Abstract
Despite the widespread consumption of nonsteroidal anti-inflammatory drugs (NSAIDs), the influence of these drugs on muscle satellite cells is not fully understood. The aim of the present study was to investigate the effect of a local NSAID infusion on satellite cells after unaccustomed eccentric exercise in vivo in human skeletal muscle. Eight young healthy males performed 200 maximal eccentric contractions with each leg. An NSAID was infused via a microdialysis catheter into the vastus lateralis muscle of one leg (NSAID leg) before, during, and for 4.5 h after exercise, with the other leg working as a control (unblocked leg). Muscle biopsies were collected before and 8 days after exercise. Changes in satellite cells and inflammatory cell numbers were investigated by immunohistochemistry. Satellite cells were identified using antibodies against neural cell adhesion molecule and Pax7. The number of Pax7+ cells per myofiber was increased by 96% on day 8 after exercise in the unblocked leg (0.14 ± 0.04, mean ± SE) compared with the prevalue (0.07 ± 0.02, P < 0.05), whereas the number of Pax7+ cells was unchanged in the leg muscles exposed to the NSAID (0.07 ± 0.01). The number of inflammatory cells (CD68+ or CD16+ cells) was not significantly increased in either of the legs 8 days after exercise and was unaffected by the NSAID. The main finding in the present study was that the NSAID infusion for 7.5 h during the exercise day suppressed the exercise-induced increase in the number of satellite cells 8 days after exercise. These results suggest that NSAIDs negatively affect satellite cell activity after unaccustomed eccentric exercise.
Keywords: muscle damage, myogenic precursor cells, Pax7, neural cell adhesion molecule, cyclooxygenase inhibitor, indomethacin
resistance training leads to skeletal muscle hypertrophy. Hypertrophy is important for both athletes and patients to benefit from training. Satellite cells (the stem cells of skeletal muscle) are essential for physiological adaptations during muscle hypertrophy and for muscle regeneration after injury. Their role is to provide new myonuclei during hypertrophy and assist in the repair of damaged muscle fiber segments for successful regeneration. Exercise has been shown in several human training studies to stimulate satellite cells to reenter the cell cycle and proliferate (as summarized in Ref. 25). More recent studies have demonstrated that as little as a single bout of exercise is sufficient to increase the number of satellite cells (13–15, 29), even in well-trained athletes (25) (as shown in Table 1). The majority of these studies have used a maximal eccentric exercise protocol.
Table 1.
Human studies investigating the satellite cell response to a single bout of exercise
| Exercise Type | Subjects' Training Status | Time After Exercise | Satellite Cell Proportion Increase |
|---|---|---|---|
| O'Reilly et al. (29) | |||
| Max eccentric: 30 × 10 reps at −180°/s | Not involved in any lower body weight training for at least 6 mo and unaccustomed to high-intensity eccentrically biased exercise | 1 and 3 days | 36% (1 day) and 80% (3 days) |
| Crameri et al. (13) | |||
| Max eccentric: 10 × 10 reps at −30°/s and 11 × 10 reps at −180°/s | Unaccustomed to high-intensity eccentric exercise and not participating in any regular exercise regimen | 4 and 8 days | >100% (4 days) and >100% (8 days) |
| Mackey et al. (25) | |||
| Endurance: 36-km run | Well-trained runners with a peak O2 consumption of 62±6 ml·kg−1·min−1 | 8 days | 27% |
| Dreyer et al. (15) | |||
| Max eccentric: 6 × 16 reps at −60°/s | Not currently participating in a resistance training program | 24 h | 141% (young) and 51% (old) |
| Crameri et al. (14) | |||
| Eccentric: 50 one-leg “drop-down” jumps; 8 × 10 reps at −30°/s and 8 × 10 reps at −180°/s | Sedentary and unaccustomed to high-intensity eccentric exercise and not participating in any regular exercise regimen | 2, 4, and 8 days | 97% (NS; 2 days), 123% (4 days), and 126% (8 days) |
| Paulsen et al. (32) | |||
| Max eccentric (elbow flexors): 14 × 5 reps at −30°/s | Physically active but not familiar with max eccentric exercise with elbow flexors | 1 h to 7 days (combined) | 19% |
The exercise type targeted thigh muscles unless otherwise indicated. The subjects' training status was as stated by the authors. The proportion of satellite cells was determined as neural cell adhesion molecule (NCAM)-positive cells as percentage of total myonuclei (increase in exercised samples vs. controls). Biopsies to measure satellite cell proliferation were from the vastus lateralis muscle except for Paulsen et al. (biceps brachii muscle), NS, not significant.
Various growth factors released during and immediately after exercise may be important for the adaptational response and for the satellite cell response to exercise. Growth factors like IGF-1 in its different splice variants (IGF-1Ea and IGF-1Ec, the latter also known as mechanogrowth factor), hepatocyte growth factor (HGF), and basic FGF have been shown in animal and cell studies to be involved at different stages of satellite cell activity (16, 17, 42). Likewise, it is known from animal and cell studies that nitric oxide (4, 42) and cytokines like IL-6 (35) and TNF-α (21) are involved in the regulation of satellite cells. According to animal studies, prostaglandins stimulate satellite cell activity, whereas nonsteroidal anti-inflammatory drugs (NSAIDs) negatively affect satellite cells by inhibiting the cyclooxygenase (COX) enzyme and thus prostaglandin synthesis. Data from animal models support the influence of prostaglandins on skeletal muscle hypertrophy (8, 9, 38), indicating that satellite cell activation, proliferation, differentiation, and fusion are dependent on the COX pathway. Furthermore, cell culture studies have specifically shown that COX-1 and COX-2 enzymes are important for satellite cell proliferation, differentiation, and fusion (26, 36) and that prostaglandins (PGE2 and PGF2α) can stimulate muscle growth and satellite cell activity (18, 30, 37). The regulation of satellite cell activity in humans is not well understood, and the effects of NSAIDs on skeletal muscle are largely unknown. Many of the above-mentioned factors are likely to be involved in satellite cell activation in humans as well.
To alleviate soreness and to recover faster, many athletes use NSAIDs both during normal training and when faced with injuries. Documentation on the extent of NSAID use among athletes is emerging slowly and suggests a high consumption. Tscholl et al. (40) reported that ∼50% of male and female World Cup soccer players consumed NSAIDs during World Cup tournaments (40); likewise, NSAIDs were used by 33% of the athletes in Atlanta and by 38% of those in Sydney (1). Warner et al. (41) reported that 15% of adolescent high school football players used NSAIDs daily. NSAIDs are also widely used by rheumatic patients to reduce pain and inflammation. It is estimated that a total of 30 million people worldwide use NSAIDs on a daily basis (14a).
However, the influence of NSAID on muscle satellite cells and adaptation is not fully understood, and NSAIDs may negatively affect the satellite cell response to exercise in humans. Mackey et al. (25) found that after a 36-km run the satellite cell number was increased by 27%, whereas this increase was blunted when NSAIDs were ingested, pointing toward a negative influence of NSAIDs on satellite cell proliferation after endurance exercise in humans. Likewise, Trappe et al. (39) showed that the rise in muscle protein synthesis observed 24 h after eccentric exercise in humans was blunted by the ingestion of NSAIDs. Given the critical role that satellite cells play in facilitating hypertrophy and regeneration in adult skeletal muscle, the present study investigated how the satellite cell response to a single bout of eccentric exercise in humans would be affected by NSAID. Furthermore, muscle inflammation is likely to be affected by anti-inflammatory medication, and it was also investigated how the inflammatory response to exercise is influenced by NSAID.
The present study investigated the role of inflammation in satellite cell activity and muscle regeneration and how NSAIDs influence the adaptation of human muscle to unaccustomed eccentric exercise in young healthy adults. Prostaglandin synthesis was blocked only during and after eccentric exercise by a controlled infusion of NSAID via microdialysis catheters (as described in Ref. 27). In this way, a local blockade was obtained only in one leg, with the other leg serving as a control within the same individual. The advantage of this design is that subjects serve as their own controls, diminishing individual variation. The primary aim of the present study was to investigate the effect of a local NSAID infusion on satellite cells and inflammation and regeneration in vivo in human muscle after unaccustomed eccentric exercise. The hypothesis was that an NSAID infusion before, during, and after exercise (7.5 h in total) affects 1) satellite cell number and activation status and 2) inflammatory cells and regeneration on day 8 after exercise.
MATERIALS AND METHODS
Subjects
Eight healthy male volunteers (means ± SD, age: 23 ± 3 yr, height: 184 ± 5 cm, weight: 81 ± 8 kg, and body mass index: 24 ± 2 kg/m2) performed maximal eccentric contractions with both legs. All subjects were generally well trained (6 h training/wk) but were unaccustomed to high-intensity eccentric exercise and had not performed leg resistance training within the last year. The Ethics Committees of the Municipalities of Copenhagen and Frederiksberg approved this study (KF 01 306773); all procedures conformed to the Declaration of Helsinki, and written informed consent was obtained from all subjects before the study.
Study Design
Pretest day.
At least 3 days before the exercise day, maximal voluntary contraction (MVC) and soreness were tested and blood samples were drawn to obtain individual prevalues.
Exercise day.
Subjects arrived in the morning, and one microdialysis catheter was positioned in the vastus lateralis muscle for the infusion of the NSAID (see below). The position was standardized based on the length of the thigh to minimize individual differences. A muscle biopsy (prevalue) was then obtained from the contralateral leg. After a minimum 1 h of rest, subjects performed 200 maximal eccentric contractions in an isokinetic dynamometer with the quadriceps muscles of each leg (see below). MVC was tested at the end of the exercise. After the exercise, a microdialysis catheter was positioned in the vastus lateralis muscle of the control leg for the infusion of placebo (and this leg served as a working control leg). Subjects rested for another 4.5 h with an infusion of NSAID or placebo, and the microdialysis catheters were then removed. The catheter for the infusion of the placebo was inserted after exercise to minimize the risk of damaging the catheter during exercise.
Eight days after exercise.
MVC and soreness were tested, and blood samples were drawn. Muscle biopsies were obtained from the leg with the previous NSAID infusion (NSAID leg) and from the unblocked leg (“no block” leg) at equal distances (1 cm) from where the microdialysis catheters had been placed.
Local NSAID Infusion
The NSAID used was indomethacin, which inhibits both COX-1 and COX-2. Microdialysis catheters were inserted under local anesthesia (1% lidocaine) in parallel to the muscle fibers in the vastus lateralis muscle. Commercially available CMA catheters (CMA 60, CMA/Microdialysis, 20-kDa molecular weight cutoff) were used for the infusion of the unspecific COX inhibitor indomethacin (Confortid, Dumex-Alpharma) to block the local synthesis of prostaglandins as described in detail by Mikkelsen et al. (27). Indomethacin was dissolved at 50 mg/ml, giving a total infusion of 45 mg of indomethacin during 7.5 h of infusion (50 mg/ml, 2 μl/min, 7.5 h).
A catheter was inserted into the contralateral leg for the infusion of placebo (Ringer-acetate solution). The microdialysis catheters were perfused at a rate of 2 μl/min with either NSAID solution or Ringer-acetate solution. Subjects were randomized to having the NSAID infusion in their right (dominant) or left leg.
Exercise Protocol and MVC
Subjects performed an eccentric exercise protocol that has previously been used in our laboratory (13, 27). In brief, a total of 200 unilateral maximal isokinetic eccentric contractions [100 at a slow contraction speed (30°/s) and 100 at a fast contraction speed (120°/s)] were performed with the quadriceps femoris muscles of each leg using an isokinetic dynamometer (KinCom KC125AP, Chattanooga Group, Harrison, TN) with a range of motion from 10° to 90° (where 0° = full extension). Subjects were instructed to resist the movement of the dynamometer arm maximally through the full range of motion in every eccentric repetition and then to relax the muscles during the concentric phase of contraction. They received real-time visual feedback on their performance on a computer screen, and they were verbally encouraged during the exercise to ensure maximal effort. It is important to note that both legs performed the exercise and comparisons were made between the blocked (NSAID) and unblocked contralateral leg. Subsequently, the total work performed during the 100 slow and fast eccentric contractions for each leg was calculated by integrating the torque-angle curves of the eccentric phase of each respective contraction. Gravity correction was performed by adding the passive work performed with the weight of the limb during the concentric phases of the cycles to the total eccentric work.
Isometric quadriceps MVC was obtained during static knee extension at a knee joint angle of 70° (where 0° = full knee extension) performed in the isokinetic dynamometer also used for the eccentric exercise. Subsequently, gravity correction was performed by subtracting the passive torque at a 70° knee joint angle from all torque values. Three maximal contractions of 3–4 s were performed separated by rest periods of 1 min (3).
Muscle Soreness
Muscle tenderness was assessed in three ways on both legs before and after the exercise bout; at 0, 0.5, and 24 h and at 2, 3, and 8 days after the exercise bout; by pressing on the vastus lateralis muscle with a blunt probe at a predetermined load (∼6 kg); when walking down stairs; and during MVC performed each test day. Subjects visually recorded their perceived pain on a visual analog scale from 1 to 10 (where 1 = no pain and 10 = extremely painful). The blunt probe was placed at a minimum of 3 cm away from any biopsy site to avoid potential pain resulting from the biopsy procedure.
Muscle Biopsies
Muscle biopsies were obtained from the vastus lateralis muscle using the percutaneous needle biopsy technique of Bergström (7) with 5-mm biopsy needles and manual suction. From each subject, one prevalue biopsy and two 8 days after exercise biopsies were obtained. Biopsies were obtained next to the microdialysis catheters at positions corresponding to “no block” (control leg) and “most block” (NSAID; next to the NSAID infusion) (27). The incision for the muscle biopsies was made in the skin 1 cm from the position of the microdialysis catheter. On extraction, the muscle specimen was aligned, embedded in OCT compound (Tissue-Tek, Sakura Finetek Europe, Zoeterwoude, The Netherlands) and frozen by immersion in isopentane precooled by liquid nitrogen. Samples were stored at −80°C pending analyses. Serial transverse sections (7 μm) were cut at −25°C using a cryostat and mounted on SuperFrost Plus slides (Menzel-Gläser, Braunschweig, Germany). All slides were coded at this point to enable the analyses being carried out in a blinded manner.
Immunohistochemistry
Staining protocols.
Antigens of interest were visualized by immunofluorescense or an enzyme color reaction. The general staining protocols are described below, and details of the antibodies are provided in Table 2.
Table 2.
Antibodies used
| Origin | Used to Visualize | Company | Catalog Number | Dilution | Incubation | |
|---|---|---|---|---|---|---|
| Primary antibodies | ||||||
| NCAM | Mouse | Satellite cells | Becton Dickinson (San Jose, CA) | 347740 | 1:100 and 1:50 | 4°C overnight |
| Ki67 | Rabbit | Active cells | Biocare Medical (Concord, CA) | CP249 | 1:200 | 2 h at room temperature |
| Pax7 | Mouse | Satellite cells | Neuromics (Edina, MN) | MO15020 | 1:100 | 4°C overnight |
| Laminin | Rabbit | Basal lamina | Dako Norden (Glostrup, Denmark) | Z0097 | 1:200 | 4°C overnight |
| CD16 | Mouse | Neutrophils | Dako Norden | M7006 | 1:200 | 4°C overnight |
| CD68 | Mouse | Macrophages | Dako Norden | M0718 | 1:200 | 4°C overnight |
| Desmin | Mouse | Desmin-negative fibers | Zymed (San Francisco, CA) | 18-0016 | 1:50 | 4°C overnight |
| Myosin heavy chain (developmental) | Mouse | Embryonic myosin | Developmental Studies Hybridoma Bank (Iowa City, Iowa) | FI.652 | 1:50 | 4°C overnight |
| Secondary antibodies | ||||||
| Anti-mouse biotinylated | Goat | Mouse primary antibodies | Dako Norden | E0433 | 1:200 | 45 min at room temperature |
| Immunoflourescent secondary antibodies | ||||||
| Alexa fluor 568 anti-mouse red | Goat | Mouse primary antibodies | Molecular Probes, Invitrogen (Taastrup, Denmark) | A11031 | 1:100 and 1:200 | 45 min at room temperature |
| Alexa fluor 488 anti-rabbit green | Goat | Rabbit primary antibodies | Molecular Probes, Invitrogen | A11034 | 1:100 and 1:200 | 45 min at room temperature |
Satellite cells in the active phases of the cell cycle were identified by immunofluorescent double staining with antibodies against neural cell adhesion molecule (NCAM) and Ki67 as described by Mackey et al. (24). Briefly, after fixation, sections were incubated overnight with an antibody against NCAM (1:50) to identify satellite cells followed by Alexa fluor 568 secondary antibody (1:200). The Ki67 antibody was subsequently applied to the same section to identify active cells. Alexa fluor 488 secondary antibody (1:200) was used to visualize the Ki67 antibody binding. 4′,6-Diamidino-2-phenylindole (DAPI) in the mounting medium (ProLong Gold Antifade reagent with DAPI, catalog no. P36931, Molecular Probes) stained the nuclei, resulting in red NCAM staining, green Ki67 staining, and blue nuclei.
Satellite cells were also identified by an antibody against Pax7. Briefly, sections were fixed in Histofix (Histolab, Gothenburg, Sweden) followed by 1 h in blocking buffer (0.01% TritonX, 1% BSA, and 1% skimmed milk). Two primary antibodies (Pax7 and laminin) were mixed in blocking buffer and applied to sections overnight. The next day, a biotinylated secondary antibody for Pax7 was applied followed by the Vector Elite ABC kit (PK-6100) and VIP substrate kit (SK-4600, Vector Laboratories, Burlingame, CA). Alexa fluor 488 secondary antibody (1:100) was used to visualize laminin antibody binding. After ethanol dehydration, sections were mounted in Pertex (Histolab, Gothenburg, Sweden).
The immunofluorescense protocol used for double stainings with antibodies against laminin and CD68 as well as CD16 and desmin involved the fixation of sections in Histofix (Histolab) followed by an incubation with two primary antibodies (diluted in 1% BSA) overnight at 4°C. The next day, sections were incubated with two fluorescent secondary antibodies (1:200) for 45 min and mounted with Prolong Gold Antifade reagent with DAPI. The result was red CD16 or CD68 staining, green staining of the basal lamina, and blue nuclei.
The enzyme color reaction used for embryonic myosin heavy chain (eMHC) involved a standard indirect staining procedure. Sections were incubated with the primary antibody overnight. The next day, biotinylated secondary antibody was applied followed by the Vector Elite ABC kit, 3,3′-diaminobenzidine substrate, and hematoxylin (60 s) to stain the nuclei. Sections were mounted in Aquamount (Merck, Darmstadt, Germany).
Quantification.
The number of satellite cells was quantified as the total number of NCAM+ cells on a biopsy cross-section. This is expressed per number of muscle fibers and as a percentage of total myonuclei [NCAM+ cells/(myonuclei + NCAM+ cells) × 100]. Myonuclei were counted from a minimum of 70 fibers. As another identification of satellite cells, Pax7+ cells were quantified in the same way. Satellite cell countings were carried out on a mean of 402 fibers (range: 159–881 fibers) at ×40 magnification using a microscope (Olympus BX51). Cells were identified as satellite cells only if they also had a visible nucleus.
To quantify satellite cells in active phases of the cell cycle, the total number of cells that were positive for both NCAM and Ki67 was counted. Numbers of NCAM+ and Ki67+ cells are expressed relative to the fiber number and as a proportion of satellite cells. The active proportion of satellite cells is the number of NCAM+ and Ki67+ cells relative to the total number of NCAM+ cells, i.e., [(no. of NCAM+-Ki67+ cells/total no. of NCAM+ cells) × 100] (24).
The total number of Ki67+ cells in the biopsies was also quantified (cells that were either positive or negative for NCAM and situated inside or outside the basal lamina) and expressed relative to fiber number.
Macrophages (and possibly other inflammatory cells) were visualized by immunofluorescent staining using a CD68 antibody double stained with laminin to visualize the basal lamina. CD68+ cells were mostly found outside the basal lamina. Fibers with infiltration of CD68+ cells into the myofibers were identified. The total number of CD68+ cells (associated with muscle fibers) was counted from images acquired at ×10 magnification using an Olympus DP71 camera with a fixed exposure time, analyzed using Cell F software, and expressed relative to fiber number. Neutrophils and other inflammatory cells were visualized by immunofluorescent staining using a CD16 antibody double stained with laminin. CD16+ cells were mostly found outside the basal lamina. Cells that were CD16+ and had a visible nucleus (DAPI) were identified using the microscope at ×40 magnification and were counted as positive. The number of CD16+ cells was expressed relative to fiber number.
The presence of desmin-negative fibers was evaluated in all muscle biopsies to assess myofiber damage.
Fibers positive for eMHC were identified, and, from the same sections, the number of fibers with one or more central nuclei was counted and expressed as a percentage of fibers.
Statistics
Results are presented as mean values ± SE. Repeated-measures analyses were performed since each subject provided samples at different time points and from both legs. Statistical analyses were performed using GraphPad Prism4 for Windows (version 4.01, GraphPad Software). Statistical significance was accepted at the P < 0.05 level. For the immunohistochemistry data, the overall difference between treatments was ascertained using a nonparametric repeated-measures Friedman test of overall difference between treatments. Dunn's multiple-comparison test was used to determine which treatments differed. On force data, parametric ANOVAs with repeated measures were performed using SAS 9.1 for Windows (SAS Institute, Cary, NC). Post hoc tests were adjusted for multiple comparisons by Tukey-Kramer.
RESULTS
Eccentric Exercise
Work.
The total work performed in the dynamometer with each leg was not significantly different between the unblocked leg and NSAID leg. At the fast (120°/s) eccentric contraction speed, the total work of the 100 repetitions was 12.9 ± 1.3 and 13.3 ± 1.5 kJ [not significant (NS)], and at the slow (30°/s) eccentric contraction speed, the total work of the 100 repetitions was 13.1 ± 2.4 and 12.9 ± 2.1 kJ (NS) for the unblocked leg and NSAID leg, respectively.
Contraction intensity.
The maximal force produced during each set of eccentric contractions declined gradually during the 10 sets. In sets 2, 5, and 10, maximal force was 328, 305, and 287 N/m in the unblocked leg and 315, 308, and 281 N/m in the NSAID leg, respectively.
Maximal Isometric Muscle Strength
MVC of the quadriceps muscle was measured isometrically at 70° of knee flexion. Isometric MVC was reduced from the prevalue (317 and 314 N/m in the unblocked leg and NSAID leg, respectively) to immediately after exercise (234 and 228 N/m, P = 0.0002) by 26% and 27% in the unblocked leg and NSAID leg, respectively, and was not significantly different from the prevalue on day 8 after exercise (319 and 298 N/m). For isometric MVC, there was a significant main effect of time (P < 0.001), with no significant effect of leg (no block or NSAID). Furthermore, there was no significant interaction effect of leg by time. Thus, the change in MVC over time was not significantly different between the unblocked leg and NSAID leg.
Soreness
Muscle soreness during MVC did not change at any of the time points measured (Fig. 1A). Soreness when pressing on the vastus lateralis muscle with a probe (Fig. 1B) was significantly increased at 24 h and 2 days after exercise compared with before exercise and returned to the prelevel on day 8. This was true for both legs, and there was no difference between the unblocked leg and NSAID leg. Likewise, thigh muscle soreness in both legs combined when walking down stairs (Fig. 1C) was increased from immediately after exercise until day 3 after exercise (compared with the prelevel) and was back at the prelevel on day 8 after exercise.
Fig. 1.
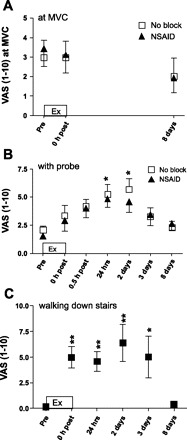
Soreness at maximal voluntary contraction (MVC; A), with probe pressing (B), and when walking down stairs (C). Soreness was measured after eccentric exercise (Ex) during a MVC (A), when pressing with a probe on the vastus lateralis muscle (B), and when walking down stairs (C). Soreness was rated on a visual analog scale (VAS; from 1 to 10, where 1 = no pain and 10 = extremely painful). No block, unblocked leg; NSAID, leg infused with a nonsteroidal anti-inflammatory drug (indomethacin). Data are presented as means ± SE; n = 8 subjects. Significant differences from the prevalue are shown (*P < 0.05 and **P < 0.01). There were no significant differences between the two legs at any time point.
Satellite Cells
Pax7.
Satellite cells were identified with the use of an antibody against Pax7, as shown in Fig. 2, and was expressed as numbers of Pax7+ cells per total myonuclei (in %). There was an overall significant difference between treatments (P = 0.02), and 8 days after exercise, the number of Pax7+ cells was significantly higher in the unblocked leg compared with the NSAID leg (P < 0.05).
Fig. 2.
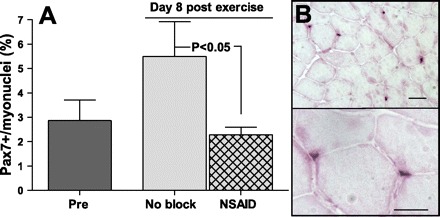
Satellite cells (Pax7+ cells) per myonuclei. A: number of Pax7+ cells expressed per number of myonuclei (in %) in muscle biopsies (vastus lateralis muscles) obtained before (pre) and 8 days after maximal eccentric exercise (no block and NSAID) from the vastus lateralis muscle. Both legs performed the exercise; in one leg, the NSAID was infused during the exercise day (NSAID leg). Data are means ± SE; n = 5 complete sets. B: immunohistochemical staining with the use of Pax7 antibody to identify satellite cells on a muscle cross-section (7 μm) taken 8 days after exercise. For illustration, the area shown is relatively densely populated with satellite cells. Bottom image shows two of the satellite cells at higher magnification. Scale bar = 50 μm.
Satellite cell counts are shown in Table 3. Pax7+ cells expressed per myofiber were borderline significant between groups (P = 0.05). The number of Pax7+ cells was increased by 96% on day 8 after exercise compared with the prevalue (P < 0.05), and at that point there was a trend toward a higher number in the unblocked leg compared with the NSAID leg (P = 0.06).
Table 3.
Satellite cells, inflammatory cells, and regeneration
| Preexercise | 8 Days After Exercise |
P Values |
||||
|---|---|---|---|---|---|---|
| Unblocked leg | NSAID leg | Friedman overall | Preexercise vs. unblocked leg | Unblocked leg vs. NSAID leg | ||
| Satellite cell numbers | ||||||
| No. of Pax7+ cells/fiber | 0.07±0.02 | 0.14±0.04 | 0.07±0.01 | 0.05 | <0.05 (96% increase from preexercise) | 0.06* |
| No. of NCAM+ cells/fiber | 0.14±0.01 | 0.17±0.02 | 0.10±0.01 | 0.04 | NS (21% increase from preexercise) | <0.05 |
| NCAM+ cells/myonuclei, % | 5.13±0.44 | 6.25±0.85 | 3.45±0.24 | 0.04 | NS (22% increase from preexercise) | <0.05 |
| No. of Myonuclei/fiber | 2.91±0.13 | 2.73±0.18 | 2.82±0.14 | NS | ||
| Satellite cell activation status | ||||||
| No. of Ki67+-NCAM+ cells/100 fibers | 0.12±0.04 | 0.80±0.30 | 0.35±0.14 | NS | ||
| Proportion of active satellite cells, % | 0.65±0.33 | 2.96±1.88 | 3.54±1.15 | NS | ||
| Total no. of Ki67+ cells/100 fibers | 0.13±0.07 | 1.51±1.21 | 0.55±0.23 | NS | ||
| Inflammatory cells | ||||||
| No. of CD16+ cells/100 fibers | 2.87±0.61 | 6.24±2.65 | 16.89±9.08 | 0.14 (NS) | 0.09* | |
| No. of CD68+ cells/100 fibers | 3.55±1.10 | 9.13±2.19 | 10.33±3.37 | 0.14 (NS) | ||
| Regeneration | ||||||
| Central nuclei, % | 0.31±0.04 | 0.71±0.20 | 0.32±0.8 | NS | ||
Values are means ± SE; n = 5–6 complete pairs for satellite cells numbers, 7 complete pairs for satellite cell activation status (except for the proportion of active satellite cells, where n = 6–7 complete pairs), 6 complete pairs for inflammatory cells, and n = 5–8 for regeneration. Counts of satellite cells, inflammatory cells, and central nuclei from muscle biopsies (vastus lateralis musles) were obtained before (preexercise) and 8 days after maximal eccentric exercise in the leg without (unblocked) and with an infusion of a nonsteriodal anti-inflammatory drug (NSAID). Central nuclei values indicate the percentage of fibers with ≥1 centrally located nucleus.
Wilcoxon matched pairs.
NCAM.
Another way of identifying satellite cells is with the use of an antibody against NCAM. As shown in Table 3, an overall difference between treatments in the number of NCAM+ cells per fiber was observed (P = 0.04). Eight days after exercise, the number of NCAM+ cells was higher in the unblocked leg than in the NSAID leg (P < 0.05). However, there was no significant change from before to 8 days after exercise in either of the legs. When the number of NCAM+ cells was expressed relative to myonuclear number (Table 3) rather than per fiber, exactly the same pattern was found. The number of myonuclei per fiber did not change during the experimental period (Table 3).
Satellite cell activation status.
Satellite cells in the active phases of the cell cycle were identified by double staining with antibodies against NCAM and Ki67. Examples of stainings are shown in Fig. 3. The number of NCAM+ and Ki67+ cells was very low, and, in almost half of the muscle biopsies, no activated satellite cells (NCAM+ and Ki67+) were found. The maximum number of NCAM+-Ki67+ cells found in one biopsy was four. In Table 3, NCAM+ and Ki67+ cells are expressed relative to fiber number and as a proportion of NCAM+ cells. There were no significant changes in satellite cell activation status at the time points sampled.
Fig. 3.
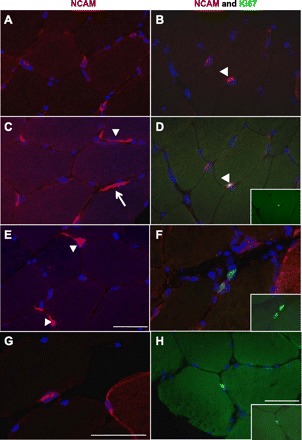
Neural cell adhesion molecule (NCAM) and Ki67 double stainings. The immunohistochemical (IHC) staining of 7-μm cross sections of muscle biopsies with the use of antibodies against NCAM (red), Ki67 (green), and 4′,6-diamidino-2-phenylindole (DAPI; blue) is shown. The left images are examples of diverse NCAM staining of (mostly) satellite cells. A and C: examples of NCAM+ satellite cells, with the typical appearance of a blue nucleus surrounded by red NCAM staining. Some satellite cells show extensions around the muscle fiber periphery (arrowhead). C: nerve staining by the NCAM antibody (arrow). E: three NCAM+ satellite cells, two with cytoplasmic extensions (arrowheads). G: an NCAM+ satellite cell and a myofiber with NCAM+ staining of the membrane and cytoplasm. The right images show combinations of NCAM (red), Ki67 (green), and DAPI (blue) staining. B and D: double staining of the same section showing, in B, two satellite cells (NCAM+, red); one is also a Ki67+ cell (green), as in the merged image in D (arrowhead). D, inset: Ki67 staining of that proliferating cell. F: two proliferating cells in the extracellular matrix. F, inset: Ki67 staining alone. H: Ki67+ cell located in the extracellular matrix. H, inset: Ki67 staining alone. Scale bars = 50 μm.
The total number of Ki67+ cells (including cells positive and negative for NCAM) was also very low, with no difference between legs and no increase after exercise (Table 3).
Damage, Inflammation, and Regeneration
Circulating creatine kinase.
Plasma creatine kinase was significantly increased by 568 ± 154% (P = 0.02) at 24 h after exercise (962 ± 413 U/l) compared with the prelevel (156 ± 28 U/l) and was elevated, but no longer significantly, on day 8 after exercise (886 ± 508 U/l).
Desmin.
No desmin-negative fibers were found in any of the biopsies analyzed.
Inflammatory cells: CD16+ and CD68+ cells.
Infiltration of CD68+ or CD16+ cells into myofibers was not observed in any of the biopsies. CD16 and CD68 staining images are shown in Fig. 4. The number of CD16+ cells per muscle fiber was not significantly changed after either exercise or the NSAID (P = 0.14; Table 3). Likewise, the number of CD68+ cells per muscle fiber was not significantly different between the treatments (P = 0.14; Table 3).
Fig. 4.
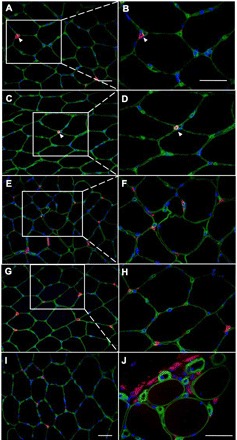
CD16 (neutrophils) and CD68 (macrophages) double stainings with laminin. IHC staining of 7-μm cross sections of muscle biopsies with the use of antibodies against CD16 (A–F) or CD68 (G–J) (red), laminin (green), and DAPI (blue) is shown. The left images are at low (×20) magnification, and the right images are at higher (×40) magnification. Part of the section on the left is enlarged on the right where indicated. A–F: CD16 staining (red). A and B: sections from a preexercise biopsy showing one CD16+ cell (arrowhead) outside the basal lamina (laminin, green) of muscle fibers. C and D: sections from a day 8 biopsy (NSAID leg) showing one CD16+ cell (arrowhead). E and F: sections from a day 8 biopsy (NSAID leg) with many CD16+ cells. All are located outside the basal lamina. Cells were counted as CD16+ cells only when they also had a visible nucleus (DAPI, blue). CD16+ cells were never observed inside muscle fibers. G–J: CD68 staining (red). G and H: sections from a preexercise biopsy showing examples of CD68+ cells. All are located outside the basal lamina (green). I: section from day 8 biopsy (no block leg) showing some CD68+ cells. J: section from day 8 biopsy (NSAID leg) showing several CD68+ cells as an example of the most extreme amount of inflammatory cells observed. CD68+ cells were never observed inside muscle fibers. Scale bars = 50 μm.
Central nuclei.
The percentage of fibers with one or more centrally located nuclei is shown in Table 3. There were no significant changes over time or differences between legs.
eMHC.
Fibers with positive staining for eMHC were rare and were found only in three of the biopsies analyzed (all on day 8, 1 “no block” leg and 2 NSAID legs), ranging from 1 to 55 fibers positive for eMHC. Positive fibers were always situated near the edge of the biopsies. As shown in Fig. 5, fibers with positive staining for eMHC often also showed NCAM+ staining in the cytoplasm of the fiber.
Fig. 5.
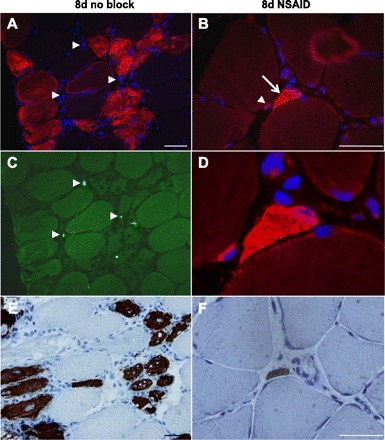
NCAM+ and embryonic MHC (eMHC)+ fibers. Examples of rarely found NCAM+ and eMHC+ myofibers are shown. IHC staining of 7-μm cross sections of muscle biopsies with the use of antibodies against NCAM (red in A, B, and D), Ki67 (green in C), and eMHC (brown in E and F) is shown. The left images are from one biopsy obtained 8 days after exercise from the unblocked leg. A: NCAM+ fibers densely populated with nuclei, some centrally located (infiltration with nuclei to this extent was seen only in this biopsy). C: double staining (with Ki67) of the same section as in A showing that some of the nuclei seen in A are in proliferative states (Ki67+, arrowheads). E: NCAM+ fibers in A are also eMHC+ (dark brown). Central nuclei are seen here as well. The right images are from one biopsy obtained 8 days after exercise from the leg with the NSAID infusion. B: NCAM+ satellite cell (arrowhead) in one fiber with a neighboring NCAM+ fiber (arrow). D: enlargement of a portion of B. None of these cells were Ki67+ (not shown). F: eMHC+ staining of the same (NCAM+) fiber as above. Scale bars = 50 μm.
DISCUSSION
Satellite Cells
The main new finding in this study was that an NSAID infusion for 7.5 h during the exercise day resulted in a reduced number of satellite cells 8 days after exercise. This is in line with the findings of Mackey et al. (25) and points to a negative influence of NSAIDs on satellite cell proliferation after exercise in human skeletal muscle. These data also support animal and cell culture studies investigating the role of prostaglandins in muscle regeneration (8, 9, 18, 26, 30, 36, 37).
Although pointing in the same direction, these two studies in humans elucidate different aspects of this effect: in the present study, subjects performed maximal unaccustomed, eccentric exercise with both legs with an NSAID infusion in one leg only during the exercise day, whereas Mackey et al. (25) studied running in two groups of endurance-trained athletes that received either systemic NSAID treatment or placebo during the entire study period. Thus, it seems that NSAIDs may exert their negative effect both when applied/infused locally for short periods and when consumed for a longer period (12 days). The results from our study suggest that NSAIDs negatively affect satellite cell proliferation during 8 days after eccentric exercise even though the infusion was done only in the hours during and after exercise. More specifically, blockade of prostaglandin synthesis only in the hours during and after hard physical work blocks some of the pathways that are necessary for satellite cell proliferation. The same nonselective COX inhibitor indomethacin was used in both studies, and, accordingly, no suggestions of the importance of the different COX isoforms can be made. The dosage of indomethacin in the present study was 45 mg administered locally (6 mg/h during 7.5 h = 45 mg) compared with the daily dose of 100 mg administered orally in Mackey et al. (25). It is difficult to determine whether these doses are comparable; however, the prostaglandin levels obtained by our local blockade during exercise [300–500 pg/ml (27)] are comparable with levels obtained by the ingestion of 100 mg indomethacin/day [equal to the dose used by Mackey et al. (25)] in a previous study by Boushel et al. (10) (400 pg/ml). The drug concentration in the blood was not measured; however, any systemic effect of the block is assumed to be minimal since the concentration of PGE2 in the unblocked leg was markedly higher than in the NSAID leg (4- to 5-fold), as has been described by Mikkelsen et al. (27).
Our results also correspond with evidence from animal and cell culture studies showing that NSAIDs negatively affect satellite cell activation, proliferation, differentiation, and fusion (8, 9, 26, 36, 38). Our results further add to the understanding of whether and how the animal data also apply to humans. The observed effect of the NSAID is probably not directly on satellite cells but possibly via several different factors affected by NSAIDs. First, the prostaglandin level may influence satellite cells, as has been shown primarily in animal and cell studies, but several other growth factors and cytokines may also be affected.
In a recent human study by Paulsen et al. (32), a COX-2 inhibitor had no effect on the satellite cell number after a single bout of eccentric exercise with the elbow flexors, which is in contrast to our findings using a nonselective COX inhibitor. This may point to some basal level of prostaglandins being necessary for satellite cell proliferation, and, when only the COX-2 enzyme is inhibited, maybe COX-1 (or other isoforms) produce sufficient levels of prostaglandins. However, this would be in contrast to animal studies showing that, specifically, the COX-2 pathway is essential in satellite cell activity (8, 9) and hypertrophy (28).
The long-term effects of NSAID consumption on satellite cells have not been investigated in humans, but the effects of ibuprofen on hypertrophy and strength was evaluated in a recent study by Krentz et al. (20). They found no effect of a moderate dose (400 mg/day) of ibuprofen on the muscle hypertrophy response to 6 wk of training. However, this dose was considerably lower than the high-ibuprofen doses used in other studies [e.g., 1,200 mg/day (39)]. A comparison of indomethacin (used in our study) with ibuprofen is difficult, but 100 mg indomethacin/day may be similar to 1,200 mg ibuprofen/day, since this is the recommended daily dosage (www.medicin.dk) and both are half of the maximal daily allowance (200 mg indomethacin/day and 2,400 mg ibuprofen/day). We consider the infused dose in our study to be higher than the 400 mg ibuprofen/day used by Krentz et al. (20).
Previously, an increase in the satellite cell number after a single bout of eccentric exercise has been consistently shown at different time points ranging from 1 to 8 days after exercise (Table 1). In the present study, increases in satellite cell numbers on day 8 after exercise in the unblocked leg ranging from 21% to 96% were observed, but, surprisingly, only the number of Pax7+ cells when expressed relative to fiber number was significantly higher than baseline (96% increase). The nonsignificant results in some of our satellite cell data is likely be due to the small sample size, since some of the subjects had one or two missing values and only complete sets of biopsies were analyzed. As shown in Table 1, the satellite cell response to a single bout of exercise observed in different human studies is highly variable. The differences between studies are primarily due to sampling at different time points. Two previous studies (13, 14) also sampled on day 8 after exercise, and both showed >100% increase in the satellite cell number per myonuclei. This discrepancy may result from differences in the exercise protocols and training status of the subjects. The study by Crameri et al. (13) used the same exercise protocol as used in the present study; however, subjects were untrained, as opposed to this study with well-trained subjects. The 27% increase in NCAM+ cells (per myonuclei, P < 0.05) found by Mackey et al. (25) corresponds with our 22% increase, although the increase was nonsignificant in the present study. All data from the previous studies shown in Table 1 were obtained with the use of NCAM to identify satellite cells, but in the study by Crameri et al. (13) Pax7 was also used. However, the increase in the number of Pax7+ cells was not significant on day 8 (13), which is in conflict with our Pax7 data. In summary, the inconsistent increases in satellite cell number in our study compared with the consistent increases shown in Table 1 illustrate the variability in immunohistochemical analyses, and is seen even though the exercise stimulus in our study was comparable with that of other studies, based on similar (or even larger) force reduction and soreness (13). Thus, a consistent increase in the number of satellite cells would have been expected.
The satellite cell activation status in our baseline biopsies (0.65%) corresponds with the findings from Mackey et al. (24) of a proportion of ∼0.5% active satellite cells in the resting state. No significant increase was observed on day 8 after exercise; however, the mean proportion of active satellite cells was ∼3% on day 8 and thus comparable with the mean values of 1.4–2.5% observed after 12 wk of resistance exercise (24). Mackey et al. (24) observed a significantly increased proportion of active satellite cells (mean: 10%) 48 h after electrical stimulation, pointing toward a substantial activation of satellite cells after electrical stimulation compared with voluntary contractions. The timing indicates that the main activation of satellite cells most likely takes place before day 8, maybe around 48 h after electrically stimulated exercise. It is unknown whether peak activation would be observed at some time point before or after 48 h after exercise, and how the time course after electrical stimulation resembles that after voluntary exercise. Compared with voluntary contractions, electrical stimulation induces more damage to the myofibers and leads to higher satellite cell numbers (13), which could influence the different proportions of activated satellite cells observed. The time course of satellite cell activation and proliferation may also differ between electrical and voluntary contraction.
The numbers of satellite cells identified by the antibody against NCAM was higher than with the Pax7 antibody. This is in line with the results from previous studies (13, 24) and would also be expected due to the different staining patterns of the two antibodies (24), with NCAM being a glycoprotein expressed at the cell surface (cell adhesion molecule) and the transcription factor Pax7 being expressed in the nucleus. Furthermore, it might be a problem if Pax7 does not stain all satellite cells, as pointed out by Reimann et al. (34), who showed that 2–15% of the satellite cells they identified by M-cadherin staining and location beneath the basal lamina were not Pax7 positive. However, most of their samples were from pathological muscle, making direct comparisons difficult. Recently, Lindström and Thornell (22) used a multiple-labeling method to identify satellite cells based on NCAM, Pax7, and laminin staining and observed that 94% of all satellite cells were both NCAM and Pax7 positive, although with large individual variability.
In any case, the observed patterns of the satellite cell response to NSAIDs and exercise were similar using the two antibodies on separate sections from the same biopsies, which suggests that both may be used for the identification of satellite cells, although the numbers of Pax7+ cells seemed to increase more than NCAM+ cells 8 days after exercise (92–96% vs. 21–22% increase compared with the prelevel).
Damage, Inflammation, and Regeneration
In line with the findings of Crameri et al. (13), no desmin-negative fibers were found in any of the biopsies analyzed; thus, we think that no gross/major damage to myofibers had occurred. Infiltration of inflammatory cells (CD68+ or CD16+ cells) into the myofibers was not found in any of the biopsies, which is also in line with the rare observations by Crameri et al. (13). Even though infiltration was not evident, inflammation could have been observed as a higher number of inflammatory cells associated with and surrounding myofibers, but these numbers were not significantly changed with exercise or the NSAID. This corresponds with the findings of Przybyla et al. (33), who found no changes in the total number of macrophages (CD68+ cells) after exercise. In the present study, the numbers of both CD68+ cells and CD16+ cells seemed to be increased on day 8 after exercise. However, this was nonsignificant despite large increases from baseline in both legs and using both CD markers (2- to 6-fold). Individual variations were large and may represent variations in the basal level or level of response between individuals or differences in timing. In some subjects, the response to exercise may be slower than in other subjects, and therefore we may sample at different relative points of their response curves. Anti-inflammatory medication was expected to reduce the number of inflammatory cells, but we found no effect on the numbers of CD68+ cells; if anything, there was even a tendency toward higher numbers (CD16+ cells, P = 0.09) in the NSAID leg. This could indicate that NSAIDs delayed the inflammatory response to exercise and, thus, that the possible rise in inflammatory cells (CD16+ cells) was over on day 8 in the unblocked leg but was still increased on day 8 after exercise in the NSAID leg.
The expression of the eMHC isoform and/or centrally located nuclei may be signs of regenerating or newly formed fibers (11). Fibers expressing eMHC were rare; likewise, there was no significant change in the number of fibers with central nuclei after the exercise and very few fibers had central nuclei, indicating that no major regeneration occurred, which is in line with the lack of major myofiber damage. The percentages of fibers with central nuclei (0.3–0.7%) were within the range observed by Mackey et al. (25). Although the number of fibers with one or more central nuclei was not significantly changed, it was twofold higher 8 days after exercise in the unblocked leg compared with the NSAID leg and preexercise.
Even though histological analyses showed that most likely no major myofiber damage and inflammation had occurred, subjects had very sore muscles (Fig. 1), supporting the view that myofiber damage is not the cause of delayed onset muscle soreness (DOMS) (13) and that eccentric exercise leading to DOMS does not necessarily induce muscle fiber degeneration, necrosis, or loss of desmin staining (43–45). All subjects had very sore muscles starting immediately after exercise and lasting 2–3 days. Soreness was back to baseline on day 8 when biopsies were analyzed for muscle damage, but we would expect that previous damage to the muscle would appear in the day 8 biopsies as signs of regeneration or that damage to the muscle would not have totally resolved on day 8. There were no differences in soreness between the legs at any time point; consequently, the infusion of the NSAID during the day of exercise did not affect muscle soreness in the days after exercise. It has to be underlined that the infusion of the NSAID was performed locally and did not include the whole muscle (27). An analgesic effect of the NSAID during the infusion may have been expected, but this was not evident immediately after exercise. This may be because the area affected by the NSAID infusion or the dose or duration of the drug infusion was not sufficient to detect any analgesic effect. There is some support in the literature for the analgesic effects of NSAIDs after exercise when soreness is substantial. Conversely, a study (5) that reported minimal soreness did not detect any significant analgesic effects of NSAIDs.
Satellite Cell Proliferation Without Damage and Inflammation
Apparently, satellite cell proliferation (and activation) can be induced by muscle loading/contraction without damage or inflammation, which is in line with the findings of Crameri et al. (13). This seems reasonable since satellite cells are not only needed for the regeneration of damaged muscle fibers but also for the general adaptation to muscle loading. Some of the satellite cells may not reach differentiation but instead be used to replenish the satellite cell pool, and, in this way, the muscle may use the acute exercise stimulus to be better prepared for further stimuli. Force itself may directly activate satellite cells by force transduction via integrins on the satellite cell surface. The activation/proliferation of satellite cells may also be stimulated indirectly via several different growth factors and/or cytokines likely produced within the muscle (muscle cells or the extracellular matrix).
Force
The force loss of 27% we observed immediately after exercise was within the range found in other studies testing acute force loss (isometrically) after similar eccentric exercise protocols with leg muscles (16–54%) (6, 12, 19, 31), although the variation between studies is large. Isometric strength was back at the prelevel 8 days after exercise, which is also in accordance with some previous studies (6, 12, 19) but not all (19, 23).
Clinical Relevance
Given the vital role that satellite cells play in skeletal muscle adaptation, any factor that interferes with their activity could counteract the anabolic effects of exercise. The data from this study indicate that the NSAID infusion, only during the day of exercise, negatively affected satellite cell proliferation during the following 8 days. As the use of NSAIDs among athletes and rheumatic patients is normally more prolonged, the negative effects may be even larger. In the treatment of DOMS or minor injuries to relieve pain to continue training, NSAIDs may reduce the adaptation to training and thus prevent some of the beneficial effects of training. During postsurgery rehabilitation and after sports injuries, effective muscle regeneration is particularly important; thus, the potential negative effects of NSAIDs could be severe. The present results warrant caution in the use of NSAIDs during training and rehabilitation in athletes in situations when NSAIDs are not necessary. However, NSAIDs are very effective for rheumatic disease treatment, and any negative effects on satellite cell activity might not be severe enough to warrant warning against their use in patients.
Given the widespread use of NSAIDs among athletes and patients, it is clinically relevant to further elucidate the potential negative effects of these drugs on muscle adaptation, and further work is required to determine any consequence for muscle function.
Conclusions
In conclusion, our results indicate that NSAIDs may negatively affect satellite cell proliferation in human muscle after eccentric exercise, supporting the view that the COX pathway is important for satellite cell activity.
GRANTS
This work was supported by grants from the H:S Research Foundation (Copenhagen University Hospitals), The Danish Rheumatism Association, and the Lundbeck Foundation. The embryonic myosin antibody (F1.652) developed by H. M. Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biology of The University of Iowa (Iowa City, IA).
ACKNOWLEDGMENTS
Monica Bayer is acknowledged for developing the staining protocol using the Pax7 antibody. Lea Larsen and Christian Have-Dall are acknowledged for participation in the NCAM/Ki67 and CD68 analyses. The subjects are acknowledged for participation in the study.
REFERENCES
- 1. Alaranta A, Alaranta H, Helenius I. Use of prescription drugs in athletes. Sports Med 38: 449–463, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Andersen LL, Andersen JL, Magnusson SP, Suetta C, Madsen JL, Christensen LR, Aagaard P. Changes in the human muscle force-velocity relationship in response to resistance training and subsequent detraining. J Appl Physiol 99: 87–94, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Anderson J, Pilipowicz O. Activation of muscle satellite cells in single-fiber cultures. Nitric Oxide 7: 36–41, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Baldwin LA. Use of nonsteroidal anti-inflammatory drugs following exercise-induced muscle injury. Sports Med 33: 177–185, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Beaton LJ, Tarnopolsky MA, Phillips SM. Contraction-induced muscle damage in humans following calcium channel blocker administration. J Physiol 544: 849–859, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975. [PubMed] [Google Scholar]
- 8. Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 287: C475–C483, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 290: C1651–C1659, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlson BM, Faulkner JA. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc 15: 187–198, 1983. [PubMed] [Google Scholar]
- 12. Child R, Brown S, Day S, Donnelly A, Roper H, Saxton J. Changes in indices of antioxidant status, lipid peroxidation and inflammation in human skeletal muscle after eccentric muscle actions. Clin Sci (Lond) 96: 105–115, 1999. [PubMed] [Google Scholar]
- 13. Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjaer M. Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583: 365–380, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crameri RM, Langberg H, Magnusson P, Jensen CH, Daa SH, Olesen JL, Suetta C, Teisner B, Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558: 333–340, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a. Danish Rheumatism Association Gigtforeningen (online). http://www.gigtforeningen.dk/ [3 September 2009].
- 15. Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33: 242–253, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549: 409–418, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 203: 89–99, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horsley V, Pavlath GK. Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J Cell Biol 161: 111–118, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hubal MJ, Chen TC, Thompson PD, Clarkson PM. Inflammatory gene changes associated with the repeated-bout effect. Am J Physiol Regul Integr Comp Physiol 294: R1628–R1637, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Krentz JR, Quest B, Farthing JP, Quest DW, Chilibeck PD. The effects of ibuprofen on muscle hypertrophy, strength, and soreness during resistance training. Appl Physiol Nutr Metab 33: 470–475, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Li YP. TNF-α is a mitogen in skeletal muscle. Am J Physiol Cell Physiol 285: C370–C376, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Lindstrom M, Thornell LE. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochem Cell Biol 132: 141–157, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Mackey AL, Donnelly AE, Turpeenniemi-Hujanen T, Roper HP. Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol 97: 197–203, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40: 455–465, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Mackey AL, Kjaer M, Dandanell S, Mikkelsen KH, Holm L, Dossing S, Kadi F, Koskinen SO, Jensen CH, Schroder HD, Langberg H. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol 103: 425–431, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Mendias CL, Tatsumi R, Allen RE. Role of cyclooxygenase-1 and -2 in satellite cell proliferation, differentiation, and fusion. Muscle Nerve 30: 497–500, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Mikkelsen UR, Helmark IC, Kjaer M, Langberg H. Prostaglandin synthesis can be inhibited locally by infusion of NSAIDS through microdialysis catheters in human skeletal muscle. J Appl Physiol 104: 534–537, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Novak ML, Billich W, Smith SM, Sukhija KB, McLoughlin TJ, Hornberger TA, Koh TJ. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol 296: R1132–R1139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38: 1434–1442, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Otis JS, Burkholder TJ, Pavlath GK. Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Exp Cell Res 310: 417–425, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Paulsen G, Crameri R, Benestad HB, Fjeld JG, Mørkrid L, Hallén J, Raastad T. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med Sci Sports Exerc. In press [DOI] [PubMed] [Google Scholar]
- 32. Paulsen G, Egner IM, Drange M, Langberg H, Benestad HB, Fjeld JG, Hallen J, Raastad T. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand J Med Sci Sports. In press [DOI] [PubMed] [Google Scholar]
- 33. Przybyla B, Gurley C, Harvey JF, Bearden E, Kortebein P, Evans WJ, Sullivan DH, Peterson CA, Dennis RA. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp Gerontol 41: 320–327, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Reimann J, Brimah K, Schroder R, Wernig A, Beauchamp JR, Partridge TA. Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res 315: 233–242, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Shen W, Li Y, Tang Y, Cummins J, Huard J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol 167: 1105–1117, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen W, Prisk V, Li Y, Foster W, Huard J. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2α. J Appl Physiol 101: 1215–1221, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Soltow QA, Betters JL, Sellman JE, Lira VA, Long JH, Criswell DS. Ibuprofen inhibits skeletal muscle hypertrophy in rats. Med Sci Sports Exerc 38: 840–846, 2006. [DOI] [PubMed] [Google Scholar]
- 39. Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E551–E556, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Tscholl P, Feddermann N, Junge A, Dvorak J. The use and abuse of painkillers in international soccer: data from 6 FIFA tournaments for female and youth players. Am J Sports Med 37: 260–265, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Warner DC, Schnepf G, Barrett MS, Dian D, Swigonski NL. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J Adolesc Health 30: 150–153, 2002. [DOI] [PubMed] [Google Scholar]
- 42. Wozniak AC, Kong J, Bock E, Pilipowicz O, Anderson JE. Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle Nerve 31: 283–300, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Yu JG, Furst DO, Thornell LE. The mode of myofibril remodelling in human skeletal muscle affected by DOMS induced by eccentric contractions. Histochem Cell Biol 119: 383–393, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Yu JG, Malm C, Thornell LE. Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem Cell Biol 118: 29–34, 2002. [DOI] [PubMed] [Google Scholar]
- 45. Yu JG, Thornell LE. Desmin and actin alterations in human muscles affected by delayed onset muscle soreness: a high resolution immunocytochemical study. Histochem Cell Biol 118: 171–179, 2002. [DOI] [PubMed] [Google Scholar]


