Abstract
Secretory IgA (SIgA) serves as the first line of defense in protecting the intestinal epithelium from enteric toxins and pathogenic microorganisms. Through a process known as immune exclusion, SIgA promotes the clearance of antigens and pathogenic microorganisms from the intestinal lumen by blocking their access to epithelial receptors, entrapping them in mucus, and facilitating their removal by peristaltic and mucociliary activities. In addition, SIgA functions in mucosal immunity and intestinal homeostasis through mechanisms that have only recently been revealed. In just the past several years, SIgA has been identified as having the capacity to directly quench bacterial virulence factors, influence the composition of the intestinal microbiota by Fab-dependent and -independent mechanisms, promote the retro-transport of antigens across the intestinal epithelium to dendritic cell (DC) subsets in gut-associated lymphoid tissue, and, finally, to down-regulate pro-inflammatory responses normally associated with the uptake of highly pathogenic bacteria and potentially allergenic antigens. This review summarizes the intrinsic biological activities now associated with SIgA and their relationships to immunity and intestinal homeostasis.
Introduction
As the most abundant class of antibody found in the intestinal lumen of humans and most other mammals, secretory IgA (SIgA) has long been recognized as a first line of defense in protecting the intestinal epithelium from enteric pathogens and toxins. SIgA production against specific mucosal antigens is dependent on the sampling by Peyer's patch M cells, processing by underlying antigen-presenting cells such as dendritic cells (DCs), T cell activation, and ultimately B cell class switch recombination in gut-associated lymphoid tissue (GALT), mesenteric lymph nodes, and possibly neighboring lamina propria (MLNs) 1, 2. Isolated lymphoid follicles (ILFs) in the small intestine also function in the induction of mucosal immune responses 3. Multiple cytokines, including IL-4, TGF-β, IL-5, IL-6, IL-10 are instrumental in intestinal stimulating SIgA production. A subset of these cytokines, notably TGF-β and IL-10, are also required for maintaining mucosal tolerance, thus establishing one of the many links between SIgA production, immunity and intestinal homeostasis.
This review highlights our current understanding of SIgA's many (recently revealed) functions in mucosal immunity and intestinal homeostasis. Because SIgA essentially resides within an external environment (i.e., the intestinal lumen), it must combat microbial infections through mechanisms that are fundamentally different than those employed by antibodies in systemic compartments. Whereas IgG, for example, promotes the killing and clearance of pathogenic bacteria through the coordinated activity of complement and Fc-mediated uptake by macrophages and neutrophils, it generally assumed that SIgA primary acts through receptor blockade, steric hindrance and/or immune exclusion. We summarize evidence for each of these activities as revealed through the use of animals models, but argue that the mechanisms underlying SIgA-mediated immunity are in fact much more complex than previously appreciated.
With respect to intestinal homeostasis, we make the case that SIgA's multifaceted roles in controlling inflammation and regulating immune responses to certain dietary antigens, commensal microflora, and enteric pathogens it is only beginning to be understood. The past several years has seen an emergence of evidence that indicates that SIgA influences the composition of the intestinal microbiota, promotes the uptake and delivery of antigens from the intestinal lumen to dendritic cell (DC) subsets located in GALT, and, influences inflammatory responses normally associated with the uptake of highly pathogenic bacteria and potentially allergenic antigens. Due to space limitations, this review will be restricted to SIgA's activities within the intestinal lumen; we will not discuss the capacity of polymeric IgA (pIgA) to neutralize pathogens intracellularly during transepithelial transport, or to promote excretion of antigens present in the lamina propria.
Multiple neutralizing properties of SIgA at gut mucosal surfaces
Blocking attachment to epithelial cells by steric hindrance and binding to receptor recognition domains
SIgA is capable of interfering with the earliest steps in the infection process by virtue of its ability to block toxins and pathogens from adhering to the intestinal epithelium 4-9. One of the best examples of this mode of protection involves cholera toxin (CT), the toxin responsible for the severe secretory diarrhea associated with Vibrio cholerae infection. In mouse models, it has been demonstrated that SIgA is essential in protecting the intestinal epithelium from the effects of luminal CT exposure 9, 10. Not surprisingly, mouse monoclonal IgA antibodies (mAbs) against the toxin's B subunit (CTB), a homopentameric molecule that binds to ganglioside GM1 on the apical surfaces of enterocytes, were sufficient to prevent CT attachment to polarized intestinal epithelial cell monolayers in vitro. These same mAbs protected neonatal mice from CT-induced secretory diarrhea, weight loss and death 11. The monoclonal antibodies did not, however, directly interact with the GM1 binding site on CTB. Therefore, it was suggested that they likely interfere with CT binding to epithelial cells through a mechanism involving steric hindrance.
SIgA has also been shown to be capable of blocking pathogens from attaching to intestinal epithelial cells by direct recognition of receptor-binding domains, as demonstrated in the case reovirus type 1 Lang (T1L). SIgA is required for full protection against intestinal reovirus T1L infection, as revealed through the use of IgA knockout mice 12. To investigate the molecular mechanism underlying SIgA-mediated immunity to reovirus, a panel of reovirus-specific monoclonal IgA antibodies was screened for those that protected mice against an oral T1L challenge. Protection was conferred by mAbs directed against the σ1 protein, an adhesin fiber known to promote viral attachment to a number of epithelial cell types 13. The exact epitope recognized by one particular IgA mAb was localized to a ∼30 amino acid region of the σ1 receptor-binding head domain, providing strong evidence that the mAb directly interferes with epithelial recognition 5. Monoclonal IgA antibodies against the other viral surface antigens, including the capsid protein, were not protective in the T1L peroral challenge model, underscoring the importance of epitope specificity in SIgA-mediated immunity 6.
Immune exclusion: agglutination, entrapment and clearance
“Immune exclusion” generally refers to the ability of SIgA to prevent microbial pathogens (and antigens) from gaining access to the intestinal epithelium through a stepwise series of events involving agglutination, entrapment in mucus, and/or clearance via peristalsis 14-16. Although immune exclusion has been recognized as a function of SIgA for nearly four decades 17, and often attributed as being an important component of protective immunity 18, very little known about the specific details of the process.
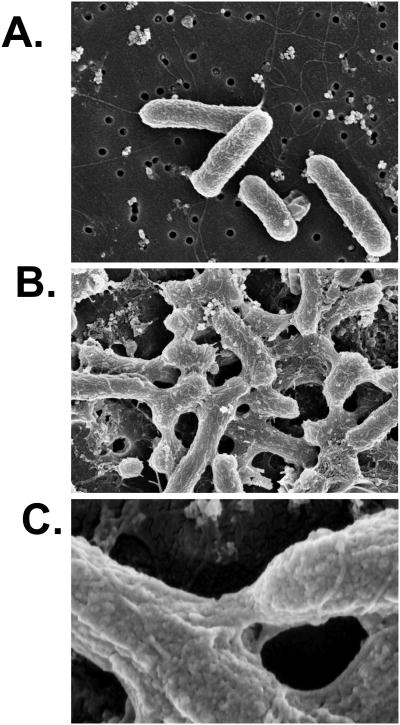
Agglutination, for example, is simply the formation of macroscopic clumps of bacteria (or viruses) as the result of antibody-mediated cross-linking via polyvalent surface antigens. There are no reports in the literature to suggest that agglutination per se has any detrimental effect on microbial physiology or virulence. On the contrary, we (and others) have reported that bacterial growth rates in culture are unaffected by agglutination 19, 20. However, scanning and transmission electron microscopy analysis of Salmonella enterica serovar Typhimurium cross-linked by a protective monoclonal IgA (“Sal4”) against the O-antigen has revealed evidence of antibody-mediated distortion of the bacterial outer membranes (Figure 1), secretion of a capsular exopolysaccharide, and alterations in the bacterial gene expression (S. Forbes, J. Dornenburg, and N. Mantis, manuscript in preparation). Cross-linking of S. Typhimurium with antibodies against the flagella did not elicit any ultrastructural changes in membrane integrity, demonstrating that agglutination is qualitatively different depending on the epitope recognized by the agglutinating antibody, and that some antibodies may have immediate effects on bacterial physiology and gene expression.
Figure 1. IgA-mediated agglutination of S Typhimurium is accompanied by gross changes in cell shape.
Mid-log phase cultures of S. Typhimurium strain 14028S were exposed to Sal4 mAb (5 μg/ml) for 45 min and then subjected to scanning electron microscopy. Panels: (A) S. Typhimurium control cells not treated with Sal4; (B and C) Cells treated with Sal4 mAb at (B) low and (C) high power. Note the gross changes in cell shape and the bridging that occurs between cells in the presence of Sal4 (panels B and C) but not in the absence of Sal4 (panel A). Figure kindly provided by Dr. Steve Forbes.
Work by Phalipon, Corthésy and colleagues has examined in mouse and rabbit model systems the capacity of SIgA to entrap bacterial pathogens in the mucus layer overlying respiratory and intestinal epithelia in vivo 21, 22. Through the use of light microscopy, immunohistochemistry, and autoradiography, it was shown that a murine IgA mAb (IgAC5) specific for the O-antigen of Shigella flexneri readily entrapped S. flexneri within a thin layer of mucus overlying the epithelium. This activity was considerably greater when the IgAC5 was complexed with secretory component (SC), because apparently the oligosaccharide side chains of SC associate with mucus. The mucus layer in the mouse and human small and large intestines is complex 23, and defining the specific molecular interactions between SIgA and individual components of the mucus layer will be necessary to fully understand the mechanisms that govern immune exclusion.
While the capacity of specific SIgAs to entrap bacteria in intestinal mucus in experimental settings is undeniable, it remains to be determined to what degree immune exclusion contributes to protective immunity to other enteropathogens, especially viruses. Indeed, it has been argued that any SIgA capable of binding to the surface of a pathogen is theoretically sufficient to intercept that pathogen in the intestinal lumen and reduce or even block its attachment to the intestinal epithelium 24. However, coating of rotavirus or reovirus with “non-neutralizing” monoclonal IgA antibodies in the intestinal lumen of mice is not sufficient to block infection 6, 25. Rather, the primary determinant of protective immunity correlated with epitope specificity; thus, challenging the importance of immune exclusion in mucosal immunity to viruses.
Direct effects of SIgA on bacterial virulence
It has been recognized for years that neither immune exclusion nor direct interference with attachment to epithelial receptors can fully account for the protective effects observed by a number of monoclonal IgA antibodies, such as those against the O-antigens of V. cholerae, S. Typhimurium and S. flexneri 11, 19, 22, 26, 27. This prompted us to examine the possibility that IgA may have a direct effect on bacterial virulence. We found that the binding of a murine monoclonal IgA (IgAC5) to the O-Ag of S. flexneri suppressed activity of the bacterial type 3-secretion (T3S) system that is necessary for S. flexneri to gain entry into intestinal epithelial cells 28. IgAC5's suppressive effect on T3S activity was rapid (5-15 min) and coincided with a partial reduction in the bacterial membrane potential and intracellular ATP levels. Although IgAC5 is neither bacteriostatic nor bactericidal, it clearly has the capacity to selectively “quench” certain virulence factors. It remains to be determined whether other IgA antibodies share this trait.
Fab-independent interaction between natural SIgA and intestinal pathogenic and commensal bacteria
SIgA is also capable of preventing pathogen and toxin attachment to epithelial surfaces, independent of the antibody variable region. In this respect, SIgA can be considered a component of the innate immune system. Both the IgA heavy chain (Fcα) and SC are heavily glycosylated. Because the oligosaccharide side chains on SIgA share a high degree of similarity with those on the luminal face of the intestinal epithelium, it has been proposed that both IgA and free or bound SC can effectively serve as competitive inhibitors (“decoys”) of pathogen binding to host cells 29-35. For example, SIgA in concentrations at or below those found in human milk inhibited the binding of Clostridium difficile toxin A to purified enterocyte brush border membrane receptors 29. Toxin A bound to free SC as strongly as it did to the heavy and light chains of IgA. Perrier and colleagues subsequently identified the galactose and sialic acid residues on free SC as being primarily responsible for blocking toxin A attachment to epithelial cell monolayers 32. SC's activities are in fact quite broad, as free SC has been shown to serve as a decoy receptor for other pathogens, including enteropathogenic E. coli via binding to intimin 32 and Streptococcus pneumoniae through interaction with choline-binding protein A {Lu, 2003 #3717. Along the same line, a recent study has shown that mannose residues present on SIgA are implicated in the inhibition of Vibrio cholerae biofilm formation contrary to serum IgG or IgM 36.
The interaction between SIgA and commensal bacteria involves Fab- and Fc-independent structural motifs, featuring bound SC as a crucial partner. Removal of glycans present on free SC or bound in SIgA resulted in a drastic drop in the interaction with Gram-positive bacteria, indicating the essential role of carbohydrates in the process. 37. Coating of commensal microorganisms by SIgA may favor gut colonization and education of the newborn's mucosal immune system towards antigens associated with symbiotic partners. For example, using human intestinal epithelial Caco-2 cell grown as polarized monolayers, we found that association of a Lactobacillus or a Bifidobacterium with nonspecific SIgA enhanced probiotic adhesion by a factor of 3.4-fold or more. Moreover, SIgA affected epithelial permeability, signaling events involved in NF-κB nuclear translocation, production of pIgR, and induction of immune mediators 38. Taken together these observations suggest that although sugar-mediated non-specific recognition occurs, its highly plastic, combinatorial nature still permits a selective interaction with commensal, non-pathogenic and pathogenic bacteria.
Role of SIgA in homeostasis of the intestinal microbiota
Induction of SIgA by neonatal exposure to commensal microorganisms
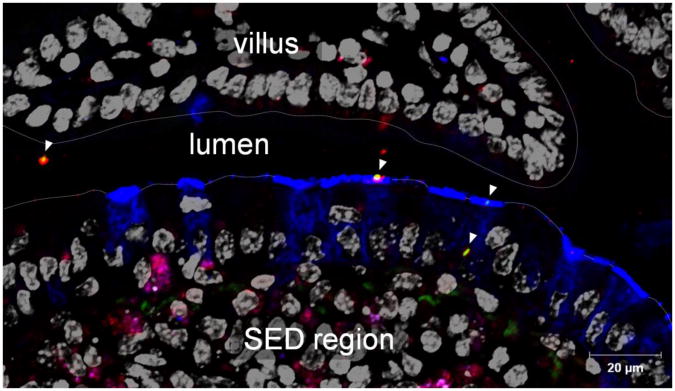
Immediately following birth, mammals are exposed to microbes associated with the external and maternal environments. The transition from a sterile environment to a highly colonized environment is accompanied by the concomitant exposure of the newborn's gastrointestinal tract to maternal IgA antibodies acquired through breast-feeding. Natural and specific SIgA antibodies in breast milk are capable of binding commensal bacteria and may be involved in the progressive, controlled establishment of the newborn's microbiota 39, 40. The microbiota, in turn, stimulates maturation of GALT, resulting in the production of IgA with both a limited affinity and repertoire to redundant epitopes on gut microorganisms 41-43. By direct visualization of a fluorescently labelled commensal bacterium administered in the form of an SIgA-based complex into the intestines of mice, we observed both preserved association with the antibody and specific targeting to, and passage across, Peyer's patch M cells (Figure 2). This observation suggests that SIgA, by virtue of its ability to associate with commensal bacteria and promote their uptake via M cells (see below), may play an important role in promoting the sampling of commensal bacteria in the form of SIgA-immune complexes by the GALT that perception of commensals by the mucosal immune system relies on this sampling site, and that the association with SIgA is essential in the process (N. Rol, L. Favre, J. Benyacoub, B. Corthésy, submitted for publication).
Figure 2. Entry of commensal bacteria coated with SIgA in a Peyer's patch via M cells.
Image acquired by laser scanning confocal microscopy. Lactobacillus rhamnosus labeled with FITC was injected in the form of SIgA-based complexes into a mouse intestinal ligated loop comprising a Peyer's patch, and in vivo incubation was performed for 2 hours. After cryosection of the tissue, M cells (blue), SIgA (red) and cell nuclei (greyish) were stained respectively, with UEA-1 lectin, anti-SC antibodies, and DAPI. Arrowheads indicate bacteria in the form of SIgA-based complexes. The appearance of SIgA-based complexes in the lumen, at the surface of M cells, and transiting through an M cell on the snapshot picture reflects the various steps in the passage from the lumen to the SED (subepithelial dome) region. Bars in insets represent 5 μm.
The past several years have yielded important information about the mechanisms involved in the intestinal IgA response against commensal microorganisms. It has been proposed that in mice a proportion of the specific SIgA against commensal bacteria are induced in a T cell-independent pathway, independent of the development of follicular lymphoid structures 44, 45. Subsequent studies in mice revealed that commensal bacteria persist in Peyer's patch DCs, contributing to induction of local specific immune responses that limit dissemination no farther than the MLNs, ultimately preventing systemic spread 46. Recently, Hapfelmeier and colleagues reported using a “reversible” germ-free mouse model that the intestinal-specific IgA response against commensal bacteria (1) requires a high threshold for induction (∼109 bacteria), (2) has a slow onset (≥ 14 days) with a long half-life (>16 weeks) and (3) constantly adapts to the predominant commensal species in the intestinal lumen 47. However, it remains an open question as to whether this type of selective SIgA response actually reflects the situation in conventionally raised mice, which encounter a complex and diverse microbiota in the context of a fully matured GALT.
Involvement of SIgA in the control of commensal microorganisms
Different model systems have been used to investigate the role of SIgA on intestinal homeostasis. Among them, mouse strains deficient in activation-induced cytidine deaminase (AID) that are unable to class switch from IgM to IgA. AID mice were shown to have profound increases in the number of non-pathogenic, anaerobic bacteria throughout their small intestines, as well as hyperplasia of isolated lymphoid follicles 48. Because AID-/- mice do not have functional SIgM, this study demonstrated that SIgA is essential in preventing hyper-stimulation of the local and non-mucosal immune systems. However, it has been noted that secretory IgM has compensatory activities in IgA-deficient mice, possibly explaining why IgA-deficient mice are “healthy” under normal laboratory conditions. These observations in mice can be compared to human IgA deficiency in which a maturation defect in B cells is commonly observed 49. IgA-deficient patients are generally asymptomatic but do exhibit a tendency to develop gastrointestinal disorders such as celiac disease 50 and allergies 51. IgA-deficient patients often have airway infections; however, these problems are mainly seen because compensatory SIgM is lacking in the airways (in contrast to the gut) 52. Together, this supports the notion that the adaptive SIgA responses may allow the host to respond to fluctuations in commensal bacteria without eliciting a deleterious response, and thus favor mucosal homeostasis 53.
The dynamics between the commensal microbiota and SIgA are likely highly complex, considering that a considerable proportion (24-74%) of the microbiota is coated with SIgA 54, 55. Peterson and colleagues used an ingenious germ-free mouse model to directly examine the impact of SIgA on host-commensal interactions 56. The authors first produced a monoclonal IgA against the capsular polysaccharide of Bacteroides thetaiotaomicron. Germ-free, immunodeficient mice secreting this particular monoclonal IgA into the intestinal lumen by virtue of a hybridoma “backpack” were then challenged with B. thetaiotaomicron and both the host's and the commensal's responses were analyzed. The authors found that B. thetaiotaomicron, in the absence of specific anti-capsular SIgA, elicited a robust oxidative stress response in the host. The presence of SIgA antibodies suppressed this response, thereby underscoring SIgA's potential to dampen deleterious host responses to commesal microflora.
An interesting observation from Peterson's work was that the capsular polysaccharide-specific monoclonal IgA reduced, but did not prevent, B. thetaiotaomicron from colonization of the mouse gut 56. Thus, under steady-state conditions, the presence of specific SIgA antibodies against surface antigens does not necessarily lead to bacterial clearance. Zitomersky and colleagues performed a longitudinal study of Bacteroidales species in 15 health adults over a period of a year and reached a similar conclusion 57. In fact, it has been argued that bacteria that bind SIgA may actually have a selective advantage in the gut 58. Bollinger and colleagues have shown that SIgA and mucin facilitate the formation of biofilms by non-pathogenic E. coli on epithelial cell monolayers grown in vitro59, 60. Biofilms have been proposed as a means by which endogenous microbiota colonize mucosal surface and ensure a steady-state growth rate in the intestinal lumen 61. The association of SIgA with biofilm formation in the gut has been demonstrated using sections from rat, baboon and human tissues 62. FISH analysis has revealed that that Lactobacilli species establish biofilms in different parts of the gastrointestinal tract, although it remains to be determined if SIgA antibodies are involved in the process 63.
Retro-transport of SIgA across the intestinal epithelium
SIgA-based transport of immune complexes by M cells
It has been known for some time that Peyer's patch M cells selectively bind SIgA and SIgA-immune complexes 64, 65. While there is evidence for a SIgA-specific receptor on M cells, this receptor has not been identified. Therefore, the exact mechanism by which M cells selectively internalize SIgA-Ag complexes in the face of a large excess of free SIgA present in the intestinal lumen remains unknown. Nonetheless, we recently provided evidence that SIgA undergoes conformational changes upon antigen engagement 66. Using specific SIgA antibodies with antigens of various sizes and complexity, we found that SIgA protease sensitivity profiles were altered upon antigen engagement, presumably reflecting differences in heavy chain backbone conformations. The conformational changes induced upon antigen interaction resulted in enhanced binding of SIgA to cellular receptors (FcαRI and pIgR), as compared to free (unbound) SIgA. These data reveal that antigen recognition by SIgA triggers structural changes in the immunoglobulin that result in enhanced receptor binding properties. It remains to be determined whether this is relevant to M cell recognition of SIgA-immune complexes.
Consequences of SIgA-immune complex uptake by M cells
The recognition that SIgA-based immune complexes are transported by Peyer's patch M cells into the subepithelial dome (SED) regions has led us to investigate the role of “retro-translocation” in the regulation of intestinal immune responses 67. Oral delivery to mice of recombinant SIgA (rSIgA) consisting of mouse pIgA and human bound SC (hSC) as a surrogate non-self antigen, triggered hSC-specific mucosal and systemic immune responses, as evidenced by antigen-specific serum and salivary antibody titers, T cell proliferation in draining MLNs and spleen, and pronounced expression of IL-10 and TGF-β by cells recovered from MLNs 67. Furthermore, analysis of IgG isotypes and cytokine profiles demonstrated the tendency of rSIgA immunization to induce a mixed Th1/Th2, tolerance-biased pattern of mucosal immune responses. It was also observed that rSIgA induced migration of DCs from the SED region to the interfollicular region, a phenomenon indicative of DC activation. Although these responses were significantly less marked when compared with responses triggered upon mixing of hSC with the prototype mucosal adjuvant CT, they nonetheless reveal that IgA can function as an immunopotentiator in the mucosal environment.
Reduction of pathogen-mediated pro-inflammatory responses by mucosal SIgA
Besides being a weak immunopotentiator, there is evidence that SIgA may actively quench the capacity certain antigens to elicit severe pro-inflammatory responses following uptake via Peyer's patch M cells. SIgA-coated S. flexneri, when injected into to ligated ileal loops are detected in the SED region in close association with myeloid CD11c+CD11b+ DCs, which are known to be tolerogenic. In the rabbit model (unlike the mouse), S. flexneri elicits a local severe acute inflammatory response that is reminiscent of that observed in human 68. This mode was used to assess the non-inflammatory capacity of protective SIgA at mucosal surfaces 21. Analysis of cytokine expression in Peyer's patches demonstrated that SIgA-coated S. flexneri results in down regulation of pro-inflammatory cytokines TNF-α, IL-6, IFN-γ, while maintaining a sustained level of regulatory IL-10. This resulted in preservation of the integrity of the intestinal barrier, and suggests that under homeostatic conditions, SIgA exerts its anti-inflammatory effects by reducing bacteria-induced pro-inflammatory circuits (rather than promoting the onset of anti-inflammatory pathways). It is therefore tempting to speculate that the retro-transport of antigen-SIgA complexes is important for the maintenance of tolerance towards innocuous proteins, including allergens.
SIgA as a scavenger of allergenic antigens
The importance of the secretory immune system in controlling allergic symptoms has been put forth in many animal and human studies, yet the phenomenon does not appear an absolute paradigm. Neutralization of allergen occurred with the abundant SIgA found in mucosal secretions and contributed towards limiting the access of allergen to the lamina propria and thus the inflammatory responses 69. Passive administration of antigen-specific or non-specific IgA reduced airways responsiveness and lung eosinophilia 70 or allergic rhinitis 71. The impact of IgA and SIgA titers during the first two years on the development of allergy was also reported 72. Reduction of fecal SIgA when compared to mice actively tolerized with the same Ag protein 73 argue in favor of the importance of SIgA in controlling allergic reactions, yet the role of IgG-based complexes with Ag cannot be excluded 74. Furthermore, pIgR KO mice, which are unable to produce SIgA and have increased intestinal permeability, display a greater immune response towards commensal bacteria, but not towards food antigens 75, a phenomenon possibly linked to increased uptake of food antigens 76. In the same knockout model, the capacity of mice to trigger oral tolerance and protect against systemic hypersensitivity by the same tolerizing antigen has also been demonstrated 77; this suggests a delicate balance between the development of secretory immunity and mucosal leakiness.
Moreover, first-line mucosal defenses were documented in grass pollen immunotherapy 78. CD89 (FcαRI) cross-linking by IgA inhibits FcεRI-dependent activation of mast cells, and diminishes allergic asthma in transgenic mice expressing the human receptor 79. Aggregated milk allergens are taken up by Peyer's patches rather than by classical epithelial cell-mediated phagocytosis 80: this resulted in the increased production of both Th2-associated IgE and SIgA. While the authors concluded on enhanced sensitization, they neglected to consider the increase in luminal SIgA, and the contribution of the Ab in neutralizing the allergens, ultimately limiting allergic responses.
However, the presence of allergen-specific SIgA is not always augmented in successfully tolerized animals, and can even be present in large amounts in sensitized ones without conferring protection 81. Moreover, the importance of SIgA against allergic diseases remains unclear with respect to recent clinical studies; patients with IgA deficiency display an increased risk of food hypersensitivity at the age of 4 years solely 51, whereas in another cohort, IgA deficiency does not show any correlation with food allergy 82. It is an open question whether the production of compensatory SIgM can explain this discrepancy. The sum of these data suggests that SIgA production is more critical for homeostasis towards commensal bacteria than food antigens. Additional studies are required to clarify the importance of SIgA in the maintenance of oral tolerance, and hence the integrity of the intestinal barrier.
Celiac disease: SIgA as a Trojan horse
An exception to the generally accepted function of immune exclusion of SIgA is the observation that the antibody acts a Trojan horse in people suffering from celiac disease (CD). In genetically susceptible individuals with CD, complexes of luminal specific SIgA antibodies and gluten-derived deamidated gliadin peptides are retro-transcytosed across epithelial cells, leading to the basal delivery of intact, highly reactive peptides that stimulate inflammatory processes via activation of target CD4+ T cells 83. This abnormal intestinal transport is mediated by the recognition of SIgA-gliadin complexes by the transferrin receptor (TfR, CD71) expressed at high levels on the apical surface of intestinal epithelial cells in CD patients. The disease appears thus as a deficient confining of SIgA-based immune complexes resulting from the misaddressing of CD71, and its subsequent fortuitous capacity to transcytose toxic gliadin peptides. However, it remains unclear why in the physiologic context the large excess of luminal SIgA displaying multiple specificities cannot prevent most of receptor-mediated endocytosis, although effective competition occurs in the presence of pIgA, SIgA or soluble CD71 in Ussing chambers in vitro.
Recognition of SIgA-antigen complexes by APCs
The SED region is the primary depot for antigens and SIgA-antigen complexes following M cells transcytosis 67, 84. While both mouse- and human-derived DCs are capable of binding and internalizing SIgA, the specific IgA receptor(s) on DCs involved in SIgA recognition have not been fully identified. Heystek and colleagues speculated the interaction of SIgA with human monocyte-derived DCs (MoDCs) occurs via a member(s) of the C-type lectin family of receptors 85. We recently tested this hypothesis and found that human colostral SIgA is recognized (and internalized) by human DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN), as well as the mannose receptor (CD206) 86. DC-SIGN (but not the MR) is expressed on myeloid DCs in the SED region, arguing for a possible role for a subset of C-type lectins in immune sampling 87. The mouse homolog of DC-SIGN, SIGNR1 (specific ICAM-3 grabbing non-integrin-related-1; CD209b) has a binding specificity similar to human DC-SIGN 88, and is similarly expressed on intestinal CD11c+CD11b+ DCs 89. On the other hand, the possibility that other receptor(s) besides DC-SIGN are involved in sampling SIgA cannot be excluded. In the mouse, for example, it was noted that the association of SIgA with Peyer's patch DCs was largely unaffected by the glycosylation state of IgA 90.
Conclusions
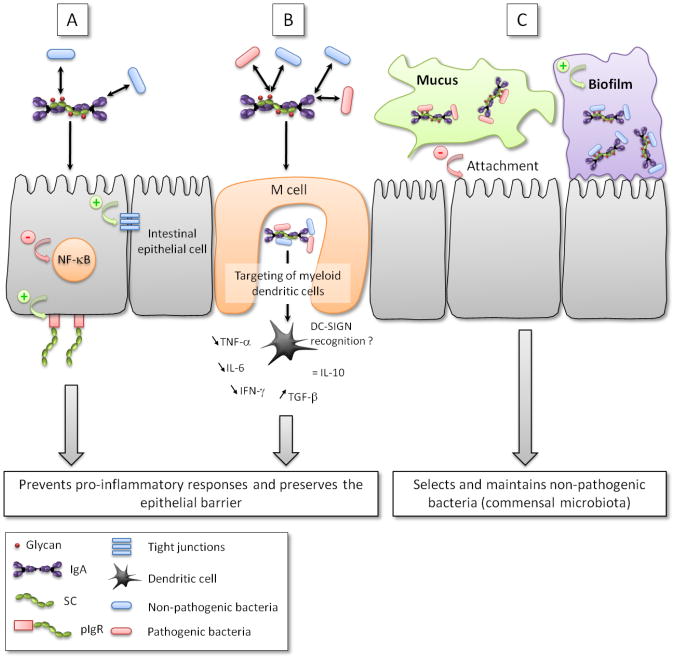
Besides its well-documented capacity to protect the intestinal epithelium from toxins, viruses and pathogenic bacteria, SIgA demonstrates an array of other activities that are integral to the maintenance of mucosal homeostasis (Figure 3). SIgA influences the composition of the intestinal microbiota, down-regulates pro-inflammatory responses normally associated with the uptake of highly pathogenic bacteria and potentially allergenic antigens, and promotes the retro-transport of antigens across the intestinal epithelium to dendritic cell (DC) subsets in the GALT. SIgA's ability to multitask is due in large part to it intrinsic complexity, particularly the diverse glycan arrays on both IgA and SC. Our increasing ability to biochemically dissect SIgA into its individual components and test then in define animal models will ultimately permit us ascribe specific tasks to SIgA in molecular detail.
Figure 3. Multi-functional interactions of between SIgA and pathogenic and nonpathogenic bacteria in the intestinal mucosa.
In all pathways, pathogenic and non-pathogenic bacteria are coated by SIgA (depicted as a dimer with bound SC) in a Fab-specific or in a Fab-independent, glycan-mediated manner. (A) Enhanced interaction between SIgA-coated non-pathogenic bacteria and the epithelium reinforces its barrier function via multiple mechanisms, including reinforcement of tight junctions, overproduction of pIgR, and reduction in nuclear translocation of NF-κB. (B) SIgA-based immune complexes with commensal and/or pathogenic bacteria are taken up by M cells where they are targeted to underlying myeloid DCs, possibly upon binding to DC-SIGN, resulting in the down regulation of local pro-inflammatory responses. (C) SIgA, as well as free SC, may play a role of “selection” by excluding pathogenic bacteria off the epithelial surface via anchoring within mucus and favoring biofilm formation of non-pathogenic bacteria in the space in close contact with epithelial cells. +: activatory effect; −: inhibitory effect.
Acknowledgments
The research topics presented in this review are supported by research grants from the Swiss Science Research Foundation (3200-122039) to B.C. and the National Institutes of Health (HD061916) to N.J.M. We are grateful to Dr. Steve Forbes and the Wadsworth Center's Electron Microscopy Core facility for Figure 1.
References Cited
- 1.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Invest. 2010;39(4-5):303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 2.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26(6):812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunol Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 4.Apter FM, Lencer WI, Finkelstein RA, Mekalanos JJ, Neutra MR. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993;61(12):5271–5278. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helander A, Miller CL, Myers KS, Neutra MR, Nibert ML. Protective immunoglobulin A and G antibodies bind to overlapping intersubunit epitopes in the head domain of type 1 reovirus adhesin sigma1. J Virol. 2004;78(19):10695–10705. doi: 10.1128/JVI.78.19.10695-10705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchings AB, Helander A, Silvey KJ, Chandran K, Lucas WT, Nibert ML, et al. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer's patches. J Virol. 2004;78(2):947–957. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006;74(6):3455–3462. doi: 10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stubbe H, Berdoz J, Kraehenbuhl JP, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. Journal of Immunology. 2000;164(4):1952–1960. doi: 10.4049/jimmunol.164.4.1952. [DOI] [PubMed] [Google Scholar]
- 9.Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. EurJImmunol. 2005;35(1):180–188. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- 10.Lycke N, Erlandsson L, Ekman L, Schon K, Leanderson T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J Immunol. 1999;163(2):913–919. [PubMed] [Google Scholar]
- 11.Apter FM, Michetti P, Winner LSd, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61(12):5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches. J Virol. 2001;75(22):10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helander A, Silvey KJ, Mantis NJ, Hutchings AB, Chandran K, Lucas WT, et al. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J Virol. 2003;77(14):7964–7977. doi: 10.1128/JVI.77.14.7964-7977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. American Journal of Clinical Nutrition. 2001;73(6):1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 15.Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19(2):315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantis NJ, Forbes SJ. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunol Invest. 2010;39(4-5):383–406. doi: 10.3109/08820131003622635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokes CR, Soothill JF, Turner MW. Immune exclusion is a function of IgA. Nature. 1975;255(5511):745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70(6):505–515. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 19.Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect Immun. 2008;76(9):4137–4144. doi: 10.1128/IAI.00416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60(5):1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183(9):5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 22.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17(1):107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 23.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 24.Kraehenbuhl JP, Neutra MR. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 25.Corthesy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, et al. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol. 2006;80(21):10692–10699. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Kalev OK, Balabanova MS, et al. Protective efficacy of IgA monoclonal antibodies to O and H antigens in a mouse model of intranasal challenge with Salmonella enterica serotype Enteritidis. Microbes Infect. 2004;6(10):901–910. doi: 10.1016/j.micinf.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Michetti P, Porta N, Mahan MJ, Slauch JM, Mekalanos JJ, Blum A, et al. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology. 1994;107:915–923. doi: 10.1016/0016-5085(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 28.Forbes SJ, Bumpus T, McCarthy EA, Corthesy B, Mantis NJ. Transient Suppression of Shigella flexneri Type 3 Secretion by a Protective O-Antigen-Specific Monoclonal IgA. MBio. 2011;2(3) doi: 10.1128/mBio.00042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. Journal of Medical Microbiology. 1998;47(10):879–888. doi: 10.1099/00222615-47-10-879. [DOI] [PubMed] [Google Scholar]
- 30.Mantis NJ, Farrant SA, Mehta S. Oligosaccharide side chains on human secretory IgA serve as receptors for ricin. J Immunol. 2004;172(11):6838–6845. doi: 10.4049/jimmunol.172.11.6838. [DOI] [PubMed] [Google Scholar]
- 31.Mestecky J, Russell MW. Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol Lett. 2009;124(2):57–62. doi: 10.1016/j.imlet.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrier C, Sprenger N, Corthesy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem. 2006;281(20):14280–14287. doi: 10.1074/jbc.M512958200. [DOI] [PubMed] [Google Scholar]
- 33.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. Journal of Biological Chemistry. 2003;278(22):20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 34.Schroten H, Stapper C, Plogmann R, Kohler H, Hacker J, Hanisch FG. Fab-independent antiadhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infection & Immunity. 1998;66(8):3971–3973. doi: 10.1128/iai.66.8.3971-3973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wold AE, Mestecky J, Tomona M, Kobata A, Ohbayashi H, Endo T, et al. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murthy AK, Chaganty BK, Troutman T, Guentzel MN, Yu JJ, Ali SK, et al. Mannose-containing oligosaccharides of non-specific human secretory immunoglobulin A mediate inhibition of Vibrio cholerae biofilm formation. PLoS One. 2011;6(2):e16847. doi: 10.1371/journal.pone.0016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathias A, Corthesy B. Recognition of Gram-positive Intestinal Bacteria by Hybridoma- and Colostrum-derived Secretory Immunoglobulin A Is Mediated by Carbohydrates. J Biol Chem. 2011;286(19):17239–17247. doi: 10.1074/jbc.M110.209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathias A, Duc M, Favre L, Benyacoub J, Blum S, Corthesy B. Potentiation of polarized intestinal Caco-2 cell responsiveness to probiotics complexed with secretory IgA. J Biol Chem. 2010;285(44):33906–33913. doi: 10.1074/jbc.M110.135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 40.Walter J, Ley RE. The Human Gut Microbiome: Ecology and Recent Evolutionary Changes. Annu Rev Microbiol. 2011 doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 41.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69(5):1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 42.Jiang HQ, Thurnheer MC, Zuercher AW, Boiko NV, Bos NA, Cebra JJ. Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine. 2004;22(7):805–811. doi: 10.1016/j.vaccine.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Stoel M, Jiang HQ, van Diemen CC, Bun JC, Dammers PM, Thurnheer MC, et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J Immunol. 2005;174(2):1046–1054. doi: 10.4049/jimmunol.174.2.1046. [DOI] [PubMed] [Google Scholar]
- 44.Gardby E, Kagrdic D, Kjerrulf M, Bromander A, Vajdy M, Hornquist E, et al. The influence of costimulation and regulatory CD4+ T cells on intestinal IgA immune responses. Dev Immunol. 1998;6(1-2):53–60. doi: 10.1155/1998/75718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288(5474):2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 46.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 47.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298(5597):1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 49.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meini A, Pillan NM, Villanacci V, Monafo V, Ugazio AG, Plebani A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann Allergy Asthma Immunol. 1996;77(4):333–336. doi: 10.1016/S1081-1206(10)63329-7. [DOI] [PubMed] [Google Scholar]
- 51.Janzi M, Kull I, Sjoberg R, Wan J, Melen E, Bayat N, et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133(1):78–85. doi: 10.1016/j.clim.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Brandtzaeg P, Karlsson G, Hansson G, Petruson B, Bjorkander J, Hanson LA. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin Exp Immunol. 1987;67(3):626–636. [PMC free article] [PubMed] [Google Scholar]
- 53.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178(1):27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 54.van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38(3):348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuruta T, Inoue R, Nojima I, Tsukahara T, Hara H, Yajima T. The amount of secreted IgA may not determine the secretory IgA coating ratio of gastrointestinal bacteria. FEMS Immunol Med Microbiol. 2009;56(2):185–189. doi: 10.1111/j.1574-695X.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 56.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Zitomersky NL, Coyne MJ, Comstock LE. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order bacteroidales in the human gut. Infect Immun. 2011;79(5):2012–2020. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friman V, Adlerberth I, Connell H, Svanborg C, Hanson LA, Wold AE. Decreased expression of mannose-specific adhesins by Escherichia coli in the colonic microflora of immunoglobulin A-deficient individuals. Infect Immun. 1996;64(7):2794–2798. doi: 10.1128/iai.64.7.2794-2798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bollinger RR, Everett ML, Palestrant D, Love SD, Lin SS, Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. 2003;109(4):580–587. doi: 10.1046/j.1365-2567.2003.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bollinger RR, Everett ML, Wahl SD, Lee YH, Orndorff PE, Parker W. Secretory IgA and mucin-mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Mol Immunol. 2006;43(4):378–387. doi: 10.1016/j.molimm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 62.Palestrant D, Holzknecht ZE, Collins BH, Parker W, Miller SE, Bollinger RR. Microbial biofilms in the gut: visualization by electron microscopy and by acridine orange staining. Ultrastruct Pathol. 2004;28(1):23–27. [PubMed] [Google Scholar]
- 63.Lebeer S, Claes IJ, Verhoeven TL, Vanderleyden J, De Keersmaecker SC. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb Biotechnol. 2011;4(3):368–374. doi: 10.1111/j.1751-7915.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169(4):1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 65.Weltzin R, Lucia-Jandris P, Michetti P, Fields BN, Kraehenbuhl JP, Neutra MR. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108(5):1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duc M, Johansen FE, Corthesy B. Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J Biol Chem. 2010;285(2):953–960. doi: 10.1074/jbc.M109.059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favre L, Spertini F, Corthesy B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J Immunol. 2005;175(5):2793–2800. doi: 10.4049/jimmunol.175.5.2793. [DOI] [PubMed] [Google Scholar]
- 68.Sansonetti PJ, Arondel J, Cantey JR, Prevost MC, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infection & Immunity. 1996;64(7):2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smits HH, Gloudemans AK, van Nimwegen M, Willart MA, Soullie T, Muskens F, et al. Cholera toxin B suppresses allergic inflammation through induction of secretory IgA. Mucosal Immunol. 2009;2(4):331–339. doi: 10.1038/mi.2009.16. [DOI] [PubMed] [Google Scholar]
- 70.Schwarze J, Cieslewicz G, Joetham A, Sun LK, Sun WN, Chang TW, et al. Antigen-specific immunoglobulin-A prevents increased airway responsiveness and lung eosinophilia after airway challenge in sensitized mice. Am J Respir Crit Care Med. 1998;158(2):519–525. doi: 10.1164/ajrccm.158.2.9801014. [DOI] [PubMed] [Google Scholar]
- 71.Heikkinen T, Ruohola A, Ruuskanen O, Waris M, Uhari M, Hammarstrom L. Intranasally administered immunoglobulin for the prevention of rhinitis in children. Pediatr Infect Dis J. 1998;17(5):367–372. doi: 10.1097/00006454-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Bottcher MF, Haggstrom P, Bjorksten B, Jenmalm MC. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp Allergy. 2002;32(9):1293–1298. doi: 10.1046/j.1365-2222.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 73.Frossard CP, Hauser C, Eigenmann PA. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J Allergy Clin Immunol. 2004;114(2):377–382. doi: 10.1016/j.jaci.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 74.Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010;3(5):461–474. doi: 10.1038/mi.2010.23. [DOI] [PubMed] [Google Scholar]
- 75.Sait LC, Galic M, Price JD, Simpfendorfer KR, Diavatopoulos DA, Uren TK, et al. Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int Immunol. 2007;19(3):257–265. doi: 10.1093/intimm/dxl142. [DOI] [PubMed] [Google Scholar]
- 76.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. Journal of Experimental Medicine. 1999;190(7):915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlsson MR, Johansen FE, Kahu H, Macpherson A, Brandtzaeg P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy. 2010;65(5):561–570. doi: 10.1111/j.1398-9995.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 78.Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178(7):4658–4666. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 79.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffie C, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22(1):31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 80.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63(7):882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 81.Perrier C, Thierry AC, Mercenier A, Corthesy B. Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clin Exp Allergy. 2010;40(1):153–162. doi: 10.1111/j.1365-2222.2009.03329.x. [DOI] [PubMed] [Google Scholar]
- 82.Aghamohammadi A, Cheraghi T, Gharagozlou M, Movahedi M, Rezaei N, Yeganeh M, et al. IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol. 2009;29(1):130–136. doi: 10.1007/s10875-008-9229-9. [DOI] [PubMed] [Google Scholar]
- 83.Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Menard S, Candalh C, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205(1):143–154. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rey J, Garin N, Spertini F, Corthesy B. Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J Immunol. 2004;172(5):3026–3033. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 85.Heystek HC, M C, WA M, G P, VK C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. Journal of Immunology. 2002;168(1):102–107. doi: 10.4049/jimmunol.168.1.102. [DOI] [PubMed] [Google Scholar]
- 86.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett. 2010;131(1):59–66. doi: 10.1016/j.imlet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76(4):1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galustian C, Park CG, Chai W, Kiso M, Bruening SA, Kang YS, et al. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol. 2004;16(6):853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Y, Kawasaki H, Hsu SC, Lee RT, Yao X, Plunkett B, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16(10):1128–1133. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kadaoui KA, Corthesy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol. 2007;179(11):7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]