Abstract
Extracorporeal membrane oxygenation (ECMO) provides essential mechanical circulatory support necessary for survival in infants and children with acute cardiac decompensation. However, ECMO also causes metabolic disturbances, which contribute to total body wasting and protein loss. Cardiac stunning can also occur, which prevents ECMO weaning, and contributes to high mortality. The heart may specifically undergo metabolic impairments, which influence functional recovery. We tested the hypothesis that ECMO alters oxidative metabolism and protein synthesis. We focused on the amino acid leucine and integration with myocardial protein synthesis. We used a translational immature swine model in which we assessed in heart 1) the fractional contribution of leucine (FcLeucine) and pyruvate to mitochondrial acetyl-CoA formation by nuclear magnetic resonance and 2) global protein fractional synthesis (FSR) by gas chromatography-mass spectrometry. Immature mixed breed Yorkshire male piglets (n = 22) were divided into four groups based on loading status (8 h of normal circulation or ECMO) and intracoronary infusion [13C6,15N]-L-leucine (3.7 mM) alone or with [2-13C]-pyruvate (7.4 mM). ECMO decreased pulse pressure and correspondingly lowered myocardial oxygen consumption (∼40%, n = 5), indicating decreased overall mitochondrial oxidative metabolism. However, FcLeucine was maintained and myocardial protein FSR was marginally increased. Pyruvate addition decreased tissue leucine enrichment, FcLeucine, and Fc for endogenous substrates as well as protein FSR. The heart under ECMO shows reduced oxidative metabolism of substrates, including amino acids, while maintaining 1) metabolic flexibility indicated by ability to respond to pyruvate and 2) a normal or increased capacity for global protein synthesis.
Keywords: extracorporeal membrane oxygenation, leucine, pyruvate, branch chain amino acids
extracorporeal membrane oxygenation (ECMO) is the principal form of mechanical circulatory support for pediatric patients suffering from acute cardiac decompensation after surgical procedures for congenital heart defects. ECMO is also instituted for heart failure in children from other causes such as sudden cardiac arrest or acute myocarditis (13, 17). Veno-arterial ECMO provides biventricular unloading by redirecting systemic venous return into a mechanical circuit where the blood is oxygenated and returned to the aorta. The ECMO circuit contains a pump that supports systemic blood flow and mean arterial pressure but reduces aortic pulsatility (13). Mortality either during or immediately after ECMO remains high, especially for infants and children with complex congenital heart disease (33). ECMO duration for cardiac indications is usually 24 to 48 h and is intended as a short-term bridge to recovery (13). When used for longer timer periods, the risks for mortality and severe morbidities including stroke and renal failure increase substantially. Therefore, early recovery from the antecedent insult and complete weaning and separation from the support circuit represent the principal goals of ECMO therapy for infants and children. However, ECMO frequently induces a cardiac stun syndrome (21). Stunning refers to sudden exacerbation of myocardial dysfunction observed in infants (21) and in animal models (31) within a few hours after instituting ECMO. The cardiac stun syndrome prevents or delays weaning from the support circuit and adversely affects survival.
Some investigators have hypothesized that inflammatory cytokines mediate or at least play an important role in the pathogenesis of ECMO-related cardiac stun. ECMO and closely related cardiopulmonary bypass procedures induce marked elevations in circulating plasma levels of multiple proinflammatory cytokines (22). Fairly modest cytokine elevations, relative to those associated with ECMO, yield metabolic abnormalities such as reductions in insulin sensitivity and shifts in substrate utilization (19). In turn, these metabolic abnormalities could affect the efficiency of cardiac ATP production and utilization, and therefore further impair the myocardial functional response to stress. Catabolic processes induced by inflammation could also modify cardiac protein balance, reduce myocardial mass, and further limit the ability of the heart to successfully wean from ECMO support. Although whole body studies in infants have documented elevations in protein turnover and amino acid oxidation during ECMO (3, 18, 34, 39), heart-specific modifications in substrate metabolism and protein synthesis have not been evaluated (3, 18, 34, 39). Thus we do not know if shifts occur in cardiac metabolism during ECMO or whether they represent potential targets for prevention of the cardiac stun syndrome.
Therefore, we formulated a hypothesis that ECMO alters amino acid oxidative metabolism and myocardial protein synthesis. To test this hypothesis we used a translational piglet model (32), which emulates ECMO in infants and children in these experiments, and pursued the fate of leucine delivered into the coronary artery, as a template for amino acid metabolism. The majority of leucine entering cardiomyocytes is either incorporated into protein or metabolized to acetoacetate and acetyl-CoA. These latter metabolites enter the citric acid cycle (CAC) and participate in oxidation, which supplies energy for ATP production. Accordingly, we measured leucine contribution to these pathways using 13Carbon (13C)-isotopomer analyses by both gas chromatography-mass spectroscopy (GCMS) and nuclear magnetic resonance spectrometry (NMR). 13C-labeled pyruvate was included as a substrate in some experiments to serve as an alternate caloric source as well as a reference for oxidation by NMR; we have previously described the inotropic and metabolic effects of pyruvate in immature swine hearts (26, 27).
MATERIALS AND METHODS
Model
We used immature mixed breed Yorkshire male piglets in these experiments. Studies were approved by the Seattle Children's Research Institute Animal Care and Use Committee and adhered to both the American Physiological Society's Guiding Principles in the Care and Use of Animals and the National Institute of Health's Guide for the Care and Use of Laboratory Animals.
In preliminary studies, we determined that mechanical circulatory support caused marked hemodilution in piglets weighing less than 7 kg. Hemodilution could be avoided by priming the support circuit with donor pig blood; however, this frequently caused devastating transfusion reactions. Pig cross-matching for blood priming of the pump is complicated by numerous serotypes (35). Accordingly, we set a lower threshold of 7 kg for pigs in this study. Male piglets between 27 and 41 days of age and weighing between 7.8 and 14.5 kg were prepared essentially as previously described (27). These pigs were beyond the neonatal age, but still immature and undergoing rapid cardiac growth. This developmental state approximates that for the majority of human infants, generally beyond the neonatal age, undergoing ECMO for cardiac indications (14). Sedation was achieved by intramuscular injection of ketamine (33 mg/kg; VEDCO, St. Joseph, MO) and xylazine (2 mg/kg; VEDCO), and the animals were placed on a circulating warming blanket. Monitors were placed for ECG, pulse oximetry (Radical SET; Masimo, Irvine, CA), and rectal temperature. A PowerLab 16/30 recorder (AD Instruments, Colorado Springs, CO) was used to continuously record data throughout all protocols. A cutdown tracheostomy was performed, facilitating mechanical ventilation and general anesthesia under inhaled isoflurane (1–3%; Baxter Healthcare, Deerfield, IL). Venous and arterial access was then obtained for continuous blood pressure monitoring, blood sampling, and infusion of maintenance fluids (5% dextrose in 0.9% saline). Arterial pH, pCO2, pO2, and hemoglobin were measured at regular intervals by Radiometer ABL 800 (Radiometer America, Westlake, OH). After the performance of a median sternotomy, the piglets were separated into two groups. The first group (LOAD) did not undergo cannulation for ECMO. For the s group (ECMO), the hemiazygous vein (which empties directly into the coronary sinus in swine) was ligated and the aorta, superior vena cava, and inferior vena cava via the right atrium were cannulated and connected to the ECMO circuit [Stockert-Shiley roller pump and hollow-fiber oxygenator (CapioX Rx 05, Terumo, and Tokyo, Japan)]. Anticoagulation was achieved with 120 units/kg of heparin (APP Pharmaceuticals, Schaumberg, IL) as a bolus followed by continuous intravenous infusion of heparin. To minimize hemodilution anemia, we used the smallest volume of priming solution possible (75 ml of 5% dextrose in 0.9% saline). In all animals, we inserted a 24-gauge BD Saf-T-Infusion catheter (BD, Franklin Lakes, NJ) into the left anterior descending artery in retrograde fashion starting just distal to the first major branch for intracoronary infusions. In the ECMO groups, pump flow was adjusted to maintain a mean arterial pressure of 50–60 mmHg, and gas sweep through the oxygenator was adjusted to maintain arterial pO2 greater than 120 mmHg and pCO2 between 35 and 45 mmHg. No inotropic or vasopressor medications were used. At the end of the infusion, the apex of the heart was rapidly excised and freeze-clamped, and then stored in liquid nitrogen for further analyses. Euthanasia at the end of each procedure was by cardiac excision with rapid exsanguination under general anesthesia.
Substrate Delivery
[13C6,15N]-L-leucine (Sigma-Aldrich, St. Louis, MO) alone or with sodium-[2-13C]-pyruvate (Sigma-Aldrich) was delivered by intracoronary infusion. The direct coronary infusion eliminated potential issues with systemic infusion relevant to hemodilution and differences in vascular volume between control pigs and those on mechanical circulatory support. We based the target concentration of leucine (3.7 mM) on prior studies by Chua et al. (7–10), which were performed in isolated perfused working rat heart. Our objective was to determine the fractional contribution of leucine (FcLeucine) to the CAC relative to another typically oxidized substrate. We previously evaluated pyruvate oxidation and anaplerotic contribution to the CAC in piglets (8, 26, 27). Those experiments showed that intracoronary pyruvate concentrations near 8 mM provided adequate NMR signal for spectral analyses. Because leucine provides twice as many carbons as pyruvate, we then adjusted the dose in mM to a near 1-to-2 molar ratio for labeled leucine and pyruvate. Accordingly, the target intracoronary concentration was 3.7 mM for [13C6,15N]-L-leucine and 7.4 mM for sodium-[2-13C]-pyruvate.
Because substrates were delivered directly into the coronary artery, we were unable to sample downstream to confirm the actual concentrations delivered. However, the dose per milligram tissue was confirmed by heart wet weight after completion of the experiments. Studies in working and Langendorff perfused rat hearts have shown that oxidation of leucine supplied at either 1 or 5 mM achieves steady-state within a few minutes (15). We performed preliminary studies using a 2-h infusion of labeled leucine in two pigs under ECMO (data not shown). In these studies we confirmed that isotopic enrichment of protein and the cytosolic leucine pool as well as the CAC intermediates was similar to that obtained with a 1 h infusion. Using this as evidence for steady-state, we set the intracoronary substrate infusion duration for the protocol to 1 h.
ECMO Duration
ECMO initiates a massive systemic inflammatory response. In human infants and neonatal pigs a rapid rise in plasma levels for multiple cytokines occurs over 6 h, followed by a plateau phase for at least 2 h in human infants and neonatal pigs (3, 22, 25, 28). Systemic hormonal and metabolic responses are attributed at least in part to the surge in cytokines. The cardiac stun phenomenon is concomitantly observed within this 8-h time frame. Therefore, we selected 8-h duration for all experimental groups as an appropriate time period for initial studies of metabolism and protein synthesis in this ECMO model.
Protocols
We had four experimental groups based on loading status and substrate(s) provided in the coronary infusate. The piglets with normal circulation (not undergoing ECMO) were termed the LOAD group and provided a control for comparison with those undergoing ECMO. Each animal received infusion of [13C6,15N]-L-leucine, and half the piglets also received sodium-[2–13C]-pyruvate, so the four groups are as follows: LOAD-LP (normal circulation, leucine and pyruvate; n = 6), LOAD-L (normal circulation, leucine only; n = 5), ECMO-LP (leucine and pyruvate; n = 6), and ECMO-L (leucine only; n = 5). In five additional piglets, we also measured coronary flow and sampled coronary venous return for oxygen content to determine the ECMO effect on myocardial oxygen consumption. Coronary flow was determined in these experiments by means of a shunt placed in the coronary sinus as previously described (26, 27) with the hemiazygous vein ligated to prevent systemic venous flow contamination.
Metabolic Analyses
Preparation of myocardial extracts.
Freeze-clamped sections of hearts in the region perfused by the left anterior descending coronary artery were pulverized under liquid nitrogen and the tissue extracted with either methanol/chloroform or 1.2 M perchloric acid, then neutralized with cold KOH to pH 7.4. The final supernatant was lyophilized overnight at −50°C. The lyophilized product was used for mass spectrophotometry or 13C-NMR spectral acquisition. For amino acid hydrolysis, tissue residue from the perchloric acid extraction was resuspended in 6N HCl and incubated overnight at 110°C, and the HCl was evaporated off at 60°C in a fume hood. The resulting product was resuspended into a 0.1M carbonate buffer to pH 7.
GCMS.
GCMS (Agilent 6890N gas chromatograph equipped with a HP-5 column coupled to 5973N mass spectrometer; Agilent Technologies, Santa Clara, CA) was performed to measure the concentrations and 13C-enrichment of leucine and CAC intermediates in apical myocardial tissue samples, as previously described (26). The molar percent enrichment (MPE) of isotope-enriched leucine was calculated from the tissue fluid free leucine enrichment of each sample. Mass isotopomers of metabolites containing 1 to n 13C-labeled atoms were identified as Mi, with i = 1, 2, . . . n, and the MPE of individual 13C-labeled mass isotopomers (Mi) of a given metabolite was calculated as follows:
where AM and AMi represent the peak areas from ion chromatograms corrected for natural abundance, corresponding to the unlabeled (M) and 13C-labeled (Mi) mass isotopomers, respectively (26). Protein fractional synthesis rates (FSR) were then calculated following the methods of Jaleel et al. (16). Briefly, the isotopic enrichment of [13C6,15N]-L-leucine in the proteins (Epro) and the free tissue fluid (Etf) were used with the following equation:
where t is the time of tracer incorporation, in hours (1 h for all groups).
CAC intermediate enrichment was evaluated via the total isotopic enrichment of several intermediates in the CAC (citrate, α-ketoglutarate, fumarate, and malate), as well as of lactate and pyruvate, from the ground tissue samples.
NMR.
NMR free-induction decays (FIDs) were acquired on a Varian Direct Drive (VNMRS) 600 MHz spectrometer (Varian, Palo Alto, CA) equipped with a Dell Precision 390 Linux workstation running VNMRJ 2.2C. The spectrometer system was outfitted with a Varian triple resonance salt-tolerant cold probe with a cold carbon preamplifier. A Varian standard one-dimensional carbon direct-observe sequence with proton decoupling was used to collect data on each sample. Final spectra were accumulations of 4,800 individual FIDs. Each FID was induced using a nonselective, 45-degree excitation pulse (7.05 μs at 58 dB), with an acquisition time of 1.3 s, a recycle delay of 3 s, and a spectral width of 224.1 ppm.
13C-glutamate spectra were analyzed in the manner of Malloy et al. (20). Fourier-transformed spectra underwent linebroadening (1 Hz) and were fitted with commercial software (NMR NUTS), and areas for the glutamate carbon complex resonances were determined. The data were run through tcaCALC analyses for determination of the fractional acetyl-CoA contribution of the labeled substrates to the CAC (tcaCALC kindly provided by the Advanced Imaging Research Center at the University of Texas, Southwestern). [2-13C]-pyruvate does label the glutamate-C5, whereas uniformly labeled leucine results in labeling at the C4 and C5 position or only C4 position. This allowed determination of relative fractional contribution to glutamate labeling via acetyl-CoA between leucine and pyruvate, as well as the unlabeled substrate contribution.
1H-NMR was also performed on extracts from two groups receiving both leucine and pyruvate, using the Varian system to determine myocardial concentration of amino acids. Preliminary data from our samples showed that methanol extraction improved signal to noise and resolution compared with standard perchloric acid extraction. A standard one-dimensional proton NOESY with presaturation (TMNNOESY) was collected on each sample, using the Chenomx standard data collection protocol: a nonselective 90° excitation pulse (∼7 μs at 53 dB), a 100-millisecond mixing time, acquisition time of 4 s, a recycle delay of 1 s, spectral width of 12 ppm, and temperature control set to 25°C. Collected spectra were analyzed using Chenomx software, with quantifications based on spectral intensities relative to 0.5 mM 2,2-dimethyl-2-silapentane-5-sulfonate, which was added as a spike to each sample. Calibration curves were developed with samples spiked with known concentrations of amino acids to assure accuracy.
IL-6.
Plasma concentrations of the porcine cytokine IL-6 were measured by ELISA (Catalog No. P6000B; R&D systems, Minneapolis, MA) according to manufacturer's protocol.
Statistical Analysis
Values are reported as means ± SE in the text, figures, and tables. Data were analyzed for significant variance with ANOVA for multiple comparisons, and we performed Student's t-tests between groups to further evaluate for significance between groups, as appropriate. Significance was defined as P < 0.05 for all comparisons. Error bars in graphs indicate SE.
RESULTS
Cardiac Function and Oxygen Consumption
Hemodynamic indexes before initiating protocol were not significantly different between the LOAD and ECMO conditions (Table 1). As expected, ECMO significantly reduced systolic and pulse pressures without a significant change in the mean arterial pressure. ECMO reduced hemoglobin by the completion of the 8-h protocols, consistent with expected hemodilution and/or hemolytic trauma from the roller pump. In the parallel studies we noted that ECMO decreased myocardial oxygen consumption by ∼40% reflecting mainly change in O2 extraction and not coronary flow (Fig. 1). ECMO also induced an inflammatory response exemplified by a surge in intraleukin-6 (Fig. 2), peaking at 4 h but then decreasing by 7 h.
Table 1.
Hemodynamic data
| Starting |
8 h |
|||||
|---|---|---|---|---|---|---|
| LOAD | ECMO | P | LOAD | ECMO | P | |
| Heart rate, beats/min | 98 ± 5 | 99 ± 4 | NS | 128 ± 9 | 129 ± 11 | NS |
| Pressure, mmHg | ||||||
| Arterial | ||||||
| Systolic | 75 ± 3 | 78 ± 2 | NS | 65 ± 1 | 55 ± 2 | <0.005 |
| Diastolic | 54 ± 2 | 56 ± 3 | NS | 40 ± 2 | 49 ± 3 | <0.01 |
| Mean | 60 ± 2 | 63 ± 2 | NS | 48 ± 1 | 51 ± 3 | NS |
| Pulse | 21 ± 2 | 21 ± 2 | NS | 25 ± 2 | 6 ± 1 | <0.001 |
| Hemoglobin, g/dL | 9.0 ± 0.6 | 9.2 ± 0.5 | NS | 8.9 ± 0.5 | 6.0 ± 0.7 | <0.01 |
Values are means ± SE; n = 11 for all groups. P values denote statistical comparisons between extracorporeal membrane oxygenation (ECMO) and piglets with normal circulation and not undergoing ECMO (LOAD) at each time point. The marked drop in pulse pressure demonstrates ventricular unloading by the ECMO circuit. NS, not significant.
Fig. 1.
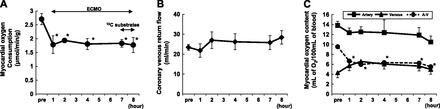
Myocardial oxygen consumption (MVO2) during extracorporeal membrane oxygenation (ECMO; n = 5). MVO2 (A) was determined through coronary sinus flow measurement (B) and systemic and coronary sinus blood sampling for O2 content (C). ECMO decreases MVO2 due to drop in A-V O2 content difference. *P < 0.05 compared with pre (baseline before ECMO).
Fig. 2.
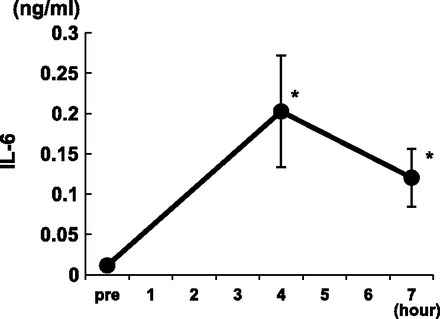
Plasma IL-6 levels during ECMO. *P < 0.05 compared with pre (baseline before ECMO).
Intracellular Free Leucine MPE
Experiments using [13C6,15N]-L-leucine as the sole labeled substrate confirmed that our protocol achieved transport of isotopic leucine into the cytoplasm. The LOAD-L group exhibited the highest total MPE for intracellular free leucine by GCMS (Fig. 3). Under these substrate provision conditions (leucine only), ECMO decreased the free leucine MPE by ∼8% (LOAD-L vs. ECMO-L; P < 0.05).
Fig. 3.
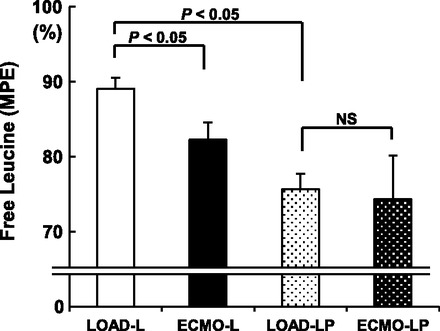
Molar percent enrichment (MPE) for isotopically labeled free intracellular leucine determined by gas chromatography-mass spectroscopy. Groups are piglets with normal circulation and not undergoing ECMO (LOAD) or ECMO either with leucine (L) or leucine plus pyruvate (LP); n = 5 or 6 per group. NS, not significant.
Addition of isotopically labeled pyruvate decreased intracellular free leucine MPE by 15% in the LOAD groups (LOAD-L vs. LOAD-LP; P < 0.005). Pyruvate also decreased free leucine MPE in the piglets that underwent ECMO, but this did not reach significance (ECMO-L vs. ECMO-LP; P = 0.08). The ECMO-induced reduction in free leucine MPE noted for LOAD-L versus ECMO-L was not observed when pyruvate was included (LOAD-LP vs. ECMO-LP). Using 1H-NMR, we also analyzed absolute free amino acid concentration in LOAD-LP and ECMO-LP, and ECMO-L groups (Table 2). These data show that loading conditions did not influence free intracellular concentration for leucine and eight other amino acids, including the other branched chain amino acids valine and isoleucine. We compared ECMO-L and a separate ECMO group (n = 5) that did not receive leucine to determine whether our supraphysiological leucine doses altered free tissue leucine concentration. Leucine provided by coronary infusion did not alter tissue levels of leucine. Leucine infusion decreased tissue concentrations for the other branched chain amino acids, valine and isoleucine.
Table 2.
Free amino acid concentration in myocardial tissues by 1H-nuclear magnetic resonance spectrometry (in μM)
| LOAD |
ECMO |
||
|---|---|---|---|
| Leucine | Leucine | No leucine | |
| Alanine | 2,823 ± 294 | 3,225 ± 284 | 3,551 ± 209 |
| Aspartate | 1,009 ± 232 | 875 ± 220 | 1,115 ± 165 |
| Glutamate | 3,893 ± 325 | 3,808 ± 315 | 5,133 ± 386 |
| Glutamine | 7,324 ± 813 | 7,699 ± 1050 | 11,014 ± 1286 |
| Glycine | 1,801 ± 209 | 1,904 ± 172 | 1,637 ± 241 |
| Isoleucine | 101 ± 21 | 134 ± 19 | 198 ± 31* |
| Leucine | 181 ± 23 | 240 ± 30 | 278 ± 25 |
| Threonine | 533 ± 167 | 853 ± 84 | 908 ± 78 |
| Valine | 178 ± 29 | 247 ± 38 | 375 ± 57* |
| Glutamate/glutamine | 0.55 ± 0.05 | 0.52 ± 0.06 | 0.50 ± 0.07 |
Values are means ± SE; n = 6 per group.
P < 0.05 vs. ECMO (leucine).
Enrichment of CAC Intermediates
Loading status alone did not significantly affect MPE of CAC intermediates determined by GCMS (for all intermediates, LOAD-L vs. ECMO-L and LOAD-LP vs. ECMO-LP; P > 0.2; Fig. 4). As expected, pyruvate increased CAC labeling by an average of 65% (LOAD-L vs. LOAD-LP, P < 0.05; ECMO-L vs. ECMO-LP, P < 0.01; Fig. 4). Loading status did not impact MPE of the pyruvate or lactate pools. As noted above, ECMO also did not affect enrichment of the free cytosolic leucine pool when pyruvate was provided. Thus the data imply that ECMO did not influence the uptake of pyruvate relative to leucine. This is subject to the assumption that sources of unlabeled pyruvate were not changed by ECMO. The systemic arterial levels of the primary alternate sources of pyruvate (glucose and lactate) were not altered by ECMO (data not shown), thereby supporting this assumption.
Fig. 4.
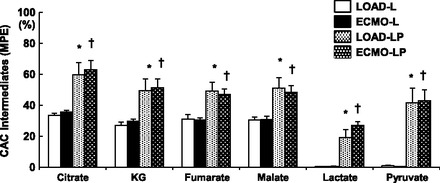
13C-MPE for citric acid cycle (CAC) intermediates. Pyruvate significantly increases MPE (* or † < 0.05 vs. L for same condition). ECMO does not significantly change MPE vs. LOAD. KG, α-ketoglutarate.
Fractional Contributions of Acetyl-Coa to the CAC
Figure 5A displays representative NMR complexes for glutamate. Our labeling strategy is such that the [2-13C]-pyruvate labels C5 for glutamate, whereas [13C6,15N]-L-leucine will label both C4 and C5 or only C4 allowing discrimination between the two (20). This labeling strategy also allows determination of the relative or fractional contributions via acetyl-CoA (Fc) to the CAC (Fig. 5B) by unlabeled endogenous substrates, labeled leucine, and labeled pyruvate by using the tcaCALC program. The loading state itself did not significantly change the 13C-leucine contribution (LOAD-L vs. ECMO-L, P = 0.69; LOAD-LP vs. ECMO-LP, P = 0.26) or the endogenous unlabeled substrate contribution (LOAD-L vs. ECMO-L, P = 0.67; LOAD-LP vs. ECMO-LP, P = 0.67). Similarly, loading status did not affect the 13C-pyruvate contribution (LOAD-LP vs. ECMO-LP; P = 0.62).
Fig. 5.
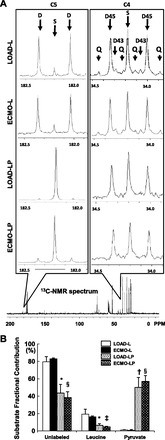
Substrate fractional contributions to CAC determined by nuclear magnetic resonance spectrometry (NMR). A: representative spectra for glutamates carbon 4 and 5. Samples were obtained from left ventricular apex extracts. Spectra are shown to demonstrate differences in peak intensities per complex. C5 spectra shows a small singlet (S) relative to doublet (D), when hearts are provided only [13C6,15N]-L-leucine. Addition of [2-13C]-pyruvate reveals the substantial fractional contribution from the labeled pyruvate compared with leucine causing the large singlet. The C4 spectrum includes S, Ds, and Quartets (Q) due to more complex carbon labeling by [13C6,15N]-leucine. Spectra peak intensities are maximized per individual panel and do not reflect intensity differences between panels. B: fractional contributions (Fc) by substrate are shown for each protocol. Piglets receiving pyruvate (LP) show substantial reductions in Fc for unlabeled substrate and leucine. Fc for substrates do not differ significantly when comparing LOAD-L or LP vs. ECMO-L, LP. All effects relate to differences caused by pyruvate. Significant effects: *P < 0.05 vs. LOAD-L; §P < 0.01 vs. ECMO-L ; ‡P < 0.05 vs. ECMO-L; †P < 0.01 vs. LOAD-L.
Under these conditions, hearts preferentially used pyruvate over leucine as an oxidative substrate, as expected. Pyruvate significantly decreased the FcLeucine in the LOAD condition by 67% (LOAD-L vs. LOAD-LP; P < 0.05) and by 71% in ECMO (ECMO-L vs. ECMO-LP; P < 0.05). Pyruvate also significantly decreased the unlabeled endogenous substrate contribution to glutamate via acetyl-CoA by 45% in LOAD (LOAD-L vs. LOAD-LP; P < 0.05) and by 54% in ECMO (ECMO-L vs. ECMO-LP; P < 0.005).
Global Protein FSR
The protein FSR was highest in the ECMO-L group (Fig. 6). ECMO increased the FSR by 25% compared with LOAD in piglets receiving leucine only, although this did not quite reach statistical significance (P = 0.06). This effect was not observed in the presence of pyruvate (LOAD-LP vs. ECMO-LP; P = 0.92). We infused leucine with targeted plasma levels, which were severalfold higher than physiological. Leucine stimulates protein synthesis in Langendorff hearts provided glucose as the sole alternative substrate and achieves maximal rates when provided at 3.7 mM (7–10). However, leucine at the same concentration does not elevate protein synthesis in working hearts when the heart is provided more robust and physiological substrates in the perfusate. We performed preliminary studies (n = 2) with the same leucine intracoronary infusion, which showed substantially higher (near 2-fold) FSRs with reloading of the ventricles by weaning from ECMO (data not shown). Therefore, we are reasonably confident that we had not reached the maximal threshold for protein synthesis under our current conditions.
Fig. 6.
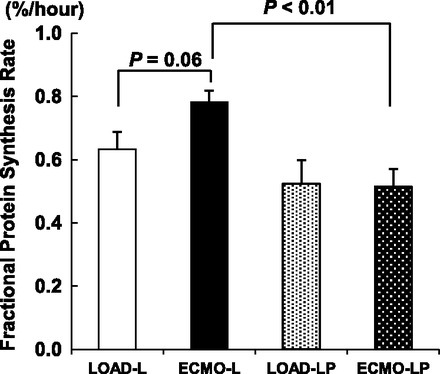
Fractional protein synthesis for each protocol expressed as percentage per hour.
Although loading condition did not alter FSR, pyruvate did show a significant effect. Pyruvate reduced the FSR by 32% in ECMO (P < 0.01 ECMO-L vs. ECMO-LP). This same comparison did not reach significance in the LOAD groups (P = 0.31 LOAD-L vs. LOAD-LP).
DISCUSSION
The principal objective of our study was to identify ECMO promoted disturbances in amino acid shuttling between oxidation and protein synthesis in myocardium. ECMO provides relatively short-term recovery for the heart, presumably through ventricular unloading. We observed that unloading substantially reduces global myocardial oxidative metabolism, reflecting the decrease in cardiac work afforded by ECMO. ECMO also initiates an inflammatory cascade over the first few hours, which has been associated with end-organ insulin resistance. These hormonal abnormalities inhibit systemic carbohydrate utilization and enhances oxidation of alternate substrate sources (34). Whole body studies in infants have shown that ECMO promotes leucine oxidation, while increasing the protein degradation rate above synthesis rate and thereby leading to a deficit in protein balance and associated skeletal muscle wasting (34). It has been proposed that this branched chain amino acid undergoes catabolism instead of incorporation into protein and bypasses the insulin sensitive reactions to provide carbon substrate for oxidation within the CAC (34). Organ specificity for these metabolic disturbances has not been previously defined. Our results show that the heart resists some of these ECMO promoted metabolic perturbations. First, ECMO preserves myocardial leucine fractional contribution to CAC. As ECMO substantially reduces the myocardial oxygen consumption rate, and by extrapolation the total CAC flux, then the overall left ventricular leucine oxidation rate is reduced in contrast with the increase observed in whole body infant studies. Due to technical limitations in this small piglet model, we could not measure protein degradation rates. However, we demonstrated that ECMO maintains or marginally increases myocardial protein synthesis depending on the particular substrate provision. These results suggest that ECMO over this time period shifts these metabolic processes in heart toward more positive protein balance by preserving branched chain amino acids for protein synthesis rather than directing them into oxidation.
We used pyruvate both as a reference substrate for validation of fractional contribution to the CAC for leucine, and as an alternate substrate for glucose and other unlabeled substrates. We observed that pyruvate inhibited both leucine oxidation and fractional protein synthesis in piglets similarly under LOAD and ECMO. The directional changes are near proportionally equivalent for these two parameters. Therefore, they do not indicate a clear direction for protein balance. Consideration of results from other studies suggests that our observed rate changes generally accompany decreases in protein degradation as well as synthesis (34). Whole body studies in infants under ECMO showed that increasing caloric supply predominantly through glucose paradoxically exacerbates negative protein balance and increases leucine oxidation. Furthermore, high dose insulin infusion under euglycemic clamp and providing substantial amounts of glucose only marginally improves protein balance in infants under ECMO (2). Thus ECMO induced insulin resistance promotes whole body amino acid oxidation, which does not respond to high dose insulin. Although the experimental conditions and substrate provisions differ substantially between the human studies and our immature pig experiments, we have made certain poignant observations regarding substrate supply influence on metabolism, which specifically relate to the heart during ECMO. The heart supported by ECMO maintains metabolic flexibility similar to the LOAD condition by preferentially oxidizing pyruvate over unlabeled substrates. The unlabeled component is undefined and can include acetyl-CoA supplied by circulating glucose and fats, as well as glycogen and triglycerides within the heart. Pyruvate utilization appears to circumvent insulin resistance, and further preserve branched chain amino acids from oxidation. However, the inability of pyruvate to stimulate protein synthesis despite shifting leucine from amino acid oxidation illustrates the complexity of these relationships. Certainly further study using alternate substrates and labeling strategies will be necessary to explore regulation of protein turnover during ECMO.
We provided high target concentrations of isotopic leucine and pyruvate in these experiments. These concentrations were required to achieve the 13C-glutamate enrichment necessary for accurate NMR isotopic analyses. Although these concentrations are supraphysiological they provide reasonable caloric alternatives for immature hearts, considering that infants on ECMO are often supplied parenteral glucose at doses which yield hyperglycemia (2, 34). As leucine stimulates cardiomyocyte protein synthesis in vitro (36), elevating free cytosolic levels of this branched chain amino acid could conceivably influence and possibly maximize protein synthesis rates in our model in vivo. However, we provided evidence that the leucine loading into the coronary artery did not increase myocardial intracellular free leucine concentration in piglets undergoing ECMO. Consistent with prior studies (40), leucine infusion did decrease concentrations for both valine and isoleucine. Because concentrations for the other amino acids did not decrease, the data conform with previous contention that leucine promotes oxidative degradation of these other branched chain amino acids. The small decreases in the pool size of those branched chain amino acids might limit but certainly would not stimulate protein synthesis. Ichihara et al. (15) provided precedent for using supraphysiological leucine concentrations (1 and 5 mM) in coronary perfusate for isolated Langendorff and working hearts. The fractional contribution of acetyl-CoA from leucine under our conditions is small, but comparable with that reported in working rat hearts supplied leucine up to 5 mM (16%) (15). Previous studies have shown that 13C-labeled pyruvate generated from glucose maximally contributes ∼60% of carbon within the CAC in heart, thereby illustrating a relative threshold for pyruvate dehydrogenase capacity (23). Our GCMS results conform to this threshold range and are further confirmed by our NMR studies showing 13C-pyruvate fractional acetyl-CoA contribution to the CAC. With operation at this threshold in our experiments, pyruvate reduces the fractional acetyl-CoA contribution of leucine to the CAC in the current experiments. This finding is consistent with results from prior studies showing pyruvate (11 mM) inhibition of leucine oxidation in ex vivo perfused rat hearts (1).
The mechanism related to preferential oxidation of pyruvate over leucine has not been previously investigated in vivo. Pyruvate can potentially inhibit leucine metabolism at multiple steps along the pathway beginning at plasma membrane and/or mitochondrial transport and extending through catabolism to acetoacetate and acetyl-CoA. The observed manipulation of fractional leucine contribution of acetyl-CoA by pyruvate could relate to trans-sarcolemmal transport, since pyruvate lowers isotopic enrichment of free intracellular leucine pool significantly in the LOAD group, although just marginally in the ECMO hearts. Potentially, an increase in protein degradation could release more unlabeled leucine into cytoplasm, thereby diluting the isotopic enriched pool, and providing an alternative interpretation for this data. As noted, whole body studies using similar 13C-leucine studies during ECMO have shown that an increase in leucine oxidation accompanies elevated rates of protein degradation (2). Instead, we found that pyruvate decreases the relative acetyl-CoA contribution from leucine. Therefore, we assumed that leucine release from protein degradation is negligible with respect to our calculations. Within these limitations, the data imply that pyruvate modification of leucine transport is responsible for at least a portion of the decrement in FcLeucine. Leucine transport into the cell is regulated by multiple amino acid transporters, predominantly by the sodium-independent system-L amino acid transporter-1. Although exercise upregulates the sodium-independent system-L amino acid transporter-1 in skeletal muscle, prior studies have suggested that myocardial transport likely depends more on undefined posttranslational modification of these amino acid transporters (12).
With the consideration that there was only a small decrement in free cytosolic leucine enrichment, it is unlikely that alteration in transport as discussed above is totally responsible for the marked pyruvate effect on leucine oxidation. Pyruvate inhibition of leucine oxidation in heart has been previously linked to reversible modifications in the branch-chain α-keto-dehydrogenase (BCKD) (38). This enzyme complex resides in the mitochondria and catalyzes oxidative decarboxylation of the α-isoketocaproate, the product of reversible leucine transamination (4–6). Pyruvate provided in low doses (0.25 mM) to isolated Langendorff perfused hearts markedly inhibited branch-chain α-keto-dehydrogenase activity with no further increase in response up to 10 mM (38). Results in our study are consistent with a mode of posttranslational modification of this enzyme, which has yet to be fully described. These same modifications could explain the ECMO promoted shift from leucine oxidation to protein incorporation.
Limitations and Experimental Considerations
Our experiments evaluated cardiac leucine oxidation and protein synthesis using ECMO, a very specific form of mechanical circulatory unloading commonly used in infants and children. We found that metabolic flexibility was maintained during ECMO. Thus we did not directly link a metabolic impairment with cardiac stunning, which often occurs during this critical time period in human infants. However, we established baseline parameters for our ECMO model, which are necessary for further study and exploration of those potential links.
Difficulties exist when trying to extrapolate our findings to other forms of therapeutic ventricular unloading. For example, ventricular assist devices (VAD) use markedly different circulatory strategies, are intended for much longer periods of support, and are generally used in older populations with chronic maladaptive left ventricular hypertrophy. Although signaling for these processes has been examined in experimental models of unloading (11, 29, 30) and in adults (37) and children with ventricular assist devices (24), no prior study has actually measured their influence on protein synthesis or amino acid oxidation. We attempted to evaluate signaling for protein synthesis in this pig model using standard immunoblot techniques. However, inconsistent antibody performance in pig myocardium as well as inherent variability for this species limited our ability to attain definitive results.
Ventricular remodeling associated with chronic unloading produces alterations in expression of specific proteins. Our objectives were to study the integration between myocardial amino acid oxidation and protein synthesis, which was relevant to short-term ECMO. Therefore, we did not attempt to perform technically difficult measures of FSRs for specific proteins. These specific types of analyses are planned for the future.
Protein turnover depends on rates of both synthesis and degradation. Our experimental design did not include the technically difficult measurements of myocardial protein degradation. Therefore, we cannot comment on total protein turnover but instead focused on integration between amino acid oxidation and CAC metabolism.
Finally, we studied metabolism after an 8-h period of ECMO. This time period is critical for pediatric patients undergoing these procedures for cardiac indications. This duration also represents the previous standard for most experimental ECMO studies in pigs, due to stability and logistical concerns. Additionally, we showed that by 8 h the inflammatory response is beginning to subside. However, the translation to the clinical scenario is limited as most patients undergo ECMO for somewhat longer time periods.
In summary, we found that ECMO decreases leucine oxidation, while preserving protein synthesis and metabolic flexibility for substrate oxidation. Pyruvate provides an alternate carbon source, which reduces protein synthesis but also decreases leucine oxidation, and possibly improving net protein balance.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant No. NIH R01-HL-60666 (to M. A. Portman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.O.P., M.K., D.L., A.K.O., and M.A.P. conception and design of research; C.M.O.P., M.K., D.L., B.B., N.I., and M.A.P. performed experiments; C.M.O.P., M.K., D.L., B.B., N.I., A.K.O., C.D.R., and M.A.P. analyzed data; C.M.O.P., M.K., D.L., B.B., N.I., C.D.R., and M.A.P. interpreted results of experiments; C.M.O.P., M.K., and M.A.P. prepared figures; C.M.O.P. and M.A.P. drafted manuscript; C.M.O.P., M.K., D.L., B.B., N.I., A.K.O., C.D.R., and M.A.P. edited and revised manuscript; C.M.O.P., M.K., D.L., B.B., N.I., A.K.O., C.D.R., and M.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
A portion of the research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory. We thank Chun Xu for assistance with perfusion.
REFERENCES
- 1. Aftring RP, Manos PN, Buse MG. Catabolism of branched-chain amino acids by diaphragm muscles of fasted and diabetic rats. Metabolism 34: 702–711, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Agus MS, Javid PJ, Piper HG, Wypij D, Duggan CP, Ryan DP, Jaksic T. The effect of insulin infusion upon protein metabolism in neonates on extracorporeal life support. AnnSurg 244: 536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, del Nido PJ, Wypij D, McGowan FX., Jr The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg 111: 1244–1251, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Buse MG, Biggers JF, Drier C, Buse JF. The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J Biol Chem 248: 697–706, 1973 [PubMed] [Google Scholar]
- 5. Buse MG, Biggers JF, Friderici KH, Buse JF. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem 247: 8085–8096, 1972 [PubMed] [Google Scholar]
- 6. Buse MG, Jursinic S, Reid SS. Regulation of branched-chain amino acid oxidation in isolated muscles, nerves and aortas of rats. Biochem J 148: 363–374, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chua B, Siehl DL, Fuller EO, Morgan HE. Effects of cardiac work and leucine on protein turnover. Adv Myocardiol 4: 115–125, 1983 [DOI] [PubMed] [Google Scholar]
- 8. Chua B, Siehl DL, Morgan HE. Effect of leucine and metabolites of branched chain amino acids on protein turnover in heart. J Biol Chem 254: 8358–8362, 1979 [PubMed] [Google Scholar]
- 9. Chua BH. Specificity of leucine effect on protein degradation in perfused rat heart. J Mol Cell Cardiol 26: 743–751, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Chua BH, Siehl DL, Morgan HE. A role for leucine in regulation of protein turnover in working rat hearts. Am J Physiol Endocrinol Metab 239: E510–E514, 1980 [DOI] [PubMed] [Google Scholar]
- 11. Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. NatMed 4: 1269, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duncan BW. Mechanical cardiac support in the young. Short-term support: ECMO. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 75–82, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J 55: 111–116, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Ichihara K, Neely JR, Siehl DL, Morgan HE. Utilization of leucine by working rat heart. Am J Physiol Endocrinol Metab 239: E430–E436, 1980 [DOI] [PubMed] [Google Scholar]
- 16. Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab 295: E1255–E1268, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joffe AR, Lequier L, Robertson CM. Pediatric outcomes after extracorporeal membrane oxygenation for cardiac disease and for cardiac arrest: a review. ASAIO J 58: 297–310, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Keshen TH, Gursoy M, Shew SB, Smith EO, Miller RG, Wearden ME, Moise AA, Jaksic T. Does extracorporeal membrane oxygenation benefit neonates with congenital diaphragmatic hernia? Application of a predictive equation. J Pediatr Surg 32: 818–822, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Krogh-Madsen R, Plomgaard P, Moller K, Mittendorfer B, Pedersen BK. Influence of TNF-α and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab 291: E108–E114, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Malloy CR, Thompson JR, Jeffrey FM, Sherry AD. Contribution of exogenous substrates to acetyl coenzyme A: measurement by 13C NMR under non-steady-state conditions. Biochemistry 29: 6756, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Martin GR, Short BL, Abbott C, O′Brien AM. Cardiac stun in infants undergoing extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 101: 607–611, 1991 [PubMed] [Google Scholar]
- 22. McILwain RB, Timpa JG, Kurundkar AR, Holt DW, Kelly DR, Hartman YE, Neel ML, Karnatak RK, Schelonka RL, Anantharamaiah GM, Killingsworth CR, Maheshwari A. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest 90: 128–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNulty PH, Cline GW, Whiting JM, Shulman GI. Regulation of myocardial [13C]glucose metabolism in conscious rats. Am J Physiol Heart Circ Physiol 279: H375–H381, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Mohapatra B, Vick GW, 3rd, Fraser CD, Jr, Clunie SK, Towbin JA, Sinagra G, Vatta M. Short-term mechanical unloading and reverse remodeling of failing hearts in children. J Heart Lung Transplant 29: 98–104, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Mu TS, Palmer EG, Batts SG, Lentz-Kapua SL, Uyehara-Lock JH, Uyehara CF. Continuous renal replacement therapy to reduce inflammation in a piglet hemorrhage-reperfusion extracorporeal membrane oxygenation model. Pediatr Res 72: 249–255, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Olson AK, Bouchard B, Ning XH, Isern N, Des Rosiers C, Portman MA. Triiodothyronine increases myocardial function and pyruvate entry into the citric acid cycle after reperfusion in a model of infant cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 302: H1086–H1093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olson AK, Hyyti OM, Cohen GA, Ning XH, Sadilek M, Isern N, Portman MA. Superior cardiac function via anaplerotic pyruvate in the immature swine heart after cardiopulmonary bypass and reperfusion. Am J Physiol Heart Circ Physiol 295: H2315–H2320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Priest JR, Slee A, Olson AK, Ledee D, Morrish F, Portman MA. Triiodothyronine supplementation and cytokines during cardiopulmonary bypass in infants and children. J Thorac Cardiovasc Surg 144: 938–943, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Razeghi P, Baskin KK, Sharma S, Young ME, Stepkowski S, Essop MF, Taegtmeyer H. Atrophy, hypertrophy, and hypoxemia induce transcriptional regulators of the ubiquitin proteasome system in the rat heart. Biochem Biophys Res Commun 342: 361, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, Taegtmeyer H. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation 108: 2536, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Shen I, Levy FH, Benak AM, Rothnie CL, O′Rourke PP, Duncan BW, Verrier ED. Left ventricular dysfunction during extracorporeal membrane oxygenation in a hypoxemic swine model. Ann Thorac Surg 71: 868–871, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Shen I, Levy FH, Vocelka CR, O′Rourke PP, Duncan BW, Thomas R, Verrier ED. Effect of extracorporeal membrane oxygenation on left ventricular function of swine. Ann Thorac Surg 71: 862–867, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Sherwin ED, Gauvreau K, Scheurer MA, Rycus PT, Salvin JW, Almodovar MC, Fynn-Thompson F, Thiagarajan RR. Extracorporeal membrane oxygenation after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2012 [DOI] [PubMed] [Google Scholar]
- 34. Shew SB, Keshen TH, Jahoor F, Jaksic T. The determinants of protein catabolism in neonates on extracorporeal membrane oxygenation. J Pediatr Surg 34: 1086, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Smith DM, Newhouse M, Naziruddin B, Kresie L. Blood groups and transfusions in pigs. Xenotransplantation 13: 186–194, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem 257: 1613–1621, 1982 [PubMed] [Google Scholar]
- 37. Uray IP, Connelly JH, Thomazy V, Shipley GL, Vaughn WK, Frazier OH, Taegtmeyer H, Davies PJ. Left ventricular unloading alters receptor tyrosine kinase expression in the failing human heart. J Heart Lung Transplant 21: 771, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Waymack PP, DeBuysere MS, Olson MS. Studies on the activation and inactivation of the branched chain alpha-keto acid dehydrogenase in the perfused rat heart. J Biol Chem 255: 9773–9781, 1980 [PubMed] [Google Scholar]
- 39. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zapalowski C, Miller RH, Dixon JL, Harper AE. Effects of perfusate leucine concentration on the metabolism of valine by the isolated rat hindquarter. Metabolism 33: 922–927, 1984 [DOI] [PubMed] [Google Scholar]


