Abstract
We hypothesize that the inner medulla of the kangaroo rat Dipodomys merriami, a desert rodent that concentrates its urine to more than 6,000 mosmol/kgH2O water, provides unique examples of architectural features necessary for production of highly concentrated urine. To investigate this architecture, inner medullary nephron segments in the initial 3,000 μm below the outer medulla were assessed with digital reconstructions from physical tissue sections. Descending thin limbs of Henle (DTLs), ascending thin limbs of Henle (ATLs), and collecting ducts (CDs) were identified by immunofluorescence using antibodies that label segment-specific proteins associated with transepithelial water flux (aquaporin 1 and 2, AQP1 and AQP2) and chloride flux (the chloride channel ClC-K1); all tubules and vessels were labeled with wheat germ agglutinin. In the outer 3,000 μm of the inner medulla, AQP1-positive DTLs lie at the periphery of groups of CDs. ATLs lie inside and outside the groups of CDs. Immunohistochemistry and reconstructions of loops that form their bends in the outer 3,000 μm of the inner medulla show that, relative to loop length, the AQP1-positive segment of the kangaroo rat is significantly longer than that of the Munich-Wistar rat. The length of ClC-K1 expression in the prebend region at the terminal end of the descending side of the loop in kangaroo rat is about 50% shorter than that of the Munich-Wistar rat. Tubular fluid of the kangaroo rat DTL may approach osmotic equilibrium with interstitial fluid by water reabsorption along a relatively longer tubule length, compared with Munich-Wistar rat. A relatively shorter-length prebend segment may promote a steeper reabsorptive driving force at the loop bend. These structural features predict functionality that is potentially significant in the production of a high urine osmolality in the kangaroo rat.
Keywords: aquaporin, ClC-K1, concentrating mechanism, urea transport
the kangaroo rat Dipodomys merriami is one of a number of mammalian species that are known to concentrate their urine to more than 6,000 mosmol/kgH2O (5), nearly twice as high as that of the laboratory rat. Over the course of about 2 mo on a dry diet (with no water), the body weight of the kangaroo rat is essentially unchanged; in contrast, within about 20 days on the same diet, the white rat loses about 50% of its body weight, primarily due to water loss (37).
The classical studies of kangaroo rat and other desert species demonstrating tolerance to low water intake underscore the potential insights these species could offer in understanding the urine-concentrating mechanism. Despite a rich history of investigations into renal tubular and vascular function (2, 5, 17), there are no medullary characteristics of desert species that have been clearly correlated with the ability to produce a highly concentrated urine. Functional and architectural features that may play a role in producing these very high osmolalities include the papilla length (an index of length of long loops of Henle), the relative medullary thickness (an index relating, among other things, the dimensions of the thick ascending limb where active NaCl transport occurs, to whole kidney size), the relative abundance of short and long loops of Henle, vascular organization, and collecting duct (CD) branching patterns (2, 12, 17). Although a correlation exists between some of these features and the ability of different species to form a high urine concentration, the relationships are often unpredictable, and a key question persists: what structural and functional features enable desert species to concentrate so robustly?
The urine-concentrating mechanism can only be fully understood by taking into consideration three-dimensional structural and functional architecture of the renal medulla. Fluid and solute flows between spatially separated compartments can be evaluated to some degree on the basis of transport proteins that are expressed within cells of neighboring epithelial structures. Indeed, the functional architecture of the outer medulla of many species, including the spatial arrangements of nephrons and blood vessels, has been well defined by many past studies (3, 24, 42). In contrast, the architecture of the inner medulla has only recently begun to be defined (33). Molecular cloning of plasma membrane fluid and solute transporters and channels during the past two decades has supported the recognition of segmental axial heterogeneity of transporter and channel expression in loops of Henle and vasculature of Munich-Wistar and Sprague Dawley rat and chinchilla medulla. Loops of Henle, blood vessels, and CDs of the laboratory rat are now known to be arranged in clearly defined, repeating patterns throughout the inner medulla (14, 29, 30, 43). These studies notwithstanding, renal functional architecture is insufficiently understood to serve as a predictor of interspecific variation in concentrating ability (2, 17, 33).
The present studies of the kangaroo rat D. merriami and our recent studies of the Munich-Wistar rat renal architecture have begun to provide the first comprehensive portrayal of the three-dimensional positional arrangements of inner medullary tubular and vascular structures, and the detailed organization of their transporters and channels. These studies provide some of the strongest and most influential data yet obtained that support, and in several ways redefine, the original passive hypothesis of the renal concentrating mechanism as proposed by Kokko and Rector (16) and Stephenson (39). The functional implications of this three-dimensional architecture have generated concepts for production of revealing mathematical models of the laboratory rat inner medullary urine-concentrating mechanism (18, 20–22) that have produced urine concentrations that are nearly equivalent to that of moderately concentrating rats. However, no computational models, most of which incorporate some version of the passive mechanism (16, 39), have yet demonstrated the feasibility of producing urine with solute concentrations approaching those of highly concentrating mammals.
A better understanding of transepithelial fluid and solute fluxes in loops of Henle, regulation of tubular and vascular flows, and compartmentation that arises from structural relationships are essential for understanding the urine-concentrating mechanism. In addition, the role of the interstitium and the papillary epithelium may play critical roles in the urine-concentrating mechanism (15, 35). The present study of the inner medulla of the desert rodent, D. merriami, builds on prior studies of renal structure and function of highly concentrating mammals, such as the chinchilla (7, 8), with the goal of identifying countercurrent systems and compartmentation that may be representative of these species and essential for the urine-concentrating mechanism.
METHODS
Animals.
Male and female Dipodomys merriami (kangaroo rat) (∼30–50 g) were obtained by live trapping at the Santa Rita Experimental Range, located ∼10 miles east of Green Valley, AZ. Kangaroo rats were housed individually in cages in the University of Arizona Health Sciences animal facility for periods up to 4 mo. Bedding used was Sakrete Play Sand, obtained from local commercial suppliers and was autoclaved prior to use. Animals were provided with 15 g Kaytee Songbird Blend wild birdseed daily and no fluids, as in captivity they do not drink free water, and they were also provided with one small leaf of spinach three times each week. Room temperature was set at 21–22°C and relative humidity was 30–70%. Young male Munich-Wistar rats (average wt, 90 g) were purchased from Harlan (Indianapolis, IN) and provided with rat chow and water ad libitum. Animals were euthanized with CO2. All experiments were conducted in accordance with The Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 1996) and approved by the University of Arizona Institutional Animal Care and Use Committee.
Tissue preparation and immunohistochemistry.
Kidneys were prepared for immunohistochemistry, as described previously (31, 32). Briefly, kidneys are perfused through the aorta with PBS (pH 7.4) for 5 min, followed by periodate-lysine-paraformaldehyde (PLP) (0.01M, 0.075M, 2%) (25) in PBS (pH 7.4) for 5 min; some kidneys were fixed without perfusion. The medulla is dissected free, immersed in PLP fixative for 3 h at 4°C, washed in PBS, and dehydrated through an ethanol series. Each medulla is trimmed to a size that measures ∼2,000 × 1,400 μm in the transverse dimension near the outer medullary-inner medullary boundary. Tissue is then immersed in a solution of Spurr's epoxy resin (Ted Pella, Redding, CA) and ethanol (1:1) for 16 h (room temperature), then in 100% Spurr's resin for 48 h (4°C), and finally embedded in 100% Spurr's resin (12 h at 60°C). Some tissue was also embedded in paraffin following ethanol dehydration. Serial transverse sections of the inner medulla were cut, beginning either at the outer medullary-inner medullary boundary, or at the papilla tip. Tissue embedded in Spurr's resin was cut into 1-μm-thick sections with a Leica EM UC6 or Leica Ultracut UCT ultramicrotome. Tissue embedded in paraffin was cut into 4-μm-thick sections with a Microm HM 355 S microtome. Serial sections lying 5 μm apart were used for tissue reconstructions.
Orthologous proteins in kangaroo rat were labeled using affinity-purified polyclonal or monoclonal antibodies against the COOH-terminal region of human water channel aquaporin 1 (AQP1, mouse monoclonal, no. 9566; Abcam, Cambridge, MA) to label descending thin limbs of Henle, rat kidney-specific chloride channel to label ascending thin limbs of Henle (ClC-K, rabbit host, no. ACL-004; Alomone Laboratories, Jerusalem, Israel), and human water channel aquaporin 2 to label collecting ducts (AQP2, goat host, no. 9882; Santa Cruz Biotechnology, Santa Cruz, CA). Descending vasa recta were labeled with a polyclonal antibody raised in rabbits against rat urea transporter B; diluted 1:200, provided by Jeff Sands and Janet Klein, Emory University). All tubules and blood vessels were labeled nonselectively with fluorescein-conjugated wheat germ agglutinin (no. FL-1021; Vector Laboratories, Burlingame, CA). Secondary antibodies conjugated to fluorescent probes [Invitrogen/Molecular Probes (Carlsbad, CA) or Jackson ImmunoResearch (Bar Harbor, ME)] were applied as described previously (31, 32). Sections were mounted with DAKO fluorescent mounting medium (DAKO, Carpinteria, CA) and were viewed with epifluorescence microscopy (Applied Precision, DeltaVision, Issaquah, Washington). These protocols are identical to those used previously for characterizing AQP1, AQP2, and ClC-K1 expression in thin limbs of Henle and CDs of Munich-Wistar rats (31, 32).
Image analysis.
Separate stacks of digitized, serial images were generated by capturing immunofluorescence from each tissue section. Continuous surface and volume representations for each tubule were constructed as described previously from serial sections no greater than 5 μm apart (29–32, 34) with Amira visualization and volume modeling software (Mercury, Chelmsford, MA). Partial three-dimensional reconstructions were created for some, but not all, tissue. Quantitative analyses were carried out on two-dimensional images using PhotoShop (Adobe) and the Image Processing Toolkit (Reindeer Graphics, Asheville, NC).
Statistical analyses.
Data combined from three or more samples are reported as means ± SE; n = number of replicates. The statistical significance of differences between means for each category of two data sets was determined with two-sample t-test (P < 0.05) and significance of differences between means for multiple data sets was determined with ANOVA and Scheffé post hoc tests (P < 0.05).
RESULTS
The functional architecture of inner medullary CDs and long loops of Henle was evaluated in serial, transverse sections by tracking CDs and loops that were labeled with primary antibodies that detect proteins involved with solute and water transport. The water channel AQP1 is expressed both in DTLs and in descending vasa recta (Fig. 1, A and B). Descending vasa recta can be readily differentiated from DTLs on the basis of wall thickness and vessel diameter in cross sections (Fig. 1A). Although the antibody that we used cannot differentiate between ClC-K1 and ClC-K2 in rat and mouse (1, 40, 41), and likely the kangaroo rat, the kidney-specific Cl channel isoform that is expressed in the ATL is ClC-K1 (Fig. 1). ClC-K1 is also expressed in prebend segments, which lie at the terminal end of the descending side of the loop, and ClC-K2 is expressed in some CDs (41) (Fig. 1). The vasopressin-sensitive water channel AQP2 is also expressed in CDs (Fig. 1). The total length of the kangaroo rat inner medulla is 6,547 ± 201 μm (means ± SE; n = 3 males), whereas the length of the Munich-Wistar male inner medulla is about 5,000 μm (34).
Fig. 1.
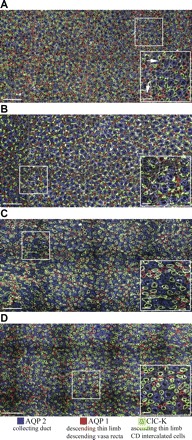
Immunolocalization of loops of Henle and collecting ducts (CDs) in inner medullary transverse sections. A and B: kangaroo rat, 500 and 2,000 μm below the outer medulla (K rat 2 in Fig. 4). C and D: Munich-Wistar rat, 500 and 2,000 μm below the outer medulla (MW rat 5 in Fig. 4). Antibodies were applied at equal concentrations, and images were acquired at equivalent intensity scaling. Structures not labeled with aquaporin 1 (AQP1), aquaporin 2 (AQP2), or the chloride channel ClC-K antibodies are shown in off-white, labeled with wheat germ agglutinin. Scale bars: 100 μm and 20 μm (inset). Boxed areas are enlarged in right corner insets. Arrows identify AQP1-positive descending vasa recta (DVR).
In transverse sections within the first 3,000 μm below the outer medulla, the AQP1-positive DTLs of the kangaroo rat are spatially separated from groups of CDs (Fig. 1, A and B). As shown in reconstructed loops of Henle and CDs (Fig. 2), as the AQP1-positive DTLs descend along the corticopapillary axis, they retain this spatial relationship to the group of CDs. In contrast, ATLs are somewhat more uniformly intermixed among the CDs (Figs. 1, A and B, and 2). These relationships are similar to those previously shown for the Munich-Wistar rat (Fig. 1, C and D) (30).
Fig. 2.
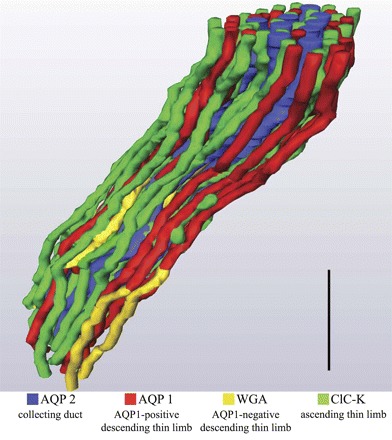
Three-dimensional reconstruction of kangaroo rat inner medullary loops of Henle and CDs. Descending thin limbs of Henle (DTLs) lie primarily at the periphery of groups of CDs, whereas ascending thin limbs of Henle (ATLs) are intermixed among the CDs. Tubules are oriented in a corticopapillary direction, with the upper edge of the image near the outer medullary-inner medullary boundary. Animal sex was female. Comparable architecture has been reported for the male Munich-Wistar rat (32). WGA, wheat germ agglutinin. Scale bar: 125 μm.
At several transverse levels along the corticopapillary axis, the number of AQP1-positive DTLs (DTLAQP1) is less than the number of ATLs as determined from AQP1 and ClC-K immunolabeling (Fig. 1, A and B). Because ClC-K1 is expressed along the entire length of each ATL in these looped structures and because descending and ascending limbs have equal lengths, it is clear that a significant length of each DTL does not express detectable AQP1. Unequal numbers of aquaporin 1-positive DTLs and ATLs are also seen in transverse sections of the Munich-Wistar rat inner medulla (Fig. 1, C and D), a species in which detectable AQP1 is expressed along no more than about 40% of its DTL (29). To estimate and compare the degree of AQP1 expression along the length of DTLs in the two species, we counted the AQP1-positive DTL (DTLAQP1) and ClC-K1-positive ATL profiles at 500 and 2,000 μm below the outer medulla in the inner medullas of kidneys from three male and three female kangaroo rats, and from four Munich-Wistar male rats. From these measurements, we calculated the DTLAQP1/ATL ratios (Fig. 3). The DTLAQP1/ATL ratio in kangaroo rat inner medullary transverse sections varies from about 0.6 to about 0.5 at 500 and 2,000 μm below the outer medulla; values are not statistically different from each other (two-way ANOVA and Scheffé tests, P < 0.05) (Fig. 3). These ratios contrast with those of the Munich-Wistar rat, which, compared with the kangaroo rat, are about 40% and 70% lower at 500 and 2,000 μm below the outer medulla, respectively (Fig. 3). Ratios at 500 and 2,000 μm below the outer medulla of the Munich-Wistar rat are statistically different from each other and are also significantly lower than the ratios of the kangaroo rat inner medulla at each level (two-way ANOVA and Scheffé tests, P < 0.05). The differences in ratios between male and female kangaroo rats were not significant.
Fig. 3.
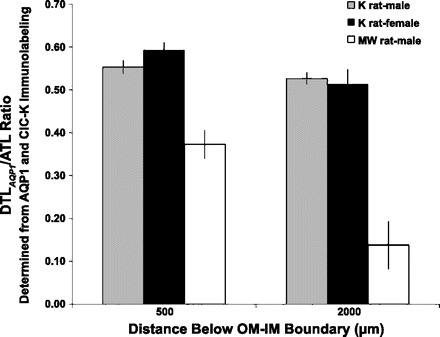
Ratios of AQP1-positive DTL and ATL profiles in transverse sections at 500 and 2,000 μm below the outer medullary-inner medullary boundary for kangaroo rat (n = 3 males, 3 females) and Munich-Wistar rat (n = 4 males). Kangaroo rat values are not significantly different from each other. Munich-Wistar rat values are significantly different from kangaroo rat values at each level and from each other at the two levels (two-way ANOVA/Scheffé tests, P < 0.05). These data are included in Fig. 4.
We also calculated the DTLAQP1/ATL ratios at a variety of different levels along the corticopapillary axis (Fig. 4), and these data indicate that AQP1 expression in the DTL declines progressively in proportion to depth, at least between the interval of 0 and 2,500 μm below the outer medulla.
Fig. 4.
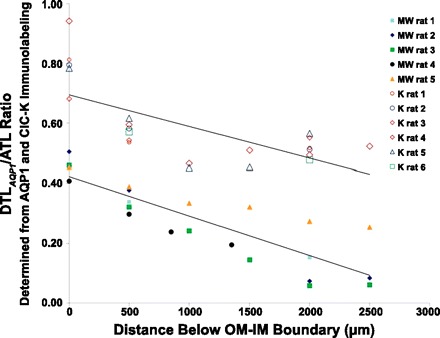
Ratios of AQP1-positive DTL and ATL profiles in transverse sections at various depths below the outer medullary-inner medullary boundary for kangaroo rat and Munich-Wistar rat. Kangaroo rat males, 1–3; females, 4–6. Lines fit by least squares method; y = −1E-04x + 0.69 (kangaroo rat, upper line); y = −1E-04x + 0.42 (Munich-Wistar rat, lower line).
A fraction of the ClC-K1-positive profiles in transverse sections are actually prebend segments and not ATLs. We determined the proportion of ClC-K1-positive profiles that are actually prebend segments by reconstructing all ATL and prebend segments within a defined region at about 2,000 μm below the outer medullary-inner medullary boundary of the inner medullas from 3 animals of each species. For these loops, we found that a mean of 9.2 ± 3.0% of ClC-K1 profiles in kangaroo rat and 7.5 ± 1.5% of ClC-K1 profiles in Munich-Wistar rat transverse sections, were actually prebend segments. With these percentages, the DTLAQP1/ATL ratios would be increased nearly equally for both species (9 and 8% for kangaroo rat and Munich-Wistar rat, respectively). Also, it has been shown for the Munich-Wistar rat that variable, periodic changes in prebend number occur at regular intervals of medullary depth (27). Although these periodic changes have not been investigated in the kangaroo rat, our prebend analyses included only sections that consisted of many groups of CDs to minimize the possible influence of these periodic changes.
The AQP1 and ClC-K1 antibodies target amino acid sequences that are highly conserved among mammalian proteins (11, 26), and we have no reason to suggest that the antibodies are substantially more or less sensitive to proteins of kangaroo rat than to those of Munich-Wistar rat. The transitions from the AQP1-positive to AQP1-negative and from ClC-K1-negative to ClC-K1-positive portions of the DTLs are relatively abrupt for both species, indicating the protein is expressed at either high (detectable) or low (undetectable) levels, in which case a difference in antibody sensitivity would be unlikely to have significant impact on the DTLAQP1/ATL ratios. To test this, we conducted immunohistochemistry using AQP1 and ClCK antibodies at 5-fold and 10-fold dilutions from those used in our ratio comparisons, and we found that the DTLAQP1 and ATL profiles, and, therefore, ratios, were identical at all dilutions for both species. These results indicate that AQP1 and ClCK antibodies were applied at saturating concentrations for our ratio measurements. Therefore, for the purpose of making DTLAQP1/ATL ratio comparisons, as we have done here, the antibody sensitivities are essentially equivalent for both species.
To obtain a preliminary estimate of the relative lengths of the AQP1-positive DTL segments, we reconstructed loops of Henle that form their bends within about 2,500 μm below the outer medulla in a single female kangaroo rat inner medulla. AQP1-negative DTL segments were visualized in transverse tissue sections by labeling them with fluorescein-conjugated wheat germ agglutinin. In this kangaroo rat inner medulla, only those loops that extend deeper than ∼500 μm below the outer medulla express detectable AQP1 in their descending segments (Fig. 5). A transition segment exists at the terminus of the AQP1-positive segment in which AQP1 is intermittently detectable, in a fashion previously reported for the Munich-Wistar rat (29). The mean length of the transition segment was 28.07 ± 5.17 μm (means ± SE for 3 male medullas, 10 DTLs from each). This length was highly variable, ranging from 5 to 190 μm. The remaining portion of the DTL, the lower segment, expresses no detectable AQP1. The fractional length of the AQP1-positive segment is variably correlated with the inner medullary loop length, at least for the initial 2,500 μm below the outer medulla; the longer the loop length, the longer the fractional length of the AQP1-positive segment (Fig. 5). The mean AQP1-positive fractional length for loops that form their bends between 2,365 and 2,615 μm below the outer medulla (the longest loops of the kangaroo rat kidney that we have reconstructed) was 0.66 ± 0.01 (mean ± SE; n = 14 loops), and ranged from 0.60 to 0.71. Loops of Henle in the Munich-Wistar rat kidney that form their bends within the same interval have a mean AQP1-positive fractional length of 0.36 ± 0.01 (means ± SE; n = 10 loops) (29). Although replicates from additional kidneys are needed for determining the variability of the AQP1-positive fractional lengths, the DTLAQP1/ATL ratios for loops of Henle confirm that AQP1 is expressed along a significantly longer inner medullary length of the kangaroo rat loop of Henle than in the Munich-Wistar rat. These species-related differences in AQP1-positive fractional lengths are supported by the loop reconstructions.
Fig. 5.
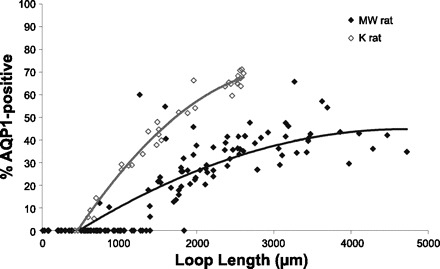
AQP1-positive fractional length of inner medullary DTLs of kangaroo rat and Munich-Wistar rat. The AQP1-positive length is variably proportional to the length of the inner medullary thin limb segment, measured from the outer medullary-inner medullary boundary to the bend of the loop. Lines are fit to exponentials; y = −9E-06x2 + 0.058x − 23.73 (kangaroo rat), y = −2E-06x2 + 0.023x − 10.52 (Munich-Wistar rat).
The prebend segment that lies at the terminal end of the descending side of the loop is believed to have a relatively high transepithelial NaCl permeability because its cell structure is identical to that of the ATL and because it expresses abundant ClC-K1 (28, 33, 36). Thus, the axial length of ClC-K1 expression along this region likely reflects characteristics of NaCl reabsorption along this segment, thereby providing insight into the role of the prebend segment in the urine-concentrating mechanism. In our study, we define the prebend segment as that region of the terminal descending limb that expresses detectable ClC-K1. To investigate prebend functionality, we reconstructed between 10 and 200 prebend segments from each of the inner medullas of five kangaroo rats and three Munich-Wistar rats. Each of these loop bends lie at an axial depth between 400 and 2,700 μm below the outer medulla. We then determined the axial length of ClC-K1 expression that occurs before the bend of each loop. These axial lengths and the depths at which their bends lie are shown in Fig. 6. Although there is variation in prebend length at each level, the mean axial length of the prebend ClC-K1-positive segment for each species appears to be a relatively fixed length for all loops of Henle, regardless of the inner medullary depth at which they form their bend, as reported previously for the Munich-Wistar rat (29). For those loops of the kangaroo rat that form their bends between the interval of 1,500 and 2,000 μm below the outer medulla, ClC-K1 is expressed for a mean axial distance of 88.9 ± 12.2 μm (means ± SE; n = 5 animals) before the bend. The prebend lengths for male and female kangaroo rats are similar so sexes were combined. For Munich-Wistar rat loops that form their bends within the same interval, ClC-K1 is expressed for a mean axial distance of 192.7 ± 25.0 μm (means ± SE; n = 3 animals) before the bend, a length comparable to our previously published measurements (29). Thus, the length of prebend ClC-K1 expression in the kangaroo rat is, on average, about 50% that of the Munich-Wistar rat (P < 0.05). The loop length-specific patterns of AQP1 and ClC-K1 expression in the DTLs of the kangaroo rat and Munich-Wistar rat are summarized in Fig. 7.
Fig. 6.
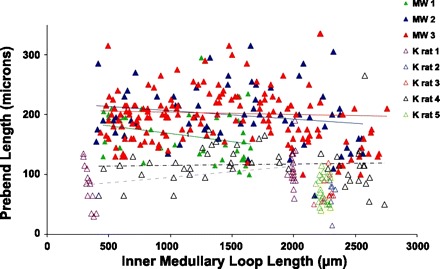
Lengths of prebend segments in kangaroo rat and Munich-Wistar rat inner medullas. Prebend segments were reconstructed from loops with inner medullary lengths between about 400 and 2,800 μm. The prebend length is relatively constant regardless of loop length. Munich-Wistar rat, filled triangles; Kangaroo rat, open triangles. Kangaroo rat males, 1–3; females, 4 and 5. Trend line equations for these five medullas are y = −0.026x + 193.99 (MW rat 1); y = −0.014x + 220.38 (MW rat 2); y = −0.004x + 207.47 (MW rat 3); y = 0.019x + 76.95 (K rat 1); y = 0.002x + 113.05 (K rat 4). See text for additional details.
Fig. 7.
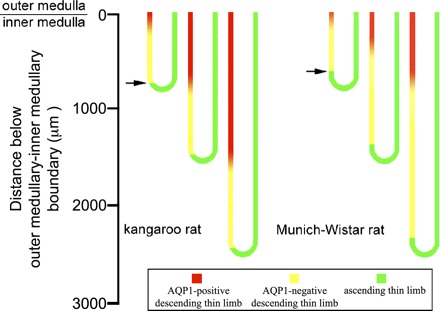
Segmentation of inner medullary loops of Henle in kangaroo rat and Munich-Wistar rat. AQP1 is expressed throughout a longer proportion of the inner medullary DTL in the kangaroo rat, compared with the Munich-Wistar rat. ClC-K1 expression begins at a lower level above the bend in the kangaroo rat, compared with the Munich-Wistar rat (arrows).
DISCUSSION
In kangaroo rat (present studies), Munich-Wistar rat (29), and chinchilla (9), AQP1 expression in long-looped DTLs gradually decreases to nearly undetectable levels at a considerable distance before the loop bend. The length of the AQP1-positive segment is correlated with loop length; that is, the longer the loop, the longer the AQP1-positive segment. Long-looped DTLs in most species that have been studied, consist of two segment types (types 2 and 3 epithelia) that have been distinguished solely on the basis of cell structure (13). These segments are distributed in a heterogeneous, species-specific manner along the corticopapillary axis (4, 28, 38), and this distribution pattern parallels that of AQP1-positive and AQP1-negative segments in Munich-Wistar rat and kangaroo rat [this study and (34)]. The AQP1-positive and AQP1-negative DTLs of Munich-Wistar rat and kangaroo rat apparently correspond to type 2 and type 3 epithelia, respectively, as previously shown for chinchilla (9). A variable-length transition segment that is intermittently AQP1-positive and negative in the Munich-Wistar rat and the kangaroo rat may correspond to a heterogeneous mix of types 2 and 3 epithelia.
In vitro perfusion studies of loops of Henle from Munich-Wistar rat (10) and chinchilla (7) indicate high osmotic water permeability in the AQP1-positive region and low, or no significant water permeability in the AQP1-negative region. As shown for the DTL of the AQP1-null mouse, a lower transepithelial water permeability leads to decreased urine concentration (6). However, even with undetectable levels of AQP1, such as in the chinchilla lower DTL (9), and in the complete absence of AQP1, such as in the AQP1-null mouse, significant transepithelial water permeability can still be detected along inner medullary DTL segments, suggesting that alternate pathways for transepithelial water flux may exist, possibly including other aquaporins, the paracellular pathway, and/or diffusion through the lipid bilayer of the plasma membrane (6).
The greater length of water channel expression in the kangaroo rat DTL, compared with the Munich-Wistar rat (Fig. 7) and other mammals, suggests that osmotic equilibration between tubule lumen and interstitium by water reabsorption may occur along a greater length, in both the outer and inner medulla, in kangaroo rat and possibly also in other species that produce a highly concentrated urine. However, since a relatively water-impermeable DTL segment exists in all species investigated, absence of substantial fluid reabsorption at the end of each loop seems obligatory. We speculate further that species-specific adaptations for efficient water removal from the inner medullary interstitium may parallel variations in AQP1 expression.
To our knowledge, the length of ClC-K1 expression along the terminal end of the descending side of the loop has been reported only for the Munich-Wistar rat (29) and kangaroo rat (this study). The prebend segments of most rodents studied have previously been distinguished on the basis of cytological criteria, and they have been shown to possess the type 4 (ATL type) epithelium (13), the chinchilla being one exception (9). However, there are no reports as to whether or not the type 4 epithelium that lies at the terminal end of the descending side of the loop expresses ClC-K1 along its entire length.
The relative length of ClC-K1 expression along the terminal end of the descending side of the loop (Fig. 7) may be relevant to the urine-concentrating mechanism. Mathematical modeling studies of the urine-concentrating mechanism have hypothesized that transepithelial solute gradients result in the most robust NaCl reabsorption along the prebend segment and postbend equivalent length portion of the ATL (20, 23). The near-bend segments of all loops of the Munich-Wistar rat lie within the intracluster region of the inner medulla (27), where, in combination with ATLs, CDs, and ascending vasa recta, they form interstitial nodal spaces, or microdomains, that appear ideally configured to mix NaCl absorbed from loops with absorbate from the CDs (which consists mostly of water, urea, and NaCl) to produce isolated sites of high osmolality that may promote water withdrawal from adjoining CDs (19). A comparable architecture exists in the kangaroo rat (Pannabecker TL, unpublished observations). The length of the ClC-K1-positive prebend segment may be a factor in optimizing the fluid and solute mixing process within the interstitial nodal spaces and this length may, therefore, be a determinant of optimal solute delivery at the near-bend region of the loop of Henle. Also, a shortening of the prebend segment would allow for proportionately longer AQP1-positive and/or AQP1-negative DTL segments (see above). Thus, prebend segment length may influence the magnitude of the transepithelial gradients for solute reabsorption by the prebend segment and by the postbend segment on the ascending side of the loop, thereby influencing formation of the corticopapillary solute gradient.
Perspectives and Significance
Studies of structure and function in kidneys of desert species offer unique perspectives on mechanisms associated with sodium and water homeostasis. Thin limbs of Henle are increasingly recognized as more than a simple pipe with unregulated transepithelial and tubular fluid and solute flows (28). On the descending side of the limb are water-permeable and water-impermeable segments of species-specific lengths. These two segments apparently correspond to two cytologically distinct segments. Significant, but variable, Na and/or urea transepithelial permeabilities have been measured along the entire DTL in rat (Dantzler WH, Evans KK, and Pannabecker TL, unpublished observations) and chinchilla (8). These data add to and support previous studies that showed transepithelial solute equilibration occurs along the DTL in the lower third of the inner medulla. A detailed understanding of spatial interactions between AQP1-positive and -negative descending segments of the inner medullary loop of Henle and other structures is essential for fully understanding the urine-concentrating mechanism.
GRANTS
This research was supported by the National Institutes of Health: National Institute of Diabetes and Digestive and Kidney Diseases, Grant DK-083338 and by the National Science Foundation, Grant IOS-0952885.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.B.U., T.I., E.J.B., and T.L.P. performed experiments; V.B.U., T.I., and T.L.P. analyzed data; V.B.U., T.I., E.J.B., W.H.D., and T.L.P. interpreted results of experiments; E.J.B., W.H.D., and T.L.P. edited and revised manuscript; E.J.B., W.H.D., and T.L.P. approved final version of manuscript; W.H.D. and T.L.P. conception and design of research; T.L.P. prepared figures; T.L.P. drafted manuscript.
REFERENCES
- 1. Akizuki N, Uchida S, Sasaki S, Marumo F. Impaired solute accumulation in inner medulla of Clcnk1−/− mice kidney. Am J Physiol Renal Physiol 280: F79–F87, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bankir L, De Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol Regul Integr Comp Physiol 249: R643–R666, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Bankir L, Kriz W. Adaptation of the kidney to protein intake and to urine concentrating activity: similar consequences in health and CRF. Kidney Int 47: 7–24, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Barrett JM, Kriz W, Kaissling B, De Rouffignac C. The ultrastructure of the nephrons of the desert rodent (Psammonys obesus) kidney. II. Thin limbs of Henle of long-looped nephrons. Am J Anat 151: 499–514, 1978 [DOI] [PubMed] [Google Scholar]
- 5. Beuchat CA. Body size, medullary thickness, and urine concentrating ability in mammals. Am J Physiol Regul Integr Comp Physiol 258: R298–R308, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Chou CL, Knepper MA, Van Hoek AN, Brown D, Ma T, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J Clin Invest 103: 491–496, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol Renal Fluid Electrolyte Physiol 263: F417–F426, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: urea and NaCl permeabilities. Am J Physiol Renal Fluid Electrolyte Physiol 264: F337–F343, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Chou CL, Nielsen S, Knepper MA. Structural-functional correlation in chinchilla long loop of Henle thin limbs: a novel papillary subsegment. Am J Physiol Renal Fluid Electrolyte Physiol 265: F863–F874, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Dantzler WH, Evans KK, Pannabecker TL. Osmotic water permeabilities in specific segments of rat inner medullary thin limbs of Henle's loops (Abstract). FASEB J 23: 970.–3., 2009 [Google Scholar]
- 11. Jentsch TJ, Friedrich T, Schriever A, Yamada H. The CLC chloride channel family. Pflügers Arch 437: 783–795, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Kaissling B, De Rouffignac C, Barrett JM, Kriz W. The structural organization of the kidney of the desert rodent Psammomys obesus. Anat Embryol 148: 121–143, 1975 [DOI] [PubMed] [Google Scholar]
- 13. Kaissling B, Kriz W. Morphology of the loop of Henle, distal tubule, and collecting duct. In: Handbook of Physiology. Sec. 8. Renal Physiology, edited by Windhager EE. New York: Oxford University Press, 1992, p. 109–167 [Google Scholar]
- 14. Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol 299: F273–F279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knepper MA, Saidel GM, Hascall VC, Dwyer T. Concentration of solutes in the renal inner medulla: interstitial hyaluronan as a mechano-osmotic transducer. Am J Physiol Renal Physiol 284: F433–F446, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Kokko JP, Rector FC. Countercurrent multiplication system without active transport in inner medulla. Kidney Int 2: 214–223, 1972 [DOI] [PubMed] [Google Scholar]
- 17. Kriz W. Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol Regul Integr Comp Physiol 241: R3–R16, 1981 [DOI] [PubMed] [Google Scholar]
- 18. Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla: II. Functional implications of three-dimensional architecture. Am J Physiol Renal Physiol 300: F372–F384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Layton AT, Gilbert RL, Pannabecker TL. Isolated interstitial nodal spaces may facilitate preferential solute and fluid mixing in the rat renal inner medulla. Am J Physiol Renal Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Two modes for concentrating urine in rat inner medulla. Am J Physiol Renal Physiol 287: F816–F839, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973–F987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Hyperfltration and inner-stripe hypertrophy may explain fndings by Gamble and co-workers. Am J Physiol Renal Physiol 298: F962–F972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Layton HE. Concentrating urine in the inner medulla of the kidney. Comments Theor Biol 1: 179–196, 1989 [Google Scholar]
- 24. Lemley KV, Kriz W. Cycles and separations: the histotopography of the urinary concentrating process. Kidney Int 31: 538–548, 1987 [DOI] [PubMed] [Google Scholar]
- 25. McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Pannabecker TL. Loop of Henle interaction with interstitial nodal spaces in the renal inner medulla. Am J Physiol Renal Physiol 295: F1744–F1751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pannabecker TL. Structure and function of the thin limbs of the loops of Henle. In: Comprehensive Physiology. Hoboken NJ: Wiley, In press [DOI] [PubMed] [Google Scholar]
- 29. Pannabecker TL, Abbott DE, Dantzler WH. Three-dimensional functional reconstruction of inner medullary thin limbs of Henle's loop. Am J Physiol Renal Physiol 286: F38–F45, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767–F774, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Pannabecker TL, Dantzler WH. Three-dimensional architecture of collecting ducts, loops of Henle, and blood vessels in the renal papilla. Am J Physiol Renal Physiol 293: F696–F704, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pannabecker TL, Henderson C, Dantzler WH. Quantitative analysis of functional reconstructions reveals lateral and axial zonation in the renal inner medulla. Am J Physiol Renal Physiol 294: F1306–F1314, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Pruitt MEC, Knepper MA, Graves B, Schmidt-Nielsen B. Effect of peristaltic contractions of the renal pelvic wall on solute concentrations of the renal inner medulla in the hamster. Am J Physiol Renal Physiol 290: F892–F896, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sands JM, Layton HE. The urine concentrating mechanism and urea transporters. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. Philadelphia, PA: Elsevier, 2007, p. 1143–1177 [Google Scholar]
- 37. Schmidt-Nielsen B, Schmidt-Nielsen K, Brokaw A, Schneiderman H. Water conservation in desert rodents. J Cell Comp Physiol 32: 331–360, 1948 [Google Scholar]
- 38. Schwartz MM, Venkatachalam MA. Structural differences in thin limbs of Henle: physiological implications. Kidney Int 6: 193–208, 1974 [DOI] [PubMed] [Google Scholar]
- 39. Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int 2: 85–94, 1972 [DOI] [PubMed] [Google Scholar]
- 40. Uchida S, Sasaki S, Furakawa T, Hiraoka M, Imai T, Hirata Y, Marumo F. Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in the kidney medulla. J Biol Chem 268: 3821–3824, 1993 [PubMed] [Google Scholar]
- 41. Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F. Localization and functional characterization of rat kidney-specific chloride channel. J Clin Invest 95: 104–113, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vimtrup BJ, Schmidt-Nielsen B. The histology of the kidney of kangaroo rats. Anat Rec 114: 515–528, 1952 [DOI] [PubMed] [Google Scholar]
- 43. Yuan J, Pannabecker TL. Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange. Am J Physiol Renal Physiol 299: F265–F272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


