morphologic features of apoptosis such as cell shrinkage and collapse, membrane blebbing, and nuclear condensation seen in other cell death settings in epithelium such as infection and ultraviolet injury (38). NF-κB translocates into nuclei of cells within outer layers of stratified epithelium (21), and this translocation is accompanied by NF-κB target gene activation (K. Hinata et al., manuscript in preparation) as seen in multiple lines of transgenic mice generated previously, in which the lacZ transgene is under control of several different types of NF-κB–responsive promoters (39). To examine a role for NF-κB in regulation of cell death within stratified epithelium, we used transgenic mice with blocked epidermal NF-κB function through keratin promoter–driven expression of IκBαM (21). IκBαM[+] epidermis exhibits regions of spontaneous premature cell death characterized by classic histologic features of apoptosis and evidence of DNA strand breakage with more than 15-fold induction of TUNEL[+] cells over normal littermates (Figure 4, a–f, and data not shown). In addition to differing from normal epidermal programmed cell death by displaying morphologic features of cellular collapse and nuclear condensation, this cell death is no longer confined to the outer epidermis but can be seen early in the outward migration process as the lower spinous layers (Figure 4c), suggesting that NF-κB may prevent a default apoptotic pathway in epithelial cells preparing to undergo more specialized forms of programmed cell death.
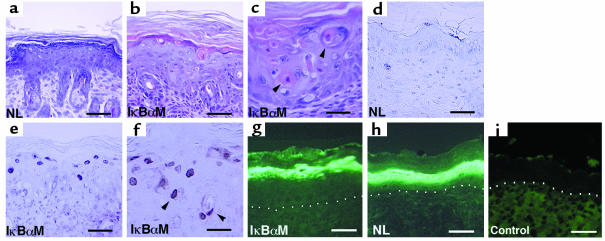
Figure 4.
Epidermal apoptosis with blockade of NF-κB function in vivo. (a–c) Histology of skin in vivo. (a) Age- and site-matched control versus (b, c) IκBαM[+] mice transgenic for loss of epidermal NF-κB function. Note the presence of apoptotic cell morphologic changes extending down to the lower spinous layers (arrows) and the complete absence of such changes in the control. (d–f) TUNEL assay in vivo. (d) Age- and site-matched control versus (e, f) mice transgenic for loss of epidermal NF-κB function. Note the presence of TUNEL-positive cells in the granular layer extending down into the lower spinous layers in IκBαM[+] mice. (a, b, d, e) Bars = 50 μm, (c, f) bars = 25 μm. Expression of the terminal differentiation marker loricrin is not inhibited by NF-κB blockade in vivo. Skin from (g) keratin promoter–driven IκBαM[+] transgenic mice (NL) (h) and littermate control were immunostained using antibody specific for loricrin. (i) Anti-mouse secondary antibody alone controls for background immunofluorescence. The dermal-epidermal boundary is highlighted by white dots. (g, h) Bars = 40 μm.
Expression of the terminal differentiation marker loricrin is not inhibited by NF-κB blockade in vivo.
Cells undergoing programmed cell death in stratified epithelium are normally going through the final phases of terminal differentiation, and there is some evidence suggesting that these processes may be linked in fetal keratinocytes (40). NF-κB blockade in transgenic epidermis has been shown previously, however, to fail to block induction of the differentiation genes keratin 10, involucrin, transglutaminase 1, and filaggrin (21). Loricrin is another terminal differentiation gene expressed at particularly high levels in the outermost cells of epidermis (41), and we examined loricrin expression in IκBαM[+] transgenic mice. Loricrin protein is present in IκBαM[+] skin at comparable levels in the correct outer epithelial layers, suggesting that induction of terminal differentiation genes and NF-κB regulation of cell death are not obligately linked in stratified epithelium (Figure 4, g and h).
NF-κB effects on expression of genes that inhibit apoptosis
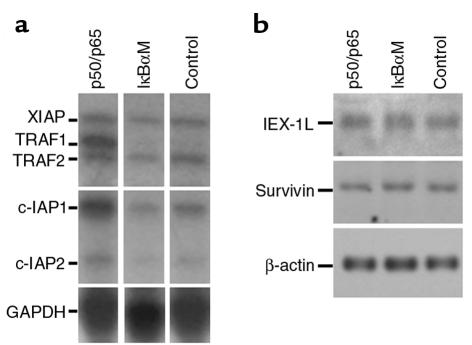
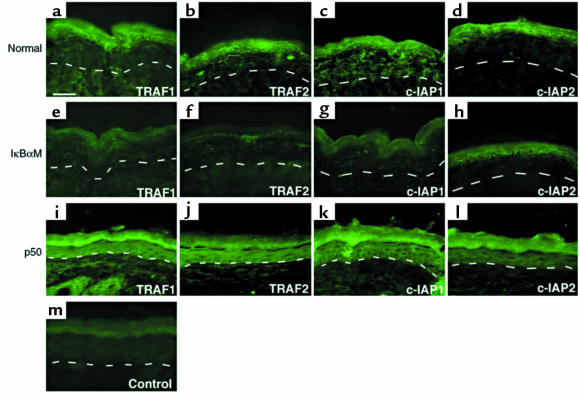
NF-κB can oppose cell death by induction of a number of antiapoptotic genes, including members of the inhibitors of apoptosis (IAP) family, which appear to prevent apoptosis by direct inhibition of specific caspases (42, 43). To study NF-κB regulation of known antiapoptotic genes in epithelial cells, ribonuclease protection analysis was performed on normal epithelial cell populations in vitro with both activated and blocked NF-κB function using probes for an array of antiapoptotic genes. Active NF-κB subunits induced TRAF1 and, more modestly, c-IAP1 (Figure 5a). These findings suggest a role for NF-κB regulation of these antiapoptotic genes in epithelial cells. NF-κB did not globally induce expression of other antiapoptotic genes, however, as seen in the case of XIAP, IEX-1L, and survivin (Figure 5, a and b). Because TRAF1, TRAF2, c-IAP1, and c-IAP2 have been shown to act in concert downstream of NF-κB in preventing cell death (11), we examined TRAF1, TRAF2, c-IAP1, and c-IAP2 protein expression in epidermis as a function of NF-κB activity. Normal controls display strong expression of these genes in a pattern striking for its localization to the outer epidermal cell layers (Figure 6). In contrast, decreased expression in each of these antiapoptotic proteins is seen to differing degrees within epidermis in the setting of NF-κB blockade (Figure 6). Consistent with NF-κB regulation of these proteins in vivo, expression of transcriptionally active NF-κB in transgenic mice, by an approach we have shown leads to active nuclear NF-κB subunits in all epidermal cell layers (21), augments TRAF1, TRAF2, c-IAP1, and c-IAP2 protein expression throughout epidermis (Figure 6). Of these proteins, c-IAP2 expression appears to be the least affected by alterations in NF-κB function in vivo (Figure 6).
Figure 5.
NF-κB effects on expression of antiapoptotic genes. (a) Ribonuclease protection. Normal human keratinocytes were transduced with retroviral expression vectors for p50 and p65 (p50/p65), IκBαM, and lacZ normal control (NL). RNA extracted 12 hours later and subjected to multi-gene ribonuclease protection assay. (b) Northern analysis. Survivin and IEX-1L mRNA expression was determined in human keratinocytes expressing p50 and p65 (p50/p65), IκBαM, and lacZ normal control.
Figure 6.
NF-κB effects on expression of antiapoptotic genes in mice transgenic for augmentation or loss of epidermal NF-κB function. (a–d) Immunofluorescence analysis of TRAF1, TRAF2, c-IAP1, and c-IAP2 in normal littermate control. (e–h) Immunofluorescence analysis in IκBαM[+] transgenic mice. (i–l) Immunofluorescence analysis in mice transgenic for constitutively active p50 NF-κB subunit; note relative hypoplasia of p50[+] epidermis. (m) Anti-rabbit secondary antibody alone control. Note decrease in detection of TRAF1, TRAF2, c-IAP1, and, to a lesser extent, c-IAP2 in IκBαM[+] epidermis, especially in the outer epithelial layers, compared with control. Note also the marked increase in TRAF1, TRAF2, c-IAP1, and, to a lesser extent, c-IAP2 expression, throughout epidermis of p50[+] skin, including cells all the way to the basal layer. The dashed line represents the epidermal basement membrane zone; all layers of epidermal morphology are highlighted in p50[+] skin. All micrographs are at the same magnification (bar = 50 μM, shown in a).
Discussion
Here we have shown that NF-κB function is necessary for normal spatial control of cell death in epidermis. Previous work had demonstrated a role for NF-κB subunits in the regulation of cell death in many cell types (6). Whereas the predominant effect of NF-κB in many tissue settings is to oppose apoptosis, NF-κB can promote cell death in some settings (15–17). The role for NF-κB in regulating cell death within epidermis was previously uncharacterized, although previous studies had demonstrated a protective role for NF-κB subunits against TRAIL-induced apoptosis in transformed keratinocytes in tissue culture (44). In addition, IKK1 knock-out mice (IKK1–/–) (22–24) display some abnormalities in epidermal homeostasis that may involve cell death. When examined at a single time point during development at embryonic day 19, IKK1–/– mice display a compacted distribution of TUNEL-positive keratinocytes in the outer layers of epidermis (23). In addition, NF-κB blockade has been shown recently to increase epidermal sensitivity to ultraviolet radiation injuryinduced apoptosis (45). To our knowledge, a role for NF-κB in regulating localization and features of cell death in uninjured, developmentally mature self-renewing epidermis, however, has not been described previously.
Our data suggest that TRAF1, TRAF2, c-IAP1, and c-IAP2, but not XIAP, IEX-1L, or survivin, are subject to some form of NF-κB regulation in epithelial cells and thus represent potential effectors of NF-κB’s newly identified antiapoptotic role in normal epidermis. Interestingly, NF-κB–induced changes in the magnitude of gene expression in cells grown in culture did not correlate in all cases with our observations in vivo, raising the possibility of more complex mechanisms of NF-κB regulation of these genes in this tissue setting. In lymphoid and other nonepithelial cell types, NF-κB has been shown to induce a number of genes that function to oppose cell death (46, 47), including TRAF1, TRAF2, c-IAP1, c-IAP2 (11, 48), IEX-1L (12), XIAP (13, 14), and A1/Bfl-1 (49, 50). IAP family proteins, including c-IAP1, c-IAP2, XIAP, and survivin (51, 52) contain a 70–amino acid domain called the baculoviral IAP repeat (BIR) domain, which is required for suppression of apoptosis and may be involved in direct binding to caspases (42, 43). NF-κB function appears necessary for normal expression of many such antiapoptotic genes. A combinatorial involvement of NF-κB–triggered genes opposing apoptosis has been demonstrated in the case of c-IAP1, c-IAP2, TRAF1, and TRAF2 where all 4 gene products are necessary for full effects (11). Based on these data, it is logical to suggest that multiple genes may mediate NF-κB-s antiapoptotic effects in epithelium. Consistent with this, our findings indicate that NF-κB function is required for appropriate expression of c-IAP1, c-IAP2, TRAF1, and TRAF2 in vivo. Consistent with our finding that c-IAP2 protein expression in vivo is not strongly repressed by NF-κB blockade, c-IAP2 protein is also not strongly induced by NF-kB subunits either, suggesting that NF-κB regulation of c-IAP2 protein expression may be less potent in vivo than that seen for TRAF1, TRAF2, and c-IAP1. Interestingly, the normal expression of these proteins is most pronounced in the outer layers of epidermis, the spatial setting in which cells approach induction of physiologic cell death and in which abnormal apoptosis occurs in transgenic mice whose epidermal NF-κB function is blocked. This suggests that active opposition of apoptosis may be important in this setting to prevent premature and abnormal cell death in cells committed to terminal differentiation. How such NF-κB regulated antiapoptotic genes interact with the normal induction of the specialized form of physiologic cell death that occurs in outer epidermis is currently unknown.
Surprisingly little is known about the signals governing cell death in outer layers of stratified epithelium. What are the likely triggers of this process? Whereas bcl-2 family genes may influence epithelial apoptosis in response to stress, they do not seem to impact physiologic epidermal cell death (53). TNFR family signal transduction remains a possibility; however, epithelia of TNFR1–/– (54) as well as Fas/CD95–deficient mice (55) do not display dramatic abnormalities in differentiation-associated cell death. Evidence in fetal epithelial cells suggests that apoptosis may be a default pathway taken in the absence of normal keratinocyte differentiation (40). However, regulation of cell death during development is complex (3) and, based on the present data and previous studies that examined normal and immortalized epithelial cells in vitro (56) and in human diseased tissue sections (57, 58), it appears clear that terminal differentiation can occur without strict linkage to normal programmed cell death. The origin of the cell death signal in stratified epithelium and the pathway by which it proceeds remain open questions for further studies.
Traditionally associated with cutaneous inflammation and cellular injury, NF-κB has recently also been identified as a potent negative growth regulator in epithelial cells (21). NF-κB regulation of cell death within a continuously self-renewing tissue identified here further expands its role in cell death control and tissue homeostasis. Although NF-κB appears necessary to inhibit premature and abnormal epidermal cell death, the role of NF-κB subunits in the physiologic epidermal cell death that lacks many classical morphologic features of apoptosis is unclear. Of interest, our data suggest that physiologic epidermal cell death proceeds via a distinct pathway that is resistant to NF-κB and its effectors c-IAP1, c-IAP2, TRAF1, and TRAF2. This possibility is supported by the fact that this death occurs in outer epithelial cell layers that display nuclear-localized NF-κB subunits (21) along with evidence of NF-κB target gene activation (K. Hinata et al., manuscript in preparation) and strong expression of c-IAP1, c-IAP2, TRAF1, and TRAF2 protein. Furthermore, overexpression of active NF-κB subunits p50 and p65 in transgenic epidermis fails to disrupt physiologic epidermal cell death as measured by changes in the number of cells undergoing cell death in the granular layer per unit surface area (ref. 21 and data not shown). Consistent with this, targeted disruption of the IκBα gene, although associated with cutaneous inflammation that may stem from induction of cytokine genes by constitutive NF-κB activation, fails to demonstrate striking abnormalities in epidermal cell death (59). This finding, taken with the current results, suggests that endogenous NF-κB subunits may not have a direct role in triggering physiologic cell death but only in preventing premature apoptosis. Such a possibility would suggest that NF-κB’s main role would be to simply promote keratinocyte viability during the process of outward migration and terminal differentiation until entry into the specialized epidermal cell death pathway occurs through other mechanisms. Implicit in such a model is the existence of a previously unappreciated default apoptotic pathway in committed epithelial cells and the fact that NF-κB function is necessary to prevent its operation. We have demonstrated previously a low basal level of NF-κB–dependent transcription in unstimulated epithelial cells (21). We believe that endogenous NF-κB promotes some degree of transcription of its antiapoptotic targets that can be inhibited by NF-κB blockade, as we report here in connection with TNFα in vitro. Combined with the recent identification of a requirement for NF-κB function in the negative growth regulation of the proliferative cells of the basal epidermal layer, this new role for NF-κB in cell death regulation positions it as an important regulator of homeostasis in stratified epithelium.
Acknowledgments
This work was supported by the Veterans Affairs Office of Research and Development and by National Institutes of Health grants AR-43799, AR-44012, and AR-45192 to P.A. Khavari. C.S. Seitz is the recipient of a postdoctoral fellowship award from Deutsche Forschungsgemeinschaft. K. Hinata is the recipient of an Alza Academia-Industry Award. We thank H. Deng for helpful early discussions, G.S. Herron for advice, and D. Vaux for the generous gift of TRAF1, TRAF2, MIHA (XIAP), MIHB (c-IAP1), and MIHC (c-IAP2) IAP cDNAs. We thank N. Griffiths and P. Bernstein for expert administrative support.
References
- 1.Tamada Y, et al. Identification of programmed cell death in normal human skin tissues by using specific labelling of fragmented DNA. Br J Dermatol. 1994;131:521–524. doi: 10.1111/j.1365-2133.1994.tb08553.x. [DOI] [PubMed] [Google Scholar]
- 2.Polakowska R, Piacentini M, Bartlett R, Goldsmith L, Haake AR. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- 3.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 4.Haake AR, Polakowska RR. Cell death by apoptosis in epidermal biology. J Invest Dermatol. 1993;101:107–112. doi: 10.1111/1523-1747.ep12363594. [DOI] [PubMed] [Google Scholar]
- 5.Hill LL, et al. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- 6.Baichwal V, Baeuerle PA. Activate NF-kappa B or die? Curr Biol. 1997;7:R94–R96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 7.Beg A, Sha W, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 10.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 11.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 12.Wu MX, Ao Z, Prasad KV, Wu R, Schlossman SF. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 13.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 14.Stehlik C, et al. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hettmann T, DiDonato J, Karin M, Leiden JM. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145–158. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumont A, et al. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene. 1999;18:747–757. doi: 10.1038/sj.onc.1202325. [DOI] [PubMed] [Google Scholar]
- 17.Schneider A, et al. NF-kB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 18.Reuther JY, Reuther G, Cortez D, Pendergast A, Baldwin AS., Jr A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 1998;12:968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C-Y, Cusack JC, Liu R, Baldwin AS. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 21.Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-kB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kB. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda K, et al. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, et al. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 26.Blank V, Kourilsky P, Israel A. Cytoplasmic retention, DNA binding and processing of the NF-kappa B p50 precursor are controlled by a small region in its C-terminus. EMBO J. 1991;10:4159–4167. doi: 10.1002/j.1460-2075.1991.tb04994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan GP, Ghosh S, Liou H, Tempst P, Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991;64:961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- 28.Logeat F, et al. Inhibition of transcription factors belonging to the rel/NF-kappa B family by a transdominant negative mutant. EMBO J. 1991;10:1827–1832. doi: 10.1002/j.1460-2075.1991.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 30.Deng H, Choate KA, Lin Q, Khavari PA. High efficiency gene transfer and pharmacologic selection of genetically engineered human keratinocytes. Biotechniques. 1998;25:274–280. doi: 10.2144/98252gt02. [DOI] [PubMed] [Google Scholar]
- 31.Choate KA, Kinsella TM, Williams ML, Nolan GP, Khavari PA. Transglutaminase 1 delivery to lamellar ichthyosis keratinocytes. Hum Gene Ther. 1996;7:2247–2253. doi: 10.1089/hum.1996.7.18-2247. [DOI] [PubMed] [Google Scholar]
- 32.Zong WX, Bash J, Gelinas C. Rel blocks both anti-Fas- and TNF alpha-induced apoptosis and an intact Rel transactivation domain is essential for this effect. Cell Death Differ. 1998;5:963–972. doi: 10.1038/sj.cdd.4400441. [DOI] [PubMed] [Google Scholar]
- 33.Viard I, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 34.Sayama K, Yonehara S, Watanabe Y, Miki Y. Expression of Fas antigen on keratinocytes in vivo and induction of apoptosis in cultured keratinocytes. J Invest Dermatol. 1994;103:330–334. doi: 10.1111/1523-1747.ep12394858. [DOI] [PubMed] [Google Scholar]
- 35.Freiberg RA, et al. Specific triggering of the Fas signal transduction pathway in normal human keratinocytes. J Biol Chem. 1996;271:31666–31669. doi: 10.1074/jbc.271.49.31666. [DOI] [PubMed] [Google Scholar]
- 36.Aragane Y, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu H, Shu H, Pan M, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNFR1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 38.Haake, A.R., and Holbrook, K. 1999. The structure and development of skin. In Dermatology in general medicine. I Freeberg et al., editors. McGraw-Hill. New York, NY. 70–114.
- 39.Schmidt-Ullrich R, et al. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- 40.Haake AR, Cooklis M. Incomplete differentiation of fetal keratinocytes in the skin equivalent leads to the default pathway of apoptosis. Exp Cell Res. 1997;231:83–95. doi: 10.1006/excr.1996.3441. [DOI] [PubMed] [Google Scholar]
- 41.Mehrel T, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 42.Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 43.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1999;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 44.Kothny-Wilkes G, et al. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 1998;273:29247–29253. doi: 10.1074/jbc.273.44.29247. [DOI] [PubMed] [Google Scholar]
- 45.van Hogerlinden M, Rozell BL, Ahrlund-Richter L, Toftgard R. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 1999;59:3299–3303. [PubMed] [Google Scholar]
- 46.Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu ZL, et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You M, Ku PT, Hrdlickova R, Bose HR., Jr ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol. 1997;17:7328–7341. doi: 10.1128/mcb.17.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grumont R, Rourke I, Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 52.Li F, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 53.Pena JC, Fuchs E, Thompson CB. Bcl-x expression influences keratinocyte cell survival but not terminal differentiation. Cell Growth Differ. 1997;8:619–629. [PubMed] [Google Scholar]
- 54.Pfeffer K, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 56.Chaturvedi V, et al. Apoptosis in proliferating, senescent, and immortalized keratinocytes. J Biol Chem. 1999;274:23358–23367. doi: 10.1074/jbc.274.33.23358. [DOI] [PubMed] [Google Scholar]
- 57.Gandarillas A, Goldsmith LA, Gschmeissner S, Leigh IM, Watt FM. Evidence that apoptosis and terminal differentiation of epidermal keratinocytes are distinct processes. Exp Dermatol. 1999;8:71–79. doi: 10.1111/j.1600-0625.1999.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 58.Mitra RS, et al. Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest. 1997;76:99–107. [PubMed] [Google Scholar]
- 59.Klement JF, et al. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]