Abstract
In the past, we have used the kinins of the cockroach Leucophaea (the leucokinins) to evaluate the mechanism of diuretic action of kinin peptides in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Now using the kinins of Aedes (the aedeskinins), we have found that in isolated Aedes Malpighian tubules all three aedeskinins (1 μM) significantly 1) increased the rate of fluid secretion (V̇S), 2) hyperpolarized the basolateral membrane voltage (Vbl), and 3) decreased the input resistance (Rin) of principal cells, consistent with the known increase in the Cl− conductance of the paracellular pathway in Aedes Malpighian tubules. Aedeskinin-III, studied in further detail, significantly increased V̇S with an EC50 of 1.5 × 10−8 M. In parallel, the Na+ concentration in secreted fluid significantly decreased, and the K+ concentration significantly increased. The concentration of Cl− remained unchanged. While the three aedeskinins triggered effects on Vbl, Rin, and V̇S, synthetic kinin analogs, which contain modifications of the COOH-terminal amide pentapeptide core sequence critical for biological activity, displayed variable effects. For example, kinin analog 1578 significantly stimulated V̇S but had no effect on Vbl and Rin, whereas kinin analog 1708 had no effect on V̇S but significantly affected Vbl and Rin. These observations suggest separate signaling pathways activated by kinins. One triggers the electrophysiological response, and the other triggers fluid secretion. It remains to be determined whether the two signaling pathways emanate from a single kinin receptor via agonist-directed signaling or from a differentially glycosylated receptor. Occasionally, Malpighian tubules did not exhibit a detectable response to natural and synthetic kinins. Hypothetically, the expression of the kinin receptor may depend on developmental, nutritional, and/or reproductive signals.
Keywords: Aedes aegypti, principal cells, aedeskinin, G protein-coupled kinin receptor, Ramsay fluid secretion assay, membrane voltage, input resistance, principal cells, α- and β-analogs of insect kinins, functionally selective agonists
epithelial secretion and absorption are the two mechanisms for regulating the volume and composition of the hemolymph in insects (4, 16). In the case of Malpighian tubules, epithelial secretion delivers a fluid to the lumen in upstream (distal) segments of the Malpighian tubule, creating a urinary space into which excess solutes, metabolic wastes, and toxins can subsequently be dumped for excretion. In downstream (proximal) segments of the tubule, in the hindgut and the rectum, reabsorptive mechanisms recover the electrolytes and water that initially served to produce the urinary space, while concentrating unwanted solutes for excretion.
Transport mechanisms of epithelial secretion and absorption are under hormonal control as Malpighian tubules execute the tasks of extracellular fluid homeostasis (4, 20). Diuretic hormones stimulate tubular secretion and urine flow, whereas antidiuretic hormones stimulate tubular reabsorption and decrease the excretion of urine.
Four families of diuretic peptides have been identified in insects: the CRF-like peptides, the calcitonin-like peptides, the CAPA peptides, and the kinin peptides (20, 22, 26). Holman et al. (30) isolated the first kinin peptides from head extracts of the cockroach Leucophaea maderae by using the motility of the cockroach hindgut as a bioassay. Thus, the isolated peptides were considered smooth muscle stimulants, i.e., myokinins, and called leucokinins (30). Agonists of smooth muscle are often secretagogues, which led our laboratory to examine the effect of leucokinin on fluid secretion in Malpighian tubules of the yellow fever mosquito, Aedes aegypti (27). We found that leucokinin-VIII from the cockroach stimulated fluid secretion in Aedes Malpighian tubules, suggesting the wide distribution of kinins in insects. Indeed, the stimulation of fluid secretion by leucokinin and other kinins has now been observed in Malpighian tubules of the house cricket (23), locust (21, 55), corn earworm (10), fruit fly (36, 54), and housefly (32). The insect subclass Pterygota, the winged insects, includes 97% of all insects, and the kinins have been found in every order of this subclass that has been examined for kinin peptides.
In the past, we have used the kinins of the cockroach (the leucokinins) to elucidate the mechanism of kinin action in Malpighian tubules of the yellow fever mosquito (5, 27, 39, 65–67). As early as 1994, the laboratory of Veenstra (57) has identified three kinins of the mosquito Aedes aegypti, the aedeskinins. In his laboratory, all three aedeskinins depolarized the transepithelial voltage in Aedes Malpighian tubules like leucokinin (58). However, only aedeskinin-I and -III stimulated fluid secretion in his laboratory, whereas aedeskinin-II had no detectable effect on fluid secretion (58).
In contrast to Veenstra's earlier work, we report here that all three aedeskinins (including aedeskinin-II) significantly increase the rate of transepithelial fluid secretion. Moreover, aedeskinin-III increases the transepithelial secretion of NaCl and KCl, like leucokinin-VIII (5, 39). Furthermore, all three aedeskinins hyperpolarize the basolateral membrane voltage (Vbl) of principal cells, while decreasing the input resistance (Rin) of principal cells, like leucokinin-VIII (5, 39). Thus, aedeskinins and leucokinins share similar, if not the same, mechanisms of action.
Our study of five additional synthetic kinin analogs that posses single or multiple substitutions of β- and/or α,α-disubstituted amino acids, both within and just outside the COOH-terminal pentapeptide core sequence critical for biological activity, reveal separate and independent signaling pathways that mediate the effects on fluid secretion (V̇S) and electrophysiology (Vbl, Rin). Some kinin analogs increase V̇S without affecting Vbl and Rin. Other analogs show the opposite: no effect on V̇S but clear effects on Vbl and Rin. Since the Malpighian tubule of Aedes aegypti is thought to express only one kinin receptor (42), different physiological effects that originate from a single receptor may reflect mechanisms of ligand-dependent signaling and/or different glycosylation states of the receptor. Thus, the G protein-coupled kinin receptor is functionally more diverse than previously assumed.
Finally, we find that some tubules do not respond to aedeskinins at all (natural or the analogs). Preliminary observations point to differences in individual mosquitoes and to differences in populations of mosquitoes.
MATERIALS AND METHODS
Mosquitoes and Malpighian Tubules
The method for rearing mosquitoes (Aedes aegypti) is described in Ref. 39, with the exception that we now feed larval mosquitoes Tetramin flakes (tropical fish food) finely ground with mortar and pestle. On the day of the experiment a female mosquito, 4–7 days posteclosion, was cold anesthetized and decapitated. Malpighian tubules were removed from the mosquito under Ringer solution by pulling gently on the rectum which freed the gut and Malpighian tubules from the abdominal cavity. A Malpighian tubule was then removed with forceps from its attachment to the gut. The proximal end of the tubule (crushed by the forceps) was subsequently teased open with sharp jeweler's broaches.
Ringer Solution and Diuretic Peptides
Ringer solution contained the following (in mM): 150 NaCl, 3.4 KCl, 25 HEPES, 1.8 NaHCO3, 1.0 MgSO4 (or MgCl2), 1.7 CaCl2, and 5.0 glucose. The pH was adjusted to 7.1 using 1 M NaOH. Table 1 lists the peptides used in the present study. Leucokinin-VIII, aedeskinins, and kinin analogs were synthesized in the laboratory of Nachman (50, 69). The identity of each peptide was confirmed by MALDI-TOF mass spectrometry, and the amount was quantified by amino acid analysis. The three aedeskinins are splice variants of the same gene (58).
Table 1.
Kinins and synthetic analogs (28)
| Peptide | Primary Structure | Reference |
|---|---|---|
| Conserved kinin sequence | Phe-X2-X3-Trp-Gly-NH2 | (56) |
| Leucokinin-VIII | Gly-Ala-Asp-Phe-Tyr-Ser-Trp-Gly-NH2 | (30) |
| Aedeskinin I | Asn-Ser-Lys-Tyr-Val-Ser-Lys-Gln-Lys-Phe-Tyr-Ser-Trp-Gly-NH2 | (57) |
| Aedeskinin II | Asn-Pro-Phe-His-Ala-Trp-Gly-NH2 | (57) |
| Aedeskinin III | Asn-Asn-Pro-Asn-Val-Phe-Tyr-Pro-Trp-Gly-NH2 | (57) |
| Analog 1460 II | Ac-Arg-Phe-Phe-[β3Pro]-Trp-Gly-NH2 | (52) |
| Analog 1547 | Phe-Phe-Phe-Ser-Trp-Gly-NH2 | (51) |
| Analog 1577 | Ac-Arg-[β3Phe]-Phe-Phe-[β3Pro]-Trp-Gly-NH2 | (52) |
| Analog 1578 | Ac-Arg-Phe-[β3Phe-β3Pro]-Trp-Gly-NH2 | (52) |
| Analog 1708, K-Aib-4 | Ac-Arg-[β3Phe]-Phe-Phe-[Aib]-Trp-Gly-NH2 | (50) |
In one set of experiments, we dissolved the peptides directly in Ringer solution and stored them at −20°C until use. In another set of experiments, the peptides were dissolved in 80% acetonitrile and 0.01% trifluoroacetic acid and stored at 4°C. On the day of the experiment, an aliquot was lyophilized and then dissolved in Ringer solution.
Ramsay Method of Fluid Secretion
The V̇S was measured at room temperature in isolated Malpighian tubules as developed by Ramsay (45) and adopted by us (29). In brief, the distal (blind) end of the tubule was bathed in a Ringer droplet of 50 μl under light mineral oil. The open end of the tubule was pulled into the oil with a glass hook so that fluid secreted by the tubule exited into the oil (Fig. 1A). The glass hook was formed on a microforge (Stoelting, Wood Dale, IL) using soft glass (R-6; Drummond Scientific, Broomall, PA). After the glass hook was acid-washed with a mixture of K2CrO4 and H2SO4, the hook was exposed to the vapor of 20 μl dimethyl-dichlorosilan for 90 s (Fluka, Buchs, Switzerland) and then baked overnight at 110°C. The silanization prevented secreted fluid from spreading out along the hook.
Fig. 1.
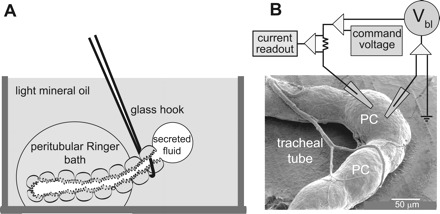
Experimental methods. Ramsay fluid secretion assay (A), and 2-electrode voltage-clamp method (B) for measurements of the basolateral membrane voltage (Vbl) and the input resistance (Rin) of a principal cell (PC) in an isolated Malpighian tubule [modified from Wu and Beyenbach (64)]. The Ramsay assay requires a minimum of 5 to 10 min before effects of kinins added to the peritubular bath are observed. The 2-electrode voltage-clamp method yields immediate data of voltage and resistance. A scanning electron micrograph of a Malpighian tubule of a female mosquito (Aedes aegypti) is shown in B.
Each tubule was used as its own control. The initial 30 min marked the control fluid secretion period. Thereafter, the control droplet (nanoliter volumes, 10−9 liter) was removed and prepared for the analysis of its ionic composition with the electron probe. Subsequently, 5 μl of Ringer solution was removed from the peritubular bath and replaced with 5 μl of Ringer solution containing one of the peptides of Table 1.
The 30-min experimental period began as soon as the peptide had been added to the peritubular bath. Rates of fluid secretion were determined by plotting cumulative volume (nl) secreted by the tubule as a function of time (30 min), each for the control and the experimental period. The plots were usually linear, yielding the rate of fluid secretion (V̇S) as the slope of the line (Microsoft Excel 2007; Microsoft, Redmond, WA).
Electron Probe Analysis of Fluid Electrolyte Composition
The ionic composition of fluid secreted by Malpighian tubules was analyzed with the electron probe as described previously (7, 62), except that we used the JEOL JXA 8900 EPMA Microprobe (Tokyo, Japan). Control and experimental fluid droplets collected in the Ramsay assay were prepared for electron probe analysis within 1 h of collection. A picoliter pipette was used to deposit a constant volume of unknowns (secreted fluid droplets) and standard solutions of known Na+, K+, and Cl− concentrations onto a polished beryllium block under light mineral oil.
Volumetric picoliter (10−12 liter) pipettes were produced using soft glass (R6; Drummond Scientific) and a microforge (Stoelting) to form a volumetric chamber of ∼60 pl from the pipette opening to an internal constriction. The exact pipette volume is not important as long as the same pipette is used for both standards and unknowns. Prior to use, the volumetric part of the pipette was vapor-silanized as described above. The silanization prevents fluid from sticking to the walls of the volumetric chamber.
After standards and unknowns were deposited on the beryllium block under oil (using the same picoliter pipette), the oil was removed in three washes with chloroform. The chloroform wash also removes water from the droplets, leaving salt crystals behind. The block is then stored in a desiccator under vacuum until analysis with the electron probe.
Wavelength dispersive spectroscopy is based on X-ray emissions specific to each element in the sample. The JEOL JXA 8900 EPMA Microprobe allows the measurement of as many as three elements in a single sample: Na+, K+, and Cl− in the present study. Seven standard solutions containing a range of Na+, K+, and Cl− concentrations were used to produce standard curves. X-ray counts from the unknowns (secreted fluid droplets) were read against the appropriate standard curve to yield the Na+, K+, and Cl− concentrations in secreted fluid. Transepithelial secretion rates of Na+, K+, and Cl− were calculated as the product of the ion concentration and the fluid secretion rate for both control and stimulated conditions.
Electrophysiological Effects of Aedeskinin and Analogs
The Vbl and the Rin of a principal cell of an isolated Malpighian tubule were measured as shown in Fig. 1B and as described previously (8, 34). In brief, Malpighian tubules were isolated and deposited on a sheet of Parafilm (American National Can, Menasha, WI) that had been stretched over the bottom of the Ringer perfusion bath of 0.5 ml volume. Malpighian tubules stick to stretched Parafilm, which secures them in place for the impalement of principal cells with microelectrodes and for flushing the Ringer bath in the washout of test substances.
Microelectrodes (Omega dot borosilicate glass capillaries, model 30–30-1; Frederick Haer, St. Bowdoinham, ME; or model 1B100F-4; World Precision Instruments, Sarasota, FL) were pulled on a programmable puller (model P-97; Sutter Instruments, Novato, CA) to yield resistances between 10 and 40 MΩ when filled with 3 M KCl. The microelectrode was bridged to the measuring hardware by using an Ag/AgCl junction. The junction was prepared by first degreasing a silver wire (OD, 0.25 mm) with alcohol, and then by Cl−-plating it in either 1) 0.1 M HCl for 20 min at a current of 50 μA or 2) in a concentrated solution of household Clorox for 15 min. The Ag/AgCl wires were inserted directly into the back of voltage and current electrodes (Fig. 1B). An Ag/AgCl wire with an OD of 0.5 mm was inserted into a 4% agar bridge of Ringer solution to serve as the ground electrode.
Vbl was measured with a Geneclamp 500 electrometer (Axon Instruments, Molecular Devices, Sunnyvale, CA) at room temperature. The Rin of the impaled principal cell was measured from current-voltage plots generated by voltage clamping the cell in a series of five increasing voltage-clamp steps: Δ5 mV, 400 ms each, starting at (Vbl - 10 mV). The voltage-stepping protocol and data acquisition were executed digitally by using a Digidata 1440 (Molecular Devices) under control of the Clampex module of the pCLAMP software package (version 10; Molecular Devices).
In the typical experiment, a single principal cell near the blind (distal) end of the Malpighian tubule was impaled with both voltage and current electrodes (Fig. 1B). The experiment was continued if both electrodes measured values of Vbl within 10 mV of each other. After recording a stable Vbl and Rin, the kinin of interest was added to the peritubular Ringer solution to yield a concentration of 10−6 M. New steady-state values of Vbl and Rin were recorded 1–2 min later as shown in Figs. 2, 4, and 5. The kinin was then washed out by flushing the 0.5 ml Ringer bath with Ringer solution at a rate of 5 ml/min.
Fig. 2.
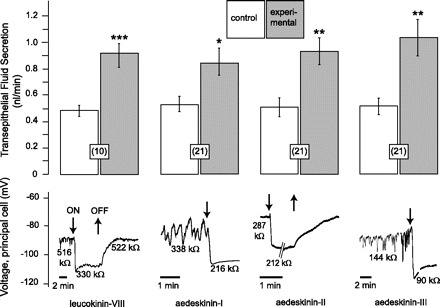
Effects of leucokinin-VIII and aedeskinins-I, -II, and -III on the rate of transepithelial fluid secretion and the Vbl and Rin of principal cells in isolated Malpighian tubules of female Aedes aegypti. All kinins were used at a concentration of 10−6 M. Data are means ± SE with the number of paired tubule experiments in parenthesis. *P < 0.05, **P < 0.001, ***P < 0.0001 paired t-test. Data of the effect of leucokinin-VIII on fluid secretion were taken from a previous study (39) for comparison with the effect of aedeskinins.
Fig. 4.
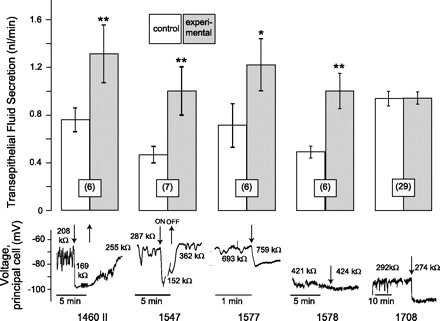
Effects of synthetic kinin analogs on the rate of transepithelial fluid secretion and the Vbl and Rin of principal cells in isolated Malpighian tubules of female Aedes aegypti. Kinin analogs were used at a concentration of 10−6 M. Analog 1708 stimulated fluid secretion in some tubules and inhibited it in other tubules, such that, on average in 29 tubules, analog 1708 had no significant effect on fluid secretion. Open and shaded bars represent means ± SE with the number of tubule experiments in parentheses; *P < 0.05, **P < 0.01, paired t-test.
Fig. 5.
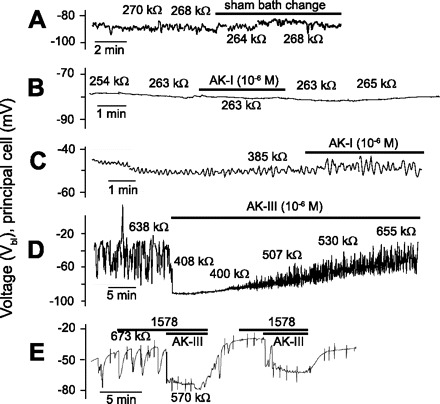
Representative electrophysiological responses of Malpighian tubules of Aedes aegypti to stimulation with kinins and analogs. Sham Ringer bath change at a Ringer flow rate of 5 ml/min (A); tubule with nonoscillating, high Vbl (B); tubule with the usual oscillating Vbl (C); aedeskinin receptor desensitization (D);and lack of effect of kinin analog 1578 on tubule electrophysiology (E). AK, aedeskinin.
Statistical Treatment of Data
Statistical significance of the data was evaluated using the paired Student t-test where each tubule was used as its own control. To construct a dose-response curve, the data were fitted using a sigmoid dose-response regression offered by the GraphPad Prism version 4.0 software for Windows (GraphPad Software, San Diego CA).
RESULTS
Effect of Leucokinin-VIII on Transepithelial Fluid Secretion and Tubule Electrophysiology
From a previous study (39), we know that leucokinin-VIII significantly increases the V̇S in isolated Malpighian tubules from 0.49 ± 0.04 nl/min to 0.91 ± 0.08 nl/min (P < 0.0001, n = 10 tubules). In the present study, leucokinin-VIII also had electrophysiological effects on principal cells of the tubule. In the representative experiment shown in Fig. 2, leucokinin-VIII hyperpolarized Vbl from about −90 mV to −108 mV, while lowering Rin from 516 to 330 kΩ. Both effects are reversible upon washout of leucokinin-VIII from the peritubular bath.
Effect of Aedeskinins on Transepithelial Fluid Secretion and Tubule Electrophysiology
All three aedeskinins tested at a concentration of 10−6 M significantly increased V̇S, hyperpolarized the Vbl, and decreased the Rin of principal cells in isolated Aedes Malpighian tubules (Fig. 2).
Aedeskinin-I significantly increased V̇S from 0.53 ± 0.06 nl/min to 0.84 ± 0.11 nl/min in 21 Malpighian tubules (P < 0.05). In electrophysiological studies, the addition of aedeskinin-I to the peritubular medium of 14 Malpighian tubules immediately and significantly hyperpolarized Vbl from −61.2 ± 6.6 mV to −69.7 ± 7.2 mV (P < 0.001, paired t-test) and significantly decreased Rin from 413.0 ± 91.6 kΩ to 326.4 ± 75.3 kΩ (P < 0.05, paired t-test). A representative experiment is shown in Fig. 2. The effects on Vbl and Rin were reversible upon washout of aedeskinin-I (data not shown).
Aedeskinin-II significantly increased V̇S from 0.51 ± 0.07 nl/min to 0.93 ± 0.10 nl/min in 21 Malpighian tubules (P < 0.001). In electrophysiological studies, the peritubular addition of aedeskinin-II immediately and significantly hyperpolarized Vbl from −70.2 ± 8.1 mV to −77.7 ± 8.2 mV in 10 principal cells of as many tubules (P < 0.001, paired t-test). In parallel, the Rin dropped significantly from 331.4 ± 41.2 kΩ to 307.0 ± 38.7 kΩ (P < 0.05, paired t-test). A representative experiment is shown in Fig. 2. The effects on Vbl and Rin were reversible upon washout of aedeskinin-II.
Aedeskinin-III significantly increased V̇S from 0.52 ± 0.06 nl/min to 1.04 ± 0.13 nl/min in 21 Malpighian tubules (P < 0.001, paired t-test). In electrophysiological studies, the peritubular addition of aedeskinin-III immediately and significantly hyperpolarized Vbl from −64.3 ± 3.7 mV to −87.8 ± 4.3 mV in 13 principal cells of as many tubules (P < 0.001, paired t-test). In parallel, the Rin dropped significantly from 343.3 ± 54.8 kΩ to 265.2 ± 41.9 kΩ (P < 0.01, paired t-test). A representative experiment is shown in Fig. 2. The effects on Vbl and Rin were reversible upon washout of aedeskinin-III (data not shown).
Invariably, if a principal cell exhibited spontaneous oscillation of Vbl under control conditions, then the peritubular addition of aedeskinin-I, -II, or -III substantially diminished or eliminated entirely these oscillations (Fig. 2).
Aedeskinin-III and Transepithelial Fluid Secretion: a Dose Response Study
To explore the diuretic mechanism of action of the aedeskinins, we studied in detail the effects of aedeskinin-III on V̇S in isolated Aedes Malpighian tubules. Aedeskinin-III was selected for this study because it was the most potent secretagogue in our laboratory (Fig. 2) and in the laboratory of Veenstra et al. (58).
Fig. 3A illustrates a dose response to aedeskinin-III. At a concentration of 10−10 M, aedeskinin-III had no effect onV̇S. At concentrations 10−8 M and higher, aedeskinin-III significantly increased V̇S. Maximal stimulation was observed at 10−6 M with a 104.50 ± 14.01% increase in V̇S in 21 tubules (P < 0.001, paired t-test) and an EC50 value of 1.5 × 10−8 M (Fig. 3A). The average control rate of V̇S in all 71 Malpighian tubules shown in Fig. 3A was 0.58 ± 0.04 nl/min.
Fig. 3.
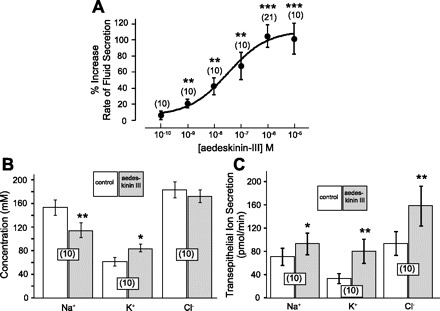
A: dose-response curve for the effects of aedeskinin-III on transepithelial fluid secretion in isolated Malpighian tubules of Aedes aegypti. The effect of aedeskinin-III (10−6 M) on the concentrations of ions in secreted fluid and the rates of transepithelial ion secretion are shown in B and C, respectively. Open and shaded bars represent means ± SE with the number of paired tubule experiments in parentheses. *P < 0.02, **P < 0.01, ***P < 0.001, paired t-test.
The effect of aedeskinin-III on transepithelial Na+, K+, and Cl− secretion was tested in 10 separate Malpighian tubules (Fig. 3, B and C). In these tubules, the average control V̇S was 0.48 ± 0.10 nl/min (data not shown). It increased significantly to 0.93 ± 0.21 nl/min in the presence of 10−6 M aedeskinin-III (P < 0.01, paired t-test). In the same 10 tubules, aedeskinin-III significantly decreased the concentration of Na+ in secreted fluid from 153.3 ± 13.1 mM to 114.3 ± 12.8 mM (P < 0.01, paired t-test), and significantly increased the concentration of K+ from 61.9 ± 7.5 mM to 83.9 ± 6.4 mM (P < 0.02, paired t-test). The change in Cl− concentration from 183.7 ± 13.2 mM to 172.1 ± 6.9 mM was not significant.
The product of the fluid secretion rate and the ion concentration in secreted fluid yields the rate of transepithelial ion secretion. As shown in Fig. 3C, aedeskinin-III significantly increased the tubular secretion rates of Na+, K+, and Cl−. The transepithelial Na+ secretion increased 32%, from 70.9 ± 14.8 pmol/min to 93.4 ± 18.9 pmol/min (P < 0.02, paired t-test). The secretion of K+ increased 140%, from 33.5 ± 8.8 pmol/min to 80.5 ± 20.5 pmol/min (P < 0.01, paired t-test), and the secretion of Cl− increased 69%, from 94.1 ± 20.6 pmol/min to 158.7 ± 34.3 pmol/min (P < 0.01, paired t-test).
Effects of Synthetic Kinin Analogs on Fluid Secretion and Tubule Electrophysiology
Analog 1460 II.
In the Ramsay fluid secretion assay, analog 1460 II (10−6 M) significantly increased V̇S from 0.76 ± 0.11 nl/min to 1.32 ± 0.24 nl/min in the six tubules studied (P < 0.01, paired t-test; Fig. 4). In the electrophysiological assay, analog 1460 II (10−6 M) significantly and reversibly hyperpolarized the Vbl from −70.0 ± 7.7 mV to −84.4 ± 6.2 mV in 8 principal cells from as many tubules (P < 0.01, paired t-test). In parallel, the Rin decreased significantly and reversibly from 323.0 ± 42.9 kΩ to 281.5 ± 34.3 kΩ (P < 0.01, paired t-test). The representative experiment shown in Fig. 4 illustrates Vbl oscillating between −65 and −85 mV under control conditions. The addition of analog 1460 II to the peritubular medium hyperpolarized Vbl to a steady-state voltage of −98 mV; in parallel, the Rin of the principal cell dropped from 208 kΩ to 169 kΩ. Upon washout of 1460 II, Vbl returned to −74 mV, while Rin increased to 255 kΩ.
Analog 1547.
In the Ramsay fluid secretion assay, analog 1547 (10−6 M) significantly increased V̇S from 0.47 ± 0.07 nl/min to 1.01 ± 0.20 nl/min in the seven tubules studied (P < 0.01, paired t-test; Fig. 4). In the electrophysiological assay, analog 1547 significantly increased Vbl from −73.2 ± 6.3 mV to −83.0 ± 7.2 mV in nine principal cells from as many tubules (P < 0.01, paired t-test), but the effects on Rin were variable. The Rin increased by as much as 131 kΩ in five tubules, but decreased by as much as 209 kΩ in four tubules even though all nine tubules recorded the hyperpolarization of Vbl. Thus, the change in mean Rin from 337.3 ± 23.4 kΩ to 305.5 ± 58.6 kΩ was not significant. In the experiment shown in Fig. 4, analog 1547 reversibly hyperpolarized Vbl together with a drop in Rin.
Analog 1577.
In the Ramsay fluid secretion assay, analog 1577 (10−6 M) significantly increased V̇S from 0.72 ± 0.18 nl/min to 1.22 ± 0.22 nl/min in the six tubules studied (P < 0.05, paired t-test; Fig. 4). In the electrophysiological assay, analog 1577 significantly hyperpolarized Vbl from −73.8 ± 2.8 mV to −85.9 ± 2.3 mV in eight principal cells from as many tubules (P < 0.01, paired t-test), but the effects on Rin were variable. In six of eight tubules the Rin decreased in the presence of analog 1577, and in two tubules Rin increased. Thus, the small decrease in the mean Rin from 372.4 ± 26.7 kΩ to 356.2 ± 26.0 kΩ did not reach statistical significance. In the experiment shown in Fig. 4, analog 1577 hyperpolarized Vbl from −62 mV to −75 mV; in parallel, the Rin increased from 693 kΩ to 759 kΩ.
Analog 1578.
In the Ramsay fluid secretion assay, analog 1578 (10−6 M) significantly increased V̇S from 0.49 ± 0.05 nl/min to 1.01 ± 0.16 nl/min in the six tubules studied (P < 0.01, paired t-test; Fig. 4). However, in the electrophysiological assay, analog 1578 (10−6 M) had no effect on Vbl and Rin. As shown in the representative experiment of Fig. 4, the usual step change in Vbl and Rin upon the addition of aedeskinins did not occur after adding analog 1578 to the peritubular bath. As such, the small change in the mean Vbl from −81.1 ± 5.9 mV to −84.1 ± 6.1 mV in 10 principal cells from as many tubules was not statistically significant. Likewise, the small drop in mean Rin from 401.2 ± 45.8 kΩ to 388.4 ± 47.4 mV kΩ was not significant.
We point out that tubules which did not respond to analog 1578 still responded to aedeskinin-III (and/or to leucokinin-VIII) at concentrations of 10−6 M (Fig. 5E). Thus, analog 1578 was remarkable in that it clearly stimulated fluid secretion in Aedes Malpighian tubules without effects on tubule electrophysiology (Fig. 4).
Analog 1708.
In the Ramsay fluid secretion assay, analog 1708 (10−6 M) is the only one that had no effect on the mean V̇S in studies of 29 tubules (Fig. 4), which stems from the fact that in 12 tubules, analog 1708 increased V̇S, whereas in 17 others it decreased V̇S. In contrast, analog 1708 significantly hyperpolarized Vbl from −73.3 ± 4.6 mV to −95.0 ± 5.0 mV in nine principal cells from as many tubules (P < 0.001, paired t-test) and significantly decreased Rin from 439.1 ± 60.0 kΩ to 351.1 ± 38.1 kΩ (P < 0.02, paired t-test). In the representative experiment shown in Fig. 4, analog 1708 hyperpolarized Vbl from −92 mV to −110 mV, while decreasing Rin from 292 kΩ to 274 kΩ. In this and another tubule experiment, the effects of analog 1708 on Vbl and Rin were irreversible upon washout of analog 1708. In the remaining seven tubules, the effects on principal cell voltage and resistance were slowly reversible upon washout of analog 1708. Thus, analog 1708 was remarkable in that it clearly affected the electrophysiology of Aedes Malpighian tubules without consistent effects on fluid secretion (Fig. 4).
Spontaneous Voltage Oscillations
Malpighian tubules of Aedes aegypti often display oscillating membrane voltages under control, unstimulated conditions (Figs. 2, 4, and 5). From previous studies, we know that oscillations of the Vbl can come about from oscillating changes in the paracellular Cl− conductance (6, 8). As the paracellular Cl− conductance increases, Vbl hyperpolarizes.
The natural aedeskinins and those synthetic kinin analogs with effects on tubule electrophysiology not only caused the hyperpolarization of Vbl together with the drop in Rin (Figs. 2 and 4), they also substantially diminished or abolished the oscillations of Vbl. Upon washout of the kinin, the oscillations usually reappeared.
Variability in the Response to Kinins
We make the occasional observation that Malpighian tubules do not respond to kinins, neither the natural aedeskinins nor the synthetic analogs (Fig. 5). We encounter nonresponding tubules under three conditions. First, Malpighian tubules with control values of Vbl more negative than −75 mV and nonoscillating, are less likely to respond to kinins than tubules with the usual control values of Vbl in the vicinity of −60 mV. In the example shown in Fig. 5B, Vbl is about −80 mV, and it does not oscillate. Aedeskinin-I (10−6 M) had no effect on Vbl and Rin in this tubule. Since kinins hyperpolarize Vbl, we hypothesize that the tubule was already kinin-activated in the mosquito from which it had been isolated. Second, the failure to respond to kinins is also observed in tubules with low and oscillating values of Vbl. In the example illustrated in Fig. 5C, Vbl oscillates around values of −50 mV. The tubule did not respond to aedeskinin-I. Third, we observe receptor desensitization in the presence of kinins. As shown in Fig. 5D, the isolated Malpighian tubule displayed the typical electrophysiological response in the presence of kinin: 1) the hyperpolarization of the Vbl, 2) the drop in the cell Rin, and 3) the cessation of Vbl oscillations. However, after 5 min in the presence of aedeskinin-III, Vbl started to repolarize and voltage oscillations returned after ∼10 min. As Vbl continued to repolarize and as voltage oscillations increased with time, the cell Rin returned to control values in the presence of aedeskinin-III. The return to control values in the presence of agonist indicates receptor desensitization.
To rule out the study of nonresponding tubules when a kinin analog had no effect, we routinely pulsed the tubule with aedeskinin-III. For example, as shown in Fig. 5E, the tubule responded to aedeskinin-III but not to kinin agonist 1578.
DISCUSSION
Aedeskinin-II Stimulates Fluid Secretion
Our experiments on the effects of the three natural aedeskinins on the fluid secretion rates and electrophysiology of isolated Aedes Malpighian tubules have confirmed an analogous study by Veenstra et al. (58) with one glaring exception: in the laboratory of Veenstra, aedeskinin-II elicits electrophysiological effects but has no effect on fluid secretion (58). In our study, we find that aedeskinin-II stimulates both fluid secretion and electrophysiology in a similar manner as aedeskinin-I and -III (Fig. 2).
The discrepancy between the results of our study and those of Veenstra may relate to our observation that occasionally Malpighian tubules do not respond to kinins at all. For example, in one series of experiments using the same hatch of mosquitoes, two out of nine tubules did not respond to aedeskinin-I in the fluid secretion assay and five of nineteen Malpighian tubules did not respond in the electrophysiological assay (Fig. 5, B and C). Similar observations can be made with aedeskinin-II and -III, as if the kinin receptor is not expressed in these Malpighian tubules.
In a review of our data back to 1989 when we first started to study insect kinins (27), the observation of nonresponding Malpighian tubules is rare, < 1% of all studies when tubules were obtained from adult mosquitoes 3–5 days old. However, that percentage can increase to 100% within some mosquito hatches (populations) where the responsiveness of the tubules to kinins is delayed for unknown reasons. Since these nonresponding tubules are not the norm, we exclude them from the present study.
Nonresponsiveness to kinins may have a developmental and/or nutritional causation. Tubules taken from imago mosquitoes shortly after eclosion may not express the kinin receptor. For example, the tubule shown in Fig. 5C had a normal, oscillating Vbl, which did not change upon stimulation with aedeskinin-I. This tubule was taken from a mosquito 2 days after eclosion, when tubules from this hatch of mosquitoes did not respond to kinins. However, a few days later, hatch mates of this mosquito responded to kinin stimulation. As a first hypothesis, the time lag in receptor expression may reflect 1) the nutritional history of the mosquito in the larval stage, 2) the continued development of Malpighian tubules after eclosion, 3) the coupling of kinin receptor expression to reproduction, blood feeding, and/or other factors. For example, the quality of larval nutrition is known to affect ovarian development in Aedes aegypti (19, 25). Females with insufficient nutrition as larvae (due to a limited food supply or larval overcrowding) emerge as undersized gono-inactive adults. These gono-inactive females require additional feeding after emerging to develop their eggs to the previtellogenic resting stage of development. In contrast, females that are sufficiently nourished as larvae emerge as large gono-active females with ovarian follicles capable of commencing vitellogenesis upon feeding on blood. Given that the diuretic responses triggered by the kinins would physiologically be most relevant to gono-active, blood-fed females, it is tantalizing to speculate that the expression of the kinin receptor in Malpighian tubules is linked to an endocrine and/or developmental axis of ovarian development.
Accordingly, development, nutrition, reproduction, blood-feeding, and other physiological processes may all influence the expression of the kinin receptor in Malpighian tubules. It is therefore conceivable that Veenstra et al. (58) observed electrophysiological effects of all three aedeskinins when he used tubules from mature and well-developed mosquitoes, but he failed to observe the effects of aedeskinin-II on fluid secretion in tubules isolated from developmentally or nutritionally compromised mosquitoes. Using mature mosquitoes in the present study, we have observed consistent effects of all three aedeskinins on both fluid secretion and electrophysiology (Fig. 2). Furthermore, Cady and Hagedorn (17) report that the injection of aedeskinin-II into the hemolymph of female Aedes aegypti increases urine production.
Aedeskinin-III Duplicates the Effects of Leucokinin
Before the aedeskinins became available, our laboratory studied the mechanism of action of the cockroach kinin, leucokinin-VIII, on Malpighian tubules of Aedes aegypti, where we made the first observation of the diuretic activity of the invertebrate family of kinins (27). We found that leucokinin-VIII increases the transepithelial secretion of water by increasing the transepithelial secretion of both NaCl and KCl (39). We have attributed the nonselective increase of both NaCl and KCl secretion to the activation of a large paracellular Cl− conductance (5, 39, 59, 65).
Like leucokinin-VIII, aedeskinin-III increases the transepithelial secretion of NaCl and KCl, and both kinins significantly decrease the Na+ concentration and increase the K+ concentration in secreted fluid with no effect on the concentration of Cl− (Fig. 3). Opposite effects on luminal Na+ and K+ concentrations (Fig. 3B) stem from the nearly complete transepithelial short circuit caused by the large increase in the paracellular Cl− conductance in the presence of kinins (5, 65). The transepithelial short circuit depolarizes the transepithelial voltage to values close to zero (39, 58) with the effect of depolarizing the apical membrane voltage and hyperpolarizing the Vbl of principal cells (Fig. 2). Since the K+ conductance of the basolateral membrane of principal cells is four times greater than the Na+ conductance (8), it follows that the hyperpolarization of Vbl increases the influx of K+ from the peritubular medium to the cytoplasm of principal cells. As a result, the concentration of cytoplasmic K+ is expected to rise, improving its competition with Na+ for extrusion across the apical membrane into the tubule lumen. Consequently, the concentration of K+ in the secreted fluid increases, while that of Na+ falls (Fig. 3B). The concentration of Cl− in secreted fluid does not change because the luminal and peritubular Cl− concentrations are already similar and high under control conditions (9, 20, 37, 61, 62). Accordingly, the kinin-induced increase in paracellular Cl− conductance merely collapses the transepithelial voltage without a change in luminal or peritubular Cl− concentration.
Voltage and Resistance Oscillations and Evidence for a Role of Ca2+
As shown in Figs. 2, 4, and 5, Malpighian tubules of Aedes aegypti often display oscillations of voltage and resistance under control conditions (6, 60, 63, 65, 67). Significantly, these oscillations are dependent on the Cl− concentrations in the peritubular medium (6, 8). Oscillations of the transepithelial voltage that are greater than the simultaneous voltage oscillations of the basolateral and apical membranes (6, 47) point to the paracellular pathway as the source of oscillations. Indeed, the oscillations derive from spontaneous cyclical Cl− conductance changes of the paracellular pathway (6). As the paracellular Cl− conductance increases, the transepithelial voltage goes to values close to 0 mV (approaching transepithelial short circuit). Consequently, the Vbl hyperpolarizes and the apical membrane voltage depolarizes (6, 47).
Studies in Drosophila Malpighian tubules have added importantly to our understanding of the spontaneous voltage and resistance oscillations observed in Malpighian tubules. Blumenthal (14) has found that the biogenic amine tyramine is a potent stimulator of transepithelial Cl− transport and a diuretic agent. The mechanism of action of tyramine, i.e., the sudden activation of a Ca2+-dependent transepithelial Cl− conductance and its kinin-like change in transepithelial voltage (depolarization) is strikingly similar to that of the kinin diuresis we have elucidated in Aedes Malpighian tubules (5, 39, 65–67). Moreover, low doses of tyramine (1–10 nM) induce oscillations of voltage and resistance in Drosophila Malpighian tubules (12, 13). What is remarkable is that Drosophila Malpighian tubules generate their own tyramine from tyrosine (12), suggesting the autoregulation of fluid secretion in Malpighian tubules. The kinins and other diuretic agents, as well as antidiuretic agents, could have overriding influences on this autoregulation. Consistent with this hypothesis is the cross-desensitization of tyramine and leucokinin that Blumenthal (11, 13) reports in Drosophila Malpighian tubules. Whether tyramine is responsible for the spontaneous oscillations in Aedes Malpighian tubules remains to be investigated.
Changes in intracellular [Ca2+] are likely to 1) mediate the spontaneous voltage oscillation and 2) signal the electrophysiological effects of kinins in Aedes and perhaps also Drosophila Malpighian tubules (11, 67). First, the Ca2+ ionophore A23187 duplicates the effects of leucokinin in Aedes Malpighian tubules by lowering the transepithelial voltage and resistance, provided that Ca2+ is present in the extracellular Ringer bath (18, 67). Second, thapsigargin, the inhibitor of Ca2+ uptake by intracellular Ca2+ stores, duplicates the electrophysiological effects of leucokinin-VIII (67). Third, the voltage oscillations disappear in the presence of kinins (Fig. 2), which are known to activate nifedipine-sensitive Ca2+ channels in the basolateral membrane of principal cells (67). Fourth, analog 1460 II is the most potent synthetic kinin in an intracellular Ca2+ fluorescence assay (aequorin) of CHO-K1 cells expressing the recombinant kinin receptor of Aedes aegypti (52) and a potent activator of the electrophysiological response in Aedes Malpighian tubules (Fig. 4). Fifth, analog 1578 elicits a weak Ca2+ response in CHO-K1 cells expressing the kinin receptor of Aedes aegypti (52), and in the present study, analog 1578 has no electrophysiological effect (Fig. 4). Furthermore, we found that analogs 1547, 1577, and 1578 progressively lose the ability to activate electrophysiological effects (Fig. 4), which is the rank order similar to that observed by Taneja-Bageshwar et al. (52) using the intracellular Ca2+ bioassay in CHO-K1 cells expressing the kinin G protein-coupled receptor (GPCR). Taken together, the above observations suggest Ca2+ mediates both the spontaneous voltage and resistance oscillations under control conditions and the electrophysiological effects of kinins. The signaling pathway(s) kinins use to increase fluid secretion are unknown.
One Receptor, Two or More Signaling Pathways
Whereas all three aedeskinins exert effects on both tubule electrophysiology and fluid secretion, not every synthetic kinin analog displays this twin effect. Analog 1708 has no effect on transepithelial fluid secretion but consistent effects on tubule electrophysiology (Fig. 4). In contrast, analogs 1547, 1577, and 1578 consistently stimulate fluid secretion, but they lose the ability to activate electrophysiological effects with increasing length of the COOH-terminal pentapeptide (Fig. 4). Since Drosophila and Aedes express only one transcript of the single kinin receptor gene, which encodes a GPCR (41–43), the question arises as to how this single GPCR can give rise to the twin effects on fluid secretion and electrophysiology on the one hand, and to separate and independent effects on fluid secretion and electrophysiology on the other hand.
As a first hypothesis, we consider ligand-dependent selectivity of receptor signaling where a single receptor can give rise to more than one physiological response (Fig. 6). The phenomenon is also known as agonist-directed signaling, ligand-directed trafficking, conformation-specific agonism, or functional selectivity (for reviews see Refs. 2 and 48). Ligand-dependent signaling is particularly well known for GPCRs (48). For example, the single mammalian serotonin GPCR can activate 1) a phospholipase C-mediated increase in inositol phosphates, and/or 2) a phospholipase A2-mediated release of arachadonic acid, depending on the ligand that binds to it (3, 49). Each ligand is thought to induce a distinct receptor conformation that targets a specific signaling pathway. For example, the binding of serotonin to the serotonin GPCR may induce one conformation that activates the inositol phosphate and arachadonic acid pathways equally, the binding of agonist (6)−1-(2,5-dimethoxy-4-iodophenyl)-2-amino propane may induce a different receptor conformation that preferentially activates the arachadonic acid pathway, and the binding of agonist 3-trifluromethylphenyl-piperazine may trigger a receptor conformation that preferentially activates the inositol phosphate pathway (48). Similarly, the three aedeskinins, leucokinin, and analog 1460 II may activate equally those signaling pathways that increase rates of fluid secretion and induce electrophysiological changes (Figs. 2 and 6A). In the case of the other synthetic analogs, the amino acid substitutions produce conformations in the ligand that allow only partial binding to the receptor, thereby activating one physiological effect but not the other (Fig. 6A). Thus, the spatial modification found in analog 1708 may allow this analog to interact with the region of the receptor, which activates the electrophysiological response while preventing interactions with the receptor region-associated fluid secretion (Fig. 6A). In contrast, the spatial modifications of analog 1578 may allow the interaction with the receptor binding sites associated with the activation of fluid secretion, but prevent the interaction with binding sites that signal electrophysiological effects (Fig. 6A).
Fig. 6.
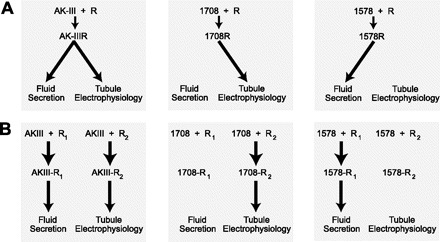
Models of ligand/receptor interactions. A: ligand-dependent signaling via a single receptor; [model adapted from Berg and Clarke (2)]. B: glycosylation-dependent signaling, where R1 and R2 represent glycosylated and nonglycosylated forms of the same receptor.
As a second hypothesis, we propose that the single kinin GPCR may control the access of ligands by way of glycosylation (Fig. 6B). GPCRs are typically glycoproteins with the glycosylation sites existing within the extracellular NH2-terminal region (46). The consensus sequence Asn-X-Ser/Thr provides for N-linked glycosylations. Although Drosophila Malpighian tubules express a single transcript encoding a kinin GPCR (consistent with one receptor), two immunochemical forms of the drosokinin GPCR are detected: one is N-glycosylated and the other is not (43). Likewise, Malpighian tubules of the Anopheles mosquito express two analogous immunochemical forms of the kinin GPCR (44). If we assume that Aedes Malpighian tubules also express two varieties of the kinin GPCR, R1 and R2, then the glycosylated receptor may signal one physiological event and the nonglycosylated receptor may signal the other. Thus, the three aedeskinins, leucokinin-VIII, and 1460 II may bind to both GPCRs, thereby triggering the twin physiological response of fluid secretion and tubule electrophysiology (Fig. 6B). However, in the case of analogs 1708 and 1578, the chemical modifications to the peptides may favor binding to the glycosylated form of the GPCR over the nonglycosylated form (or vice versa), resulting in one physiological response that dominates over the other. Glycosylation-dependent ligand binding of GPCRs and signaling has been observed. The glycosylation can influence 1) receptor-receptor self-association (24), 2) stabilization of the GPCR in the plasma membrane (53), 3) folding of the GPCR into a high-affinity conformation (68), 4) membrane trafficking and surface expression of the GPCR (15, 40), and 5) ligand binding and the ensuing signal transduction response (1, 35).
The phenomena of agonist-directed or glycosylation-dependent signaling now sheds light on an observation that has been enigmatic to us for the past 21 years: leucokinin-VIII produces different dose response curves of its effects on 1) the transepithelial voltage, and 2) rates of fluid secretion in Aedes Malpighian tubules (27). The voltage dose-response curve is steep with an EC50 of 2.4 × 10−9 M, whereas the fluid secretion dose response curve is shallow with an EC50 of 3.0 × 10−8 M (27), similar to that observed in the present study using aedeskinin-III (Fig. 3A). Veenstra and colleagues (58) made similar observations finding that the aedeskinins have different threshold concentrations when assayed for the depolarizing activity of the transepithelial voltage and also different potencies on fluid secretion. Veenstra et al. (58) suggested more than one type of kinin receptor to account for the differences observed in electrophysiological and fluid secretion assays. The present study suggests agonist-directed signaling of kinins, or alternatively, a differentially glycosylated receptor that can activate electrophysiological or fluid secretion responses.
Perspectives and Significance
In the present study, we have used two bioassays (electrophysiology and fluid secretion) to probe kinin/receptor interactions in Malpighian tubules of the yellow fever mosquito Aedes aegypti. We have observed that the three natural aedeskinins activate both physiological responses. In contrast, synthetic kinin analogs containing spatial modifications in the critical pentapeptide core region may trigger just one or both effect(s). Separate and independent effects on electrophysiology and fluid secretion may reflect ligand-directed signaling and/or glycosylation-dependent functional states of the single kinin receptor expressed in Aedes Malpighian tubules. Separate effects of kinin analogs on electrophysiology and fluid secretion suggest that natural kinins may be physiologically more diverse than previously assumed. For example, dose-dependent effects on tubule electrophysiology without an effect on fluid secretion may indicate the renal response to a purely ionic challenge, whereas effects on fluid secretion without an effect on electrophysiology may indicate the renal (electroneutral) response to a volume load. More than one bioassay should be consulted to fully evaluate the structure/function relations of insect kinins and their GCPRs. The reliance on only one bioassay (as in high-throughput screens of voltage effects or changes in intracellular Ca2+ concentrations) would have excluded analog 1578 as a potential diuretic agent. By incorporating the Ramsay fluid secretion assay, we have proven otherwise.
GRANTS
The National Science Foundation Award IBN-0078058 made this work possible in the Beyenbach laboratory, and the U.S. Department of Agriculture/Department of Defense, Deployed War Fighter Protection Initiative No. 0500-32000-001-01R supported work in the Nachman laboratory.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Marcus Huss at the University of Osnabrück for erudite discussions.
APPENDIX: ON CHANGES IN VOLTAGE AND FLUID SECRETION
In response to a reviewer's inquiry about how voltage effects can be independent from effects on ion transport and fluid secretion in Malpighian tubules, it is instructive to compare the quantities of ions (charge) needed to change a voltage with the quantities of ions secreted across the epithelium into the tubule lumen. Figure 3 illustrates that in the presence of aedeskinin-III the tubule secretes cations or anions at a rate of ∼165 pmol/min. The secretion is mediated by the 1.5-mm tubule segment suspended in the Ringer droplet of the Ramsay fluid secretion assay (Fig. 1A). Since the average electrical diameter of the Aedes Malpighian tubule determined by cable analysis is ∼22 μm (38), the transepithelial cation or anion secretion can be normalized to an epithelial surface area, i.e., of 2.7 × 10−9 mol/s − cm2. Applying the Faraday constant yields an electrical equivalent of 2.6 × 10−4 coul/s − cm2, or 260 μA/cm2.
The electrical capacitance of epithelia is ∼3.5 μF/cm2 (33). The quantity of charge q is the product of capacitance (C) and voltage (V). Accordingly, the kinin-induced depolarization of the transepithelial voltage of Aedes Malpighian tubule from 59 mV to 6 mV (39) requires a charge redistribution of only 1.9 × 10−7 coul/cm2 which takes only 0.72 ms at the transepithelial charge secretion rate of 2.6 × 10−4 coul/s − cm2. In that short time the tubule secretes a volume of only 1.2 × 10−14 liters of fluid, far too small for detection in the Ramsay fluid secretion assay. The above calculations illustrate that a change of voltage requires very little ion flux. Accordingly, a change in voltage may not reflect a significant change in ion and fluid secretion.
The kinin-induced drop in transepithelial voltage from 59 mV to 6 mV together with the drop in transepithelial resistance from 58 Ω/cm2 to 10 Ω/cm2 (39) indicates the switch from a moderately tight epithelium to a leaky epithelium. At the extremes, leaky epithelia are specialized for massive transepithelial transport of solute and water (often in isosmotic proportions as in renal proximal tubules), and tight epithelia (with low rates of transepithelial transport) are specialized for storage functions (such as the urinary bladder). In tight epithelia, changes in transepithelial ion transport are reflected in changes in voltage and resistance; however, in leaky epithelia large changes in transepithelial ion transport may take place with little effect on voltage and resistance, because leaky epithelia operate near the short-circuit condition (where short-circuit is the condition of zero voltage).
REFERENCES
- 1. Assil IQ, Abou-Samra AB. N-glycosylation of CRF receptor type 1 is important for its ligand-specific interaction. Am J Physiol Endocrinol Metab 281: E1015–E1021, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Berg KA, Clarke WP. Development of functionally selective agonists as novel therapeutic agents. Drug Discov Today Ther Strateg 3: 421–428, 2006 [Google Scholar]
- 3. Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54: 94–104, 1998. [PubMed] [Google Scholar]
- 4. Beyenbach KW. Extracellular fluid homeostasis in insects? In: Molecular Comparative Physiology, edited by Kinne RHK, Kinne-Saffran E, Beyenbach KW. Basel: Karger, 1993, p. 146–173 [Google Scholar]
- 5. Beyenbach KW. Regulation of tight junction permeability with switch-like speed. Curr Opin Nephrol Hypertens 12: 543–550, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Beyenbach KW, Aneshansley JD, Pannabecker TL, Masia R, Gray D, Yu MJ. Oscillations of voltage and resistance in Malpighian tubules of Aedes aegypti. J Insect Physiol 46: 321–333, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Beyenbach KW, Dantzler WH. Comparative kidney tubule sources, isolation, perfusion, and function. Methods Enzymol 191: 167–226, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Beyenbach KW, Masia R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J Insect Physiol 48: 375–386, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Beyenbach KW, Piermarini PM. Osmotic and ionic regulation in insects. In: Osmotic and Ionic Regulation: Cells and Animals, edited by Evans DH. Boca Raton, FL: CRC, 2009, p. 231–293 [Google Scholar]
- 10. Blackburn MB, Wagner RM, Shabanowitz J, Kochansky JP, Hunt DF, Raina AK. The isolation and identification of three diuretic kinins from the abdominal ventral nerve cord of adult Helicoverpa zea. J Insect Physiol 41: 723–730, 1995 [Google Scholar]
- 11. Blumenthal EM. Characterization of transepithelial oscillations in the Drosophila Malpighian tubule. J Exp Biol 204: 3075–3084, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Blumenthal EM. Isoform- and cell-specific function of tyrosine decarboxylase in the Drosophila Malpighian tubule. J Exp Biol 212: 3802–3809, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Blumenthal EM. Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am J Physiol Cell Physiol 289: C1261–C1267, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol 284: C718–C728, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Boudin H, Lazaroff B, Bachelet CM, Pelaprat D, Rostene W, Beaudet A. Immunologic differentiation of two high-affinity neurotensin receptor isoforms in the developing rat brain. J Comp Neurol 425: 45–57, 2000. [PubMed] [Google Scholar]
- 16. Bradley TJ. The excretory system: structure and physiology. In: Comprehensive Insect Physiology: Biochemistry and Pharmacology, edited by Kerkut GA, Gilbert LI. Oxford: Pergamon, 1985, p. 421–439 [Google Scholar]
- 17. Cady C, Hagedorn HH. The effect of putative diuretic factors on in vivo urine production in the mosquito, Aedes aegypti. J Insect Physiol 45: 317–325, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Clark TM, Hayes TK, Holman GM, Beyenbach KW. The concentration-dependence of CRF-like diuretic peptide: mechanisms of action. J Exp Biol 201: 1753–1762, 1998. [DOI] [PubMed] [Google Scholar]
- 19. Clements AN. The Biology of Mosquitoes. London: Chapman and Hall, 1992 [Google Scholar]
- 20. Coast G. The endocrine control of salt balance in insects. Gen Comp Endocrinol 152: 332–338, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Coast GM. The influence of neuropeptides on Malpighian tubule writhing and its significance for excretion. Peptides 19: 469–480, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Coast GM. Neuropeptides implicated in the control of diuresis in insects. Peptides 17: 327–336, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Coast GM, Holman GM, Nachman RJ. The diuretic activity of a series of cephalomyotropic neuropeptides, the achetakinins, on isolated Malpighian tubules of the house cricket Acheta domesticus. J Insect Physiol 36: 481–488, 1990 [Google Scholar]
- 24. Fernandes H, Cohen S, Bishayee S. Glycosylation-induced conformational modification positively regulates receptor-receptor association: a study with an aberrant epidermal growth factor receptor (EGFRvIII/DeltaEGFR) expressed in cancer cells. J Biol Chem 276: 5375–5383, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Foster WA, Walker ED. Mosquitoes (Culicidae) In: Medical and Veterinary Entomology, edited by Mullen G, Durden L. San Diego: Academic, 2002, p. 203–262 [Google Scholar]
- 26. Gaede G, Hoffmann KH, Spring JH. Hormonal regulation in insects: facts, gaps, and future directions. Physiol Rev 77: 963–1032, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Hayes TK, Pannabecker TL, Hinckley DJ, Holman GM, Nachman RJ, Petzel DH, Beyenbach KW. Leucokinins, a new family of ion transport stimulators and inhibitors in insect Malpighian tubules. Life Sci 44: 1259–1266, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Hayes TK, Strey A, Belk S, Holman GM, Nachman RJ, Petzel D, Readio J, Meola R, Pannabecker T, Beyenbach K. Biochemical characterization of mosquito kinin and related receptors. Ann NY Acad Sci 814: 342–345, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Hegarty JL, Zhang B, Pannabecker TL, Petzel DH, Baustian MD, Beyenbach KW. Dibutyryl cAMP activates bumetanide-sensitive electrolyte transport in Malpighian tubules. Am J Physiol Cell Physiol 261: C521–C529, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Holman GM, Cook BJ, Nachman RJ. Isolation, primary structure and synthesis of leucokinin-VII and VIII: the final members of the new family of cephalomyotropic peptides isolated from head extracts of Leucophaea maderae. Comp Biochem Physiol [C] 88: 31–34, 1987 [Google Scholar]
- 31. Holman GM, Nachman RJ, Coast GM. Isolation, characterization and biological activity of a diuretic myokinin neuropeptide from the housefly, Musca domestica. Peptides 20: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Iaboni A, Holman GM, Nachman RJ, Orchard I, Coast GM. Immunocytochemical localisation and biological activity of diuretic peptides in the housefly, Musca domestica. Cell Tissue Res 294: 549–560, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Krug SM, Fromm M, Gunzel D. Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys J 97: 2202–2211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masia R, Aneshansley D, Nagel W, Nachman RJ, Beyenbach KW. Voltage clamping single cells in intact Malpighian tubules of mosquitoes. Am J Physiol Renal Physiol 279: F747–F754, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Nehring RB, Richter D, Meyerhof W. Glycosylation affects agonist binding and signal transduction of the rat somatostatin receptor subtype 3. J Physiol (Paris) 94: 185–192, 2000 [DOI] [PubMed] [Google Scholar]
- 36. O'Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in Malpighian tubules of Drosophila melanogaster. J Exp Biol 199: 1163–1175, 1996 [DOI] [PubMed] [Google Scholar]
- 37. O'Donnell MJ, Spring JH. Modes of control of insect Malpighian tubules: synergism, antagonism, cooperation and autonomous regulation. J Insect Physiol 46: 107–117, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Pannabecker TL, Aneshansley DJ, Beyenbach KW. Unique electrophysiological effects of dinitrophenol in Malpighian tubules. Am J Physiol Regul Integr Comp Physiol 263: R609–R614, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol 132: 63–76, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem 275: 13727–13736, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Pietrantonio PV, Gibsona GE, Streya AA, Petzel D, Hayesa TK. Characterization of a leucokinin binding protein in Aedes aegypti (Diptera: Culicidae) Malpighian tubule. Insect Biochem Mol Biol 30: 1147–1159, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Pietrantonio PV, Jagge C, Taneja-Bageshwar S, Nachman RJ, Barhoumi R. The mosquito Aedes aegypti (L.) leucokinin receptor is a multiligand receptor for the three Aedes kinins. Insect Mol Biol 14: 55–67, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Radford JC, Davies SA, Dow JA. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 277: 38810–38817, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Radford JC, Terhzaz S, Cabrero P, Davies SA, Dow JA. Functional characterisation of the Anopheles leucokinins and their cognate G-protein coupled receptor. J Exp Biol 207: 4573–4586, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Ramsay JA. Active transport of potassium by the Malpighian tubules of insects. J Exp Biol 30: 358–369, 1953 [Google Scholar]
- 46. Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450: 383–387, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Sawyer DB, Beyenbach KW. Dibutyryl-cAMP increases basolateral sodium conductance of mosquito Malpighian tubules. Am J Physiol Regul Integr Comp Physiol 248: R339–R345, 1985 [DOI] [PubMed] [Google Scholar]
- 48. Simmons MA. Functional selectivity, ligand-directed trafficking, conformation-specific agonism: what's in a name? Mol Interv 5: 154–157, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Stout BD, Clarke WP, Berg KA. Rapid desensitization of the serotonin(2C) receptor system: effector pathway and agonist dependence. J Pharmacol Exp Ther 302: 957–962, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Taneja-Bageshwar S, Strey A, Isaac RE, Coast GM, Zubrzak P, Pietrantonio PV, Nachman RJ. Biostable agonists that match or exceed activity of native insect kinins on recombinant arthropod GPCRs. Gen Comp Endocrinol 2008 [DOI] [PubMed] [Google Scholar]
- 51. Taneja-Bageshwar S, Strey A, Zubrzak P, Pietrantonio PV, Nachman RJ. Comparative structure-activity analysis of insect kinin core analogs on recombinant kinin receptors from Southern cattle tick Boophilus microplus (Acari: Ixodidae) and mosquito Aedes aegypti (Diptera: Culicidae). Arch Insect Biochem Physiol 62: 128–140, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Taneja-Bageshwar S, Strey A, Zubrzak P, Williams H, Reyes-Rangel G, Juaristi E, Pietrantonio P, Nachman RJ. Identification of selective and nonselective, biostable β-amino acid agonists of recombinant insect kinin receptors from the southern cattle tick Boophilus microplus and mosquito Aedes aegypti. Peptides 29: 302–309, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Tansky M, Pothoulakis C, Leeman S. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc Natl Acad Sci USA 104: 10691–10696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JAT. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J Exp Biol 202: 3667–3676, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Thompson KS, Rayne RC, Gibbon CR, May ST, Patel M, Coast GM, Bacon JP. Cellular colocalization of diuretic peptides in locusts: a potent control mechanism. Peptides 16: 95–104, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Torfs P, Nieto J, Veelaert D, Boon D, van de Water G, Waelkens E, Derua R, Calderon J, de Loof A, Schoofs L. The kinin peptide family in invertebrates. Ann NY Acad Sci 897: 361–373, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Veenstra JA. Isolation and identification of three leucokinins from the mosquito Aedes aegypti. Biochem Biophys Res Commun 202: 715–719, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Veenstra JA, Pattillo JM, Petzel DH. A single cDNA encodes all three Aedes leucokinins, which stimulate both fluid secretion by the Malpighian tubules and hindgut contractions. J Biol Chem 272: 10402–10407, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Wang S, Rubenfeld A, Hayes TK, Beyenbach KW. Leucokinin increases paracellular permeability in insect Malpighian tubules. J Exp Biol 199: 2537–2542, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Weng XH, Piermarini PM, Yamahiro A, Yu MJ, Aneshansley DJ, Beyenbach KW. Gap junctions in Malpighian tubules of Aedes aegypti. J Exp Biol 211: 409–422, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Whittembury G, Paz-Aliaga A, Biondi A, Carpi-Medina P, Gonzalez E, Linares H. Pathways for volume flow and volume regulation in leaky epithelia. Pflügers Arch 405, Suppl 1: S17–S22, 1985 [DOI] [PubMed] [Google Scholar]
- 62. Williams JC, Beyenbach KW. Differential effects of secretagogues on Na and K secretion in the Malpighian tubules of Aedes aegypti (L.). J Comp Physiol 149: 511–517, 1983 [Google Scholar]
- 63. Williams JC, Beyenbach KW. Differential effects of secretagogues on the electrophysiology of the Malpighian tubules of the yellow fever mosquito. J Comp Physiol [B] 154: 301–309, 1984 [Google Scholar]
- 64. Wu DS, Beyenbach KW. The dependence of electrical transport pathways in Malpighian tubules on ATP. J Exp Biol 206: 233–243, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Yu M, Beyenbach KW. Leucokinin and the modulation of the shunt pathway in Malpighian tubules. J Insect Physiol 47: 263–276, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Yu MJ, Beyenbach KW. Effects of leucokinin-VIII on Aedes Malpighian tubule segments lacking stellate cells. J Exp Biol 207: 519–526, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Yu MJ, Beyenbach KW. Leucokinin activates Ca2+-dependent signal pathway in principal cells of Aedes aegypti Malpighian tubules. Am J Physiol Renal Physiol 283: F499–F508, 2002 [DOI] [PubMed] [Google Scholar]
- 68. Zhang R, Cai H, Fatima N, Buczko E, Dufau M. Functional glycosylation sites of the rat luteinizing hormone receptor required for ligand binding. J Biol Chem 270: 21722–21728, 1995 [DOI] [PubMed] [Google Scholar]
- 69. Zubrzak P, Williams H, Coast GM, Isaac RE, Reyes-Rangel G, Juaristi E, Zabrocki J, Nachman RJ. β-Amino acid analogs of an insect neuropeptide feature potent bioactivity and resistance to peptidase hydrolysis. Biopolymers 88: 76–82, 2007 [DOI] [PubMed] [Google Scholar]


