Abstract
Humanin is a small endogenous antiapoptotic peptide, originally identified as protective against Alzheimer's disease, but subsequently also found on human endothelium as well as carotid artery plaques. Endothelial dysfunction is a precursor to the development of atherosclerotic plaques, which are characterized by a highly proinflammatory, reactive oxygen species, and apoptotic milieu. Previous animal studies demonstrated that humanin administration may improve endothelial function. Thus the aim of this study was to test the hypothesis that patients with coronary endothelial dysfunction have reduced systemic levels of humanin. Forty patients undergoing coronary angiography and endothelial function testing were included and subsequently divided into two groups based on coronary blood flow (CBF) response to intracoronary acetylcholine (normal ≥ 50% increase from baseline, n = 20 each). Aortic plasma samples were obtained at the time of catheterization for the analysis of humanin levels and traditional biomarkers of atherosclerosis including C-reactive protein, Lp-Pla2, and homocysteine. Baseline characteristics were similar in both groups. Patients with coronary endothelial dysfunction (change in CBF = −33 ± 25%) had significantly lower humanin levels (1.3 ± 1.1 vs. 2.2 ± 1.5 ng/ml, P = 0.03) compared with those with normal coronary endothelial function (change in CBF = 194 ± 157%). There was a significant and positive correlation between improved CBF and humanin levels (P = 0.0091) not seen with changes in coronary flow reserve (P = 0.76). C-reactive protein, Lp-Pla2, and homocysteine were not associated with humanin levels. Thus we observed that preserved human coronary endothelial function is uniquely associated with higher systemic humanin levels, introducing a potential diagnostic and/or therapeutic target for patients with coronary endothelial function.
Keywords: endothelial dysfunction, humanin, coronary
humanin is a 24-amino-acid peptide known to be associated with improved neuronal cell survival in patients with familial Alzheimer's disease (13) via interference with pro- and antiapoptotic proteins such as Bid, Bax, ceramide (12, 27, 28), and IGFBP-3 (15). Previous in vitro work by our lab has shown humanin to be expressed in both the vascular endothelium as well as the unstable carotid atheromatous plaque (2, 29). Moreover, humanin introduced to cultured endothelial cells wards off damage related to reactive oxygen species (ROS) production, typically incurred by the inflammatory stress of oxidized low-density lipoproteins LDL (Ox-LDL) (2). In vivo work from our group also demonstrated that humanin administration preserves endothelial function and reduces atheromatous plaque burden in ApoE knockout mice (22). These effects are believed to be achieved through improved nitric oxide (NO) bioavailability (22), regulation of anti-apoptotic factors (19), and AMP-kinase signaling (21). Recent work has also shown that humanin attenuates remodeling of the renal vasculature in a murine model of atherosclerosis mainly by downregulating proteins involved in angiogenesis, apoptosis, fibrosis, and inflammation (30). Taken together, preclinical data suggest beneficial structural and functional effects of humanin on the vascular system; however, there is a paucity of data on the functional correlation in humans.
Endothelial dysfunction is defined as an inappropriate vasomotor response to physiological or pharmacological stress (4, 7, 23) and has been linked to various cardiovascular pathologies such as obesity, diabetes, hypertension, coronary artery disease, and stroke (1, 3–5, 8, 24, 26). Endothelial dysfunction is thought to be a harbinger of atheromatous damage to the vasculature contributing to increased cardiovascular morbidity and mortality (4, 18, 24, 26). Classically, atheromatous plaques are rich in lipids and Ox-LDL, inflammatory cells such as monocytes/macrophages, as well as necrotic cores, all initiating and feeding a cycle of increased ROS production, inflammation, and apoptosis.
While humanin expression in the endothelium and in atherosclerotic plaques, and its ability to alleviate the deleterious effects of ROS-induced endothelial injury (21, 22) as well as inflammation (30) in animal models, are well documented, it is unknown if circulating endogenous levels of humanin are linked to endothelial dysfunction in humans. The aim of this study was to test the hypothesis that coronary endothelial dysfunction is associated with altered humanin levels in humans.
METHODS
Human subjects and recruitment.
The study was approved by the Institutional Review Board of Mayo Foundation, and all study subjects provided written, informed consent. We enrolled a total of 40 patients undergoing coronary angiography and coronary endothelial function testing for clinical indications of recurrent angina with or without a positive stress test who met the criteria for inclusion into the two study groups. Patients with acute coronary syndromes (unstable angina or acute myocardial infarction), heart failure (ejection fraction <50%), or severe renal or liver disease were excluded. The patients were classified as having normal endothelial function, n = 20; or endothelial dysfunction as assessed by intracoronary acetylcholine challenge (see below), and the absence of significant structural coronary lesions on angiography, n = 20 (10, 11).
Study protocol/hemodynamic measurements.
Patients underwent a diagnostic coronary angiography using standard clinical protocols. Arterial blood (20 ml) was drawn from a catheter for biochemical analyses (16, 17). All subjects underwent assessment of endothelium-dependent coronary vasoreactivity, as previously described (10, 11). In brief, 5,000 units of heparin were given intravenously to all patients and a Doppler guidewire (Flowire, Volcano) within a coronary-infusion catheter (Ultrafuse, SciMed Life System) was positioned into the midportion of the left anterior descending coronary artery. Acetylcholine (ACh) at increasing concentrations (10−6 to 10−4 M) was infused into the left anterior descending coronary artery to assess endothelium-dependent vasoreactivity. Haemodynamic data, Doppler measurements, and a coronary angiogram were obtained after each infusion. Coronary artery diameter was measured by an independent investigator in the segment 5 mm distal to the tip of the Doppler wire using a computer-based image analysis system. Average peak velocity (APV) was derived from the Doppler flow velocity spectra and CBF determined as π(coronary artery diameter/2)2 × (APV/2). As previously described, microvascular endothelial dysfunction was defined as an increase in CBF of <50% and epicardial endothelial dysfunction (macrovascular function) was defined as a decrease in epicardial coronary artery diameter of more than 20% in response to the maximal dose of acetylcholine (10−4 M) (25). Endothelium-independent microvascular function was determined by the coronary flow reserve (CFR), which is the ratio of the APV at maximal hyperemia [induced by intracoronary adenosine (24–60 μg)] to the APV at baseline (9). The study population, in total, was analyzed by both coronary microvascular and epicardial vessel function as well as coronary flow reserve (CRF).
Humanin levels.
For human plasma humanin levels, samples were collected from 40 volunteers undergoing angiography. Plasma humanin was measured using an in-house ELISA assay after acetonitrile/HCl extraction as reported previously (20).
Biochemical assays.
Serum lipids were measured using enzymatic colorimetry and LDL cholesterol calculated. Hemoglobin A1c was measured using ion-exchange high-performance chromatography (BIO-RAD Variant II Turbo Hemoglobin A1c program, Hercules, CA). Free insulin levels were measured by an automated chemiluminescent immunoenzymatic assay (ACCESS, Beckman-Coulter, Fullerton, CA). High-sensitivity C-reactive protein (hs-CRP) levels were measured using a latex particle-enhanced immunoturbidemetric assay on a Hitachi 912 automated analyzer. Serum creatinine, Lp-Pla2, homocysteine, and complete blood counts were measured as described before (9).
Statistical analyses.
Data were expressed as means ± SD. Baseline comparisons were made by Student's t-test for continuous data or Fischer's Exact Comparison for binary measures. Comparison of different groups was performed by one-way ANOVA followed by post hoc tests for parametric and nonparametric distribution. Comparisons between the two groups were made by Student's t-test or Mann-Whitney rank sum test. A value of P < 0.05 was considered significant. Correlations were analyzed using Spearman tests, and all tests were performed using JMP (9.0.1, SAS Institutes).
RESULTS
General characteristics are listed in Table 1. There were no differences in baseline variables between groups divided into normal and abnormal coronary microvascular endothelial function (<50% increase in CBF to ACh). Furthermore, there were no statistical differences in medication profiles between the two groups. Compared with controls, patients with microvascular endothelial dysfunction had significantly lower humanin levels (Table 2 and Fig. 1). No specific level of humanin could be used to demarcate one group of either endothelial function or humanin levels from the other, likely owing to the smaller sample size and relative novelty of the humanin assay. Within-group analysis (not shown) still demonstrated positive correlations between humanin levels and percent changes in CBF, thus reinforcing the relationship between the two and negating the possibility that statistical differences could be an artifact from between-group differences. Furthermore, linear regression analysis revealed a positive and significant relationship between humanin and CBF response to intracoronary ACh (R2 = 0.17, P = 0.01; Fig. 2A). There was also a positive relationship between coronary artery diameter response to ACh and humanin (R2 = 0.09, Fig. 2B), but this did not reach statistical significance (P = 0.053). CFR and humanin levels were not correlated (R2 = 0.002; P = 0.76). Humanin levels directly correlated to percent changes in coronary blood flow with acetylcholine challenge (P = 0.009, Fig. 2A) and tended to correlate with changes in coronary artery diameter (P = 0.05, Fig. 2B). Levels of circulating leukocytes (7.2 ± 1.8 × 109/l vs. 6.0 ± 1.4 × 109/l, P = 0.03), monocytes (0.6 ± 0.2 × 109/l vs. 0.4 ± 0.2 × 109/l, P = 0.03), and lymphocytes (2.1 ± 0.6 × 109/l vs. 1.6 ± 0.6 × 109/l, P = 0.02) were all significantly reduced in patients with endothelial dysfunction (Table 2). Humanin levels also positively correlated with body mass index (BMI) (P = 0.042), and increased with increasing monocytes numbers (P = 0.014), but not with leukocytes (P = 0.34).
Table 1.
Patient characteristic statistics
| Normal Endothelial Function | Poor Endothelial Function | P Value | |
|---|---|---|---|
| Male | 10/20 (50%) | 10/20 (50%) | 0.62 |
| Female | 10/20 (50%) | 10/20 (50%) | 0.62 |
| Age, yr | 46.6 (±10.8) | 46.4 (±9.7) | 0.81 |
| Currently smoking | 5/20 (75%) | 4/20 (20%) | 0.78 |
| Body mass index, kg/m2 | 29.8 (±6.4) | 27.0 (±4.8) | 0.14 |
| Family history of CVD | 14/20 (70%) | 13/20 (65%) | 0.75 |
| Diabetes mellitus type 2 | 2/20 (10%) | 1/20 (5%) | 0.89 |
| Hypertension | 8/20 (40%) | 9/20 (45%) | 0.36 |
| Total cholesterol, mg/dl | 190.1 (±55.2) | 189.1 (±47.7) | 0.95 |
| LDL cholesterol, mg/dl | 108.4 (±47.8) | 115.0 (±40.0) | 0.64 |
| HDL cholesterol, mg/dl | 53.6 (±15.4) | 52.4 (±9.7) | 0.78 |
| Triglycerides, mg/dl | 140.6 (±76.0) | 110.2 (±66.0) | 0.19 |
| %Change in coronary perfusion to maximal ACh | 194.6 (±157.6) | −32.0 (±25.3) | >0.001 |
| %Change in coronary diameter to maximal ACh | 0.7 (±16.1) | −36.0 (±23.7) | >0.001 |
Values are means (±SD). CVD, cardiovascular disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Table 2.
Comparisons of serum surrogate markers for cardiovascular disease between those with normal and poor endothelial function
| Normal Endothelial Function | Abnormal Endothelial Function | P Value | |
|---|---|---|---|
| Humanin, ng/ml | 2.2 (±1.5) | 1.3 (±1.1) | 0.03∗ |
| Insulin, micro-IU/ml | 6.2 (±4.6) | 5.8 (±3.9) | 0.79 |
| Glucose, mg/dl | 118.7 (±89.2) | 96.9 (±9.8) | 0.28 |
| Hemoglobin A1c, % | 5.3 (±0.7) | 5.2 (±0.3) | 0.46 |
| l-Arginine, ng/ml | 63.7 (±16.6) | 71.0 (±17.1) | 0.18 |
| hs-CRP, mg/l | 1.5 (±1.9) | 1.6 (±2.3) | 0.80 |
| Homocysteine, μmol/l | 8.0 (±2.3) | 8.0 (±2.6) | 0.95 |
| Hemoglobin, ×1012/l | 14.3 (±1.1) | 14.2 (±1.6) | 0.90 |
| Leukocytes, ×109/l | 7.2 (±0.4) | 6.0 (±1.4) | 0.03∗ |
| Neutrophils, ×109/l | 4.2 (±1.6) | 3.7 (±1.2) | 0.29 |
| Monocytes, ×109/l | 0.6 (±0.2) | 0.4 (±0.2) | 0.03∗ |
| Lymphocytes, ×109/l | 2.1 (±0.6) | 1.6 (±0.6) | 0.02∗ |
| Platelets, ×109/l | 261.3 (±96.1) | 252.6 (±64.7) | 0.74 |
| Uric acid, mg/dl | 4.9 (±1.9) | 4.6 (±1.6) | 0.53 |
| BNP, pg/ml | 20.5 (±11.2) | 29.7 (±43.3) | 0.36 |
| Fibrinogen, mg/dl | 259.4 (±68.9) | 279.6 (±73.4) | 0.37 |
| Total cholesterol, mg/dl | 190.1 (±55.2) | 189.3 (±46.5) | 0.96 |
| LDL-cholesterol, mg/dl | 108.4 (±47.8) | 114.0 (±39.2) | 0.68 |
| HDL-cholesterol, mg/dl | 53.6 (±15.4) | 52.6 (±9.5) | 0.81 |
| Triglycerides, mg/dl | 140.6 (±76.0) | 115.3 (±15.2) | 0.27 |
Values are means (±SD). hs-CRP, high-sensitivity C-reactive protein; BNP, brain natriuretic peptide.
Significant difference.
Fig. 1.
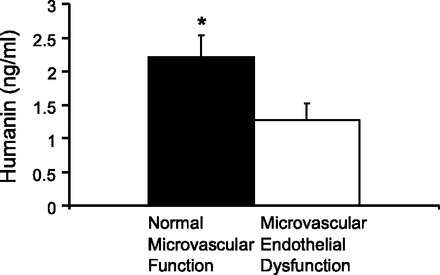
Serum humanin levels (ng/ml) in patients with normal microvascular endothelial function vs. poor endothelial function (*P = 0.03).
Fig. 2.
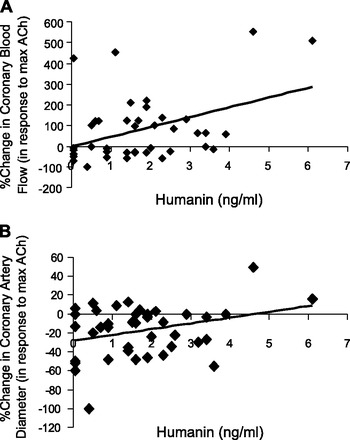
A: coronary microvascular endothelial function is significantly correlated with serum humanin levels (R2 = 0.17, P = 0.009). B: coronary epicardial endothelial function displays a trend toward a positive correlation with serum humanin levels but failed to reach statistical significance in a Spearman correlation model (R2 = 0.09, P = 0.053).
DISCUSSION
The current study demonstrates that circulating humanin levels are associated with attenuated microvascular coronary endothelial dysfunction in humans. This relationship holds irrespective of most other significant factors relating surrogate markers for cardiovascular disease, inflammation, or cardiac damage. As microvascular function is usually the first affected in the disease state, it is possible that humanin levels could be affected early in the atherotic disease process in concert with microvascular changes, and before deleterious macrovascular injury has taken hold. This finding linking an anti-apoptotic agent to preserved endothelial function could possibly hint toward novel mechanisms involved in the regulation of vascular function.
It could be speculated that in patients with a predilection toward endothelial dysfunction caused by mechanisms independent of inflammation, humanin serves to abrogate the effects of increased cellular inflammation. This hypothesis is underscored by the observation in the current study as humanin appears to be linked to BMI based on our data. Perhaps there is a mechanistic association between increased inflammation caused by obesity and resultant potentially protective increase by monocytes, lymphocytes, and/or humanin. We describe a direct association between leukocyte numbers and coronary endothelial function, and a concomitant positive correlation between humanin levels, BMI, and circulating monocytes plus lymphocytes. This reflex could serve as a protective response in populations at higher risk for atherosclerosis. Thus there is a unique, and potentially diagnostic and/or therapeutic, relationship between low endogenous, circulating humanin levels and coronary endothelial dysfunction in humans.
Humanin is thought to originate from mitochondria in tissues such as the brain, vasculature, skeletal muscle, testes, and pancreas (14). Previous work has shown a particular expression in endothelial surfaces and unstable atheromatous plaques (2, 29). Our data present a few interesting possibilities pertinent to cardiovascular disease and humanin levels. From a diagnostic and/or prognostic point of view, humanin could either be underproduced by dysfunctional mitochondria or overly sequestered in endothelial surfaces in response to early atherosclerotic plaques. Therefore, humanin could potentially be used as a marker for mitochondrial function in cardiovascular disease or as a treatment strategy to stabilize atherosclerosis in patients found to have endothelial dysfunction. In support of this stipulation, prior work in animal models supports the ability of humanin to ameliorate early vascular disease in hyperlipidemic mice (30).
Indeed, given the few differences observed between patients with normal endothelial function and those with endothelial dysfunction the underlying mechanisms linking humanin to endothelial function remain to be elucidated, especially in view of the limited associations we found between humanin levels and other inflammatory mediators commonly associated with regulation of endothelial function such as l-arginine, insulin, homocysteine, hs-CRP, fibrinogen, lipoprotein (a), or lipids. One limitation involves including only arterial samples from the coronary catheterization instead of peripheral venous samples. However, one benefit of this method is that arterial samples reach the coronary circulation first, perhaps furthering its endothelial protective role. These findings are supported by previous animal work in our lab showing that endothelial function can be preserved with the long term administration of humanin without concomitant alterations in other traditional molecular surrogates for vascular health such as VEGF, insulin, resistin, tPAI-1, or IL-6 (22). Along these lines, we found that l-arginine levels were not allied with humanin levels, arguing against, but not completely discounting, a link between NO bioavailability and humanin levels and administration (29).
Interestingly, we found an increase in the counts of white blood cells—particularly monocytes and lymphocytes—in patients with coronary endothelial dysfunction. This somewhat diverges from the lack of association observed in this and previous studies (6), between any other markers of increased oxidative stress or inflammation with humanin levels in patients with abnormal endothelial function. The relationship with leukocytes was also highlighted by significant positive correlations with humanin levels in this same group of patients. This observation is somewhat intriguing considering the abundant data indicating that humanin reduces inflammation. Based on prior work (30), one would surmise that humanin levels would be associated with oxidative stress, which should reduce cellular inflammatory responses. Possibly, in patients with elevated numbers of leukocytes who are predisposed to develop endothelial dysfunction, humanin increases as a defense mechanism stabilizing previously unstable disease. This view is underscored by recent in vivo work showing that in the renal vasculature of an atherosclerotic murine model the expression of MCP-1 and osteopontin, which both induce an influx of inflammatory cells such as monocytes/macrophages into the endothelium, were reduced after 16 wk of intraperitoneal injections of humanin (30). Thus it is not unlikely that humanin serves to stabilize the endothelium by negating the potential wayward consequences of vascular inflammation.
In conclusion, we show for the first time that circulating endogenous humanin levels are associated with preserved coronary endothelial function. This augmentation is not associated with other overt markers of vascular inflammation or cardiovascular disease surrogates, yet circulating levels of humanin are positively correlated with certain subsets of leukocytes. Future studies should elucidate the mechanisms underlying these findings and explore the diagnostic and/or therapeutic implications of humanin in cardiovascular disease.
GRANTS
This study was partly supported by National Institutes of Health Grants 1R01AG034430, 1R01GM090311, and 1R01ES020812.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.J.W. analyzed data; R.J.W. interpreted results of experiments; R.J.W. prepared figures; R.J.W. drafted manuscript; A.F., J.H., M.R.-P., J.W., P.C., and L.O.L. edited and revised manuscript; P.C. and A.L. conception and design of research.
REFERENCES
- 1. Avogaro A, de Kreutzenberg SV. Mechanisms of endothelial dysfunction in obesity. Clin Chim Acta 360: 9–26, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bachar A, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res 88: 360–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 44: 2137–2141, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bonetti P, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109: 1127–1132, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Elkind MSV, Sciacca RR, Boden-Albala B, Tondella MLC, Feikin DR, Fields BS, Sacco RL, Tullio MRD, Homma S. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis 181: 329–338, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Flammer A, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghiadoni L, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation 102: 2473–2478, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol 52: 1314–1325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han S, Bae JH, Holmes DR, Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J 29: 1359–1369, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 96: 3390–3395, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J Neurosci 21: 9235–9245, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA 98: 6336–6341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoang P, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P, Lee KW. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism 59: 343–349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA 100: 13042–13047, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 115: 2715–2721, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 51: 127–133, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 111: 363–368, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, Jia Y, Hwang D, Cobb LJ, Cohen P, Wang C. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology 151: 350–357, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Muzumdar R, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One 4: e6334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muzumdar R, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, Nemkal A, Atzmon G, Klein L, Gundewar S, Ji SY, Lavu M, Predmore BL, Lefer DJ. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol 30: 1940–1948, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh Y, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, Lerman LO, Cohen P, Lerman A. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis 219: 65–73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reriani M, Flammer AJ, Jama A, Lerman LO, Lerman A. Novel functional risk factors for the prediction of cardiovascular events in vulnerable patients following acute coronary syndrome. Circ J 76: 778–783, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 31: 1142–1148, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Suwaidi J, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Targonski P, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation 107: 2805–2809, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Xu XCC, Gao J, Chua KW, Wang H, Hamdy RC, Chua BH. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res 1227: 12–18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu XCC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke 37: 2613–2619, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Zacharias D, Kim SG, Massat AE, Bachar AR, Oh YK, Herrmann J, Rodriguez-Porcel M, Cohen P, Lerman LO, Lerman A. Humanin, a cytoprotective peptide, is expressed in carotid artherosclerotic plaques in humans. PLoS One 7: e31065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci 91: 199–206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


