Abstract
The mammalian homolog of the fish calcium regulatory hormone stanniocalcin-1 (STC1) is ubiquitously expressed and likely functions in an autocrine/paracrine fashion. Mammalian STC1 does not appear to exert significant effects on serum calcium, and its physiological role remains to be determined. In macrophages, STC1 decreases intracellular calcium and cell mobility; attenuates the response to chemoattractants; and diminishes superoxide generation through induction of uncoupling protein-2 (UCP2). In cytokine-treated endothelial cells, STC1 attenuates superoxide generation and the activation of inflammatory pathways [c-Jun NH2-terminal kinase (JNK) and NF-κB]; maintains the expression of tight junction proteins, preserving the endothelial monolayer seal; and decreases transendothelial migration of leukocytes. Combined, the effects of STC1 on endothelial cells and macrophages predict potent anti-inflammatory action. Indeed, application of the anti-glomerular basement membrane (GBM) glomerulonephritis model to STC1 transgenic mice that display increased expression of STC1 transgene in endothelial cells and macrophages yields renal protection. Our data suggest that STC1 activates antioxidant pathways in endothelial cells and macrophages and displays cytoprotective and anti-inflammatory actions.
Keywords: monocytes, mitochondria, uncoupling proteins, free radicals, lipopolysaccharide
this focused review highlights newly recognized antioxidant and anti-inflammatory actions of stanniocalcin-1 (STC1) and briefly discusses the pertinent literature.
What is Stanniocalcin-1?
STC1 is an important calcium regulatory hormone in fish, in which elevation of serum calcium triggers the release of STC1 from the corpuscles of Stannius (75) (organs associated with the kidneys) to inhibit calcium influx through the gill and intestine (48, 77) and maintain stable calcium concentrations in the blood. Fish and mammals express two stanniocalcin genes, STC1 and STC2 (81). Mammalian stanniocalcin genes are similarly structured; both have four exons and display conserved exon-intron boundaries (38). The fish STC1 gene contains five exons (57); exons 3 and 4 correspond to exon 3 in mammalian STC1 and STC2, suggesting that mammalian STC1 and STC2 are derived from a common ancestral gene (53). Mammalian STC1 and STC2 contain N-glycation motifs, PKC/PKA consensus sequences, a signal peptide sequence of ∼24 amino acids, and a prosequence of ∼15 amino acids that are subsequently cleaved to yield the mature proteins; consistently, mammalian STC1 and STC2 are secreted phosphoglycoproteins (60). STC1 and STC2 have moderately conserved primary amino acid sequences, especially at their NH2-terminal domains with spatial conservation of cysteine residues, suggesting that they might have similar biological functions, although recombinant STC2 protein does not displace STC1 from its putative receptor (83).
In humans, STC1 is a protein of 247 amino acids that is 90% similar to salmon STC1 over the first 200 NH2-terminal amino acids (64). Mammalian STC1 mRNA is ubiquitously expressed, and the highest level of expression is found in the ovary, kidney, prostate, and thyroid (12, 64, 81). It was previously suggested that STC1 protein does not circulate in the blood of mammals (19) except during pregnancy and lactation (22); however, recent reports suggest that mammalian STC1 is blood borne (35, 40), likely attached to a soluble protein (40). The distributions of STC1 mRNA and protein are not always parallel. For example, in situ hybridization studies in the kidney reveal restricted expression of STC1 mRNA in the cortical and medullary collecting ducts, yet the protein is detected along the entire nephron (18, 87). Similarly, the expression of STC1 mRNA does not parallel the expression of the protein in cells of the ovaries (81) and uterus (76). The ubiquitous distribution of STC1 mRNA in mammalian organs, the wider tissue distribution of STC1 protein compared with the mRNA, and the fact that STC1 is a secreted protein suggest that STC1 functions in an autocrine/paracrine manner. The function of mammalian STC1 is yet to be defined, but the diverse schemes regulating its expression (32, 34, 41, 65, 73, 89, 90) and its dysregulation in malignancies (28, 86) suggest that STC1 plays important roles in the normal physiology of many organs.
Like STC1, mammalian STC2 is ubiquitously expressed; in humans, the primary site of STC2 production is the pancreas (60), where the protein localizes to a subpopulation of islet cells, suggesting involvement of STC2 in glucose and energy metabolism (60). Consistent with this, deletion of STC2 produces overweight mice (11). Abnormalities in the expression of STC2 have also been associated with various malignancies (5, 13).
Stanniocalcin-1 Receptors
There are results that suggest the existence of STC1-binding protein (receptor). This conclusion is based on experiments using stanniocalcin-alkaline phosphatase fusion protein, which was used in binding assays yielding results consistent with the presence of high-affinity (0.25–0.8 nM), saturable, and displaceable binding sites for STC1 in cells of the kidney, liver, breast, and ovaries (56, 58, 66, 70). Analysis of membrane fractions suggested that 90% of the binding sites are mitochondrial, while ∼10% are located at the plasma membrane (56). It was suggested that STC is produced by one cell type, secreted, and targeted to neighboring cells, where it is bound to cell surface receptors, trafficked, and sequestered in the mitochondria (56). Membrane and mitochondrial receptors have similar binding affinities, suggesting that they are identical (56). As to targeting of STC1 to the mitochondria, two models have been proposed by Sazonova et al. (70): one model envisions the membrane receptor acting first as a signal transducer and second as a chaperone to facilitate translocation of STC1; alternatively, the membrane-bound receptor may serve as a signal transducer, which then passes STC1 to a unique mitochondrial receptor for internalization. Consistent with these models, we have recently shown (85) internalization of recombinant STC1 from the medium and subsequent sequestration of the protein in the mitochondria of freshly isolated murine peritoneal macrophages within 10 min.
Phenotype of Transgenic Stanniocalcin-1 Mice
To date, two transgenic STC1-overexpressing mice have been generated (27, 80). Muscle-specific overexpression of STC1, driven by the myosin light chain-2 promoter (27), produced dwarf mice that displayed normal blood pressure and normal reproductive capacity when transgenic males were mated with wild-type (WT) females. Serum phosphate levels were normal, but serum calcium levels were elevated; the latter finding was attributed to stimulated osteoclast activity (27). Notably, compared with WT littermates, transgenic mice were hyperphagic (32% higher food intake per g body wt), consumed more oxygen (14% more per g body wt), and had leaner fat pads and faster clearance of glucose (27), yet transgenic mice displayed normal serum free fatty acids, IGF-binding protein-1, thyroxine (T4), and growth hormone (GH) (27). These mice were noted to have mitochondrial swelling (27), suggesting that STC1 affects mitochondrial function and/or structure.
In the second transgenic mouse line(s), STC1 expression is driven by the metallothionein I minimal promoter (80) and displays strong preferential expression of the transgene in the liver, heart, brain, endothelial cells, and macrophages (35, 80); serum calcium levels are normal, while serum phosphate levels are slightly higher. Here, too, transgenic mice are smaller compared with WT littermates yet display no abnormalities in serum GH and IGF-I or steady-state mRNA levels of pituitary TSH β-subunit, FSH β-subunit, LH β-subunit, or α-glycoprotein subunit (80).
In light of observations by others and data from our lab, I would like to propose a plausible explanation for the dwarf phenotype in both transgenic mice. A report by James and coworkers (25) suggested uncoupling of the mitochondria by STC1. In agreement with this observation, our data suggest that STC1 upregulates the expression of mitochondrial uncoupling protein-2 (UCP2) in macrophages (85) and UCP3 in cardiomyocytes (52a). And it is highly likely that STC1 upregulates uncoupling proteins in other tissues. This is interesting because transgenic overexpression of UCP3 in skeletal muscle produces hyperphagic mice that are smaller in size compared with WT littermates and display leaner fat pads and faster clearance of glucose (17), a phenotype similar to that of STC1 transgenic mice (27). Since both transgenic mouse lines display high serum levels of STC1 (27, 80), it is highly likely that the dwarf phenotype of these transgenic mice results from STC1-mediated upregulation of uncoupling proteins in different tissues; as a consequence, the mice are hyperphagic and consume more oxygen—to compensate for the hypermetabolic state caused by uncoupling of the mitochondria (see below).
A STC1 knockout (KO) mouse line has been generated and displays normal phenotype (10), yet this mouse line has not been challenged (e.g., applying a disease model) to determine the impact of STC1 deletion on disease states.
STC1 Suppresses Superoxide Generation in Macrophages Through Induction of Mitochondrial Uncoupling Proteins: A New Paradigm for Regulation of Macrophage Function
We have shown that STC1 inhibits macrophages through several interrelated pathways: STC1 attenuates intracellular second messenger signals through calcium and decreases macrophage mobility (42); in addition, it inhibits macrophage response to chemoattractants (42) and induces UCP2 expression [in freshly isolated mouse peritoneal macrophages and cultured murine macrophages (Raw 264.7 cells)]—decreasing mitochondrial membrane potential and superoxide generation (85). Superoxides are important for macrophage function and viability. As discussed below, UCP2 plays a critical role in the regulation of superoxide generation in macrophages; hence the recognition that STC1 decreases superoxide generation in macrophages (through induction of UCP2) places it in the forefront of immunity and cytoprotection.
As shown in Fig. 1, transfer of electrons (e−) from high-energy molecules (NADH and FADH2; products of the Krebs cycle in the mitochondrial matrix) along the respiratory chain (complexes I–IV) generates a higher H+ ion concentration in the mitochondrial intermembrane space compared with the matrix, increasing mitochondrial membrane potential; concomitantly, superoxide production (formed by electron reduction of oxygen during electron transport) is also increased. H+ ions travel back to the matrix through the ATP synthase complex, facilitated by the favorable electrochemical gradient, generating ATP in the process (39, 71, 82). Alternatively, H+ ions may leak back to the matrix through uncoupling proteins (localized at the inner mitochondrial membrane and serving as H+ channels), bypassing ATP synthase—dissipating the H+ gradient. While oxygen consumption and the utilization of NADH and FADH2 continue, ATP generation diminishes, hence the term “uncoupled phosphorylation” referring to the dissociation between oxidation (of NADH and FADH2) and phosphorylation of ADP to generate ATP; this translates into reduced efficiency of the mitochondria [less ATP generated per oxidized high-energy molecule (NADH and FADH2)] but with the added benefit of reduced superoxide generation (7, 68).
Fig. 1.
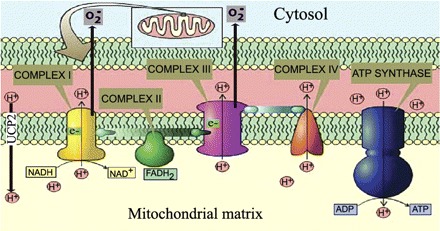
Mitochondrial uncoupling decreases ATP and superoxide generation. Transfer of electrons (e−) from high-energy molecules (NADH and FADH2) by complexes of the respiratory chain (I–IV) generates H+ ion gradient (pH difference) between the matrix and the intermembrane space, while producing superoxide (O2−). Hydrogen ion gradient is utilized by the ATP synthase to generate ATP. Alternatively, H+ ion gradient may be dissipated by uncoupling proteins such as uncoupling protein (UCP)2, bypassing ATP synthase; this diminishes ATP synthesis and superoxide generation. NAD, nicotinamide adenine dinucleotide.
The best known uncoupling protein is UCP1. It is highly abundant in the mitochondria of brown adipocytes. It uncouples phosphorylation, producing a futile cycle, which dissipates oxidative energy as heat (thermoregulation; Ref. 79). A group of UCP1 homologs have been discovered recently [UCP2 in the lymphoid system; UCP3 in the heart and skeletal muscle; UCP4 and UCP5 in the brain (7, 44)], and, like pharmacological uncouplers (45), they diminish superoxide generation (7, 44, 68). The physiological impact of mitochondrial uncoupling depends on the biological system or cell type undergoing uncoupling. For example, increased UCP2 expression in macrophages diminishes their function (see below), while increased UCP2 expression in pancreatic cells decreases insulin secretion (9). UCP2 is highly expressed in macrophages, where it plays an important role in immunity (2, 63). Data from Arsenijevic et al. (2) suggested that mice null for UCP2 are resistant to infection by Toxoplasma gondii. On exposure to Toxoplasma, macrophages from UCP2−/− mice generated 80% more reactive oxygen species (ROS) compared with macrophages from WT mice and had fivefold greater toxoplasmacidal activity in vitro. Notably, the benefits of UCP2 deletion in macrophages were abolished by a quencher of ROS (2). Similar observations were made by Bai et al. (3), demonstrating that mitochondrially generated ROS in UCP2−/− macrophages potentiate NF-κB activity, resulting in a “primed state” that amplifies subsequent inflammatory responses (3). Since activation of NF-κB in macrophages stimulates chemokines, immune receptors, adhesion molecules, and cell cycle regulators (78), one can envision that STC1-mediated induction of UCP2—with the resultant suppression of superoxide generation–is expected to diminish macrophage function (85) and have a profound impact on the immune/inflammatory response. The effect of STC1 on superoxide is independent of NADPH oxidase, as suppression of superoxide generation by STC1 is observed in gp91phox-null macrophages (85). Conventional wisdom has it that the production of physiologically relevant ROS in macrophages occurs exclusively via the NADPH oxidase system. Thus the identification of a physiologically relevant and STC1/UCP2-regulatable ROS-generating system in macrophage mitochondria introduces a new paradigm for the regulation of immunity and inflammation.
Moreover, recent data suggest that lipopolysaccharide (LPS) increases mitochondrial superoxide generation through downregulation of UCP2 (26). Since STC1 upregulates, while LPS downregulates, UCP2 in macrophages (26) we hypothesized that STC1 acts as a naturally occurring LPS antagonist. Indeed, STC1 blunts the rise in superoxide generation in LPS-treated peritoneal macrophages (85). This finding is critically important, as it suggests a role for STC1 in regulating innate immunity. Finally, superoxides and other ROS damage proteins and nucleic acids and have been implicated in aging and neurodegenerative disease (6, 59); thus STC1 may potentially delay aging-related organ dysfunction.
As mentioned above, uncoupled mitochondria are less efficient in ATP generation (7, 45, 68). Indeed, we find lower ATP levels in STC1-treated macrophages (85). The decline in cellular ATP levels can lead to cell death (69). On the other hand, the reduction in ROS generation can reduce apoptosis (46, 54). Hence, induction of UCP2 by STC1 could potentially decrease cell viability (due to the decrease in ATP) or increase it (due to suppression of superoxide generation). Of interest, we find increased cell viability in STC1-treated macrophages; however, STC1 induces cell cycle arrest at the G1 phase (85). Consistent with this, a recent report suggested that lower cellular ATP levels may promote cell survival by inducing cell cycle arrest (55). Thus, despite the reduction in ATP levels in STC1-treated macrophages, cell survival is enhanced, suggesting cytoprotection likely due to the combined reduction in ATP levels and superoxide generation. Our findings are in agreement with data from Zhang et al. (92) showing that transfection of Paju cells with STC1-expressing plasmid increased cell resistance to ischemic challenge, or elevated intracellular free calcium, induced by treatment with thapsigargin (92). Similarly, in multipotent stem cells, STC1 reduces apoptosis of lung cancer epithelial cells under conditions of low pH and hypoxia (4). In contrast, Law et al. (52)suggested that STC1 may promote apoptosis in human colon adenoma cells, while Nguyen et al. (62) suggested that STC1 reduces proliferation of mouse embryonic fibroblasts following oxidative injury, likely through suppression of MEK/ERK. The response to STC1 may be cell specific. In addition, cancer cells have escaped cell cycle regulatory signaling, and hence one should carefully interpret their response to stimuli that affect cell survival. Thus the response of colon cancer cells to STC1 does not negate a cytoprotective role for STC1 as we and others have reported (4, 92).
STC1 Opposes Action of Cytokines on Endothelium: A New Paradigm for Regulation of Transendothelial Permeability
It is well established that cytokines increase endothelial permeability, enhancing transmigration of inflammatory cells (21, 84). Attachment between neighboring cells is facilitated by tight junctions and adherence junctions (20). Tight junctions contain the transmembrane protein occludin, which is bound to zonula occludens-1 (ZO-1) (29). Adherence junctions are formed by clusters of transmembrane proteins that belong to the cadherin family—linked intracellularly to catenins, which promote anchorage to the actin cytoskeleton (30, 43). Through cytoskeletal association, the tight junctions and adherence junctions form the apical junction complex, which plays an important role in regulating leukocyte transmigration (24). Stimulation of endothelial cells by cytokines, frequently referred to as “activation,” involves the convergence of signaling mediators that include ROS, stress-activated protein kinases [c-Jun NH2-terminal kinase (JNK)], and NF-κB (16, 31, 47). This “activation” is associated with increased expression of adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and increased permeability—facilitating transendothelial migration of leukocytes (1, 67, 74).
We previously showed expression of STC1 mRNA in endothelial cells in vivo (72) and localization of the protein at the apical surface (8). In cultured endothelial cells STC1 regulates gene expression (8), and in TNF-α-treated endothelial cells STC1 stabilizes permeability through a number of interrelated mechanisms (14): 1) it attenuates the generation of ROS and the activation of signaling pathways that promote inflammation (JNK and NF-κB); 2) it opposes the effects of cytokines on the expression of tight junction proteins (ZO-1, occludin, claudin-1) (14); and 3) importantly, it inhibits cytokine-induced- but fails to affect VEGF-induced endothelial permeability (14). A proposed scheme for the action of STC1 in endothelial cells is shown in Fig. 2. Is the effect of STC1 on the endothelium relevant to endothelial biology? The answer is yes, as STC1 dose-dependently diminishes the migration of macrophages and T cells across cytokine (IL-1β)-treated endothelial monolayer (8). Translating our in vitro observations to the in vivo setting, STC1 is expected to diminish transendothelial migration of macromolecules and leukocytes in the setting of inflammation.
Fig. 2.
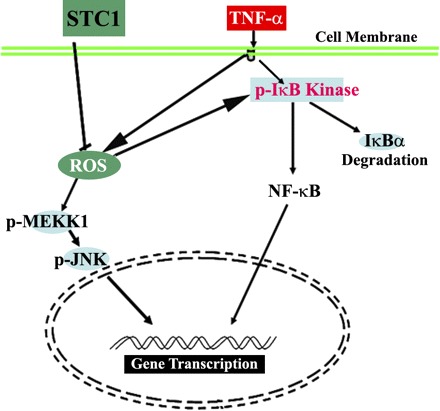
Proposed scheme for stanniocalcin-1 (STC1) action in endothelial cells. TNF-α activates NF-κB (increased IκB phosphorylation) and increases free radical production [reactive oxygen species (ROS)]. Activities of NF-κB and c-Jun NH2-terminal kinase (JNK) pathways are redox sensitive. STC1 diminishes free radical production (increasing GSH/GSSG), providing a mechanism for downregulation of NF-κB and JNK.
Transgenic Overexpression of STC1 Protects from Experimental Anti-GBM Glomerulonephritis
As discussed above, STC1 suppresses macrophage mobility and function (42), inhibits cytokine-induced rise in endothelial permeability (14), and diminishes transendothelial migration of inflammatory cells (8): through these combined and unique actions, STC1 is predicted to possess potent anti-inflammatory action. To test this hypothesis, we applied the anti-GBM disease model to STC1 transgenic mice that display preferential expression of STC1 transgene in endothelial cells and macrophages (35, 80). Experimental anti-GBM glomerulonephritis (GN) is an inflammatory disease characterized by proteinuria, macrophage and T-cell infiltration, glomerular crescent formation, and Th1 antibody and cytokine responses; it leads to progressive renal failure over days to weeks (37, 49, 50). The expression of proinflammatory cytokines (such as IL-1β) is markedly elevated in anti-GBM GN (51, 61, 88, 91); hence, increased endothelial permeability is expected. Our data show striking protection from anti-GBM-induced kidney injury in STC1 transgenic mice (35). Compared with WT mice, STC1 transgenic mice exhibited 1) diminished infiltration of inflammatory macrophages into the glomeruli; 2) marked reduction in crescent formation and sclerotic glomeruli (75% and 90%, respectively); 3) decreased interstitial fibrosis (80%); 4) preservation of kidney function and lower blood pressure; and 5) diminished C3 deposition in the glomeruli and reduced expression of macrophage inflammatory protein-2 (MIP-2) and transforming growth factor-β2 (TGF-β2) in the kidney. Moreover, compared with baseline, WT mice, but not STC1 transgenic mice, had more proteinuria and marked reduction in urine output. STC1 had no effect on the expression of cytokines characteristic of either Th1 or Th2 activation. Additionally, STC1 overexpression does not appear to affect antibody response or complement activation in the context of anti-GBM GN. Recent reports suggest that macrophages are common effectors for both CD4 and CD8 T cell-dependent injury in anti-GBM (36) and that macrophage depletion diminishes the recruitment of T cells to the kidney and provides renal protection (23, 33). Thus inhibition of macrophage infiltration into the glomeruli as we found in STC1 transgenic mice appears to be necessary and sufficient to ameliorate anti-GBM disease. Our data are consistent with these reports and support our hypothesis that through direct inhibition of macrophages and attenuation of transendothelial migration of inflammatory cells, STC1 provides potent anti-inflammatory action. Indeed, this is what we find.
Collectively, our observations identify STC1 as a key regulator of macrophage and endothelial function and may potentially lead to the development of new therapeutic targets to modulate immunity/inflammation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases O'Brien Kidney Center Grant P50-DK-64233-01, DK-062828, DK-080306, and Fellowship Training Grant DK-62703-04.
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- 1. Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 50: 197–263, 1998 [PubMed] [Google Scholar]
- 2. Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26: 435–439, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S. Persistent nuclear factor-kappa B activation in Ucp2-/- mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem 280: 19062–19069, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, Dimattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 27: 670–681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouras T, Southey MC, Chang AC, Reddel RR, Willhite D, Glynne R, Henderson MA, Armes JE, Venter DJ. Stanniocalcin 2 is an estrogen-responsive gene coexpressed with the estrogen receptor in human breast cancer. Cancer Res 62: 1289–1295, 2002 [PubMed] [Google Scholar]
- 6. Brand MD, Buckingham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA, Echtay KS. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp 203–213, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2: 85–93, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chakraborty A, Brooks H, Zhang P, Smith W, McReynolds MR, Hoying JB, Bick R, Truong L, Poindexter B, Lan H, Elbjeirami W, Sheikh-Hamad D. Stanniocalcin-1 regulates endothelial gene expression and modulates transendothelial migration of leukocytes. Am J Physiol Renal Physiol 292: F895–F904, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes 48: 1482–1486, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Chang AC, Cha J, Koentgen F, Reddel RR. The murine stanniocalcin 1 gene is not essential for growth and development. Mol Cell Biol 25: 10604–10610, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang AC, Hook J, Lemckert FA, McDonald MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW, Reddel RR. The murine stanniocalcin 2 gene is a negative regulator of post-natal growth. Endocrinology 149: 2403–2410, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Chang AC, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol 112: 241–247, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Chang AC, Jellinek DA, Reddel RR. Mammalian stanniocalcins and cancer. Endocr Relat Cancer 10: 359–373, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Chen C, Jamaluddin MS, Yan S, Sheikh-Hamad D, Yao Q. Human stanniocalcin-1 blocks TNF-alpha-induced monolayer permeability in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 28: 906–912, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen XL, Zhang Q, Zhao R, Medford RM. Superoxide, H2O2, and iron are required for TNF-alpha-induced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol 286: H1001–H1007, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, Piercy V, Carter SA, Lehner I, Smith SA, Beeley LJ, Godden RJ, Herrity N, Skehel M, Changani KK, Hockings PD, Reid DG, Squires SM, Hatcher J, Trail B, Latcham J, Rastan S, Harper AJ, Cadenas S, Buckingham JA, Brand MD, Abuin A. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 406: 415–418, 2000 [DOI] [PubMed] [Google Scholar]
- 18. De Niu P, Olsen HS, Gentz R, Wagner GF. Immunolocalization of stanniocalcin in human kidney. Mol Cell Endocrinol 137: 155–159, 1998 [DOI] [PubMed] [Google Scholar]
- 19. De Niu P, Radman DP, Jaworski EM, Deol H, Gentz R, Su J, Olsen HS, Wagner GF. Development of a human stanniocalcin radioimmunoassay: serum and tissue hormone levels and pharmacokinetics in the rat. Mol Cell Endocrinol 162: 131–144, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J 9: 910–918, 1995 [PubMed] [Google Scholar]
- 21. Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost 86: 308–315, 2001 [PubMed] [Google Scholar]
- 22. Deol HK, Varghese R, Wagner GF, DiMattia GE. Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology 141: 3412–3421, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 167: 1207–1219, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edens HA, Parkos CA. Modulation of epithelial and endothelial paracellular permeability by leukocytes. Adv Drug Deliv Rev 41: 315–328, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Ellard JP, McCudden CR, Tanega C, James KA, Ratkovic S, Staples JF, Wagner GF. The respiratory effects of stanniocalcin-1 (STC-1) on intact mitochondria and cells: STC-1 uncouples oxidative phosphorylation and its actions are modulated by nucleotide triphosphates. Mol Cell Endocrinol 264: 90–101, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J 402: 271–278, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filvaroff EH, Guillet S, Zlot C, Bao M, Ingle G, Steinmetz H, Hoeffel J, Bunting S, Ross J, Carano RA, Powell-Braxton L, Wagner GF, Eckert R, Gerritsen ME, French DM. Stanniocalcin 1 alters muscle and bone structure and function in transgenic mice. Endocrinology 143: 3681–3690, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Fujiwara Y, Sugita Y, Nakamori S, Miyamoto A, Shiozaki K, Nagano H, Sakon M, Monden M. Assessment of stanniocalcin-1 mRNA as a molecular marker for micrometastases of various human cancers. Int J Oncol 16: 799–804, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127: 1617–1626, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol 8: 307–332, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175–2183, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Groves TC, Wagner GF, DiMattia GE. cAMP signaling can antagonize potent glucocorticoid post-transcriptional inhibition of stanniocalcin gene expression. J Endocrinol 171: 499–516, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Holdsworth SR, Neale TJ, Wilson CB. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest 68: 686–698, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Honda S, Kashiwagi M, Ookata K, Tojo A, Hirose S. Regulation by 1alpha,25-dihydroxyvitamin D3 of expression of stanniocalcin messages in the rat kidney and ovary. FEBS Lett 459: 119–122, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Huang L, Garcia G, Lou Y, Zhou Q, Truong LD, DiMattia G, Lan XR, Lan HY, Wang Y, Sheikh-Hamad D. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol 174: 1368–1378, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D'Souza M, Milton G, Holdsworth SR. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol 109: 134–142, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Ishibashi K, Miyamoto K, Taketani Y, Morita K, Takeda E, Sasaki S, Imai M. Molecular cloning of a second human stanniocalcin homologue (STC2). Biochem Biophys Res Commun 250: 252–258, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Jain S, Nath S. Kinetic model of ATP synthase: pH dependence of the rate of ATP synthesis. FEBS Lett 476: 113–117, 2000 [DOI] [PubMed] [Google Scholar]
- 40. James K, Seitelbach M, McCudden CR, Wagner GF. Evidence for stanniocalcin binding activity in mammalian blood and glomerular filtrate. Kidney Int 67: 477–482, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A, Lewin DA, Gerritsen ME. Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol 156: 1887–1900, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanellis J, Bick R, Garcia G, Truong L, Tsao CC, Etemadmoghadam D, Poindexter B, Feng L, Johnson RJ, Sheikh-Hamad D. Stanniocalcin-1, an inhibitor of macrophage chemotaxis and chemokinesis. Am J Physiol Renal Physiol 286: F356–F362, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9: 317–321, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Kim-Han JS, Dugan LL. Mitochondrial uncoupling proteins in the central nervous system. Antioxid Redox Signal 7: 1173–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15–18, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Kubota Y, Takahashi S, Sato H, Suetomi K. Radiation-induced apoptosis in peritoneal resident macrophages of C3H mice: selective involvement of superoxide anion, but not other reactive oxygen species. Int J Radiat Biol 81: 459–472, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Lafeber FP, Flik G, Wendelaar Bonga SE, Perry SF. Hypocalcin from Stannius corpuscles inhibits gill calcium uptake in trout. Am J Physiol Regul Integr Comp Physiol 254: R891–R896, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int 48: 753–760, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Lan HY, Nikolic-Paterson DJ, Mu W, Atkins RC. Local macrophage proliferation in the pathogenesis of glomerular crescent formation in rat anti-glomerular basement membrane (GBM) glomerulonephritis. Clin Exp Immunol 110: 233–240, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lan HY, Nikolic-Paterson DJ, Zarama M, Vannice JL, Atkins RC. Suppression of experimental crescentic glomerulonephritis by the interleukin-1 receptor antagonist. Kidney Int 43: 479–485, 1993 [DOI] [PubMed] [Google Scholar]
- 52. Law AY, Lai KP, Lui WC, Wan HT, Wong CK. Histone deacetylase inhibitor-induced cellular apoptosis involves stanniocalcin-1 activation. Exp Cell Res 314: 2975–2984, 2008 [DOI] [PubMed] [Google Scholar]
- 52a. Liu DJ, Huang L, Wang Y, Bujak M, Frangogiannis N, Sheikh-Hamad D. Stanniocalcin-1 is a naturally occurring anti-oxidant and may function as cordioprotectant in ischemic or hypertrophic cardiomyopathy (Abstract). J Am Soc Nephrol 19: 160–161A, 2008 [Google Scholar]
- 53. Luo CW, Pisarska MD, Hsueh AJW. Identification of a stanniocalcin paralog, stanniocalcin-2, in fish and the paracrine actions of stanniocalcin-2 in the mammalian ovary. Endocrinology 146: 469–476, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol 170: 1079–1090, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell 9: 843–854, 2005 [DOI] [PubMed] [Google Scholar]
- 56. McCudden CR, James KA, Hasilo C, Wagner GF. Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 277: 45249–45258, 2002 [DOI] [PubMed] [Google Scholar]
- 57. McCudden CR, Kogon MR, DiMattia GE, Wagner GF. Novel expression of the stanniocalcin gene in fish. J Endocrinol 171: 33–44, 2001 [DOI] [PubMed] [Google Scholar]
- 58. McCudden CR, Majewski A, Chakrabarti S, Wagner GF. Co-localization of stanniocalcin-1 ligand and receptor in human breast carcinomas. Mol Cell Endocrinol 213: 167–172, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Melov S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann NY Acad Sci 908: 219–225, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Moore EE, Kuestner RE, Conklin DC, Whitmore TE, Downey W, Buddle MM, Adams RL, Bell LA, Thompson DL, Wolf A, Chen L, Stamm MR, Grant FJ, Lok S, Ren H, De Jongh KS. Stanniocalcin 2: characterization of the protein and its localization to human pancreatic alpha cells. Horm Metab Res 31: 406–414, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Neugarten J, Feith GW, Assmann KJ, Shan Z, Stanley ER, Schlondorff D. Role of macrophages and colony-stimulating factor-1 in murine antiglomerular basement membrane glomerulonephritis. J Am Soc Nephrol 5: 1903–1909, 1995 [DOI] [PubMed] [Google Scholar]
- 62. Nguyen A, Chang AC, Reddel RR. Stanniocalcin-1 acts in a negative feedback loop in the prosurvival ERK1/2 signaling pathway during oxidative stress. Oncogene 28: 1982–1992, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Nishio K, Qiao S, Yamashita H. Characterization of the differential expression of uncoupling protein 2 and ROS production in differentiated mouse macrophage-cells (Mm1) and the progenitor cells (M1). J Mol Histol 36: 35–44, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Olsen HS, Cepeda MA, Zhang QQ, Rosen CA, Vozzolo BL. Human stanniocalcin: a possible hormonal regulator of mineral metabolism. Proc Natl Acad Sci USA 93: 1792–1796, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paciga M, DiMattia GE, Wagner GF. Regulation of luteal cell big stanniocalcin production and secretion. Endocrinology 15: 4204–4212, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Paciga M, McCudden CR, Londos C, DiMattia GE, Wagner GF. Targeting of big stanniocalcin and its receptor to lipid storage droplets of ovarian steroidogenic cells. J Biol Chem 278: 49549–49554, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Pober JS, Lapierre LA, Stolpen AH, Brock TA, Springer TA, Fiers W, Bevilacqua MP, Mendrick DL, Gimbrone MA., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol 138: 3319–3324, 1987 [PubMed] [Google Scholar]
- 68. Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes 53: S130–S135, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17: 3401–3415, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Sazonova O, James KA, McCudden CR, Segal D, Talebian A, Wagner GF. Stanniocalcin-1 secretion and receptor regulation in kidney cells. Am J Physiol Renal Physiol 294: F788–F794, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Schultz BE, Chan SI. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct 30: 23–65, 2001 [DOI] [PubMed] [Google Scholar]
- 72. Sheikh-Hamad D, Bick R, Wu GY, Christensen BM, Razeghi P, Poindexter B, Taegtmeyer H, Wamsley A, Padda R, Entman M, Nielsen S, Youker K. Stanniocalcin-1 is a naturally occurring L-channel inhibitor in cardiomyocytes: relevance to human heart failure. Am J Physiol Heart Circ Physiol 285: H442–H448, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Sheikh-Hamad D, Rouse D, Yang Y. Regulation of stanniocalcin in MDCK cells by hypertonicity and extracellular calcium. Am J Physiol Renal Physiol 278: F417–F424, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol 57: 827–872, 1995 [DOI] [PubMed] [Google Scholar]
- 75. Stannius H. Ueber Nebennieren bei Knochenfischen. Arch Anat Physiol 6: 97–101, 1939 [Google Scholar]
- 76. Stasko SE, DiMattia GE, Wagner GF. Dynamic changes in stanniocalcin gene expression in the mouse uterus during early implantation. Mol Cell Endocrinol 174: 145–149, 2001 [DOI] [PubMed] [Google Scholar]
- 77. Tagaki Y, Hirano T, Yamada J. Effects of the removal of the corpuscles of Stannius on the transport of calcium across the intestine of rainbow trout. Zool Sci 2: 523–530, 1985 [Google Scholar]
- 78. Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol 38: 1647–1653, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Thompson MP, Kim D. Links between fatty acids and expression of UCP2 and UCP3 mRNAs. FEBS Lett 568: 4–9, 2004 [DOI] [PubMed] [Google Scholar]
- 80. Varghese R, Gagliardi AD, Bialek PE, Yee SP, Wagner GF, DiMattia GE. Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology 143: 868–876, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Varghese R, Wong CK, Deol H, Wagner GF, DiMattia GE. Comparative analysis of mammalian stanniocalcin genes. Endocrinology 139: 4714–4725, 1998 [DOI] [PubMed] [Google Scholar]
- 82. Wada Y, Sambongi Y, Futai M. Biological nano motor, ATP synthase FoF1: from catalysis to gammaepsilonc(10–12) subunit assembly rotation. Biochim Biophys Acta 1459: 499–505, 2000 [DOI] [PubMed] [Google Scholar]
- 83. Wagner GF, DiMattia GE. The stanniocalcin family of proteins. J Exp Zoolog A Comp Exp Biol 305: 769–780, 2006 [DOI] [PubMed] [Google Scholar]
- 84. Walsh SV, Hopkins AM, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev 41: 303–313, 2000 [DOI] [PubMed] [Google Scholar]
- 85. Wang Y, Huang L, Abdelrahim M, Cai Q, Truong A, Bick R, Poindexter B, Sheikh-Hamad D. Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol 86: 981–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Welcsh PL, Lee MK, Gonzalez-Hernandez RM, Black DJ, Mahadevappa M, Swisher EM, Warrington JA, King MC. BRCA1 transcriptionally regulates genes involved in breast tumorigenesis. Proc Natl Acad Sci USA 99: 7560–7565, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wong CK, Ho MA, Wagner GF. The co-localization of stanniocalcin protein, mRNA and kidney cell markers in the rat kidney. J Endocrinol 158: 183–189, 1998 [DOI] [PubMed] [Google Scholar]
- 88. Yang N, Nikolic-Paterson DJ, Ng YY, Mu W, Metz C, Bacher M, Meinhardt A, Bucala R, Atkins RC, Lan HY. Reversal of established rat crescentic glomerulonephritis by blockade of macrophage migration inhibitory factor (MIF): potential role of MIF in regulating glucocorticoid production. Mol Med 4: 413–424, 1998 [PMC free article] [PubMed] [Google Scholar]
- 89. Yeung HY, Chan DK, Mak NK, Wagner GF, Wong CK. Identification of signal transduction pathways that modulate dibutyryl cyclic adenosine monophosphate activation of stanniocalcin gene expression in neuroblastoma cells. Endocrinology 144: 4446–4452, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CK. Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 146: 4951–4960, 2005 [DOI] [PubMed] [Google Scholar]
- 91. Yu XQ, Fan JM, Nikolic-Paterson DJ, Yang N, Mu W, Pichler R, Johnson RJ, Atkins RC, Lan HY. IL-1 up-regulates osteopontin expression in experimental crescentic glomerulonephritis in the rat. Am J Pathol 154: 833–841, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang K, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC. Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci USA 97: 3637–3642, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]


