Abstract
The purpose of this investigation was to partially remove feedback from type III/IV skeletal muscle afferents and determine how this feedback influences the central and peripheral hemodynamic responses to passive leg movement. Heart rate (HR), stroke volume (SV), cardiac output (CO), mean arterial pressure, leg vascular conductance (LVC), and leg blood flow (LBF) were measured during 2 min of passive knee extension in eight young men before and after intrathecal fentanyl injection. Passive movement increased HR by 14 beats/min from baseline to maximal response during control (CON) (65 ± 4 to 79 ± 5 beats/min, P < 0.05), whereas HR did not significantly increase with the fentanyl block (BLK). LBF and LVC increased in both conditions; however, these increases were attenuated and delayed during BLK [%change from baseline to maximum, LBF: CON 295 ± 109 vs. BLK 210 ± 86%, (P < 0.05); LVC: CON 322 ± 40% vs. BLK 231 ± 32%, (P < 0.04)]. In CON, HR, SV, CO, and LVC increased contributing to the hyperemic response. However, under BLK conditions, statistically insignificant increases in HR and SV combined to yield a small, but significant, increase in CO and an attenuated hyperemic response. Therefore, partially blocking skeletal muscle afferent feedback blunts the central hemodynamic response due to passive limb movement, which then results in an attenuated and delayed movement-induced hyperemia. In combination, these findings provide evidence that limb movement-induced hyperemia has a significant central hemodynamic component induced by peripheral nerve activation.
Keywords: afferent nerve fiber, blood flow, hemodynamics
central and peripheral hemodynamic factors contribute to the hyperemic response at the onset of exercise. These factors include the muscle pump (24, 38) mechanically induced vasodilation (6, 19, 41), mechanical deformation of arterioles (37), flow-mediated vasodilation (22, 34), and cardioacceleration (26, 30–33, 43) due to stimulation of afferent mechanosensitive and metabosensitive fibers in the working muscles (25, 36). Isolating the relative importance of these numerous mechanisms is difficult and requires a systematic approach to understand the nature of any single mechanism.
One approach to study the movement-induced central and peripheral hemodynamic responses to the onset of exercise is to perform passive movement, thereby minimizing the metabolic contribution of voluntary exercise. Recently, our group (26, 43) and others (30, 31, 33) have highlighted the role of cardioacceleration in the hyperemic responses to passive movement. Wray et al. (43) reported that passive knee extension resulted in significant tachycardia and increased leg blood flow (LBF) during the first 5 s of movement. However, in this study, stroke volume (SV) was not measured, and cardiac output (CO) was assumed to remain constant. Despite an increase in LBF with passive movement, Gonzalez-Alonso et al. (12) reported no increase in CO, whereas Ter Woerds et al. (40) reported no increase in either CO or LBF. McDaniel et al. (26) carefully examined the temporal responses of central and peripheral factors and found that an increase in CO was indeed associated with the hyperemic response to passive movement. Critical for the interpretation of these findings is the observation that, in the absence of hyperemia, accomplished by cuff occlusion of the passively moved leg, significant increases in CO, heart rate (HR), and SV were still evident at the onset of movement (26). This finding indicates that the HR-driven increase in CO is not dependent on, but is likely a primary initiator of, movement-induced hyperemia. Feedback arising from skeletal muscle afferent fibers (type III and IV fibers) has been implicated as the mechanism responsible for the cardioacceleration at the onset of limb movement (28, 30, 33); however, direct manipulation of this neural feedback mechanism has yet to be performed. To partially block lower limb afferent nerve fiber activity and diminish the influence of the exercise pressor reflex, the opioid receptor agonist, fentanyl, was used. Fentanyl, when bound to the opioid receptor, attenuates the cortical projection of the afferent nerve fibers, most of which synapse on cells in the lumbar dorsal horn of the spinal cord (15, 29). Stimulation of the spinal opioid receptors inhibits transmission of nociceptive, mechanosensitive, and metabosensitive input from group III/IV afferents to the spinal cord (10, 16, 27, 44–46), thereby reducing the group III/IV afferent-dependent cardiovascular and ventilatory responses to exercise in animal models (14, 27, 35).
Given the circumstantial evidence supporting the role of cardioacceleration in the onset of muscle hyperemia, the purpose of this investigation was to partially block the feedback from group III and IV afferent muscle fibers utilizing the opioid receptor agonist, fentanyl, and examine the impact this intervention has on limb movement-induced hyperemia. We hypothesized that, under conditions in which the feedback from the mechanoreceptors and metaboreceptors is partially blocked, the hyperemic response to passive movement will be both reduced and delayed, implicating HR as the initiating factor in the transient increase in LBF.
METHODS
Subjects
Eight recreationally active men volunteered to participate in this research study (age 27 ± 4 yr, body mass 77 ± 13 kg, stature 1.8 ± 0.1 m). Written, informed consent was obtained from each participant, and all procedures were approved by the Institutional Review Board of both the University of Utah and the Veteran Affairs Medical Center of Salt Lake City, Utah.
Experimental Protocol
Before the experimental trials, all subjects reported to the laboratory for a familiarization trial. During this session, passive and active knee extension were performed, and Doppler ultrasound imaging of the femoral artery was performed to ensure that acceptable images could be obtained at rest and during exercise. Upon arrival at the laboratory, the right femoral artery and vein were catheterized (18-gauge central venous catheter, Arrow International, Reading, PA) using the Seldinger technique. Following a 30-min period of recovery, subjects moved to the knee-extensor ergometer and underwent a modified hypercapnic ventilatory response test (HCVR), and baseline hemodynamic measurements were made with the subject in the upright seated position, which was maintained throughout the experimental protocol. Single-leg passive exercise was achieved by moving the leg through the range of motion permitted by the knee-extensor ergometer (90 to 180° knee joint angles) at 1 Hz with the ergometer set at 15 W. Setting the workload at 15 W during passive movement allowed us to 1) directly compare the O2 cost of passive and active exercise, and 2) maintain a smooth and constant cadence during passive exercise. Real-time feedback was provided to the researcher by a digital display of cadence. Before the start and throughout the protocol, subjects were encouraged to remain passive and resist the urge to assist with leg movement. To avoid the startle reflex and active resistance to the passive movement, subjects were made aware that passive movement would take place in ∼1 min, but, to minimize the chance of an anticipatory response, they were not informed of exactly when this movement would initiate. Passive movement was performed for 2 min, followed by 3 min of active knee-extensor exercise at 15 W to determine the O2 consumption (V̇o2; pulmonary and leg V̇o2) for this workload. Following a 2-h period of recovery, the intrathecal fentanyl injection was performed. The HCVR test and baseline hemodynamic measures followed by the passive movement protocol, as described, were then repeated. Therefore, control (CON) trials were always performed prior to the fentanyl blocking (BLK) trials.
Measurements
HCVR test.
To assess the effect of intrathecal injection of fentanyl on cerebral opioid receptors, a modified HCVR test was performed, as a direct and unwanted effect of fentanyl on the cerebral opioid receptors would be evident by attenuated CO2-dependent respiratory drive [i.e., CO2 sensitivity (23)]. The HCVR test was performed using a steady-state, open-circuit technique (5). In addition to eupneic air breathing (5 min), ventilatory responses to two different hyperoxic-hypercapnic gas mixtures (1: 70% O2, 27% N2, 3% CO2, and 2: 70% O2, 24% N2, 6% CO2) were measured in each subject. The subjects breathed each gas for 4 min, and the tests were separated by at least 5 min of exposure to room-air to allow ventilatory variables to return to baseline levels. Arterial blood gases were collected during the final 30 s of each condition and analyzed for arterial partial pressure of CO2. Breathing frequency (fR) and tidal volume (Vt) were assessed and averaged over the final minute of each condition.
Femoral blood flow.
Measurement of femoral arterial blood velocity and vessel diameter were performed in the passively moved leg distal to the inguinal ligament and proximal to the deep and superficial femoral bifurcation with a Logic 7 ultrasound system (General Electric Medical Systems, Milwaukee, WI). The ultrasound system was equipped with a linear transducer operating at an imaging frequency of 10 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel. Arterial diameter was measured, and mean velocity (Vmean) (angle corrected, and intensity-weighted area under the curve) was automatically calculated (Logic 7). Using arterial diameter and Vmean, blood flow in the femoral artery was calculated as blood flow = Vmean π(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute.
Central hemodynamic variables.
HR, SV, and CO were determined with a finometer (Finapres Medical Systems, Amsterdam, the Netherlands). SV was calculated from beat-by-beat pressure waveforms assessed by photoplethysmography using the Modelflow method (Beatscope version 1.1; Finapres Medical Systems), which, in combination with HR, has been documented to accurately estimate CO during a variety of experimental protocols (4, 8, 9, 39, 42). Intravascular systolic and diastolic arterial and mean venous pressures (MVP) were determined from in-line pressure transducers (Baxter, Deerfield, IL) placed at the level of the catheters. Mean arterial pressure (MAP) was calculated as diastolic + 1/3(systolic − diastolic).
Leg and systemic O2 transport and utilization.
Throughout all studies, pulmonary V̇o2 and ventilation (V̇e) were measured using a metabolic cart (True One 2400, ParvoMedics, Salt Lake City, UT). Blood samples were obtained from femoral artery and vein catheters and measured using a combined blood-gas analyzer and co-oximeter (GEM 4000, Instrumentation Laboratories, Bedford, MA). To calculate leg V̇o2, femoral blood flow was multiplied by the difference between arterial and venous O2 content.
Intrathecal fentanyl injection.
With subjects in an upright seated position with a slight flexion of the torso, the skin and subcutaneous tissue were anesthetized at the L3-L4 vertebral interspace using 2–4 ml of 1% (10 mg/ml) lidocaine. A 25-gauge, 9-cm Pecan needle was advanced to the subarachnoid space. Free-flowing cerebrospinal fluid (CSF) confirmed subarachnoid positioning of the needle tip. A small amount of CSF was aspirated, and 1 ml of fentanyl (0.05 mg/ml) injected to block feedback arising from group III and IV afferent skeletal muscle fibers. The needle was then removed, and the subjects remained in an upright seated position throughout the remainder of the study to minimize the potential risk of cephalad movement within the CSF. The efficacy and extent of the block was tested by neurological exam (cutaneous hypoaesthesia to pinprick and cold perception on the torso, upper and lower limbs) before limb movement.
Data acquisition.
Throughout each protocol, HR, SV, CO, MAP, MVP, and ECG signals underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) using a data acquisition system, (AcqKnowledge; Biopac Systems, Goleta, CA). In addition, this data acquisition system acquired the audio antegrade and retrograde signals (10,000 Hz) from the Doppler ultrasound system that served as a qualitative indicator of blood velocity changes facilitating the temporal processing of all variables.
Data and Statistical Analysis
The data acquisition software allowed second-by-second analyses of HR, SV, CO, MAP, and MVP. All analyses were performed using a 5-s moving average. The second-by-second antegrade and retrograde velocities were analyzed by the Doppler ultrasound system (GE Logic 7) for the first 60 s of movement, and 12 s averages were employed from 60 to 120 s of movement. With the use of the antegrade and retrograde velocities and femoral artery diameters, antegrade, retrograde, and total LBF were calculated. Pulmonary V̇o2, V̇e, and blood-gas variables were obtained during baseline and the final minute of passive movement. Two-way repeated-measures ANOVA were utilized to determine significant differences between CON and BLK conditions. When a significant main effect (interaction of treatment × time) was observed, further analysis was performed to determine whether a significant change over time occurred within a treatment. When a significant change over time was observed within a treatment, the change from baseline to peak was determined. Significance was set at an α-level of 0.05, and data are presented as means ± SE.
RESULTS
HCVR Test and Cutaneous Hypoaesthesia
The ventilatory responses to CO2 at each of the three levels of inspired CO2 (room air, 0.03, and 0.06) revealed that there were no differences between CON and BLK for fR, Vt, and arterial partial pressure of CO2 (data not reported). Neurological examination just before limb movement indicated cutaneous hypoaesthesia below T6 in all subjects.
Central Responses to Passive Movement
The central responses to passive movement are presented in Fig. 1 and Table 1. At baseline, HR was not different between conditions (CON 65 ± 4 vs. BLK 62 ± 5 beats/min, P = 0.32). Passive movement resulted in a peak increase of 14 beats/min (21%) in HR above baseline during CON. In contrast, during BLK, HR varied nonmonotonically over time as a result of the passive movement.
Fig. 1.

Central hemodynamic responses to passive limb movement. Values are means ± SE for heart rate, stroke volume, cardiac output (CO), mean arterial pressure, and mean leg venous pressure. One minute of baseline data was collected before passive movement. The transition from baseline to passive movement occurred at time 0 on the x-axis. Note that the responses represented in this figure are an average response of all subjects; therefore, here the maximal peak values are underestimated. CON, control; BLK, fentanyl blocking; bpm, beats/min.
Table 1.
Summary of the resting and maximal individual responses to passive movement
| CON | BLK | |
|---|---|---|
| HR, beats/min | ||
| Baseline | 65 ± 4 | 62 ± 5 |
| Max | 79 ± 5 | 72 ± 4 |
| SV, ml/beat | ||
| Baseline | 98 ± 7 | 84 ± 7 |
| Max | 115 ± 9* | 93 ± 7 |
| CO, l/min | ||
| Baseline | 6.3 ± 0.6 | 5.1 ± 0.4† |
| Max | 8.4 ± 0.9* | 6.2 ± 0.5*† |
| MAP, mmHg | ||
| Baseline | 122 ± 3 | 116 ± 4 |
| Max | 110 ± 3* | 107 ± 4 |
| MVP, mmHg | ||
| Baseline | 20 ± 1 | 20 ± 1 |
| Max | 22 ± 2* | 22 ± 1* |
| LBF, ml/min | ||
| Baseline | 457 ± 59 | 446 ± 44 |
| Max | 1,756 ± 251* | 1,364 ± 168* |
| LVC, ml·min−1·mmHg−1 | ||
| Baseline | 3.7 ± 0.4 | 4 ± 0.7 |
| Max | 15.4 ± 2.0* | 12.8 ± 1.7* |
Values are means ± SE. CON, control; BLK, fentanyl blocking; HR, heart rate; SV, stroke volume; CO, cardiac output; MAP, mean arterial pressure; MVP, mean leg venous pressure; LBF, leg blood flow; LVC, leg vascular conductance. Note that these data represent peak values on an individual basis, and for this reason they do not match the average responses illustrated in Figs. 1 and 2.
Difference from baseline,
difference from CON: P < 0.05. As the main effect of time for HR, SV, and MAP were not significant in BLK, the significance of the change from baseline to maximum was not determined.
SV was not significantly different between trials during baseline (CON 98 ± 7 vs. BLK 84 ± 7 ml/beat, P = 0.07). Under CON conditions, SV increased by 17 ml/beat (18%); however, during BLK, SV did not increase significantly.
While neither HR nor SV exhibited significant changes during baseline or passive movement in the BLK condition, the product of these variables, the CO response, was altered by BLK. Specifically, BLK reduced baseline CO (CON 6.3 ± 0.6 vs. BLK 5.1 ± 0.4 l/min, P = 0.01) and attenuated the increase in CO due to passive movement. CO increased by 2.1 ± 0.4 l/min during CON (34 ± 5%) and by 1.1 ± 0.2 l/min during BLK (22 ± 4%; P = 0.02; see Fig. 3). CO returned toward baseline values at the end of the 2 min of passive movement, as CO was no longer greater than baseline in either CON (7.1 ± 1.0 l/min, P = 0.08) or BLK (5.5 ± 0.5 l/min, P = 0.10).
Fig. 3.
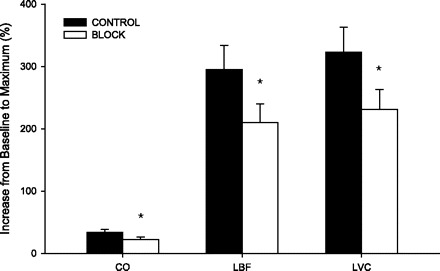
Percent increase from baseline to maximum for CO, LBF, and LVC. Values are means ± SE. *Different from CON: P < 0.05.
MAP was not altered by BLK during baseline (CON 122 ± 3 vs. BLK 116 ± 4 mmHg, P = 0.09; Fig. 1 and Table 2). In CON, MAP exhibited a progressive reduction during the first 20 s of passive movement and reached a nadir of 110 ± 3 mmHg (P < 0.01). In contrast, during BLK, MAP fluctuated inconsistently around the baseline value (P = 0.89). MVP was not different between conditions at baseline (CON 20 ± 1 vs. BLK 20 ± 1 mmHg, P = 0.25) and increased gradually during the first 35 s of passive movement and was then maintained at this elevated level (CON 22 ± 2 and BLK 22 ± 1 mmHg, both P < 0.05) throughout the remaining portion of the movement in both CON and BLK.
Table 2.
Systemic and leg oxygen transport and utilization
| CON |
BLK |
|||
|---|---|---|---|---|
| Baseline | Passive | Baseline | Passive | |
| V̇e, l/min | 8.1 ± 0.5 | 10.4 ± 0.3* | 8.4 ± 0.4 | 9.8 ± 0.3 |
| fR, breaths/min | 13.6 ± 1.3 | 16.8 ± 1.1 | 14.6 ± 1.2 | 16.8 ± 1.0 |
| Vt, liter | 0.63 ± 0.05 | 0.65 ± 0.05 | 0.60 ± 0.31 | 0.59 ± 0.15 |
| Pulmonary V̇o2, l/min | 0.26 ± 0.02 | 0.30 ± 0.01* | 0.26 ± 0.01 | 0.30 ± 0.01* |
| Leg V̇o2, l/min | 0.03 ± 0.01 | 0.05 ± 0.01* | 0.03 ± 0.00 | 0.05 ± 0.01* |
| Leg (a-v)Do2, ml/dl | 7.2 ± 1.0 | 5.9 ± 0.9 | 6.7 ± 0.4 | 7.4 ± 1.0 |
Values are means ± SE. V̇e, ventilation; fR, breathing frequency; Vt, tidal volume, V̇o2, O2 consumption; (a-v)Do2, arterial-to-venous O2 difference. It should be noted that these values were obtained during the final minute of the passive movement and do not correspond to the transient increases that occurred during the first minute of passive movement.
Significant difference from baseline, P < 0.05.
Pulmonary V̇o2 and V̇e are presented in Table 2. Passive limb movement resulted in a significant increase in V̇e during CON and, although not achieving statistical significance, tended to increase V̇e during BLK (P = 0.06). Pulmonary V̇o2 increased by 0.04 ± 0.01 l/min during both CON and BLK (P < 0.03). Passive movement did not result in a change in fR or Vt in either CON or BLK (Table 2).
Peripheral Responses to Passive Movement
The peripheral responses to passive movement are presented in Fig. 2 and Table 1. Retrograde LBF at baseline was reduced by BLK (CON 135 ± 18 vs. BLK 97 ± 16 ml/min, P = 0.004). During CON, retrograde LBF fell below baseline values during the first 20 s of movement, increased from 20 to 40 s, and reached 260 ± 6 ml/min at the end of passive movement (Fig. 2). In contrast, during the BLK trial, retrograde LBF increased gradually over time and reached 199 ± 35 ml/min at the end of passive knee extension. Despite these significantly different patterns of change in retrograde LBF, the overall impact of retrograde LBF to total LBF in the upright seated position was minimal, as the predominant contributor to LBF was an increase in antegrade LBF (Fig. 2). LBF before passive movement was not different between conditions (CON 457 ± 59 vs. BLK 446 ± 44 ml/min, P = 0.79). Under CON conditions, LBF increased to a peak of 1,299 ± 616 ml/min (P < 0.01) above baseline values. Although the initial increase in LBF was transient, the clear peak in LBF was followed by a steady fall, yet remained elevated by 350 ± 233 ml/min (P < 0.01) above baseline at the end of the 2 min of limb movement. During BLK, LBF increased to a peak of 919 ± 407 ml/min (P < 0.01) above baseline and then decayed to a similar level as CON during the second minute of movement (302 ± 221 ml/min above baseline, P < 0.01). The percent increase in peak LBF above baseline was reduced during BLK by ∼30% (CON 295 ± 109 vs. BLK 210 ± 86%, P = 0.05, Fig. 3).
Fig. 2.
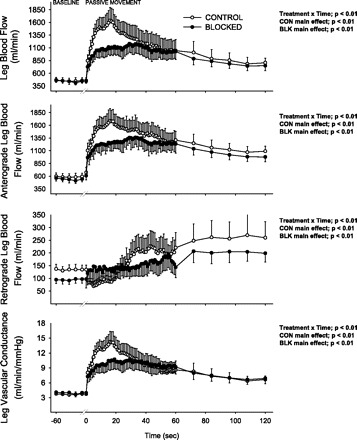
Peripheral hemodynamic responses to passive limb movement. Values are means ± SE for leg blood flow (LBF), antegrade LBF, retrograde LBF, and leg vascular conductance (LVC). One minute of baseline data was collected before passive movement. The transition from baseline to passive movement occurred at time 0 on the x-axis. Note that the responses represented in this figure are an average response of all subjects; therefore, here the maximal peak values are underestimated.
Leg vascular conductance (LVC) was unaffected by BLK at baseline (CON 3.7 ± 0.4 vs. BLK 4.0 ± 0.7 ml·min−1·mmHg−1, P = 0.68). During passive movement in CON, LVC increased by 322 ± 40% above baseline and peaked at 15.4 ± 2.0 ml·min−1·mmHg−1 (P < 0.01). LVC remained elevated above baseline at the end of the limb movement period. During passive movement in BLK, the increase in LVC was significant compared with baseline (12.8 ± 1.7 ml·min−1·mmHg−1, P < 0.01), yet less than the increase during CON (231 ± 32%, P = 0.04). Similar to CON, LVC during BLK remained elevated compared with baseline throughout the second minute of passive limb movement.
Leg V̇o2 increased by 0.02 ± 0.01 l/min in both CON and BLK (P ≤ 0.03) (Table 2). Leg arterial-to-venous O2 difference from baseline to movement did not change in either the CON or BLK conditions.
Timing of Central and Peripheral Responses
Of the variables measured, only CO, LBF, and LVC revealed significant changes in response to limb movement for both CON and BLK conditions, with the increases being attenuated during BLK (Fig. 3). During passive movement, the time to peak CO between conditions was not different (CON 40 ± 7 vs. BLK 37 ± 6 s, P = 0.73), while the time at which peak LBF (CON 14 ± 9 vs. BLK 33 ± 15 s, P < 0.01) and LVC (CON 14 ± 2 vs. BLK 28 ± 6 s, P < 0.04) occurred were delayed during BLK.
DISCUSSION
In the present study, we sought to remove the immediate increase in HR associated with the onset of passive limb movement to determine the role of cardioacceleration in the hyperemic response to exercise. To this end, an opioid receptor agonist (fentanyl) was used to block the cortical projection of opioid-mediated muscle afferents from the lower limb. The first major finding of this study was that partially blocking the afferent feedback significantly altered both the central and peripheral responses to passive limb movement, as evidenced by the blunted changes in HR, SV, CO, MAP, LBF, and LVC. This approach revealed that 1) intact, afferent feedback from skeletal muscle is obligatory for the normal hyperemic response to limb movement; and 2) cardioacceleration is a primary contributor to this hyperemic response and sets in motion a cascade of peripheral hemodynamic events, which are attenuated in the BLK state. Specifically, the attenuation of the HR and SV response not only decreased both peak LBF and LVC, but these responses occurred later during passive limb movement. These findings provide evidence that limb movement-induced hyperemia has a significant component that originates with the activation of afferent fibers and possesses a central hemodynamic component.
Contribution of CO and Analytic Approach to the Hyperemic Response
Passive exercise serves as an experimental model for studying the mechanisms governing exercise hyperemia in the absence of elevated skeletal muscle metabolism associated with voluntary exercise. Using this experimental approach, previous investigations have implicated cardioacceleration (30, 31) and CO in the hyperemic response to passive limb movement (26, 30, 31, 33, 43). By pharmacologically altering the HR response to passive movement, the present study supports these previous findings and provides further evidence that the immediate increase in HR is obligatory for the initial magnitude and timing of the hyperemic response to limb movement. While feedback from mechanoreceptors has been documented to result in cardioacceleration, it is important not to overlook the role of SV on the central hemodynamic response, which likely contributes to the CO and hyperemic response. During CON, the increase in LBF was greater, despite the same movement being performed between the CON and BLK conditions. Given this increase in LBF, it is reasonable to assume that venous return to the heart was increased, leading to the elevated SV. Likewise, during CON, the transient decline in MAP likely reduced ventricular afterload, concomitantly contributing to the increase in SV.
Similar to previous work from our group (26), high temporal resolution of the central and peripheral hemodynamic responses was achieved by acquiring second-by-second data during the first 60 s of passive movement. A different outcome would have been observed, if the first minute of data had not been collected, or if only steady-state values were reported (31), as many of the variables (HR, CO, SV, and MAP) returned toward baseline during the 2 min of passive movement. Other variables, such as LBF and LVC, although slightly elevated above baseline, were not different between the two conditions during the second minute of movement. It is our contention that studies that failed to document a significant increase in LBF or contribution of CO to the hyperemic response likely did not have adequate time resolution for the measurements of LBF and CO, as the first measurements were made several minutes after the onset of passive movement (12, 31, 40).
O2 Transport and Utilization During Passive Movement
During passive movement, pulmonary and leg V̇o2 increased. However, the documented “work” performed by the subject was small. During the passive movement protocol, the work rate on the ergometer was set at 15 W, meaning that the researcher responsible for moving the leg was working at this work rate. Following the passive movement protocol, the subject transitioned to a 3-min period of knee-extensor exercise at this 15-W work rate. This allowed us to directly determine the V̇o2 (both pulmonary and leg) required to perform the knee-extensor exercise at 15 W and compare this to the small increase in pulmonary and leg V̇o2 recorded during the passive movement. Under both CON and BLK conditions, the increase in pulmonary V̇o2 due to passive movement was only 0.04 l/min compared with 0.49 l/min when knee extension was actively performed at 15 W. Similarly, leg V̇o2 increased by only 0.02 l/min during passive limb movement compared with 0.32 l/min from baseline to active exercise at 15 W. Therefore, the increases in pulmonary V̇o2 and leg V̇o2 were only 8 and 6%, respectively, of the total increase compared with active exercise. Previous work using a similar passive exercise protocol reported nearly identical and apparently unavoidable increases in both pulmonary and leg V̇o2 (12, 13). Thus, while passive movement appears to result in a minor increase in metabolism, the contribution of work performed by the subject was <6% of the actual V̇o2 required to perform active knee extension at 15 W. The increase in pulmonary and leg V̇o2 may be due to joint and limb stabilization during passive movement.
Partitioning the Central and Peripheral Hemodynamic Contributors to Hyperemia
During CON conditions, group III/IV muscle mechanoreceptors and metaboreceptors were intact and able to feedback to the cardiovascular control center to increase HR and CO, which then contributed to the increase in LBF. Alternatively, the immediate drop in MAP may have stimulated the baroreceptor reflex to increase HR, which would then result in the central hemodynamic and hyperemic response. Indeed, direct stimulation of group III mechanoreceptors has been demonstrated to induce a depressor response (7). Concomitant with the initial increase in HR, limb movement resulted in vessel deformation, possibly leading to increased LBF flow via mechanically induced vasodilation (6, 19), likely indicative of a peripherally derived mechanism for the increase in LBF. Further vasodilation, evidenced by the initial reduction in retrograde LBF and increases in LVC, may have occurred as a result of flow-mediated dilation within the passively moved limb, which then resulted in a transient reduction in MAP. As the passive movement continued, MAP returned toward baseline values, as did HR and CO. Coinciding with the return of MAP, HR, and CO to baseline, input from the afferent fibers likely accommodated to the limb movement, thereby relaying less sensory information to the cardiovascular control center. An inability of mechanoreceptor feedback to sustain the central hemodynamic response to passive exercise has been reported (30, 31).
In contrast, under BLK conditions, limb movement again resulted in vessel deformation and a subsequent increase in LBF; however, feedback from the group III and IV afferents was partially blocked, and the ability of the mechanoreceptors (primarily group III fibers) to increase HR was blunted. Additionally the potential role of the baroreceptor reflex in the BLK condition was diminished as the reduction in MAP was no longer significant. The effectiveness of the BLK to alter the HR response to passive movement was apparent as the transient increase in HR under CON conditions was absent in the BLK state (Fig. 1). As the afferent block was only partial (i.e., not all feedback from group III and IV fibers was blocked), minor and nonsignificant fluctuations in HR and SV resulted in a significant, yet attenuated, increase in CO. As a consequence of these differences from the CON condition, the increase in LBF during BLK was attenuated, occurring without a concomitant reduction in MAP and coupled with an attenuated increase in LVC compared with CON. Furthermore, the times at which peak LBF and LVC occurred were delayed during BLK, indicating that the time course of peripheral responses to passive limb movement was also affected by diminished feedback from the moving limb. After the first minute of passive limb movement, the peripheral hyperemic response was similar between conditions, likely due to accommodation of afferent fibers during the CON condition.
During the transient period of elevated femoral blood flow in the CON condition, there was a substantial 11-mmHg decrease in MAP (Fig. 1), which may be attributable to acute vasodilation in the passively moved leg, which outstrips the drive to raise pressure by increasing CO. With all other variables held constant, a decrease in pressure should act to reduce blood flow, but the concomitant influence of an increase in CO and the local increase in LVC as a consequence of mechanically induced vessel dilation (6, 19), and/or flow-mediated dilation resulting from increased shear stress (20–22, 34), more than offset the decreased MAP, resulting in elevated LBF and LVC in the passively moved leg. Under BLK conditions, the increase in CO and concomitant increase in LBF was reduced compared with CON, and the hyperemic response occurred without a significant reduction in MAP and an attenuated increase in LVC.
Afferent Skeletal Muscle Feedback and the Hemodynamic Responses
To our knowledge, this is the only study to block afferent feedback during passive movement in humans and subsequently observe a significant alteration in both the central and peripheral hemodynamics. Previous studies have demonstrated that group III afferent fibers respond to tendon stretch (11, 17, 18), whereas group IV fibers are primarily thought to be nociceptors and metaboreceptors (1, 28). Previous work blocking (25) or stimulating (36) the group III and IV fibers in cats revealed a crucial role of these fibers in regards to the cardiovascular and/or respiratory response to exercise. Work in humans during passive cycling has determined that peripheral afferent reflexes from exercising muscles are likely involved in cardioacceleration at the onset of passive movement (30, 31, 33); however, these studies did not pharmacologically inhibit the afferent fibers.
Given that opioid receptors are present in the cardiovascular control center in the brain stem, direct activation of these receptors may result in modified cardiovascular control, independent of the reflex originating in the skeletal muscle afferents during passive movement. Localization of the opioid receptor block to muscle afferent fibers in the present study was confirmed via CO2 sensitivity testing (modified HCVR test) (3), which was not altered by fentanyl. Furthermore, neurological examination indicated cutaneous hypoaesthesia below T6 in all subjects, indicating that the fentanyl did not migrate cephalad from the injection site. Recently, Amann et al. (2) used the same fentanyl approach to partially block group III/IV afferent nerve fibers from the lower limbs and reported an attenuation in the ventilatory, HR, and pressor responses during leg cycling exercise. These findings indicated that the block was effective at the expected location (i.e., lower limbs) and that group III/IV muscle afferents are required for the normal exercise response. No change in the cardioventilatory response was observed during arm cycling exercise, confirming that the blockade was effective and specific to the lower limbs. Based on these recent findings and the present results, we are confident that the fentanyl administered in this study remained low in the spinal cord and acted by partially blocking group III and IV skeletal muscle afferent fibers and not via opioid receptors in the cardiovascular control center in the brain stem.
In the present study, baseline CO was slightly reduced following fentanyl injection. The cause of this reduction in baseline CO is not known; however, as described, it is unlikely that fentanyl acted on the opioid receptors in the brain stem, as the CO2 sensitivity and aesthesia sensory tests ruled out this possibility. This baseline offset in CO is not likely to be the driving factor for the attenuated central and peripheral hemodynamic responses to passive movement in the BLK condition, as no other variables were altered at baseline. Furthermore, the magnitude of change in HR, SV, LBF, MAP, and LVC due to passive movement was greater in CON than BLK, supportive of the benign baseline reduction in CO.
Conclusion
In summary, this study has identified a critical role for skeletal muscle afferent feedback in the central and peripheral hemodynamic responses to passive limb movement. Under CON conditions, when afferent feedback was intact, passive movement resulted in cardioacceleration, characterized by a significant transient increase in HR. In addition to the initial rise in HR, SV, CO, and LVC were all increased in response to passive limb movement. Partially blocking limb afferent feedback via an opioid receptor agonist attenuated the central hemodynamic contribution to limb movement by blunting the initial HR response. While CO, LBF, and LVC increased during limb movement in the BLK state, the magnitude of increase of each of these variables was reduced compared with CON. It is concluded that skeletal muscle afferent fiber activation leading to cardioacceleration is a key factor that sets in motion a series of central hemodynamic and peripheral responses ultimately contributing to the limb hyperemia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (First published July 15, 2010). doi:10.1152/japplphysiol.00462.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci (Lond) 106: 365–369, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Berkenbosch A, Ward DS, Olievier CN, DeGoede J, VanHartevelt J. Dynamics of ventilatory response to step changes in Pco2 of blood perfusing the brain stem. J Appl Physiol 66: 2168–2173, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coote JH, Perez-Gonzalez JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol 208: 261–278, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Vaal JB, de Wilde RBP, van den Berg PCM, Schreuder JJ, Jansen JRC. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth 95: 326–331, 2005 [DOI] [PubMed] [Google Scholar]
- 9. de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia 64: 762–769, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature 284: 351–353, 1980 [DOI] [PubMed] [Google Scholar]
- 11. Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540: 1095–1102, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68: 2466–2472, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Kalia M, Mei S, Kao F. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary response to static exercise. Circ Res 48: I48–I62, 1981 [PubMed] [Google Scholar]
- 16. Kalliomaki J, Luo XL, Yu YB, Schouenborg J. Intrathecally applied morphine inhibits nociceptive C fiber input to the primary somatosensory cortex (SI) of the rat. Pain 77: 323–329, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koller A, Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol Heart Circ Physiol 258: H916–H920, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Koller A, Kaley G. Flow velocity-dependent regulation of microvascular resistance in vivo. Microcirc Endothelium Lymphatics 5: 519–529, 1989 [PubMed] [Google Scholar]
- 22. Kooijman M, Thijssen DHJ, de Groot PCE, Bleeker MWP, van Kuppevelt HJM, Green DJ, Rongen GA, Smits P, Hopman MTE. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol 253: H993–H1004, 1987 [DOI] [PubMed] [Google Scholar]
- 25. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 108: 76–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meintjes AF, Nobrega ACL, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex: effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res 77: 326–334, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain 54: 241–289, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Mense S, Craig AD. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience 26: 1023–1035, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25: 37–41, 1993 [PubMed] [Google Scholar]
- 31. Nobrega AC, Williamson JW, Friedman DB, Araujo CG, Mitchell JH. Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc 26: 709–714, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Nobrega AC, Williamson JW, Mitchell JH. Left ventricular volumes and hemodynamic responses at onset of dynamic exercise with reduced venous return. J Appl Physiol 79: 1405–1410, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Nurhayati Y, Boutcher SH. Cardiovascular response to passive cycle exercise. Med Sci Sports Exerc 30: 234–238, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Pomeroy G, Ardell JL, Wurster RD. Spinal opiate modulation of cardiovascular reflexes in the exercising dog. Brain Res 381: 385–389, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Sato A, Sato Y, Schmidt RF. Heart rate changes reflecting modifications of efferent cardiac sympathetic outflow by cutaneous and muscle afferent volleys. J Auton Nerv Syst 4: 231–247, 1981 [DOI] [PubMed] [Google Scholar]
- 37. Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol 265: H1227–H1234, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Ter Woerds W, De Groot PCE, van Kuppevelt DHJM, Hopman MTE. Passive leg movements and passive cycling do not alter arterial leg blood flow in subjects with spinal cord injury. Phys Ther 86: 636–645, 2006 [PubMed] [Google Scholar]
- 41. Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004 [DOI] [PubMed] [Google Scholar]
- 42. van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol 90: 131–137, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yaksh TL. Spinal opiate analgesia: characteristics and principles of action. Pain 11: 293–346, 1981 [DOI] [PubMed] [Google Scholar]
- 45. Yaksh TL, Noueihed R. The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol 25: 433–462, 1985 [DOI] [PubMed] [Google Scholar]
- 46. Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther 202: 411–428, 1977 [PubMed] [Google Scholar]


