Abstract
The objectives of the present study were to examine the effects of intermittent hypoxia (IH) on arterial baroreflex function and assess the underlying mechanism(s). Experiments were performed on adult male rats treated with 14 days of IH (15 s of hypoxia, 5 min of normoxia; 8 h/day) or normoxia (control). Arterial blood pressures were elevated in IH-treated rats, and this effect was associated with attenuated heart rate and splanchnic sympathetic nerve responses to arterial baroreflex activation. In IH-treated rats, carotid baroreceptor responses to elevated sinus pressures were attenuated. Endothelin-1 (ET-1) levels were elevated in the carotid sinus region of IH-treated rats, and this effect was associated with increased endothelin converting enzyme (ECE) activity, which generates biologically active ET-1. ETA receptor antagonist prevented the effects of IH on carotid baroreceptor activity. In IH-treated rats, reactive oxygen species (ROS) levels were elevated in the carotid sinus region, and antioxidant treatment prevented the effects of IH on ET-1 levels, ECE activity, carotid baroreceptor activity, and baroreflex function. These results demonstrate that 1) IH attenuates arterial baroreflex function, which is in part due to reduced carotid baroreceptor responses to elevated carotid sinus pressure, and 2) IH-induced carotid baroreceptor dysfunction involves reactive oxygen species-dependent upregulation of ET-1 signaling in the carotid sinus region.
Keywords: intermittent hypoxia, baroreflex sensitivity, carotid baroreceptors, reactive oxygen species, heart rate, splanchnic sympathetic nerve activity, blood pressure
sleep-disordered breathing with recurrent apneas is a leading cause of morbidity and mortality in adult humans (25). Recurrent apneas are characterized by transient, repetitive cessations of breathing resulting in periodic decreases in arterial blood O2 levels or intermittent hypoxia (IH). Recurrent apnea patients exhibit abnormalities of the autonomic nervous system including persistent elevation of sympathetic nerve activity (16). Arterial baroreflex is a major regulator of the sympathetic tone. Several studies reported attenuated arterial baroreflex function in recurrent apnea patients (1, 2, 4, 14), and continuous positive airway pressure (CPAP) treatment restored baroreflex function (1). Rats treated with IH also exhibit reduced baroreflex as determined by spectral analysis of heart rate responses to changes in blood pressure (13). The effect of IH on baroreflex regulation of sympathetic nerve activity in rodents has not been determined.
The altered baroreflex function by IH is likely due to its effects on multiple sites in the baroreflex pathway including baroreceptors in the aortic arch and the carotid sinus region, brain stem neurons associated with processing of afferent information from baroreceptor afferents, as well as reactivity of blood vessels. A previous study reported that IH had no significant effect on aortic baroreceptor activity, and the reduced heart rate response to baroreflex activation was attributed in part to the impaired processing of baroreceptor afferent information in the central nervous system (8). However, whether IH affects carotid baroreceptor activity has not been examined. The objectives of the present study were to examine the effects of IH on heart rate and sympathetic nerve responses to baroreflex activation, on carotid baroreceptor afferent nerve activity, and to assess the underlying mechanism(s). Our results showed that IH reduces baroreflex regulation of heart rate as well as sympathetic activity, and these effects were associated with attenuated carotid baroreceptor responses to elevated carotid sinus pressure. Our data further demonstrated that IH-induced changes in carotid baroreceptor function involve reactive oxygen species-dependent upregulation of endothelin-1 (ET-1) signaling.
MATERIALS AND METHODS
Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago. Experiments were performed on adult, male Sprague-Dawley rats weighing between 200 and 300 g.
Exposure to IH
The protocols for IH treatment were essentially the same as previously described (18, 19). Briefly, conscious rats were placed in a specialized chamber flushed with alternating cycles of nitrogen gas and room air. During hypoxia, the inspired O2 level reached a nadir of 5% O2 and was maintained at this level for 15 s. This was followed by room air (21% O2), which was maintained for 5 min. The gas flows were regulated by timer-controlled solenoid valves. Animals were exposed to IH every day between 9:00 AM and 5:00 PM (9 episodes/h; 8 h/day) for 14 days. Ambient O2 and CO2 levels in the chamber were continuously monitored, and CO2 levels were maintained between 0.2 and 0.5%. Blood oxygen saturation levels were determined by small animal pulse oxymeter (MouseQx Plus, Starr Life Sciences). Control experiments were performed on rats exposed to alternating cycles of room air instead of hypoxia. In the experiments where the contribution of ROS was examined, rats were given either manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP; 5 mg·kg−1·day−1 ip; Alexis Biochemicals), a cell permeable antioxidant, or vehicle every day before they were exposed to IH or normoxia.
Measurements of blood pressure and baroreflex regulation of heart rate in conscious rats.
Rats were anesthetized with intraperitoneal administration of a 10:1 mixture of ketamine/xylazine (ketamine, 70 mg/kg body wt; xylazine, 7 mg/kg body wt). The abdomen was exposed, and a biocompatible radio-telemetry probe (Data Science International, Arden Hills, MN) was surgically implanted into the abdominal aorta. A sterile catheter was placed in the external jugular vein for systemic administration of phenylephrine (PE) for assessing baroreflex function of heart rate. Following 1 wk of postoperative recovery, baseline heart rate and arterial pressures and their responses to systemic administration of PE (3 μg/kg body wt iv) were recorded. The dose of PE was chosen from preliminary experiments. The protocols were repeated after 14 days of IH or normoxia (controls). All measurements were made in the same rat before and after exposure to IH such that the same animal served as its own control, thus minimizing the intra-animal variation. The data were analyzed with the Dataquest A.R.T. Version 4.1 (Data Sciences International). The heart rate responses to PE were expressed as ratio of change in heart rate (beats/min) to change in blood pressure (mmHg).
Assessment of Baroreflex Regulation of Sympathetic Nerve Activity
Same rats as above were anesthetized with urethane (1.2 g/kg body wt ip). The core body temperature was maintained at 37 ± 0.5°C by a heating blanket (re-circulating warm water). Rats were mechanically ventilated with room air supplemented with O2. Arterial blood samples were collected to determine the blood gases. Arterial Po2 and Pco2 were maintained at 150 ± 7 and 38 ± 4 Torr, respectively, by adjusting the rate and tidal volume of the respirator. The splanchnic nerve was isolated on the left side by using a retroperitoneal approach, cut above the celiac ganglion, and de-sheathed, and the cut central end was placed on bipolar platinum-iridium electrodes for recording electrical activity as described previously (6). The nerve along with the electrode was covered with warm mineral oil to prevent drying. In all experiments, a Grass amplifier (P511) was used, and the amplifications of nerve signals were set at ×10, 000, and the low- and high-frequency settings were set at 100 and 1,000 Hz, respectively. The analog signals of sympathetic nerve discharges were integrated (Paynter Filter, 50-ms time constant; CWE, Ardmore, PA). The baseline nerve activity was recorded for 20 min while ventilating the rat with room air. The splanchnic nerve activity (SNA) was recorded in response to PE (3 μg/kg body wt iv). At the end of the experiment, hexamethonium (25 mg/kg body wt iv), a ganglion blocking drug, was administered systemically, which caused silencing of SNA activity. Nerve activity, integrated signals, as well as blood pressure signals were stored in a computer via an analog-to-digital translation board (PowerLab/8P; AD Instruments) for further analysis. The hexamethonium-induced inhibition of SNA was considered the “zero” electrical activity, and changes in SNA in response to PE were presented relative to the zero activity.
Measurement of Carotid Baroreceptor Activity
The carotid baroreceptor activity was recorded from an ex vivo carotid sinus “pouch” preparation as described (3). Briefly, carotid bifurcations (common carotid artery and external, internal carotid arteries along with intact sinus nerve) were harvested from anesthetized rats. After cleaning the connective tissue, the common carotid artery was cannulated and connected to a home-made automated device that generated a step increase in the sinus pressure. The internal carotid artery was ligated, and a catheter was placed in the external carotid artery for monitoring carotid sinus pressure using a pressure transducer. The carotid bifurcation was placed in a recording chamber (250-μl volume) and continuously superfused with a medium having the following composition (in mM): 125 NaCl, 5 KCl, 1.8 CaCl2, 2 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, 10 d-glucose, and 5 sucrose maintained at 36°C, and the medium was bubbled continuously with 95% O2-5% CO2. To facilitate recording of clearly identifiable action potentials, the sinus nerve was treated with 0.1% collagenase for 5 min. “Single” unit action potentials were recorded from one of the nerve bundles with a suction electrode and stored in a computer via an analog-to-digital translation board (PowerLab/8P; AD Instruments). The following criteria were employed for identifying baroreceptor activity: 1) increased action potential frequency in response to step increase in sinus pressure and 2) no change in action potential frequency in response to stagnant hypoxia induced by stopping the superfusion. Baseline carotid sinus pressure was set at 20 mmHg in all experiments. Carotid sinus pressure was increased at 20-mmHg steps from 20 to 200 mmHg, and each pressure step was maintained for 20 s. In the experiments assessing the role of ET-1, endothelin receptor antagonists (ETA and ETB) were added to the reservoir containing the medium used for irrigating the carotid sinus preparation.
Analysis of baroreceptor activity.
Response of the “single” carotid baroreceptor unit discharge (impulses/s) to increase in sinus pressure was analyzed by the following equation: Y = P1 + (P2 − P1)/[1 + eP3(P4 − X)], where Y is the carotid baroreceptor activity at a given pressure X, P1 is the lower plateau of Y, P2 is the upper plateau of Y, P3 is the slope parameter, P4 is the pressure at which Y is halfway between P1 and P2, and X is the sinus pressure. The threshold pressure Pth (P1) was defined as the pressure at which the baroreceptor begins to show a significant increase in pressure-related discharge based on the shape of the entire pressure-response curve. Furthermore, the operating pressure (Poper) shown in Fig. 4H represents a range defined as the pressure over which baroreceptors could effectively modulate their discharge rate in response to a change in pressure, i.e., Poper = P2 − P1 (27).
Fig. 4.
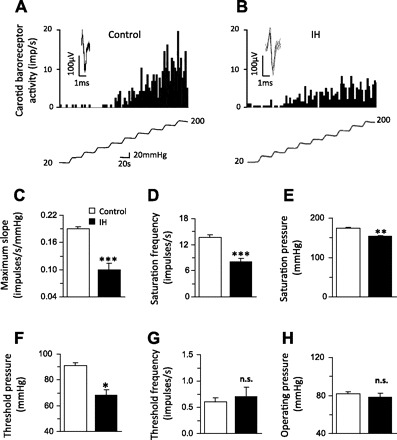
IH attenuates carotid baroreceptor responses to increased carotid sinus pressures. Examples of carotid baroreceptor response to step increase in carotid sinus pressure (20-mmHg steps) in a control (A) and IH-treated (B) rat. Bottom in A and B represents step increase in intra-carotid sinus pressure. Superimposed action potentials of a single fiber from which the data were derived are shown in the inset of A and B. C–H: average data of functional parameters of carotid baroreceptor activity in control and IH-treated rats. Data are means ± SE; n = 18 baroreceptors from 6 rats each in control and IH. Significant difference compared with control: *P < 0.05; **P < 0.01; ***P < 0.001; n.s. denotes P > 0.05 compared with control.
Immunocytochemistry
Anesthetized rats (urethane; 1.2 g/kg body wt) were perfused transcardially with 4% paraformaldehyde. The carotid bifurcations were dissected and immersed in 25% sucrose solution in distilled water at 4°C for 16–24 h. The tissues were mounted in OCT compound, and 8- to 10-μm-thick sagittal sections were cut and processed for immunofluorescence. The tissue sections were treated with 20% normal goat serum (NGS) and 0.2% Triton X-100 in PBS for 2 h, followed by incubation with polyclonal ET-1 primary antibody (1:200 dilution; Peninsula Laboratories) in PBS with 1% NGS and 0.2% Triton X-100. Immunostained regions were visualized with Alexa Red (Molecular Probes) fluorescently labeled secondary antibodies. To assess the specificity of endothelial staining, the sections were co-stained with 1:20 dilution of biotinylated Griffonia simplicifolia isolectin B4 (Vector Laboratories), which selectively binds to endothelial cells (11). Signal from the bound isolectin was detected using avidin conjugated to FITC probe.
Measurement of ET-1 Content by Enzyme Immunoassay
Carotid sinus regions were dissected under dissecting microscope and homogenized in 10 volume of a mixture of 1 M acetic acid and 20 mM HCl. The homogenate was boiled at 100°C and centrifuged at 13,000 g for 10 min at 4°C. The supernatant was removed and stored at −80°C until further analysis. ET-1 levels were determined with a commercially available ET-1 enzyme immunoassay (EIA) kit (Assay Designs) following the manufacturer's instructions. All measurements were performed in duplicate. The detection limit of ET-1 by EIA was 0.41 pg/ml. ET-1 levels were expressed as picograms of ET-1 per milligram of protein. The protein content was determined by Bio-Rad DC protein assay using bovine serum albumin as the standard.
Measurements of Pre-Pro ET-1 and ET-1 Receptor mRNAs
Carotid sinus regions were dissected and homogenized, and RNA was extracted using TRIZOL (Invitrogen) according to the manufacture's instructions. For quantitative real-time PCR analysis, 1 μl of RNA was reverse transcribed using superscript III reverse transcriptase (Invitrogen). Primer sequences for real-time RT-PCR amplification were as follows: pre-pro ET-1 (133 bp), forward CCGAGCCCAAAGTACCATGC and reverse GCTGATGGCCTCCAACCTTC; ETA receptor (119 bp), forward CTTCTGCATGCCCTTGGTGT and reverse CTCGACGCTGCTTGAGGTGT; ETB receptor (117 bp), forward AAGTCGTGTTTGTGCTGCTGGTG and reverse GCTGGAGCGGAAGTTGTCGT; and 18S rRNA (151 bp), forward GTAACCCGTTGAACCCCATT and reverse CCATCCAATCGGTAGTAGCG. Real-time PCR was carried out using a MiniOpticon system (Bio-Rad Laboratories, Hercules, CA) with SYBR green as a fluorogenic binding dye (Invitrogen). The reaction mixtures were incubated at 50°C for 2 min (action of uracil DNA glycosylase) then at 95°C for 8 min and 30 s (uracil DNA glycosylase inactivation and DNA polymerase activation), followed by 40 two-step cycles of 15 s at 95°C (first step) and 1 min at 60°C (second step). The products were analyzed by Opticon Monitor software, using a standard curve. The values were normalized to its 18S rRNA. The relative expression was determined by the ΔΔCt method where, first, the level of gene of interest (GOI) is normalized to a housekeeping gene (HKG) 2−ΔCt = 2−[Ct(GOI) − Ct(HKG)], and fold change in gene expression was determined by 2−ΔΔCt = 2−[ΔCt(+/+) − ΔCt (+/−)]. The purity and specificity of all products were confirmed by omitting the template and by appropriate size and single melting temperature.
Measurement of Endothelin-Converting Enzyme Activity
Tissues (carotid sinus region and control regions) were homogenized in 100 μl of 50 mM HEPES buffer, pH 7.4, containing 100 mM NaCl and 20 mM CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) and incubated at 4°C overnight. The extract was centrifuged at 18,000 g for 30 min at 4°C, and the supernatant was used for endothelin-converting enzyme (ECE) activity assay. ECE activity was determined using the procedure as described previously (35) with few modifications. Briefly, tissue extract, which is equivalent to 1–2 μg of protein, was incubated with 100 pmol of pre-pro ET-1, 50 mM HEPES buffer, pH 7.4 containing 100 mM NaCl and 20 mM CHAPS at 37°C for 4 h. At the end of incubation, the reaction was stopped by the addition of phosphoramidon (10 μM). In parallel experiments, the same amount of tissue extract was pre-incubated with phosphoramidon (10 μM) for 15 min before the addition of pre-pro ET-1. The amount of ET-1 formed in both reactions was determined using an ET-1 assay kit (Assay Designs, Ann Arbor, MI) following the manufacturer's instructions. ECE activity was expressed as the amount of phosphoramidon-inhibitable ET-1 formed per milligram of protein per hour.
Measurement of Malondialdehyde Levels
Carotid bifurcation along with the carotid artery was harvested from anesthetized rats. Carotid sinus region and nearby common carotid artery (control) were dissected under a dissecting microscope. Tissues were frozen in liquid nitrogen and stored at −80°C until further analysis. Malondialdehyde (MDA) levels were determined as described previously (22) with a few modifications. Briefly, tissues were homogenized in 10 volumes of 20 mM phosphate buffer (pH 7.4) at 4°C. Either an aliquot of the sample (12.5 μl) or MDA standard was added to 8.1% (wt/vol) SDS (6.25 μl), 20% (vol/vol) acetic acid (47 μl), and 0.8% (wt/vol) thio-barbituric acid (47 μl). The samples were heated for 60 min, cooled, and centrifuged at 3,000 g for 15 min. The fluorescence intensity of the supernatant was determined using excitation and emission wavelengths of 530 and 550 nm, respectively. The results were expressed as nanomoles of MDA formed per milligram of protein.
Data Analysis
All data are presented as means ± SE. Statistical significance was assessed by two-way ANOVA with repeated measures followed by Tukey's test and unpaired t-test when appropriate. P values of <0.05 were considered significant.
RESULTS
Effects of IH on Blood Pressure and Heart Rate Response to Baroreflex Activation in Conscious Rats
An example of ambient O2 and arterial blood O2 saturations during exposure to IH in a conscious rat is shown in Fig. 1A. During each episode of hypoxia, ambient O2 levels decreased from 20 to ∼5% and blood O2 saturations fell from 97 to ∼80%. Following 14 days of IH exposure, mean blood pressures (MBP) were elevated on average by 11 ± 1.7 mmHg (range of 4–20 mmHg; P < 0.01; Fig. 1B). The increase in MBP was due to significant increases in both systolic as well as diastolic blood pressures (P < 0.01; data not shown).
Fig. 1.
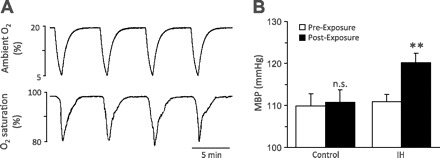
Intermittent hypoxia (IH) elevates arterial blood pressure in rats. A: example illustrating ambient oxygen (top) and arterial blood oxygen saturation (bottom) levels in an unrestrained, conscious rat during exposure to IH. B: average data of mean blood pressure (MBP) in rats treated with either normoxia (control) or IH for 14 days. Values are means ± SE from control (n = 6) and IH (n = 10) rats. **Denotes P < 0.01; n.s., denotes P > 0.05 compared with MBP measured before exposures to either normoxia or IH for 14 days.
Previous studies reported that anesthetics depress baroreflex responses (4, 26). Therefore, heart rate response to baroreflex activation by PE-induced increase in blood pressure was determined in conscious rats by telemetry. Figure 2A illustrates heart rate responses to PE in the same rat before (left) and after IH treatment (right). Before IH, PE evoked marked bradycardia, and this response was attenuated after IH. Average data showed a significant reduction in heart rate response to baroreflex activation following IH treatment (P < 0.01; Fig. 2B). Analysis of heart rate responses to baroreflex activation vs. changes in blood pressure in IH-treated rats showed a significant correlation (r2 = 0.6; P < 0.01; Fig. 2C).
Fig. 2.
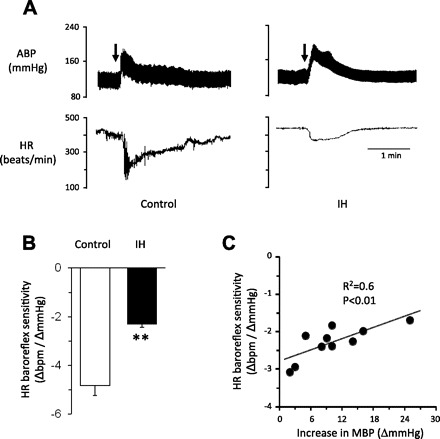
IH decreases heart rate responses to arterial baroreflex activation. A: examples of heart rate (HR) responses to phenylephrine (PE; 3 μg/kg body wt; I.V. at arrows)-induced increase in blood pressure in a conscious rat before (left) and after 14 days of IH (right). B: average data (means ± SE) of heart rate responses to baroreflex activation (n = 10 rats). C: correlation of heart rate responses to baroreflex activation and changes in blood pressure in IH-treated rats. Values are means ± SE from 10 rats. **Significant difference compared with control (P < 0.01).
Effect of IH on Sympathetic Nerve Responses to Baroreflex Activation in Anesthetized Rats
Splanchnic nerve activity (SNA) responses to baroreflex activation were determined in anesthetized, paralyzed, and mechanically ventilated rats (same rats as above). Anesthetized preparation was chosen for these experiments because measurement of SNA in conscious rats was technically difficult. Control experiments were performed on age-matched rats treated with normoxia. Figure 3A illustrates examples of SNA response to PE (3 μg/kg body wt iv) in a control (left) and an IH-treated rat (right). In the control rat, systemic administration of PE inhibited SNA, and this response was attenuated in the IH-treated rat. Average data showed significantly reduced PE-induced SNA inhibition in IH-treated rats compared with control rats (P < 0.01; Fig. 3B).
Fig. 3.
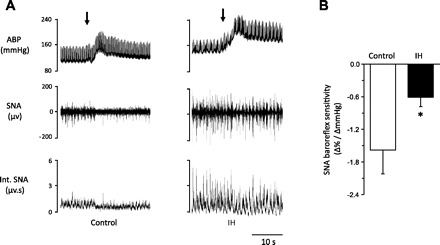
Effects of IH on sympathetic nerve response to arterial baroreflex activation. A: examples of splanchnic sympathetic nerve activity (SNA) response to phenylephrine-(PE; 3 μg/kg body wt; I.V. at arrows)-induced increase in blood pressure in a control (left) and IH-treated rat (right). B: average data (means ± SEM) of changes in SNA baroreflex sensitivity. Values are means ± SE from control (n = 6) and IH (n = 6) rats. ABP, arterial blood pressure; SNA, splanchnic nerve activity; Int. SNA, integrated splanchnic nerve activity. *Significant difference compared with control (P < 0.05).
Effect of IH on Carotid Baroreceptor Activity
The following experiments were performed to determine the effects of IH on carotid baroreceptor activity. Since basal blood pressures were elevated in IH-treated rats, an ex vivo rat carotid sinus preparation was used, which allowed us to set the initial carotid sinus pressure to the same level in all preparations. Figure 4, A and B, illustrates examples of “single unit” baroreceptor response to step increase in carotid sinus pressure in a control and an IH-treated rat, respectively. Baroreceptor activity increased progressively in response to step increases in sinus pressure in both control and IH-treated preparations. However, in IH-treated preparations, the magnitude of baroreceptor activation at higher sinus pressures was attenuated (Fig. 4, A and B). Responses of individual baroreceptor fibers were qualitatively the same in all preparations. Average data showed that the maximum slope of the stimulus-response, saturation frequency, saturation, and threshold pressures were significantly reduced in IH-treated preparations (Fig. 4, C–F), whereas threshold frequencies and operating pressure ranges were comparable to controls (Fig. 4, G and H).
ET-1 Mediates the Effects of IH on Carotid Baroreceptor Activity
Previous studies have shown that carotid baroreceptor activity can be modulated by a variety of vasoactive agents (9), including ET-1 (5). Since IH upregulates ET-1 expression in the nearby carotid chemoreceptors (23), its role in IH-induced decrease in carotid baroreceptor activity was determined. ET-1 levels were elevated by ∼2.5-fold in the carotid sinus region of IH-treated rats (P < 0.01; Fig. 5A). In the common carotid artery, ET-1 levels were below the detection limit of EIA in both the control and the IH-treated rats. The increased ET-1 expression in the carotid sinus region was localized to endothelial cells, as evidenced by co-localization of ET-1-like immunoreactivity with isolectin, an established marker of endothelial cells (Fig. 5B) (7).
Fig. 5.
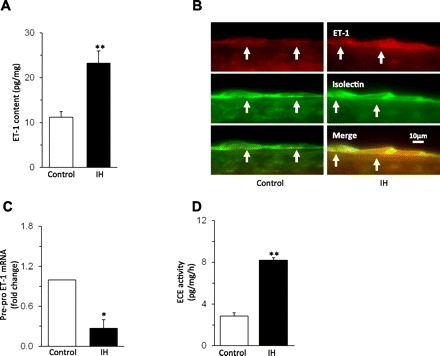
Effect of IH on endothelin-1 (ET-1) expression in the carotid sinus region. A: average data of ET-1 content measured by enzyme immunoassay (EIA) in the carotid sinus region from control and IH-exposed rats. B: example illustrating the immunocytochemical localization of ET-1-like immunoreactivity and isolectin labeling of endothelial cells in the carotid sinus region (at arrows) from control (left) and IH-treated (right) rat. C: effect of IH on pre-pro ET-1 mRNA levels in the carotid sinus region. D: IH increased endothelin converting enzyme (ECE) activity in the carotid sinus region. Data are means ± SE from control and IH rats; n = 6 rats in each group. Significant difference compared with normoxia-treated rats (control): *P < 0.05; **P < 0.01.
The elevated ET-1 levels may be due to either activation of pre-pro ET-1 mRNA and/or increased processing of biologically active ET-1 from its precursor, pre-pro ET-1, by ECE. To assess these possibilities, pre-pro ET-1 mRNA and ECE activity were determined. Pre-pro ET-1 mRNA was downregulated (Fig. 5C), and ECE activity increased by approximately threefold in the carotid sinus region of IH-treated rats (Fig. 5D).
To assess whether ET-1 mediates the effects of IH, carotid baroreceptor activity was determined in the presence of ETA and ETB receptor antagonists. In control preparations, 1 μM BQ-610, an ETA receptor antagonist, had no significant effect on carotid baroreceptor responses to increased sinus pressures (P > 0.05; Fig. 6, A and C–F), whereas it completely prevented the effects of IH on baroreceptor responses to increased sinus pressure (Fig. 6, B and C–F). On the other hand, 1 μM BQ-788, an ETB receptor antagonist, depressed baroreceptor response to increases in sinus pressure in two of the IH-treated preparations, and it had no effect in the remaining four preparations. Average data showed that BQ-788 had no significant effect on carotid baroreceptor responses to increases in sinus pressure in IH-treated preparations (P > 0.05).
Fig. 6.
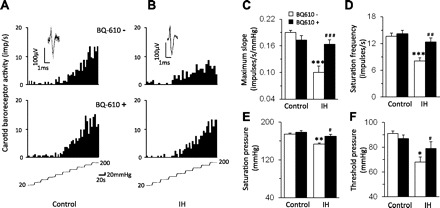
ETA receptor antagonist prevents the effects of IH on carotid baroreceptor activity. A and B: examples of carotid baroreceptor response to increase in carotid sinus pressure in the presence of vehicle (top) and BQ-610 (1 μM), an ETA receptor antagonist (bottom) in a control (left) and IH-treated (right) rat. The intra-carotid pressure is shown at bottom of A and B. C–F: average data of functional parameters of carotid baroreceptors in presence of vehicle and BQ-610 in control and IH-treated rats. Data are means ± SE; n = 16–18 baroreceptors from control and IH-treated rats (n = 6 rats in each group). Significant difference compared with controls: *P < 0.05; **P < 0.01; ***P < 0.001. Significant difference compared with IH-treated rats: #P < 0.05; ##P < 0.01; ###P < 0.001.
Reactive Oxygen Species (ROS) Mediate Upregulation of ET-1 by IH
Previous studies reported that reactive oxygen species (ROS) mediates systemic and cellular responses to IH (20, 30, 32, 37). The role of ROS signaling in IH-induced ET-1 upregulation was determined. MDA levels were measured as an index of ROS. MDA levels increased by approximately threefold in the carotid sinus region but not in the near by carotid artery of IH-treated rats, and this effect was prevented by MnTMPyP, an antioxidant (Fig. 7A). MnTMPyP treatment prevented the effects of IH on ET-1 levels and ECE activity in the carotid sinus region (Fig. 7, B–D). In the carotid sinus region from control rats, MnTMPyP had no effect on ET-1 levels (control = 12 ± 2 pg/mg, and control + MnTMPyP = 13 ± 4 pg/mg; P > 0.05) and ECE activity (control = 3.1 ± 0.3 pg·mg−1·h−1, and control + MnTMPyP = 2.9 ± 0.4 pg·mg−1·h−1; P > 0.05). Furthermore, MnTMPyP prevented the effects of IH on carotid baroreceptor activity and heart rate as well as SNA responses to baroreflex activation (Fig. 8, E–G).
Fig. 7.
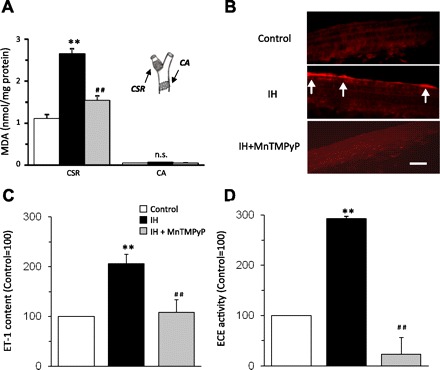
Reactive oxygen species (ROS) mediate upregulation of ET-1 in the carotid sinus region by IH. A: malondialdehyde (MDA) levels, an index of ROS, were determined in the carotid sinus region (CSR) and common carotid artery (CA). IH increased ROS levels in the CSR but not in the CA, and this response was prevented by MnTMPyP, an antioxidant. Inset: the CSR and CA regions from which the data were derived. B: IH increased ET-1-like immunoreactivity in the carotid sinus region, and MnTMPyP treatment abolished this response. The scale bar represents 10 μm. C and D: MnTMPyP prevents the effects of IH on ET-1 (C) and ECE activity in the carotid sinus region (D). Data are means ± SE from control and IH-treated rats (n = 6 in each group). **Significant difference compared with control tissues (P < 0.01). ##Significant difference compared with IH-treated tissues (P < 0.01).
Fig. 8.
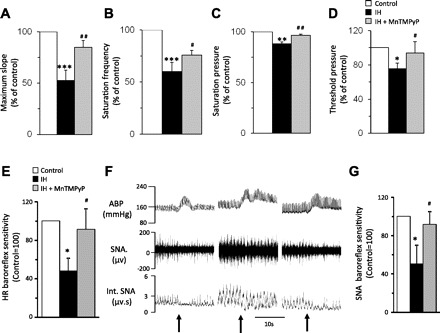
Effects of antioxidant treatment on carotid baroreceptor activity and baroreflex function in IH-treated rats. A–D: MnTMPyP treatment prevents the effects of IH on carotid baroreceptor activity. E: effect of MnTMPyP on heart rate responses to baroreflex activation in IH-treated rats. F: examples of splanchnic nerve activity (SNA) responses to phenylephrine (PE; 3 μg/kg body wt iv at arrows)-induced increase in blood pressure in control (left), IH (middle)-, and IH + MnTMPyP (right)-treated rats. G: average data (means ± SE) of SNA responses to PE (3 μg/kg body wt iv; control = 100%) in control, IH-, and IH + MnTMPyP-treated rats. Data are means ± SE; n = 6 rats in each group. ABP, arterial blood pressure; Int. SNA, integrated SNA. Signficant difference compared with control: *, **, and *** denote *P < 0.05; **P < 0.01; **P < 0.001. Significant difference compared with IH-treated preparations: #P < 0.05; ##P < 0.01.
DISCUSSION
The present study examined the impact of IH on arterial baroreflex and assessed the underlying mechanisms. Our results showed that arterial baroreflex is attenuated in IH-treated rats, as evidenced by reduced heart rate responses to baroreflex activation, a finding consistent with earlier studies on rats (8, 13), and further demonstrate that IH attenuates baroreflex regulation of SNA. Baroreflex response to increase vs. decrease in blood pressure shows hysteresis in humans (31). Since baroreflex function was assessed by analyzing the response only to increases but not to decreases in blood pressure in the present study, it could be argued that we have overestimated the changes in baroreflex function. However, studies on rats have shown that both pressor and depressor trials reflect similar trends in baroreflex sensitivity (15, 33, 36). Although the effects of depressor trials on baroreflex sensitivity in IH-treated rats need to be established in future studies, present results demonstrate that IH reduces baroreflex responses of heart rate as well as SNA. Consistent with other studies, we also found elevated blood pressures in IH-treated rats. The magnitude of blood pressure changes correlated with changes in baroreflex function (Fig. 2C). The depressed baroreflex might be the consequence of changes in blood pressure.
It is well known that activation of baroreceptors in the carotid sinus and aortic arch trigger the baroreflex. Present results demonstrate that IH impairs carotid baroreceptor function. Although we realize that it is ideal to assess the baroreceptor function in intact rats with pulsatile blood flow, our studies were performed on an ex vivo carotid sinus preparation due to elevated basal blood pressures in IH-treated rats that could confound interpretation of results derived from intact rats. Despite this limitation, ex vivo carotid sinus preparation has the following advantages: 1) it allowed us to set the initial pressures in the sinus to the same level in control and IH-treated rats and 2) by controlling sinus pressures, it facilitated the analysis of various parameters of baroreceptor function, including the slope of the response, saturation and threshold pressures, etc. It is known that carotid baroreceptors are comprised of both medullated and non-medullated fibers, and their sensitivities and adaptive properties to a given sinus pressure differ considerably (10, 27). Although we monitored “single” unit baroreceptor afferent nerve activity, because of the short length of the sinus nerve, we could not measure the conduction velocities to classify them either as medullated or non-medullated fibers, which is a limitation of this study. A previous study reported that IH had no significant impact on aortic baroreceptor activity (8). Therefore, present results suggest that impaired carotid baroreceptor function contributes in part to the attenuated arterial baroreflex in IH-treated rats in addition to the recently reported effects of IH on central baroreflex regulation (29). The contribution of depressed baroreflex to IH-induced hypertension, however, remains to be established.
How might IH affect carotid baroreceptor function? It is known that a variety of vasoactive hormones modulate barorecetor activity (9). The following observations implicate a role for ET-1, a potent vasoconstrictor peptide in mediating the effects of IH on carotid baroreceptor activity. These include that 1) ET-1 expression was elevated in the carotid sinus region and 2) ETA receptor antagonist prevented the effects of IH on carotid baroreceptor activity. A role for ET-1 in IH-induced reduced carotid baroreceptor activity is further supported by an earlier report showing that exogenous application of ET-1 attenuates carotid baroreceptor function in dogs (3). Interestingly, the increased ET-1 expression was confined to endothelial cells in the carotid sinus region, as evidenced by co-localization of ET-1-like immunoreactivity with isolectin, an established marker of this cell type (7). Interestingly, IH-induced upregulation of ET-1 was confined to the endothelial cells in the carotid sinus region, and this effect was not seen in the nearby carotid artery. The absence of detectable levels of ET-1 in the common carotid artery is consistent with a study by Wang et al. (34), who reported very low levels of pre-pro ET-1 and ECE mRNAs and no discernable ET-1-like immunoreactivity in the common carotid artery. It follows that the effects of IH on endothelial cells are not uniform among the blood vessels. Such a possibility is further supported by reported differences in the responses of endothelial cells to a given stimulus among various blood vessels (5). It is likely that ET-1 released from the endothelial cells activates ETA receptors and changes vascular compliance, which in turn reduces the baroreceptor responses to increased sinus pressures. Alternatively, ET-1 may directly affect the excitability of the baroreceptor afferent nerves. Further studies, however, are needed to assess these possibilities.
Since our results implicate ET-1 in mediating the effects of IH on carotid baroreceptor activity, we analyzed the mechanisms underlying the upregulation of ET-1 by IH. It is unlikely that enhanced transcription would account for IH-induced upregulation of ET-1 because pre-pro ET-1 mRNA levels decreased in the carotid sinus region from IH-treated rats. On the other hand, our data suggest that posttranslational mechanisms involving activation of ECE resulting in increased processing of ET-1 from its precursor contribute to elevated ET-1 levels by IH in the carotid sinus region. How might IH activate ECE? The following findings suggest a role for ROS in mediating the effects of IH on ECE. First, ROS levels were elevated in IH-treated carotid sinus region as evidenced by increased MDA levels. Second, antioxidant treatment prevented the effects of IH on MDA levels, ECE activity, and ET-1 expression. Previous studies identified NADPH oxidases (Nox) and decreased mitochondrial complex I activity as major sources of ROS generation under the setting of IH (12, 18, 30, 37), which may contribute to the enhanced ROS production in the carotid sinus region of IH-treated rats. Nonetheless, the finding that antioxidant treatment prevented the effects of IH on carotid baroreceptor activity and baroreflex function suggests that ROS-dependent recruitment of ET-1 signaling contributes in part to the attenuated baroreflex by reducing carotid baroreceptor response to elevated sinus pressures. In addition, the effects of MnTMPyP might involve central neurons associated with regulation of baroreflex function since it can enter the brain (21, 28).
The findings of the present study might be of relevance in further understanding regulation of sympathetic tone under the setting of IH in experimental models and in humans experiencing IH as a consequence of recurrent apneas. Increased sympathetic activity is well documented in recurrent apnea patients (16) and in IH-treated rodents (Fig. 3; Refs. 13, 19). Arterial baro- and chemoreflexes contribute to the regulation of sympathetic tone, wherein baroreflex inhibits and chemoreflex facilitates the sympathetic tone. Under the setting of IH, ROS-dependent recruitment of ET-1 peptide leads to enhanced arterial chemoreflex by sensitizing the carotid chemoreceptors as shown previously (17, 24), whereas ET-1, by attenuating the carotid baroreceptor activity, contributes to the attenuated baroreflex function. The imbalance between the chemo- and baroreflexes might account in part to the persistent elevation of sympathetic activity under the conditions of IH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-76537 and HL-90554.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.-J. Peng, J. Nanduri, X. Zhang, N. Wang, and G. Raghuraman performed experiments; Y.-J. Peng, J. Nanduri, and G. K. Kumar analyzed data; Y.-J. Peng prepared figures; J. Nanduri, J. Seagard, G. K. Kumar, and N. R. Prabhakar interpreted results of experiments; N. R. Prabhakar conception and design of research; N. R. Prabhakar edited and revised manuscript; N. R. Prabhakar approved final version of manuscript.
REFERENCES
- 1. Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S, Di Rienzo M, Mancia G, Bonsignore G. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med 166: 279–286, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154: 1490–1496, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Chapleau MW, Hajduczok G, Abboud FM. Suppression of baroreceptor discharge by endothelin at high carotid sinus pressure. Am J Physiol Regul Integr Comp Physiol 263: R103–R108, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Coleman TG. Arterial baroreflex control of heart rate in the conscious rat. Am J Physiol Heart Circ Physiol 238: H515–H520, 1980 [DOI] [PubMed] [Google Scholar]
- 5. Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res 55: 65–76, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Ernst C, Christie BR. Isolectin-IB 4 as a vascular stain for the study of adult neurogenesis. J Neurosci Methods 150: 138–142, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster R, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 293: H2809–H2818, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Holmes AE, Ledsome JR. Effect of norepinephrine and vasopressin on carotid sinus baroreceptor activity in the anesthetized rabbit. Experientia 40: 825–827, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Jones JV, Thoren PN. Characteristics of aortic baroreceptors with non-medullated afferents arising from the aortic arch of rabbits with chronic renovascular hypertension. Acta Physiol Scand 101: 286–293, 1977 [DOI] [PubMed] [Google Scholar]
- 11. Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93: 1074–1081, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol 100: 1974–1982, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 574: 605–613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mostarda C, Moraes-Silva IC, Moreira ED, Medeiros A, Piratello AC, Consolim-Colombo FM, Caldini EG, Brum PC, Krieger EM, Irigoyen MC. Baroreflex sensitivity impairment is associated with cardiac diastolic dysfunction in rats. J Card Fail 17: 519–525, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 177: 385–390, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R735–R742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal 9: 1397–1403, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Raghuraman G, Rai V, Peng YJ, Prabhakar NR, Kumar GK. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid Redox Signal 11: 1777–1789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem 93: 47–52, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rey S, Corthorn J, Chacon C, Iturriaga R. Expression and immunolocalization of endothelin peptides and its receptors, ETA and ETB, in the carotid body exposed to chronic intermittent hypoxia. J Histochem Cytochem 55: 167–174, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res 1086: 152–159, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Roth T. An overview of the report of the national commission on sleep disorders research. Eur Psychiatry 10, Suppl 3: 109s–113s, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Seagard JL, Elegbe EO, Hopp FA, Bosnjak ZJ, von Colditz JH, Kalbfleisch JH, Kampine JP. Effects of isoflurane on the baroreceptor reflex. Anesthesiology 59: 511–520, 1983 [DOI] [PubMed] [Google Scholar]
- 27. Seagard JL, van Brederode JF, Dean C, Hopp FA, Gallenberg LA, Kampine JP. Firing characteristics of single-fiber carotid sinus baroreceptors. Circ Res 66: 1499–1509, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Sharma SD, Raghuraman G, Lee MS, Prabhakar NR, Kumar GK. Intermittent hypoxia activates peptidylglycine alpha-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing. J Appl Physiol 106: 12–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 589: 1463–1476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci 30: 10763–10772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 587: 2049–2057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol 293: H2971–H2976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valenti VE, Abreu LC, Ferreira C. Sidestream cigarette smoke exposure effects on baroreflex in adult rats. Arq Bras Cardiol 96: 148–153, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Douglas SA, Louden C, Vickery-Clark LM, Feuerstein GZ, Ohlstein EH. Expression of endothelin-1, endothelin-3, endothelin-converting enzyme-1, and endothelin-A and endothelin-B receptor mRNA after angioplasty-induced neointimal formation in the rat. Circ Res 78: 322–328, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Warner TD, Mitchell JA, D'Orleans-Juste P, Ishii K, Forstermann U, Murad F. Characterization of endothelin-converting enzyme from endothelial cells and rat brain: detection of the formation of biologically active endothelin-1 by rapid bioassay. Mol Pharmacol 41: 399–403, 1992 [PubMed] [Google Scholar]
- 36. Xie F, Sun C, Sun LH, Li JY, Chen X, Che H, Lu GY, Yang BF, Ai J. Influence of fluvastatin on cardiac function and baroreflex sensitivity in diabetic rats. Acta Pharmacol Sin 32: 321–328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuan G, Adhikary G, McCormick AA, Holcroft JJ, Kumar GK, Prabhakar NR. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol 557: 773–783, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]


