Abstract
Scratching inhibits pruritogen-evoked responses of neurons in the superficial dorsal horn, implicating a spinal site for scratch inhibition of itch. We investigated if scratching differentially affects neurons depending on whether they are activated by itchy vs. painful stimuli, and if the degree of inhibition depends on the relative location of scratching. We recorded from rat lumbar dorsal horn neurons responsive to intradermal (id) microinjection of serotonin (5-HT). During the response to 5-HT, scratch stimuli (3 mm, 300 mN, 2 Hz, 20 s) were delivered at the injection site within the mechanosensitive receptive field (on-site), and 4–30 mm away, outside of the receptive field (off-site). During off-site scratching, 5-HT-evoked firing was significantly attenuated followed by recovery. On-site scratching excited neurons, followed by a significant post-scratch decrease in 5-HT-evoked firing. Most neurons additionally responded to mustard oil (AITC). Off-site scratching had no effect, while on-site scratching excited the neurons. These results indicate that scratching exerts a state-dependent inhibitory effect on responses of spinal neurons to pruritic but not algesic stimuli. Moreover, on-site scratching first excited neurons followed by inhibition, while off-site scratching immediately evoked inhibition of pruritogen-evoked activity. This accounts for the suppression of itch by scratching at a distance from the site of the itchy stimulus.
Keywords: rat, serotonin, scratch, itch dorsal horn neuron, inhibition
Introduction
Itch is often defined as an unpleasant sensation associated with the desire to scratch. Itch sensation presumably provides a warning signal that an organism or plant spicule has invaded the skin surface, and triggers scratching to remove the offending stimulus. Scratching and a variety of other noxious counterstimuli relieve itch sensation in humans (Murray & Weaver, 1975; Ward et al., 1996; Yosipovitch et al., 2005). The mechanism by which scratching the skin surface relieves itch is thought to involve inhibition of spinal itch-signaling neurons (Davidson et al., 2009; Akiyama et al., 2011, 2012).
We have presently investigated the effect of scratching on presumptive itch-signaling neurons in the rat spinal cord. Sprague-Dawley rats exhibit pain-like responses to the prototypical itch mediator, histamine (Carstens, 1997; Klein et al., 2011), but exhibit itch-related hindlimb scratching behavior following intradermal (id) injection of serotonin (5-hydroxytramptamine= 5-HT)( Berendsen & Broekkamp, 1991; Thompsen et al., 2001; Jinks & Carstens, 2002). 5-HT-evoked scratching involves 5-HT2 receptors (Nojima & Carstens, 2003a) and is inhibited by μ-opioid antagonists but not agonists (Nojima et al., 2003; Nojima & Carstens, 2003b; Spradley et al., 2012). A subpopulation of rat dorsal root ganglion (DRG) neurons with primary afferent C-fibers gave prolonged responses to peripheral cutaneous application of 5-HT (Hachisuka et al., 2010). Superficial dorsal horn neurons in the rat lumbar spinal cord respond to id injection of 5-HT (Carstens, 1997; Jinks & Carstens, 2002; Jinks et al., 2002; Nojima et al., 2003) over a time course matching that of 5-HT-evoked scratching (Jinks & Carstens, 2002). 5-HT-responsive neurons also responded to a variety of algogens (Carstens, 1997; Jinks & Carstens, 2002). Substance P participates as a spinal neuropeptide transmitter in 5-HT-evoked scratching, which was significantly attenuated following neurotoxic destruction of neurons in the superficial medullary and cervical dorsal horn that express neurokinin-1 (NK-1) receptors (Carstens et al., 2010). Gastrin releasing peptide is another neuropeptide implicated in the spinal transmission of itch (Sun & Chen, 2007; Sun et al., 2009). Thus, using 5-HT as a pruritogen, the Sprague-Dawley rat presents an attractive animal model to investigate spinal mechanisms of itch and its modulation. In the present study, we have investigated the effect of scratching on responses of presumptive itch-signaling spinal neurons to cutaneous application of 5-HT and the algogen, allyl isothiocyanate (AITC; mustard oil) which elicits a burning pain sensation in humans.
Experimental Procedures
Experiments were performed using 13 adult male Sprague-Dawley rats (Simonsen, Gilroy, CA; 420–660 g) under a protocol approved by the UC Davis Animal Care and Use Committee. The single-unit recording from the lumbar spinal cord was conducted as previously detailed (Jinks & Carstens, 2002). Anesthesia was induced by sodium pentobarbital (60 mg/kg ip) and maintained by intravenous infusion of pentobarbital (10–20 mg/kg/hr). A tungsten microelectrode recorded single-unit activity in the lumbosacral spinal cord. A chemical search strategy (Jinks & Carstens, 2002) identified and isolated 5-HT-responsive units. Briefly, a small (~0.1 μl) intradermal (id) microinjection of 5-HT (1%; 47 mM; Sigma-Aldrich, St. Louis MO) was made in the ventral hindpaw and a unit in the superficial lumbar dorsal horn (depth < 300 um) exhibiting ongoing activity was isolated. After the ongoing activity subsided, 1 μl of 5- HT was injected through the same needle. Only units exhibiting an increase of >30% in firing were selected for further study.
During a period of relatively stable elevated firing following id 5-HT, a series of scratch stimuli was delivered either directly at the injection site (on-site), or at a site 4–30 mm away (off-site). Scratch stimuli consisted of back-and-forth movements of a brush bristle resembling a rat claw across the hindpaw skin at a constant frequency of 2 Hz, excursion of 3 mm, force of 300 mN, and duration of 20 sec. The scratch stimulus was applied either on- or off-site, in randomized order with at least 60 sec between scratching at either site.
5-HT-evoked activity usually decreased toward pre-injection levels after 1 hr. In most cases we were able to test the effect of scratching on neuronal activity following id injection of vehicle (id saline, n=6) and topical (2 μl) application of 75% AITC (n=9) or mineral oil (n=6). Ten of 12 5-HT-responsive neurons responded to AITC, similar to our previous report (Jinks & Carstens, 2002).
After testing effects of scratching on evoked responses, the neurons were tested for mechanosensitivity. Using a von Frey filament (bending force, 55 mN), we determined the border between locations at which the mechanical stimulus either did, or did not, elicit a reproducible response to at least 3 of 5 stimulus applications. This border was taken as the perimeter of the receptive field. As noted above, on-site scratching was delivered at the injection site which was always located within the receptive field, while off-site scratching delivered at distances of 4–30 mm from the injection site which was always outside the perimeter of the receptive field. Units were classified as wide dynamic range (WDR) if they gave graded responses to progressively stronger stimuli, or nociceptive-specific (NS) if they responded to noxious pinch but not to low-threshold von Frey, cotton wisp or brush stimuli.
Action potentials were recorded to computers running Spike2 (CED, Cambridge UK) and Chart software (AD Instruments, Colorado Springs CO) and usually quantified as spikes/sec. Mean firing rate was calculated over 1 min periods before and after injection of 5-HT, AITC or vehicles. Ongoing responses were summed over 20-sec intervals before, during and after on- or off-site scratching, and compared by use of repeated-measures analysis of variance (ANOVA) followed by a post hoc Bonferroni test, with P < 0.05 set as significant. At the conclusion, an electrolytic lesion was made at the spinal cord recording site. The spinal cord was postfixed in 10% buffered formalin, cut in 50 μm frozen sections, and examined under the light microscope to identify lesions.
Results
Data were collected from 13 5-HT-responsive neurons located in the superficial dorsal horn (Fig. 3E) at a mean depth of 146.4 +/− 33.7 (SE) μm from the surface. All units responded to scratching. Twenty percent responded at lower frequency to low-threshold mechanical stimuli and was classified as wide dynamic range (WDR), while the remainder were classified as nociceptive-specific (NS).
Fig. 3.
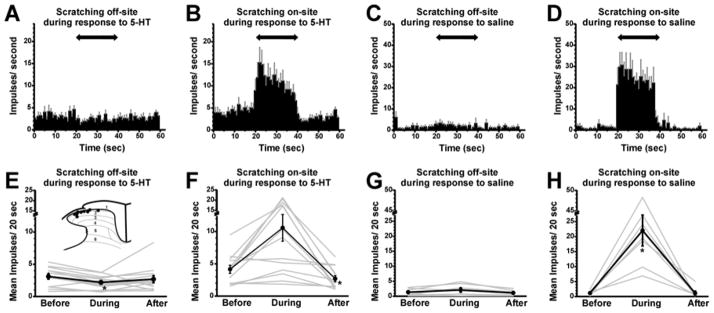
Effects of scratching on 5-HT-evoked firing. A: PSTH of averaged neuronal firing (n=13) following id 5-HT. Two-headed arrow indicates duration of off-site scratching. Gray error bars: SEM. B: as in A for on-site scratching. C: PSTH of averaged neuronal firing (n=6) following id saline, with off-site scratching. D: as in C for on-site scratching. E: Graph plots mean 5-HT-evoked firing rate before, during and after off-site scratching. Inset: spinal recording sites (dots) plotted on schematic of lumbar dorsal horn. Numbers indicate laminae. *: significantly different from before (p<0.05, Bonferroni following one-way repeated measures ANOVA). F: graph as in E for on-site scratching. During is significantly different from before (p<0.05, Bonferroni following One-way repeated measures ANOVA). After is significantly different from before (p<0.05, paired t-test). Please add different symbol at during. G: mean firing rate following id saline, with off-site scratching. *: significantly different from before (p<0.05, Bonferroni following One-way repeated measures ANOVA). H: graph as in G for on-site scratching. *: significantly different from before (p<0.05, Bonferroni following One way repeated measures ANOVA).
5-HT increased firing to a level that was significantly higher compared to the pre-injection baseline or to vehicle- (saline-) evoked firing (Fig. 1). Ten of 12 (83%) units tested responded to AITC, which elicited a significant increase in firing compared to the pre-injection baseline and to vehicle (mineral oil) application (Fig. 1). An example is shown in Fig. 2. This unit was located in the superficial dorsal horn and responded to id 5-HT. During the 5-HT-evoked response, on-site scratching further excited the neuron, while off-site scratching inhibited ongoing activity (Fig. 1, left-hand PSTH). Saline did not elicit a response. Following id saline, on-site scratching also excited the unit while off-site scratching had no effect (Fig. 2, middle PSTH). The unit responded to a second id injection of 5-HT (Fig. 2, 3rd PSTH from left). Application of mineral oil (AITC vehicle) had no effect, and on- (but not off-) site scratching again exited the unit (Fig. 2, 4th PSTH from left). Application of AITC excited the unit, and AITC-evoked firing was not inhibited by either on- or off-site scratching (Fig. 2, right-hand PSTH).
Fig. 1.
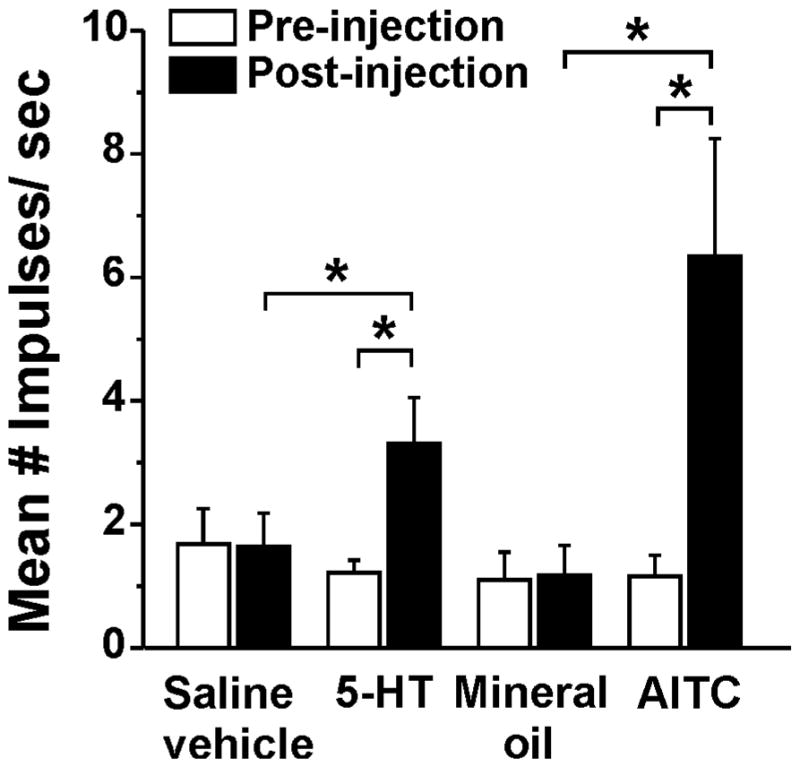
Activation of superficial dorsal horn neurons by 5-HT, AITC and vehicles. Bar graph plots mean firing rate (calculated over 60 sec) pre- (open bars) and post-injection (filled bars). Error bars: SEM. *: significantly different (p<0.05, paired t-test).
Fig. 2.
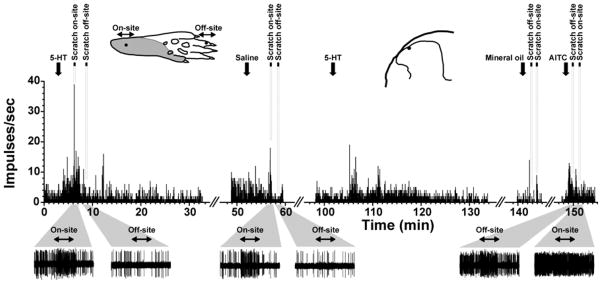
Individual example. Upper: peristimulus-time histogram (PSTHs; bin width: 1 sec) of unit located in superficial dorsal horn. Downward arrows indicate time of application of indicated stimuli. Times of on- and off-site scratching are indicated by black bars with dashed lines. Upper left inset: drawing of hindpaw showing receptive field (gray) and locations of on-site and off-site scratching (dots and horozintal two-headed arrows). Upper right inset: recording site (dot) on drawing of lumbar spinal section. Lower: Gray trapezoids beneath PSTH indicate 60-sec time windows that have been expanded to show spike traces (bottom row) of neuron’s response before, during and after scratching. Scratch period indicated by two-headed arrows.
Effects of scratching on 5-HT-evoked firing are summarized in Fig. 3. Fig. 3A presents an averaged peristimulus-time histogram (PSTH) showing that 5-HT-evoked ongoing activity was suppressed during off-site scratching. Fig. 3E plots responses before, during and after off-site scratching for individual units (gray lines) and for the population average (thick black line with error bars). Firing was significantly reduced during off-site scratching (from a mean of 3.1 +/− 0.4 [SEM] impulses/20 sec to 2.19 +/− 0.29 impulses/20 sec) followed by recovery (2.69 +/− 0.54). Fig. 3B and F show that firing was enhanced during on-site scratching (from 4.15 +/− 0.62 to 10.58 +/− 2.07 impulses/20 sec), followed by a significant reduction in firing post-scratching (2.7 +/− 0.46 impulses/20 sec). The low level of neuronal activity following id saline injection was not significantly affected by off-site scratching (Figs. 3C, G; before: 1.57 +/− 0.45, during: 2.42 +/− 0.77; after: 1.3 +/− 0.35 impulses/20 sec) but was significantly enhanced during on-site scratching (Figs. 3D, H; before: 1.33 +/− 0.32; during: 21.2 +/− 5.6; after 1.3 +/− 0.71 impulses/20 sec).
Fig. 4 similarly presents data following application of AITC or its vehicle, mineral oil. Off-site scratching had no significant effect on AITC-evoked firing (Fig. 4A, E; before: 4.78 +/− 1.7, during: 4.5 +/− 1.3; after: 3.96 +/− 1.19 impulses/20 sec), whereas activity was enhanced during on-site scratching (Fig. 4B, F; before: 6.29 +/− 1.7; during: 14.27 +/− 4.6; after: 3.93 +/− 1.27 impulses/20 sec). The low level of activity following mineral oil application was not significantly affected by off-site scratching (Fig. 4C, G; before: 0.93 +/− 0.28; during: 1.3 +/− 0.61; after 1.21 +/− 0.64 impulses/20 sec) and was significantly enhanced by on-site scratching (Fig. 4D, H; before: 1.31 +/− 0.67, during: 18.61 +/− 4.8; after: 2.53 +/− 1.72 impulses/20 sec).
Fig. 4.
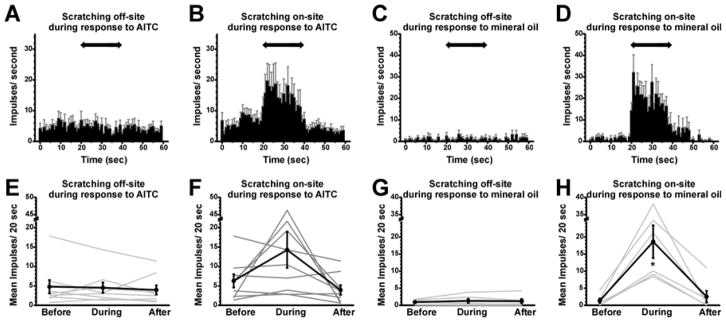
Effects of scratching on AITC-evoked firing. A: PSTH of averaged neuronal firing (n=9) following application of AITC (format as in Fig. 3A). B: as in A for on-site scratching. C: PSTH of averaged neuronal firing (n=6) following application of mineral oil, with off-site scratching. D: as in C for on-site scratching. E: Graph plots mean AITC-evoked firing rate before, during and after off-site scratching (format as in Fig. 3E). F: graph as in E for on-site scratching. G: mean firing rate following application of mineral oil, with off-site scratching. H: graph as in G for on-site scratching. *: significantly different from before (p<0.05, Bonferroni following one-way repeated measures ANOVA).
Discussion
The present results are consistent with previous studies reporting that scratching inhibited the responses of primate spinothalamic tract neurons to id histamine (Davidson et al., 2009) and inhibited responses of mouse superficial dorsal horn neurons to id histamine and chloroquine (Akiyama et al., 2012). Both of these prior studies reported that the scratch inhibition was state-dependent: scratching inhibited responses to pruritogens but not to the algogen, capsaicin, a finding that is confirmed in the present study for a different algogen, AITC. Moreover, we previously observed that pruritogen-evoked firing of murine dorsal horn neurons was suppressed during off-site scratching, and after the termination of on-site scratching (Akiyama et al., 2012), as confirmed in the present study for 5-HT-responsive neurons in the rat.
The neurons were presently identified using a 5-HT search strategy. It is possible that our recording methods, using tungsten microelectrodes, may have had a bias toward isolating relatively larger neurons although we cannot prove this. We did not presently attempt to identify the projection status of the recorded neurons, and it is possible if not likely that some were ascending projection neurons. In the rat, spinothalamic projections from neurons in the superficial dorsal horn are more sparse from mid-lumbar compared to mid-cervical segments, while spinoparabrachial projections from lamina I are relatively more dense from mid-lumbar compared to mid-cervical segments (Burstein et al., 1990; Al-Khater & Todd, 2009). Thus, some of the present 5-HT-sensitive neurons may have projected to the parabrachial nucleus while fewer would be expected to project to thalamus. An unknown percentage of 5-HT-sensitive neurons may have been local interneurons without ascending projections. Our present approach did not allow us to distinguish between interneurons and projection neurons.
It should be noted that the present lumbar 5-HT-sensitive units received afferent input from the hindlimb, whereas most prior studies of itch-related scratching behavior have involved injections of pruritogens into skin at the nape of the neck, or the cheek. However, intradermal injection of 5-HT into the mouse hindpaw elicited biting directed toward the injection site (Hagiwara et al., 1999). The 5-HT-evoked biting was suppressed by naloxone, supporting the argument that biting is an itch-related behavior. We and others have provided additional data supporting the idea that biting directed toward a hindlimb site of pruritic stimulation reflects itch (Akiyama et al., 2010; LaMotte et al., 2011). Thus, we believe that our present investigation of 5-HT-sensitive lumbar neurons is relevant to mechanisms of itch arising from the hindlimbs.
The majority of pruritogen-sensitive spinal neurons in cats, rats, mice and primates also respond to algogens such as capsaicin or AITC (Andrew & Craig, 2001; Jinks & Carstens 2002; Simone et al., 2004; Davidson et al., 2007, 2012; Akiyama et al., 2009a, b). We postulated that neurons responsive to both pruritogens and algogens selectively signal itch, while nociceptive neurons that are unresponsive to pruritogens selectively signal pain (Akiyama et al., 2009a, b). This concept is supported by recent evidence showing that primary afferent neurons expressing MrgprA3, which responds to the itch mediator chloroquine (Liu et al., 2009), coexpress TRPV1 and also respond to capsaicin (Han et al., 2013). Cutaneous application of capsaicin elicited itch-, rather than pain-, related behavior in global TRPV1 knockout mice in whom TRPV1 was selectively re-expressed in MrgprA3-expressing primary sensory neurons (Han et al., 2013). If neurons sensitive to both pruritogens and algogens signal itch, then the itch-relieving effect of scratching might be explained by the selective inhibition of the pruritogen-evoked responses of such neurons. Off-site scratching presumably excited spinal inhibitory interneurons that inhibited pruritogen-evoked neuronal activity. On-site scratching presumably activated nociceptors that directly excited the pruritogen-responsive neurons, as well as spinal inhibitory interneurons that inhibited the pruritogen-sensitive neurons after scratching had ceased (Fig. 2B). The post-scratch inhibition observed presently is consistent with previous electrophysiological studies (Davidson et al., 2009; Akiyama et al., 2012) as well as human studies in which scratching suppressed histamine- or cowhage-evoked itch, followed by a return of itch sensation within 6–12 sec after scratching ceased (Kosteletzky et al., 2009). However, an older study reported that scratching suppressed cowhage-evoked itch sometimes for several minutes (Chapmann et al., 1960). The enhanced neuronal firing during on-site scratching implies increased itch, which may be masked by concurrent pain, with itch relief occurring at the termination of scratching when inhibition dominates.
It is unclear why scratching failed to inhibit the AITC- or capsaicin-evoked responses of pruritogen-sensitive neurons. One possibility is that the nociceptors activated by skin scratching do not excite spinal itch-inhibitory interneurons. However, this is not supported by recent studies. “Bhlhb5” knockout mice lacking a subpopulation of inhibitory interneurons exhibited significantly enhanced spontaneous and pruritogen-evoked scratching behavior (Ross et al., 2010). Such interneurons may release GABA and/or glycine as neurotransmitters to inhibit itch-signaling spinal neurons (Akiyama et al., 2011). Moreover, genetic deletion of the vesicular glutamate transporter-2 (VGLUT-2) in nociceptors, which reduced central nociceptive neurotransmission, resulted in enhanced scratching behavior (Lagerstrom et al., 2010; Liu et al., 2010). These studies suggest that nociceptors do excite spinal itch-inhibitory interneurons, and that removal of the nociceptive input and/or inhibitory spinal interneurons disinhibits itch transmission. Further studies are needed to understand the mechanism by which a pruritic stimulus allows scratching to excite spinal inhibitory interneurons to inhibit pruriceptive spinal transmission, while an algesic stimulus apparently prevents scratching from exciting itch-inhibitory interneurons.
Although the present data support a spinal site for scratch inhibition of itch transmission, they do not rule out an additional supraspinal contribution. We previously reported that scratch-evoked inhibition of ongoing spinal neuronal activity in a mouse model of dry skin pruritus was reduced by approximately 50% following disruption of descending spinal pathways at the upper cervical level (Akiyama et al., 2011), implicating a partial role for descending pathways in scratch inhibition of itch. Currently little is know about how descending pathways modulate spinal itch transmission, and how they may contribute to the state-dependency of scratch-evoked inhibition.
5-HT-evoked neuronal activity was inhibited during off-site scratching, consistent with our previous study in mice (Akiyama et al., 2012). The inhibitory effect of scratching at a distance from the site of 5-HT injection is consistent with older human studies showing that itch could be relieved by scratching skin within the same dermatome at distances of up to 24 mm from the site of the experimental pruritic stimulus (Graham et al., 1951; Chapmann et al., 1960). It is also noteworthy that patients with chronic itch exhibited scratch marks that were aligned along dermatomal lines, with the length of the scratch mark correlated with tactile discrimination (Cornbleet 1953; Savin et al., 1999).
Conclusions
Scratching inhibited responses of superficial dorsal horn neurons to the pruritogen, 5-HT, but not the algogen AITC, indicating that the effect of scratching is state (i.e., stimulus) -dependent. Off-site scratching inhibited 5-HT- but not AITC-evoked firing. On-site scratching facilitated neuronal responses to both 5-HT and AITC, with only 5-HT-evoked firing being significantly inhibited following the end of scratching. These data indicate that the effect of scratching is also site-dependent. The present results are consistent with observations that itch can be relieved by scratching at or distant from a focal site of pruritic stimulation, and support a spinal cord site of action by which scratching inhibits the transmission of itch signals.
Highlights.
Off-site scratching inhibited spinal neuronal responses to 5-HT but not AITC
On-site scratching facilitated neuronal responses to both 5-HT and AITC
5-HT (but not AITC) -evoked firing was inhibited after on-site scratching
Results support a spinal cord site for scratch-inhibition of itch transmission
Acknowledgments
Supported by grants from the National Institutes of Health (DE013685 and AR057194). T. Akiyama received a postdoctoral fellowship from the Japan Society for the Promotion of Science.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- AITC
allyl isothiocyanate
- ANOVA
analysis of variance
- DRG
dorsal root ganglion
- Mrgpr
mas-related G-protein-coupled receptor
- NK-1
neurokinin-1
- NS
nociceptive-specific
- PSTH
peristimulus-time histogram
- TRPV1
transient receptor potential vanilloid-1
- VGLUT-2
vesicular glutamate transporter-2
- WDR
wide dynamic range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol. 2009a;102:2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett. 2010;484(1):62–65. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PloS One. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci. 2009b;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Carstens MI, Carstens E. Site-dependent and state-dependent inhibition of pruritogen-responsive spinal neurons by scratching. Eur J Neurosci. 2012;36(3):2311–2316. doi: 10.1111/j.1460-9568.2012.08136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III, and IV of rat spinal cord to thalamus, periaqueductal gray matter, and lateral parabrachial area. J Comp Neurol. 2009;515(6):629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4(1):72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Broekkamp CLE. A peripheral 5-HT1D–like receptor involved in serotonergic induced hindlimb scratching in rats. Eur J Pharmacol. 1991;194:201–208. doi: 10.1016/0014-2999(91)90106-z. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Giesler GJ., Jr The cells of origin of the spinothalamic tract of the rat: a quantitative reexamination. Brain Res. 1990;511(2):329–337. doi: 10.1016/0006-8993(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Carstens E. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol. 1997;77(5):2499–2514. doi: 10.1152/jn.1997.77.5.2499. [DOI] [PubMed] [Google Scholar]
- Carstens E, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21(4):303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapmann L, Goodell H, Wolff HG. Structures and processes involved in the sensation of itch. In: Montagna W, editor. Advances in biology of skin. Vol. 1. New York: Pergamon; 1960. pp. 161–188. [Google Scholar]
- Cornbleet T. Scratching patterns. 1. Influence of site. J Investig Dermatol. 1953;20:105–110. [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108(6):1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nature Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27(37):10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DT, Goodell H, Wolff HG. Neural mechanisms involved in itch, itchy skin, and tickle sensations. J Clin Investig. 1951;30:37–49. doi: 10.1172/JCI102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachisuka J, Furue H, Furue M, Yoshimura M. Responsiveness of C neurons in rat dorsal root ganglion to 5-hydroxytryptamine-induced pruritic stimuli in vivo. J Neurophysiol. 2010;104(1):271–279. doi: 10.1152/jn.00938.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Nojima H, Kuraishi Y. Serotonin-induced biting of the hind paw is itch-related response in mice. Pain Res. 1999;14:53–59. [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16(2):174–82. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87(3):1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Simons CT, Dessirier JM, Carstens MI, Antognini JF, Carstens E. C-fos induction in rat superficial dorsal horn following cutaneous application of noxious chemical or mechanical stimuli. Exp Brain Res. 2002;145(2):261–269. doi: 10.1007/s00221-002-1128-3. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011;106(3):1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Handwerker HO, Torebjork HE. The ability of humans to localise noxious stimuli. Neuroscience Lett. 1993;150:219–222. doi: 10.1016/0304-3940(93)90540-2. [DOI] [PubMed] [Google Scholar]
- Kosteletzky F, Namer B, Forster C, Handwerker HO. Impact of scratching on itch and sympathetic reflexes induced by cowhage (Mucuna pruriens) and histamine. Acta Dermato-Venereol. 2009;89:271–277. doi: 10.2340/00015555-0624. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Lind AL, Olund C, Smith C, Mendez JA, Wallén-Mackenzie Å, Wood JN, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol. 2011;20(10):778–782. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray FS, Weaver MM. Effects of ipsilateral and contralateral counterirritation on experimentally produced itch in human beings. J Comp Physiol Psychol. 1975;89:819–826. doi: 10.1037/h0077028. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. 5-Hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J Pharmacol Exp Ther. 2003a;306(1):245–252. doi: 10.1124/jpet.103.049239. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. J Neurosci Meth. 2003b;126(2):137–143. doi: 10.1016/s0165-0270(03)00074-8. [DOI] [PubMed] [Google Scholar]
- Nojima H, Cuellar JM, Simons CT, Carstens MI, Carstens E. Spinal c-fos expression associated with spontaneous biting in a mouse model of dry skin pruritus. Neurosci Lett. 2004;361(1–3):79–82. doi: 10.1016/j.neulet.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, Carstens E. Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci. 2003;23(34):10784–10790. doi: 10.1523/JNEUROSCI.23-34-10784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin JA, Aoki T, Bielska CA. Some observations on the direction of scratch marks. J Amer Acad Dermatol. 1999;41:1035–1039. doi: 10.1016/s0190-9622(99)70271-9. [DOI] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91(1):213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Spradley JM, Davoodi A, Carstens MI, Carstens E. Opioid modulation of facial itch- and pain-related responses and grooming behavior in rats. Acta Derm Venereol. 2012;92(5):515–520. doi: 10.2340/00015555-1364. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448(7154):700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol. 2001;81:250–254. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Wahlgren CF. Itch and atopic dermatitis: an overview. J Dermatol. 1999;26:770–779. doi: 10.1111/j.1346-8138.1999.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64(1):129–138. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G, Fast K, Bernhard JD. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Investig Dermatol. 2005;125:1268–1272. doi: 10.1111/j.0022-202X.2005.23942.x. [DOI] [PubMed] [Google Scholar]


