Abstract
The bile duct system of the liver is lined by epithelial cells (i.e., cholangiocytes) that respond to a large number of neuroendocrine factors through alterations in their proliferative activities and the subsequent modification of the microenvironment. As such, activation of biliary proliferation compensates for the loss of cholangiocytes due to apoptosis and slows the progression of toxic injury and cholestasis. Over the course of the last three decades, much progress has been made in identifying the factors that trigger the biliary epithelium to remodel and grow. Because a large number of autocrine factors have recently been identified as relevant clinical targets, a compiled review of their contributions and function in cholestatic liver diseases would be beneficial. In this context, it is important to define the specific processes triggered by autocrine factors that promote cholangiocytes to proliferate, activate neighboring cells, and ultimately lead to extracellular matrix deposition. In this review, we discuss the role of each of the known autocrine factors with particular emphasis on proliferation and fibrogenesis. Because many of these molecules interact with one another throughout the progression of liver fibrosis, a model speculating their involvement in the progression of cholestatic liver disease is also presented.
Keywords: biliary epithelium, cholangiopathies, gastrointestinal hormones, neuroendocrine factors, proliferation
the liver is comprised of two epithelia cell types: hepatocytes, which initiate secretion of bile at the bile canaliculus, and cholangiocytes, which line the bile ducts and modify ductal bile during transport to the duodenum in response to a series of spontaneous and hormone-regulated events (3, 53). The biliary system, which is lined by cholangiocytes, forms a three-dimensional network extending from the proximal branch called the canals of Hering to the extrahepatic ducts (4, 5, 54). The canals of Hering are lined by both cholangiocytes and hepatocytes along with bipotential hepatic progenitor cells (103, 107), which bridge the bile canaliculus with bile ductules that merge to form interlobular ducts that continue merging to form the ducts of larger sizes. Cholangiocytes possess specific surface-transport systems for secreting a large number of substrates such as electrolytes and bicarbonate. A number of factors have been shown to play key roles in the regulation of ductal secretion such as the autonomic nervous system, gastrointestinal hormones, and peptides (9).
In the liver, only cholangiocytes express the secretin receptor (SR) (7). The biological action of secretin on cholangiocytes occurs via a series of coordinated events (3, 5, 53). First, secretin binds to the basolateral SR of cholangiocytes causing an adenylyl cyclase-dependent increase in cAMP levels and activation of protein kinase A (PKA) (5, 53). Second, PKA phosphorylates the cystic fibrosis transmembrane conductance regulator at the apical membrane of cholangiocytes triggering the release of Cl− (6, 53). The resulting Cl− gradient activates the apically located Cl−/HCO3− anion exchanger 2 to secrete bicarbonate into ductal bile (31, 53). Additionally, cAMP contributes to Cl− conductance through exchange proteins activated directly by cyclic AMP, which is a PKA-independent pathway (74).
Cholangiocytes in the adult liver are normally mitotically dormant (1). Constitutive expression of proteins involved in cell cycle, such as p27, and members of the Bcl-2 family of proteins have been shown to be important for holding cholangiocytes in a resting state (46, 101). The importance of cAMP signaling in the regulation of cholangiocyte proliferation was evidenced by administration of forskolin (an adenylate cyclase activator) to rats. In this study, forskolin increased the number of bile ducts, cAMP levels, and provided the first evidence for the PKA-Src-MEK-ERK1/2 pathway in biliary hyperplasia (30). Activation of cAMP-dependent signaling pathways plays also a key role in pathologies such as autosomal recessive polycystic kidney disease through exchange proteins activated directly by cyclic AMP and PKA-dependent mechanisms (13). In addition, many forms of cell damage, disruption of cell matrix, or release of cytokines may trigger proliferation by evoking cAMP, phosphoinositide 3-kinase (PI3K)/AKT, Src and Ca2+ signaling pathways (13, 32). A summary of the molecular pathways regulating cholangiocyte proliferation is illustrated in Fig. 1.
Fig. 1.
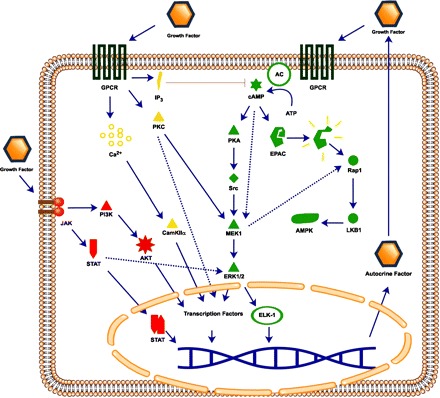
Major molecular pathways mediating cholangiocyte proliferation. Regulation of cholangiocyte proliferation occurs through 1) G protein-coupled receptors (GPCR)-induced cAMP production and downstream PKA and/or exchange proteins activated directly by cyclic AMP (EPAC) activation; 2) GPCR activation of Ca2+, or PKC pathway, or inhibition by inositol 1,4,5-trisphosphate (IP3) pathway; and 3) tyrosine kinase activation and JAK/STAT or phosphoinositide 3-kinase (PI3K)/AKT pathway. Induction of these pathways can activate transcription factors for genes involved in proliferation and the synthesis of growth factors that participate in an autocrine loop. AC, adenylyl cyclase; LKB1, liver kinase B1.
Cholangiocytes are the primary targets of human diseases termed cholangiopathies (4, 59). These diseases can be genetic or acquired and lead to cholestasis, chronic liver injury, and eventually liver failure (4, 59). The progression of cholangiopathies is characterized by enhanced apoptosis and absence of proliferative responses to liver injury (4, 59). Elevated apoptosis and reduced proliferation results in loss of bile ducts (ductopenia), which is a unifying event in cholangiopathies (4, 59). Cholangiocytes respond to injury by activating compensatory proliferative and secretory mechanisms. The proliferative compensatory mechanism opposes cholangiocyte death and sustains bile ducts (61, 67). The increased proliferation of bile ductules, termed “ductular reaction,” together with an epithelial-mesenchymal transition (EMT) and periportal fibrosis leads to cirrhosis (90). It has been suggested that proliferating cholangiocytes contribute to the ductular reaction in the liver by undergoing EMT that is associated with the activation of other cell types in the liver (39). During EMT, mature epithelial cells lose their normal cell-to-cell contacts, which may be necessary for biliary proliferation (i.e., ductular reaction) to occur (39). However, controversy exists for the existence of EMT in biliary epithelia. For example, in animal models of liver fibrosis, lineage tracing did not demonstrate evidence of EMT in cholangiocytes (23, 91). Several studies have shown that cholangiocytes express EMT markers (82, 84, 86). Although, it is clear that cholangiocytes can express putative markers of EMT in models of biliary damage, there remains uncertainty as to whether these cells undergo full phenotypic change to mesenchymal cell type in pathogenesis of human liver diseases.
A number of animal models of cholestasis and human cholangiopathies have demonstrated neuroendocrine-like changes in cholangiocytes during cholangiopathies (4, 9, 39). These changes represent an adaptive response to biliary damage and are necessary for survival and repair of the biliary tree. However, adaptive changes can be a major determinant for disease progression and the fibrogenic process. Chromogranin A, glycolipid A2-B4, S-100 protein, neural cell adhesion molecule, and neuroendocrine granules are phenotypic markers that have been described in proliferating cholangiocytes that display neuroendocrine phenotypes (9). These activated/proliferating cells with neuroendocrine characteristics secrete factors that affect the progression of biliary diseases. Here, the known autocrine factors and their roles in the progression of biliary disease and fibrosis are reviewed. These autocrine factors are highlighted in Table 1. With the growing numbers of recently identified autocrine factors that participate in the regulation of cholangiocyte proliferation and progression of biliary diseases, we provide a comprehensive review of each factor and the specific mechanisms they regulate.
Table 1.
Autocrine factors secreted by cholangiocytes and their role in proliferation or fibrogenesis
| Autocrine Factor | Mechanism/Outcome | ↑/↓ | References | |
|---|---|---|---|---|
| Proliferation | Secretin | cAMP | ↑ | 1, 5, 6, 30, 41 |
| Glucagon-like peptide | cAMP, Ca2+, PI3K | ↑ | 70 | |
| Leptin | JAK/STAT/ERK1/2 | ↑ | 25 | |
| Insulin-like growth factor 1 | PI3K | ↑ | 10, 34 | |
| Progesterone | Ca2+/PKC | ↑ | 38 | |
| Prolactin | cAMP, JAK/STAT, PKC, Src | ↑ | 102 | |
| Follicle-stimulating hormone | cAMP, PKC | ↑ | 68 | |
| Testosterone | cAMP | ↑ | 111 | |
| Serotonin | Inhibits proliferation through IP3 | ↓ | 72 | |
| Met-enkephalin | Inhibits proliferation through IP3 | ↓ | 71 | |
| Nerve growth factor | PI3K, ERK1/2 | ↑ | 37, 110 | |
| CGRP | cAMP | ↑ | 42 | |
| VEGF | PKC, ERK 1/2 | ↑ | 26, 36, 69, 96 | |
| Fibrogenesis | Renin-angiotensin | TGF-β1, NADPH oxidase, TNF-α, procollagen | 14, 51, 77, 88, 112 | |
| Platelet-derived growth factor | EMT, stellate cell activation | 45, 56 | ||
| Transforming growth factor-β | αVβ6 integrin, collagen, laminin, fibronectin, others | 66, 75, 87, 99, 101 | ||
| αVβ6 integrin | TGF-β1, collagen, fibronectin | 87, 100 | ||
| Connective tissue growth factor | TGF-β1, collagen, activates fibroblasts | 20, 92 | ||
| Endothelin 1 | collagen, apoptosis, decreases VEGF | 26, 66 | ||
| Hedgehog | osteopontin, cxcl16, EMT, activates MF-HSCs | 82–86 | ||
| Substance P | collagen, α-SMA | 40 | ||
CGRP, calcitonin gene-regulated peptide; VEGF, vascular endothelial growth factor; PI3K, phosphoinositide 3-kinase; IP3, inositol 1,4,5-trisphosphate; EMT, epithelial-mesenchymal transition; MF-HSCs, myofibroblast-hepatic progenitor cells; SMA, smooth muscle actin.
Peptide Hormones
Secretin/SR axis.
Secretin is a hormone produced by S cells that reside within the crypts of Lieberkun of the duodenum. Secretin stimulates biological actions in multiple organs including the pancreas, stomach, and biliary epithelium. The secretin/SR axis has been shown to play a key role in the regulation of biliary proliferation (7). SR is only expressed by cholangiocytes in the liver, and its expression is closely coupled to both proliferative and secretory activity. SR expression is upregulated during extrahepatic cholestasis induced by bile duct ligation (BDL) (7). The importance of the secretin/SR axis is highlighted in recent studies that demonstrated that there is a significant reduction of large cholangiocyte proliferation in the SR(−/−) knockout mouse model with BDL (41). These effects were dependent on the activation of the cAMP/PKA/ERK1/2 intracellular signaling pathway (41). In addition, in vitro studies showed that the basal proliferative rate of cholangiocytes was decreased after stable knockdown of SR by short hairpin RNA (41), indicating autocrine regulation of biliary growth by secretin. Preliminary data (S. Glaser and G. Alpini, unpublished observations) from our laboratory demonstrates that cholangiocytes express secretin mRNA and secrete secretin, which indicates a possible autocrine role of secretin in the regulation of biliary function. This autocrine mechanism underscores the fundamental role of secretin as a trophic factor for cholangiocytes and illustrates the importance of autocrine regulation of biliary function in the pathogenesis of cholestatic liver diseases.
Glucagon-like peptide 1.
Glucagon-like peptide 1 (GLP-1) is secreted by enteroendocrine L cells of the gastrointestinal (GI) system. The endocrine effects of GLP-1 are stimulated through its interaction with a specific G protein-coupled receptor (GLP-1R). The finding that GLP-1 induces pancreatic ductal cells to acquire a neuroendocrine phenotype is of great interest as it provides a new function for GLP-1 in differentiation of ductal epithelia (21) and the rationale for evaluating the role of GLP-1 in the regulation of biliary function. A recent study on biliary adaptive responses to cholestasis has shown that cholangiocytes are locally regulated by GLP-1 (70). In BDL rats, GLP-1R was expressed by cholangiocytes and stimulation with GLP-1 activated the PI3K, cAMP-PKA, and Ca2+-CamKIIα pathways. Proliferating cholangiocytes expressed the GLP-1 mRNA transcripts, which were diminished by adding the GLP-1R antagonist exendin 9–39 to the culture medium (70). The study suggests that GLP-1 signaling from the GI tract and continued GLP-1-autocrine signaling by cholangiocytes is a driving factor for the neuroendocrine transdifferentiation of cholangiocytes (70).
Leptin.
Leptin is a peptide hormone secreted by adipocytes that acts on the hypothalamus to regulate satiety and caloric homeostasis. Serum leptin is increased in obesity and a wide range of cancers including cholangiocarcinoma (33, 109). Studies have shown that the interaction of leptin with its receptor OBRI stimulates the JAK/STAT pathway through STAT3 and downstream ERK1/2, resulting in angiogenesis and cancer growth. The expression of leptin and the leptin receptor was demonstrated in normal cholangiocytes as well as cholangiocarcinoma cells (25). This study demonstrated that leptin stimulates cholangiocarcinoma growth and that local leptin secretion is involved in cholangiocyte growth (25).
Insulin-like growth factor 1.
Insulin-like growth factor 1 (IGF1) is a circulating hormone and local growth factor that is secreted primarily from the liver (3). Through its interaction with the specific receptor IGF1-R, IGF1 is important for cell survival and prevention of apoptosis in a number of epithelia. Recent studies exploring the effect of local secretion of IGF1 in bile ducts have demonstrated that selective siRNA silencing of local IGF1 secretion potentiated the cytotoxic effects of the bile acid glycochenodeoxycholic acid more potently than circulating IGF1 (10, 34). Furthermore, IGF1 mRNA was expressed by both cholangiocytes and hepatocytes. A paracrine mechanism may also play a role in injury/repair because both hepatocytes and cholangiocytes secrete and respond to IGF1.
Reproductive and Steroid Hormones
Estrogen.
Steroid hormones (i.e., sex hormones) regulate cell growth in a variety of organs. The most well-known of these, estrogen, has been shown to have a profound effect on cell growth. Estradiol (E2), the primary estrogen, targets the uterus, cardiovascular, nervous, and digestive systems (11). Recent studies have shown that estradiol is a mitogen for the liver. One study demonstrated that chronic administration of estradiol causes an increase in total liver mass (27). Another study reported that estradiol caused a rapid but transient growth in the liver and suggested that estrogens stimulate at a converging point for different growth factor signal-transduction pathways such as hepatocyte growth factor, transforming growth factor-α, epidermal growth factor, and acidic fibroblast growth factor (79). However, these mitogenic effects in the liver have not been fully elucidated. Cholangiocytes are among the cells targeted by estrogens as evidenced by studies showing that estrogen affects proliferation, repair, fibrosis, and angiogenesis. Each of these processes is regulated by autocrine/paracrine factors released from cholangiocytes. Data suggest that estrogen is a potent activator of an autocrine/paracrine loop involving vascular endothelial growth factor (VEGF)-A/C (8, 69). An autocrine loop involving nerve growth factor (NGF) and its receptor tyrosine kinase receptor A (TrkA) is also stimulated by estrogens (37). It is likely that other autocrine/paracrine factors are released in response to estrogen, but this remains to be determined.
Progesterone.
Progesterone is a steroid hormone secreted by the ovaries, adrenal glands, and the corpus luteum. The two main receptors of progesterone, PR-A and PR-B, are expressed in multiple organ systems such as the reproductive and nervous systems and mammary tract, where they modulate cell growth. A recent immunohistochemical study in paraffin-embedded gallbladder sections has demonstrated that many more patients (46%) with secondary hepatolithiasis show positivity for PR of gallbladder specimens than patients with primary hepatolithiasis (93). Unfortunately, in this study the immunohistochemical expression of PR was not evaluated in healthy control specimens (93). Progesterone has been shown to impair gallbladder emptying in response to cholecystokinin (105). Cholangiocytes are also the target of progesterone. In liver sections from normal and BDL rats, cholangiocytes were positive for PR (38). Chronic administration of progesterone increased the number of bile ducts of normal rats, whereas an antiprogesterone antibody inhibited cholangiocyte hyperplasia during BDL, suggesting that progesterone stimulates cholangiocyte proliferation during disease and nondisease states (38). In addition, cholangiocytes were shown to express the key proteins required for steroidogenesis (StAR, P450scc, and 3β-HSD) and synthesized and secreted progesterone (38).
Prolactin.
Prolactin (PRL) is a hormone secreted by the anterior pituitary that promotes cellular differentiation and survival in a number of cell types. PRL receptors are well characterized in the mammary glands and ovaries but are also present in the adrenal glands, gonads, and the central nervous system (CNS). PRL receptors are expressed as either short or long isoforms, having different tissue distribution, and may signal through similar or different STATs. Binding of PRL to its receptors results in dimerization and activation of JAK/STAT-1, MAPK and Src signaling, whereas the long form also is associated with Ca2+. Recent studies by Bogorad et al. (18, 19) have shown that the long isoform of PRL predominates in rat intrahepatic bile ducts and its expression increases under obstructive cholestasis. We have recently shown that normal cholangiocytes express both isoforms (short and long) of PRL, expression that was increased by BDL (102). The difference between the expression of long and short forms of PRL in cholangiocytes is likely due to the different strain of rats used in our studies (female Fischer 344) (102) and the studies (albino mongrel) from Bogorad et al. (18). Administration of PRL stimulated proliferation of normal and BDL female cholangiocytes through a Ca2+/PKC pathway (102). Our studies did not establish which isoform of PR mediates the stimulatory effects of PRL on cholangiocyte growth (102). Because PRLs activate different signaling pathways (see above), further studies are necessary to pinpoint the specific PRL isoform regulating biliary proliferation and to determine whether these receptor subtypes regulate other biliary functions such as secretion and response to damage. It is also necessary to establish whether these receptors differentially target small and/or large cholangiocytes that function by Ca2+- and cAMP-dependent mechanisms, respectively (1, 5, 6, 29, 30, 54). Similar to other cell types (44), normal cholangiocytes expressed the message and protein for PRL and secrete PRL that was increased in cholestatic rats. Administration of an anti-PRL antibody to BDL female rats decreased cholangiocyte proliferation (102). Taken together, the data suggest that PRL stimulates biliary growth by autocrine mechanisms. One study found high PRL levels in three of five patients with nonalcoholic chronic liver diseases (50). Understanding how PRL is induced and how it regulates cholangiocyte growth may be useful in the future management and evaluation of various liver diseases and clearly warrants further exploration.
Follicle-stimulating hormone.
Another hormone of the anterior pituitary and the central hormone of reproduction is follicle-stimulating hormone (FSH). The primary function of FSH is to stimulate germ cells. A recent study has shown that FSH induces cholangiocyte proliferation via an autocrine mechanism (68). The mRNA for FSH was found in cholangiocytes, and the protein was secreted into culture media (68). Administration of anti-FSH antibody to BDL rats decreased ductal mass, and secretin-induced cAMP-dependent phosphorylation of ERK1/2 and ELK-1. There is a striking parallel between the findings that both FSH and PRL regulate cholangiocyte growth in an autocrine fashion. In the normal pituitary gland, autocrine signals from growth factors induce gonadotrophs to produce FSH and lactotrophs to produce PRL. These initial autocrine stimulators include gonadotropin-releasing hormone, angiotensin II, leptin, vasoactive intestinal peptide, and IGF. Intriguingly, most of these autocrine factors are discussed in this review and may act as early factors in the neuroendocrine transdifferentiation of cholangiocytes. We speculate that release of these autocrine factors in a step-wise manner allows new intracellular pathways to be activated and contribute to the growth and antiapoptotic phenotype seen in cholangiocytes in chronic biliary disease and cholangiocarcinoma (Fig. 2).
Fig. 2.
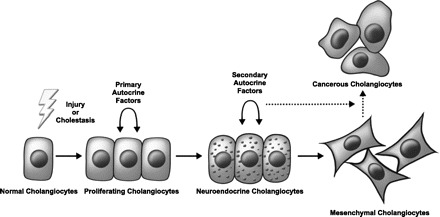
Cholangiocyte progression through the course of biliary damage. Cholangiocytes are activated by injury or cholestasis resulting in their proliferation and secretion of primary autocrine factors. Stimulation by these factors invokes a neuroendocrine change and the production of other (secondary) autocrine factors eventually leading to an epithelial-mesenchymal transition. In time, activated cells may progress into cancer.
Testosterone.
Testosterone is an anabolic steroid hormone secreted by the testes in males. Testosterone stimulates development of the Wolffian ducts during male embryogenesis and spermatogenesis in male adults through binding to the androgen receptor (AR). ARs are expressed in a number of metastatic adenocarcinomas, hepatocellular carcinoma, and cholangiocarcinoma (47, 81, 94). We recently evaluated the effects of testosterone on cholangiocyte growth in rats and found that testosterone stimulated cAMP-dependent biliary proliferation and secretin-stimulated ductal secretion. Chemical castration using flutamide or immunoneutralization of testosterone with an antitestosterone antibody in vivo reduced testosterone levels and decreased biliary proliferation and intrahepatic bile duct mass (111). In vitro, basal proliferative rates of cholangiocytes were inhibited by flutamide (AR antagonist). Decreased testosterone serum levels have been observed in models of cholestasis, which is thought to be the result of hypotrophy of seminal vesicles (55, 113). In fact, testicular atrophy and lowered testosterone serum levels have been shown in patients with primary biliary cirrhosis (PBC) and cirrhosis (28, 106). In our study, the synthesis of testosterone by cholangiocytes was also assessed. Expression of 17β-HSD3, the enzyme regulating testosterone synthesis, was present in cholangiocytes from rats and in normal rat intrahepatic cholangiocyte cultures in culture, and testosterone hormone was present in the supernatant of cholangiocytes in culture (111). Cholangiocyte expression of 17β-HSD3 increased when testosterone was administered to rats, indicating testosterone may induce its own synthesis via an autocrine mechanism. Increased biliary secretion of testosterone is postulated to compensate locally for lower testosterone levels systemically during cholestasis (111).
Neuropeptides and Neurotransmitters
Serotonin.
Serotonin is a neuroendocrine hormone, which in the GI tract acts as a potent messenger to nearby epithelial cells (64). Physiologically, serotonin induces fluid and ion secretion from the intestinal mucosa and potentiates the effect of intestinal hormones on pancreatic secretion (64). Serotonin has been found to modulate cell proliferation in a number of cells including kidney epithelial cells and hepatocytes (12). Serotonin has been shown to abolish fasting-induced bile flow in canine by acting directly on the biliary epithelium (58). We have recently demonstrated the role of serotonin modulation of cholangiocyte proliferation (72). In this study, the receptors for serotonin were expressed on the basolateral domain of cholangiocytes and upregulated in BDL rats (72). Stimulating the serotonin receptor with the serotonin agonists 8-hydroxy-DPAT and anpirotline, decreased cholangiocyte proliferation through increased inositol 1,4,5-trisphosphate, and downstream inactivation of the effecter molecules, Src and ERK1/2 (72). Strong evidence points toward the role of serotonin in autocrine regulation of biliary function. Serotonin is synthesized and secreted by normal rat cholangiocytes. However, there was an upregulation in serotonin secretion in rats with extrahepatic cholestasis induced by BDL (72). Immunoneutralization of the endogenous serotonin secreted by cholangiocytes using an antiserotonin antibody was shown to enhance the growth of the biliary epithelium (72). These findings suggest that in normal cholangiocytes serotonin has an important role in neutralizing physiological levels of proliferative signals such as secretin and certain bile acids and may curtail the strong proliferative signals from high levels of secretin, bile acids, and growth factors in the cholestatic liver (72). It is worth noting, however, that, although serotonin decreased the proliferation of noncancerous cholangiocytes, the opposite effect was found in cholangiocarcinoma (2). We speculate that cholangiocytes may escape the inhibitory action of serotonin through a change in the expression pattern of serotonin receptor subtypes.
Opioids.
Endogenous opioids (endorphins, enkephalins, dynorphins, endomorphins) are found in high concentration in the CNS, where they are involved in processes such as pain and addiction, but are also found peripherally (62, 97). In the gastrointestinal tract, endogenous opioids are known to stimulate receptors on a variety of different cell types (49). In fact, studies in the mouse and human have shown that endogenous opioids modulate proliferation in peripheral organs such as pancreas, colon, and bile ducts (71, 114, 115). Furthermore, fetal human and rat livers and the liver of rats with cholestasis express the preproenkephalin mRNA that encodes for the endogenous opioid peptide Met-enkephalin. Also, Met-enkephalin immunoreactivity is detected in hepatocytes and in proliferating bile ductules in cholestatic liver. Nicoll and colleagues (80) suggested that endogenous opioids may have an autocrine effect during cholestasis. A recent study has shown that cholangiocytes secrete and respond to opioids during cholestasis (71). This study demonstrated the presence of three opioid receptors on cholangiocytes: δOR, μOR, and κOR (71). Stimulating δOR strongly diminished proliferation, whereas activation of μOR slightly increased proliferation (71). Additionally, expression of Met-enkephalin was found in the bile of normal rats, and the levels were tripled when cholangiocytes were stimulated with secretin (71). It was suggested that this increase in Met-enkephalin secretion was a regulatory mechanism to limit the excessive growth of the biliary tree by interacting with the δOR (71). The concept that liver cells including bile ducts are sources of endogenous opioids during cholestasis is supported by the fact that hepatic Met-enkephalin immunoreactivity is enhanced in patients with PBC (16). Hepatic concentrations of proenkephalin-derived opioids are also increased in cholestatic rats (17). In cholangiocarcinoma, the autocrine production of endogenous opioids was shown to inhibit the growth and migration of malignant cholangiocytes (73). This study outlined a self-limiting mechanism, whereby the secretion of endogenous opioids protects cholangiocytes from malignant transformation (73).
NGF.
NGF is a member of the neurotrophin family, which acts on cells expressing the specific tyrosine kinase receptors of the Trk family. NGF is known for its vital role in the maintenance of sympathetic and sensory nerve growth but recently has been shown to regulate proliferation, differentiation, and remodeling in experimental models of various diseases affecting nonnervous tissues (63, 95). NGF is a putative growth factor and acts in an autocrine fashion in tissues such as the pancreas and in a number of cancers (78, 108). During cholestasis, NGF has been shown to modulate the proliferative responses of bile ducts through ERK 1/2 and PI3K pathways (37). Interestingly, stimulating cholangiocyte growth with estrogen had an additive effect on NGF-induced proliferation and increased autocrine signaling of the NGF-TrkA loop (37). Because stellate cells also produce NGF and express TrkA, it is possible that cholangiocytes and stellate cells cross talk through a NGF-paracrine mechanism. NGF-β overexpression is associated with lymph node metastasis and nerve infiltration in human hilar cholangiocarcinoma (110). The paracrine and autocrine roles of NGF have also been suggested in promoting the development of liver cirrhosis and hepatocellular carcinoma (89).
Sensory neuropeptides.
Autonomic and sensory neural pathways regulate the biliary proliferation and function during cholestatic states (42). Both types of neurons possess efferent functions and can release sensory neuropeptides, calcitonin gene-regulated peptide (CGRP), and substance P (SP), locally in the organs that they innervate (42, 48, 76). In rodent liver, CGRP-positive nerves are present as dense networks in the fibromuscular layer of the biliary epithelium, surrounding the portal vein, and in the stromal compartment of portal areas (43). Circulating levels of sensory neuropeptides such as CGRP and SP are increased in human and animal models of cirrhosis (15, 60). CGRP signals via calcitonin-like receptors (CLR). CLR requires a receptor activity-modifying protein (RAMP) to be functionally active. Both α- and β-CGRP signal through the CLR/RAMP1 receptor. We have recently evaluated the role of CGRP on cholangiocyte proliferation (42). Cholangiocytes expressed α-CGRP, β-CGRP, and CGRP receptor components. CGRP activated proliferation of murine cholangiocytes through cAMP/PKA/CREB-dependent signaling (42). The supernatant of isolated cholangiocytes from 3-day CGRP-KO mice had reduced proliferation compared with wild-type mice, suggesting that CGRP may act via an autocrine mechanism (42). In support of this, a CGRP-receptor antagonist reduced the proliferation of cholangiocytes in culture (42). Another sensory neuropeptide, SP, is a member of the tachykinin peptide family, which is made up by SP, neurokinin A, neurokinin B, neuropeptide K, neuropeptide γ, and hemokinin. The tachykinin receptor family consists of neurokinin-1, -2, and -3 receptors (NK-1R, NK-2R, and NK-3R). SP binds and signals via the NK-1R (40). Our recent study provided evidence that 1) cholangiocytes express NK-1R and 2) the SP-NK-1R axis sustains the proliferation of cholangiocytes by activation of cAMP signaling. Knockdown of the NK-1R gene in cholestatic mice decreases intrahepatic bile duct mass concomitant with reduced PKA phosphorylation compared to controls (40).
Angiogenic Factors
VEGF.
The VEGF family is well known for its role in angiogenesis. Members of this family include the cytokines VEGF-A, -B, -C, -D, -E, angiopoietin-1 (Ang-1), and placenta growth factor. These peptide signals function as mitogens for vascular endothelial cells by regulating vascular dilation, permeability, migration, and survival. In the liver, VEGF-A has been shown to prevent bile-duct damage caused by hepatic artery ligation (HAL) (35). We provided the first evidence that cholangiocytes express the message and secrete VEGF-A/C and VEGF-A/C stimulates biliary growth by interacting with VEGF-R2/R3 by activation of autocrine signaling (36). Blocking VEGF expression/secretion in vivo by anti-VEGF-A or anti-VEGF-C antibodies decreased biliary hyperplasia during cholestasis (36). These findings suggest that VEGF mediates the adaptive proliferative response of cholangiocytes to cholestasis (36). In BDL rats, HAL induced the disappearance of the peribiliary vascular plexus, increased biliary apoptosis, decreased cholangiocyte proliferation, and increased cholangiocyte angiogenesis (35). HAL-mediated effects were prevented by administration of recombinant VEGF-A, which suggested that VEGF stimulated biliary growth by an autocrine mechanism (35). Release of VEGF-A from cholangiocytes in primary culture was decreased after HAL in BDL rats, which was restored during VEGF-A administration (35). Furthermore, Fabris et al. (24) have shown that VEGF and Ang-1 supports biliary growth via autocrine mechanisms in cholangiocytes from polycystic liver disease (ADPKD) (24). VEGF stimulated proliferation in cholangiocytes and had a paracrine effect on the portal vasculature (24). It was postulated that this mechanism plays a role in promoting the growth of liver cysts and their vascular supply in ADPKD (24). A recent study has also shown that autocrine and paracrine VEGF signaling promotes the growth of liver cysts in polycystic-2-knockout (Pkd2KO) mice (96). Treatment with the mTOR inhibitor, rapamycin, significantly slowed the progression of cystic disease in Pkd2KO mice by decreased cyst area, and liver/body weight ratio (96).
Autocrine/Paracrine Factors Regulating Biliary Fibrogenesis
Renin-Ang system.
The renin-Ang system (RAS) is well known for its functions in regulating blood pressure, electrolyte balance, and fluid homeostasis. When blood volume is low, juxtaglomerular cells in the kidney secrete renin, which is cleaved by the liver enzyme angiotensinogen to form Ang I, which is further cleaved by Ang-converting enzyme (ACE) to form Ang II, the major physiologically active component of the RAS. The two receptors for Ang II (AT1 and AT2) are expressed in virtually all organs and often induce pathways that counteract the actions of each other. An abundance of animal and human studies on liver fibrosis have looked into the activation of the RAS after ACE/Ang receptor blocker (ARB) inhibition and point to the RAS as an important step in the pathogenesis of liver fibrosis (14, 51, 88, 112). In rats, ACE/ARB inhibitors decreased a number of fibrogenic processes such as collagen deposition, inflammation, oxidative stress, transforming growth factor (TGF)-β1 expression, matrix metalloproteinase (MMP)2/9 activity, and accumulation of myofibroblasts. In patients treated with ACE/ARB inhibitors, a decrease in collagen expression, fibrotic stage, and activation of hepatic stellate cells (HSCs) was seen. Munshi et al. (77), in a comprehensive review, discussed these findings and provided a mechanistic explanation for the RAS in liver fibrosis (77). The authors stated that proliferating biliary epithelium serves as a neuroendocrine compartment for autocrine/paracrine signaling during liver diseases by secreting RAS components. These cause downstream activation of TGF-β1, NADPH oxidase, TNF-α, and procollagen production. It is important to note that these downstream signaling pathways involve either the production or activation by reactive oxygen species-linking the fibrogenic and inflammatory processes.
Platelet-derived growth factor.
Platelet-derived growth factor (PDGF) drives the differentiation of mesenchymal cell growth and differentiation during development and is released by platelets during coagulation. PDGF-β is involved in the fibro-proliferative response of chronic inflammatory disorders such as scleroderma, rheumatoid arthritis, and atherosclerosis. One study reported that, in rats, cholangiocytes responded to BDL by secreting PDGF-β (45). BDL livers had increased expression of PDGF-β mRNA and protein demonstrated by in situ hybridization and immunohistochemistry. PDGF-β secretion by the biliary epithelium was thought to induce the fibrogenic response and participate in EMT. The study suggests that PDGF-β secretion acts by autocrine/paracrine mechanisms because the PDGF-β chain and its receptor increased after BDL and expression of PDGF-R was demonstrated on nearby stellate cells. PDGF-mediated chemoattraction of hepatic progenitor cells by bile ducts has been shown in cholestatic rats (57). It is proposed that peribiliary cells distinct from HSC undergo a PDGF-mediated conversion into myofibroblasts, thus contributing to the initial formation of biliary-type liver fibrosis (56).
TGFs and integrins.
TGF-β1 and -β2 are important cytokines for controlling proliferation and differentiation in a broad spectrum of tissues and extracellular matrix (ECM)-synthesis in the mesenchyme. Their activity increases the synthesis of ECM proteins such as laminin, collagen, fibronectin, and tissue inhibitors of metalloproteinases (TIMPs) while decreasing the expression of ECM degradative enzymes. In the case of wound healing and fibrosis, TGF-β cooperates with αVβ6 integrin-a profibrogenic integrin that is notable for its profibrogenic role in the activation of latent TGF-β1. In the normal liver, TGF-β cytokines and αVβ6 integrin are not expressed at high levels but increase during cholestasis. Several studies have analyzed the relationship between liver fibrosis, αVβ6 integrin, and TGF-β (75, 87, 99, 100). The expression of αVβ6 integrin transcript levels increased 100-fold in proliferating cholangiocytes during advanced liver fibrosis (87). Inhibition of the αVβ6 integrin gene and its receptor retarded progression of biliary fibrosis by reducing activation of TGF-β1 and collagen synthesis and adhesion of cholangiocytes to fibronectin. It is worth noting that, although αVβ6 integrin induces the expression of TGF-β1, the reverse pathway is also present. Sullivan et al. (99) demonstrated in human MMNK-1 cholangiocytes that the mRNA expression of the αVβ6 integrin gene (Itgβ6) is induced by TGF-β1/p38/MAPK pathway through activation of transcription factors SMAD and AP-1 (99). One study compared the normal and fibrotic liver expression of RNA transcripts for TGF-β1 and TGF-β2 (75). In normal liver tissue, expression of the TGF-β1 transcript was high in HSCs, myofibroblasts, inflammatory cells, but not in cholangiocytes. However, the levels of TGF-β1 increased significantly during fibrosis. In contrast to TGF-β1, TGF-β2 was expressed in high levels in cholangiocytes.
Connective tissue growth factor.
Connective tissue growth factor (CTGF) is a hormone that exhibits chemotactic and mitogenic actions on fibroblasts. CTGF is induced by TGF-β in endothelial cells and fibroblasts and shares biological actions on target cells such as fibrosis, chemotaxis, and proliferation. During wound healing and fibrosis, CTGF stimulates epithelial cells and fibroblasts to produce ECM components via autocrine/paracrine mechanisms (20, 65). Several studies have evaluated the production of CTGF in BDL rats and animal models of biliary fibrosis (92, 98). One study reported a sevenfold increase in CTGF mRNA expression in the bile duct epithelial cells of rats with biliary fibrosis. Another study evaluated a DNA microarray of genes expressed in early fibrogenesis as a time-course following BDL and found increased expression of TGF-β, collagen-I, CTGF, and TIMP-1 and a decrease in MMPs 42 days after BDL-induced liver fibrosis (98).
Endothelin.
Endothelin (ET-1) is an important mediator of vascular function and is well known for its potent effects on vasoconstriction. During normal conditions, ET-1 is tonically inhibited by nitric oxide (NO), but during low NO bioavailability (as in oxidative stress and inflammation) ET-1 production increases. In animal models of liver disease, ET-1 has been shown to be an important mediator of liver injury and collagen synthesis (22, 104). In rats with cholangiocarcinoma, ET-1 increased collagen synthesis, apoptosis, and decreased VEGF (26). One study evaluated the role of cholangiocyte ET-1 production in hepatopulmonary syndrome (66). After BDL, hepatic ET-1 levels in tissue increased, and staining for ET-1 coincided with the cholangiocyte marker CK19. After 1-wk BDL, isolated cholangiocytes increased expression of ET-1 mRNA 3.5-fold compared to hepatocytes, which remained unchanged. A relationship between ET-1 and TGF-β1 was demonstrated as administering TGF-β1 stimulated promoter activity, expression and production of ET-1 in normal rats.
Hedgehog signaling.
Hedgehog (Hh) signaling regulates remodeling and migration during embryogenesis and wound healing in adults. Cells expressing the receptor patched are responsive to Hh ligand through intracellular Smo and the Gli family of transcription factors. Activation of these transcription factors causes tissue remodeling and chemotaxis of nearby cells through the production of specific chemokines. Several studies on rats have evaluated the involvement of the activation and production of Hh ligand in the bile duct epithelium (52, 83, 85, 86). In the bile duct epithelium, IHC demonstrated expression of IHh (member of Hh family) and Gli2 (52). Hh was shown to accumulate and promote cholangiocyte EMT and chemokine production through an autocrine/paracrine mechanism involving myofibroblastic HSC (84, 85). On the other hand, neutralizing Hh with an anti-Hh antibody decreased cholangiocyte proliferation and expression of the EMT marker S100A4 (84). Furthermore, Hh induced the activation of divergent autocrine pathways including cxcl16 and osteopontin (85). This cxcl16, produced in cholangiocytes, increased the chemotaxis of natural killer T cells in culture and recruited them to the portal tracts (85).
Summary
In this comprehensive review, we highlighted the various autocrine factors that participate in the regulation of cholangiocyte proliferative and adaptive responses during the progression of chronic cholestatic liver diseases. During the early stages of biliary disease, cholangiocytes respond to both biliary pressure and other contributing factors induced by cholestasis through the activation of proliferation and the switching off of apoptosis. Activation of biliary proliferation triggers the production of a variety of autocrine factors by cholangiocytes resulting in the development of a neuroendocrine phenotype. These activated cholangiocytes begin secreting autocrine factors normally seen in development, sexual reproduction, cell migration, and the nervous system. Together these autocrine factors regulate biliary proliferation in a concerted fashion during cholestasis and also the activation of cells (i.e., hepatic stellate cells, vascular endothelial cells, hepatocytes, immune cells) in the nearby microenvironment, which results in the activation of angiogenesis, fibrogenesis, and inflammatory process and cumulates as biliary fibrosis and subsequent liver cirrhosis. In the late stages of disease, neuroendocrine cells participate in the irreversible stages of biliary fibrosis although the precise mechanism is still unknown. The autocrine/paracrine factors secreted by cholangiocytes are clearly important for orchestrating not only the proliferation of cholangiocytes but also the pathogenesis pathways that result in biliary fibrosis.
Future Perspectives
As our understanding of the critical autocrine factors that regulate biliary proliferation and the potential role of these factors in the cross talk between neighboring cells in the portal tract microenvironment expands, so to will the potential to develop novel therapeutic interventions for chronic cholestatic liver diseases such as primary sclerosing cholangitis, PBC, and biliary atresia. Clearly additional studies are warranted to determine the translational potential of many of the factors discussed in this review. In particular, future studies are needed to evaluate mechanistically the cross-talk that occurs between cholangiocytes and other cells in the portal microenvironment, which is certainly an important aspect of the pathogenesis of cholestatic liver diseases. In addition, studies are needed to determine the cohort of factors that might be beneficial for controlled stimulation of biliary proliferation during conditions of ductopenia and perhaps in biliary atresia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.J. prepared figures; K.J. and S.G. drafted manuscript; K.J., M.M., M.K.M., S.A., G.A., and S.G. edited and revised manuscript; M.M., G.A., and S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was partially supported by National Institute of Diabetes and Digestive and Kidney Diseases RO1 Grant DK-081442 a VA CDA2 Career Development Grant awarded to S. Glaser. This work was also partially supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a Veterans Affairs Research Career Scientist Award, a Veterans Affairs Merit Award, and National Institutes of Health Grants DK054811, DK58411, and DK76898 awarded to G. Alpini.
REFERENCES
- 1. Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 274: G767–G775, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, Franchitto A, Coufal M, Frampton G, Alvaro D, Lee SP, Marzioni M, Benedetti A, DeMorrow S. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res 68: 9184–9193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 81: 569–578, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: The Liver: Biology & Pathobiology, edited by Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA. Philadelphia: Lippincott Williams & Wilkins, 2001, p. 421–435 [Google Scholar]
- 5. Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110: 1636–1643, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage G, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 272: G289–G297, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Alpini G, Ulrich CD, 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 266: G922–G928, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser S, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, Onetti Muda A, Dostal DE, De Santis A, Attili AF, Benedetti A, Gaudio E. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol 169: 877–888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser S, Francis H, Cantafora A, Blotta I, Attili AF, Gaudio E. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol 43: 875–883, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Ascenzia P, Bocedic A, Marino M. Structure-function relationship of estrogen receptor α and β: Impact on human health. Mol Aspects Med 27: 299–402, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56: 413–424, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49: 160–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, Brenner DA, Rippe RA. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology 41: 1046–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Bendtsen F, Schifter S, Henriksen JH. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J Hepatol 12: 118–123, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Bergasa NV, Liau S, Homel P, Ghali V. Hepatic Met-enkephalin immunoreactivity is enhanced in primary biliary cirrhosis. Liver 22: 107–113, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Bergasa NV, Vergalla J, Swain MG, Jones EA. Hepatic concentrations of proenkephalin-derived opioids are increased in a rat model of cholestasis. Liver 16: 298–302, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Bogorad RL, Ostroukhova TY, Orlova AN, Rubtsov PM, Smirnova OV. Long isoform of prolactin receptor predominates in rat intrahepatic bile ducts and further increases under obstructive cholestasis. J Endocrinol 188: 345–354, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Bogorad RL, Ostroukhova TY, Orlova AN, Rubtsov PM, Smirnova OV. Prolactin receptors in rat cholangiocytes: regulation of level and isoform ratio is sex independent. Biochemistry (Mosc) 71: 178–184, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 5: 153–165, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Bulotta A, Hui H, Anastasi E, Bertolotto C, Boros LG, Di Mario U, Perfetti R. Cultured pancreatic ductal cells undergo cell cycle re-distribution and beta-cell-like differentiation in response to glucagon-like peptide-1. J Mol Endocrinol 29: 347–360, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Cho JJ, Hocher B, Herbst H, Jia JD, Ruehl M, Hahn EG, Riecken EO, Schuppan D. An oral endothelin-A receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis. Gastroenterology 118: 1169–1178, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53: 1685–1695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43: 1001–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, Candelaresi C, Venter J, Di Sario A, Marzioni M, Bearzi I, Glaser S, Alvaro D, Marucci L, Francis H, Svegliati-Baroni G, Benedetti A. Leptin enhances cholangiocarcinoma cell growth. Cancer Res 68: 6752–6761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fava G, DeMorrow S, Gaudio E, Franchitto A, Onori P, Carpino G, Glaser S, Francis H, Coufal M, Marucci L, Alvaro D, Marzioni M, Horst T, Mancinelli R, Benedetti A, Alpini G. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int 29: 1031–1042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fisher B, Gunduz N, Saffer EA, Zheng S. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Res 44: 2410–2415, 1984 [PubMed] [Google Scholar]
- 28. Floreani A, Paternoster D, Mega A, Farinati F, Plebani M, Baldo V, Grella P. Sex hormone profile and endometrial cancer risk in primary biliary cirrhosis: a case-control study. Eur J Obstet Gynecol Reprod Biol 103: 154–157, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 295: C499–C513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Francis H, LeSage G, DeMorrow S, Alvaro D, Ueno Y, Venter J, Glaser S, Mancino MG, Marucci L, Benedetti A, Alpini G. The α2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol 293: C1252–C1262, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci USA 108: 1403–1408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol 207: 12–22, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Gatto M, Drudi-Metalli V, Torrice A, Alpini G, Cantafora A, Blotta I, Alvaro D. Insulin-like growth factor-1 isoforms in rat hepatocytes and cholangiocytes and their involvement in protection against cholestatic injury. Lab Invest 88: 986–994, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Franchitto A, Onori P, Ueno Y, Marzioni M, Fava G, Venter J, Reichenbach R, Summers R, Alpini G. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol 291: G307–G317, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, DeMorrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser S, Francis H, Mancino MG, Ueno Y, Barbaro B, Benedetti A, Attili AF, Alvaro D. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 127: 1198–1209, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Glaser S, DeMorrow S, Francis H, Ueno Y, Gaudio E, Vaculin S, Venter J, Franchitto A, Onori P, Vaculin B, Marzioni M, Wise C, Pilanthananond M, Savage J, Pierce L, Mancinelli R, Alpini G. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol 295: G124–G136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glaser S, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 11: e7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glaser S, Gaudio E, Renzi A, Mancinelli R, Ueno Y, Venter J, White M, Kopriva S, Chiasson V, DeMorrow S, Francis H, Meng F, Marzioni M, Franchitto A, Alvaro D, Supowit S, Dipette DJ, Onori P, Alpini G. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol 301: G297–G305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M, Ueno Y, Dostal D, Carpino G, Mancinelli R, Butler W, Chiasson V, DeMorrow S, Francis H, Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 52: 204–214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glaser S, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, Francis HL, Dickerson IM, DiPette DJ, Supowit SC, Alpini GD. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest 87: 914–926, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Goehler LE, Sternini C. Calcitonin gene-related peptide innervation of the rat hepatobiliary system. Peptides 17: 209–217, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol 64: 47–67, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol 31: 100–109, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Harnois DM, Que FG, Celli A, LaRusso NF, Gores GJ. Bcl-2 is overexpressed and alters the threshold for apoptosis in a cholangiocarcinoma cell line. Hepatology 26: 884–890, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Hinchliffe SA, Woods S, Gray S, Burt AD. Cellular distribution of androgen receptors in the liver. J Clin Pathol 49: 418–420, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holzer P. Efferent-like roles of afferent neurons in the gut: Blood flow regulation and tissue protection. Auton Neurosci 125: 70–75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 155: 11–17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ilan Y, Oren R, Tur-Kaspa R. Elevated growth hormone levels in patients with non-alcoholic chronic liver disease. J Gastroenterol Hepatol 8: 448–450, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 121: 148–155, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology 45: 1091–1096, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 281: G612–G625, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology 31: 555–561, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Kiani S, Valizadeh B, Hormazdi B, Samadi H, Najafi T, Samini M, Dehpour AR. Alteration in male reproductive system in experimental cholestasis: roles for opioids and nitric oxide overproduction. Eur J Pharmacol 615: 246–251, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 83: 163–173, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest 80: 697–707, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Kortz WJ, Schirmer BD, Nashold JR, Jones RS, Meyers WC. Effects of serotonin on canine bile formation. Surgery 98: 907–913, 1985 [PubMed] [Google Scholar]
- 59. Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Lee FY, Lin HC, Tsai YT, Chang FY, Lu RH, Hou MC, Li CP, Chu CJ, Wang SS, Lee SD. Plasma substance P levels in patients with liver cirrhosis: relationship to systemic and portal hemodynamics. Am J Gastroenterol 92: 2080–2084, 1997 [PubMed] [Google Scholar]
- 61. LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol 276: G1289–G1301, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Lesniak A, Lipkowski AW. Opioid peptides in peripheral pain control. Acta Neurobiol Exp (Warsz) 71: 129–138, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Levi-Montalcini R. The nerve growth factor and the neuroscience chess board. Prog Brain Res 146: 525–527, 2004 [PubMed] [Google Scholar]
- 64. Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology 118: 1197–1207, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Lim M, Goldstein MH, Tuli S, Schultz GS. Growth factor, cytokine and protease interactions during corneal wound healing. Ocul Surf 1: 53–65, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Luo B, Tang L, Wang Z, Zhang J, Ling Y, Feng W, Sun JZ, Stockard CR, Frost AR, Chen YF, Grizzle WE, Fallon MB. Cholangiocyte endothelin 1 and transforming growth factor beta1 production in rat experimental hepatopulmonary syndrome. Gastroenterology 129: 682–695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, DeMorrow S, Carpino G, Kopriva S, White M, Fava G, Alvaro D, Alpini G. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol 176: 1790–1800, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, Kopriva S, White M, Kossie A, Savage J, Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol 297: G11–G26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, Onori P, Franchitto A, Ginanni-Corradini S, Gaudio E, Alvaro D. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis 41: 156–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marzioni M, Alpini G, Saccomanno S, Candelaresi C, Venter J, Rychlicki C, Fava G, Francis H, Trozzi L, Glaser S, Benedetti A. Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 133: 244–255, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Marzioni M, Alpini G, Saccomanno S, de Minicis S, Glaser S, Francis H, Trozzi L, Venter J, Orlando F, Fava G, Candelaresi C, Macarri G, Benedetti A. Endogenous opioids modulate the growth of the biliary tree in the course of cholestasis. Gastroenterology 130: 1831–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, Venter J, Fava G, LeSage G, Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 128: 121–137, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Marzioni M, Invernizzi P, Candelaresi C, Maggioni M, Saccomanno S, Selmi C, Rychlicki C, Agostinelli L, Cassani B, Miozzo M, Pasini S, Fava G, Alpini G, Benedetti A. Human cholangiocarcinoma development is associated with dysregulation of opioidergic modulation of cholangiocyte growth. Dig Liver Dis 41: 523–533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, LaRusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 295: G725–G734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol 139: 1221–1229, 1991 [PMC free article] [PubMed] [Google Scholar]
- 76. Munshi MK, Priester S, Gaudio E, Yang F, Alpini G, Mancinelli R, Wise C, Meng F, Franchitto A, Onori P, Glaser SS. Regulation of biliary proliferation by neuroendocrine factors: implications for the pathogenesis of cholestatic liver diseases. Am J Pathol 178: 472–484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Munshi MK, Uddin MN, Glaser SS. The role of the renin-angiotensin system in liver fibrosis. Exp Biol Med (Maywood) 236: 557–566, 2011 [DOI] [PubMed] [Google Scholar]
- 78. Navarro-Tableros V, Sanchez-Soto MC, Garcia S, Hiriart M. Autocrine regulation of single pancreatic beta-cell survival. Diabetes 53: 2018–2023, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Ni N, Yager JD. Comitogenic effects of estrogens on DNA synthesis induced by various growth factors in cultured female rat hepatocytes. Hepatology 19: 183–192, 1994 [PubMed] [Google Scholar]
- 80. Nicoll J, Axiotis CA, Bergasa NV. The delta opioid receptor 1 is expressed by proliferating bile ductules in rats with cholestasis: implications for the study of liver regeneration and malignant transformation of biliary epithelium. Med Hypotheses 65: 1099–1105, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Ohnishi S, Murakami T, Moriyama T, Mitamura K, Imawari M. Androgen and estrogen receptors in hepatocellular carcinoma and in the surrounding noncancerous liver tissue. Hepatology 6: 440–443, 1986 [DOI] [PubMed] [Google Scholar]
- 82. Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, Choi SS, Alpini G, Schwarz KB, Diehl AM, Whitington PF. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology 53: 1246–1258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 57: 1275–1282, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 118: 3331–3342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology 50: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest 87: 499–514, 2007 [DOI] [PubMed] [Google Scholar]
- 87. Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology 135: 660–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ramalho LN, Ramalho FS, Zucoloto S, Castro-e-Silva Junior O, Correa FM, Elias Junior J, Magalhaes JF. Effect of losartan, an angiotensin II antagonist, on secondary biliary cirrhosis. Hepatogastroenterology 49: 1499–1502, 2002 [PubMed] [Google Scholar]
- 89. Rasi G, Serafino A, Bellis L, Lonardo MT, Andreola F, Zonfrillo M, Vennarecci G, Pierimarchi P, Sinibaldi Vallebona P, Ettorre GM, Santoro E, Puoti C. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J Gastroenterol 13: 4986–4995, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol 15: 259–269, 1998 [PubMed] [Google Scholar]
- 91. Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 139: 987–998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol 158: 1239–1244, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sheen-Chen SM, Ho HT, Chen WJ, Sheen CW, Eng HL, Chou FF. Progesterone receptor in patients with hepatolithiasis. Dig Dis Sci 46: 2374–2377, 2001 [DOI] [PubMed] [Google Scholar]
- 94. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. Diagn Pathol 1: 34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Skaper SD. Nerve growth factor: a neurokine orchestrating neuroimmune-endocrine functions. Mol Neurobiol 24: 183–199, 2001 [DOI] [PubMed] [Google Scholar]
- 96. Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, Tian X, Somlo S, Strazzabosco M. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 51: 1778–1788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stein C, Zollner C. Opioids and sensory nerves. Handb Exp Pharmacol 495–518, 2009 [DOI] [PubMed] [Google Scholar]
- 98. Sugihara T, Koda M, Matono T, Maeda K, Yamamoto S, Ueki M, Murawaki Y. Extracellular matrix metabolism-related gene expression in bile duct-ligated rats. Mol Med Report 2: 345–351, 2009 [DOI] [PubMed] [Google Scholar]
- 99. Sullivan BP, Kassel KM, Manley S, Baker AK, Luyendyk JP. Regulation of transforming growth factor-beta1-dependent integrin beta6 expression by p38 mitogen-activated protein kinase in bile duct epithelial cells. J Pharmacol Exp Ther 337: 471–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sullivan BP, Weinreb PH, Violette SM, Luyendyk JP. The coagulation system contributes to αVβ6 integrin expression and liver fibrosis induced by cholestasis. Am J Pathol 177: 2837–2849, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tadlock L, Yamagiwa Y, Hawker J, Marienfeld C, Patel T. Transforming growth factor-beta inhibition of proteasomal activity: a potential mechanism of growth arrest. Am J Physiol Cell Physiol 285: C277–C285, 2003 [DOI] [PubMed] [Google Scholar]
- 102. Taffetani S, Glaser S, Francis H, DeMorrow S, Ueno Y, Alvaro D, Marucci L, Marzioni M, Fava G, Venter J, Vaculin S, Vaculin B, Lam IP, Lee VH, Gaudio E, Carpino G, Benedetti A, Alpini G. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol 7: 6, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology 30: 1425–1433, 1999 [DOI] [PubMed] [Google Scholar]
- 104. Thirunavukkarasu C, Yang Y, Subbotin VM, Harvey SA, Fung J, Gandhi CR. Endothelin receptor antagonist TAK-044 arrests and reverses the development of carbon tetrachloride induced cirrhosis in rats. Gut 53: 1010–1019, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tierney S, Nakeeb A, Wong O, Lipsett PA, Sostre S, Pitt HA, Lillemoe KD. Progesterone alters biliary flow dynamics. Ann Surg 229: 205–209, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tsega E, Nordenfelt E, Hansson BG, Mengesha B, Lindberg J. Chronic liver disease in Ethiopia: a clinical study with emphasis on identifying common causes. Ethiop Med J 30: 1–33, 1992 [PubMed] [Google Scholar]
- 107. Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, Reid LM. Human hepatic stem cell and maturational liver lineage biology. Hepatology 53: 1035–1045, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang ZY, Ding Y, Miki T, Warita K, Matsumoto Y, Takeuchi Y, Wang SJ, Feng JG, Liu W, Wang YD, Wang XL, Wang YH, Liu Y, Shan BE. Nerve growth factor and receptors are significantly affected by histamine stimulus through H1 receptor in pancreatic carcinoma cells. Mol Med Report 3: 103–109, 2010 [DOI] [PubMed] [Google Scholar]
- 109. Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 5: 1221–1228, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xu LB, Liu C, Gao GQ, Yu XH, Zhang R, Wang J. Nerve growth factor-beta expression is associated with lymph node metastasis and nerve infiltration in human hilar cholangiocarcinoma. World J Surg 34: 1039–1045, 2010 [DOI] [PubMed] [Google Scholar]
- 111. Yang F, Priester S, Onori P, Venter J, Renzi A, Franchitto A, Munshi MK, Wise C, Dostal DE, Marzioni M, Saccomanno S, Ueno Y, Gaudio E, Glaser S. Castration inhibits biliary proliferation induced by bile duct obstruction: novel role for the autocrine trophic effect of testosterone. Am J Physiol Gastrointest Liver Physiol 301: G981–G991, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34: 745–750, 2001 [DOI] [PubMed] [Google Scholar]
- 113. Yoshioka K, Sasaki M, Imai S, Tsujio M, Taniguchi K, Mutoh K. Testicular atrophy after bile duct ligation in chickens. Vet Pathol 41: 68–72, 2004 [DOI] [PubMed] [Google Scholar]
- 114. Zagon IS, Hytrek SD, McLaughlin PJ. Opioid growth factor tonically inhibits human colon cancer cell proliferation in tissue culture. Am J Physiol Regul Integr Comp Physiol 271: R511–R518, 1996 [DOI] [PubMed] [Google Scholar]
- 115. Zagon IS, Smith JP, McLaughlin PJ. Human pancreatic cancer cell proliferation in tissue culture is tonically inhibited by opioid growth factor. Int J Oncol 14: 577–584, 1999 [DOI] [PubMed] [Google Scholar]


