Abstract
Reperfusion injury to tissue following an ischemic event occurs as a consequence of an acute inflammatory response that can cause significant morbidity and mortality. Components of both the innate (complement, immunoglobulin, monocytes, and neutrophils) and adaptive (B and T lymphocytes) immune systems have been demonstrated to mediate tissue injury. Spleen tyrosine kinase (Syk) is responsible for membrane-mediated signaling in various cell types including B lymphocytes, macrophages, and T cells. We investigated the ability of a small drug Syk inhibitor, R788, to protect mice against mesenteric ischemia-reperfusion (I/R)-induced local (intestine) and remote lung injury. Mice were fed with chow containing a Syk inhibitor for 6 days before the performance of intestinal I/R, which resulted in silencing of the expression of the active phosphorylated Syk. Syk inhibition significantly suppressed both local and remote lung injury. The beneficial effect was associated with reduced IgM and complement 3 deposition in the tissues and significant reduction of polymorphonuclear cell infiltration. Our data place Syk upstream of events leading to the binding of natural antibodies to the ischemia-conditioned tissues and urge the consideration of the use of Syk inhibitors in the prevention or improvement of tissue injury of organs exposed to ischemia or hypoperfusion.
Keywords: Syk, Syk inhibition, R788
ischemia-reperfusion (I/R) injury and its sequelae represent a leading cause of morbidity and mortality and are a major clinical problem. Reperfusion of organs subjected to ischemia or hypoperfusion of tissues elicits an inflammatory response that results in organ damage and malfunction (12). During I/R injury, various mediators are produced in ischemic areas that enter the circulation and result in remote organ damage. Different organs can be affected, including liver, kidney, and lung (14, 33).
I/R injury occurs in various clinical conditions including shock, organ transplantation, reestablishing coronary artery flow after myocardial infarction, and systemic autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (16). Oxidative stress and excessive complement activation occur during ischemia and subsequent reperfusion-induced intestinal mucosal injury (12, 33). Reactive oxygen species are generated after reperfusion, and they stimulate the release of cytokines and the expression of adhesion molecules on damaged cells in the reperfused tissues (12, 23). The inflammatory reaction triggered by mesenteric ischemia is characterized by neutrophil infiltration, activation, and local mucosal injury, in which complement activation plays a major role (1). Various molecules and cells have been implicated in I/R injury, and these include components of the innate immune system, such as complement, natural antibodies, and neutrophils, and also various chemokines and cytokines (16). Recent studies have indicated that the adaptive immune response, namely T cells and B cells, also participates in I/R injury (7, 13).
Tyrosine phosphorylation is an important process in initiating signals from antigen receptors, integrins, and cytokine receptors in cells of the immune system (18). The activity of tyrosine kinases is central to many cellular processes, and their role in inflammation is important (24). The nonreceptor spleen tyrosine kinase (Syk) is involved in signal transduction in a variety of cell types. It is a key mediator of immune receptor signaling in host inflammatory cells (B cells, mast cells, macrophages, and neutrophils) (25, 28). Syk-mediated phosphorylation of several adaptor proteins and the formation of signaling complex at the cell surface membrane leads to activation of downstream pathways followed by a functional response (4, 29). Syk phosphorylation plays a central role in the activation of immune cells and thereby plays a critical role in the pathogenesis of different autoimmune diseases (22). Syk also plays an important role in complement-mediated phagocytosis (27, 29).
Given the importance of Syk in immune cell signaling, a number of small drug inhibitors have been developed in an effort to suppress the autoinflammatory response in diseases. The Syk inhibitor R406 potently inhibits IgE- and IgG-mediated activation of Fc receptor signaling and reduces immune complex-mediated inflammation (5). The prodrug of R406, R788, claims the same effects (6) and has been considered in the treatment of allergic and autoimmune diseases including arthritis and SLE (3, 11, 17, 34). In a phase II clinical trial in patients with RA, Syk proved its clinical promise with acceptable side effects and significant clinical improvement (30). These results suggest that R406 has potent anti-inflammatory activity and therefore may be of clinical value in the prevention and treatment of mesenteric I/R-induced tissue damage.
In this communication we demonstrate that inhibition of Syk activation limits local and remote organ damage in mice subjected to intestinal I/R. Prevention of tissue injury is associated with limited complement deposition and infiltration of tissues by polymorphonuclear cells. We propose that Syk inhibitors undergo consideration for the treatment or prevention of injury in conditions associated with reperfusion of ischemic tissues.
MATERIALS AND METHODS
Mice.
Adult, 8- to 12-wk-old C57BL/6J mice were obtained from Jackson Laboratory (Boston, MA). Mice underwent at least a 7-day acclimatization before experimentation. All mice used in this study were maintained in specific pathogen-free conditions in the animal research facility at the Beth Israel Deaconess Medical Center (BIDMC).
Administration of Syk inhibitor R788 to mice.
Syk inhibitor R788 was provided by Rigel Pharmaceuticals. Mice were fed food containing R788 [3 g/kg chow (n = 6) or 5 g/kg chow (n = 3)], control chow (n = 6), or regular mouse chow (n = 6). Food was prepared by Research Diets (New Brunswick, NJ). C57BL/6J mice were fed the R788-containing chow ad libitum for 6 days before experimentation in order to determine the effect of R788 on I/R injury.
I/R protocol.
Mice were randomly assigned to the following experimental groups: 1) Mouse chow (MC) + sham procedures, 2) MC + I/R procedures, 3) Control chow (CC) + sham, 4) CC + I/R, 5) R788 (3 g/kg) + sham, 6) R788 (3 g/kg) + I/R, and 7) R788 (5 g/kg) + I/R (n = 3 mice/group).
Mice were anesthetized with 10 mg/kg ketamine, 20 mg/kg xylazine, and 3 mg/kg acepromazine given intraperitoneally. In addition, 5 mg/kg ketamine and 3 mg/kg xylazine were given intramuscularly during the experiment when necessary. All procedures were performed on anesthetized, spontaneously breathing animals with body temperature maintained at 37°C with a controlled heating pad. All experiments were performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) of the BIDMC.
Animals underwent I/R as described previously (8, 15). Briefly, we performed a midline laparotomy before a 30-min equilibration period. The superior mesenteric artery was identified and isolated, and then a small nontraumatic microvascular clip delivering ∼85 g of pressure was applied for 30 min. The clip was removed after this ischemic phase, and the intestines were allowed to reperfuse for 3 h. Sham-operated mice underwent the above-described surgical intervention without artery occlusion. The laparotomy incisions were sutured with 4-0 SofSilk, and the mice were resuscitated with 1.0 ml of prewarmed sterile PBS subcutaneously and monitored during the reperfusion period.
At the conclusion of the reperfusion period, mice were euthanized by carbon dioxide asphyxiation, following the IACUC Guidelines of the BIDMC. The small intestine was isolated, and a 20-cm section distal to the gastroduodenal junction was removed and flushed with ice-cold PBS, followed by ice-cold 10% phosphate-buffered formalin before overnight fixation in 10% phosphate-buffered formalin. Lung removal consisted of intact extraction of the bronchial tree after expansion with 200–300 μl of 10% phosphate-buffered formalin and fixing overnight in 10% phosphate-buffered formalin. Formalin-fixed intestine and lung tissues were extensively washed in PBS, processed, and embedded in paraffin for histological, immunohistochemical (IHC), and immunofluorescence (IF) analysis described below.
Injury score.
For histological analysis, 20-cm segments of small intestine specimens were fixed in 10% phosphate-buffered formalin immediately after euthanasia. In the next step, tissues were embedded in paraffin, sectioned transversely in 5-μm sections, and stained with hematoxylin and eosin. For each section, we graded 50 villi on a six-tiered scale as described previously (8). To summarize, a normal villus was assigned a score of 0; villi with tip distortion were scored as 1; villi without goblet cells and with Guggenheims' spaces were scored as 2; villi with patchy disruption of the epithelial cells were scored as 3; villi with exposed but intact lamina propria and sloughing of epithelial cells were given a score of 4; villi with exuding lamina propria were assigned a score of 5; and villi with hemorrhage or denudation were scored as 6.
Alveolar and periluminal injury scores in each lung section were calculated based on the method of Cooke et al. (10). For each lung section, we examined 10–20 fields at high-power field magnification (×400) and scored for alveolar infiltration on a three-tiered scale. The calculation of alveolar scores was as follows: when no infiltrate was present, a score of 0 was given; when the infiltrate could be visualized easily only at ×400, the score was 1; when infiltrates were readily visible, a score of 2 was assigned; and the score for consolidation was 3. Similarly, each section was scored for periluminal damage (airway or blood vessel) at ×100. The calculation for periluminal scores was as follows: when there was no infiltrate, a score of 0 was assigned; when the infiltrate was between 1 and 3 cell layers thick, the score was 1; for infiltrates ranging from 4 to 10 cell layers thick, a score of 2 was assigned; and infiltrates >10 cell layers thick were scored as 3. On the basis of the overall involvement of the section, a severity score was calculated: the severity score for 0–25% involvement was 1; a severity score of 2 was assigned for 25–50% involvement; and the severity score for >50% involvement was 3. For calculation of the total lung injury score, the means of alveolar and periluminal scores for each section were summed up and multiplied by the severity score, which gave a final score ranging from 0 to 18.
Immunohistochemistry and immunofluorescence staining.
To perform IHC staining, formalin-fixed paraffin sections of intestine and lung were subjected to rehydration and antigen retrieval by a standard protocol. For IHC studies, the following reagents were used: rabbit anti-mouse Syk (N-19; Santa Cruz), rabbit anti-mouse phosphorylated (p-)Syk (phospho Y323; Abcam), and rabbit anti-mouse complement 3 (C3) (B-9; Santa Cruz, CA). The RB6–8C5 antibody was used to detect granulocyte receptor 1 (GR-1), which is a member of the Ly6 gene family, Ly6G and Ly6C (Santa Cruz).
For IF staining, formalin-fixed paraffin-embedded sections were blocked with 10% BSA-PBS at room temperature for 1 h and then incubated with FITC-labeled primary or secondary antibodies at room temperature for 1 h. The slides were washed, and coverslips were mounted with Dako Fluorescent mounting medium and analyzed by confocal microscopy (Nikon Eclipse Ti, Nikon Instruments, Melville, NY). FITC-labeled goat anti-mouse IgG (Santa Cruz, sc2010), FITC-labeled rat anti-mouse C3 (11H9; Santa Cruz, sc-58926), FITC-labeled goat anti-mouse IgM (Southern Biotech, 1021-02), and FITC-labeled rat anti-mouse Ly6G and Ly6C (clone R6B-8C5; BD Pharmingen) were the antibodies used.
Statistical analysis.
Data are presented as means ± SE. The comparison of data was performed by one-way ANOVA with post hoc analysis by Tukey test. When P < 0.05, the differences were considered significant.
RESULTS
Syk inhibitor limits intestinal I/R injury.
To determine the effect of Syk inhibition on mesenteric I/R injury, we evaluated intestinal tissues. I/R groups had significantly more intestinal mucosal damage compared with sham-operated mouse groups. After 3 h of reperfusion, the I/R groups exhibited significant mucosal injury (Fig. 1). The intestinal injury scores in the MC-I/R (2.7 ± 0.53) and CC-I/R (2.43 ± 0.34) groups were significantly higher than those in MC-Sham (0.44 ± 0.06), CC-Sham (0.32 ± 0.04) and Syk inhibitor (Syk inh)-Sham (0.42 ± 0.33) groups (all P values ≤ 0.001). Mice pretreated with Syk inhibitor had significantly attenuated mucosal injury scores [3 g/kg R788: 0.58 ± 0.08 vs. MC-I/R and CC-I/R (P values 0.001 and 0.002, respectively); 5 g/kg R788: 0.92 ± 0.14 vs. MC-I/R and CC-I/R (P values 0.003 and 0.01, respectively)]. The injury scores in the Syk inhibitor-treated I/R mouse group were similar to those in MC-Sham, CC-Sham and Syk inh-Sham mouse groups (P values >0.05) (Fig. 1). We did not observe any differences in the preventive effect of Syk inhibitor at 3 g/kg and 5 g/kg doses.
Fig. 1.
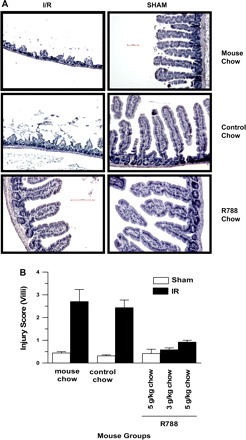
Spleen tyrosine kinase (Syk) inhibitor therapy attenuates ischemia-reperfusion (I/R) injury in the intestine. A: representative hematoxylin and eosin (H & E)-stained slides from the intestine of mice subjected to I/R or sham operation and treated with R788 (3 g/kg and 5 g/kg for 6 days) were visualized and captured under a light microscope. Each image is representative of 3 mice/group. All photomicrographs are ×200 magnification. B: H & E-stained intestinal sections from different treatment groups were scored for intestinal mucosal damage as described in materials and methods. Each bar is the average ± SE for 3 mice/group.
Syk inhibitor limits remote lung I/R injury.
To evaluate whether Syk inhibitor could also prevent I/R-induced remote lung injury, we examined lung tissues. The average pulmonary damage score in the MC-I/R mice (6.83 ± 2.2) was significantly higher than those in the MC-Sham (1.11 ± 0.1), CC-Sham (0.67 ± 0.17), and Syk inh-Sham (1.03 ± 0.26) groups (all P values <0.01). The CC-I/R mouse group (6.07 ± 0.52) displayed more serious pulmonary injury than all of the Sham groups (P values <0.05). Treatment with Syk inhibitor (3 g/kg R788: 1.36 ± 0.18, 5 g/kg R788: 1.1 ± 0.46) resulted in reduced total lung injury scores compared with the MC-I/R and CC-I/R groups (P values <0.05). The injury scores in the Syk inhibitor-treated I/R mouse group were similar to those in MC-Sham, CC-Sham, and Syk inh-Sham mouse groups (P values >0.05) (Fig. 2).
Fig. 2.
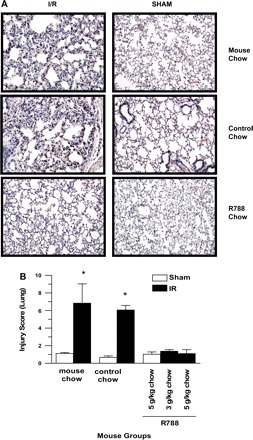
Syk inhibitor therapy reduces remote lung injury following mesenteric I/R. A: representative H & E-stained paraffin sections of lung tissue were subjected to the same treatment as in Fig. 1. Each image is representative of 3 mice/group. All photomicrographs are ×200 magnification. B: lung injury scores from each group were determined based on the criteria outlined in materials and methods. Each bar is the average ± SE for 3 mice/group. Data were compared with ANOVA followed by Tukey's multiple comparison test. *P < 0.05, MC and CC-I/R groups vs. all other groups.
Intestine and lung Syk and p-Syk expression.
Syk expression in the intestine and lung were evaluated by IHC. Syk staining in intestines and lungs was more prominent in I/R groups than in sham-operated groups. However, pretreatment with Syk inhibitor did not lead to any changes in Syk staining in either the intestine or the lung (Fig. 3, A and B).
Fig. 3.
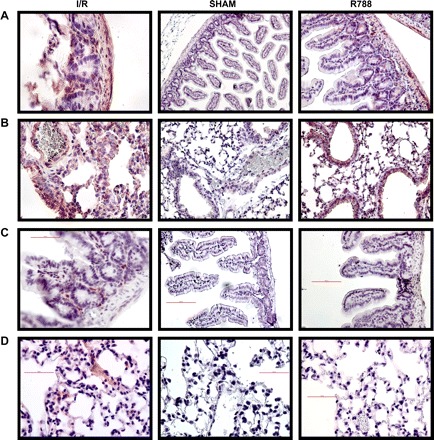
Syk staining in intestinal and lung tissues. Tissue sections were stained as described in materials and methods. A and B: Syk staining was assessed in intestinal (A) and lung (B) tissues by immunohistochemistry staining. Magnification: ×400 for I/R and R788 in A, ×200 for Sham in A and all of B. C and D: phosphorylated (p-)Syk staining was performed in intestinal (C) and lung (D) tissues by immunohistochemistry. All photomicrographs are ×200 magnification.
Because the Syk inhibitor suppresses the expression of p-Syk, the active form of Syk, we performed p-Syk staining by using IHC and IF. We observed staining in I/R group mice. There was no staining in sham-operated mouse groups. In the I/R mouse group, Syk inhibitor treatment eliminated p-Syk staining in the lung and intestine (Fig. 3, C and D). There was only faint staining for p-Syk in the lung and intestine. We believe that Syk phosphorylation is an early event in I/R-induced damage. After a 3-h reperfusion, we detected only slight p-Syk staining.
IgM and IgG staining.
Reperfusion after ischemia induces alteration of the endothelial membrane and allows neoantigen expression. Circulating natural IgM antibodies bind to these antigens and may trigger complement-mediated injury (31). Therefore, we performed IgM staining by IF. There was increased IgM staining in I/R intestinal and lung samples compared with the sham-operated tissues. In addition, we observed that Syk inhibitor reduced IgM deposition and hence detection by IF staining in both tissues (Fig. 4). Unlike the differences detected for natural IgM staining, we were only able to detect minimal IgG staining in all groups (data not shown).
Fig. 4.
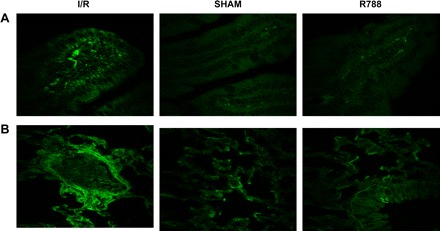
Syk inhibitor reduces IgM staining. IgM staining was performed in intestinal (A) and lung (B) tissues by immunofluorescence. All photomicrographs are ×400 magnification.
Syk inhibitor limits C3 deposition in mouse intestine and lung.
It has been reported that I/R activates complement, as indicated by C3 deposition on the intestine (31). To determine whether Syk inhibitor prevents complement deposition, we performed IHC (Fig. 5, A and B) and IF (Fig. 5, C and D) staining. Compared with the staining in sham-operated groups, mice subjected to I/R had prominent deposition of C3 on the surface of epithelial cells. Treatment of mice with Syk inhibitor attenuated C3 staining in the intestinal mucosa.
Fig. 5.
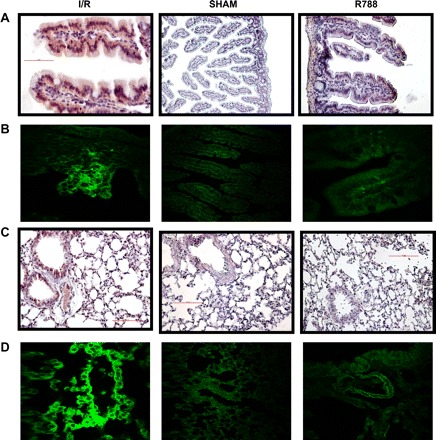
Syk inhibitor reduces I/R-exacerbated complement 3 (C3) deposition in the intestine and lung. A and B: C3 deposition in intestinal tissue was assessed by immunohistochemistry (A) and immunofluorescence (B) staining. Magnification: ×400 for I/R and R788 in A and all of B, ×200 for Sham in A. C and D: C3 deposition in lung tissue was assessed by immunohistochemistry staining (C) and immunofluorescence staining (D) and confocal microscopy. All photomicrographs are ×200 magnification.
Similar to our observation in the intestine, there was significant deposition of C3 in the lungs of mice subjected to I/R compared with animals subjected to the sham operation. Mice treated with Syk inhibitor displayed limited C3 deposition in the lungs.
GR-1 staining.
It was suggested that infiltration by neutrophils mediates local tissue damage in response to I/R (9). Therefore, we evaluated the presence of neutrophils in the intestine and lung after sham and I/R procedures in mice treated with Syk inhibitor. To this end we used an anti-GR-1 antibody, because GR-1 is predominantly expressed on neutrophils and to a much lesser degree on monocytes. Mice subjected to I/R had more prominent GR-1 staining within the intestinal tissue after 3-h reperfusion compared with the sham-operated mouse groups. The administration of Syk inhibitor to mice prevented I/R-induced neutrophil infiltration. In addition, Syk inhibitor treatment decreased infiltration by in the lung tissues (Fig. 6).
Fig. 6.
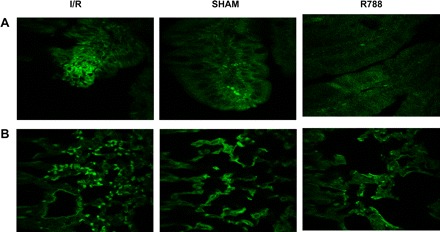
Syk inhibitor decreases neutrophil infiltration. Granulocyte receptor 1 (GR-1) staining to determine polymorphonuclear neutrophil numbers was performed in intestinal (A) and lung (B) tissues and assessed by immunofluorescence. Magnification: ×400 (A), ×200 (B).
DISCUSSION
In this study we demonstrate that pretreatment of mice with a small drug Syk inhibitor significantly reduces I/R injury in both intestinal and lung tissues. The R788 Syk inhibitor was effective at both 3 g/kg and 5 g/kg doses. This is the first study to evaluate the effect of Syk inhibition on I/R injury. Our finding that inhibition of Syk function is protective against I/R injury supports the idea that Syk signaling pathways are active during the inflammatory process that occurs during I/R injury-mediated tissue injury.
Syk inhibitor was found to protect against different autoimmune, allergic diseases in animal models and in humans (11, 17). The most likely effect of this inhibitor comes from targeting Fcγ receptor (Fcrγ) alone and many other Syk-dependent receptors on hematopoietic and intrinsic tissue cells. Previous studies in Syk-deficient murine macrophages indicated that Syk played a central role in Fcrγ-mediated phagocytosis but not in complement-mediated phagocytosis. However, recent studies have further demonstrated that Syk plays an important role in complement-mediated phagocytosis as well (27, 29). An important component of the inflammatory response to mesenteric I/R has been shown to be complement (15, 31), since complement-deficient mice are protected from this type of injury. Inhibition of complement by various inhibitors similarly results in protection against I/R injury (5, 31). Many studies have shown that natural antibodies present in the serum of normal mice bind to neoantigens expressed on injured tissues, activate complement, and initiate damage (15, 31). Activation of the complement pathway also increases the expression of adhesion molecules required for neutrophil homing, therefore contributing to remote organ injury, including the lungs (14). In our study, we confirmed significant C3 and moderate IgM deposition in the intestinal mucosa and endothelial lung tissues after 3-h reperfusion. Treatment with Syk inhibitor decreased this mucosal tissue injury and inhibited C3 and IgM deposition. However, we did not observe prominent IgG deposition in any tissues or changes after Syk inhibition. Therefore, the effect of Syk inhibition in our study may be explained partly by complement inhibition.
Mesenteric I/R induces neutrophil infiltration and serves as a priming bed where systemic neutrophils become activated as they traffic through the vasculature of the injured tissue (9). The oxidative burst induced by anti-IgG in human neutrophils primed with TNF-α was inhibited by R406 (2). Fc receptor signaling in neutrophils was also specifically inhibited by R406 (5). In our study, Syk inhibition decreased intestinal and remote lung I/R-induced infiltration by GR-1-positive cells. The effect of Syk inhibitor on neutrophil function has been evaluated in previous studies. Their conclusion was that R406 did not affect negatively the ability of neutrophils to clear bacteria (5). In I/R injury, complement activation generates C5a and causes mast cell degranulation and neutrophil accumulation (19). In our study, Syk inhibitor may prevent the accumulation of neutrophils by inhibiting upstream events such as complement activation. In addition, it was shown that Syk inhibition blocked mast cell degranulation, and so it is used for allergic diseases (32). This effect of Syk inhibition might also be associated with its protective effect on I/R injury.
Syk phosphorylates itself and downstream proteins predominantly at tyrosine residues, although serine and threonine residues may also be phosphorylated. The Syk inhibitor R406 inhibits all phosphorylation events downstream of Syk signaling (5, 6). Although we observed the efficacy of Syk inhibition in I/R-induced lung and intestinal injury, we could only demonstrate moderate p-Syk staining in injured tissues. It is most likely that Syk is phosphorylated during the period of reperfusion injury when immune cells of both the innate and adaptive immune systems are activated, and therefore we detected a moderate degree of p-Syk staining during the reperfusion period. One study demonstrated rapid phosphorylation in SLE T cells, suggesting that peak phosphorylation in normal T cells occurs by 120 s (21). While this is true, we believe that Syk phosphorylation may occur in different cells throughout the reperfusion period, hence our finding of a moderate increase in p-Syk-positive cells. Alternatively, another study mentioned that intracellular signaling molecules like Syk were colocalized with the initial B-cell receptor microclusters and that maximal spreading occurred within ∼3 min (20). The above-mentioned conditions might have contributed to our inability to detect more prominent p-Syk staining in I/R-injured tissue samples. In vitro studies are controlled and limited to single cell types, and this contrasts with ongoing activation events in vivo. Nonetheless, the in vitro studies described above lend support to our findings that recruitment of different immune cells to the sites of local and remote injury is an ongoing process, as is p-Syk expression.
A recent study revealed that ischemia-mediated aggregation of the actin cytoskeleton plays a crucial role in mediating the deposition of C3 and IgM as well as I/R injury (26). Syk was reported to be effective on actin polymerization and dynamics during complement-mediated phagocytosis (27). In addition, it was shown that Syk inhibition slowed the rapid kinetics of actin polymerization (20). The beneficial effect of Syk inhibition in our study on C3 deposition and intestinal I/R may be at least partly explained by its ability to prevent actin polymerization.
During the short period of administration of Syk inhibitor to the mice (1 wk) we did not observe any clinical side effects. Syk inhibition in patients with RA (30) for 6 mo was reported to be associated with the development of nausea, diarrhea, gastritis, mild neutropenia, and hypertension. Although these side effects may develop after prolonged use of Syk inhibitors, it is suspected that even milder or no side effects will develop in trauma patients who may require shorter treatment.
In conclusion, the administration of a Syk inhibitor limited damage in both intestinal and lung tissues of mice subjected to I/R injury. Based on our observation that Syk inhibitor conferred reduced C3 deposition and polymorphonuclear neutrophil infiltration in local and remote tissues, we propose that Syk inhibitor scavenges complement products and limits local complement activation and tissue injury. The use of a Syk inhibitor may therefore be of clinical value in I/R injury even in humans.
GRANTS
Research was supported by Grant W81XWH-09-1-0530 from Medical Research and Material Command of the Department of the Army. O. N. Pamuk was supported by TUBITAK Scholar.
DISCLOSURES
P. Pine is employed by Rigel Pharmaceuticals. G. C. Tsokos received a grant from Rigel to determine whether Syk inhibition is of value to lupus nephritis.
ACKNOWLEDGMENTS
The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting the views of the United States Department of the Army or the Department of Defense.
REFERENCES
- 1. Anderson J, Fleming SD, Rehrig S, Tsokos GC, Basta M, Shea-Donohue T. Intravenous immunoglobulin attenuates mesenteric ischemia-reperfusion injury. Clin Immunol 114: 137–146, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Asman B, Gustafsson A, Bergstrom K. Priming of neutrophils with tumor necrosis factor-alpha measured as Fcgamma receptor-mediated respiratory burst correlates with increased complement receptor 3 membrane density. Int J Clin Lab Res 26: 236–239, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S, Chang B, Zhao FF, Payan DG, Grossbard EB, Daikh DI. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum 58: 1433–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol 26: 209–213, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, Qu K, Herlaar E, Lau A, Young C, Wong BR, Lovell S, Sun T, Park G, Argade A, Jurcevic S, Pine P, Singh R, Grossbard EB, Payan DG, Masuda ES. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther 319: 998–1008, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Cha HS, Boyle DL, Inoue T, Schoot R, Tak PP, Pine P, Firestein GS. A novel spleen tyrosine inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther 317: 571–578, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Crispin JC, Tedder TF, Lucca JD, Tsokos GC. B cells contribute to ischemia/reperfusion-mediated tissue injury. J Autoimmun 32: 195–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. Arch Surg 101: 478–483, 1970 [DOI] [PubMed] [Google Scholar]
- 9. Conner WC, Gallagher CM, Miner TJ, Tavaf-Mutamen KM, Wolcott H, Shea-Donohue T. Neutrophil priming state predicts capillary leak after gut ischemia in rats. J Surg Res 84: 24–30, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawforf JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. I. The roles of minor H antigens and endotoxin. Blood 8: 3230–3239, 1996 [PubMed] [Google Scholar]
- 11. Deng GM, Liu L, Bahjat R, Pine PR, Tsokos GC. Inhibition of spleen tyrosine kinase suppresses skin and kidney disease in lupus prone mice. Arthritis Rheum (March 10, 2010). doi:10.1002/art.27452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diepenhorst GMP, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury. Lesson learned from animal and clinical studies. Ann Surg 249: 889–899, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Edgerton C, Crispin JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang YT, Tsokos GC. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol 130: 313–321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleming SD, Anderson J, Wilson F, Shea-Donohue T, Tsokos GC. C5 is required for CD49d expression on neutrophils and VCAM expression on vascular endothelial cells following mesenteric ischemia/reperfusion. Clin Immunol 106: 55–64, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Fleming SD, Shea-Donohue T, Gutheridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol 169: 2126–2133, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun Rev 5: 89–92, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Ghosh D, Tsokos GC. Spleen tyrosine kinase: an Src family of non-receptor kinase has multiple functions and represents a valuable therapeutic target in the treatment of autoimmune and inflammatory diseases. Autoimmunity 43: 48–55, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Johnson P, Cross JL. Tyrosine phosphorylation in immune cells: direct and indirect effects on toll-like receptor-induced proinflammatory cytokine production. Crit Rev Immunol 29: 347–367, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Karpel-Massler G, Fleming SD, Kirschfink M, Tsokos GC. Human C1 esterase inhibitor attenuates murine mesenteric ischemia/reperfusion induced local organ injury. J Surg Res 115: 247–256, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Krishnan S, Juang YT, Chowdhury B, Magilavy A, Fisher CU, Nguyen H, Nambiar MP, Kyttaris V, Weinstein A, Bahjat R, Pine P, Rus V, Tsokos GC. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol 181: 8145–8152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurosaki T, Hikida M. Tyrosine kinase and their substrates in B lymphocytes. Immunol Rev 228: 132–148, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Kyttaris VC, Tsokos GC. Syk kinase as a treatment target for therapy in autoimmune diseases. Clin Immunol 124: 235–237, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: C227–C241, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Page TH, Smolinska M, Gillespie J, Urbaniak AM, Foxwell BM. Tyrosine kinases and inflammatory signaling. Curr Mol Med 9: 69–85, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Ruzza P, Biondi B, Calderan A. Therapeutic prospect of Syk inhibitors. Expert Opin Ther Pat 19: 1361–1376, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Shi T, Moulton VR, Lapchak PH, Deng GM, Lucca JJD, Tsokos GC. Ischemia-mediated aggregation of the actin cytoskeleton is one of the major initial events resulting in ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 296: G339–G347, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Shi Y, Tohyama Y, Kadono T, He J, Miah SM, Hazama R, Tanaka C, Tohyama K, Yamamura H. Protein-tyrosine kinase Syk is required for pathogen engulfment in complement-mediated phagocytosis. Blood 107: 4554–4562, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Tohyama Y, Yamamura H. Complement-mediated phagocytosis—the role of Syk. IUBMB Life 58: 304–308, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Tohyama Y, Yamamura H. Protein tyrosine kinase Syk: a key player in phagocytic cells. J Biochem 145: 267–273, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-torres JL, Murphy FT, Musser TK, Straniero N, Vicente-Gonzales AV, Grossbard E. Treatment of rheumatoid arthritis with a Syk inhibitor. A twelve-week, randomized, placebo-controlled trial. Arthritis Rheum 58: 3309–3318, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Williams JP, Pechet TTV, Weiser MR, Reid R, Kobzik L, Moore FD, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol 86: 938–942, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA 101: 3886–3891, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao H, Montalto MC, Pfeiffer KJ, Hao L, Stahl GL. Murine model of gastrointestinal ischemia associated with complement-dependent injury. J Appl Physiol 93: 338–345, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Zhu Y, Herlaar E, Masuda ES, Burleson GR, Nelson AJ, Grossbard EB, Clemens GR. Immunotoxicity assessment for the novel spleen tyrosine kinase inhibitor R406. Toxicol Appl Pharmacol 221: 268–277, 2007 [DOI] [PubMed] [Google Scholar]


