Abstract
Central chemoreception is the mechanism by which CO2/pH-sensitive neurons (i.e., chemoreceptors) regulate breathing in response to changes in tissue pH. A region of the brain stem called the retrotrapezoid nucleus (RTN) is thought to be an important site of chemoreception (23), and recent evidence suggests that RTN chemoreception involves two interrelated mechanisms: H+-mediated activation of pH-sensitive neurons (38) and purinergic signaling (19), possibly from pH-sensitive glial cells. A third, potentially important, aspect of RTN chemoreception is the regulation of blood flow, which is an important determinate of tissue pH and consequently chemoreceptor activity. It is well established in vivo that changes in cerebral blood flow can profoundly affect the chemoreflex (2); e.g., limiting blood flow by vasoconstriction acidifies tissue pH and increases the ventilatory response to CO2, whereas vasodilation can wash out metabolically produced CO2 from tissue to increase tissue pH and decrease the stimulus at chemoreceptors. In this review, we will summarize the defining characteristics of pH-sensitive neurons and discuss potential contributions of pH-sensitive glial cells as both a source of purinergic drive to pH-sensitive neurons and a modulator of vasculature tone.
Keywords: control of breathing, central chemoreceptor, vascular control, neural-glial interactions
central chemoreception is the mechanism by which the brain regulates breathing in response to changes in tissue pH, wherein CO2/H+ chemoreceptors sense pH changes to regulate the depth and frequency of breathing. Several brain regions are thought to participate in chemoreception including the nucleus tractus solitarius, locus coeruleus, medullary raphe, prebotzinger complex, fastigial nucleus of the cerebellum, hypothalamus, and retrotrapezoid nucleus (RTN) (14, 22). Although the relative contributions of these putative chemoreceptor regions remains controversial (24, 42), there is compelling evidence that the RTN is an important site of chemoreception (1, 6, 26, 38, 43). In this review, we will summarize the properties of chemosensitive RTN neurons; further details on this topic can be found in several recent reviews (23, 24, 41). Surprisingly, pH-sensitive RTN glial cells, first described by Fukuda et al. (16) more than 30 years ago, have received relatively little attention (9–11, 46, 53) despite evidence from other brain regions that glial cells can function as important modulators of both neural network activity and vascular tone (25). Therefore, we will also describe general characteristics of glial cells and propose an expanded model of chemoreception that includes a role for RTN glia as a source of excitatory drive to pH-sensitive neurons as well as a regulator of local vascular tone.
THE RTN IS AN IMPORTANT SITE OF CENTRAL CHEMORECEPTION
The early work of Mitchell and colleagues more than 40 years ago established that acidification of the ventral medullary surface (area M) stimulates breathing (34); therefore, they concluded that pH-sensitive neurons in this region, later defined as the RTN (57), are able to provide the chemical drive to breathe. This possibility has been strengthened by a series of in vivo experiments by Nattie and colleagues who showed that chemical activation of the RTN led to increased respiratory drive and the ventilatory response to CO2 (6, 30, 43), whereas chemical inhibition of the RTN had the opposite effect (40, 44). These studies also found that the contribution of the RTN to respiratory drive is more pronounced in anesthetized animals compared with conscious animals. These observations together with those described below led to the working hypothesis that the RTN provides a tonic excitatory drive to the central pattern generator: during sleep or anesthesia, input from the RTN provides an important chemical drive to breathe; however, during wakefulness, the respiratory central pattern generator as well as upstream chemoreceptor regions (e.g., RTN) receive excitatory input from many sources (e.g., hypothalamus, medullary raphe), and so input from any one source, including the RTN, becomes less essential to respiratory drive (22, 23).
More recently, together with Patrice Guyenet and Douglas Bayliss, we were able to determine the cellular identity of RTN chemoreceptors (38). Some of the properties of RTN chemoreceptors are summarized in Fig. 1. In anesthetized rats ventilated to maintain an end-expiratory CO2 level approximating the physiological range, CO2/H+-sensitive RTN neurons exhibit a low tonic level of activity and are robustly activated by hypercapnia (Fig. 1, A1 and A2); the CO2/H+ sensitivity of these neurons remains intact when inputs from peripheral chemoreceptor are removed or when respiratory neuronal activity is blocked with morphine (38), indicating that the CO2/H+ sensitivity of these cells is centrally mediated and not dependent on feedback from respiratory centers. As traditionally expected for central respiratory chemoreceptors (34, 56), CO2/H+-sensitive RTN neurons have extensive dendrites within the marginal layer of the ventral medullary surface (Fig. 1A3), are glutamatergic, as evidenced by expression of the vesicular glutamate transporter VGLUT2 (Fig. 1A5), and project directly to key pontomedullary respiratory centers, including the pre-Bötzinger complex (38). In addition, CO2/H+-sensitive RTN neurons express the transcription factor Phox2b (Fig. 1A5) (39, 59). Mutations in the gene that encodes PHOX2B have been shown to cause severe respiratory deficits that characterize a condition known as congenital central hypoventilation syndrome (CCHS) (7), the principal symptom of which is hypoventilation during sleep and reduced (or absent) chemical drive to breathe. In addition, a transgenic animal model of CCHS developed by creating a knock-in of the most frequent CCHS-causing mutation (Phox2b27Ala/+) show reduced ventilatory response to CO2 at birth, apparently due to a massive reduction in the number of RTN neurons since other respiratory regions appeared normal in these animals (7). Furthermore, conditional Phox2b mutations that selectively disrupt RTN development also reduced CO2 sensitivity at approximately embryonic day 15 measured in vitro using the brain stem-spinal cord preparation (8). The presence of Phox2b in RTN neurons further supports the possibility that they function as important chemoreceptors. Perhaps the most convincing evidence that Phox2b-expressing RTN neurons contribute to respiratory drive was obtained in the elegant work of Abbot et al. (1), who used a lentivirus to target expression of the light-activated cationic channel channelrhodopsin-2 in Phox2b-expressing cells. Photo-stimulation of even a small percentage of Phox2b-expressing RTN neurons caused a marked increase in phrenic nerve activity (1).
Fig. 1.
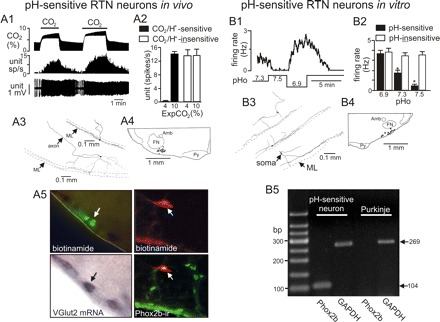
Defining characteristics of RTN chemoreceptors in vivo and in vitro. A1: the typical firing rate response of a CO2/H+-sensitive neuron in vivo to changes in end expiratory CO2; increasing Exp CO2 increased neuronal activity in a reversible and repeatable manner. A2: average firing rate of CO2/H+-sensitive (n = 26) and CO2/H+-insensitive (n = 39) neurons in vivo under control (4% CO2) and hypercapnic (10% CO2) conditions. After recording cells were labeled with biotinamide for later conformation of location, morphology, and neurochemical phenotype. A3: the structure of two CO2/H+-sensitive neurons labeled in vivo (ML, marginal layer). A4: plots the location of 17 CO2/H+-sensitive neurons (black dots) recorded in vivo. Bregma level −11.4 mm (Amb, nucleus ambiguous; FN, facial nucleus; ML, marginal layer; Py, pyramidal tracts). A5: a biotinamide (Alexa Fluor 488 fluorescence)-labeled CO2/H+-sensitive RTN neuron recorded in vivo (top) and the same cell expresses vesicular glutamate transporter-2 (VGLUT2) mRNA (bright-field illumination; bottom). A6: top shows a CO2/H+-sensitive RTN neuron recorded in vivo and labeled with biotinamide (Cy-3, red), and bottom shows that the same cell is immunoreactive for Phox2b (Alexa 488, green), biotinamide with Cy-3 (red); colocalization is shown in yellow. B1: trace of firing rate and bath pH show characteristic responses of a pH-sensitive RTN neuron to pH values ranging from 6.9 to 7.5; these cells are spontaneously active at control pH 7.3, nearly silent at pH 7.5, and robustly active at pH 6.9. B2: average firing rate of pH-sensitive (n = 40) and pH-insensitive (n = 47) neurons recorded in vitro at varying pH. *P <0.01 for effect of pH. B3: structure of three biocytin-filled pH-sensitive neurons that are reminiscent of CO2/H+-sensitive RTN neurons recorded in vivo. B4: composite map shows the location of 11 pH-sensitive neurons (black dots) recorded in vitro (Amb, nucleus ambiguous; FN, facial nucleus; ML, marginal layer; Py, pyramidal tracts). B5: agarose gel of single cell RT-PCR for Phox2b and GAPDH; the chemosensitive RTN neuron expresses Phox2b, but the Purkinje cell does not. This figure is composed of previously published data (38, 39, 59) and presented here with permission from the appropriate journals.
In brain stem slices, we identified a population of RTN neurons in a similar location and of a similar morphology and neurochemical phenotype as CO2/H+-sensitive RTN neurons identified in vivo (38, 39). The cells were characterized as being pH-sensitive by making cell-attached current clamp recordings of membrane potential during exposure to acidic (pH 6.9) and alkaline (pH 7.5) perfusate (Fig. 1, B1 and B2). The structure of these cells is strikingly similar to that of pH-sensitive RTN neurons recorded in vivo (38); specifically, the locations of their soma and long secondary dendrites, which project along the marginal layer of the surface of the ventrolateral medulla, were nearly identical between preparations (Figs. 2, B3 and B4). In addition, we used single-cell RT-PCR to demonstrate that pH-sensitive RTN neurons recorded in vitro represent the cellular correlate of the glutamatergic and Phox2b-expressing chemoreceptors characterized in vivo (Fig. 1B5; Ref. 39).
Fig. 2.
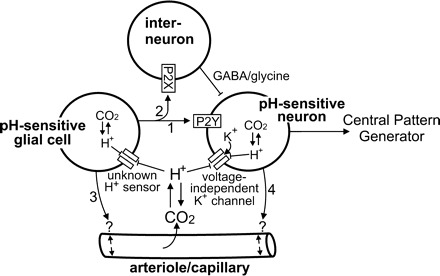
Working model of chemoreception by the retrotrapezoid nucleus (RTN). The RTN contains a population of CO2/H+-sensitive neurons that appear to function as respiratory chemoreceptors (see text and Fig. 1 for more details). The RTN also contains a population of pH-sensitive glial cells (12, 16, 53) that may contribute to chemoreception by releasing ATP during hypercapnia. Evidence indicates that ATP can contribute to chemoreception by 1) activating pH-sensitive neurons through a P2Y-receptor-dependent mechanism (arrow 1); 2) inhibiting pH-sensitive neurons by activation of P2X-receptors on interneurons (arrow 2); 3) modulating vascular tone to increase or decrease tissue pH (arrow 3). It is also possible that pH-sensitive neurons influence activity of pH-sensitive glial cells by the release of excitatory neurotransmitters or increased extracellular K+ (arrow 4) or regulate vascular tone directly (arrow 5).
The mechanism by which RTN neurons sense pH remains unresolved. These cells appear to be intrinsically pH-sensitive because their firing-rate response to pH changes persisted after blocking ionotropic glutamate receptors with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) and antagonist 2-amino-5-phosphovalerate (APV, 20 μM) and also when P2-receptors were blocked with pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS; 100 μM), an ATP-receptor antagonist (36, 39). In addition, voltage-clamp experiments (in the presence of 0.1 μM tetrodotoxin to block action potentials) showed that RTN chemoreceptors express a pH-sensitive voltage-independent K+ conductance that likely confers pH sensitivity to these cells (39). The properties of this pH-sensitive current suggest involvement of the TASK family of background K+ channels (i.e., TASK-1 and TASK-3). However, the pH-sensitive current in RTN neurons is not sensitive to halothane (39), a volatile anesthetic known to activate TASK channels. In addition, central chemoreception and pH sensitivity of RTN neurons was retained in TASK-1, TASK-3, and double TASK-1/3 knockout animals (39), indicating that these channels do not confer pH sensitivity RTN neurons or are required for central chemoreception. Similar results were obtained by Trapp et al. (60), using independently generated TASK-1 and TASK-3 knockout animals. The properties of the pH-sensitive K+ current expressed by RTN neurons (i.e., high pH sensitivity in the physiological range and a lack of halothane sensitivity) are not consistent with the known properties of other background K+ channels. Note that TWIK-1 channels are inhibited by H+, but their response to halothane has not been described.
A number of alternative pH-sensitive K+ channels have been proposed to contribute to respiratory chemoreception (for review, see Ref. 51). For example, several pH-sensitive inward rectifying K+ channels are expressed in brain stem neurons. However, we find no evidence for inward rectification in I-V relationships recorded from pH-sensitive RTN neurons (38, 39). Large conductance Ca2+-dependent K+ channels are also pH-sensitive and thought to contribute to chemoreception, but we found that inhibition of these channels had no effect on the pH sensitivity of RTN neurons (Mulkey DK, Bayliss DA, unpublished observations). Several members of the voltage-dependent K+ (Kv) family of channels are pH-sensitive (51) and may exhibit a constitutively active “window” current that could contribute to the background pH-sensitive K+ current in RTN neurons.
In addition to responding to pH changes, the activity of RTN neurons can also be modulated by various neurotransmitters. For example, the RTN received extensive innervation from medullary serotonergic neurons, and it was shown both in vivo and in vitro that exposure to serotonin increased baseline activity of RTN chemoreceptors and caused an upward parallel shift in their CO2/H+ sensitivity (37). These results suggest that serotonergic raphe neurons contribute to chemoreception at least in part by modulating excitability of RTN neurons. In a similar fashion, RTN glia may also contribute to chemoreception by modulating activity of pH-sensitive RTN neurons. For example, the RTN also contains pH-sensitive glial cells (12, 16, 53). Since tetrodotoxin will not inhibit glial transmitter release, it remains possible that local paracrine mechanisms, including excitatory input from pH-sensitive glial cells, could contribute to pH sensitivity of RTN neurons and respiratory drive. Based on this possibility, we have expanded our working model of RTN chemoreception to include possible contributions from pH-sensitive glial cells (Fig. 2).
GENERAL CHARACTERISTICS OF GLIAL CELLS
The term “glia” was coined in 1856 by Rudolf Virchow because these cells appeared to “glue” the nervous system together and currently refers to all nonaction potential forming cells of the nervous system. During the decades following their discovery, glial cells have been classified based on their location, morphology, and reactivity to different staining methods. Recently, more functional roles for glial cells have been established including myelination, immune response, ion and transmitter homeostasis, and active participation in synaptic plasticity and neuronal excitability. Although many subpopulations of glial cells have been described, they all can be classified into one of four categories: microglia, oligodendrocytes, polydendrocytes, and astrocytes. Each of these subtypes differs in proposed function, immunoreactivity, and electrophysiology.
Microglia are immunocompetent cells of the central nervous system that can be identified immunohistochemically by expression of the cluster differentiation marker CD11b (a β-integrin marker of microglia). In their “resting” state microglia can interact with all elements of the CNS and can respond to a variety of physiological signals, including pH (13). However, microglia have a resting membrane potential (approximately −40 mV) that is considerably more depolarized than the resting membrane potential of pH-sensitive glia (approximately −70 mV) (16, 53), suggesting that pH-sensitive RTN glia are probably not microglia. Other characteristics of resting microglia include high membrane resistance and the presence of inward rectifying K+ (Kir) channels, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors, and glutamate transporters (3, 55). Once activated by injury or infection, microglia increase expression of outward rectifying K+ channels (3).
Oligodendrocytes and polydendrocytes belong to the same lineage. Oligodendrocytes are myelin-producing cells usually identified by antibody labeling for myelin basic protein (17). Oligodendrocytes are also electrically distinct in that they have low membrane resistance, exhibit mostly voltage-independent K+ currents, and express glutamate transporters but not AMPA receptors (17). Polydendrocytes are glia progenitors present in all brain regions throughout life (45); they are identified immunohistochemically by expression of NG2 (45), an integral membrane chondroitin sulfate proteoglycan. The electrical profile of polydendrocytes includes high membrane resistance, outward rectifying K+ currents, distinctive inward Na+ currents, and presence of AMPA receptors but not glutamate transporters (31, 64).
Astrocytes are the largest class of glial cells and can be further classified based on location, morphology, and association with vasculature, e.g., protoplasmic astrocytes are located in the gray matter and fibrous astrocytes are located in white matter. Both cell types have numerous fine processes and form close associations with neurons and blood vessels. Astrocytes are commonly differentiated from other glial cell types based on expression of glial fibrillary acidic protein (GFAP) or S100β (a Ca2+ binding protein expressed by astrocytes) (15). However, both GFAP and S100β activity are found in glial progenitor cells (45), and protoplasmic astrocytes can be GFAP-negative (45). Recent transcriptome analysis has revealed aldehyde dehydrogenase 1L1 to be a more specific marker of protoplasmic astrocytes (4). In addition, electrophysiological studies have identified two subtypes of astrocytes in the hippocampus (33, 64) and brain stem (20): one type has passive electrical properties, low membrane resistance, and expresses glutamate transporters (e.g., passive astrocytes); the second type has voltage-dependent channels, comparatively higher membrane resistance, and preferentially expresses AMPA receptors over glutamate transporters (e.g., variable rectifying astrocytes). The distribution of these astrocyte subtypes vary across development (64) and is thought to reflect distinct cell types with unique functions, but this has yet to be demonstrated.
POSSIBLE ROLES OF GLIA IN CENTRAL CHEMORECEPTION
Extracellular pH Regulation
Originally, it was proposed that glial cells in the RTN contribute to the mechanism of chemoreception by potentiating CO2-induced extracellular acidosis (11). It is well known that astrocytes and oligodendrocytes help regulate extracellular K+ by taking up extracellular K+ ions during times of increased neuronal activity (47). This process effectively buffers changes in extracellular K+; however, it can also increase Na+/HCO3− cotransport into glia (5), which would enhance the CO2-induced fall in extracellular pH and potentiate activation of RTN chemoreceptors (Fig. 2, arrow 4). This possibility is supported by a series of in vivo experiments in which glial cells were artificially depolarized by microinjection of fluorocitrate, a glia-specific metabolic toxin, into the RTN. The fluorocitrate-mediated glia depolarization, presumably by depletion of ATP and consequent loss of cytoplasmic K+ (28), caused extracellular acidification followed by increased respiratory frequency and ventilatory sensitivity to CO2 (11, 26). It is important to note that these studies used subtoxic doses of fluorocitrate; therefore, the glia had responded to fluorocitrate-mediated depolarization by either exacerbating extracellular acidification or releasing excitatory transmitters and consequently increasing the activity of pH-sensitive neurons.
Purinergic Signaling and Central Chemoreception
There is increasing evidence that ATP, possibly released by astrocytes (58), is an important mediator of central chemoreception (18, 19). For example, in vivo studies showed that exposure to high CO2 (i.e., hypercapnia) caused discrete release of ATP within the RTN (19). In addition, application of ATP into the RTN stimulated respiratory output, whereas application of the ATP receptor antagonist (PPADS) to the same region lowered CO2 respiratory responsiveness (19).
It was proposed that purinergic P2X-receptors mediate the effects of ATP on ventral surface neurons (18); however, mice lacking P2X2-receptors exhibit normal CO2 sensitivity (54), and this theory still lacks evidence at the cellular level. It was shown in the brain stem-spinal cord preparation that exposure to ATP stimulated respiratory output but that CO2-mediated respiratory drive was unaffected by block of P2 receptors (32), suggesting that purinergic signaling can modulate respiratory activity without directly affecting chemosensitivity. At the level of the RTN, we found that purinergic signaling can have opposing effects on excitability of pH-sensitive RTN neurons in the brain slice preparation. For example, activation of P2X receptors, presumably on interneurons, leads to GABAA- or glycine-mediated inhibition of pH-sensitive neurons; conversely, activation of P2Y receptors directly activates pH-sensitive RTN neurons even when glutamatergic transmission was blocked by CNQX (10 μM) and APV (20 μM) (36). As mentioned above, the pH sensitivity of RTN neurons in vitro was retained when P2 receptors were blocked with PPADS (36, 38). These results suggest that CO2/H+-evoked release of ATP can modulate the activity of pH-sensitive neurons and contribute to the integrated output of the RTN (i.e., respiratory drive) during hypercapnia (see Fig. 2, arrows 1–2).
The source of ATP release in the RTN during hypercapnia is poorly understood. In other brain regions, it is well established that activated astrocytes can release excitatory transmitters (e.g., ATP, glutamate) (25). Furthermore, observations first made three decades ago showed that a subset of nonspiking RTN cells are CO2/H+-sensitive (16), suggesting that these pH-sensitive glia could be the source of ATP released during hypercapnia (Fig. 2, arrows 1–3). However, the identity of these pH-sensitive glial cells, the mechanism by which they sense changes in pH, and their potential contribution to respiratory drive remain unknown.
Vascular Control and Central Chemoreception
There is a close relationship between the mechanism of chemoreception and blood flow. The same stimulus that drives breathing (e.g., CO2/H+) also acts as a potent vasodilator to coordinate blood flow with the metabolic needs of tissues (2). The effects of CO2/H+ on blood flow buffers tissue pH and stabilizes chemoreceptor activity during fluctuations in arterial CO2. For example, CO2/H+-mediated vasodilation increases blood flow and accelerates removal of metabolically produced CO2, thereby increasing tissue pH and limiting chemoreceptor activation for a given increase in arterial CO2 (2). The opposite has been shown to occur with a drop in arterial CO2. An additional factor to consider is that changes in blood flow affect tissue O2 levels and so may affect the formation of reactive oxygen species (ROS). This is relevant because ROS can exert direct effects on cerebral vasculature (i.e., vasodilation; Ref. 48), and ROS have been shown to activate putative chemoreceptors in certain brain stem regions (35). These effects of vascular CO2/H+ reactivity on respiration have been demonstrated in several in vivo preparations, including in humans, and it is thought that disruption of this mechanism contributes to breathing instability observed in patients with central sleep apnea (2, 61).
The cellular and molecular mechanisms underlying CO2/H+-mediated cerebral vasodilation are not fully known. It is generally thought that the vascular response to CO2/H+ is an intrinsic property of blood vessels (2). However, astrocytes are also known to regulate blood flow in response to neural activity (25), and evidence suggests that astrocytes in the glia limitans contribute to CO2/H+-mediated vasodilation (62). Astrocytes can release a number of vasodilatory signals, including ATP (21), which can initiate arteriole dilation by activation of P2Y receptors on smooth muscle or endothelial cells (27). Alternatively, ATP can be readily hydrolyzed by ectonucleotidases to adenosine, which has been shown to mediate vasodilation by activation of P1 receptors (63). This latter possibility may contribute to CO2-mediated cerebral vasodilation because inhibition of the A2A subtype of P1 receptors has been shown to attenuate the effects of CO2 on blood flow (50). Therefore, it is possible that CO2/H+-evoked ATP release by pH-sensitive RTN glial cells initiates vasodilation, via P1-receptors, to buffer tissue pH and effectively “fine tune” chemoreceptor activity.
An interesting alternative possibility is that chemosensitive regions regulate blood flow and tissue pH differently from nonchemosensitive regions. It has been shown in brain slices from rat pups <11 days postnatal that putative chemosensitive neurons in the nucleus tractus solitarius and RTN regulate pHi differently from neurons in nonchemosensitive regions; the Na+/H+ exchanger is more sensitive to inhibition by extracellular acidification in neurons from chemosensitive areas than in neurons from nonchemosensitive areas (46, 52). These differences in pHi regulation are thought to reflect the unique function of chemoreceptors as pH sensors (52). By analogy, perhaps hypercapnia causes constriction of vasculature in chemosensitive regions rather than vasodilation that is typically observed in other brain regions (2). This unique vascular response could be mediated by the discrete release of ATP in the RTN. In this case, ATP may mediate capillary vasoconstriction by activation of P2Y-receptors on pericytes (contractile cells that regulate blood flow through capillaries) (49). The effects of purinergic signaling on vascular tone in the RTN have not been determined, and so they are depicted in Fig. 2 as a single arrow (arrow 3) representing vasoconstriction or vasodilation. In addition, recent evidence indicates that Phox2b-expressing neurons directly innervate vascular pericytes (29), implying that chemosensitive neurons may directly regulate capillary diameter (Fig. 2, arrow 4). To understand how vascular control contributes to chemoreception, it will be important for future experiments to determine the effects of CO2/H+ on vascular tone in chemosensitive and nonchemosensitive regions.
CONCLUSIONS
Current evidence supports the possibility that the RTN is an important site of central chemoreception. Despite the apparent intrinsic pH sensitivity of RTN neurons, evidence also suggests that purinergic signaling also contributes to RTN chemoreception (19). Although the source of CO2-evoked ATP release in the RTN has not been determined, these findings raise the interesting possibility that pH-sensitive glia contribute to chemoreception by providing a purinergic drive to pH-sensitive neurons. Purinergic signaling can stimulate activity of pH-sensitive neurons by activation of P2Y receptors or decrease activity of pH-sensitive neurons by activation of P2X receptors on inhibitory interneurons. It is also conceivable that glial cells contribute to the mechanism of chemoreception indirectly by regulating vascular tone. For these reasons, understanding the physiological significance of RTN glial cells will be an important topic in future studies of central chemosensitivity.
GRANTS
This work was supported by a large faculty grant from the University of Connecticut Research Foundation.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Abbott SB, Stornetta RL, Fortuna MG, DePuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 29: 5806–5819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Boucsein C, Kettenmann H, Nolte C. Electrophysiological properties of microglial cells in normal and pathologic rat brain slices. Eur J Neurosci 12: 2049–2058, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cream CL, Li A, Nattie EE. RTN TRH causes prolonged respiratory stimulation. J Appl Physiol 83: 792–799, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105: 1067–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci 29: 14836–14846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erlichman JS, Cook A, Schwab MC, Budd TW, Leiter JC. Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am J Physiol Regul Integr Comp Physiol 286: R289–R302, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A, Leiter JC. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci 28: 4888–4896, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol 85: 1599–1604, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Erlichman JS, Putnam RW, Leiter JC. Glial modulation of CO2 chemosensory excitability in the retrotrapezoid nucleus of rodents. Adv Exp Med Biol 605: 317–321, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Faff L, Ohlemeyer C, Kettenmann H. Intracellular pH regulation in cultured microglial cells from mouse brain. J Neurosci Res 46: 294–304, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity.Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friend WC, Clapoff S, Landry C, Becker LE, O'Hanlon D, Allore RJ, Brown IR, Marks A, Roder J, Dunn RJ. Cell-specific expression of high levels of human S100 beta in transgenic mouse brain is dependent on gene dosage. J Neurosci 12: 4337–4346, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflügers Arch 376: 229–235, 1978 [DOI] [PubMed] [Google Scholar]
- 17. Gipson K, Bordey A. Analysis of the K+ current profile of mature rat oligodendrocytes in situ. J Membr Biol 189: 201–212, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signalling in the medullary mechanisms of respiratory control in the rat: respiratory neurones express the P2X2 receptor subunit. J Physiol 552: 197–211, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Grass D, Pawlowski PG, Hirrlinger J, Papadopoulos N, Richter DW, Kirchhoff F, Hulsmann S. Diversity of functional astroglial properties in the respiratory network. J Neurosci 24: 1358–1365, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci 19: 520–528, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol 168: 59–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86: 1009–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Holleran J, Babbie M, Erlichman JS. Ventilatory effects of impaired glial function in a brain stem chemoreceptor region in the conscious rat. J Appl Physiol 90: 1539–1547, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles. Stroke 34: 1473–1478, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Largo C, Ibarz JM, Herreras O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78: 295–307, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Lazarenko RM, Milner TA, DePuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol 517: 69–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol 87: 910–919, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol 31: 537–549, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro. Respir Physiol Neurobiol 142: 27–42, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci 23: 1750–1758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitchell RA. Respiratory chemosensitivity in the medulla oblongata. J Physiol 202: 3P–4P, 1969 [PubMed] [Google Scholar]
- 35. Mulkey DK, Henderson RA, III, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol 95: 910–921, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci 26: 7230–7233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol 89: 153–162, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci 126–127: 332–338, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nattie E, Shi J, Li A. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat. Respir Physiol 124: 179–193, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10: 9–22, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Nottingham S, Leiter JC, Wages P, Buhay S, Erlichman JS. Developmental changes in intracellular pH regulation in medullary neurons of the rat. Am J Physiol Regul Integr Comp Physiol 281: R1940–R1951, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107: 589–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paravicini TM, Sobey CG. Cerebral vascular effects of reactive oxygen species: recent evidence for a role of NADPH-oxidase. Clin Exp Pharmacol Physiol 30: 855–859, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phillis JW, Lungu CL, Barbu DE, O'Regan MH. Adenosine's role in hypercapnia-evoked cerebral vasodilation in the rat. Neurosci Lett 365: 6–9, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Ritucci NA, Chambers-Kersh L, Dean JB, Putnam RW. Intracellular pH regulation in neurons from chemosensitive and nonchemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 275: R1152–R1163, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289: R851–R861, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23: 11315–11321, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schilling T, Eder C. Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Res 1186: 21–28, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Schlafke ME, Pokorski M, See WR, Prill RK, Loeschcke HH. Chemosensitive neurons on the ventral medullary surface. Bull Physiopathol Respir (Nancy) 11: 277–284, 1975 [PubMed] [Google Scholar]
- 57. Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989 [DOI] [PubMed] [Google Scholar]
- 58. Spyer KM. To breathe or not to breathe? That is the question. Exp Physiol 94: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci 28: 8844–8850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol 106: 850–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of the glia limitans on pial arteriolar relaxation in the rat. Am J Physiol Heart Circ Physiol 287: H331–H339, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol 92: 647–651, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Zhou M, Schools GP, Kimelberg HK. Development of GLAST+ astrocytes and NG2+ glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95: 134–143, 2006 [DOI] [PubMed] [Google Scholar]


