Abstract
Glucose in the gut lumen activates gut endocrine cells to release 5-HT, glucagon-like peptide 1/2 (GLP-1/2), and glucose-dependent insulinotropic polypeptide (GIP), which act to change gastrointestinal function and regulate postprandial plasma glucose. There is evidence that both release and action of incretin hormones is reduced in type 2 diabetes (T2D). We measured cellular activation of enteroendocrine and enterochromaffin cells, enteric neurons, and vagal afferent neurons in response to intestinal glucose in a model of type 2 diabetes mellitus, the UCD-T2DM rat. Prediabetic (PD), recent-diabetic (RD, 2 wk postonset), and 3-mo diabetic (3MD) fasted UCD-T2DM rats were given an orogastric gavage of vehicle (water, 0.5 ml /100 g body wt) or glucose (330 μmol/100 g body wt); after 6 min tissue was removed and cellular activation was determined by immunohistochemistry for phosphorylated calcium calmodulin-dependent kinase II (pCaMKII). In PD rats, pCaMKII immunoreactivity was increased in duodenal 5-HT (P < 0.001), K (P < 0.01) and L (P < 0.01) cells in response to glucose; glucose-induced activation of all three cell types was significantly reduced in RD and 3MD compared with PD rats. Immunoreactivity for GLP-1, but not GIP, was significantly reduced in RD and 3MD compared with PD rats (P < 0.01). Administration of glucose significantly increased pCaMKII in enteric and vagal afferent neurons in PD rats; glucose-induced pCaMKII immunoreactivity was attenuated in enteric and vagal afferent neurons (P < 0.01, P < 0.001, respectively) in RD and 3MD. These data suggest that glucose sensing in enteroendocrine and enterochromaffin cells and activation of neural pathways is markedly impaired in UCD-T2DM rats.
Keywords: 5-hydroxytryptamine, intestinal glucose, incretin
type 2 diabetes mellitus (T2DM) is associated with the development of obesity and characterized by the impaired ability of gut hormones to regulate plasma levels of glucose and alterations in energy balance (10). A substantial portion of insulin secretion in response to oral ingestion of glucose is mediated via release of the incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide (GLP-1). GIP and GLP-1 are released from K and L gut enteroendocrine cells (EECs), respectively, in response to luminal glucose and augment glucose-stimulated insulin secretion (20, 25). However, patients with T2D demonstrate an impaired incretin response resulting in insufficient insulin secretion from the pancreatic β-cell in response to ingestion of glucose (27). Whether this impairment of the incretin response in T2D is due to altered release of incretins and impaired incretin action at the pancreatic β-cell has been an area of controversy. There is evidence that plasma levels of GIP following oral glucose are not different between healthy controls and patients with T2D, but that there is a decrease in the release of GLP-1 (39). However, other evidence suggests that decrease β-cell response to incretin hormones in T2D patients (9). Thus altered function in gut endocrine cells may play a role in the pathogenesis of T2DM; however, in vivo studies looking at direct activation of these cell populations have been limited, in part, due to the difficulty in studying this cell population in vivo.
Glucose-sensing mechanisms by K and L cells have been extensively studied using in vivo models and EEC lines and have identified a number of possible mechanisms. Recent studies using cultures of highly purified K or L cell culture have suggested that KATP channels and possibly the sodium-glucose cotransporter SGLT-1 play a predominant role in glucose sensing (14, 26, 35). There is also evidence that taste transduction elements including the G protein-coupled sweet taste receptors T1R1/3 may also sense glucose in the gut (18).
Luminal glucose also induces release of 5-hydroxytryptamine (5-HT) from enterochromaffin (EC) cells in the gut wall. Receptors for 5-HT are expressed by many different cell types in the gut wall, and 5-HT activates intrinsic reflexes to regulate motor and secretory function. 5-HT also activates extrinsic, vagal afferent terminals located in the gut wall via 5-HT3 receptors (5-HT3Rs) to activate a vago-vagal reflex to inhibit gastric emptying and stimulate pancreatic exocrine secretion (19, 24). Interestingly, there is evidence that vagal efferent outflow can also influence the secretion of incretin hormones to maintain glucose homeostasis (4, 29, 37). Loss of vagal function also results in a reduced incretin effect, demonstrating the importance of peripheral neuroendocrine loop to regulate plasma glucose in the postprandial period (29).
The UCD-T2DM rat is a relatively new animal model of human T2DM demonstrating polygenic adult-onset obesity and insulin resistance accompanied by β-cell dysfunction. It more closely models the pathophysiology of adult-onset T2DM in humans than other rodent models of the disease (7). We hypothesized that in T2DM, glucose sensing in the gut wall would be impaired. During the onset and progression of T2DM, cellular activation of ECs and EECs in response to luminal glucose would be reduced along with diminished activation of the gut-brain axis. We have recently developed a method for measuring activation of gut EECs and ECs in situ, avoiding the use of either cell lines or isolated cell preparation (40). This technique uses detection of phosphorylated calcium calmodulin-dependent kinase II (pCaMKII) by immunohistochemistry as a marker of cellular activation. Moreover, the ability to conduct experiments in unanesthetized rats allows us to assess the activation of intrinsic and extrinsic neurons along the gut-brain axis.
MATERIALS AND METHODS
Animals.
The UCD-T2DM model was developed by breeding Sprague-Dawley rats with adult-onset obesity and insulin resistance with Zucker diabetic fatty-lean rats. UCD-T2DM rats have a pancreatic β-cell function defect in combination with polygenic adult onset obesity that results in development of T2DM, which closely resembles the pathophysiology of T2DM in humans (7). These rats have no defect in leptin receptor signaling. Male UCD-T2DM rats classified as prediabetic (PD), recently diabetic (RD, 2 wk postonset), or long-term diabetic (3MD, 3-mo postonset) were given ad libitum access to standard chow and water and housed in a controlled environment with a 10-h:14-h light/dark cycle. Diabetes onset is defined as a nonfasted blood glucose concentration greater than 200 mg/dl on two consecutive weeks. Animals were maintained and handled in accordance with protocols approved by the institutional Animal Care and Use Committee (University of California, Davis).
Experiments and tissue collection.
Body weight was recorded throughout the experimental period. Rats were fasted overnight in wire-bottom cages with free access to water. Rats were given an oral gavage of vehicle (0.5 ml water/100 g body wt) or glucose (0.5 ml of 330 μmol/100 g body wt); 6 min following gavage, rats were deeply anesthetized with pentobarbital sodium (100 mg/kg ip) and transcardially perfused with 0.9% NaCl with 0.1% heparin at 4°C followed by 1 ml/g body wt of 4% paraformaldehyde dissolved in PBS (PFA-PBS) at 4°C. Small intestine and nodose ganglia were rapidly removed and postfixed for 2 h in 4% PFA-PBS before transfer and storage at 4°C in 25% sucrose dissolved in PBS until processing.
Immunohistochemistry.
A portion of the duodenum, ∼7 mm × 7 mm, was taken about 2 cm distal of the pyloric-duodenal junction. Frozen horizontal sections of 8 μm thickness from duodenal crypts were cut by cryostat and mounted onto coated slides (Fisher Superfrost Plus, Fisher Scientific, Pittsburgh, PA). Six to ten tissue sections for each antibody combination were cut from tissue from each rat. Slides were desiccated on a warming table and washed in 0.1 M phosphate buffer (PBS, 3× 10 min each), then blocked with 20% goat serum dissolved in PBS for 30 min at 37°C incubation.
The following antibodies were employed in this study: anti-pCaMKII (1:200 dilution, pT286 rabbit IgG polyclonal antibody, V111A, Promega, Madison, WI); anti-serotonin (5-HT) (1:1,000 dilution, mouse monoclonal antibody, MO758, Dako, Carpinteria, CA); anti-GIP (1:100 dilution, rabbit polyclonal antibody, Chemicon, Temecula, CA); and anti-GLP-1 (diluted 1:100, rabbit polyclonal antibody, ab22625, Abcam, Cambridge, MA). All primary antibodies were incubated at 37°C for 2.5 h. After 3× 10-min PBS washes, tissues were incubated with secondary antibodies (goat anti-rabbit IgG AlexaFluor 488, A11034; goat anti-mouse AlexaFluor 546, A11030; or goat anti-rabbit IgG AlexaFluor 546, A11035, Molecular Probes, Eugene, OR). Secondary antibodies were diluted 1:500 in 2% GS-PBS and incubated at 37°C for 30 min.
Tissue sections were double labeled with two different primary antibodies. If primary antibodies were from two different species, antibodies were coincubated. If the antibodies were both rabbit polyclonal antibodies, the incubations were sequential. The anti-5-HT and pCaMKII antibodies were made in different species, incubated together, and identified with anti-mouse 546 and anti-rabbit 488 secondary antibodies, respectively. The anti-pCaMKII and anti-GIP/anti-GLP-1 are all rabbit polyclonal antibodies, thus requiring a sequential immunohistochemistry procedure. One complete immunohistochemistry protocol was completed for the first primary antibody (anti-pCaMKII), which was followed immediately by a complete protocol for the second primary antibody (anti-GIP or anti-GLP-1). The first primary antibody (anti-pCaMKII) was incubated at 37°C for 2.5 h followed by 3× 10-min PBS washes. The first secondary antibody (anti-rabbit 488) was added and incubated for 30 min at 37°C. Slides were then washed with 0.1 M PBS 1× 10 min at room temperature followed by an overnight wash at 4°C in PBS. The second blocking step consisted of two steps; tissue was blocked with 20% normal rabbit serum (S-5000,Vector Labs, Burlingame, CA) in PBS for 30 min at 37°C followed with 3× PBS washes. Second, slides were incubated with monovalent unconjugated goat anti-rabbit F(ab) (111–007-003, Jackson ImmunoResearch, West Grove, PA) diluted 20 μg/ml for 30 min at 37°C. These steps prevent nonspecific binding of the second primary and secondary antibodies. After 3× PBS washes, the second primary antibody, either anti-GIP or anti-GLP-1, was incubated for 2.5 h at 37°C. After 3× PBS washes, the second secondary antibody goat anti-rabbit 546 was incubated for 30 min at 37°C. Slides were then washed with 0.1 M PBS 1× 10 min at room temperature followed by an overnight wash at 4°C in PBS.
Slides were coverslipped using GelMount (Biomeda, Foster City, CA) and dried in the dark at room temperature. Slides were stored at −20°C until imaging. Fixed neural tissues of nodose ganglia had identical slide preparation as gut tissue; the primary antibody was pCaMKII and goat anti-rabbit IgG conjugated AlexaFluor 488 secondary antibody.
Specificity of the anti-pCaMKII antibody was confirmed with a CaMKII (phosphor Thr286) blocking peptide from GenScript (RP 19957), which blocks labeling by the Promega anti-pCaMKII antibody under the experimental conditions outlined above.
Image acquisition and analysis.
Images were made with a confocal microscope (Bio-Rad, Radiance System 2100, Hercules, CA; Olympus Confocal Microscope, Center Valley, PA) using exactly the same acquisition parameters for each image for a specific tissue or cell type. Images were changed to a gray-level image in Photoshop (Adobe Systems, San Jose, CA) and then analyzed in Scion Image (Scion, Frederick, MD). Pixels above a threshold brightness were considered to be immunopositive and were then summed by the computer software. The threshold level was determined by the investigator and applied uniformly. The threshold value was typically chosen at a brightness level three times that of background for a specific cell type or tissue.
K cells (GIP), L cells (GLP-1), and 5-HT cells were analyzed as individual cells. Scion software was used to draw around the plasma membrane of the cell; this defined a region of interest to be analyzed. This threshold value was kept constant for all analyzed cells. Pixels at or above the threshold were counted as immunopositive. The number of immunopositive pixels was summed by the software for the entire cell. The whole area of the cell, in pixels, was determined by adjusting the threshold so that the entire area of the cell could be determined. Next the region of the cell's nucleus was drawn around, and the number of labeling pixels in the nucleus and the number of pixels in the entire nucleus was determined. With the use of these values, the percent labeled pixels in the whole cell and in the nucleus can be calculated. The number of labeled pixels in the nucleus was subtracted from the whole cell to give the number of labeled pixels in the cytoplasm. For proteins such as pCaMKII, which is not found in the nucleus, any labeling in the nucleus was considered as background labeling. If the nucleus has 0.2% pCaMKII labeling and the cytoplasm has 5% labeling, the labeling in the cytoplasm was normalized by subtracting the percent labeling in the nucleus from the cytoplasmic labeling. In this example it would be 4.8%.
For the submucosal and myenteric plexus, the number of pixels for the entire plexus was determined by using the software to draw around the margin of the plexus, raising the threshold to saturation and measuring the total number of pixels in the plexus. Dividing the area of labeled pixels by the total area of the plexus × 100 then results in an index of pCaMKII expression in terms of percent labeled pixels for the plexus.
Quantification of immunoreactivity is presented as either percent labeled pixels or percent labeled cells over total area normalized by a set baseline threshold value as previously described (40). A cell was considered to be a labeled, reactive, cell if 20% or more of its cytoplasmic area was immunopositive for pCaMKII.
Statistical analysis.
A total of 27 rats were used in this study; n = 9 PD, RD, and 3MD, respectively. Data were analyzed using Scion Image software (NIH Image for Windows, Beta 4.0.2 Scion, 2000) for individual neuron images and data analyzed by Prism GraphPad (v.5.02) and are represented as mean values ± SE and analyzed by one-way ANOVA to determine statistical significance (P < 0.05), followed by Bonferroni's post-hoc test.
RESULTS
Metadata.
The mean age of the rats at time of experiment were 128 ± 5, 122 ± 4, and 222 ± 20 days old, with body weights of 594 ± 16, 582 ± 18, and 562 ± 18 g and nonfasting glucose concentration of 145 ± 6, 432 ± 35, and 476 ± 23 mg/dl for PD, RD, and 3MD, respectively. The RD rats had been diabetic for 10 ± 1 days and the 3MD for 93 ± 1 days.
pCaMKII immunoreactivity in duodenal 5-HT, GIP, and GLP-1 immunoreactive cells in response to glucose in T2DM rats.
Gavage of glucose produced a significant increase in pCaMKII expression in 5-HT-immunoreactive cells in PD rats compared with vehicle treatment. This increase in immunoreactive pCaMKII to glucose was significantly reduced in RD and 3MD rats to near nadir levels (Fig. 1, A and B; P < 0.001). In addition, there was a reduction in 5-HT immunoreactivity in 3MD but not RD compared with PD rats (Fig. 1C), although this did not reach statistical significance. Immunoreactivity for pCaMKII in duodenal K cells was significantly increased in response to intestinal glucose in PD rats compared with vehicle treatment, but there was no increase in response to glucose in RD and 3MD rats (Fig. 2, A and B, P < 0.01). Similarly, glucose-induced expression of pCaMKII in duodenal L cells was increased in PD rats but not in RD and 3MD rats (Fig. 3, A and B; P < 0.01). There was a significant reduction in immunoreactivity for GLP-1 in L cells of both RD and 3MD rats compared with PD rats (32% and 33% of PD levels, respectively) (Fig. 3, A and C; P < 0.01); however, there was no change in GIP immunoreactivity with the three diabetic groups (Fig. 2C).
Fig. 1.
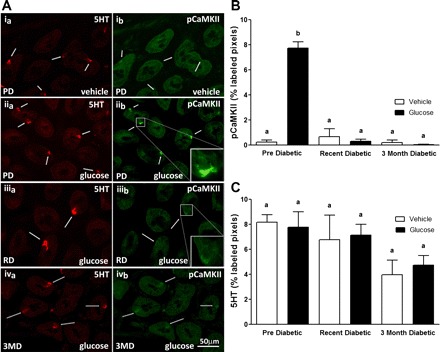
A: photomicrographs to show immunoreactivity for 5-HT (a; left, red) and phosphorylated CaMKII (b; right, green) in sections of duodenum following oral gavage of water (vehicle) (i) in prediabetic (PD) rats or glucose in PD (ii), recently diabetic (RD; iii), or 3 mo diabetic (3MD; iv) rats. In PD rats, orogastric gavage of glucose induced a significant increase in pCaMKII immunoreactivity compared with administration of water as indicated by numerous pCaMKII-labeled cells (ib vs. iib). The level of immunoreactive pCaMKII in enterochromaffine cells (ECs) following administration of glucose in RD and 3MD rats is much reduced compared with PD rats (iib vs. iiib and ivb). B: quantitative analysis of pCaMKII immunoreactivity in EC cells; pCaMKII in response to glucose gavage was significantly reduced in RD and 3MD rats compared with PD rats. C: quantitative analysis of 5-HT immunoreactivity in ECs from vehicle-treated PD, RD, and 3MD rats; there was a trend for a decrease in 5-HT immunoreactivity in the duodenum of 3MD rats compared with PD, but this did not reach statistical significance. Data are expressed as means ± SE, n = 3–5 rats per group, 48–67 cells/analyzed per treatment group. Significant differences between PD and RD or 3MD denoted by different letters; P < 0.001.
Fig. 2.
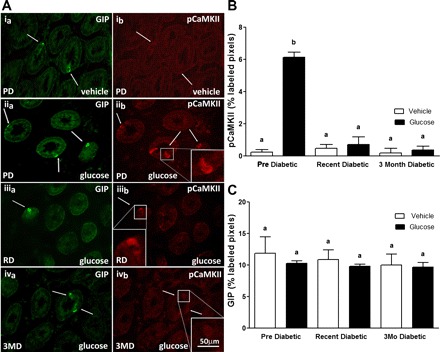
A: photomicrographs to show immunoreactivity for glucose-dependent insulinotropic polypeptide (GIP) (a; left, green) and pCaMKII (b; right, red) in sections of duodenum following oral gavage of vehicle (i) in PD rats or glucose in PD (ii), RD (iii), or 3MD (iv) rats. In PD rats, orogastric gavage of glucose induced a significant increase in pCaMKII immunoreactivity compared with administration of water as indicated by pCaMKII-labeled cells (ib vs. iib). After administration of glucose in RD and 3MD rats, levels of pCaMKII immunoreactivity in GIP cells were much reduced compared with PD rats (iib vs. iiib and ivb). B: quantitative analysis of pCaMKII-IR in GIP cells; pCaMKII-IR in response to glucose gavage was significantly reduced in RD and 3MD rats compared with PD rats. C: quantitative analysis of GIP immunoreactivity showed no significant difference in GIP expression between PD, RD, and 3MD rats. Data are expressed as means ± SE, n = 3–4 rats per group; 31–44 cells analyzed/treatment group. Significant differences between PD and RD or 3MD denoted by different letters; P < 0.01.
Fig. 3.
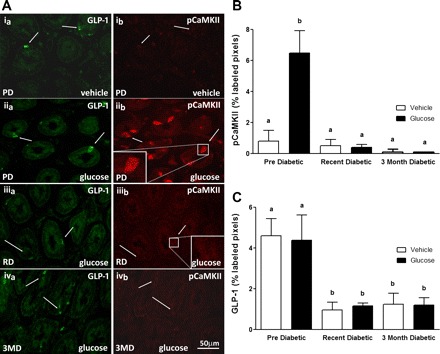
A: photomicrographs to show immunoreactivity for glucagon-like peptide-1 (GLP-1) (a; left, green) and pCaMKII (b; right, red) in sections of duodenum following oral gavage ofvehicle (water) (i) in PD rats or glucose in PD (ii), RD(iii) or 3MD (iv) rats. In PD rats, orogastric gavage of glucose induced a significant increase in pCaMKII immunoreactivity compared with administration of water as indicated by pCaMKII-labeled cells (ib vs. iib). After administration of glucose in RD and 3MD rats, levels of immunoreactive pCaMKII in GLP-1 cells are much reduced compared with PD rats (iib vs. iiib and ivb). B: quantitative analysis of pCaMKII immunoreactivity in GLP-1 cells; pCaMKII expression in response to glucose gavage was significantly reduced in RD and 3MD rats compared with PD rats. C: quantitative analysis of GLP-1 immunoreactivity shows a significant decrease in levels in RD and 3MD rats compared with PD controls. Data are expressed as means ± SE, n = 3–4 rats per group; 32–44 cells analyzed per group. Significant differences between PD and RD or 3MD denoted by different letters a and b; P < 0.01.
pCaMKII immunoreactivity in enteric and vagal afferent neurons in response to glucose in T2DM rats.
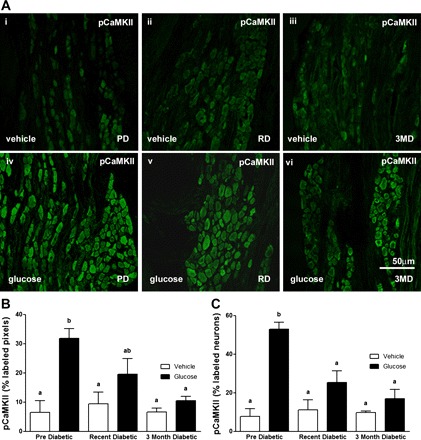
There was minimal neuronal activation in the submucosal or myenteric plexus following orogastric gavage with vehicle (Figs. 4A and 5A); glucose treatment induced a significant increase in pCaMKII expression in both the submucosal and myenteric plexus from PD rats (Figs. 4 and 5). Glucose-induced pCaMKII immunoreactivity was significantly decreased in both the submucosal and myenteric plexus in RD and 3MD compared with PD rats (Figs. 4 and 5; P < 0.01).
Fig. 4.
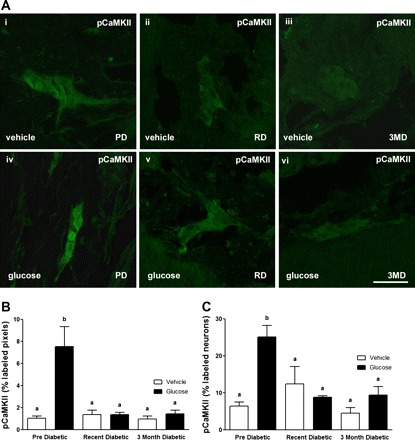
A: photomicrographs to show immunoreactivity for pCaMKII in the submucosal plexus of the duodenum from PD, RD, and 3MD rats following orogastric gavage with vehicle (i–iii, top) or glucose (iv–vi, bottom). B and C: quantification of immunoreactivity for pCaMKII in submucosal neurons in response to orogastric gavage or vehicle administered to PD, RD, and 3MD rats. B: data expressed as percent labeled pixels. C: data expressed as percent labeled neurons. Data are expressed as means ± SE, n = 4–5 rats per group; 39–50 plexuses imaged and 197–361 neurons/treatment group analyzed. Significant differences between PD and RD or 3MD denoted by different letters; P < 0.01. Scale bar = 50 μm.
Fig. 5.
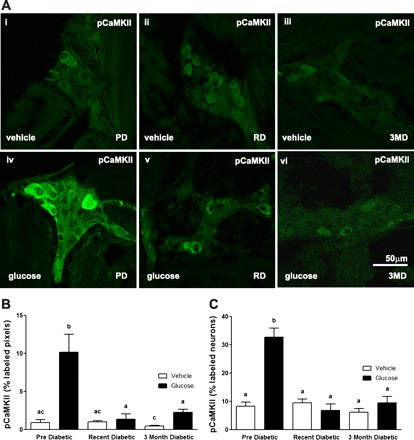
A: photomicrographs to show immunoreactivity for pCaMKII in the myenteric plexus of the duodenum from PD, RD, and 3MD rats following orogastric gavage with vehicle (ddH2O) (top) or glucose (bottom). B: quantification of photomicrographs for immunoreactivity for pCaMKII in myenteric neurons in response to orogastric gavage of glucose or vehicle administered to PD, RD, and 3MD rats. B: data expressed as percent labeled pixels. C: data expressed as percent labeled neurons. Data are expressed as means ± SE, n = 3–4 rats per group; 30–40 plexuses imaged and 467–516 neurons/treatment group analyzed. Significant differences between PD and RD or 3MD denoted by different letters; P < 0.01. Scale bar = 50 μm.
Similarly, activation of vagal afferent neurons in response to intragastric gavage with glucose was significantly attenuated with the onset and progression of diabetes. pCaMKII expression in nodose neurons was significantly increased in PD rats treated with glucose compared with vehicle treatment; this was significantly reduced in RD and 3MD rats to a level that was not significantly different from PD rats treated with vehicle (Fig. 6, A and B; P < 0.001).
Fig. 6.
A: photomicrographs to show immunoreactivity for pCaMKII in nodose ganglia from PD, RD, and 3MD rats following orogastric gavage with vehicle or glucose. B: quantification of photomicrographs for immunoreactivity for pCaMKII in nodose neurons in response to orogastric gavage of glucose. Data are expressed as means ± SE, n = 3–6 rats per group; 477–1656 cells imaged/treatment. Significant differences between PD and RD or 3MD denoted by different letters P < 0.001. Scale bar = 50 μm.
DISCUSSION
In the present study, we investigated whether glucose sensing in gut EECs and ECs and in intrinsic and vagal afferent neurons is impaired in a rodent model of T2DM. The data show that glucose-induced activation of 5-HT, GIP, GLP-1 containing cells, as well as neurons of the ENS and the vagal pathway are all markedly impaired in UCD-T2DM rats. This suggests that T2DM is associated with impaired glucose sensing in the gut wall and transmission of this information via the vagus nerve to the central nervous system. The inability of gut ECs and EECs to respond to glucose and mount an adequate incretin response or reflex changes regulate motor and secretory function likely impairs the postprandial response to a meal and may lead to dysregulation of postprandial levels of plasma glucose.
In this study, we used pCaMKII immunoreactivity as an index of cellular activation following in vivo treatment of rats with intragastric glucose (31). This technique has the clear advantage of being able to study the response of EECs and ECs in situ, where the cells remain part of an intact and polarized epithelium. Moreover, this technique allows for the study of activated neuronal pathways downstream to the activation of gut epithelial cells. Thus the possible consequences of defective glucose sensing in the gut EECs and ECs on activation of intrinsic and extrinsic reflex pathways can be determined. One possible confounding factor may be differences in gastric emptying between prediabetic and diabetic rats; we have not determined the rate of gastric emptying in this model. There is conflicting data on the rate of gastric emptying of liquids from patients with T2D, with studies showing no difference in emptying rates of glucose in T2D patients and healthy controls and another showing more rapid emptying of glucose in T2D patients (2, 17). Similarly, OLETF rats demonstrate no deficit in gastric emptying relative to control rats (8), whereas streptozotocin-induced diabetic mice have accelerated gastric emptying (38).
Serotonin is an amine mediator that is abundant in the gut wall. We have previously shown that glucose induces the release of 5-HT from ECs; 5-HT subsequently binds to 5-HT3Rs on vagal afferent nerve terminals in the gut mucosa to activate vago-vagal reflexes important in regulation of gastric emptying as well as mediating glucose-induced reductions in food intake (11, 30, 42). Previous work has suggested that 5-HT cell content of the gut is reduced in rodent models of obesity and diabetes compared with control animals (3, 32, 33), but activation of these cells in vivo in the UCD-T2DM model has not been previously investigated. We observed that activation of 5-HT cells is markedly reduced in RD and 3MD rats. The mechanisms leading to this attenuated cellular activation in response to glucose is not clear and was not investigated in this study but may involve alterations in the sensing mechanism itself, such as changes in expression of SGLTs, sweet-taste receptors or other proteins, or may be due to more nonselective changes in intracellular pathways involved in secretion from the gut EECs. Reduced activation of EECs may contribute to the overall reduced ability to sense luminal glucose, leading to the hyperphagia observed in untreated T2DM.
Impaired incretin action of GIP and GLP-1 has been implicated as a large contributing factor in the development and progression of T2DM (5). This is supported by observations that nutrient-stimulated insulin secretion is blunted with reduced incretin function in T2DM (1, 16). However, the mechanisms by which GLP-1 and GIP activity are attenuated appear to be different since diabetic subjects remain sensitive to exogenously administered GLP-1 but become insensitive to exogenous GIP (23). Our finding that glucose-induced activation of K cells as measured by pCaMKII is reduced in rats with T2DM suggests that GIP cells may have an impaired ability to respond to luminal glucose. This is consistent with observations that insulinotropic activity of GIP is reduced in T2DM (21), suggesting that there may be a defect in K cell signaling and secretion.
Our finding that GLP-1 hormone content and L cell activation is reduced in UCD-T2DM rats is in line with previous reports showing reduced plasma GLP-1 activity in animal models of T2DM and in diabetic humans (34, 39). GLP-1 secretion from the distal ileum is dependent, at least in part, on a GIP-driven enteroendocrine reflex loop (28). Under diabetic conditions, reduced GIP release from K cells may lead to a decrease in stimulation of GLP-1 release from distal L cells. We did not find any differences in ileal GLP-1 content (unpublished observations) between pre- and diabetic rats, suggests that there may be differences in the population of L cells in the proximal versus the distal gut. Overall, the impairment of duodenal K and L cell activity could be major factors contributing to impaired regulation of postprandial plasma glucose in the UCD-T2DM rat.
The enteric nervous system is essential for monitoring gut function and glucose homeostasis and has been shown to be compromised in diabetes (15, 36, 41). Here, neuronal activation in response to glucose was significantly reduced within both the submucosal and myenteric plexuses of rats with T2DM. Whether the impaired neuronal response is due to decreased release of 5-HT, for example, is not clear. However, we cannot rule out the possibility that reduced neuronal response may be due to compromised neuronal function. Chemically induced diabetes has been shown to be associated with altered enteric neuron size, population, and neurodegenerative changes in rodent models (6, 22); there was no evident change in the enteric neurons observed in the present study, but this was not systemically examined.
Vagal efferent neuronal dysfunction has been demonstrated in rodents and patients with T2DM (12, 33), although little attention has previously been paid to afferent neural response to nutrient stimuli. This is the first study to demonstrate impaired activation along the vagal afferent pathway in a model of T2DM. pCaMKII immunoreactivity was significantly elevated in nodose ganglia of prediabetic UCD-T2DM rats following glucose gavage, a response previously shown to be induced by glucose and not caused by an osmotic effect (40); around 26% of nodose neurons were activated by glucose, which is higher than in our previous study. It should be noted that not only are these a different strain of rats but that all of the “prediabetic” controls would eventually go on to develop T2D and thus there maybe some alteration in signaling or neuronal function even in the prediabetic group. This activation may be due to release of 5-HT or GLP-1 from gut EECs and ECs, as vagal afferents express receptors for both. In addition, there is some evidence for direct sensing of glucose by vagal afferent neurons (13). Reduced vagal afferent neuron activity in T2DM may be due, in part, to decreased 5-HT and incretin hormone release, leading to impaired vagal afferent response to glucose in the UCD-T2DM rat.
Perspectives and Significance
We have demonstrated that diabetes progression in the UCD-T2DM rat is associated with reductions in 5-HT, L, and K cell signaling, and subsequent decreases in activity of the enteric nervous system and vagus nerve. This decreased ability to detect luminal glucose and to mount appropriate humoral, enteric, and vago-vagal reflex will likely contribute to altered regulation of glucose homeostasis, insulin secretion, and food intake in the postprandial period in T2DM.
Table 1.
Number of rats, enteric plexuses, nodose ganglia, and epithelial cells used for image analysis
| Prediabetic |
Recent Diabetic |
3 Mo Diabetic |
||||
|---|---|---|---|---|---|---|
| H2O | Glucose | H2O | Glucose | H2O | Glucose | |
| 5HT | ||||||
| rats | 4 | 4 | 3 | 5 | 3 | 4 |
| cells | 52 | 57 | 48 | 67 | 48 | 56 |
| GIP | ||||||
| rats | 4 | 4 | 3 | 3 | 3 | 3 |
| cells | 44 | 42 | 33 | 31 | 31 | 34 |
| GLP-1 | ||||||
| rats | 4 | 4 | 3 | 3 | 3 | 3 |
| cells | 44 | 43 | 39 | 32 | 36 | 33 |
| Submucosal Plexus | ||||||
| rats | 4 | 4 | 4 | 5 | 4 | 4 |
| plexuses | 40 | 40 | 39 | 50 | 40 | 40 |
| cells | 197 | 215 | 201 | 361 | 202 | 274 |
| Myenteric Plexuses | ||||||
| rats | 3 | 4 | 3 | 4 | 3 | 4 |
| plexuses | 32 | 40 | 31 | 40 | 30 | 40 |
| cells | 617 | 496 | 401 | 653 | 620 | 467 |
| Nodose Ganglia | ||||||
| rats | 3 | 6 | 3 | 6 | 3 | 6 |
| cells | 1,202 | 1,548 | 1,656 | 1,118 | 477 | 813 |
GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L., J.W.S., P.J.H., and H.E.R. conception and design of research; J.L., B.P.C., E.M., J.W.S., J.L.G., and K.L.S. performed experiments; J.L., E.M., and J.W.S. analyzed data; J.L., E.M., J.W.S., and H.E.R. interpreted results of experiments; J.L. and J.W.S. prepared figures; J.L., J.W.S., and H.E.R. drafted manuscript; J.L., B.P.C., J.W.S., K.L.S., P.J.H., and H.E.R. edited and revised manuscript; J.L., B.P.C., E.M., J.W.S., J.L.G., K.L.S., P.J.H., and H.E.R. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was funded by National Institutes of Health (NIH) DK 58588 (to H. E. Raybould) and DK-087307 (to P. J. Havel), and AT-002993 (to P. J. Havel). P. J. Havel's research program also receives support from NIH Grants HL-091333, DK-063616, and a multicampus award from the University of California, Office of the President (MRPI-5998SC).
REFERENCES
- 1. Ayala JE, Bracy DP, Hansotia T, Flock G, Seino Y, Wasserman DH, Drucker DJ. Insulin action in the double incretin receptor knockout mouse. Diabetes 57: 288–297, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsboll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 96: 737–745, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Belai A, Lincoln J, Milner P, Burnstock G. Progressive changes in adrenergic, serotonergic, and peptidergic nerves in proximal colon of streptozotocin-diabetic rats. Gastroenterology 95: 1234–1241, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19: 1–19, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 145: 2653–2659, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil 19: 951–960, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 295: R1782–R1793, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Jonghe BC, Hajnal A, Covasa M. Decreased gastric mechanodetection, but preserved gastric emptying, in CCK-1 receptor-deficient OLETF rats. Am J Physiol Gastrointest Liver Physiol 291: G640–G649, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53: 624–632, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24–32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freeman SL, Glatzle J, Robin CS, Valdellon M, Sternini C, Sharp JW, Raybould HE. Ligand-induced 5-HT3 receptor internalization in enteric neurons in rat ileum. Gastroenterology 131: 97–107, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Gaddipati KV, Simonian HP, Kresge KM, Boden GH, Parkman HP. Abnormal ghrelin and pancreatic polypeptide responses in gastroparesis. Dig Dis Sci 51: 1339–1346, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Grabauskas G, Song I, Zhou S, Owyang C. Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia. J Physiol 588: 617–632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Guo C, Quobatari A, Shangguan Y, Hong S, Wiley JW. Diabetic autonomic neuropathy: evidence for apoptosis in situ in the rat. Neurogastroenterol Motil 16: 335–345, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hansotia T, Baggio LL, Delmeire D, Hinke SA, Yamada Y, Tsukiyama K, Seino Y, Holst JJ, Schuit F, Drucker DJ. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 53: 1326–1335, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Horowitz M, O'Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med 19: 177–194, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104: 15069–15074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masuda M, Miyasaka K, Funakoshi A. Involvement of 5-hydroxytryptamine (5-HT)3 receptor mechanisms in regulation of basal pancreatic secretion in conscious rats. J Auton Nerv Syst 62: 58–62, 1997 [DOI] [PubMed] [Google Scholar]
- 20. McCullough AJ, Miller LJ, Service FJ, Go VL. Effect of graded intraduodenal glucose infusions on the release and physiological action of gastric inhibitory polypeptide. J Clin Endocrinol Metab 56: 234–241, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Meier JJ, Nauck MA. Incretins and the development of type 2 diabetes. Curr Diab Rep 6: 194–201, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Monckton G, Pehowich E. Autonomic neuropathy in the streptozotocin diabetic rat. Can J Neurol Sci 7: 135–142, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284: G367–G372, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 51: 2757–2763, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532–539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rijkelijkhuizen JM, McQuarrie K, Girman CJ, Stein PP, Mari A, Holst JJ, Nijpels G, Dekker JM. Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism 59: 502–511, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 133: 233–240, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140: 1687–1694, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Savastano DM, Covasa M. Intestinal nutrients elicit satiation through concomitant activation of CCK(1) and 5-HT(3) receptors. Physiol Behav 92: 434–442, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Soderling TR, Chang B, Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 276: 3719–3722, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Spangeus A, Kand M, El-Salhy M. Gastrointestinal endocrine cells in an animal model for human type 2 diabetes. Dig Dis Sci 44: 979–985, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Takahara H, Fujimura M, Taniguchi S, Hayashi N, Nakamura T, Fujimiya M. Changes in serotonin levels and 5-HT receptor activity in duodenum of streptozotocin-diabetic rats. Am J Physiol Gastrointest Liver Physiol 281: G798–G808, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86: 3717–3723, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 587: 27–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tougas G, Hunt RH, Fitzpatrick D, Upton AR. Evidence of impaired afferent vagal function in patients with diabetes gastroparesis. Pacing Clin Electrophysiol 15: 1597–1602, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Uneyama H, Torii K. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology 137: 262–273, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Verhulst PJ, De Smet B, Saels I, Thijs T, Ver Donck L, Moechars D, Peeters TL, Depoortere I. Role of ghrelin in the relationship between hyperphagia and accelerated gastric emptying in diabetic mice. Gastroenterology 135: 1267–1276, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50: 609–613, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol Motil 23: e282–e293, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yagihashi S, Sima AA. Diabetic autonomic neuropathy in the BB rat. Ultrastructural and morphometric changes in sympathetic nerves. Diabetes 34: 558–564, 1985 [DOI] [PubMed] [Google Scholar]
- 42. Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 530: 431–442, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


