Abstract
Previous studies have shown that pharmacological inhibition of the phosphoinositol-3 (PI3) kinase disrupts the activation of mast cells. Through phosphoinositide-dependent kinase PDK1, PI3 kinase activates the serum- and glucocorticoid-inducible kinase 3 (SGK3). The present study explored the role of SGK3 in mast cell function. Mast cells were isolated and cultured from bone marrow (BMMCs) of gene-targeted mice lacking SGK3 (sgk3−/−) and their wild-type littermates (sgk3+/+). BMMC numbers in the ear conch were similar in both genotypes. Stimulation with IgE and cognate antigen triggered the release of intracellular Ca2+ and entry of extracellular Ca2+. Influx of extracellular Ca2+ but not Ca2+ release from intracellular stores was significantly blunted in sgk3−/− BMMCs compared with sgk3+/+ BMMCs. Antigen stimulation further led to a rapid increase of a K+-selective conductance in sgk3+/+ BMMCs, an effect again blunted in sgk3−/− BMMCs. In contrast, the Ca2+ ionophore ionomycin activated K+ currents to a similar extent in sgk3−/− and in sgk3+/+ BMMCs. β-Hexosaminidase release, triggered by antigen stimulation, was also significantly decreased in sgk3−/− BMMCs. IgE-dependent anaphylaxis measured as a sharp decrease in body temperature upon injection of DNP-HSA antigen was again significantly blunted in sgk3−/− compared with sgk3+/+ mice. Serum histamine levels measured 30 min after induction of an anaphylactic reaction were significantly lower in sgk3−/− than in sgk3+/+ mice. In conclusion, both in vitro and in vivo function of BMMCs are impaired in gene targeted mice lacking SGK3. Thus SGK3 is critical for proper mast cell function.
Keywords: allergy, anaphylaxis, calcium ion channels, degranulation, potassium ion channels, Kca3.1, phosphoinositol-3 kinase
mast cells play a decisive role in IgE-dependent allergic reactions (45), including allergic rhinitis (65), asthma (18), anaphylactic shock, and delayed hypersensitivity reactions (5, 35, 78). Through cytokine release they regulate the function of other inflammatory cells, such as neutrophils and T cells (9, 35, 36, 59).
Mast cell function is governed by the activity of Ca2+ channels (20, 22, 25, 28, 29, 44, 57, 72), K+ channels (19, 20, 27, 55), and Cl− channels (28, 29).
Mast cell ion channel activity (49) and function (2, 4, 38, 81) are dependent on phosphoinositol-3 (PI3) kinase. PI3 kinase-dependent signaling involves 3-phosphoinositide (PIP3)-dependent kinase PDK1, which phosphorylates and thus activates the protein kinase B (PKB) and serum- and glucocorticoid- inducible kinase (SGK) isoforms (1, 10, 47, 64). Recently, SGK1 has been demonstrated to play a critical role in mast cell activation (71).
The serum- and glucocorticoid-inducible kinase isoform SGK3 has originally been identified as a gene closely related to the serum- and glucocorticoid-inducible kinase SGK1 (48). SGK3 shares 80% of the amino acid sequence identity in its catalytic domain with SGK1 (48). SGK3 is identical to the cytokine-independent survival kinase CISK (52). The “SGK-like” gene (SGKL) in chromosome 8q12.3 (24) encodes a protein whose predicted amino acid sequence is virtually identical to that of human SGK3. SGK3 has been shown to be expressed in all tissues tested thus far (48). Unlike SGK1 (33, 50), SGK3 is not under transcriptional control of serum and glucocorticoids (50). While SGK1 is upregulated by cell stress and a wide variety of hormones and mediators, it is particularly important during stress conditions. SGK3, on the other hand, is constitutively expressed and serves basal functions (50). For instance, radiation leads to marked upregulation of SGK1 transcription (70) but decreases SGK3 transcript levels (76).
Neither knockout of SGK3 alone (58) nor of both SGK1 and SGK3 (39) leads to a severe phenotype, even though SGK1 (50, 70) and SGK3 (50, 79, 82) participate in the maintenance of cell survival. SGK3 further participates in the regulation of a variety of carriers and ion channels (50). Gene-targeted mice lacking functional SGK3 have a strikingly delayed hair growth (23, 56, 58, 60), which has been attributed to apoptosis of keratinocytes (3). Interestingly, SGK3 and Akt2 appear to have partially redundant roles in that the hair loss phenotype of mice deficient in both is markedly augmented than in mice lacking either one (56). Moreover, SGK3-deficient mice show decreased basal intestinal glucose transport (66) and a subtle decrease of locomotion (50).
In the present study, experiments were performed on gene-targeted mice lacking SGK3 (sgk3−/−) and their wild-type littermates (sgk3+/+) to elucidate whether mast cell ion channel regulation and degranulation involves SGK3.
MATERIALS AND METHODS
Mice.
All animal experiments were conducted according to the German law for the welfare of animals and were approved by local authorities.
The targeting strategy for disruption of the Sgk3 gene has been described earlier (58). To generate mice homozygous for the targeted allele, the resulting heterozygote (sgk3+/−) males and females were interbred to yield SGK3-deficient mice (sgk3−/−) and their wild-type littermates (sgk3+/+).
Culture of bone marrow-derived mast cells.
Mast cells were isolated from femoral bone marrow of 6- to 8-wk-old naive sgk3+/+ and sgk3−/− mice and cultured for 4 wk in RPMI 1640 (Invitrogen Life Technologies) containing 10% FCS, 1% penicillin-streptomycin, 20 ng/ml IL-3 (R&D Systems), and 100 ng/ml of the c-kit ligand stem cell factor (PeproTech). Bone marrow mast cell (BMMC) maturation was confirmed by flow cytometry (FACSCalibur; BD Biosciences) using the following specific fluorescent-labeled Abs: PE-labeled anti-FcεRI (eBioscience), allophycocyanin-labeled anti-CD117 (BD Pharmingen), and FITC-labeled anti-CD34 (BD Pharmingen). Cells were kept in culture 4–6 wk before the experiments. For experiments, BMMCs were sensitized for 1 h with monoclonal mouse anti- dinitrophenyl (DNP) mouse IgE (anti-DNP IgE, 5–10 μg/ml per 1 × 106 cells, clone SPE-7; Sigma-Aldrich) in culture medium and challenged with DNP-human serum albumin (DNP-HSA; 50 ng/ml; Sigma-Aldrich).
Determination of mast cell numbers in the ear conches.
Anesthetized mice were euthanized by cervical dislocation, and the skin was cleansed with 70% ethanol. Ear conches were cut off at the base, fixed in 4% paraformaldehyde overnight, and finally embedded in paraffin. Tissue sections (4-μm thick) taken from the middle of the conches were prepared, deparaffinized, and stained with toluidine blue. Mast cell numbers of 10 different areas on different slices per conch of 4 sgk3+/+ and 3 sgk3−/− mice were determined using a Zeiss Axiovert 200 microscope with a LD Achroplan ×20 lens in brightfield mode.
Patch clamp.
Patch-clamp experiments were performed at room temperature in voltage-clamp, fast-whole cell mode (41). BMMCs were continuously superfused by a flow system inserted into the dish. The bath was grounded via a bridge filled with NaCl-Ringer solution containing (in mM) 145 NaCl, 5 KCl, 2 MgCl2, 1 CaCl2, 10 glucose, and 10 HEPES/NaOH (pH 7.4, 300 mosM). Borosilicate glass pipettes (2–4 MΩ tip resistance; GC 150 TF-10, Harvard Apparatus, March-Hugstetten, Germany) manufactured by a microprocessor-driven DMZ puller (Zeitz, Augsburg, Germany) were used in combination with a MS314 electrical micromanipulator (MW, Märzhäuser, Wetzlar, Germany). The currents were recorded by an EPC-9 amplifier (HEKA, Lambrecht, Germany) using Pulse software (HEKA) and an ITC-16 Interface (Instrutech, Port Washington, NY). Whole cell currents were determined as 10 successive 200-ms square pulses from a −35 mV holding potential to potentials between −115 and +65 mV. The currents were recorded with an acquisition frequency of 10 and 3 kHz low-pass filtered.
The pipette solution contained (in mM) 140 K-gluconate, 5 KCl, 1.2 MgCl2, 2 EGTA, 1.26 CaCl2 (pCa 7), 2 Na2ATP, and 10 HEPES/KOH (pH 7.2, 280 mosM) and was used in combination with NaCl-Ringer bath solution. Where indicated the antigen DNP-HSA, (50 ng/ml, Sigma-Aldrich, Germany) and the Ca2+ ionophore ionomycin (1 μM, Sigma-Aldrich) were added to the bath solution.
The offset potentials between both electrodes were zeroed before sealing. The potentials were corrected for liquid junction potentials as estimated according to Barry and Lynch (8). The original whole cell current traces are depicted without further filtering, and currents of the individual voltage square pulses are superimposed. The applied voltages refer to the cytoplasmic face of the membrane with respect to the extracellular space. The inward currents, defined as flow of positive charge from the extracellular to the cytoplasmic membrane face, are negative currents and depicted as downward deflections of the original current traces.
Intracellular calcium measurements.
Intracellular Ca2+ measurements were performed as described (69). Briefly, BMMCs were sensitized with IgE (10 μg/ml) for 1 h at 37°C and subsequently loaded with fura-2 AM (2 μM, Molecular Probes, Goettingen, Germany) for 20 min at 37°C. Fluorescence measurements were carried out with an inverted phase-contrast microscope (Axiovert 100, Zeiss, Oberkochen, Germany). Cells were excited alternatively at 340 and 380 nm, and the light was deflected by a dichroic mirror into either the objective (Fluar ×40/1.30 oil, Zeiss, Oberkochen) or a camera. Emitted fluorescence intensity was recorded at 505 nm and data acquisition was performed by using specialized computer software (Metafluor, Universal Imaging, Downingtown). Intracellular Ca2+ was measured before and following addition of DNP-HSA to IgE-sensitized BMMCs in the absence or presence of extracellular Ca2+.
As a measure for the increase of cytosolic Ca2+ activity, the slope and peak of the changes in the 340/380 nm ratio were calculated for each experiment. For intracellular calibration purposes, ionomycin (10 μM) was applied at the end of each experiment. Experiments were performed with Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1.2 MgSO4, 2 CaCl2, 2 Na2HPO4, 32 HEPES, and 5 glucose, pH 7.4. To reach nominally Ca2+-free conditions, experiments were performed using Ca2+-free Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1.2 MgSO4, 2 Na2HPO4, 32 HEPES, 0.5 EGTA, and 5 glucose, pH 7.4.
Measurement of degranulation.
Mature BMMCs were seeded on 96-well plates in fresh medium with anti-DNP IgE antibody (5 μg/ml) for 1 h. Afterwards cells were washed in Tyrode salt solution (Sigma-Aldrich) and challenged with DNP-HSA (50 ng/ml). Twenty microliters of supernatant and 20 μl of 2 mM 4-nitrophenyl N-acetyl-β-d-glucosaminide (Sigma-Aldrich), diluted in 0.2 M citrate buffer, pH 4.5, were added to each well of the 96-well plate, and color was developed for 2 h at 37°C. The reaction was terminated with 1 M Tris buffer, pH 9.0, and the absorbance was measured at 405 nm in an ELISA microplate reader. The data are expressed as the percentage of the total release (Triton X-100 0.1%) and are corrected for spontaneous release.
Passive systemic anaphylaxis/antigen-induced anaphylaxis and serum histamine concentrations.
Mice were sensitized with 30 μg/250 μl anti-DNP IgE by intraperitoneal application. Five hours later, mice were challenged with either DNP-HSA (100 μg/200 μl) or PBS. Body temperature was monitored before and every minute after antigen challenge with an 8-Channel USB Thermometer (Tübingen, Germany) during the midportion of the light phase of the light cycle. Mice were placed with the tail raised, and the Vaseline-covered probe was inserted a standardized distance of 2 cm until a stable temperature reading was obtained. Baseline temperature was measured after mice were habituated to rectal probe insertion. Ambient room temperature was 23°C, and the animals were exposed to a 12-h light and 12-h dark cycle (7 am to 7 pm). Data are expressed as a change in body temperature following treatment (Δ°C). Histamine levels were analyzed in the blood of male sgk3+/+ and sgk3−/− mice 30 min after induction of an anaphylactic reaction. Histamine was measured by ELISA according to the instructions of the manufacturer (IBL-Hamburg, Hamburg, Germany).
Statistics.
Data are provided as means ± SE; n represents the number of animals/independent experiments. All data were tested for significance using Student's unpaired two-tailed t-test or ANOVA (Dunnets test), where applicable. P < 0.05 was considered to indicate statistical significance.
RESULTS
Cells were derived from the bone marrow (BMMCs) of SGK3 knockout mice (sgk3−/−) and their wild-type littermates (sgk3+/+), and expression of the mast cell surface markers CD117, CD34, and FcεRI (Fig. 1A) was determined. No significant difference in the abundance of any of the three markers for mast cell maturation was observed between BMMCs of the two genotypes (Fig. 1B).
Fig. 1.
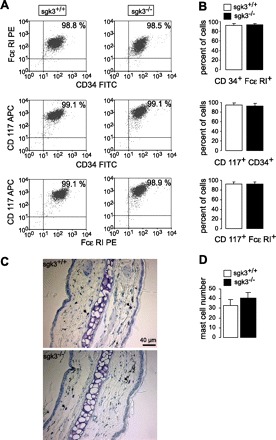
Maturation of bone marrow mast cells (BMMCs) from sgk3+/+ and sgk3−/− mice. A: original dot plots of CD117-, CD34-, and FcεRI-positive BMMCs from sgk3+/+ and sgk3−/− mice. Numbers depict the percentage of cells in the respective quadrant, acquired within the mast cell gate. B: frequency of mast cells in primary culture. Mean percent (± SE; n = 6 individual BMMC cultures) of sgk3+/+ (open bars) and sgk3−/− (closed bars) BMMCs acquired within the mast cell gate. C: ear conche sections of sgk3+/+ (top) and sgk3−/− (bottom) mice stained with toluidine blue for mast cell detection (mast cells are indicated by black arrows). D: number of mast cells (±SE) in skin, analyzed by staining of ear conche sections with toluidine blue. Mean mast cell numbers of toluidine blue-positive cells in one area (×200 magnification) as calculated from 10 different areas on different slices per conch of 4 sgk3+/+ (open bar) and 3 sgk3−/− (closed bar) mice (P = 0.43, two-tailed unpaired t-test).
The number of mast cells in the skin, analyzed by staining of ear conch sections with toluidine blue (Fig. 1, C and D), was similar in sgk3+/+ and sgk3−/− mice (P = 0.43).
Stimulation with IgE and cognate antigen was followed by a sharp increase of cytosolic Ca2+ in sgk3+/+ cells, an effect significantly blunted in sgk3−/− cells (Fig. 2, A and B). Before addition of antigen the basal Ca2+ level was not different between sgk3+/+ and sgk3−/− BMMCs (fluorescence ratio: 1.40 ± 0.07 in sgk3+/+ vs. 1.35 ± 0.14 in sgk3−/− BMMCs). To further assess the effect of SGK3 on Ca2+ mobilization, the cells were sensitized with IgE and challenged with antigen in the absence of extracellular Ca2+ (Fig. 2C). As a result, in the nominal absence of extracellular Ca2+, the exposure to IgE and antigen was followed by a transient increase in intracellular Ca2+, an effect not significantly different between sgk3+/+ and sgk3−/− cells (Fig. 2D). Thus lack of SGK3 predominantly impaired the entry of extracellular Ca2+.
Fig. 2.
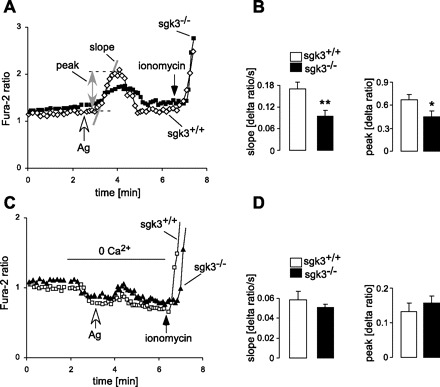
Antigen-induced Ca2+ entry into BMMCs from sgk3+/+ and sgk3−/− mice. A: representative original tracings showing the fura-2 fluorescence ratio of 340 over 380 nm in fura-2/AM-loaded BMMCs from sgk3+/+ and sgk3−/− mice before and following addition of antigen (Ag, 50 ng/ml). At the end of each experiment, ionomycin (10 μM) was added for calibration. For quantification of the Ca2+ entry into the BMMCs, the slope (Δratio/ms) and peak (Δratio) were calculated following addition of Ag as indicated in the figure. B: means (±SE) of the slope (left) and peak (right) of the fluorescence ratio change for sgk3+/+ (n = 9, open bars) and sgk3−/− (n = 8, closed bars) BMMCs following stimulation with Ag (50 ng/ml). *P < 0.05 and **P < 0.01, significant difference between both groups (two-tailed unpaired t-test). C: representative original tracings showing the ratio of 340/380 nm fura-2 fluorescence in fura-2-loaded sgk3+/+ and sgk3−/− BMMCs before and after addition of Ag (50 ng/ml) in the absence of extracellular Ca2+. To reach a Ca2+-free environment, EGTA (0.5 mM) was added to the Ca2+-free bath solution. D: means (±SE) of the slope (left) and peak value (right) of the fluorescence ratio change for sgk3+/+ (n = 5, open bars) and sgk3−/− (n = 5, closed bars) BMMCs upon stimulation with antigen in a Ca2+-free solution.
BMMCs are known to express Ca2+-activated K+ channels KCa3.1, which are important amplifiers of Ca2+ entry upon IgE-antigen-dependent stimulation (69). The K+ currents of BMMCs upon receptor stimulation were measured in patch-clamp experiments (Fig. 3). Addition of antigen to the bath solution resulted in a rapid increase of a K+-selective conductance. The current amplitude was growing after antigen application and reached its maximum in about 3 min. The maximal amplitude was significantly blunted in sgk3−/− if compared with sgk3+/+ cells (Fig. 3). However, when the cells were stimulated with a Ca2+ ionophore ionomycin, no difference in measured K+ currents was detected between the genotypes (Fig. 3C). Accordingly, the surface expression and maximal activity of the Ca2+-activated K+ channel did not differ between sgk3+/+ and sgk3−/− cells; i.e., SGK3 deficiency primarily decreased the Ag-stimulated Ca2+ entry.
Fig. 3.
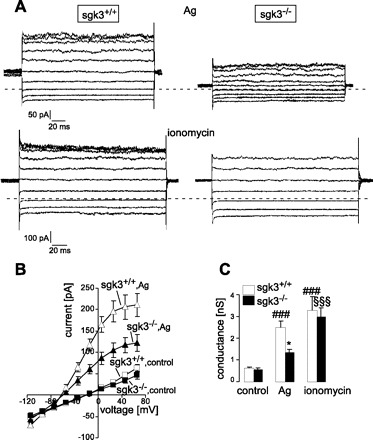
Antigen-induced K+ currents are reduced in sgk3−/− BMMCs. A: representative whole cell currents from sgk3+/+ (left) and sgk3−/− (right) BMMCs elicited by 200-ms pulses ranging from −115 to +65 mV in 20-mV increments from a holding potential of −35 mV. Currents were recorded in standard NaCl bath solution 3 min after stimulation with either Ag (50 ng/ml, top) or ionomycin (1 μM, bottom). The dotted line indicates the zero current value. B: mean current-voltage relationships (±SE, n = 7) in sgk3+/+ (open symbols) and sgk3−/− (closed symbols) BMMCs before (control, squares) and 3 min after stimulation with antigen (Ag, 50 ng/ml, triangles). C: mean whole cell conductance (± SE) of sgk3+/+ (open bars) and sgk3−/− (closed bars) BMMCs as recorded in B before (control) and after stimulation with either Ag (50 ng/ml) or ionomycin (1 μM). Data were calculated by linear regression between −55 and +5 mV. *P < 0.05, significant difference between sgk3+/+ and sgk3−/− cells (ANOVA); ###P < 0.001, significant difference from sgk3+/+ cells under control conditions (ANOVA); §§§P < 0.001, significant difference from sgk3−/− cells under control conditions (ANOVA).
Decreased Ca2+ entry in sgk3−/− BMMCs could result in decreased antigen-induced mediator release. To determine whether SGK3 deficiency influences mast cell degranulation, the release of β-hexosaminidase was measured in sgk3+/+ and sgk3−/− cells. As shown in Fig. 4, β-hexosaminidase release was significantly reduced in sgk3−/− BMMCs.
Fig. 4.
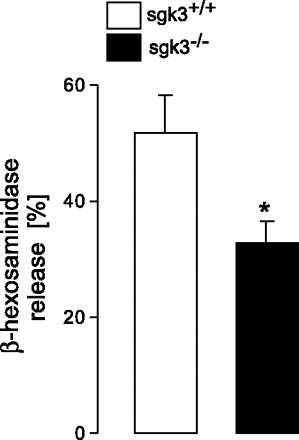
Degranulation of antigen-stimulated sgk3−/− and sgk3+/+ BMMCs. β-Hexosaminidase release from cultured sgk3−/− BMMCs (closed bars) and their wild-type littermates sgk3+/+ (open bars) stimulated for 15 min with 50 ng/ml antigen (±SE, n = 5 individual experiments). Release in the supernatant was calculated as percentage of total cellular (0.1% Triton X-100) β-hexosaminidase. The stimulated β-hexosaminidase release in each experiment was corrected for the spontaneous release. *Significant difference between genotypes (P < 0.05; two-tailed unpaired t-test).
To determine whether this defect in Ag-stimulated Ca2+ entry into sgk3−/− BMMCs affects mast cell function in vivo, we tested sgk3+/+ and sgk3−/− mice for passive systemic anaphylaxis (Fig. 5). Mice were sensitized with anti-DNP IgE intraperitoneally, and after 5 h rest, they received DNP-HSA antigen or saline as a control by intraperitoneal injection, and body temperature was monitored over time. The measured drop in body temperature following antigen treatment was reduced in sgk3−/− mice (Fig. 5, A and B). Serum histamine levels measured 30 min after induction of the anaphylactic reaction were significantly lower in sgk3−/− than those levels in sgk3+/+ mice (Fig. 5C), thus pointing to an impairment of sgk3-deficient mast cell function in vivo.
Fig. 5.
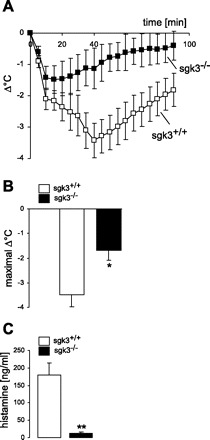
Systemic anaphylactic reaction in sgk3+/+ and sgk3−/− mice. A: changes in body temperature (Δ°C) of sgk3−/− mice (n = 5, closed squares) and their wild-type littermates sgk3+/+ (n = 6, open squares) following induction of anaphylaxis (±SE). Mice were given intraperitoneal anti- dinitrophenyl (DNP) IgE (30 μg) and challenged with 100 μg DNP- human serum albumin (HSA) after 5 h. B: arithmetic means (± SE) of maximal changes in body temperature (Δ°C) of sgk3−/− mice (n = 5, closed bars) and their wild-type littermates sgk3+/+ (n = 6, open bars) following induction of anaphylaxis. *Significant difference between genotypes (P < 0.05; two-tailed unpaired t-test). C: serum histamine levels 30 min after induction of anaphylaxis in sgk3−/− mice (closed bar) and their wild-type sgk3+/+ littermates (open bar) (n = 3). **Significant difference between genotypes (P < 0.01; two-tailed unpaired t-test).
DISCUSSION
The present study unravels a novel function of the serum- and glucocorticoid-inducible kinase SGK3. Specifically, Ca2+ entry, Ca2+-activated K+ channel activity, and degranulation are blunted in BMMCs from SGK3 knockout mice (sgk3−/−) compared with BMMCs from their wild-type littermates (sgk3+/+). Accordingly, sgk3−/− mice appear to be more resistant to anaphylactic shock.
As shown earlier (6, 20, 22, 25, 28, 29, 44, 57, 72), Ca2+ entry through Ca2+ channels is critically important for the regulation of mast cell degranulation. The Ca2+ entry depends on the potential difference across the cell membrane (63) and is thus influenced by the activity of Ca2+-activated K+ channels (19, 20, 27, 55, 69).
The stimulation of K+ channels is blunted, but not completely inhibited, in the nominal absence of extracellular Ca2+ (71). Accordingly, the activation of K+ channels largely depends on extracellular Ca2+. However, the present data do not rule out additional mechanisms involved in the regulation of K+ channels.
In mast cells, PI3 kinase has been shown to regulate cell proliferation, adhesion, and migration, as well as antigen-IgE-induced degranulation and cytokine release (2). Moreover, PI3 kinase was suggested to target the TRPV2 Ca2+ channel in these cells (75). In the Xenopus oocyte heterologous expression system, SGK1 and SGK3 have previously been shown to increase the cell membrane abundance and activity of the Ca2+ channel TRPV5 (32) and TRPV6 (17). It is conceivable that SGK1 has a similar stimulating effect on TRPV2 and/or other Ca2+ channels important for Ca2+ entry into mast cells.
In the Xenopus oocyte system, SGK3 has been shown to stimulate the activity of several ion channels including the epithelial Na+ channel ENaC (34, 62), the renal and cochlear Cl− channel complex ClC-Ka/barttin (30), the cell volume-regulated Cl− channel ClC2 (61), the cardiac voltage-gated Na+ channel SCN5A (16), the cardiac K+ channels KCNE1/KCNQ1 (31) and HERG (54), the glutamate receptor GluR1 (73) as well as the voltage-gated K+ channels Kv1.3 (37, 42, 80), Kv1.5 (77), and Kv4.3 (7). SGK3 further stimulates the activity of a wide variety of transporters including the Na+-glucose cotransporter SGLT1 (26), the glutamine transporter SN1 (13), the glutamate transporters EAAT1 (12), EAAT2 (14), EAAT3 (67), and EAAT5 (15), the dicarboxylate cotransporter NaDC-1 (11), the creatine transporter CreaT (SLC6A8) (68), the myoinositol transporter SMIT (46), and the Na+-K+-ATPase activity (43). Both, SGK1 and SGK3 regulate the function of channels and transporters by influencing expression, trafficking, and degradation of the channel and transport proteins (50).
SGK3 has been shown in vitro to confer cell survival (52, 83), an effect that may be related to the effect of SGK3 on Kv1.3 channel activity. In human embryonic kidney cells and Jurkat lymphocytes, Kv1.3 is involved in the regulation of cell proliferation (37, 51) and apoptosis (40, 51, 74). The antiapoptotic effect may further be secondary to phosphorylation of forkhead transcription factors (21, 52, 83). Moreover, SGK3 has been shown to phosphorylate and thus inactivate Bad (52, 83). Phosphorylated Bad binds to the chaperone 14–3-3 and is thus prevented from traveling to the mitochondria, where it triggers apoptosis (53).
At first glance, it may be surprising that both the knockout of SGK1 (71) and knockout of SGK3 (this study) disrupt antigen-induced Ca2+ entry into and activation of mast cells. Accordingly, even though both kinases obviously serve similar functions in mast cells, they cannot fully replace each other and lack of one of the two kinases disrupts mast cell function. It must be kept in mind that SGK3 is constitutively expressed, while SGK1 is genomically upregulated following cell stress. Thus SGK3 may be required for the stimulation of channel or carrier expression in unstressed conditions; i.e., before antigen exposure, while SGK1 may be required for the full-blown entry of Ca2+ during stimulation.
In conclusion, SGK3 participates in antigen-stimulated Ca2+ entry and subsequent activation of Ca2+-activated K+ channels. The latter leads to augmentation of Ca2+ entry and thus similarly participates in the stimulation of mast cell degranulation. Those cellular effects are critical for anaphylactic response in vivo.
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, SFB 766) and NIH R01-DK56695 (to D. Pearce).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the meticulous preparation of the manuscript by Tanja Loch, Sari Rübe, and Lejla Subasic.
REFERENCES
- 1. Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol 7: 776–789, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature 431: 1007–1011, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Alonso L, Okada H, Pasolli HA, Wakeham A, You T, Mak TW, Fuchs E. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol 170: 559–570, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol 172: 7254–7262, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Askenase PW, Van Loveren H, Kraeuter-Kops S, Ron Y, Meade R, Theoharides TC, Nordlund JJ, Scovern H, Gerhson MD, Ptak W. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol 131: 2687–2694, 1983 [PubMed] [Google Scholar]
- 6. Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol 9: 81–88, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Baltaev R, Strutz-Seebohm N, Korniychuk G, Myssina S, Lang F, Seebohm G. Regulation of cardiac shal-related potassium channel Kv 4.3 by serum- and glucocorticoid-inducible kinase isoforms in Xenopus oocytes. Pflügers Arch 450: 26–33, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol 121: 101–117, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hultner L, Rocken M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med 192: 1441–1452, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J 20: 4380–4390, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boehmer C, Embark HM, Bauer A, Palmada M, Yun CH, Weinman EJ, Endou H, Cohen P, Lahme S, Bichler KH, Lang F. Stimulation of renal Na+ dicarboxylate cotransporter 1 by Na+/H+ exchanger regulating factor 2, serum and glucocorticoid inducible kinase isoforms, and protein kinase B. Biochem Biophys Res Commun 313: 998–1003, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid-inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem 86: 1181–1188, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Boehmer C, Okur F, Setiawan I, Broer S, Lang F. Properties and regulation of glutamine transporter SN1 by protein kinases SGK and PKB. Biochem Biophys Res Commun 306: 156–162, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4–2 is impacted by SGK kinases. J Neurochem 97: 911–921, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Boehmer C, Rajamanickam J, Schniepp R, Kohler K, Wulff P, Kuhl D, Palmada M, Lang F. Regulation of the excitatory amino acid transporter EAAT5 by the serum and glucocorticoid dependent kinases SGK1 and SGK3. Biochem Biophys Res Commun 329: 738–742, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Boehmer C, Wilhelm V, Palmada M, Wallisch S, Henke G, Brinkmeier H, Cohen P, Pieske B, Lang F. Serum and glucocorticoid inducible kinases in the regulation of the cardiac sodium channel SCN5A. Cardiovasc Res 57: 1079–1084, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Bohmer C, Palmada M, Kenngott C, Lindner R, Klaus F, Laufer J, Lang F. Regulation of the epithelial calcium channel TRPV6 by the serum and glucocorticoid-inducible kinase isoforms SGK1 and SGK3. FEBS Lett 581: 5586–5590, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Bradding P. The role of the mast cell in asthma: a reassessment. Curr Opin Allergy Clin Immunol 3: 45–50, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Bradding P. Mast cell ion channels. Chem Immunol Allergy 87: 163–178, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Bradding P, Okayama Y, Kambe N, Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukoc Biol 73: 614–620, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buess M, Engler O, Hirsch HH, Moroni C. Search for oncogenic regulators in an autocrine tumor model using differential display PCR: identification of novel candidate genes including the calcium channel mtrp6. Oncogene 18: 1487–1494, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Campagna DR, Custodio AO, Antiochos BB, Cirlan MV, Fleming MD. Mutations in the serum/glucocorticoid regulated kinase 3 (Sgk3) are responsible for the mouse fuzzy (fz) hair phenotype. J Invest Dermatol 128: 730–732, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Dai F, Yu L, He H, Zhao Y, Yang J, Zhang X, Zhao S. Cloning and mapping of a novel human serum/glucocorticoid regulated kinase-like gene, SGKL, to chromosome 8q12.3-q131. Genomics 62: 95–97, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Dernick G, Alvarez dT, Lindau M. Exocytosis of single chromaffin granules in cell-free inside-out membrane patches. Nat Cell Biol 5: 358–362, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4–2 and kinases SGK1, SGK3, and PKB. Obes Res 12: 862–870, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Duffy SM, Cruse G, Lawley WJ, Bradding P. β2-adrenoceptor regulation of the K+ channel iKCa1 in human mast cells. FASEB J 19: 1006–1008, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Duffy SM, Lawley WJ, Conley EC, Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol 167: 4261–4270, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Duffy SM, Leyland ML, Conley EC, Bradding P. Voltage-dependent and calcium-activated ion channels in the human mast cell line HMC-1. J Leukoc Biol 70: 233–240, 2001 [PubMed] [Google Scholar]
- 30. Embark HM, Bohmer C, Palmada M, Rajamanickam J, Wyatt AW, Wallisch S, Capasso G, Waldegger P, Seyberth HW, Waldegger S, Lang F. Regulation of CLC-Ka/barttin by the ubiquitin ligase Nedd4–2 and the serum- and glucocorticoid-dependent kinases. Kidney Int 66: 1918–1925, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Embark HM, Bohmer C, Vallon V, Luft F, Lang F. Regulation of KCNE1-dependent K(+) current by the serum and glucocorticoid-inducible kinase (SGK) isoforms. Pflügers Arch 445: 601–606, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Embark HM, Setiawan I, Poppendieck S, van de Graaf SF, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun CC, Bindels RJ, Lang F. Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem 14: 203–212, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Firestone GL, Giampaolo JR, O'Keeffe BA. Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem 13: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Friedrich B, Feng Y, Cohen P, Risler T, Vandewalle A, Broer S, Wang J, Pearce D, Lang F. The serine/threonine kinases SGK2 and SGK3 are potent stimulators of the epithelial Na+ channel alpha,beta,gamma-ENaC. Pflügers Arch 445: 693–696, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23: 749–786, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Galli SJ, Nakae S. Mast cells to the defense. Nat Immunol 4: 1160–1162, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Gamper N, Fillon S, Huber SM, Feng Y, Kobayashi T, Cohen P, Lang F. IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1.Pflügers Arch 443: 625–634, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol 6: 218–230, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick JA, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F. Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol 290: R945–R950, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Gulbins E, Szabo I, Baltzer K, Lang F. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci USA 94: 7661–7666, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamill OP, Marty A, Neher E, Sakmann B, Sigeorth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 42. Henke G, Maier G, Wallisch S, Boehmer C, Lang F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 199: 194–199, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Henke G, Setiawan I, Bohmer C, Lang F. Activation of Na+/K+-ATPase by the serum and glucocorticoid-dependent kinase isoforms. Kidney Blood Press Res 25: 370–374, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Kahr H, Schindl R, Fritsch R, Heinze B, Hofbauer M, Hack ME, Mortelmaier MA, Groschner K, Peng JB, Takanaga H, Hediger MA, Romanin C. CaT1 knock-down strategies fail to affect CRAC channels in mucosal-type mast cells. J Physiol 557: 121–132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J Immunol 175: 4167–4173, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klaus F, Palmada M, Lindner R, Laufer J, Jeyaraj S, Lang F, Boehmer C. Up-regulation of hypertonicity-activated myo-inositol transporter SMIT1 by the cell volume-sensitive protein kinase SGK1. J Physiol 586: 1539–1547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339: 319–328, 1999 [PMC free article] [PubMed] [Google Scholar]
- 48. Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344: 189–197, 1999 [PMC free article] [PubMed] [Google Scholar]
- 49. Lam RS, Shumilina E, Matzner N, Zemtsova IM, Sobiesiak M, Felder E, Dietl P, Huber SM, Lang F. Phosphatidylinositol-3-kinase regulates mast cell ion channel activity. Cell Physiol Biochem 22: 169–176, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol 205: 147–157, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Liu D, Yang X, Songyang Z. Identification of CISK, a new member of the SGK kinase family that promotes IL-3-dependent survival. Curr Biol 10: 1233–1236, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J 349: 547–557, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Maier G, Palmada M, Rajamanickam J, Shumilina E, Bohmer C, Lang F. Upregulation of HERG channels by the serum and glucocorticoid inducible kinase isoform SGK3. Cell Physiol Biochem 18: 177–186, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Mark DS, Berger P, Cruse G, Yang W, Bolton SJ, Bradding P. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J Allergy Clin Immunol 114: 66–72, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Mauro TM, McCormick JA, Wang J, Boini KM, Ray L, Monks B, Birnbaum MJ, Lang F, Pearce D. Akt2 and SGK3 are both determinants of postnatal hair follicle development. FASEB J 23: 3193–3202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mazurek N, Berger G, Pecht I. A binding site on mast cells and basophils for the anti-allergic drug cromolyn. Nature 286: 722–723, 1980 [DOI] [PubMed] [Google Scholar]
- 58. McCormick JA, Feng Y, Dawson K, Behne MJ, Yu B, Wang J, Wyatt AW, Henke G, Grahammer F, Mauro TM, Lang F, Pearce D. Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell 15: 4278–4288, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 176: 2238–2248, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Okada T, Ishii Y, Masujin K, Yasoshima A, Matsuda J, Ogura A, Nakayama H, Kunieda T, Doi K. The critical roles of serum/glucocorticoid-regulated kinase 3 (SGK3) in the hair follicle morphogenesis and homeostasis: the allelic difference provides novel insights into hair follicle biology. Am J Pathol 168: 1119–1133, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palmada M, Dieter M, Boehmer C, Waldegger S, Lang F. Serum and glucocorticoid inducible kinases functionally regulate ClC-2 channels. Biochem Biophys Res Commun 321: 1001–1006, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Palmada M, Dieter M, Speil A, Bohmer C, Mack AF, Wagner HJ, Klingel K, Kandolf R, Murer H, Biber J, Closs EI, Lang F. Regulation of intestinal phosphate cotransporter NaPi IIb by ubiquitin ligase Nedd4–2 and by serum- and glucocorticoid-dependent kinase 1. Am J Physiol Gastrointest Liver Physiol 287: G143–G150, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18: 3024–3033, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pawankar R. Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy 87: 111–129, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Sandu C, Rexhepaj R, Grahammer F, McCormick JA, Henke G, Palmada M, Nammi S, Lang U, Metzger M, Just L, Skutella T, Dawson K, Wang J, Pearce D, Lang F. Decreased intestinal glucose transport in the sgk3-knockout mouse. Pflügers Arch 451: 437–444, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Schniepp R, Kohler K, Ladewig T, Guenther E, Henke G, Palmada M, Boehmer C, Rothstein JD, Broer S, Lang F. Retinal colocalization and in vitro interaction of the glutamate transporter EAAT3 and the serum- and glucocorticoid-inducible kinase SGK1. Invest Ophthalmol Vis Sci 45: 1442–1449, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Shojaiefard M, Christie DL, Lang F. Stimulation of the creatine transporter SLC6A8 by the protein kinases SGK1 and SGK3. Biochem Biophys Res Commun 334: 742–746, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Shumilina E, Lam RS, Wölbing F, Matzner N, Zemtova IM, Sobiesiak M, Mahmud H, Sausbier U, Biedermann T, Ruth P, Sausbier M, Lang F. Blunted IgE-mediated activation of mast cells in mice lacking the Ca2+ activated K+ channel KCa3.1. J Immunol 180: 8040–8047, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Simon P, Schneck M, Hochstetter T, Koutsouki E, Mittelbronn M, Merseburger A, Weigert C, Niess A, Lang F. Differential regulation of serum- and glucocorticoid-inducible kinase 1 (SGK1) splice variants based on alternative initiation of transcription. Cell Physiol Biochem 20: 715–728, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Sobiesiak M, Shumilina E, Lam RS, Wolbing F, Matzner N, Kaesler S, Zemtsova IM, Lupescu A, Zahir N, Kuhl D, Schaller M, Biedermann T, Lang F. Impaired mast cell activation in gene-targeted mice lacking the serum- and glucocorticoid-inducible kinase SGK1. J Immunol 183: 4395–4402, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med 200: 137–147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strutz-Seebohm N, Seebohm G, Korniychuk G, Baltaev R, Ureche O, Striegel M, Lang F. Additive regulation of GluR1 by stargazin and serum- and glucocorticoid-inducible kinase isoform SGK3. Pflügers Arch 452: 276–282, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Szabo I, Gulbins E, Apfel H, Zhang X, Barth P, Busch AE, Schlottmann K, Pongs O, Lang F. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem 271: 20465–20469, 1996 [DOI] [PubMed] [Google Scholar]
- 75. Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry 43: 11701–11708, 2004 [DOI] [PubMed] [Google Scholar]
- 76. Turtoi A, Brown I, Oskamp D, Schneeweiss FH. Early gene expression in human lymphocytes after gamma-irradiation-a genetic pattern with potential for biodosimetry. Int J Radiat Biol 84: 375–387, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack AF, Chao CM, Su J, Nitschke R, Alexander D, Friedrich B, Wulff P, Kuhl D, Lang F. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 54: 1090–1099, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Van Loveren H, Meade R, Askenase PW. An early component of delayed-type hypersensitivity mediated by T cells and mast cells. J Exp Med 157: 1604–1617, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, Dunn IF, Schinzel AC, Sandy P, Hoersch S, Sheng Q, Gupta PB, Boehm JS, Reiling JH, Silver S, Lu Y, Stemke-Hale K, Dutta B, Joy C, Sahin AA, Gonzalez-Angulo AM, Lluch A, Rameh LE, Jacks T, Root DE, Lander ES, Mills GB, Hahn WC, Sellers WR, Garraway LA. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 16: 21–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Warntges S, Friedrich B, Henke G, Duranton C, Lang PA, Waldegger S, Meyermann R, Kuhl D, Speckmann EJ, Obermuller N, Witzgall R, Mack AF, Wagner HJ, Wagner A, Broer S, Lang F. Cerebral localization and regulation of the cell volume-sensitive serum- and glucocorticoid-dependent kinase SGK1. Pflügers Arch 443: 617–624, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Wymann MP, Bjorklof K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans 31: 275–280, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Xu J, Liao L, Qin J, Xu J, Liu D, Songyang Z. Identification of Flightless-I as a substrate of the cytokine-independent survival kinase CISK. J Biol Chem 284: 14377–14385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xu J, Liu D, Gill G, Songyang Z. Regulation of cytokine-independent survival kinase (CISK) by the Phox homology domain and phosphoinositides. J Cell Biol 154: 699–705, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]


