Abstract
Adenosine has potent effects on both the cardiovascular and immune systems. Exposure of tissues to adenosine results in increased vascular permeability and extravasation of serum proteins. The mechanism by which adenosine brings about these physiological changes is poorly defined. Using mice deficient in the A3 adenosine receptor (A3AR), we show that increases in cutaneous vascular permeability observed after treatment with adenosine or its principal metabolite inosine are mediated through the A3AR. Adenosine fails to increase vascular permeability in mast cell–deficient mice, suggesting that this tissue response to adenosine is mast cell–dependent. Furthermore, this response is independent of activation of the high-affinity IgE receptor (FcεR1) by antigen, as adenosine is equally effective in mediating these changes in FcεR1 β-chain–deficient mice. Together these results support a model in which adenosine and inosine induce changes in vascular permeability indirectly by activating mast cells, which in turn release vasoactive substances. The demonstration in vivo that adenosine, acting through a specific receptor, can provoke degranulation of this important tissue-based effector cell, independent of antigen activation of the high-affinity IgE receptor, supports an important role for this nucleoside in modifying the inflammatory response.
Introduction
Adenosine and its primary metabolite inosine are ubiquitous nucleosides that can be released in substantial quantities from ischemic tissue and stimulated mast cells (1–4). Adenosine acts through cell-surface adenosine receptors to orchestrate numerous physiological events in multiple tissues. Few physiological roles have been ascribed to inosine, and only recently has it been shown that inosine can participate in receptor-mediated signaling (5). To date, 4 adenosine receptors have been identified, A1, A2A, A2B, and A3, each with unique tissue distributions, ligand affinity, and signal transduction mechanisms (6). A1 and A2A receptors are activated by submicromolar concentrations of adenosine, whereas A2B and A3 receptors become activated only when adenosine levels rise into the micromolar range. Inosine has been shown to bind to A3 receptors in the range of 10–50 μM (5). These differences in ligand affinity have important physiological implications, as tissue levels of adenosine and inosine are believed to increase into the micromolar range during periods of inflammation, hypoxia, or ischemia (1, 3, 4, 7). Hence, the A2B and A3 receptors have become attractive pharmacologic targets for modifying the inflammatory response.
Changes in microvascular permeability are crucial to the development and perpetuation of the inflammatory response. It has been recognized for many years that adenosine can produce vasodilatation and increases in capillary permeability in numerous vascular beds (8–11). Although there is evidence that cAMP-coupled A2 receptors on vascular smooth muscle are responsible for adenosine-mediated vasodilatation (12), the precise mechanism involved and the receptors mediating vascular permeability changes are not as well established (13–15). Furthermore, it is not clear whether adenosine acts directly on endothelial cells, or if the observed increase in vascular permeability is indirect, resulting from mediator release by stimulated immune cells.
A number of lines of evidence suggest that adenosine receptors are present on endothelial cells and that direct activation of these receptors results in alteration of endothelial permeability. Although the permeability of in vitro monolayers of macrovascular aortic endothelial cells decreases in response to adenosine, microvascular endothelial cells demonstrate adenosine-induced increases in permeability (16, 17). Several whole-vessel preparations and in vivo studies in multiple tissues have shown increases in microvascular permeability after exposure to adenosine; however, these experiments are confounded by adenosine’s effects on perivascular immune cells (9–11).
Mast cells are present in most tissues and are often found in close proximity to blood vessels, including capillaries and postcapillary venules (18–20). Upon stimulation, these cells release a number of mediators, including leukotrienes, histamine, and serotonin, that act directly on the vasculature to produce vasodilatation, increased permeability, and subsequent plasma protein extravasation into surrounding tissue (21). The ability to release these vasoactive substances suggests that the mast cell may play a key role in regulating these physiological processes.
Modulation of mast-cell function by adenosine has been extensively investigated. In vitro studies have shown that adenosine can potentiate mediator release from stimulated mast cells but cannot provoke mediator release alone (22). However, a number of studies suggest that adenosine can initiate mast-cell degranulation in vivo in the absence of additional stimuli (23, 24). Because adenosine fails to initiate degranulation of mast cells in culture, it is not clear that this in vivo response is mediated by binding of adenosine to a particular receptor and, moreover, which of the adenosine receptors found on mast cells is required for this response.
Efforts to define the adenosine receptor responsible for these effects on mast-cell degranulation has been difficult owing to incomplete specificity of receptor agonists and antagonists. Nevertheless, most studies suggest that A2B and A3 receptors are predominantly involved (24–32). The differential importance assigned to these receptors in mast-cell degranulation may reflect the fact that mast cells examined were from different species and tissues.
To further delineate the mechanisms by which adenosine and its principal metabolite inosine mediate proinflammatory effects on tissues, we examined the vascular response to these nucleosides using 3 mutant mouse lines; namely, mice lacking the A3 adenosine receptor, mice lacking mast cells, and mice lacking a functional high-affinity receptor for IgE. These animals have enabled us both to define the mechanism by which adenosine induces changes in cutaneous vascular permeability in vivo and to determine the receptor through which adenosine and inosine exert these physiological effects.
Methods
Animal welfare.
The use of experimental animals was in accordance with the Institutional Animal Care and Use Committee guidelines of the University of North Carolina at Chapel Hill.
A3AR-deficient, FcεR1 β chain–deficient, and mast cell–deficient mice.
Gene targeting in murine embryonic stem cells was used to generate mice deficient in the A3AR and in the β chain of the FcεR1 high-affinity IgE receptor as described previously (32, 33). Mast cell–deficient mice (WBB6F1/J-W/WV) and their congenic normal litter mates (WBB6F1/J-W/W+) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). Experiments were carried out with A3AR–/– mice of various ages, ranging from 16 to 98 weeks old. Age-matched littermates were used as A3AR+/+ controls in all experiments. Mast cell–deficient mice and their controls ranged from 10 to 16 weeks old. FcεR1 β chain–deficient mice and their controls ranged from 10 to 14 weeks old.
Isolation of bone marrow–derived mast cells.
Bone marrow–derived mast cells (BMMCs) were isolated from the femurs of 3- to 6-month-old mice and placed in culture for at least 4 weeks in the presence of murine IL-3 to select for pure populations of mast cells as described previously (34). Culture media consisted of RPMI 1640 medium supplemented with 8% FCS, 8% murine IL-3 culture supplement (Collaborative Biomedical Products, Bedford, Massachusetts, USA), 20 mM HEPES, 4 mM L-glutamine, 0.08 U/mL penicillin, 0.08 U/mL streptomycin, 800 μM nonessential amino acids, 800 μM sodium pyruvate, 0.04 mg/mL gentamicin, and 92 μM β-mercaptoethanol. Cell cultures were maintained at a constant 37°C in a humidified chamber containing 5% CO2. Adherent cells (macrophages, monocytes) were depleted from culture by transferring cells in suspension to fresh media weekly at a concentration of 1 × 105 to 5 × 105 cells/mL.
Hexosaminidase assay.
Mast cells were incubated overnight at 37°C with monoclonal murine anti-DNP IgE (clone SPE-7; Sigma Chemical Co., St. Louis, Missouri, USA) at a concentration of 100 ng/mL per million cells to give 100% occupancy of IgE receptors. Cells were washed twice with a glucose-saline, Pipes-buffered medium containing 1 mM calcium (Siraganian buffer; also with KCl, MgCl2, BSA [pH 7.2]) and transferred to 96-well microtiter plates at a concentration of 500,000 cells per 75 μL. Cells were incubated with or without adenosine for 1 minute before stimulation with antigen (DNP-albumin, 10 ng/mL; Sigma Chemical Co.). Reactions were terminated after 20 minutes by centrifuging samples at 4°C at 2,000 g for 5 minutes. Hexosaminidase secretion was determined by incubating supernatant and cell lysate with 1 mM p-nitrophenyl-N-acetyl-β-D-glucosaminide (p-NAG, molecular weight 342.3; Sigma Chemical Co.). After a 1-hour incubation at 37°C, 0.1 M Na2CO3/NaHCO3 was added and absorbance was read at 405 nm. Hexosaminidase release was expressed as a percentage of the total amount of hexosaminidase present in the cells.
Intradermal adenosine and inosine.
Mice were injected with 100 μL of 1% Evans blue (Sigma Chemical Co.) in PBS intravenously by tail vein. One hour later, they were anesthetized and given 20 μL of adenosine (10–3 –10–4 M) or inosine (10–4 M) and 20 μL of PBS into the right and left ears, respectively. One hour after intradermal injections, mice were sacrificed by cervical dislocation, and 7-mm ear punches were obtained from each ear. The Evans blue dye was extracted by incubation in 0.5 mL of formamide at 55°C for 48 hours and was quantitated by measuring the absorbance of Evans blue at 610 nm with a spectrophotometer (35).
Histological analysis.
Organs were harvested from A3AR+/+ and A3AR–/– mice and fixed in 10% formalin. Tissue was embedded in paraffin, and 5-μm sections were cut and stained with toluidine blue. Mast-cell numbers in each tissue were quantitated in a blinded fashion by counting the number of positively staining cells in each tissue from 3 animals of each genotype.
Passive cutaneous anaphylaxis.
Passive cutaneous anaphylaxis was performed as described previously (36). Briefly, animals were lightly anesthetized and injected intradermally in the right ear with 20 ng of murine monoclonal anti-DNP IgE diluted in 20 μL of PBS. The left ear was injected with PBS alone. Twenty-four hours later, the animals were injected intravenously with 100 μg of DNP-albumin in 100 μL of 0.9% PBS; 1% Evans blue dye was added to permit visual localization of increased vascular permeability. The reaction was quantitated at 90 minutes after injection as already described here.
Passive systemic anaphylaxis.
Passive systemic anaphylaxis was performed as described previously (37). Briefly, animals were injected intravenously with 200 μL of PBS containing 20 μg of a murine monoclonal anti-DNP IgE, and 24 hours later, with 200 μL of PBS containing 1% Evans blue dye and 1 mg DNP-albumin. Control animals received antigen only or IgE only. Baseline temperature was established for each animal using a rectal probe before injection of the antigen. Temperature drops due to anaphylactic response were recorded at 20, 30, 40, and 50 minutes after the injection of antigen. Ear edema was quantitated as already described here.
Statistical analysis.
Data are presented as mean ± SEM. Statistical significance was assessed by using the unpaired Student’s t test.
Results
Effect of adenosine and inosine on degranulation of BMMCs.
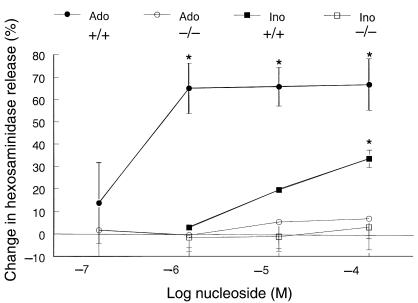
To compare the effect of adenosine and its metabolite inosine on mast-cell function, in particular mast-cell degranulation, BMMCs were prepared from wild-type and A3AR–/– mice. Wild-type and A3AR–/– BMMCs were exposed to increasing concentrations of either adenosine or inosine, and degranulation was assessed by measuring the release of hexosaminidase, an enzyme present in mast-cell granules. No increase in hexosaminidase was seen upon exposure of up to 100 μM adenosine or inosine, indicating that neither nucleoside alone provoked degranulation of BMMCs. To compare the ability of these 2 agents to potentiate antigen-mediated BMMC degranulation, mast cells were loaded with IgE and subsequently exposed to antigen and various concentrations of adenosine and inosine. IgE-dependent degranulation without adenosine or inosine was similar between genotypes (27 ± 3% vs. 27 ± 5%). As shown in Figure 1, adenosine and inosine both enhanced antigen-mediated degranulation of wild-type mast cells. However, a maximum increase in potentiation of this response by adenosine was seen at concentrations approximately 10-fold lower than that seen with inosine. At a dose of 100 μM, inosine increased hexosaminidase release by 34 ± 4% (P = 0.008), whereas adenosine enhanced release by 67.3 ± 11% (P = 0.028). To determine whether inosine mediates its action through one of the adenosine receptors, we examined the ability of inosine to potentiate degranulation of A3AR–/– mast cells. Similar to adenosine, inosine failed to potentiate antigen-induced degranulation in BMMCs from A3AR–/– mice (Figure 1). Antigen-induced degranulation in A3AR–/– cells could, however, be potentiated by other secretagogues such as PGE2 to similar extents as that seen in BMMCs from wild-type animals (26 ± 6% release with antigen; 53 ± 2% release with antigen and PGE2). These results indicate that the ability of both adenosine and inosine to enhance antigen-induced degranulation of murine BMMCs occurs exclusively through activation of the A3 adenosine receptor.
Figure 1.
Potentiation of antigen-induced degranulation of BMMCs from A3AR–/– and A3AR+/+ mice by adenosine and inosine. IgE-loaded mast cells were incubated with antigen (DNP-albumin) in the presence or absence of increasing concentrations of adenosine or inosine. The amount of degranulation was determined by measuring the percent hexosaminidase released from cells. Data represent the mean percent increase in hexosaminidase release with adenosine and inosine, ± SEM from 3 experiments, each performed in duplicate. Ado = adenosine, Ino = inosine. *P < 0.03.
Changes in vascular permeability in response to adenosine and inosine in A3AR+/+ and A3AR–/– mice.
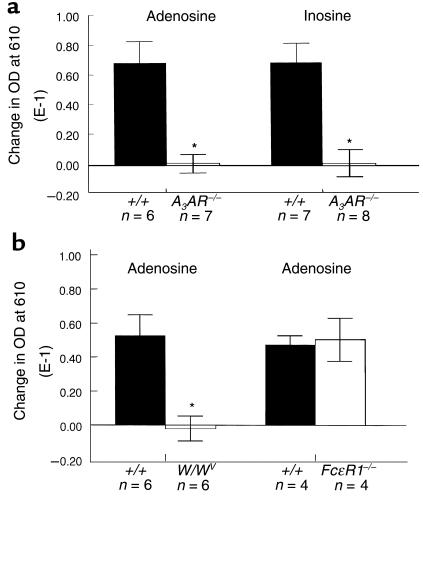
We examined the effects of adenosine and inosine on microvascular permeability and plasma protein extravasation in the skin by intradermally injecting these nucleosides into the pinna of the mouse ear. Evans blue dye binds to serum proteins and remains confined to the intravascular space unless vascular permeability is altered. Quantitation of the dye present in the ear can therefore be used to quantify the levels of plasma protein extravasation into tissue. As shown in Figure 2a, intradermal adenosine caused significant extravasation of plasma proteins into the ears of A3AR+/+ mice. Inosine was also effective at producing protein extravasation. However, in contrast to our in vitro data, inosine was at least as effective as adenosine in producing this response. To determine whether these effects were mediated through a specific receptor, we measured the ability of these agents to cause plasma protein extravasation in A3AR–/– mice. Neither adenosine nor inosine evoked dye extravasation in any of the A3AR–/– animals tested, even at concentrations as high as 1 mM (Figure 2a).
Figure 2.
Plasma protein extravasation after intradermal adenosine and inosine in wild-type (+/+), A3AR-deficient (A3AR–/–), mast cell–deficient (W/WV), and FcεR1 β-chain–deficient mice (FcεR1–/–). Mice were injected intravenously with 100 μL of 1% Evans blue in PBS. One hour later, 20 μL of adenosine 10–3 M or inosine 10–4 M (a), or adenosine 10–4 M (b), was injected intradermally into the right ear of each animal. Control ears (left ears) were injected with 20 μL of PBS. One hour after intradermal injections, 7-mm ear biopsies were obtained and placed in formamide at 57°C for 48 hours to extract dye. Plasma protein extravasation was quantitated by measuring OD at 610 nm. Data represent the mean difference in OD between PBS-treated ears and nucleoside-treated ears, ± SEM. n = number of animals in each group. *P < 0.005.
Adenosine-induced edema in mast cell–deficient mice.
To determine whether the plasma protein extravasation in response to adenosine was due to direct effects on the endothelium or secondary to mast cell–mediator release, adenosine was delivered intradermally into the pinna of the ears of mast cell–deficient mice and their wild-type littermate controls. Although edema formation was seen in the wild-type animals, it was not observed in the mast cell–deficient mice (Figure 2b). These findings suggest that A3AR activation of mast cells is the physiological mechanism behind adenosine-induced changes in vascular permeability.
Mast-cell histology.
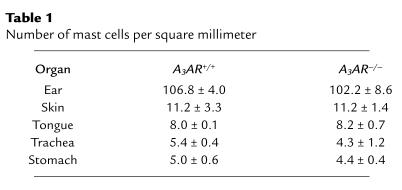
To verify that the absence of the A3 receptor did not affect mast-cell development, mast cells were examined from A3AR–/– animals and controls. Morphologically, BMMCs isolated from A3AR–/– mice were indistinguishable from those obtained from A3AR+/+ animals. Tissue mast cells stained with toluidine blue also appeared morphologically similar between genotypes. Table 1 summarizes the number of resident mast cells present in various tissues from A3AR–/– and A3AR+/+ mice. No differences in cell numbers were found between genotypes in any of the tissues examined. Quantification of mast cells in the stomach was based on counting mast cells in the submucosa and muscularis propria and did not include mucosal mast cells.
Table 1.
Number of mast cells per square millimeter
Passive cutaneous and systemic anaphylaxis.
Previous work has demonstrated that increases in plasma protein extravasation seen during a passive anaphylaxis response in the mouse results from activation of mast cells by antigen and IgE via the FcεR1 receptor (37). Therefore, to determine whether tissue mast cells of the A3AR–/– animals could respond to other stimuli and whether the magnitude of this response was altered as a result of loss of the A3AR, we examined plasma protein extravasation after the induction of passive cutaneous and systemic anaphylaxis. The cutaneous anaphylactic response was elicited by loading mast cells intradermally with murine monoclonal IgE specific for DNP-albumin. Twenty-four hours later, the antigen was administered intravenously. Similar to A3AR+/+ mice, intravenously administered antigen led to localized plasma protein extravasation at the site of intradermal IgE administration in A3AR–/– animals (data not shown). While many of the A3AR–/– mice displayed a slightly blunted response, the observed differences between genotypes were not statistically significant (P = 0.21).
A systemic anaphylactic response was elicited in wild-type and A3AR–/– mice by intravenously loading tissue mast cells with monoclonal IgE specific for DNP-albumin. Twenty-four hours later, the DNP antigen was administered intravenously. In wild-type mice, passive systemic anaphylaxis is characterized by profound shock with hypotension, hypothermia, and increased vascular permeability leading to generalized fluid extravasation. We quantified the anaphylactic response in 2 ways. First, temperature change was monitored rectally at various times after administration of antigen; and second, changes in vascular permeability were determined by measuring Evans blue dye extravasation into ear tissue. Antigen administration resulted in progressive hypothermia in both wild-type and A3AR–/– animals over the time interval measured (data not shown). The body temperature dropped significantly in the animals that received IgE and antigen compared with control animals treated with either IgE alone or antigen alone (P < 0.05). No significant differences were detected between genotypes at any time point measured. Vascular permeability also increased in both wild-type and A3AR–/– animals after antigen challenge (data not shown). Again, no significant differences were observed between genotypes (P = 0.31).
Adenosine-mediated edema formation in FcεR1-deficient mice.
It is possible that the ability of adenosine to initiate degranulation of tissue mast cells, but not BMMCs, is dependent on occupancy, in vivo, of the high-affinity IgE receptor, FcεR1, with subthreshold levels of IgE and antigen. To test this hypothesis, the response of mice lacking the β chain of the FcεR1 receptor to adenosine was examined. It has been shown previously that expression of functional FcεR1 in murine mast cells is dependent on the expression of the β chain (33). As shown in Figure 2b, a similar degree of plasma protein extravasation was seen in wild-type and FcεR1-deficient animals in response to adenosine (P = 0.84). These results suggest that mast-cell activation by adenosine in vivo occurs independent of any FcεR1 receptor–mediated signal transduction.
Discussion
Adenosine and inosine can have dramatic effects on vascular permeability, with edema formation occurring rapidly upon cutaneous exposure to these nucleosides. We show here that these physiological changes are mediated through the binding of adenosine and inosine to the A3 adenosine receptor. Lack of plasma protein extravasation after the intradermal administration of adenosine in mast cell–deficient mice fails to support a model in which adenosine induces changes in vascular permeability by acting directly on adenosine receptors expressed by endothelial cells. Rather, these findings are consistent with the hypothesis that adenosine and inosine mediate changes in vascular permeability indirectly by activation of tissue mast cells, which in turn release secondary mediators that act on endothelial cells. The experiments described here also show that adenosine and inosine alone can provide, through activation of the A3 receptor, sufficient signal for degranulation of intradermal mast cells in vivo. These findings contrast with in vitro studies using BMMCs, in which exposure to adenosine or inosine alone does not result in mast-cell degranulation.
Previous work has shown that adenosine can influence vascular permeability in multiple tissues, and our results showing edema formation in the mouse after the intradermal injection of adenosine are consistent with these studies (9–11, 17). It has previously been difficult to definitively assign a specific adenosine receptor to a given physiological response owing to the incomplete selectivity of adenosine receptor agonists and antagonists. Our studies show that plasma protein extravasation in response to adenosine is mediated entirely through activation of the A3 receptor, as adenosine cannot provoke this physiological response in mice lacking this receptor. These findings are consistent with reports showing that pharmacologic reagents that preferentially bind to the A3 receptor are effective at inducing edema formation (30, 38).
To determine whether adenosine-mediated increases in cutaneous vasopermeability are due to direct effects on the vasculature or occur indirectly through mast cell–mediator release, we used mast cell–deficient mice and their congenic littermate controls. Control mice showed the expected increase in vascular permeability in response to adenosine, whereas mast cell–deficient mice showed no response. These results suggest that mast cells are required for the induction of plasma protein extravasation by adenosine and that adenosine does not act directly on the vasculature to produce these physiological changes. Earlier in vitro studies using microvascular endothelial cells have shown adenosine to have direct effects on permeability (17). It is possible that these discrepancies reflect a difference in the site and cell type exposed to adenosine. In the experiments described here, adenosine was delivered to the interstitial space. It is possible that high levels of adenosine delivered to the luminal surface of the endothelial cell may directly alter postcapillary permeability. In vivo studies have also suggested that adenosine may have direct effects on the vasculature; however, the results of most of these studies are confounded by the presence of perivascular mast cells (9, 10). Recently, studies of vascular permeability in the skin of conscious rats after exposure to adenosine analogues have suggested an indirect action through mediator release from mast cells, as plasma protein extravasation after the intradermal administration of an adenosine analogue was nearly eliminated when animals were pretreated with either a histamine and serotonin antagonist or compound 48/80 that depletes mast-cell mediators (30). Our results are in agreement with these observations and provide evidence that adenosine’s effects on cutaneous vascular permeability are mast-cell mediated. Formal proof that these effects are indeed mast-cell mediated awaits successful and unsuccessful restoration of the response to adenosine in mast cell–deficient mice reconstituted with A3AR+/+ and A3AR–/– BMMCs, respectively.
Given our findings that the edema response to adenosine is mast cell–dependent, and that this response is absent in the A3AR-deficient mice, 2 possible explanations need to be considered. One possibility is that the failure to see a response in the A3AR-deficient mice is due to the nonresponsiveness of mast cell to adenosine because of the absence of A3AR receptor expression. An alternative hypothesis is that the A3AR plays an important role in the development, migration, or overall survival of mast cells and that loss of this receptor renders mice mast-cell deficient. Two lines of experimental data support the former hypothesis. First, mast cells were found in approximately normal numbers in all tissues examined and could not be distinguished from wild-type controls based on morphological criteria. Perhaps more convincing data supporting the normal development of mast cells in A3AR–/– mice are the demonstration that loss of this receptor has only a minimal impact on systemic and cutaneous IgE-mediated anaphylaxis in these animals. A small decrease in response to cutaneous anaphylaxis was observed, but this difference did not reach statistical significance. It is possible that this trend may be related to the in vitro demonstration that adenosine, acting through the A3 receptor, potentiates IgE-mediated mast-cell degranulation. The role of adenosine in this response may be difficult to measure when mast cells are maximally stimulated in passive anaphylaxis, and further studies with suboptimal doses of antigen or antibody may reveal a greater role for adenosine in these mast cell–mediated inflammatory responses. Moreover, further insight into the contribution of adenosine to disease processes such as asthma, in which mast-cell activation is believed to play a role, may be gained by examining A3AR-deficient mice in established models of this disease.
Previous investigations using murine BMMCs have shown adenosine’s potentiation of degranulation in these cells to be partially pertussis-toxin sensitive (39). These same investigators also showed that adenosine-mediated rises in inositol trisphosphate (IP3) and intracellular calcium were pertussis-toxin insensitive (39). Because A3-mediated signal transduction has been shown to be sensitive to pertussis toxin (40, 41), it has been speculated that another adenosine receptor, such as the A2B receptor, plays a role in adenosine’s actions on BMMC degranulation. Our results showing the potentiation of antigen-stimulated BMMC degranulation by adenosine through the A3 receptor do not support these observations. A3 receptors have been shown to interact with multiple G proteins including Gi and Gq, and it has been suggested that A3AR stimulation of phospholipase C may have a pertussin toxin–insensitive component in some systems (42).
While adenosine can potentiate antigen-induced mast-cell degranulation in vitro, it cannot initiate degranulation independent of an additional stimulus. In contrast, adenosine alone appears to be sufficient to activate mast cells in vivo. Studies carried out in several species support this observation (23, 24, 29–31). Several different hypotheses can explain the profound effect of adenosine on mast-cell function in vivo. First, BMMCs in tissue culture may be immature and lack the necessary signaling mechanisms required to initiate degranulation in response to adenosine. Second, the ability of adenosine to initiate degranulation may vary between different mast-cell types. Tissue mast cells can be classified into either connective tissue or mucosal mast cells based on certain morphological and histochemical characteristics, and it has been suggested that BMMCs more closely resemble the latter (21). The in vivo studies described here examine the cutaneous response to adenosine and, therefore, reflect activation of A3AR on connective tissue mast cells. Finally, in vivo, low levels of antigen may be bound to IgE receptors occupied by circulating IgE, providing the additional signaling necessary for activation of mast cells by adenosine. The availability of mouse lines lacking a functional FcεR1 receptor has enabled us to test this hypothesis directly. Intradermal administration of adenosine to these mice results in a similar degree of plasma protein extravasation as observed in wild-type controls, establishing that adenosine-induced mast-cell degranulation in vivo occurs independently of the presence of signal transduction by the high affinity IgE receptor.
Although it is well accepted that adenosine is a paracrine and autocrine mediator in a broad spectrum of physiological responses, less information is available concerning the functions of inosine, a primary metabolite of adenosine. Two different routes for metabolism of adenosine have been described. First, adenosine can be used as a substrate for nucleotide synthesis producing ADP and ATP, which themselves have potent receptor-mediated biologic reactions (43). Alternatively, adenosine can be converted by adenosine deaminase to inosine. The activity of these 2 pathways is believed to be regulated, at least in part, by the substrate availability (44). When levels of adenosine are low, most adenosine is converted to AMP by adenosine kinase. However, when adenosine levels increase as a result of trauma, shock, exercise, hypoxia, or endotoxin, adenosine deamination predominates, leading to significant increases in inosine production and resultant interstitial levels of inosine that can rise to greater than 1 mM (44, 45). The biologic significance of these high tissue levels of inosine has not been established, nor is it known whether this metabolite mediates it actions solely by binding to adenosine receptors or whether it mediates its effects through yet undescribed inosine receptors. In vitro studies have shown that inosine can potentiate antigen-induced degranulation of both rat serosal mast cells and rat RBL-2H3 mastlike cells (5, 46). We show here that inosine can also potentiate the degranulation of BMMCs. Furthermore, this response is not seen in A3AR-deficient mast cells, demonstrating that inosine’s actions on BMMCs are mediated through the A3 receptor. These findings are consistent with recent pharmacologic studies that showed inosine to preferentially bind to recombinant rat A3 receptors (5). These studies also suggest that adenosine is more effective than inosine in mediating this response and are consistent with earlier studies with rat mast cells that showed inosine to be some 10 times less potent that adenosine at enhancing antigen-induced degranulation (46).
Intradermal injection of inosine was at least as effective as adenosine at eliciting an edema response. This finding is consistent with pharmacologic studies that suggested that inosine can activate the A3 receptor (5). Thus, the studies reported here support a physiological role for inosine in the acute inflammatory response and show that these effects of inosine are mediated solely through an adenosine receptor, specifically the A3 receptor. This does not rule out the possibility that other biologic responses of inosine may be mediated by other adenosine receptors or yet uncharacterized receptors.
In summary, we have shown that both adenosine and its principal metabolite inosine promote plasma protein extravasation through activation of A3 adenosine receptors. Lack of any changes in vascular permeability in mast cell–deficient mice after exposure to adenosine suggests that adenosine acts indirectly through A3 receptors on mast cells to produce these physiological changes. These actions of adenosine in vivo occur independently of the presence of the expression of the high-affinity IgE receptor, suggesting a more profound role for adenosine as a modifier of the inflammatory response.
Acknowledgments
The authors thank S. Galli and C. Williams for helpful advice, and M. Solle for critical review of the manuscript. This work was supported by an American Lung Association Research Training Fellowship Award (S.L. Tilley) and a grant from the National Institutes of Health (HL58554 to B.H. Koller).
References
- 1.Mentzer RM, Rubio R, Berne RM. Release of adenosine by hypoxic canine lung tissue and its possible role in pulmonary circulation. Am J Physiol. 1975;229:1625–1631. doi: 10.1152/ajplegacy.1975.229.6.1625. [DOI] [PubMed] [Google Scholar]
- 2.Marquardt DL, Gruber HE, Wasserman SI. Adenosine release from stimulated mast cells. Proc Natl Acad Sci USA. 1984;81:6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrader J, Gerlach E. Compartmentation of cardiac adenine nucleotides and formation of adenosine. Pflugers Arch. 1976;367:129–135. doi: 10.1007/BF00585148. [DOI] [PubMed] [Google Scholar]
- 4.Rubio R, Berne RM, Bockman EL, Curnish RR. Relationship between adenosine concentration and oxygen supply in rat brain. Am J Physiol. 1975;228:1896–1902. doi: 10.1152/ajplegacy.1975.228.6.1896. [DOI] [PubMed] [Google Scholar]
- 5.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 7.Mills GC, Schmalstieg FC, Trimmer KB, Goldman AS, Goldblum RM. Purine metabolism in adenosine deaminase deficiency. Proc Natl Acad Sci USA. 1976;73:2867–2871. doi: 10.1073/pnas.73.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collis MG. The vasodilator role of adenosine. Pharmacol Ther. 1989;41:143–162. doi: 10.1016/0163-7258(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 9.Gawlowski DM, Duran WN. Dose-related effects of adenosine and bradykinin on microvascular permselectivity to macromolecules in the hamster cheek pouch. Circ Res. 1986;58:348–355. doi: 10.1161/01.res.58.3.348. [DOI] [PubMed] [Google Scholar]
- 10.Overholser KA, Harris TR. Effect of exogenous adenosine on resistance, capillary permeability-surface area and flow in ischemic canine myocardium. J Pharmacol Exp Ther. 1984;229:148–152. [PubMed] [Google Scholar]
- 11.Sollevi A, Fredholm B. Role of adenosine in adipose tissue circulation. Acta Physiol Scand. 1981;112:293–298. doi: 10.1111/j.1748-1716.1981.tb06819.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiener HL, Thalody GP. Kinetic characterization of adenosine A2 receptor-mediated relaxation in isolated rabbit aorta. Eur J Pharmacol. 1993;238:65–74. doi: 10.1016/0014-2999(93)90506-d. [DOI] [PubMed] [Google Scholar]
- 13.Abiru T, Endo K, Machida H. Differential vasodilatory action of 2-octynyladenosine (YT-146), an adenosine A2 receptor agonist, in the isolated rat femoral artery and vein. Eur J Pharmacol. 1995;281:9–15. doi: 10.1016/0014-2999(95)00219-b. [DOI] [PubMed] [Google Scholar]
- 14.Haynes J, Jr, Obiako B, Thompson WJ, Downey J. Adenosine-induced vasodilation: receptor characterization in pulmonary circulation. Am J Physiol. 1995;268:H1862–H1868. doi: 10.1152/ajpheart.1995.268.5.H1862. [DOI] [PubMed] [Google Scholar]
- 15.Elliott J, Brady FE. Characterization of vasodilatory adenosine receptors in equine digital veins. J Vet Pharmacol Ther. 1998;21:74–81. doi: 10.1046/j.1365-2885.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 16.Haselton FR, Alexander JS, Mueller SN. Adenosine decreases permeability of in vitro endothelial monolayers. J Appl Physiol. 1993;74:1581–1590. doi: 10.1152/jappl.1993.74.4.1581. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, Kuhne W, Schwartz P, Piper HM. A2-adenosine receptor stimulation increases macromolecule permeability of coronary endothelial cells. Am J Physiol. 1992;262:H1174–H1181. doi: 10.1152/ajpheart.1992.262.4.H1174. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- 19.Rakusan K, Sarkar K, Turek Z, Wicker P. Mast cells in the rat heart during normal growth and in cardiac hypertrophy. Circ Res. 1990;66:511–516. doi: 10.1161/01.res.66.2.511. [DOI] [PubMed] [Google Scholar]
- 20.Rhodin JAG, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989;21:1–34. [PubMed] [Google Scholar]
- 21.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 22.Marquardt DL, Parker CW, Sullivan TJ. Potentiation of mast cell mediator release by adenosine. J Immunol. 1978;120:871–878. [PubMed] [Google Scholar]
- 23.Doyle MP, Linden J, Duling BR. Nucleoside-induced arteriolar constriction: a mast cell-dependent response. Am J Physiol. 1994;266:H2042–H2050. doi: 10.1152/ajpheart.1994.266.5.H2042. [DOI] [PubMed] [Google Scholar]
- 24.Hannon JP, Pfannkuche HJ, Fozard JR. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br J Pharmacol. 1995;115:945–952. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquardt DL, Walker LL, Heinemann SF. Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol. 1994;150:4508–4515. [PubMed] [Google Scholar]
- 26.Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. J Clin Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2b receptor. Mol Pharmacol. 1997;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 28.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 29.Fozard JR, Pfannkuche HJ, Schuurman HJ. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur J Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- 30.Reeves JJ, Jones CA, Sheehan MJ, Vardey CJ, Whelan CJ. Adenosine A3 receptors promote degranulation of rat mast cells both in vitro and in vivo. Inflamm Res. 1997;46:180–184. doi: 10.1007/s000110050169. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd RK, Linden J, Duling BR. Adenosine-induced mast vasoconstriction in vivo. Circ Res. 1996;78:627–634. doi: 10.1161/01.res.78.4.627. [DOI] [PubMed] [Google Scholar]
- 32.Salvatore, C.A., et al. Disruption of the A3 adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J. Biol. Chem. In press. [DOI] [PubMed]
- 33.Dombrowicz D, et al. Allergy-associated FcRβ is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–529. doi: 10.1016/s1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 34.Rottem M, Barbieri S, Kinet J-P, Metcalfe DD. Kinetics of the appearance of FcεRI-bearing cells in interleukin-3-dependent mouse bone marrow cultures: correlation with histamine content and mast cell maturation. Blood. 1992;79:972–980. [PubMed] [Google Scholar]
- 35.Jancso-Gabor A, Szolcsanyi J, Jancso N. A simple method for measuring the amount of azovan blue exuded into the skin in response to an inflammatory stimulus. J Pharm Pharmacol. 1967;19:486–487. doi: 10.1111/j.2042-7158.1967.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 36.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcRγ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 37.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor α chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 38.Yamawaki I, Tamaoki J, Takeda Y, Nagai A. Effect of adenosine and its analogues on microvascular leakage in the rat trachea. Nihon Kokyuki Gakkai Zasshi. 1998;36:231–235. [PubMed] [Google Scholar]
- 39.Marquardt DL, Walker LL. Alteration of mast cell responsiveness to adenosine by pertussis toxin. Biochem Pharmacol. 1988;37:4019–4025. doi: 10.1016/0006-2952(88)90088-3. [DOI] [PubMed] [Google Scholar]
- 40.Ali H, Cunha-Melo JR, Saul WF, Beaven MA. Activation of phospholipase c via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. J Biol Chem. 1990;265:745–753. [PubMed] [Google Scholar]
- 41.Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A2b adenosine receptors: radioligand binding, Western blotting, and coupling to Gq in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol. 1999;56:705–713. [PubMed] [Google Scholar]
- 42.Palmer TM, Gettys TW, Stiles GL. Differential interaction with and regulation of multiple G-proteins by the rat A3 adenosine receptor. J Biol Chem. 1995;270:16895–16902. doi: 10.1074/jbc.270.28.16895. [DOI] [PubMed] [Google Scholar]
- 43.Silverman, E.S., Gerritsen, M.E., and Collins, T. 1997. Metabolic functions of the pulmonary endothelium. In The lung: scientific foundations. R.G. Crystal, J.B. West, E.R. Weibel, and P.J. Barnes, editors. Lippincott-Raven. Philadelphia, PA. 629–651.
- 44.Hellewell PG, Pearson JD. Metabolism of circulating adenosine by the porcine isolated perfused lung. Circ Res. 1983;53:1–7. doi: 10.1161/01.res.53.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Jones CE, Thomas JX, Devous MD, Norris CP, Smith EE. Positive inotropic response to inosine in the in situ canine heart. Am J Physiol. 1977;233:H438–H443. doi: 10.1152/ajpheart.1977.233.4.H438. [DOI] [PubMed] [Google Scholar]
- 46.Church MK, Hughes PJ, Vardey CJ. Studies on the receptor mediating cyclic AMP-independent enhancement by adenosine of IgE-dependent mediator release from rat mast cells. Br J Pharmacol. 1986;87:233–242. doi: 10.1111/j.1476-5381.1986.tb10176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]