Abstract
Objective
Edema formation, inflammation and ileus in the intestine are commonly seen in conditions like gastroschisis, inflammatory bowel disease and cirrhosis. We hypothesized that early enteral feeding would improve intestinal transit. We also wanted to study the impact of early enteral feeding on global gene expression in the intestine.
Design
Rats were divided into Sham or Edema ± immediate enteral nutrition (IEN). At 12 hrs, small intestinal transit via FITC-Dextran and tissue water were measured. Ileum was harvested for total RNA to analyze gene expression using cDNA microarray with validation using real-time PCR. Data are expressed as mean ± SEM, n=4-6 and *, ** = p < 0.05 vs. all groups using ANOVA.
Results
IEN markedly improved intestinal transit with minimal genetic alterations in Edema animals. Major alterations in gene expression were detected in primary, cellular and macromolecular metabolic activities. Edema also altered more genes involved with the regulation of the actin cytoskeleton.
Conclusions
Intestinal edema results in impaired small intestinal transit and globally increased gene expression. Early enteral nutrition improves edema-induced impaired transit and minimizes gene transcriptional activity.
Keywords: intestinal edema, ileus, enteral feeding, micorarray
Introduction
The gut is particularly susceptible to injury, which can result in gut edema, ileus, and failure of intestinal defense mechanisms. These factors can delay recovery, increase morbidity and hospital length of stay (1-4). Intestinal edema formation, inflammation and ileus are commonly seen in patients affected with gastroschisis, inflammatory bowel disease and cirrhosis. Excessive intraoperative crystalloid fluid administration during gastrointestinal surgery results in intestinal edema and ileus (5). Fluid overload has been postulated to contribute to feeding intolerance and ileus and bacterial translocation associated with gut edema (6-7). Another major risk factor associated with intestinal ileus is inflammation. Pro-inflammatory mediators like inducible nitric oxide synthase, cyclo-oxygenase-2, interleukin-6 and recruitment of leukocytes to the intestine have been shown to impair gut motility in animal models (8-11). Our group has focused on defining the role gut edema plays in intestinal dysfunction by using a reproducible edema model of acute mesenteric venous pressure elevation and intravenous fluid resuscitation. We have previously shown that gut edema promotes intestinal ileus, depresses intestinal smooth muscle contractility and increases mucosal permeability without ischemia/reperfusion injury (12-13).
Malnutrition is recognized as a cause of increased morbidity and mortality in surgical patients and intestinal dysmotility creates a major dilemma in delivering enteral nutrition in critically ill patients. The timing and route of administration of feeding patients with ileus has not been optimized to date. Moore et al. (14), evaluated tolerance of enteral nutrition after major injury. This study found that 50% of patients undergoing surgery had fair to poor tolerance to early enteral nutrition. However, in this study early enteral nutrition was not given until 24 hours after the last surgery. The purpose of this study was to investigate if administration of immediate enteral nutrition (IEN) would improve intestinal transit and modulate gene expression in intestinal edema.
Materials and Methods
All procedures were approved by the University of Texas Animal Welfare Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animal.
Operative procedures and experimental groups
Male Sprague-Dawley rats, weighing between 300-350 grams (Harlan Labs, Indianapolis, Indiana, USA), were fasted 16-18 hours prior to surgery and given free access to water. Under general anesthesia with isoflurane using aseptic technique, an intravenous catheter was placed by surgical exposure and direct placement of catheter into the jugular vein. Next, a midline laparotomy incision was made and a silastic catheter introduced into the mid-duodenum via needle puncture and secured with a 6-0 silk purse-string suture. The catheter was brought through the musculature of the left abdominal wall and subcutaneous tissue toward the back of the neck where it was exteriorized through an interscapular incision and fixed to the skin with 4-0 silk suture. Prior to closure of mid-line incision, a 4-0 silk ligature was placed around the superior mesenteric vein (SMV) and tied over a PE-10 silastic tube, which was removed after ligature was secured and 80cc/kg body weight of 0.9% saline administered via the jugular venous catheter. This acute elevation in SMV pressure allowed for the creation of acute intestinal edema (Edema). The experiment included four groups: Sham, Sham given IEN, Edema and Edema given IEN. A micro-pump was attached to a backpack and connected to the duodenal catheter and the enteral formula was started at 0.4 cc/kg/hour and run continuously for 12 hours. Table 1 depicts the enteral formula given to the animals in this study. This formula was adapted and modified to keep the formula isotonic (15).
Table 1.
Composition of nutrient solution
| Nutrient solution | mL/L* |
|---|---|
| Travasol 10%† | 500 |
| D50W | 500 |
| Electrolytes‡ | 20 |
| Potassium phosphates‡ | 6 |
| MVI-12° | 5 |
Amount of the respective solutions added
Contains the following: essential amino acids leucine (730 mg), isoleucine (600 mg), lysine (580 mg), valine (580 mg), phenylalanine (560 mg), histidine (480 mg), threonine (420 mg), methionine (400 mg), and tryptophan (180 mg); non-essential amino acids alanine (2.07 g), arginine (1.15 g), glycine (1.03 g), proline (680 mg), serine (500 mg), and tyrosine (40 mg).
Abbott Laboratories, North Chicago, IL, USA.
Astra Pharmaceuticals, Westborough, MA, USA.
D50W, 50% dextrose injection, USP; TPN, total parenteral nutrition.
Intestinal Transit
The effect of IEN on intestinal motility in Sham and Edema animals was measured in vivo using 0.1 ml of a 5 mM solution of non-absorbable FITC-Dextran injected into the duodenal catheter. Intestinal transit for each study was measured at the specified time points above; 0.1 mL of a 5-mmol/L solution of nonabsorbable fluorescein isothiocyanate (FITC)-dextran (molecular weight, 9,400; FITC content, 0.008 mol/mol glucose; Sigma-Aldrich, St. Louis, MO) was injected into the duodenal catheter and flushed with 0.1 mL of normal saline. Twenty-five minutes after FITC-dextran injection, rats were anesthetized with an intraperitoneal injection of ketamine and the entire small intestine was removed and divided into 10 equal segments. The intraluminal contents of each segment were flushed with 3 mL of 5-mmol/L Tris-buffer (pH 10.3) to recover the FITC-dextran. The FITC-dextran concentration was measured using an optical scanner to read the optical density of the FITC-dextran (STORM model 860, Amersham Biosciences, Piscataway, NJ) and expressed as a fraction of total tracer recovered and presented as the geometric center of distribution as described previously in the literature (9,16-17). The distal ileum was harvested and snap frozen and stored at -80°C for RNA extraction. Intestinal transit for each study was measured at the specified time points above
Intestinal Water Determination
The small intestine from the duodenum to the ileum at the ileocecal valve was harvested at 12 hours to determine the tissue water content. Intestinal samples were opened along the antimesenteric border and blotted dry. Wet weight was determined prior to placing tissue in an oven set to 60°C. Tissues were dried until a constant dry weight was obtained over 2-3 days and dry weights were measured and used to determine tissue water content using the following formula: ((wet weight) – (dry weight)/dry weight).
Gene Expression Studies
Rat 30K-Microarray Analysis
The effect of IEN on global gene expression in Sham and Edema animals was measured using a dual-labeled rat 30-K Agilent (Agilent Technologies, Palo Alto, CA) microarray slide. Total RNA was isolated from the distal ileum using RNAzol Bee (Tel-Test, Friendswood, TX), precipitated and washed with chloroform and isopropanol then stored in DEPC-treated water. The RNA samples were checked for degradation and DNA contamination on an Agilent 2100 Bioanalyser using an Agilent RNA 6000 Nano Kit as recommended by the manufacturer. The RNA was labeled with an Agilent Fluorescent Direct Labeling Kit and Perkin Elmer Cyanine-3 or Cyanine-5 dye (Perkin Elmer Life Sciences, Boston, MA). The experiment included three groups (n=4-5 animals pooled RNA): 1) Sham versus 2) Sham given IEN (Sham/IEN), 3) Edema and 4) Edema given IEN (Edema/IEN). The dual-fluorescently labeled cDNA were combined and purified using the Agilent recommended procedure for each microarray chip. Hybridization and post-hybridization washes were performed on an Agilent cDNA microarray using the manufacturers recommended procedure except the hybridization temperature was lowered to 60°C. Following hybridization and washing, microarrays were scanned using an Axon 4200-Two Channel Scanner (Axon, Sunnyvale, CA). The scanned microarrays were analyzed and significant fold changes were determined using GenePix 5.1 software from Axon. Significant fold changes for genes were determined by taking the log2 ratio of the expression between Edema with or without IEN and Sham/IEN vs. Sham animals (log2 ratio = Experimental Group/Sham Group). Genes with a log2 ratio ≥ 1 (> 2-fold up) were considered up-regulated and ≤ 1 (< 2 fold down) were considered down-regulated. Following filtration and normalization of each qualified gene, hierarchical clustering and visualization were carried out using Cluster 3.0 and TreeView software (Eisen Lab, Stanford, CA). Genes were further categorized using the gene ontology DAVID-EASE software from the National Institute of Health (http://david.abcc.ncifcrf.gov/). This software enables the visualization of molecular interactions from our gene expression data set by overall functional classification and interactive gene maps developed by various gene consortiums in the field of genomics.
SYBR Green Real-Time Quantitative Polymerase Chain Reaction
Microarray validation was performed by using real time- polymerase chain reaction (RT-PCR) for selected gene pathways identified from the DAVID-EASE analysis from the same animals used in the microarray experiment. The primer sequences used for the validation of the microarray experiment are shown in Table 2. cDNA was generated from the total RNA (10 ng) from the distal ileum in the microarray experiment using SuperScript™ First-Strand Synthesis kit (Invitrogen, Carslbad, CA). Primer sequences were designed using AmplifX 1.1 program directed at both the 3′ and 5′ end of the gene sequence. Gene-specific primers used in this study are listed in Table 2.
Table 2.
Primer Sequences
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Actin | 5′-TCT GGA GAA GAG CTA TGA GCT GCC TG -3′ | 5′-TCG TGC CAC CAG ACA GCA CTG TGT TG -3′ |
| SMOTH-26 | 5′-TAA GTG CGT CTT CAC CTA CGT GCA-3′ | 5′-TGC ACG TAG GTG AAG ACG CAC TTA-3′ |
| HDAC5 | 5′-CCT GTT CGC TGA GTT CCA GAA ACA-3′ | 5′-TGT TTC TGG AAC TCA GCG AAC AGG-3′ |
| v-CRK(Crkll) | 5′-ATT CCT GTC CCT TAC GTG GAG AAG-3′ | 5′-CTT CTC CAC GTA AGG GAC AGG AAT-3′ |
| TGF-B2 | 5′-TGA AAC GGA AGC GCA TCG AA-3′ | 5′-TT CGA TGC GCT TCC GTT TCA-3′ |
| TRADD | 5′-TGA GCT CTG CAA ACT GAC GTG T-3′ | 5′-CAC GTC AGT TTG CAG AGC TCA-3′ |
| ITGB4 | 5′-ATT CAT CCA ACA TCG TGG AGC TGC-3′ | 5′-GCA GCT CCA CGA TGT TGG ATG AAT-3′ |
| Cdnk1 | 5′-TTG GAG AAG CAC TGC CGA GAT ATG-3′ | 5′-CAT ATC TCG GCA GTG CTT CTC CAA-3′ |
| BAD | 5′-AAC ACA GAT GCG ACA AAG CGC-3′ | 5′-GCG CTT TGT CGC ATC TGT GTT-3′ |
| TGF-B1 | 5′-TGT TCG TGA CGT GAG GGA GTT TTG-3′ | 5′-CAA AAC TCC CTC ACG TCA CGA ACA-3′ |
Real-time quantitative polymerase chain reaction (RT-PCR) was performed using a Sybr Green protocol to confirm microarray results. Due to the inherent inaccuracies in quantifying cDNA by absorbance, the amount of cDNA added to an RT-PCR from each sample was more accurately determined by measuring a housekeeping transcript level in each sample (β-Actin). Selected candidate genes were identified (n=3 per group, randomly selected from the study samples). Relative mRNA expression (CT) was quantified in triplicate and normalized to β-actin and calculated relative to Sham using the 2(−ΔΔCT) method (18-20).
Data analysis
All data for transit, tissue water and histology are expressed as mean ± SEM using a commercial statistical software program (NCSS, Kaysville, UT). Statistical significance of differences among groups was determined by one way analysis of variance (ANOVA) followed by Duncan's multiple comparison test. A P-value <0.05 was considered significant. n=4-6. Significant fold changes were determined by using the GenePix 5.1 software (Axon). Significant fold changes for genes were determined by taking the log2 ratio of the expression between Edema with or without IEN and Sham/IEN vs. Sham animals (log2 ratio = Experimental Group/Sham Group). Genes with a log2 ratio ≥ 1 (> 2-fold up) were considered up-regulated and ≤ 1 (< 2 fold down) were considered down-regulated.
Results
Intestinal Transit
The effect of IEN on intestinal transit in Sham and Edema animals was measured in vivo using FITC-Dextran. As depicted in Figure 1, the average mean geometric center (MGC) in Edema (3.4 ± 0.1*) was significantly decreased compared to Sham (4.7 ± 0.2**) animals. IEN significantly improved intestinal transit in Edema (Edema/IEN: 4.6 ± 0.3**) animals and was comparable to Sham animals with or without administration of IEN (Sham/IEN: 5.8 ± 0.4*) (ANOVA: F statistic = 13.9, p value = 0.00004).
Figure 1. Intestinal transit.
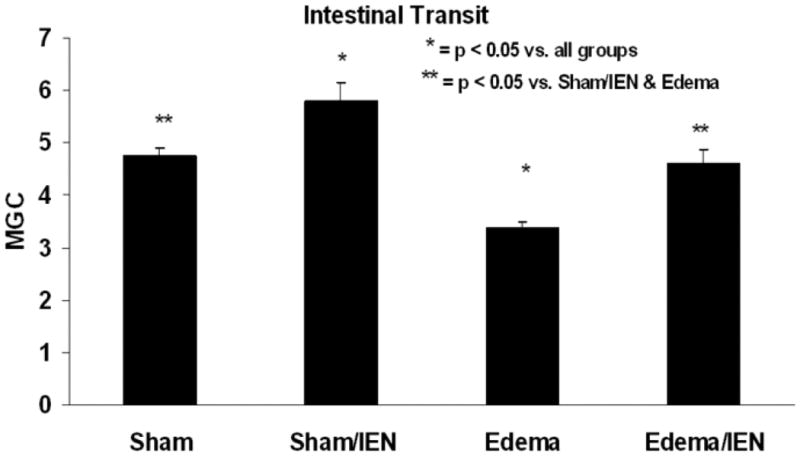
Rats were subjected to Sham surgery with and without IEN (Sham and Sham/IEN), acute elevation of SMV pressure and crystalloid resuscitation with and without IEN (Edema and Edema/IEN). Intestinal transit of tracer along intestinal segments was then measured as detailed in “Methods.” The mean geometric center (MGC) of tracer is presented. *P < 0.05 for Edema versus all other groups, using one-way ANOVA. n = 5-6.
Intestinal Water Determination
We next sought to determine the tissue water content in our acute intestinal edema model and the effects of IEN on intestinal water balance. The tissue wet to dry weight ratio in Edema (3.8 ±0.0) animals was greater than Sham (3.2 ± 0.1*) animals (Figure 2). However, immediate enteral nutrition (3.6 ± 0.1) only slightly decreased the fluid accumulation in the intestine associated with our acute intestinal edema model. IEN also significantly increased the tissue water weight in Sham/IEN (3.6 ± 0.1) animals compared to Sham animals (ANOVA: F statistic = 5.9, p value = 0.005).
Figure 2. Tissue water weight.
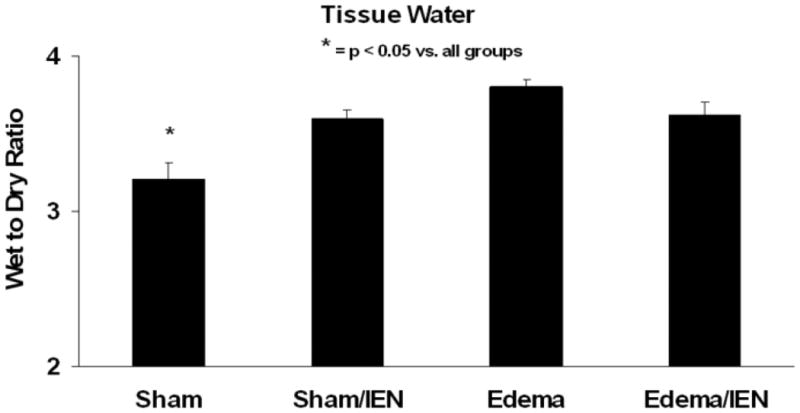
Rats were subjected to Sham surgery with and without IEN (Sham and Sham/IEN), acute elevation of SMV pressure and crystalloid resuscitation with and without IEN (Edema and Edema/IEN). Tissue water weight is calculated as a wet weight to dry weight ratio and expressed. *P < 0.05 for Sham versus Sham/IEN, Edema and Edema/IEN, using one-way ANOVA. n = 5-6.
Gene Expression Studies
Feeding effects on global gene expression analysis
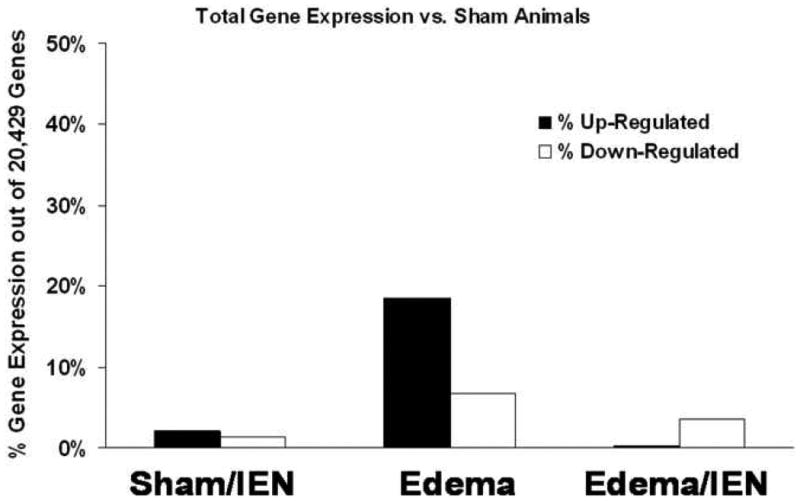
The effect of IEN on global gene expression in Sham and Edema animals was measured using a dual-labeled rat 30-K microarray slide. As shown in Figure 3, the global gene changes in Edema animals was greater with a 18% up regulated and 7% down regulated gene effect compared with Edema animals receiving IEN with a 0.2% up regulated and 4% down regulated gene effect. IEN did not alter global gene changes among the Sham animals with a 2% up regulated and 1% down regulated gene effect.
Figure 3. Global gene expression versus Sham animals.
Differentially expressed genes: Cluster and functional classification analysis
Genes identified to be significantly up or down regulated using the Gene 5.1 software from Axon were examined using the Eisen Lab Cluster software. Three major gene clusters were identified and underwent hierarchical clustering analysis using the TreeView software (Figure 4). Cluster 1 contained 608 genes with interactions in the following pathways: oxidative phosphorylation, apoptosis and tight junctions. Figure 5 reflects the differentially regulated genes in the apoptotic gene pathway. The TNF-associated via death domain (TRADD, log2 ratio: 1.0), Interleukin-3 receptor (IL-3R, log2 ratio: 1.1), Nuclear factor kappa-B p105 subunit (NF-κB, log2 ratio: 2.0) and Bcl-2 associated death agonist (BAD, log2 ratio: 1.1) genes in Edema were up-regulated, but IEN did not alter these genes in Edema or Sham animals. Edema also down-regulated Baculovirus inhibitor of apoptosis (IAP, log2 ratio: -1.2), Calpain (log2 ratio: -1.4), Phosphatidylinositol 3-kinase (PI3K, log2 ratio: -1.6), Caspase 7 (Casp 7, log2 ratio: -1.2) and Apoptotic death agonist BID (log2 ratio: -1.2) genes, which were also not changed in the animals receiving IEN. Cluster 2 contained 7 genes with genes from the following functional categories: receptor activity and ion binding. Cluster 3 contained 622 genes with interactions in following pathways: Mitogen activated protein kinase (MAPK), Transforming growth factor-beta (TGF-β) and Janus kinase-signal transducer activating transcription factor (JAK-STAT) signaling. Edema down-regulated several genes: Actin-related protein 3 homolog (ARP3, log2 ratio: 2.0), Rho A - binding serine/threonine kinase (ROCK, log2 ratio: -1.7), v-crk avian sarcoma virus CT10 oncogene homolog (CrkII, log2 ratio: -1.3) and Myosin light chain kinase (MLCK, log2 ratio: -1.7) involved in the regulators of actin cytoskeleton pathway as depicted in Figure 6. IEN did not change the expression levels above genes except for MLCK, which was up-regulated in Edema/IEN animals. p21 (CDKN1A)-activated kinase 2 (PAK) was down regulated in Edema (log ratio: -2.5) and Edema/IEN (log2 ratio: -1.2) animals and up regulated in Sham/IEN (log2 ratio: 1.2) animals. Thymosin beta-4 (Tmsb4) was up regulated in the Sham/IEN and Edema animals, but not changed in the Edema/IEN animals. Figure 7 depicts the DAVID-EASE analysis obtained from the differentially expressed genes among the groups in other functional categories from all three clusters. Primary, cellular and macromolecular metabolic activities accounted for > 50% of the genes among all the groups in the gene ontology analysis. There was a very minimal response in the immune response genes among groups (Figure 7).
Figure 4. Hierarchical clustering of differentially regulated genes.
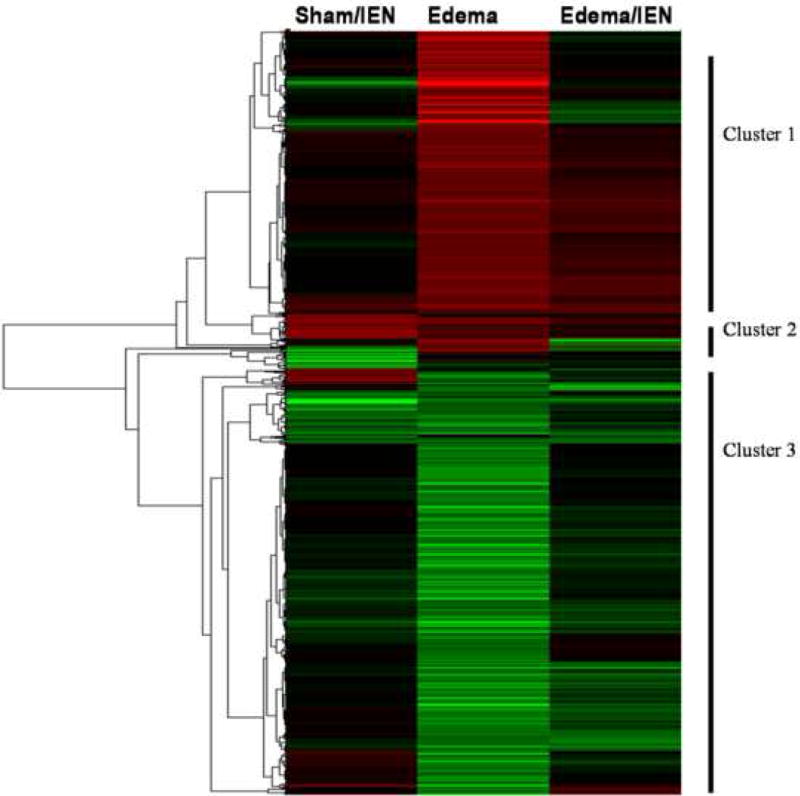
Cluster was performed by Treeview software. Intensity in the red and green color spectrum denotes up-regulated and down-regulated genes respectively. The black intensity denotes no change in gene expression levels compared to Sham animals. n = 4-5.
Figure 5. Apoptosis pathway differentially regulated genes.
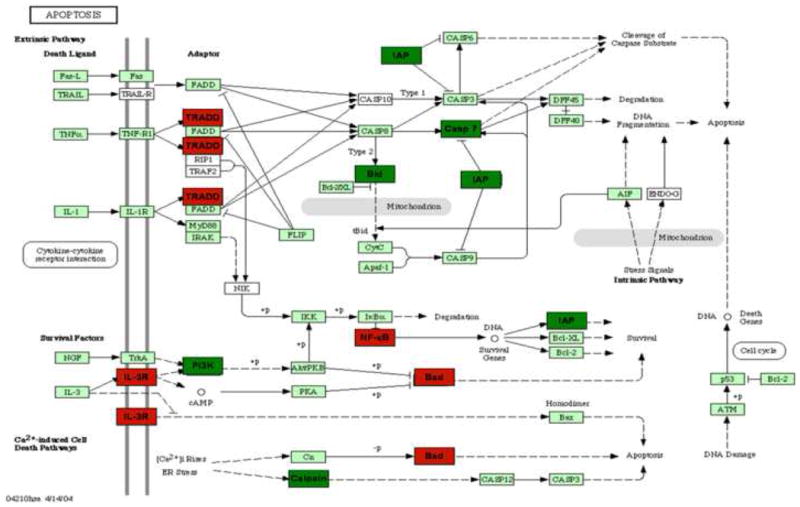
Intensity in the red and green color spectrum denotes up-regulated and down-regulated genes respectively. n = 4-5.
Figure 6. Regulators of Actin Cytoskeleton differentially regulated genes.
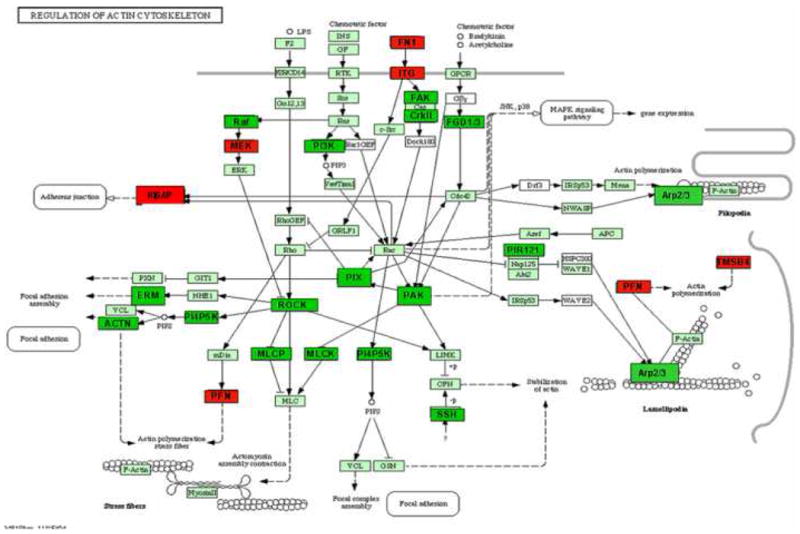
Intensity in the red and green color spectrum denotes up-regulated and down-regulated genes respectively. n = 4-5.
Figure 7. DAVID-EASE Gene Ontology of differentially regulated genes.
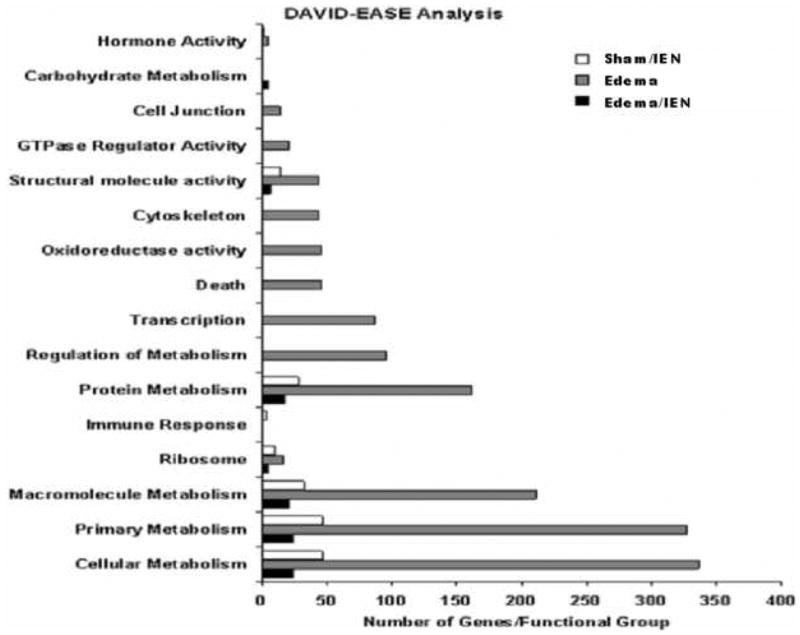
Gene ontology was performed using the DAVID-EASE software. The y-axis denotes the functional category assigned to individual gene ID numbers. The x-axis denotes the total number of genes both up and down-regulated in the groups. n = 4-5.
Quantitative Validating Real-Time PCR
Microarray validation was performed by using real time- polymerase chain reaction (RT-PCR) for selected gene from cluster 1 and 3 identified from the DAVID-EASE analysis. The quantitative PCR results compared to the microarray experiment for this study shown are shown in Figure 8.
Figure 8. A-C. Real Time QT-PCR.
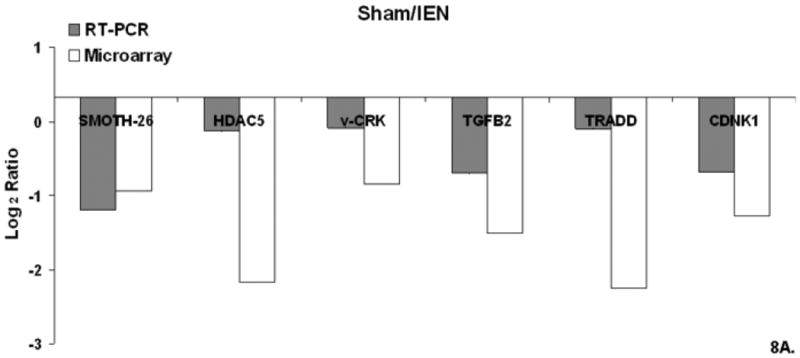
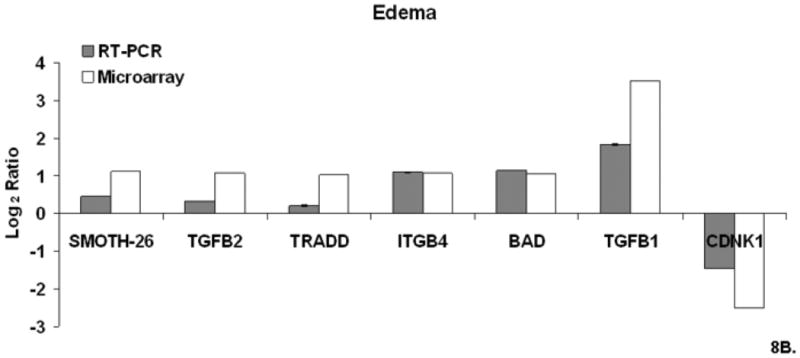
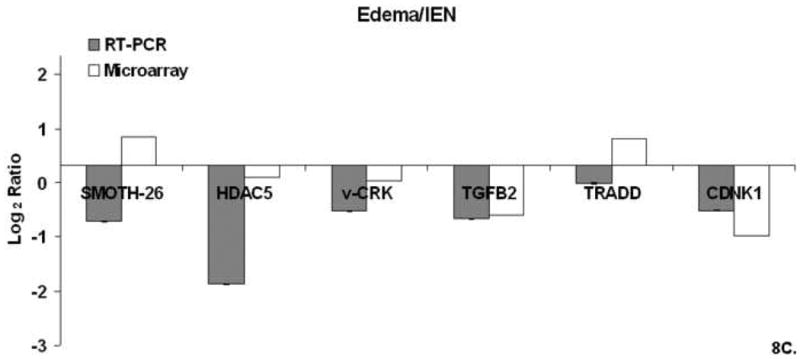
Relative mRNA expression (CT) was quantified in triplicate and normalized to β-actin and calculated relative to Sham using the 2(−ΔΔCT) method. Figure 8A. Sham/IEN QT-PCR. Figure 8B Edema QT-PCR. Figure 8C Edema/IEN QT-PCR.
Discussion
Our study shows that early enteral nutrition improved intestinal transit associated with gut edema and returned a majority of genes back to baseline expression. Although the majority of studies have focused on surgical manipulation and ischemia/reperfusion role in ileus, our group has focused on defining the role gut edema plays in intestinal dysfunction by using a reproducible acute intestinal edema model of acute mesenteric venous pressure elevation and intravenous fluid resuscitation. Intestinal transit has been used by our group and others (9, 11-12) as a surrogate marker for evaluating ileus. In this present study intestinal edema delayed intestinal transit. Delivering an intraluminal volume of a nutrient dense solution improved delayed intestinal transit associated with intestinal edema and only slightly improved intestinal tissue water. Acceleration in intestinal transit is also demonstrated in Sham animals receiving IEN. Owens et al. demonstrated in their neonatal enteral nutritional pup model that intestinal water weight increased as the % of enteral nutrition was increased and the migrating motor complex was greater in pups receiving more enteral nutrition compared to pups that did not receive enteral nutrition (21). This may account for the similar results since in our Sham/IEN group receiving enteral nutrition. Grossie et al. showed similar improvements in intestinal transit with delivery of enteral nutrition and intraluminal delivery of saline in a model of ischemia/reperfusion injury (15). We have also show in prior studies of resuscitation-induced gut edema and ischemic preconditioning (12, 22), that there was a threshold level of edema that needed to be generated before there was a delay in intestinal transit. In our resuscitation-induced gut edema model, gut edema was produced by acute mesenteric venous hypertension and crystalloid resuscitation, intestinal transit was shown to be depressed (12). In this same study, fluid resuscitation alone was enough to create intestinal edema, but there was a threshold level of edema that needed to be generated before there was a delay in intestinal transit. Late IPC produced comparable levels of fluid accumulation (edema) seen in the group of rats receiving fluid resuscitation only; however, in the setting of IPC, gut edema did not reach the threshold level necessary to delay intestinal transit seen in that study. In our current study of IEN, the Sham/IEN group produced tissue water levels comparable to the animals from our prior gut edema study that received crystalloid resuscitation without partial venous occlusion. Similar to the crystalloid group, the intestinal edema seen in our Sham/IEN did not reach the threshold level necessary to delay intestinal transit. Most of the prospective studies on early enteral feeding showed that early enteral feeding into the small intestine is well tolerated and reduces the duration of postoperative ileus (23-24); however all these studies consider ‘early’ > 24 hours after insult. Limited data exists on immediate enteral feeding in critically ill patients.
Smooth muscle contractility plays an important role in intestinal motility. The majority of studies evaluating ileus in the setting of intestinal injury have focused on inflammation in models of hemorrhagic shock, ischemia/reperfusion and surgical manipulation; yet few reports have investigated the role of cytoskeletal stress fiber alterations relationship to ileus after intestinal injury. Newer research on smooth muscle cell signaling and remodeling has provided evidence for mechanical stress induced alterations in smooth muscle contractility. We have previously shown that our edema model results in a 4-fold interstitial pressure within the submucosa (mechanical stress), which results in approximately a 4-fold increase in engineering stress applied to the smooth muscle (25). Radhakrishnan et al. performed detailed studies on the effects of intestinal edema on the tissue elastic modulus of intestinal tissue and its relationship to cytoskeletal proteins. As intestinal edema formation increased, elasticity increased with a concomitant delay in intestinal transit. Intermediate filaments like calponin and vimentin and cytoskeletal proteins like globular actin are the main tissue determinants of intestinal stiffness and they were decreased in the muscular layers of edematous intestine (25-26). Smooth muscle contractility is also affected by both extracellular matrix components and myosin light chain phosphorylation. Uray et al. demonstrated that intestinal edema decreased myosin-light chain contractility by decreasing phosphorylation of the myosin light chain (13). In this study we used cDNA microarray to help explore the differentially regulated genes related to contractile force proteins that may contribute to the delay in intestinal transit seen in our edema model. Our microarray data showed that edema had the greatest effect on cytoskeletal gene expression (Figure 7) with decreased expression of myosin light chain kinase mRNA (Figure 5) and increased calponin expression. IEN had the opposite effects on both MLCK and calponin mRNA expression, which may play a role in maintenance of the contractile apparatus in Edema/IEN animals.
Mechanical stress not only effects stress fiber formation, but has also been found to inhibit smooth muscle cell proliferation, promoting apoptosis and up-regulation of TGF-β transcription stimulating collagen deposition (28-30). In our study, intestinal edema altered more genes in the apoptotic pathway. Edema up-regulated the death domain adaptor molecule (Figure 6), TRADD which interacts with the tumor necrosis factor receptor mediating programmed cell death signaling and nuclear factor-kappa b (NFκ-B) activation (31-32). TRADD also reduces recruitment of proteins known as inhibitor-of apoptosis, these genes were also down-regulated by edema. The finding that NFκ-B is up-regulated is consistent with recent data from our group demonstrating that NFκ-B is activated in intestinal edema and associated with decreased smooth muscle contraction; however, pharmacological inhibition restores intestinal smooth muscle contractility in the setting of intestinal edema.
One limitation of our study is the use of full-thickness intestinal tissue for the gene expression experiments. There may be a difference in gene expression in the intestinal mucosa, submucosa or muscularis layers and/or local immune cells. These shortcomings could be ultimately overcome by immunohistochemistry and separating mucosa from the muscularis of the intestine. Nevertheless, these data begin to explore the global effects of acute intestinal edema and early enteral nutrition on gene expression in the intestine. Further studies are needed in our edema model to explore alterations in the pathways identified in our study on intestinal dysfunction.
In conclusion, we have shown that early delivery of nutrients to the intestine helps to resolve ileus associated with gut edema. Administration of immediate, early enteral nutrition helped to restore the delayed intestinal motility pattern seen in intestinal edema. These data are important for not only establishing a link between cellular homeostasis and intestinal dysmotility, but may lead to further insight into how intestinal edema delays intestinal transit. These data provide further evidence that edema can alter gene expression patterns in the intestine promoting dysfunction.
Acknowledgments
The authors would like to thank Dr. Loose for his technical assistance with the microarray experiments.
Funding: Supported by NIGMS grants T32 GM 08792, P50 GM38529, 5K08 GM00675 and CDC-620069, NHLBI grant GM36115; NIDDK P30 GM56338
Footnotes
Competing Interests: No competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raeburn CD, Moore EE, Biffl WL, et al. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg. 2001;182:542–546. doi: 10.1016/s0002-9610(01)00821-2. [DOI] [PubMed] [Google Scholar]
- 2.Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Cox CS, Moore FA. Patients with impending abdominal compartment syndrome do not respond to early volume loading. Am J Surg. 2003;186(6):602–7. doi: 10.1016/j.amjsurg.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hassoun HH, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Postinjury multiple organ failure: The role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Balogh Z, McKinley BA, Cox CS, Jr, et al. Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock. 2003;20(6):483–92. doi: 10.1097/01.shk.0000093346.68755.43. [DOI] [PubMed] [Google Scholar]
- 5.Prien T, Backhaus N, Pelster F, Pircher W, Bunte H, Lawin P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J Clin Anesth. 1990 Sep-Oct;2(5):317–23. doi: 10.1016/0952-8180(90)90077-g. [DOI] [PubMed] [Google Scholar]
- 6.Miedema BW, Schillie S, Simmons JW, Burgess SV, Liem T, Silver D. Small bowel motility and transit after aortic surgery. J Vasc Surg. 2002;36(1):19–24. doi: 10.1067/mva.2002.124368. [DOI] [PubMed] [Google Scholar]
- 7.Bauer AJ, Schwarz NT, More BA, Turler A, Kalff JC. Ileus in critical illness: mechanisms and management. Curr Opin Crit Care. 2002;8(2):152–157. doi: 10.1097/00075198-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz NT, Kalff JC, Turler A, Speidel N, Grandis JR, Billiar TR, Bauer AJ. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology. 2004 Jan;126(1):159–69. doi: 10.1053/j.gastro.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 9.Hassoun HT, Weisbrodt NW, Mercer DW, Kozar RA, Moody FG, Moore FA. Inducible nitric oxide synthase mediates gut ischemia/reperfusion-induced ileus only after severe insults. J Surg Res. 2001;97:150–154. doi: 10.1006/jsre.2001.6140. [DOI] [PubMed] [Google Scholar]
- 10.Hassoun HT, Zou L, Moore FA, Kozar RA, Weisbrodt NW, Kone BC. α-Melanocyte stimulating hormone protects against mesenteric ischemia-reperfusion injury. Amer J Physiol. 2002;282:G1059–G1068. doi: 10.1152/ajpgi.00073.2001. [DOI] [PubMed] [Google Scholar]
- 11.Attuwaybi B, Kozar RA, Gates KS, Moore-Olufemi S, Sato N, Weisbrodt NW, Moore FA. Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma. 2004;56(4):749–56. doi: 10.1097/01.ta.0000119686.33487.65. [DOI] [PubMed] [Google Scholar]
- 12.Moore-Olufemi SD, Xue H, Attuwaybi BO, Fischer U, Harari Y, Oliver DH, Weisbrodt N, Allen SJ, Moore FA, Stewart R, Laine GA, Cox CS., Jr Resuscitation-induced gut edema and intestinal dysfunction. J Trauma. 2005;58(2):264–70. doi: 10.1097/01.ta.0000133571.64393.d2. [DOI] [PubMed] [Google Scholar]
- 13.Uray KS, Laine GA, Xue H, Allen SJ, Cox CS., Jr Intestinal edema decreases intestinal contractile activity via decreased myosin light chain phosphorylation. Crit Care Med. 2006 Oct;34(10):2630–7. doi: 10.1097/01.CCM.0000239195.06781.8C. [DOI] [PubMed] [Google Scholar]
- 14.Moore FA, Cocanour CS, McKinley BA, Kozar RS, DeSoignie RC, Von-Maszewski ME, Weisbrodt NW. Migrating motility complexes persist after severe traumatic shock in patients who tolerate enteral nutrition. J of Trauma. 2001;51:1075–1082. doi: 10.1097/00005373-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Grossie VB, Jr, Weisbrodt NW, Moore FA, Moody F. Ischemia/reperfusion-induced disruption of rat small intestine transit is reversed by total enteral nutrition. Nutrition. 2001 Nov-Dec;17(11-12):939–43. doi: 10.1016/s0899-9007(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 16.Moore-Olufemi SD, Xue H, Allen SJ, Moore FA, Stewart RH, Laine GA, Cox CS., Jr Inhibition of intestinal transit by resuscitation-induced gut edema is reversed by L-NIL. J Surg Res. 2005 Nov;129(1):1–5. doi: 10.1016/j.jss.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981 Nov;6(3):211–7. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 18.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Research. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 19.Gibson UEM, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Research. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 20.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymase reaction assay. J Mol Endo. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 21.Owens L, Burrin DG, Berseth CL. Minimal Enteral Feeding Induces Maturation of Intestinal Motor Function but Not Mucosal Growth in Neonatal Dogs. J Nutr. 2002;132:2717–2722. doi: 10.1093/jn/132.9.2717. [DOI] [PubMed] [Google Scholar]
- 22.Moore-Olufemi SD, Kozar RA, Moore FA, Sato N, Hassoun HT, Cox CS, Jr, Kone BC. Ischemic preconditioning protects against gut dysfunction and mucosal injury after ischemia/reperfusion injury. Shock. 2005 Mar;23(3):258–63. [PubMed] [Google Scholar]
- 23.Cothren CC, Moore EE, Ciesla DJ, Johnson JL, Moore JB, Haenel JB, Burch JM. Postinjury abdominal compartment syndrome does not preclude early enteral feeding after definitive closure. Am J Surg. 2004;188(6):653–8. doi: 10.1016/j.amjsurg.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 24.Kaur N, Gupta MK, Minocha VR. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World J Surg. 2005 Aug;29(8):1023–7. doi: 10.1007/s00268-005-7491-z. discussion 1027-8. [DOI] [PubMed] [Google Scholar]
- 25.Radhakrishnan RS, Xue H, Weisbrodt N, Moore FA, Allen SJ, Laine GA, Cox CS., Jr Resuscitation-induced intestinal edema decreases the stiffness and residual stress of the intestine. Shock. 2005;24(2):165–70. doi: 10.1097/01.shk.0000168873.45283.4c. [DOI] [PubMed] [Google Scholar]
- 26.Radhakrishnan RS, Radhakrishnan HR, Xue H, Moore-Olufemi SD, Mathur AB, Weisbrodt NW, Moore FA, Allen SJ, Laine GA, Cox CS., Jr Hypertonic saline reverses stiffness in a Sprague-Dawley rat model of acute intestinal edema, leading to improved intestinal function. Crit Care Med. 2007 Feb;35(2):538–43. doi: 10.1097/01.CCM.0000254330.39804.9C. [DOI] [PubMed] [Google Scholar]
- 27.Moore BA, Otterbein LE, Turler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003 Feb;124(2):377–91. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 28.Kalff JC, Hierholzer C, Tsukada K, Billiar TR, Bauer AJ. Hemorrhagic shock results in intestinal muscularis intercellular adhesion molecule (ICAM-1) expression, neutrophil infiltration, and smooth muscle dysfunction. Arch Orthop Trauma Surg. 1999;119(1-2):89–93. doi: 10.1007/s004020050363. [DOI] [PubMed] [Google Scholar]
- 29.LSumpio BE, Banes AJ. Response of porcine aortic smooth muscle cells to cyclic tensional deformation in culture. J Surg Res. 1988 Jun;44(6):696–701. doi: 10.1016/0022-4804(88)90103-5. [DOI] [PubMed] [Google Scholar]
- 30.Sotoudeh M, Li YS, Yajima N, Chang CC, Tsou TC, Wang Y, Usami S, Ratcliffe A, Chien S, Shyy JY. Induction of apoptosis in vascular smooth muscle cells by mechanical stretch. Am J Physiol Heart Circ Physiol. 2002 May;282(5):H1709–16. doi: 10.1152/ajpheart.00744.2001. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez JA, Perr HA. Mechanical stretch modulates TGF-beta1 and alpha1 (I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol. 1999 Nov;277(5 Pt 1):G1074–80. doi: 10.1152/ajpgi.1999.277.5.G1074. [DOI] [PubMed] [Google Scholar]
- 32.Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005 May;12(5):473–81. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]


