Abstract
Total knee arthroplasty (TKA) utilizes a tourniquet to reduce blood loss, maintain a clear surgical “bloodless” field, and to ensure proper bone-implant cementing. In 2007, over 600,000 TKAs were performed in the United States, and this number is projected to increase to 3.48 million procedures performed annually by 2030. The acute effects of tourniquet-induced ischemia-reperfusion (I/R) on human skeletal muscle cells are poorly understood and require critical investigation, as muscle atrophy following this surgery is rapid and represents the most significant clinical barrier to long-term normalization of physical function. To determine the acute effects of I/R on skeletal muscle cells, biopsies were obtained at baseline, maximal ischemia (prior to tourniquet release), and reperfusion (following tourniquet release). Quadriceps volume was determined before and 2 wk post-TKA by MRI. We measured a 36% decrease in phosphorylation of Akt Ser473 during ischemia and 37% during reperfusion (P < 0.05). 4E-BP1 Thr37/46 phosphorylation decreased 29% during ischemia and 22% during reperfusion (P < 0.05). eEF2 Thr56 phosphorylation increased 25% during ischemia and 43% during reperfusion (P < 0.05). Quadriceps volume decreased 12% in the TKA leg (P < 0.05) and tended to decrease (6%) in the contralateral leg (P = 0.1). These data suggest cap-dependent translation initiation, and elongation may be inhibited during and after TKA surgery. We propose that cap-dependent translational events occurring during surgery may precipitate postoperative changes in muscle cells that contribute to the etiology of muscle atrophy following TKA.
Keywords: atrophy, muscle, tourniquet, 4E-binding protein 1, activating transcription factor 4, growth, arrest, DNA damage
osteoarthritis (oa) of the knee is among the most prevalent chronic diseases in the United States (10) affecting 60% of adults over the age of 65 (44) and is nearly 40% more prevalent in women than in men (25). The impact on disability due to knee OA is fourth behind ischemic heart disease, cerebrovascular disease, and musculoskeletal disease (48). Total knee arthroplasty (TKA) is the most commonly performed surgical remediation for patients with long-standing OA. In 2005, nearly 500,000 TKAs were performed in the United States at a cost of over $11 billion (33), and the number of surgeries performed annually in the United States is projected to increase to 3.48 million by 2030 (30).
Despite the universal success of TKA in eliminating knee pain due to OA, it remains to be determined whether physical function normalizes over time. Physical function is directly linked to muscle mass and strength of the lower extremities, the quadriceps in particular. However, a key finding of several studies on TKA, is the persistent inability to restore quadriceps muscle volume and strength (20, 37, 38, 40, 57, 60), leading many to conclude that the single greatest clinical barrier following TKA is long-term muscle atrophy. Further, quadriceps muscle atrophy has been shown to be the single greatest contributor to strength deficits, explaining 77% of the strength loss, at 1 to 3 years post-TKA (36).
Ischemia/reperfusion (I/R) occurs whenever a tourniquet is used during surgeries, such as TKA, to control blood loss, maintain a clear surgical field, and facilitate proper bone-implant cementing. Unfortunately, there is a gap in our understanding of the acute effects of tourniquet-induced I/R injury on human skeletal muscle cells during TKA. Previous studies report that skeletal muscle is negatively affected by periods of ischemia lasting 15 (2), 30 (2, 51, 53, 54), and 60 min (2, 18, 46, 51, 52) with damage progressing as the duration of ischemia increases. Among the cellular responses to ischemia is conservation of ATP for use to stabilize the cell. As such, protein synthesis, which uses significant amounts of ATP on a percentage basis, is acutely inhibited at protein control points, eukaryotic initiation factor 4E binding protein (4E-BP1) and eukaryotic initiation factor 2 (eIF2), which are primarily responsible for regulating cap-dependent translation initiation (59). Over 95% of mRNA is translated in a cap-dependent process (24, 26).
Assimilated, it is reasonable to conclude that tourniquet-induced I/R injury may precipitate a cascade of cellular events that, in part, help to explain the significant and rapid loss of muscle that is observed clinically following TKA. The overarching goal of this study was to characterize the cellular events during I/R that may help to explain postoperative changes in muscle cells that may contribute to the etiology of muscle atrophy following TKA. Specifically, the purpose of this study was to test the hypothesis that I/R in skeletal muscle would alter cellular events involved with controlling cap-dependent translation initiation and elongation such as 4E-BP1, eIF2α, and eukaryotic elongation factor-2 (eEF2), in a manner consistent with downregulation of protein synthesis. Further, this study also sought to test the hypothesis that quadriceps muscle volume would decrease following TKA.
METHODS
Ethics approval.
This study was approved by the PeaceHealth Institutional Review Board, Sacred Heart Medical Center, at Riverbend and the Biomedical Institutional Review Board for the University of Oregon and conducted in accordance with the Declaration of Helsinki. All subjects gave informed written consent prior to study participation. This study is registered with ClinicalTrials.gov (identifier: NCT00760383).
Subjects.
We studied 13 subjects (10 females and 3 males) who were recruited from surgical candidates from the Slocum Center for Orthopedics and Sports Medicine. All subjects were between 60 and 80 years of age and scheduled to have primary TKA. Excluded from the study were patients with untreated endocrine disease, significant heart, kidney, liver, blood or respiratory disease, peripheral vascular disease, active cancer, recent treatment with anabolic steroids, or oral corticosteroids for greater than 1 wk, and alcohol or drug abuse. Details for each subject are provided in Table 1.
Table 1.
Subject charateristics and treatment
| Sex | Age | Ht, cm | Wt, kg | BMI | Dx | Medications | Tourniquet Time, min | Reperfusion Time, min | Anesthesia |
|---|---|---|---|---|---|---|---|---|---|
| F | 70 | 174 | 73 | 24 | OA | Aspirin*, Osteo-Biflex, I-caps, Protonix, Crestor, Tylenol | 42 | 16 | FNB and Gen |
| F | 76 | 173 | 59 | 20 | Deg Arth | Benazepril, hydrochlorothiazide, Diltiazem, aspirin, Lipitor, ibuprofen as needed, alendronate, NitroQuick | 35 | 18 | FNB and Gen |
| F | 65 | 160 | 80 | 31 | Deg Arth | Aleve*, Lexapro, Wellbutrin | 42 | 17 | FNB and Spinal |
| M | 66 | 185 | 101 | 29 | OA | Aspirin*, Tylenol, Glucosamine | 52 | 16 | FNB and Gen |
| F | 73 | 183 | 122 | 36 | OA | Estradiol, Levothyroxine, Pirixicam, Potassium, Triamterene, Amlopidine, Darvocet | 40 | 19 | FNB and Gen |
| F | 72 | 168 | 65 | 23 | Deg Arth | Advair, Ambien, aspirin*, Calcium, Cardizem, Fosamax, Lipitor, Mirapex, Paroxetine, Spironolactone, Triamterene, Vicodin, or Percocet | 35 | 13 | FNB and Gen |
| F | 62 | 163 | 64 | 24 | OA | Amitriptyline, Zolpidem, Vitamin D | 47 | 16 | FNB and Spinal |
| F | 70 | 157 | 100 | 41 | OA | Betimol, Caltrate, HCTZ, Levothyroxine, Monopril, Tylenol, Naprosyn, Loratidine, Meclizine | 49 | 13 | FNB and Spinal |
| M | 64 | 178 | 97 | 31 | OA | Diltiazem, Benazepril, Glucosamine, Ibuprofen* | 48 | 16 | FNB and Spinal |
| M | 73 | 171 | 269 | 42 | OA | Calcium with vitamin D, Combigan, Enalapril, Furosemide, Glucosamine, Norvasc, Prozac, Simvastatin, Travatan, Vicodin | 45 | 22 | FNB, Gen, and muscle relaxant |
| F | 76 | 165 | 88 | 32 | OA | Aspirin*, Coreg, Fosinopril, Isosorbide, Lasix, Vytorin, Aleve*, Nitroglycerin | 40 | 14 | FNB and Spinal |
| F | 71 | 147 | 77 | 36 | OA | Estradiol, Advair Diskus, Triamterene/HCTZ, Celexa, Diclofenac, Prevacid, Glucosamine, Darvocet and Claritin as needed | 32 | 15 | FNB and Gen |
| F | 73 | 170 | 73 | 25 | OA | Spiriva, Advair Diskus, Coreg, Simvastatin, Alendronate, vitamin D, Omeprazole, Benicar, calcium, Albuterol inhaler as needed | 35 | 13 | FNB and Epd |
Medication withheld week prior to surgery.
Dx, diagnosis, Deg Arth, degenerative arthritis; Epd, epidural anesthesia; FNB, femoral nerve block; Gen, general anesthesia; OA, osteoarthritis; Spinal: spinal anesthesia.
Femoral nerve block indicates a single injection of 30 ml of 0.25% to 0.5% bipivicaine or ropivacaine. For general anesthesia, intravenous administration of propofol was maintained by inhalation of either desflurane or sevoflurane. For spinal anesthesia, 0.75% bupivicaine +20 μg of fentanyl was administered. For epidural anesthesia, 0.25% bupivicaine was administered. The muscle relaxant was administered by local injection of rocuronium bromide.
Study design.
Two weeks prior to surgery, subjects had a MRI of the mid-thigh region at the Slocum Center for Orthopedics and Sports Medicine MRI facility equipped with a Philips Achieva 8C 1.5 T system. A T1-weighted, fast-spin echo-pulse sequence using the following parameters were used to obtain slice images of the mid-thigh region of interest: transmit times of ∼500 ms, and a echo time of ∼12 ms; fields of view were variable to match patient size, with slice thickness of 5 mm and acquisition matrix size of 256 × 512 mm. Two weeks after TKA, each subject returned to the Slocum Center for Orthopedics and Sports Medicine MRI facility for a repeat scan. The prescan image was used by the operator to align postscan parameters to determine changes in muscle tissue volume in the identical region of interest. Coded images were transferred electronically via encrypted electronic data transfer to the laboratory computer for analysis. Three preoperative midthigh slice images were matched to three identical postoperative midthigh slice images for analysis using Analyze v.10 software package with semiautomated delineation of muscle borders, and through the use of thresholding methods, the software can auto-differentiate muscle from fat using voxel intensity within each border region for quantitative determination of muscle volume. One researcher was responsible for analyzing each image. Interclass correlation coefficients for that operator are nonoperative (contralateral) quadriceps: rectus femoris (RF), ICC = 0.96, vastus (vasti), including all three vasti, i.e., intermedius, lateralis, and medialis, ICC = 0.99; and operative (TKA) quadriceps: RF, ICC = 0.96, vasti, ICC = 0.99.
On the day of surgery, each subject was admitted in the morning to Sacred Heart Medical Center, at Riverbend, in the fasted state. Following standardized preoperative procedures for TKA, each subject was prepped for surgery. Anesthesia was administered with either an epidural, spinal, or general anesthetic, along with a preoperative femoral nerve block placed for postoperative analgesia. Induction of general anesthesia was accomplished with intravenous propofol, and anesthesia maintenance was with inhalational anesthetic (either desflurane or sevoflurane), with or without muscle relaxant (rocuronium bromide) (Table 1). After induction of anesthesia in the operating room (OR), a 10-cm-wide Zimmer tourniquet was placed at the proximal third of the thigh (but not inflated), and standard lower extremity sterilization procedures were performed. This was followed by the first of three muscle biopsies obtained from the vastus lateralis muscle on the operative (TKA) leg, at the level of middle-third of the thigh, using a 5-mm Bergström biopsy needle with applied suction. After the first muscle biopsy was obtained, the operative leg was exsanguinated using an Esmarch bandage, and the tourniquet was inflated for the first time to 300 mmHg or greater depending on systolic blood pressure to ensure minimal blood flow to the operative (TKA) leg. Just prior to tourniquet deflation, following the main components of the surgery, a second muscle biopsy was obtained. After the second muscle biopsy was obtained the tourniquet was deflated for the later components of the surgery, allowing for reperfusion of the limb with blood. The third and final muscle biopsy was obtained during the reperfusion phase in the OR prior to being moved to postoperative recovery (Fig. 1). Muscle biopsy samples were immediately blotted, and adipose tissue was removed before being frozen in liquid nitrogen (<1 min) and stored at −80°C until analysis.
Fig. 1.

Study design. Bilateral midthigh MRI was performed 2 wk prior to and 2 wk post-TKA by the same technician. Arrows indicate skeletal muscle biopsies. A baseline muscle biopsy was acquired in the OR ∼1 h after a single-injection femoral nerve block was given and following anesthesia but before inflation of the tourniquet (see Table 1 for details). The second muscle biopsy was obtained immediately prior to tourniquet deflation. The third muscle biopsy was obtained following deflation of the tourniquet (reperfusion), just prior to the subject leaving the operating room.
Muscle tissue homogenization.
Details of the homogenization procedures have been previously published (16), with specific modifications implemented for this study. Frozen muscle samples (20–30 mg) were ground using Heidolph Brinkmann's Silent Crusher M in homogenized (1:9, wt/vol) on ice in a buffer containing: 50 mM Tris·HCL, 250 mM mannitol, 50 mM NaF, 5 mM Na pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1.0% Triton X-100, pH 7.4, 1 mM benzamidine, 1 mM DTT, 0.1 mM PMSF, and 5 μg/ml soybean trypsin inhibitor. Aliquots were collected after centrifugation at 6,000 rpm (2,817 g) at 4°C for 10 min. Separate aliquots to isolate 4E-BP1 were subjected to an additional 10 min of boiling at 100°C followed by centrifugation at 10,000 rpm (7,826 g) at 4°C for 30 min before being expelled of solid protein fragments. Protein concentration (mg/ml) was determined in duplicate using a Bradford assay (1:5 Bio-Rad protein assay concentrate) on a Bio-Rad SmartSpec Spectrophotometer. All samples were stored at −80°C until further analysis.
SDS PAGE and immunoblotting.
Details of the immunoblotting procedures have been previously published (15, 16), with specific modifications implemented for this study. Homogenates were loaded in duplicate (equal micrograms per lane) into precast Bio-Rad Criterion Tris·HCL 7.5%, 15%, or TGX all kDa gels using electrode buffer (0.3% Tris base, 14.4% glycine, 1% SDS in dd-H2O) for analysis of various proteins of interest. A single muscle homogenate was repeatedly loaded onto each membrane as an internal control.
Following SDS PAGE, proteins were transferred to Bio-Rad Immuno-Blot PVDF membranes, which were soaked in 100% methanol for 1 min prior to transfer at 50 V for 60 min in transfer buffer for 7.5% and 15% gels and at 100 V for 60 min for TGX gels [10% N-cyclohexyl-3-aminopropanesulfonic acid (CAPS) buffer (2.21% CAPS and 2% 2 N NaOH in ultra-pure H2O) and 10% methanol in ultra-pure H2O].
Upon transfer, PVDF membranes were blocked in Blotto [5% nonfat dry milk powder in TBST] under constant agitation for 30–60 min. Following overnight incubation with primary antibodies, membranes were rinsed in TBS-Tween twice, followed by 60 min incubation in Blotto containing secondary antibody (donkey anti-rabbit) at room temperature. Following serial washes, the membranes were incubated in chemiluminescent reagent (ECL+ Western blotting detection system; Amersham Biosystems, Piscataway, NJ) for 5 min to detect horseradish peroxidase activity. Images were obtained with a ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA). Quantity One 1-D Software (version 4.6.9; Bio-Rad) was used to quantify the densitometry analysis of proteins. All results were made relative to our loading control (one lane per gel) to account for variance between gels and total actin, which was probed for in each lane, serving as an internal loading control.
Antibodies.
The primary antibodies p-Akt Ser473 (no. 9271), Akt (no. 9272), p-4E-BP1 Thr37/46 (no. 9459), 4E-BP1 (no. 9452), p-eIF4G Ser1108 (no. 2441), eIF4G (no. 2498), p-eIF4E Ser209 (no. 9741), eIF4E (no. 9742), p-eIF2α Ser51 (no. 3597), p-eEF2 Thr56 (no. 9459), eEF2 (no. 9452), p-Mnk1 (no. 2111), and Mnk1 (no. 2195) were purchased from Cell Signaling (Beverly, MA). Additional primary antibodies, t-eIF2α (sc-11386), CREB-2/activating transcription factor 4 (ATF4) (sc-22800), and GADD34 (sc-824) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Monoclonal mouse anti-actin (no. 691002) was purchased from MP Biomedicals (Solon, OH) for use as our protein control. ECL anti-rabbit IgG and horseradish peroxidase from donkey and sheep were purchased from GE Healthcare (New York, NY) and used as our secondary antibodies.
Total RNA isolation.
Details for RNA isolation have been previously published (17) with slight modifications implemented for this study. Skeletal muscle samples (20–30 mg) were homogenized in 1 ml TRI Reagent using Heidolph Brinkmann's Silent Crusher M at 10,000–15,000 rpm in Eppendorph RNase-free tubes. Separation was achieved by adding 0.2 ml of chloroform and precipitated using 0.5 ml of isopropanol. The RNA pellet was washed twice and dried in 75% ethanol, and dissolved in 1.5 μl of 0.1 mM EDTA for each 1 mg of starting tissue.
RNA concentrations were determined using an OD260/280 ratio on a Bio-Rad SmartSpec Spectrophotometer. All samples met the minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines of OD260/280 with an average of 1.91 ± 0.03, indicating the RNA quality was without protein or phenol contamination (8, 55). Microfluidic capillary electrophoresis analysis (RNA standard sensitivity chips, Experion, software version 3.0; Bio-Rad) was used in accordance with RNA StdSens kit directions to establish an RNA quality index (RQI) based on the ribosomal RNA profile of each sample. Samples run in triplicate averaged an RQI of 8.52 ± 0.2 (highly degraded RNA to intact RNA on a 1–10 scale) and a 28S:18S ratio of 1.21 ± 0.02, a ratio between 1 and 2, indicating intact RNA.
Reverse transcriptase and cDNA synthesis.
One microgram of RNA was reversed transcribed into cDNA on a CFX96 real-time PCR Detection System (Bio-Rad) using iScript Reaction Mix (Bio-Rad), according to manufacturer's instructions. Samples were then stored at −80°C for future analysis.
Oligonucleotide primers for quantitative PCR.
Oligonucleotide primers were designed using Beacon Design software (version 7.91), specific to our Bio-Rad CFX96 Real-Time PCR Detection System, based on NCBI Entrez Gene ID search results. Primer efficiencies were run in triplicate across four dilutions: 1 μg, 0.11 μg, 0.012 μg, and 0.0014 μg. In accordance with MIQE guidelines (8), primer efficiencies were 95–105% efficient when analyzed on the log linear scale: PCR efficiency = 10−1/slope−1. Primers were designed for GAPDH, NM_002046, forward: CTCTGGTAAAGTGGATATTGT and reverse: GGTGGAATCATATTGGAACA; EIF4G1, NM_001194946, forward: TATTCCAGCCAACTTGTC and reverse: CAACAGCCTCCTTCTTAT; EIF4G2, NM_001042559, forward: CACATTCAGACGCCTCCTAAT and reverse: TCACGCTTATCATAGACATCAACA; EIF4G3, NM_001198801, forward: TTATAGCCGACTCTTCTA and reverse: TTCCTTCTCTGTATCTGA; and GADD45A, NM_001199741, forward: ATCCACATTCATCTCAAT and reverse: GTAACTACAAAGGTATTTCA.
mRNA quantitation by quantitative PCR.
Details on quantitative PCR (qPCR) have been previously published (17), with specific modifications employed for this study. Samples of cDNA were analyzed using SYBR Green fluorescence (iQ SYBR Green Supermix, Bio-Rad). Each reaction within a 96-well plate contained 12.5 μl SYBR Green, 9.5 μl of DEPC-treated, nuclease-free water, 0.5 μl forward, and 0.5 μl reverse primers, and 2 μl of cDNA template. A mastermix of SYBR Green, water, and primers was first made and distributed into each 96-well reaction tube before final addition of the cDNA template to diminish variance between samples. All samples were run in triplicate. An initial 5-min cycle at 95°C was used to denature the cDNA. This was followed with 50 PCR cycles consisting of denaturation at 95°C for 10 s followed by 30 s of primer annealing at the optimized primer pair annealing temperature. All PCR cycles were followed by a melt analysis. Each qPCR run had its cycle threshold (CT) arbitrarily set to 200 RFU to account for intrarun variability. All genes of interest (GOI) were normalized to GAPDH, our gene of reference (GOR). mRNA results for all GOIs are expressed as fold changes relative to the GOR using the Livak method, also known as the 2−ΔΔCT method (32): ΔCT(test) = CT(GOI, test) − CT(GOR, test), ΔCT(baseline) = CT(GOI, baseline) − CT(GOR, baseline), ΔΔCT = ΔCT(test) − ΔCT(baseline), and 2−ΔΔCT = fold change from baseline.
Statistical analysis.
All values are expressed as means ± SE. Statistical evaluation of our data was performed using paired-samples t-test to compare ischemic and reperfusion samples to baseline, which is a test of gain scores (27). Differences between means were considered statistically significant at P ≤ 0.05. With 44 paired t-tests tests, we expect between 0 and 5 false positives (95% confidence bound). Specifically, we anticipate an 18% chance of four or more errors but only a 6.7% chance of five or more errors and a 2.2% chance of six or more errors. Analysis for all variables were performed using SAS Institute (2009), Base SAS 9.2.
RESULTS
Demographics.
Male and female subject characteristics were not different for age, height, weight, and body mass index (P > 0.05; data for all subjects are included in Table 1).
Gene expression.
Adequate tissue samples for mRNA analysis were available for 12 subjects. Compared with baseline, eIF4G1 mRNA increased 19% [P = 0.048, 80% confidence interval (CI) (9%, 30%)] during reperfusion (Fig. 2A). Compared with baseline, eIF4G2 mRNA increased 19% [P = 0.04, 80% CI (10%, 29%)] during reperfusion (Fig. 2B). Compared with baseline, eIF4G3 mRNA increased 28% [P = 0.01, 80% CI (14%, 42%)] during reperfusion (Fig. 2C). Compared with baseline, a downstream transcription target of ATF4, GADD45A, increased 37% [P = 0.02, 80% CI (17%, 56%)] during ischemia and remained elevated by 30% during reperfusion, but was not significant (P = 0.11) (Fig. 2D).
Fig. 2.
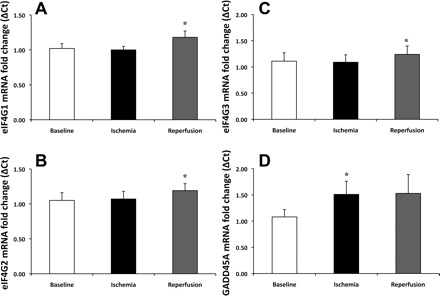
Increased gene expression of eIF4G and the activating transcription factor 4 (ATF4) transcription target growth arrest and DNA damage 45A (GADD45A). Transcripts for eukaryotic initiation factor 4 gamma 1 (eIF4G1) increased 19% (P = 0.048) (A), eIF4G2 increased 19% (P = 0.04) (B), and eIF4G3 increase 28% (P = 0.01) (C) from baseline to reperfusion. The transcription target of ATF4, GADD45A mRNA, significantly increased during ischemia 37% (P = 0.02) and showed a trend to increase, by 30% (P = 0.11) above baseline, during reperfusion (D). Results were normalized to GAPDH. Data are expressed as mean fold change ± SE (n = 12). *P ≤ 0.05 vs. baseline.
Cell signaling.
Immunoblot data were assessed for Akt Ser473, 4E-BP1 Thr37/46, eIF2α Ser51, ATF4, and GADD34 using all 13 subjects. Adequate tissue sample quantities for Mnk1, Thr197/202, eIF4E Ser209, eIF4G Ser1108, and eEF2 T56 were available for only 11 subjects.
Compared with baseline, Akt phosphorylation at Ser473 decreased by 35% [P = 0.01, 80% CI (−52%, −20%)] during ischemia and 37% [P = 0.02, 80% CI (−48%, −26%)] during reperfusion (Fig. 3A). Total Akt protein remained unchanged (0.64 ± 0.13, 0.78 ± 0.09, and 0.62 ± 0.09, during baseline, ischemia, and reperfusion, respectively; P > 0.05). Compared with baseline, 4E-BP1 phosphorylation at Thr37/46 decreased by 29% [P = 0.04, 80% CI (−41%, −16%)] during ischemia and 22% [P = 0.046, 80% CI (−42%, −3%)] during reperfusion (Fig. 3B). Similarly to Akt, total 4E-BP1 protein remained unchanged [1.22 ± 0.25, 0.90 ± 0.19, and 0.84 ± 0.16 (AU), during baseline, ischemia, and reperfusion, respectively; P > 0.05].
Fig. 3.
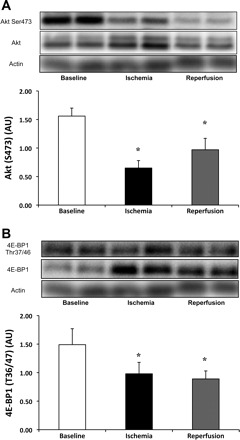
Dephosphorylation of Akt and 4E-BP1 during ischemia and reperfusion. Phosphorylation status of Akt at Ser473 was significantly decreased by 36% (P = 0.01) during ischemia and 37% (P = 0.02) during reperfusion (A). 4E-BP1 Thr36/47 was hypophosphorylated during ischemia by 29% (P = 0.04) and 22% (P = 0.046) during reperfusion (B). 4E-BP1 hypophosphorylation is indicative of blunted cap-dependent translation, which will block protein synthesis. Phosphorylation status was made relative to total protein. Actin served as a loading control. Data are expressed as means ± SE (n = 13). *P ≤ 0.05 vs. baseline.
While phosphorylation of Mnk1 Thr197/202 did not significantly change (1.91 ± 0.38, 1.68 ± 0.27, and 1.66 ± 0.27 AU, during baseline, ischemia, and reperfusion, respectively; P > 0.05), compared with baseline its total protein content increased 54% [P = 0.03, 80% CI (34%, 73%)] during ischemia and returned to baseline during reperfusion (Fig. 4A). We did not see a significant increase in phosphorylation of eIF4E Ser209 (2.86 ± 0.53, 2.57 ± 0.91, and 2.56 ± 0.69 AU, during baseline, ischemia, and reperfusion, respectively; P > 0.05), although compared with baseline, total protein content of eIF4E increased 51% [P = 0.03, 80% CI (18%, 84%)] during ischemia and returned to baseline during reperfusion (Fig. 4B). We did not see significant changes in phosphorylation of eIF4G Ser1108 (0.79 ± 0.28, 0.48 ± 0.15, and 0.80 ± 0.16 AU, during baseline, ischemia, and reperfusion, respectively; P > 0.05) or total protein of eIF4G (1.08 ± 0.20, 1.03 ± 0.17, and 0.80 ± 0.10 AU, during baseline, ischemia, and reperfusion, respectively; P > 0.05).
Fig. 4.
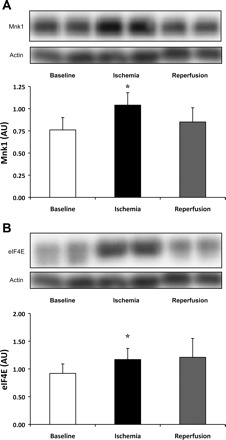
Increased Mnk1 and eIF4E total protein during ischemia. Mnk1 total protein content significantly increased 54% (P = 0.03) during ischemia and returned to baseline during reperfusion (A). eIF4E total protein content significantly increased 51% (P = 0.03) during ischemia and returned to baseline during reperfusion (B). Actin served as a loading control. Data are expressed as means ± SE (n = 11). *P ≤ 0.05 vs. baseline.
Deactivation of the eEF2 was assessed by phosphorylation at eEF2 Thr56 which, compared with baseline increased 25% [P = 0.04, 80% CI (12%, 39%) during ischemia and 43% [P = 0.01, 80% CI (27%, 59%)] during reperfusion (Fig. 5). Total protein for eEF2 was unchanged during the study (0.35 ± 0.07, 0.49 ± 0.09, and 0.41 ± 0.07 AU for baseline and ischemia, respectively; P > 0.05).
Fig. 5.
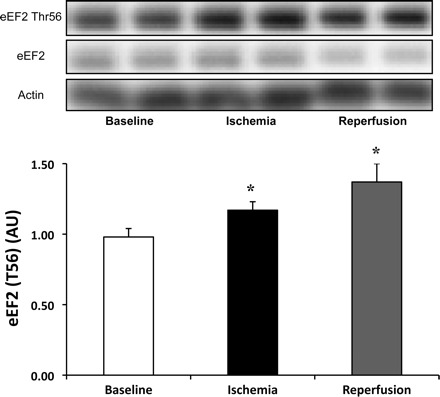
Increased phosphorylation of eukaryotic elongation factor-2 (eEF2) during ischemia and reperfusion. Phosphorylation status of eEF2 Thr36 significantly increase by 25% (P = 0.04) during ischemia and 43% (P = 0.01) during reperfusion compared with baseline. Phosphorylation status was made relative to total protein. Actin served as a loading control. Data are expressed as means ± SE (n = 11). *P ≤ 0.05 vs. baseline.
Endoplasmic reticulum (ER) stress was assessed by the phosphorylation status of eIF2α Ser51 and its downstream targets. eIF2α phosphorylation and total protein content were not altered relative to baseline (phospho/total; 1.64 ± 0.31, 1.27 ± 0.18, and 1.83 ± 0.49, and total protein; 1.22 ± 0.22, 1.59 ± 0.39, and 0.95 ± 0.20 AU, during baseline, ischemia, and reperfusion, respectively; P > 0.05); however, two downstream targets were upregulated during ischemia. ATF4 protein levels, compared with baseline increased 96% [P = 0.01, 80% CI (50%, 143%)] during ischemia but returned to baseline during reperfusion (Fig. 6A), and GADD34 protein levels compared with baseline increased 83% [P = 0.03, 80% CI (32%, 134%)] during ischemia, returning to baseline during reperfusion (Fig. 6B).
Fig. 6.
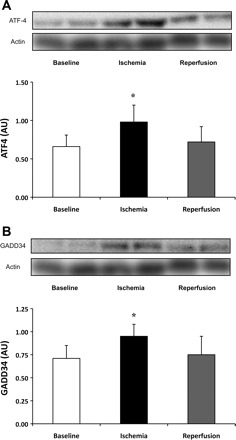
Downstream targets of eIF2α Ser51. Total protein content of ATF4 increased 96% during ischemia (P = 0.01) and returned to baseline during reperfusion. A: total protein content of GADD34 increase 83% during ischemia (P = 0.03) and returned to baseline during reperfusion (B). Actin served as a loading control. Data are expressed as means ± SE (n = 13). *P ≤ 0.05 vs. baseline.
Quadriceps atrophy.
Pre- and post-TKA MRIs were available for nine subjects. Compared with before surgery, midthigh quadriceps volume in the TKA leg decreased 12% [P = 0.012, 80% CI (−17%, −8%)] 2 wk after surgery. A similar reduction (6% decrease) was observed in the nonoperative (contralateral) midthigh quadriceps volume 2 wk after surgery, but was not significant (P = 0.11) (Fig. 7). Before surgery, midthigh quadriceps volume was less 7% [P = 0.05, 80% CI (12%, −2%)] in the operative vs. nonoperative lower extremity; i.e., the TKA quadriceps volume was less than the contralateral leg.
Fig. 7.
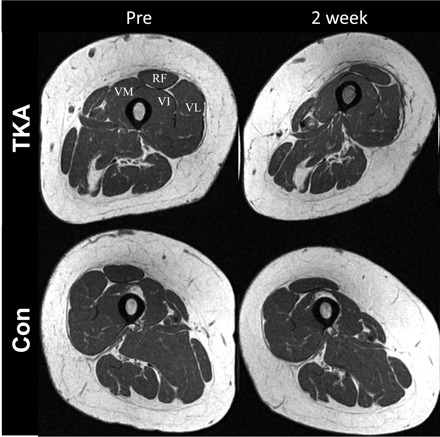
Representative MRI cross section showing acute quadriceps muscle atrophy 2 wk post-TKA. Representative cross section of the midthigh before (Pre) and post-TKA (2 wk) of the (TKA) leg and the contralateral, nonoperative, (Con) leg. Compared with before surgery, midthigh quadriceps volume in the TKA leg (TKA) decreased 12% 2 wk after surgery (P = 0.012). Similar muscle atrophy (6% decrease) was observed in the nonoperative (Con) lower extremity 2 wk after surgery but was not significant (P = 0.11). Before surgery, midthigh quadriceps volume was smaller, by 7% in the operative vs. nonoperative lower extremity (P = 0.05). Pre- and post-TKA images are obtained at the exact midthigh region of interest. Differences in appearances of the TKA leg are due to issues related to reduced range of motion postoperatively (knee flexion contracture), preventing full extension of the knee. RF, rectus femoris; VL, vastus lateralis; VI, vastus intermedius; and VM, vastus medialis.
DISCUSSION
This study reveals several novel and important findings regarding the acute effects of I/R in skeletal muscle on transcriptional, translational, and cell signaling pathways known to be involved in cap-dependent translation initiation and elongation. First, we report that 4E-BP1 was hypophosphorylated, which suggests that cap-dependent translation initiation was inhibited during I/R. Second, we determined that eEF2 phosphorylation was increased, supporting the hypothesis that translation elongation was similarly attenuated during I/R. Third, we measured significant quadriceps muscle atrophy occurring acutely, 2 wk post-TKA, in the operative (−12%) and a trend for similar declines in muscle volume (−6%) in the nonoperative lower extremity. To our knowledge, this study is the first to measure changes in key regulatory proteins important for controlling cap-dependent translation initiation and elongation during TKA surgery and subsequent muscle loss occurring within 2 wk of surgery.
Translation initiation begins with the formation of the ternary initiation complex (eIF4F), consisting of eIF4A, eIF4G, and eIF4E, which binds with the 40S ribosomal subunit, stabilizes the preinitiation complex, and binds to the 7-methylguanosine cap structure located at the 5′ end of eukaryotic mRNA. Although the formation of eIF4F is critical for cap-dependent translation initiation, the binding protein 4E-BP1 can inhibit formation of the eIF4F complex by binding to eIF4E, thus preventing cap-dependent translation initiation (21, 24). Activation of Akt has been shown to stimulate downstream effectors of the mTOR pathway, 4E-BP1, and S6K1, under anabolic conditions (16), while overexpression of a hypophosphorylation form of Akt has been shown to block 4E-BP1 phosphorylation (22). Here we show that during ischemia and reperfusion, Akt and its downstream effector, 4E-BP1, are hypophosphorylated. The observed dephosphorylation of 4E-BP1 allows for strong binding of, and inactivation of, initiation factor eIF4E and is consistent with a reduced formation of the cap-binding complex eIF4F. The downregulation of 4E-BP1 measured in our study is similar to the results from Arsham et al. (3), who observed hypophosphorylation of 4E-BP1 under hypoxic conditions. These data show that 4E-BP1 is dephosphorylated during and immediately after TKA, which is consistent with cap-dependent translation initiation being downregulated (21).
When phosphorylated at Ser209, eIF4E decreases its binding affinity for 5′ capped mRNA (50). In this study, we did not see a significant change in phosphorylation of eIF4E; however, we did observe a significant increase in Mnk1 and eIF4E total protein content. Saghir et al. (49) observed overexpression of Mnk1 was sufficient to increase eIF4E phosphorylation. In our current study, Mnk1 may not have reached sufficient physiological levels to phosphorylate eIF4E, which is supported by data from Martin et al. (35), who measured a significant increase in bound 4E-BP1-eIF4E during ischemia and a subsequent decrease in protein synthesis in PC12 cells, while eIF4E phosphorylation remained unaltered. Apart from changes in phosphorylation status and protein levels of eIF4E, the binding and unbinding from 4E-BP1 are the primary determinants for the availability of eIF4E to form the eIF4F complex (45). It remains to be determined the degree to which increases in Mnk1 and eIF4E levels play a role in skeletal muscle I/R during TKA.
Phosphorylation of eIF4G Ser1108 fully activates eIF4G and enhances the formation of the eIF4G-eIF4E complex (47). While we did not observe changes in phosphorylation or total protein of eIF4G, myocardial ischemia has been shown to upregulate caspase-3-induced cleavage of eIF4G (12), to suppress protein synthesis, and to attenuate an antiapoptotic response (11). It is possible that the increase in gene expression of all three isoforms of eIF4G (eIF4G1, eIF4G2, and eIF4G3) during reperfusion may be the cell's response to proteolytic degradation of eIF4G and/or cleavage by caspases as Marissen et al. (34) have shown that this disruption alters the ability for complex formation (eIF4F) and binding of the capped mRNA with the 40S ribosomal subunit, resulting in significant translation inhibition. However, while eIF4G1 and eIF4G3 promote translation, eIF4G2 may act as a translational repressor, and we have measured elevations in transcripts for all three. These findings potentially suggests that multiple levels of cellular responses are occurring that may involve transcription, translation, and/or posttranslational modifications. Further work is necessary to determine the contribution of each of these mechanisms to restore muscle cell homeostasis during I/R associated with TKA.
After eIF2 delivers the initiator methionyl tRNA (met-tRNAi) to the AUG start codon on the mRNA, eIF2 hydrolyzes GTP to GDP and dissociates from ribosomal complex. Phosphorylation of eIF2α blocks the GTP exchange for eIF2-GDP, effectively binding eIF2α with the guanine exchange factor eIF2B, and inhibits the formation of a ternary complex consisting of methionine-tRNA and eIF2 coupled to GTP (21). While eIF2α Ser51 phosphorylation was not altered in our study, this has been reported to be necessary for the acute response to hypoxic stress (29). It may be that eIF2α was phosphorylated at an earlier time point during ischemia, prior to the second biopsy. This is supported by our data showing an increase in ATF4 protein, a distal substrate of eIF2α whose cap-independent translation is upregulated during hypoxic stress, including ER stress, which can precipitate the unfolded protein response (4, 23).
Additional evidence for the potential involvement of eIF2α at an earlier time point is provided by the measured increase in GADD45A transcript levels, a downstream target of ATF4 (19), and is translated via cap-independent mechanisms, preferentially during cell stress (58). Further, the downstream target of eIF2α, GADD34, can increase in response to skeletal muscle cell stress (58). Additionally, eIF2α may not require complete phosphorylation to initiate down-regulation of translation initiation (59). This may explain our findings that downstream effectors of eIF2α were upregulated (i.e., ATF4 and GADD34), while phosphorylation of eIF2α at Ser51 remained unchanged during our sampling times. Alternatively, GADD34, which was upregulated during ischemia, may have been present in sufficient quantities to facilitate the assembly of an eIF2α phosphatase and subsequent dephosphorylation of eIF2α (7, 41), restoring cap-dependent initiation and cell homeostasis (42). Arsham et al. (3) were unable to detect a change in eIF2α Ser51 following 30 min of hypoxia, despite significant downregulation of components of the mTORC1 pathway, including 4E-BP1. Lastly, eIF2α does not require complete phosphorylation to initiate downregulation of translation initiation due to the lower level of expression of eIF2B (59), and its activity may respond differently under anoxic (29) vs. hypoxic conditions (3, 31). Further work is required to unravel the potential contributions of eIF2α during I/R in our model.
Following initiation of cap-dependent translation, additional regulation of translation elongation can facilitate protein synthesis. The translocation of the peptidyl-tRNA and the growing polypeptide during the elongation phase of translation from the A-site to the P-site of the ribosome is promoted by eEF2, which loses its binding affinity for the ribosome when phosphorylated (9). The eukaryotic elongation factor-2 (eEF2) can thus attenuate translation, and subsequent protein synthesis, when phosphorylated (28). Our results for eEF2 are consistent with previous data (6) and provide further evidence for blunted translation during ischemia and reperfusion. Earlier work from rat models suggests that elongation is not impaired by sepsis in skeletal muscle (56); however, more recent work in myocardial I/R model has shown an increased phosphorylation of eEF2 at Thr56 during I/R (14). Our results show that phosphorylation of eEF2 Thr56 increased during ischemia and reperfusion. Not only may translation elongation be inhibited during low energy levels, such as ischemia, but reperfusion failed to elicit a reversal of eEF2 phosphorylation in our clinical model of I/R in skeletal muscle. Further work is needed to determine whether phosphorylation of eEF2 at Thr56 is sufficient to attenuate translation elongation and if this translates to an attenuation of protein synthesis (6).
Typical of clinical translational research, our study design has inherent limitations. For example, we were unable to control for medications, physical activity, the anesthetics/muscle relaxants, and we do not have a direct measure of protein synthesis or degradation. As such, we acknowledge the critical need for further studies designed to quantify the magnitude of the contribution of I/R injury on muscle protein anabolism and catabolism during and after TKA.
Perspectives and Significance
By far, the most significant clinical barrier following TKA surgery is persistent muscle atrophy and weakness (20, 37, 57). Specifically, quadriceps muscle strength has been shown to be as much as 40% weaker than age-matched healthy controls 2 mo following surgery (43) and has been singled out as the main contributor to diminished strength and poor return of physical function after TKA (36). While there exists the strong possibility that the preoperative single injection femoral nerve block and/or the anesthetics used during surgery may be playing a role in altering proteins regulating cap-dependent translation, it stands to reason that acute alterations in muscle cells that we have measured in this study may initiate a cascade of events that culminate in the eventual loss of muscle that is a known sequelae of TKA and are similar in extent to the rapid alterations measured in critically ill patients (13). Indeed, we have found that quadriceps muscle atrophy occurs acutely, within 2 wk post-TKA, in the operative leg (−12%), which is similar to what has been found by others at ∼27 days post-TKA (39), but it was double that of the nonoperative leg (−6%), which is similar in the extent of atrophy observed by researchers modeling 2 wk of immobilization in healthy subjects (1). It remains that additional research is necessary to better delineate the potential impact of preoperative analgesics and tourniquet use on muscle metabolism and whether or not the clinical recommendation to apply continuous tourniquet for no more than 2 h needs to be revisited (5). Additionally, further research is necessary to elucidate the interactions of anesthetics and I/R injury and the evolution of atrophy in our clinical model, i.e., nonoperative disuse atrophy vs. operative disuse atrophy and I/R injury, we believe this work brings us one step closer to understanding the fundamental changes occurring acutely in muscle cells and begin the process of bridging the gap between cause and effect of muscle loss following an increasingly common surgery performed on older adults.
In the end, we found that key regulators of “global” translation initiation and elongation are altered in a way that is consistent with reductions in protein synthesis in skeletal muscle subjected to I/R during a commonly performed surgical procedure, which may exacerbate immobilization-induced reductions in muscle volume (Fig. 8). Further, multiple factors associated with translational regulation were found to be modified at both transcriptional and translational levels and lend support to our hypothesis that downregulation of cap-dependent translation initiation occurs in these patients. This study, to our knowledge, is the first to provide evidence of such events taking place in muscle cells of older adults during total knee replacement surgery.
Fig. 8.
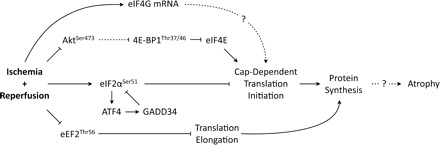
Ischemia-reperfusion inhibits multiple proteins regulating cap-dependent translation. The schematic diagram of proteins regulating signaling pathways controlling cap-dependent mRNA translation initiation and elongation. Ischemia-reperfusion inhibits cap-dependent translation initiation via inhibition of Akt-mTOR pathway and availability of 4E-BP1 to bind to and inhibit eIF4E association with eIF4G to form an active mRNA cap-binding complex (eIF4F). Ischemia-reperfusion also activates endoplasmic reticulum (ER) stress via eIF2α, which inhibits cap-dependent translation initiation. ATF4 and GADD34, each downstream components of eIF2α, are proteins involved in recovery from cell stress and are upregulated during ischemia and provide inhibitory feedback onto eIF2α. Alterations due to ischemia/reperfusion (I/R) additionally stimulate mRNA for all three isoforms of eIF4G. Together, these data may potentially provide some insight into the dramatic quadriceps atrophy (−12%) occurring within 2 wk post-TKA.
GRANTS
This study was supported by the National Center for Medical Rehabilitation and Research, Eunice Kennedy Shriver National Institute of Child Health & Human Development, NIH grant K01HD57332 (H.C. Dreyer). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.M.R., A.N.B., H.A.S., B.A.L., B.A.J., and H.C.D. performed experiments; S.M.R., A.N.B., H.A.S., K.S., and H.C.D. analyzed data; S.M.R., A.D.H., and H.C.D. prepared figures; S.M.R., A.N.B., H.A.S., B.A.J., J.S.G., and H.C.D. drafted manuscript; S.M.R., A.N.B., A.D.H., K.S., B.A.L., J.S.G., and H.C.D. edited and revised manuscript; S.M.R., A.N.B., H.A.S., A.D.H., K.S., B.A.L., B.A.J., J.S.G., and H.C.D. approved final version of manuscript; A.N.B., A.D.H., K.S., J.S.G., and H.C.D. interpreted results of experiments; H.C.D. conception and design of research.
ACKNOWLEDGMENTS
We thank Crystal Mills, Alicia Morrison, Brian Pifer, and Sam Hannah of the Slocum Center for Orthopedics & Sports Medicine for her help with the recruitment, scheduling, and MRIs. We would also like to thank surgical staff at Sacred Heart Medical Center at Riverbend and the Anesthesiologists from Northwest Anesthesia Physicians group for their assistance with the study. Finally, we thank Dr. Andrew Lovering for critical reading of the manuscript.
REFERENCES
- 1. Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, Phillips SM, Tarnopolsky M. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLos One 4: e6518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appell HJ, Gloser S, Duarte JA, Zellner A, Soares JM. Skeletal muscle damage during tourniquet-induced ischaemia. The initial step towards atrophy after orthopaedic surgery? Eur J Appl Physiol Occup Physiol 67: 342–347, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem 278: 29655–29660, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol 24: 7469–7482, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 10: 620–630, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Brush MH, Shenolikar S. Control of cellular GADD34 levels by the 26S proteasome. Mol Cell Biol 28: 6989–7000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Carlberg U, Nilsson A, Nygard O. Functional properties of phosphorylated elongation factor 2. Eur J Biochem 191: 639–645, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention Prevalence and most common causes of disability among adults—United States, 2005. MMWR Morb Mortal Wkly Rep 58: 421–426, 2009 [PubMed] [Google Scholar]
- 11. Clemens MJ, Bushell M, Morley SJ. Degradation of eukaryotic polypeptide chain initiation factor (eIF) 4G in response to induction of apoptosis in human lymphoma cell lines. Oncogene 17: 2921–2931, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Connolly EP, Thuillier V, Rouy D, Bouetard G, Schneider RJ. Inhibition of Cap-initiation complexes linked to a novel mechanism of eIF4G depletion in acute myocardial ischemia. Cell Death Differ 13: 1586–1594, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol 589: 3883–3895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crozier SJ, Vary TC, Kimball SR, Jefferson LS. Cellular energy status modulates translational control mechanisms in ischemic-reperfused rat hearts. Am J Physiol Heart Circ Physiol 289: H1242–H1250, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol 106: 1403–1411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duarte JA, Gloser S, Remiao F, Carvalho F, Bastos ML, Soares JM, Appell HJ. Administration of tourniquet. I. Are edema and oxidative stress related to each other and to the duration of ischemia in reperfused skeletal muscle? Arch Orthop Trauma Surg 116: 97–100, 1997 [PubMed] [Google Scholar]
- 19. Ebert SM, Monteys AM, Fox DK, Bongers KS, Shields BE, Malmberg SE, Davidson BL, Suneja M, Adams CM. The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol Endocrinol 24: 790–799, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finch E, Walsh M, Thomas SG, Woodhouse LJ. Functional ability perceived by individuals following total knee arthroplasty compared to age-matched individuals without knee disability. J Orthop Sports Phys Ther 27: 255–263, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5: 827–835, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev 12: 502–513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318–327, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Hootman JM, Sniezek JE, Helmick CG. Women and arthritis: burden, impact and prevention programs. J Womens Health Gend Based Med 11: 407–416, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Johannes G, Sarnow P. Cap-independent polysomal association of natural mRNAs encoding c-myc, BiP, and eIF4G conferred by internal ribosome entry sites. RNA 4: 1500–1513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanji GK. 100 Statistical Tests. Thousand Oaks, CA: Sage, 1999 [Google Scholar]
- 28. Kaul G, Pattan G, Rafeequi T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct 29: 227–234, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, Lambin P, Koumenis C, Sonenberg N, Wouters BG. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J 25: 1114–1125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21: 521–531, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, Holt HL, Solomon DH, Yelin E, Paltiel AD, Katz JN. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med 169: 1113–1121; discussion 1121–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marissen WE, Gradi A, Sonenberg N, Lloyd RE. Cleavage of eukaryotic translation initiation factor 4GII correlates with translation inhibition during apoptosis. Cell Death Differ 7: 1234–1243, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Martin ME, Munoz FM, Salinas M, Fando JL. Ischaemia induces changes in the association of the binding protein 4E-BP1 and eukaryotic initiation factor (eIF) 4G to eIF4E in differentiated PC12 cells. Biochem J 351: 327–334, 2000 [PMC free article] [PubMed] [Google Scholar]
- 36. Meier WA, Marcus RL, Dibble LE, Foreman KB, Peters CL, Mizner RL, LaStayo PC. The long-term contribution of muscle activation and muscle size to quadriceps weakness following total knee arthroplasty. J Geriatr Phys Ther 32: 35–38, 2009 [PubMed] [Google Scholar]
- 37. Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther 35: 424–436, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol 32: 1533–1539, 2005 [PubMed] [Google Scholar]
- 39. Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am 87: 1047–1053, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res 23: 1083–1090, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153: 1011–1022, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J 22: 1180–1187, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ouellet D, Moffet H. Locomotor deficits before and two months after knee arthroplasty. Arthritis Rheum 47: 484–493, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Parsley BS, Bertolusso R, Harrington M, Brekke A, Noble PC. Influence of gender on age of treatment with TKA and functional outcome. Clin Orthop Relat Res 468: 1759–1764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem 269: 5338–5349, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Racz IB, Illyes G, Sarkadi L, Hamar J. The functional and morphological damage of ischemic reperfused skeletal muscle. Eur Surg Res 29: 254–263, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Raught B, Gingras AC, Gygi SP, Imataka H, Morino S, Gradi A, Aebersold R, Sonenberg N. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J 19: 434–444, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reginster JY, Khaltaev NG. Introduction and WHO perspective on the global burden of musculoskeletal conditions. Rheumatology (Oxford) 41, Suppl 1: 1–2, 2002 [PubMed] [Google Scholar]
- 49. Saghir AN, Tuxworth WJ, Jr, Hagedorn CH, McDermott PJ. Modifications of eukaryotic initiation factor 4F (eIF4F) in adult cardiocytes by adenoviral gene transfer: differential effects on eIF4F activity and total protein synthesis rates. Biochem J 356: 557–566, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem 269: 5350–5359, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sexton WL, Korthuis RJ, Laughlin MH. Microvascular injury after ischemia and reperfusion in skeletal muscle of exercise-trained rats. J Appl Physiol 68: 2329–2336, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Sternbergh WC, 3rd, Adelman B. The temporal relationship between endothelial cell dysfunction and skeletal muscle damage after ischemia and reperfusion. J Vasc Surg 16: 30–39, 1992 [PubMed] [Google Scholar]
- 53. Suval WD, Duran WN, Boric MP, Hobson RW, 3rd, Berendsen PB, Ritter AB. Microvascular transport and endothelial cell alterations preceding skeletal muscle damage in ischemia and reperfusion injury. Am J Surg 154: 211–218, 1987 [DOI] [PubMed] [Google Scholar]
- 54. Suval WD, Hobson RW, 2nd, Boric MP, Ritter AB, Duran WN. Assessment of ischemia reperfusion injury in skeletal muscle by macromolecular clearance. J Surg Res 42: 550–559, 1987 [DOI] [PubMed] [Google Scholar]
- 55. Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines. Methods 50: S1–S5, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol Cell Physiol 262: C1513–C1519, 1992 [DOI] [PubMed] [Google Scholar]
- 57. Walsh M, Woodhouse LJ, Thomas SG, Finch E. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther 78: 248–258, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Whitney ML, Jefferson LS, Kimball SR. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem Biophys Res Commun 379: 451–455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wouters BG, van den Beucken T, Magagnin MG, Koritzinsky M, Fels D, Koumenis C. Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol 16: 487–501, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech 23: 320–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


