Abstract
Secretory IgA (SIgA), the predominant class of antibody found in intestinal secretions. While SIgA’s role in protecting the intestinal epithelium from the enteric pathogen and toxins has long been recognized, surprisingly little is known about the molecular mechanisms by which this is achieved. The present review summarizes the current understanding of how SIgA functions to prevent microbial pathogens and toxins from gaining access to the intestinal epithelium. We also discuss recent work from our laboratory examining the interaction of a particular protective monoclonal IgA with Salmonella and propose, based on this work, that SIgA has a previously unrecognized capacity to directly interfere with microbial virulence at mucosal surfaces.
1. Vulnerability of the intestinal epithelium to microbial infections
As the largest mucosal surface in the human body, the intestinal epithelium is highly vulnerable to microbial attack. Indeed, infections acquired through the consumption of contaminated food and water are a leading cause of morbidity and mortality worldwide. Children under the age of 5, in developing countries, are especially susceptible to such infectious caused by rotavirus (Franco et al., 2006), Shigella species (Levine et al., 2007), and Salmonella enterica (Petri et al., 2008). Infections caused by S. enterica serovar Typhi (S. Typhi) alone are estimated to cause more than 16 million cases of typhoid fever, and more than half a million deaths, each year (Levine, 2006). In developed countries such as the United States, Escherichia coli O157:H7 and S. enterica serovar Typhimurium (S. Typhimurium) infections are increasingly associated with food processing and handling, and they therefore represent an emerging public health threat (Maki, 2009). In 2009, for example, an outbreak of S. Typhimurium associated with contaminated peanut butter affected hundreds of persons and resulted in at least 8 deaths. Additionally, many enteric pathogens including S. Typhimurium, as well as bacterium- and plant-derived toxins, are considered by the Centers for Disease Control and Prevention (CDC) as bioterrorism agents that could be (or have already been) deliberately used to contaminate food and water supplies with the intent of inducing illness at a local, regional or national level (Mantis et al., in press).
The intestinal epithelium is equipped with a number of innate defense mechanisms, to deter microbial pathogens and toxins from gaining access to epithelial cells receptors (Mantis and Bry, 2008; Turner, 2009). First, the epithelium itself is a barrier, consisting of a single layer of columnar epithelial cells arranged side-by-side and joined at their apical aspects by tight junctions. In the small intestine, absorptive enterocytes are the predominant epithelial cell type, and are responsible for the bulk of nutrient absorption from the gut lumen. The apical surfaces of these cells consist of rigid, closely packed microvilli rich in membrane-bound glycolipids and stalked glycoproteins, some of which are known receptors for pathogens (e.g., rotavirus) and toxins (e.g., cholera toxin, or CT). The tips of these microvilli are coated with a 400–500 nm thick glycoprotein meshwork known as the filamentous brush border glycocalyx (FBBG), which is proposed to function in epithelial defense by limiting the access of virus- and bacterium-sized particles to membrane-bound receptors (Frey et al., 1996; Mantis et al., 2000). Second, interspersed with the adsorptive enterocytes are a number specialized epithelial cell types that assist in innate immunity, including mucin-producing goblet cells and crypt-situated Paneth cells, which produce antimicrobial compounds including lysozyme and alpha-defensins (Deplancke and Gaskins, 2001; Lievin-Le Moal and Servin, 2006; Ouellette, 2006). Finally, epithelial cells, upon infection or toxin exposure, “call for help” by secreting pro-inflammatory chemokines and lipid mediators that trigger the recruitment and activation of leukocytes (e.g., polymorphonuclear cells) that aid in the phagocytosis of extracellular pathogens (Tam et al., 2008).
However, the innate immune system is of only limited efficacy in deterring the most virulent pathogens and toxins. S. Typhimurium, for example, defends itself against antimicrobial compounds in the intestinal lumen by altering its own gene expression, reducing permeability of its outer membrane, and activating extracellular scavenging pathways (Bader et al., 2003; Haraga et al., 2008; Raffatellu et al., 2009). Certain enterotoxins, of both plant and microbial origins, are so potent that even a small number of molecules are sufficient to induce epithelial dysfunction and/or host cell death (Mantis, 2005; Matsumura et al., 2007). Immunity against these agents necessitates the involvement of the body’s adaptive immune system, specifically the gut-associated lymphoid tissues (GALT). In the small intestine, the GALT consists of aggregates of lymphoid follicles known as Peyer’s patches (Fig. 1). These organized lymphoid follicles contain germinal centers enriched in B cells whose differentiation and somatic cell hypermutation are driven in response to antigens present in the intestinal lumen (Brandtzaeg and Johansen, 2005; Cerutti and Rescigno, 2008; Suzuki and Fagarasan, 2009). By virtue of the local cytokine environment, B cells within the Peyer’s patches not only undergo class switch recombination preferentially to IgA, but they also express J chain, the 15 kDa protein that serves to promote immunoglobulin dimerization (Brandtzaeg, 1974; Brandtzaeg and Johansen, 2005). As a result, mucosa-derived plasma cells secrete IgA in a dimeric or polymeric form.
Figure 1. SIgA: Induction, transport and function in the intestinal lumen.
Particulate antigens (e.g., pathogens and toxins) present in the intestinal lumen are actively sampled by M cells present in the follicle-associated epithelium (FAE) overlying gut-associated lymphoid tissues (GALT). Following M cell transport, antigens are sampled by a network of sub-epithelial dendritic cells, which in turn present antigens to B cells within the regional lymphoid follicle. Following class-switch recombination and affinity maturation in the germinal center (dashed line), IgA-positive B cells emigrate from the GALT and seed the surrounding lamina propria. Dimeric IgA in the lamina propria is vectorially transported across the epithelium by the pIgR. Once in the intestinal lumen, SIgA can engage with cognate antigens on the surfaces of pathogens.
The epithelium overlying organized lymphoid follicles, the so-called follicle-associated epithelium (FAE), is an integral component of the GALT (Fig. 1). Probably the most distinctive feature of the FAE is the presence of M cells, a unique epithelial cell type that is specialized for the uptake and transepithelial transport of particulate antigens, including macromolecules (Neutra et al., 1987; Pappo and Ermak, 1989), viruses (Amerongen et al., 1991; Sicinski et al., 1990; Wolf et al., 1981), bacteria (Jones et al., 1994; Owen et al., 1986; Sansonetti et al., 1996), and parasites (Marcial and Madara, 1986). Indeed, M cells have been considered the ‘gateway’ to the GALT (Neutra et al., 2001). The apical and basolateral surfaces of M cells have distinctive features that enable these cells to rapidly and efficiently deliver mucosal antigens from the lumen to underlying lymphoid follicles. For example, M cells lack the well-developed brush border and FBBG that is present on enterocytes. Consequently, M cell apical membranes are more accessible to particles, viruses, and bacteria than are the apical surface of adjacent enterocytes (Frey et al., 1996; Mantis et al., 2000). In mice and humans, the apical surfaces of M cells have a pattern of glycosylation that is distinct from that seen on the FAE enterocytes and villus enterocytes (Clark et al., 1993; Giannasca et al., 1994; Giannasca et al., 1999). M cells also selectively express an IgA receptor, as well as Toll-like receptors and other pattern recognition receptors on their apical membranes: such receptors probably facilitate antigen recognition and contribute to signaling in the local environment (Chabot et al., 2006; Lo et al., 2003; Mantis et al., 2002; Tohno et al., 2005; Weltzin et al., 1989).
2. SIgA: Structure, production and transepithelial transport
Dimeric IgA secreted by plasma cells in the lamina propria is transported across the epithelium into intestinal secretions by the polymeric immunoglobulin receptor (pIgR). The pIgR is a transmembrane glycoprotein expressed exclusively on the basolateral surfaces of columnar epithelial cells, especially those in the crypts (Kaetzel, 2005; Mostov, 1994). The extracellular region of pIgR consists of five immunoglobulin-like domains that recognize dimeric IgA (and IgM). The receptor, in complex with dimeric IgA, is internalized by endocytosis at the cell’s basolateral surface, and is then transported vectorially to the apical membrane. Once facing the intestinal lumen, the receptor is proteolytically cleaved, such that the 70 kDa extracellular domain of pIgR, known as secretory component (SC), is released in covalent association with IgA, giving rise to SIgA. Thus, SIgA is effectively a polypeptide complex that consists of two (or more) IgA monomers joined at their C-termini by J chain in association with SC. SC has a number of proposed functions besides assisting with the transport and release of IgA into the intestinal lumen; these functions include protecting IgA from proteolytic degradation and anchoring IgA in the mucus layer overlying the mucosal surfaces (see below) (Crottet and Corthesy, 1998; Ma et al., 1998; Phalipon et al., 2002; Phalipon and Corthesy, 2003).
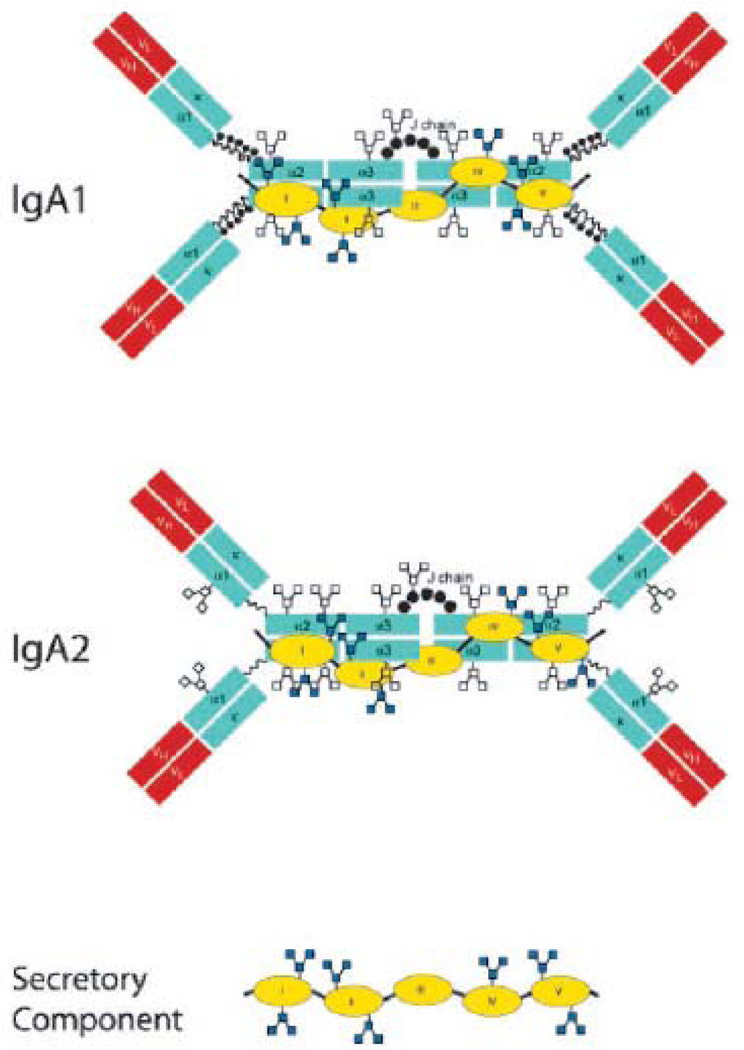
Whereas mice have a single type of IgA, humans have two distinct IgA subclasses, IgA1 and IgA2. The primary difference between the two subclasses occurs in the hinge region between domains Cα1 and Cα2: IgA1 has a 20-amino acid hinge whereas IgA2 has a 7-amino acid hinge (Mestecky et al., 2005). Mouse IgA has a short hinge region and most closely resembles human IgA2. To date, no differences in specific effector functions have been ascribed to the two human IgA subclasses. IgA1 and IgA2 are found in roughly equal proportions in secretions, except in the large intestine where IgA2 is more prevalent (Crago et al., 1984; Mestecky and Russell, 2009).
Human IgA1, IgA2, and SC are heavily glycosylated, a characteristic which is integral to their capacity to function in intestinal secretions. Human IgA1 has two N-glycosylation sites, and nine potential O-glycosylation sites within the serine- and threonine-rich hinge between Cα1 and Cα2 (Royle et al., 2003; Tomana et al., 1976). Human IgA2 has three (and in some isoforms, four) N-linked side chains, but no O-linked side chains. On average, oligosaccharide side chains account for ~10% of the molecular mass of the IgA. In contrast, glycans comprise only ~3% of the mass of human IgG. SC has seven N-glycosylation sites and approximately 15% of the molecular mass of the molecule is attributable to carbohydrates side chains, containing bi- and tri-antennary and Lewis-type structures (Hughes et al., 1999; Perrier et al., 2006). Recent molecular modeling of SIgA vividly demonstrates the spatial extent to which N- and O-linked glycan side chains project from Fcα and SC, rendering them highly accessible to lectins and viral or bacterial adhesins (Royle et al., 2003). In marked contrast, the glycans on IgG are proposed to face inward and to be sequestered between the H chains (Mattu et al., 1998).
3. SIgA’s mechanisms for protecting the epithelium
The present review will summarize the current understanding of how SIgA functions to prevent microbial pathogens and toxins from gaining access to the intestinal epithelium. Our focus will be limited to SIgA’s activities within the intestinal lumen; we will not discuss the capacity of IgA to neutralize pathogens intracellularly during transepithelial transport (Burns et al., 1996; Corthesy et al., 2006; Fernandez et al., 2003; Mazanec et al., 1995; Mazanec et al., 1992), or to promote excretion of antigens present in the lamina propria (Kaetzel et al., 1991; Kaetzel et al., 1994). Specific examples of two well-established mechanisms by which SIgA inhibits toxin, virus and bacterial contact with epithlelial cells will be given. Last, we will discuss recent work from our laboratory in which we have demonstrated that a protective monoclonal IgA, known as Sal4, has the capacity to directly interfere with S. Typhimurium virulence factors, and effectively “arrest” the bacterium at the epithelial border.
Blocking attachment to epithelial cell receptors
SIgA is capable of interfering with the earliest steps in the infection process by binding to epitopes on the surfaces of pathogens and toxins involved epithelial receptor recognition (Apter et al., 1993a; Mantis et al., 2006; Stubbe et al., 2000) (Fig. 1). SIgA’s ability to block epithelial receptor interactions can occur through antibody variable region (Fv)-dependent as well as Fv-independent mechanisms, as will be discussed below.
Probably the best example of Fv-dependent interference involves the interaction of SIgA with cholera toxin (CT). CT is a member of the A–B family of enterotoxins, which includes botulinum neurotoxins (BoNT), shiga toxins (Stx), and the plant-derived toxin, ricin. CT is the toxin primarily responsible for the secretory diarrhea associated with Vibrio cholerae infection (Butler and Camilli, 2005). The toxin’s A subunit (CTA) catalyzes the NAD-ribosylation of the regulatory GTPase, Gαs, which in turn activates adenylate cyclase and cyclic AMP-dependent chloride secretion in crypt epithelial cells (Lencer and Tsai, 2003). The B subunit (CTB) oligomerizes to form a pentamer that binds specifically to the ganglioside GM1, and promotes toxin internalization. The toxin then traffics in a retrograde manner from the plasma membrane to the endoplasmic reticulum (ER), after which CTA is retrotranslocated into the cytoplasm (Lencer and Tsai, 2003). The effects of CT on intestinal epithelial cells can be studied in vitro using well-differentiated human intestinal cell lines such as T84 (Lencer et al., 1992).
It is now well established that SIgA is required for immunity to CT, and that protection is mediated primarily by antibodies that block toxin attachment to the epithelial cell receptor GM1. The requirement for SIgA in conferring immunity to CT was first demonstrated experimentally in a vaccine setting by Lycke and colleagues, who reported that J-chain knockout mice, following vaccination with CT, remained vulnerable to the effects of the toxin, whereas wild type control animals were immune (Lycke et al., 1999). Because J chain knockout mice had wild-type levels of anti-toxin IgA-producing B cells in the lamina propria, but severely reduced levels of SIgA levels in the intestinal lumen, it was concluded that antibodies in secretions were essential for full protection against the effects of CT, at least in the mouse model employed in this study. This conclusion was further supported by Uren and colleagues who reported a number of years later that CT-vaccinated pIgR knock-out mice, which are effectively devoid of SIgA but have normal to elevated levels of IgA in serum, were susceptible to cholera toxin challenge (Uren et al., 2005).
To investigate the mechanism by which the SIgA protects the epithelium from CT, Apter and colleagues produced a collection of anti-toxin monoclonal IgA antibodies from the Peyer’s patches of CT-immunized mice (Apter et al., 1993a). Three anti-CTB dimeric IgA MAbs were characterized in detail, and each was shown to block CT attachment to the apical surfaces of T84 cell monolayers in vitro. The three MAbs were also capable of functioning in vivo, as evidenced by the fact that neonatal mice treated passively with the MAbs were immune to CT-induced secretory diarrhea, weight loss and death (Apter et al., 1993b). It was proposed that the MAbs did not directly interact with the GM1 binding site on RTB, but, rather, functioned by steric hindrance. This conclusion was based on the observation that purified GM1, when added exogenously in an ELISA, did not competitively inhibit the antibodies from recognizing CTB.
SIgA has also been shown to prevent viral infections by blocking virus adhesion to epithelial cells. One notable example involves reovirus type 1 Lang (T1L), a murine enterovirus that initially infects the intestinal mucosa via attachment to Peyer’s patch M cells (Wolf et al., 1981). Silvey and colleagues demonstrated that SIgA is required for full protection against reovirus, a conclusion based on the observation that IgA knockout mice are susceptible secondary intestinal infections with reovirus, whereas wild type animals are immune (Silvey et al., 2001). To investigate the molecular mechanism underlying SIgA-mediated immunity to reovirus, Hutchings and colleagues examined the capacity of monoclonal IgA antibodies directed against viral surface antigens, including an adhesin and the capsid, to protect mice against oral T1L challenge (Hutchings et al., 2004). It was determined that protection was conferred by only one of the monoclonal antibodies tested, known as 1E1. 1E1 was determined to bind to the σ1 protein, an adhesin fiber known to promote viral attachment to a number of epithelial cell types, including M cells (Helander et al., 2003). The epitope recognized by 1E1 was localized to the receptor-binding head domain of σ1 (Helander et al., 2004). Monoclonal IgA antibodies against the other viral surface antigens, including the capsid protein, were not protective in the T1L peroral challenge model (Hutchings et al., 2004). The above studies demonstrate that protection against enteropathogenic viruses such as reovirus is conferred most effectively by SIgA antibodies directed against epitopes on the viral surfaces involved in attachment to epithelial cell receptors.
Fv-independent blocking of pathogen attachment to the epithelium
SIgA is also capable of preventing pathogen and toxin attachment to epithelial surfaces, independent of the antibody variable region. In this respect, SIgA can be considered a component of the innate immune system. As noted above, both the IgA H chain (Fcα) and SC are heavily glycosylated. Because the oligosaccharide side chains on SIgA share a high degree of similarity with those on the luminal face of the intestinal epithelium, it has been proposed that both IgA and SC can effectively serve as competitive inhibitors (“decoys”) of pathogen binding to host cells (Dallas and Rolfe, 1998; Mantis et al., 2004; Mestecky and Russell, 2009; Perrier et al., 2006; Royle et al., 2003; Schroten et al., 1998; Wold et al., 1990).
This role for SIgA was recently demonstrated in the case of ricin toxin (Mantis et al., 2004). The B subunit (RTB) of ricin toxin contains two primordial galactose binding domains that are conserved among the large family of so-called R-type lectins (Dodd and Drickamer, 2001 ; Rutenber et al., 1987). That family of lectins includes the BoNT accessory protein HA1 (Inoue et al., 2003), as well as the galactose-/N-acetylgalactosamine (GalNAc)-binding lectin from the protozoan parasite Entamoeba histolytica (Frederick and Petri, 2005). Like RTB, HA1 and the protozoan GalNAc lectin are involved in promoting toxin/pathogen attachment to intestinal epithelial cells.
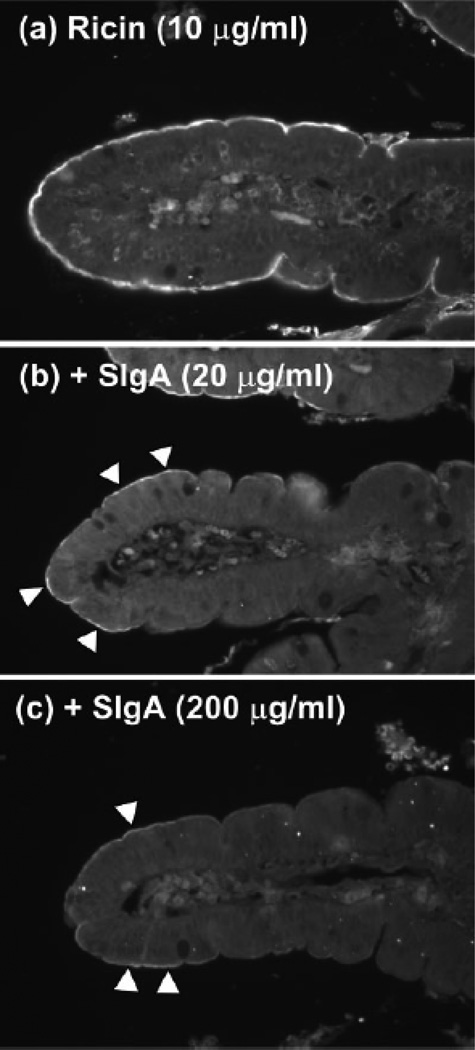
By lectin blot overlay and ELISA, we demonstrated that ricin recognizes the galactose-containing oligosaccharide side chains on human IgA1, IgA2, and SC (Mantis et al., 2004). Ricin attachment to the apical surfaces of T84 intestinal epithelial cell monolayers was reduced >10-fold by the addition of human colostral SIgA, and this interaction was dependent on the immunoglobulin oligosaccharide side chains, not the antibody binding domain (Mantis et al., 2004). To examine whether this interaction could be relevant in vivo, we overlaid ricin onto tissue sections of human duodenum. The apical surfaces of enterocytes in vivo are coated with a 500-nm thick FBBG that is rich in glycoproteins, that we postulated may serve as a potential high avidity binding site for ricin (Frey et al., 1996; Mantis et al., 2000). Indeed, when applied to human intestinal tissue sections, ricin stained the luminal surfaces of intestinal villi strongly and uniformly (Fig. 2). The addition of molar excess amounts of SIgA reduced toxin binding to the intestinal villi in a dose-dependent manner. These data demonstrate that SIgA, in an FV-independent fashion, is sufficient to block ricin, and probably other pathogen associated R-type lectins, from attaching to epithelial cell surfaces.
Fig. 2. SIgA competitively inhibits ricin toxin binding to the luminal surfaces of human intestinal villi.
Biotinylated ricin (10 µg/ml) was incubated for 1 h with PBS (a) or indicated concentrations of sIgA (b and c), and then applied to deparaffinized tissue sections of human duodenum. Sections were then labeled with streptavidin-FITC and visualized by fluorescence microscopy. a, Ricin labeled the luminal aspects of intestinal villi strongly and uniformly. Ricin also weakly labeled cells within the lamina propria. B and c, Preincubation of ricin with indicated concentrations of sIgA Abs reduced toxin attachment to luminal aspects of the intestinal villi. Arrowheads highlight areas of visible toxin labeling. Figure reproduced from (Mantis et al., 2004) with permission from the American Association of Immunologists.
Interestingly, it has been proposed that SC itself, by virtue of its N- and O-linked carbohydrate side chains, also plays an important role in SIgA-mediated innate immunity in the intestine. Dallas and Rolfe, for example, demonstrated that purified SIgA in concentrations at or below those found in human milk inhibited the binding of Clostridium difficile toxin A to purified enterocyte brush border membrane receptors (Dallas and Rolfe, 1998). Toxin A bound to SC significantly more strongly than to it did to the heavy and light chains of IgA. Moreover, purified SC alone was sufficient to inhibit toxin binding to receptors. That initial report was recently confirmed and extended by Perrier and colleagues who identified the galactose and sialic acid residues on SC as being primarily responsible for blocking toxin A attachment to epithelial cell monolayers (Perrier et al., 2006). Furthermore, SC may serve as a decoy receptor for other pathogens, including enteropathogenic E.coli (Perrier et al., 2006).
Immune exclusion
Immune exclusion is one of the most commonly proposed mechanisms by which SIgA blocks microbes from attaching to, colonizing and invading mucosal epithelia (Brandtzaeg, 2003; Kraehenbuhl and Neutra, 1992; Michetti et al., 1992; Michetti et al., 1994). In the context of the gut, immune exclusion is defined as the ability of SIgA, through its recognition of polyvalent antigens on the surfaces of viruses and bacteria, to agglutinate (i.e., cross-link) microbial pathogens in the intestinal lumen and thereby retard or abrogate their ability to adhere to and/or penetrate the epithelium. Moreover, the mucus layer overlying the epithelium is proposed to not only limit diffusion of SIgA-immune aggregates, but to also actively entrap them and promote their clearance from the gut via peristalsis (Deplancke and Gaskins, 2001; Lievin-Le Moal and Servin, 2006) (Fig. 1).
The first report of immune exclusion is generally attributed to Williams and Gibson (Williams and Gibbons, 1972). Those investigators examined whether SIgA, through binding to and aggregation of bacteria, is sufficient by itself to prevent or reduce bacterial colonization on mucosal surfaces. In their experiments, Streptococcus species indigenous to the oral mucosa were mixed with SIgA purified from parotid saliva, and then examined by light microscopy for the ability to adhere to human buccal epithelial cells. It was observed that agglutinated bacteria were 3–4 times less capable of adhering to human epithelial cells than were mock-treated bacteria. While SIgA was used in these studies, the authors noted that this effect would probably occur with any class of antibody capable of promoting bacterial agglutination.
Nonetheless, subsequent studies have substantiated immune exclusion as a bona fide mechanism by which SIgA can protect the intestinal epithelium against infection. The most thoroughly documented example involves immunity to the enteropathogenic bacterium Shigella flexneri (Boullier et al., 2009; Phalipon et al., 2002; Phalipon et al., 1995). S. flexneri is the causative agent of shigellosis, or bacillary dysentery, a disease that is endemic in developing countries (Levine et al., 2007). S. flexneri infects the colonic mucosa, and like reovirus, first gains entry into the mucosa via M cells (Cossart and Sansonetti, 2004). To investigate the role of secretory antibodies in immunity to S. flexneri, Phalipon and colleagues produced a murine monoclonal dimeric IgA that was directed against the O-antigen of serotype 5a strain (Phalipon et al., 1994). The O-antigen, the serotype specific carbohydrate on the surface of S. flexneri, is known to be a primary target of antibodies in convalescent individuals (Ferreccio et al., 1991; Levine et al., 2007; Oberhelman et al., 1991; Phalipon et al., 1995; Van de Verg et al., 1990). The protective capacity of the anti-O antigen-specific monoclonal antibody, known as IgAC5, was demonstrated in a mouse pulmonary infection model that mimics inflammatory lesions (e.g., neutrophil influx) induced during natural intestinal infection (Phalipon et al., 1994). IgAC5 was protective against S. flexneri infection when instilled intranasally into mice 1 hr before bacterial challenge, or when administered passively using the so-called “backpack” tumor model (Kraehenbuhl and Neutra, 1992). Light microscopy, in conjunction with autoradiography, revealed that IgAC5 entraps S. flexneri within a thin layer of mucus overlying the respiratory epithelium (Phalipon et al., 2002). Similar results were obtained when comparable studies were performed in a rabbit ligated ileal loop model of shigellosis, demonstrating that immune exclusion occurs in the intestinal lumen, and not solely in the respiratory tract (Boullier et al., 2009). Another important facet of the above-described studies is the demonstration that IgAC5-mediated localization and protection against S. flexneri is dependent on SC. For example, IgAC5 in complex with recombinant human SC was ~10 fold more protective than was IgAC5 alone (Phalipon et al., 2002). Moreover, was shown that the carbohydrate moieties on SC are not only be responsible for enhancing IgAC5’s protective effects, but are also necessary for mucus localization (Phalipon et al., 2002).
Unfortunately, it is often assumed, based in part on early work by Stokes and colleagues (Stokes et al., 1975), that the simple association of SIgA with an epitope on the surface of a pathogen is sufficient to promote immune exclusion, and thereby confer protection in vivo. However, this is not always the case. In the case of reovirus, for example, IgA antibodies that bound the head region of the adhesion-like σ1 protein were protective, whereas antibodies against the viral capsid were not (Helander et al., 2004). These data reveal that coating reovirus with SIgA is itself not sufficient to confer protective immunity; rather SIgA must associate with specific functional epitopes on the viral surface. This has implications vaccine for design, especially against HIV-1. As noted by Kozlowski and Neutra, conventional neutralizing antibodies against HIV-1, as defined by their ability to block viral entry into CD4+ CCR5/CXCR4 T cells, are rare (Kozlowski and Neutra, 2003). It is therefore important to determine whether coating the virus with “non-neutralizing” antibodies are in fact sufficient to reduce (or prevent) transmission across mucosal surfaces. We and other have produced human monoclonal IgAs against surface expressed epitopes that will permit this to be tested in in vitro and/or ex vivo models of HIV-1 mucosal transmission (Mantis et al., 2007; Wallace et al., 2009; Wolbank et al., 2003).
Direct effects of SIgA on bacterial virulence
Neither receptor blocking nor immune exclusion mechanisms can explain SIgA-mediated protection against a number of virulent enteropathogenic bacteria, such as S. Typhimurium and the closely-related S. Enteriditis. Like S. flexneri, S. Typhimurium initially infects the intestinal mucosa via Peyer’s patch M cells (Carter and Collins, 1974; Clark et al., 1994; Jones et al., 1994; Martinoli et al., 2007), although infection also occurs via villus enterocytes (Takeuchi, 1967; Vazquez-Torres et al., 1999). Contact with the epithelium is mediated by a number of fimbrial adhesins (Baumler et al., 1996a; Baumler et al., 1996b; Giannasca et al., 1996) and is augmented by bacterial motility (Marchetti et al., 2004). Motility of S. Typhimurium is driven by flagella, which are whip-like filaments (>10 µm in length) that rotate coordinately to promote movement through viscous environments such as mucus (Berg, 2003). Once in contact with the intestinal surface, S. Typhimurium employs the so-called Salmonella pathogenicity island-1 (SPI-1) type three secretion system (T3SS) to invade Peyer’s patch M cells (Cossart and Sansonetti, 2004 ; Galan and Wolf-Watz, 2006). The SPI-1 T3SS is a syringe-like structure projecting from the bacterial surface that upon contact with host cells injects an array of bacterial proteins into the host cell cytosol, resulting in cytoskeletal rearrangements and bacterial macropinocytosis (Cossart and Sansonetti, 2004; Galan and Wolf-Watz, 2006). Strains of S. Typhimurium that lack certain SPI-1-encoded effector proteins, or that are unable to display the SPI-1 T3SS on their surfaces, are unable to invade M cells and are avirulent by the oral route (Ellermeier et al., 2005; Galan, 2001; Jones et al., 1994). Infection of the intestinal mucosa by S. Typhimurium is associated also with an intense inflammatory response (McCormick, 2007; Raffatellu et al., 2008).
Following infection, the SIgA response to S. Typhimurium is directed almost exclusively against the O-antigen component of lipopolysaccharide (LPS) (Iankov et al., 2001; Iankov et al., 2002a; Michetti et al., 1992). In S. Typhimurium, the O-antigen consists of 20–35 repeats of tri- or tetrasaccharide units are anchored to the bacterial outer membrane via lipid A. It is estimated that more than 75% of the bacterial outer surface is covered with O-antigen (Nikaido, 1996). While the mucosal immune response following Salmonella infection is primarily directed against the O-antigen, the flagella are also a target of SIgA (Iankov et al., 2002a). Flagella-specific SIgA antibodies are primary directed against flagellin (FliC), the protein that polymerizes to form the flagellar filaments.
It is well established that protection against S. Typhimurium and S. Enteritidis infection is conferred by SIgA antibodies directed against the O-antigen, but not those against the flagella (Carter and Collins, 1974; Iankov et al., 2004; Michetti et al., 1992). A role for O-antigen specific IgA in protective was demonstrated experimentally by Michetti and colleagues who produced a collection of dimeric monoclonal IgA antibodies directed against the S. Typhimurium O-antigen (Michetti et al., 1992). One of these monoclonal antibodies, Sal4, was characterized in detail (Michetti et al., 1992; Michetti et al., 1994). When delivered into the intestinal lumen by normal receptor-mediated transepithelial transport, Sal4 was sufficient to protect mice against a lethal oral challenge with S. Typhimurium (Michetti et al., 1992). Sal4 markedly reduced the numbers of bacteria that gained entry into the Peyer’s patches following oral challenge, suggesting that the antibody interferes with bacterial attachment to and/or invasion of intestinal epithelial cells (Michetti et al., 1992). In agreement with the hypothesis, it was subsequently shown that the presence of Sal4 is sufficient to block invasion of polarized intestinal epithelial cell monolayers by S. Typhimurium in vitro (Michetti et al., 1994).
Similar findings were reported by Iankov and colleagues using S. Enteritidis as a model system (Iankov et al., 2004; Iankov et al., 2002b). Those investigators produced a collection of monoclonal IgAs against S. Enteritidis flagella (anti-H) and O-antigens (serotype O9) (Iankov et al., 2001; Iankov et al., 2002a). The O-antigen specific IgAs reduced S. Enteritidis invasion of HEp-2 intestinal epithelial cell monolayers, whereas the anti-flagellin antibodies did not (Iankov et al., 2002b). Similar outcomes were reported in vivo, in a model of generalized infection after intranasal inoculation of BALB/c mice. Specifically, the anti-O antigen monoclonal antibodies were protective, whereas the anti-flagellin antibodies were not (Iankov et al., 2004).
While both the above studies established that IgA antibodies against the O-antigen are protective against Salmonella infection, neither resolved the question of exactly how these antibodies interfered with the ability of the bacteria to invade intestinal epithelial cells. Nevertheless, a number of lines of evidence argue against a role for agglutination and, therefore, immune exclusion. For example, anti-flagella IgAs are more efficient than anti-O antigen specific antibodies at promoting Salmonella agglutination (a prerequisite for immune exclusion), yet flagella-specific IgAs are not protective in vitro or in vivo (Carter and Collins, 1974; Iankov et al., 2004; Iankov et al., 2002b). To directly address the role of agglutination in protection, we performed S. Typhimurium invasion assays in the presence of sub-agglutinating concentrations of Sal4 (Forbes et al., 2008). Under these conditions, Sal4 reduced the ability of S. Typhimurium to invade polarized epithelial cells monolayers by ~20 fold. Sal4’s protective effects were not augmented when the experiments were repeated under agglutinating conditions. Finally, as further evidence that agglutination is not integral to Sal4’s protective effects, we performed invasion assays in the presence of O-antigen specific Fab fragments (Forbes et al., 2008). Monovalent Fabs, while unable to induce bacterial agglutination, blocked S. Typhimurium entry into epithelial cells almost as effectively as did Sal4. We conclude from these studies that agglutination, and by extension immune exclusion, cannot account for the effects of O-antigen specific IgA on Salmonella’s entry into epithelial cells.
Based on the above data, we hypothesized that O-antigen specific IgA antibodies, specifically Sal4, must interfere with S. Typhimurium’s invasion of epithelial cells by another mechanism, possibility by one entailing bacterial motility and/or SPI-1 entry into epithelial cells. To investigate the impact of Sal4 on motility, we exposed S.Typhimurium to Sal4 at a range of concentrations and then monitored bacterial movement by video light microscopy. Digital video tracking analysis revealed that Sal4 induces a time- and dose-dependent arrest in bacterial motility (Forbes et al., 2008). Sal4’s inhibitory effects were rapid (<15 min), and they were independent of the antibody’s ability to promote bacterial agglutination. Moreover, Sal4-treated bacteria were completely “paralyzed” following antibody treatment. This is in contrast to what is observed when the bacteria are treated with anti-flagella (H) antibodies. In that case, the bacteria twitched and tumbled before ceasing to swim (Forbes et al., 2008). These experiments revealed that Sal4 is a potent inhibitor of bacteria motility, and that the mechanisms by which anti-flagella and anti-O antibodies alter S. Typhimurium motility are distinct.
Because non-motile mutants of S.Typhimurium are 30–50 fold less invasive than their parental wild type strains (Jones et al., 1992), we speculated that Sal4’s effects on motility could account for the antibody’s observed protective capacity. To examine this possibility, we performed bacterial invasion assays, in the absence and presence of Sal4, in which centrifugation was employed to artificially promote bacterium-host cell contact. To our surprise, Sal4-treated bacteria remained non-invasive, even though they adhered to the epithelial cell monolayers as effectively as untreated cells. These data reveals that Sal4 interferes with at least two distinct steps in the infection of epithelial cells by S. Typhimurium: it inhibits flagellum-based motility and blocks SPI-1 T3SS-dependent epithelial entry.
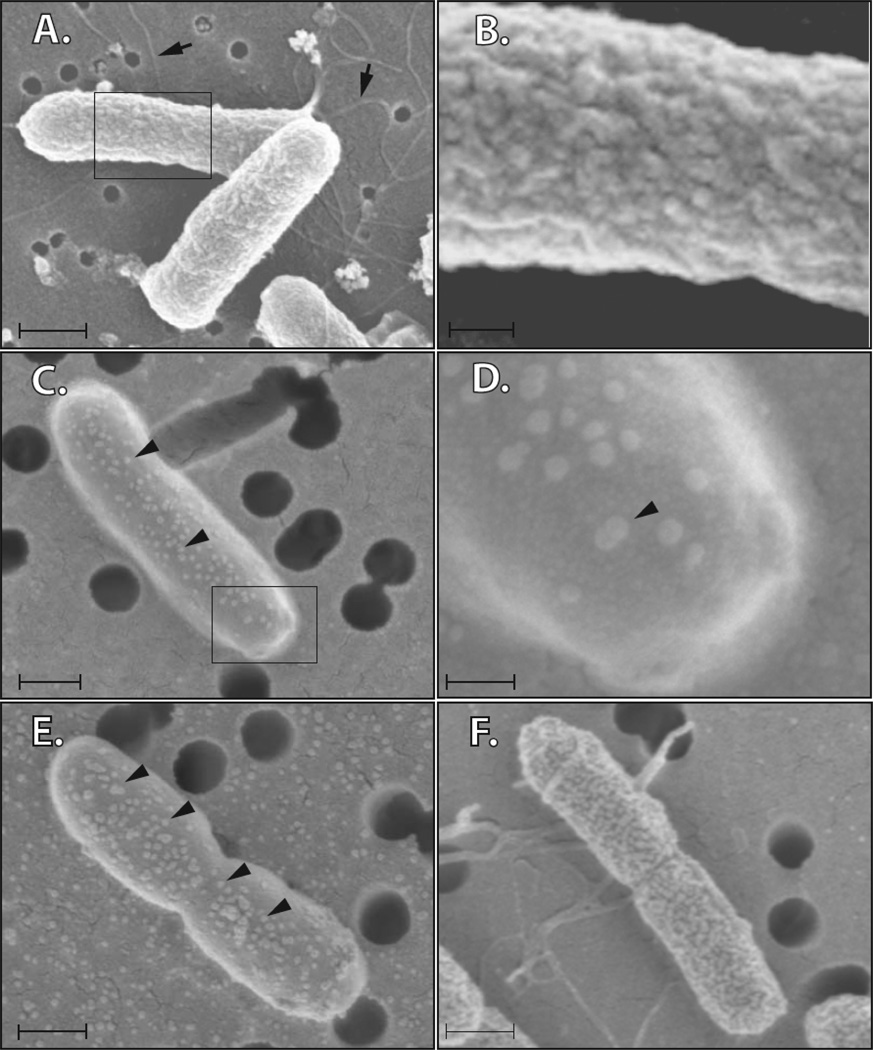
How might a Sal4 be interfering with both flagellum-based motility and SPI-1 T3S in S. Typhimurium? We hypothesized that Sal4, through its ability to associate with the O-antigen, perturbs the inherent organization of the bacterial LPS layer, and thereby destabilizes the integrity outer membrane. To investigate this possibility, we examined control- and Sal4-treated bacteria by scanning electron microcopy (SEM). Whereas the surfaces of control bacterial cells were uniformly grooved and ridged, the surfaces of Sal4-treated cells appear blistered (Fig. 3). The blisters (or blebs) were present on what were otherwise uncharacteristically smooth cell surfaces. IgAC5 had a similar effect on the surface of S. flexneri (S. Forbes and N. Mantis, manuscript in preparation). While the molecular basis of these changes remains unknown, it is clear that the association of IgA with LPS is sufficiently profound as to induce changes in the surface topology of the bacterial outer membrane and affect both motility and SPI-1 T3SS. These findings fundamentally challenge the previous assumptions as to how SIgA functions to combat microbial pathogens in the intestinal lumen (Fig. 4). Future studies in our laboratory will be aimed at understanding in molecular detail the sequence of events that occur at the bacterial cell surface following the association of Sal4 with the O-antigen.
Figure 3. Sal4 induce changes in the surface topology of the outer membrane of S. Typhimurium.
S. Typhimurium cells were incubated with TEPC-15 (a control monoclonal IgA of irrelevant antigen specificity), Sal4, anti-O or anti-H antibodies for 15 min, then captured on a porous (0.2 or 0.4 µm) filters, fixed with 2% gluteraldehyde in 0.1 M sodium cacodylate buffer, dehydrated, critical-point dried, and then attached to an aluminum stud with carbon paste and sputter coated with gold. The specimens were observed at 5.00 kV using a LEO 1550VP FEG scanning electron microscope (Carl Zeiss SMT). Panels A–B: TEPC-15 (5 µg/ml); Panels C–D: Sal4 (5 µg/ml); Panel E; polyclonal anti-O antiserum (1:200 dilution); Panel F: polyclonal anti-flagella antiserum (1:200 dilution). Note flagella (Panel A, arrows). Membrane ‘blebs’ were observed on Sal4-treated cells (Panel C, arrowheads). In Panels A,C,E,F, the scale bars are equivalent to 0.5 µm. In panels B,D the scale bars are 0.1 µm. Polyclonal anti-sera were purchased from BD Difco (Franklin Lakes, NJ). The data presented in this figure are part of a manuscript in preparation by the authors of this review.
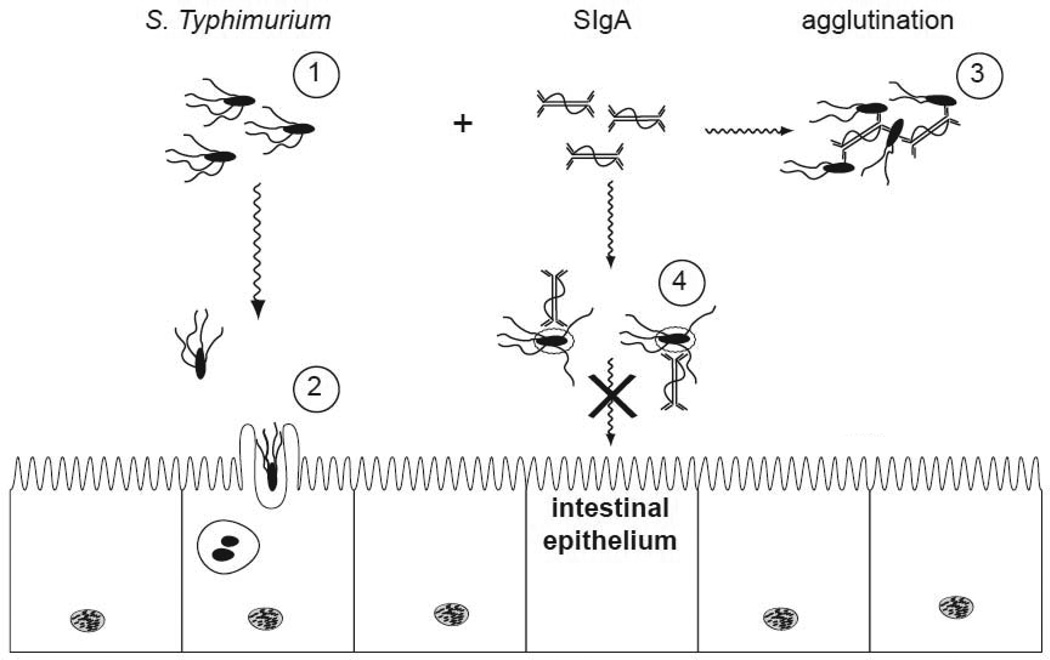
Figure 4. Proposed mechanisms by which O-antigen specific SIgA protects against S. Typhimurium infection.
(1) S. Typhimurium proliferates within the intestinal lumen and propels itself via flagella-based motitity; (2) Upon contact with intestinal epithelial cells, the bacteria promote their own uptake and engulfment. This process is mediated by the SPI-1 T3S system; (3) Agglutination of S. Typhimurium in the presence of LPS specific SIgA antibodies, such as Sal4. This process would occur only at optimal antibody-bacteria ratios; (4) An alternative model by which Sal4 may arrest S. Typhimurium. In this case, the association of Sal4 with the bacterial LPS inhibits flagella-based motility, reduces SPI-1 T3S activity, and induces alterations in the bacterial outer membrane (depicted as a halo surrounding the bacterial cells).
4. Summary and perspective
In this review, we have summarized the current understanding of the three proposed mechanisms by which SIgA prevents pathogens from gaining access to the intestinal epithelium. The three mechanisms are (i) interference with epithelial receptor binding; (ii) immune exclusion; and (iii) direct impact on the activation or expression of virulence determinants (e.g., motility and T3S). As these mechanisms are not necessarily mutually exclusive, it would not be surprising to discover that immunity against most viral and bacterial pathogens involves engagement of more than one of SIgA’s activities. A more thorough understanding of SIgA’s role in intestinal immunity will enable rational design of more effective mucosal vaccines, as well as the development more sophisticated surrogate markers that are necessary to evaluate these vaccines in human clinical trials (Levine, 2006).
Fig 5.
ACKNOWLEDGEMENTS
We gratefully acknowledge William Samsonoff of the Wadsworth Center’s Electron Microscopy Core facility for assistance with the SEM. This work was supported in part by NIH grants HD061916 and AI082059 to NJM.
REFERENCES
- Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: A mechanism for transmission of AIDS. J Acq Immun Def Synd. 1991;4:760–765. [PubMed] [Google Scholar]
- Apter FM, Lencer WI, Finkelstein RA, Mekalanos JJ, Neutra MR. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993a;61:5271–5278. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter FM, Michetti P, Winner LSd, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993b;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader MW, Navarre WW, Shiau W, Nikaido H, Frye JG, McClelland M, Fang FC, Miller SI. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol Microbiol. 2003;50:219–230. doi: 10.1046/j.1365-2958.2003.03675.x. [DOI] [PubMed] [Google Scholar]
- Baumler A, Tsolis R, Bowe F, Kusters J, Hoffmann S, Heffron F. The pef Fimbrial Operon of Salmonella typhimurium Mediates Adhesion to Murine Small Intestine and Is Necessary for Fluid Accumulation in the Infant Mouse. Infection and Immunity. 1996a;64:61–68. doi: 10.1128/iai.64.1.61-68.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumler A, Tsolis R, Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer’s patches. Proc Natl Acad Sci USA. 1996b;93:279–283. doi: 10.1073/pnas.93.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, Phalipon A. Secretory IgA-Mediated Neutralization of Shigella flexneri Prevents Intestinal Tissue Destruction by Down-Regulating Inflammatory Circuits. J Immunol. 2009 doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature. 1974;252:418–420. doi: 10.1038/252418a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003;293:3–15. doi: 10.1078/1438-4221-00241. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effects of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Butler SM, Camilli A. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol. 2005;3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176:4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- Clark MA, Jepson MA, Simmons NL, Hirst BH. Differential expression of lectin-binding sites defines mouse intestinal M cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- Clark MA, Jepson MA, Simmons NL, Hirst BH. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Research in microbiology. 1994;145:543–552. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Corthesy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, Schwartz-Cornil I. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol. 2006;80:10692–10699. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- Crago SS, Kutteh WH, Moro I, Allansmith MR, Radl J, Haaijman JJ, Mestecky J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. Journal of Immunology. 1984;132:16–18. [PubMed] [Google Scholar]
- Crottet P, Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’)2: a possible implication for mucosal defense. Journal of Immunology. 1998;161:5445–5453. [PubMed] [Google Scholar]
- Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. Journal of Medical Microbiology. 1998;47:879–888. doi: 10.1099/00222615-47-10-879. [DOI] [PubMed] [Google Scholar]
- Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. American Journal of Clinical Nutrition. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Dodd RB, Drickamer K. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology. 2001;11:71R–79R. doi: 10.1093/glycob/11.5.71r. [DOI] [PubMed] [Google Scholar]
- Ellermeier CD, Ellermeier JR, Slauch JM. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- Fernandez MI, Pedron T, Tournebize R, Olivo-Marin JC, Sansonetti PJ, Phalipon A. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity. 2003;18:739–749. doi: 10.1016/s1074-7613(03)00122-5. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. American journal of epidemiology. 1991;134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect Immun. 2008;76:4137–4144. doi: 10.1128/IAI.00416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Frederick JR, Petri WA., Jr. Roles for the galactose-/N-acetylgalactosamine-binding lectin of Entamoeba in parasite virulence and differentiation. Glycobiology. 2005;15:53R–59R. doi: 10.1093/glycob/cwj007. [DOI] [PubMed] [Google Scholar]
- Frey A, Giannasca KT, Weltsin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: Implications for microbial attachment and vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Giannasca KT, Giannasca PJ, Neutra MR. Adherence of Salmonella typhimurium to Caco-2 Cells: Identification of a glycoconjugate receptor. Infect Immun. 1996;64:135–145. doi: 10.1128/iai.64.1.135-145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannasca PJ, Giannasca KT, Falk P, Gordon JI, Neutra MR. Regional differences in glycoconjugates of intestinal M cells in mice: Potential targets for mucosal vaccines. Am J Physiol. 1994;267:G1108–G1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 1999;67:946–953. doi: 10.1128/iai.67.2.946-953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- Helander A, Miller CL, Myers KS, Neutra MR, Nibert ML. Protective immunoglobulin A and G antibodies bind to overlapping intersubunit epitopes in the head domain of type 1 reovirus adhesin sigma1. J Virol. 2004;78:10695–10705. doi: 10.1128/JVI.78.19.10695-10705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Silvey KJ, Mantis NJ, Hutchings AB, Chandran K, Lucas WT, Nibert ML, Neutra MR. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J Virol. 2003;77:7964–7977. doi: 10.1128/JVI.77.14.7964-7977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GJ, Reason AJ, Savoy L, Jaton J, Frutiger-Hughes S. Carbohydrate moieties in human secretory component. Biochimica et Biophysica Acta. 1999;1434:86–93. doi: 10.1016/s0167-4838(99)00168-5. [DOI] [PubMed] [Google Scholar]
- Hutchings AB, Helander A, Silvey KJ, Chandran K, Lucas WT, Nibert ML, Neutra MR. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer’s patches. JVirol. 2004;78:947–957. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Ivanova R, Sechanova L, Mitov IG. Monoclonal antibodies of IgA isotype specific for lipopolysaccharide of Salmonella enteritidis: production, purification, characterization and application as serotyping reagents. FEMS Microbiol Lett. 2001;196:215–221. doi: 10.1111/j.1574-6968.2001.tb10567.x. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Ivanova R, V RV, Mitov IG. Production and characterization of monoclonal immunoglobulin A antibodies directed against Salmonella H:g,m flagellar antigen. FEMS Immunol Med Microbiol. 2002a;33:107–113. doi: 10.1111/j.1574-695X.2002.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Kalev OK, Balabanova MS, Mitov IG. Protective efficacy of IgA monoclonal antibodies to O and H antigens in a mouse model of intranasal challenge with Salmonella enterica serotype Enteritidis. Microbes Infect. 2004;6:901–910. doi: 10.1016/j.micinf.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Mitov IG. Lipopolysaccharide-specific but not anti-flagellar immunoglobulin A monoclonal antibodies prevent Salmonella enterica serotype enteritidis invasion and replication within HEp-2 cell monolayers. Infect Immun. 2002b;70:1615–1618. doi: 10.1128/IAI.70.3.1615-1618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Sobhany M, Transue TR, Oguma K, Pedersen LC, Negishi M. Structural analysis by X-ray crystallography and calorimetry of a haemagglutinin component (HA1) of the progenitor toxin from Clostridium botulinum. Microbiology. 2003;149:3361–3370. doi: 10.1099/mic.0.26586-0. [DOI] [PubMed] [Google Scholar]
- Jones B, Ghori N, Falkow S. Salmonella typhimurium initiated murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s Patches. Journal Experimental Medicine. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BD, Lee CA, Falkow S. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect Immun. 1992;60:2475–2480. doi: 10.1128/iai.60.6.2475-2480.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel CS, Robinson JK, Lamm ME. Epithelial transcytosis of monomeric IgA and IgG cross-linked through antigen to polymeric IgA. A role for monomeric antibodies in the mucosal immune system. Journal of Immunology. 1994;152:72–76. [PubMed] [Google Scholar]
- Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr Mol Med. 2003;3:217–228. doi: 10.2174/1566524033479852. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl JP, Neutra MR. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Delp C, Neutra MR, Madara JL. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. Journal of Cell Biology. 1992;117:1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer WI, Tsai B. The intracellular voyage of cholera toxin: going retro. Trends Biochem Sci. 2003;28:639–645. doi: 10.1016/j.tibs.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Levine MM. Enteric infections and the vaccines to counter them: future directions. Vaccine. 2006;24:3865–3873. doi: 10.1016/j.vaccine.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D, Tynan W, Dickerson J, Mendy J, Chang HW, Scharf M, Byrne D, Brayden D, Higgins L, Evans C, et al. Peptidoglycan recognition protein expression in mouse Peyer’s Patch follicle associated epithelium suggests functional specialization. Cell Immunol. 2003;224:8–16. doi: 10.1016/s0008-8749(03)00155-2. [DOI] [PubMed] [Google Scholar]
- Lycke N, Erlandsson L, Ekman L, Schon K, Leanderson T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J Immunol. 1999;163:913–919. [PubMed] [Google Scholar]
- Ma JK-C, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventative immunotherapy in humans. Nature Medicine. 1998;4:601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- Maki DG. Coming to grips with foodborne infection--peanut butter, peppers, and nationwide salmonella outbreaks. The New England journal of medicine. 2009;360:949–953. doi: 10.1056/NEJMp0806575. [DOI] [PubMed] [Google Scholar]
- Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57:1424–1439. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Mantis NJAF, Neutra MR. Accessibility of glycolipid and glycoprotein epitopes on rabbit villus and follicle-associated epithelium. American journal of physiology. 2000;278:G915–924. doi: 10.1152/ajpgi.2000.278.6.G915. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Bry L. The Intestinal Epithelium: The Interface Between Host and Pathogen. In: Vajdy M, editor. Immunity Against Mucosal Pathogens. San Diego: Springer; 2008. pp. 3–22. [Google Scholar]
- Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. Journal of Immunology. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Farrant SA, Mehta S. Oligosaccharide side chains on human secretory IgA serve as receptors for ricin. JImmunol. 2004;172:6838–6845. doi: 10.4049/jimmunol.172.11.6838. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006;74:3455–3462. doi: 10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Morici L, Roy C. Mucosal Vaccines for Biodefense. In: Kozlowski P, editor. Mucosal Vaccines: Moderns concepts, strategies, and challenges. Berlin/Heidelbeg: Springer-Verlag; in press. [Google Scholar]
- Mantis NJ, Palaia J, Hessell AJ, Mehta S, Zhu Z, Corthesy B, Neutra MR, Burton DR, Janoff EN. Inhibition of HIV-1 Infectivity and Epithelial Cell Transfer by Human Monoclonal IgG and IgA Antibodies Carrying the b12 V Region. J Immunol. 2007;179:3144–3152. doi: 10.4049/jimmunol.179.5.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M, Sirard JC, Sansonetti P, Pringault E, Kerneis S. Interaction of pathogenic bacteria with rabbit appendix M cells: bacterial motility is a key feature in vivo. Microbes Infect. 2004;6:521–528. doi: 10.1016/j.micinf.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marcial MA, Madara JL. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90:583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- Martinoli C, Chiavelli A, Rescigno M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Jin Y, Kabumoto Y, Takegahara Y, Oguma K, Lencer WI, Fujinaga Y. The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. Journal of Biological Chemistry. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. Journal of Virology. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA. Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. Febs J. 2007;274:3513–3518. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Moro I, Kerr MA, Woof JM. Mucosal Immunoglobulins. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Burlington, MA: Academic Press; 2005. pp. 153–181. [Google Scholar]
- Mestecky J, Russell MW. Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol Lett. 2009;124:57–62. doi: 10.1016/j.imlet.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti P, Porta N, Mahan MJ, Slauch JM, Mekalanos JJ, Blum A, Kraehenbuhl JP, Neutra MR. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology. 1994;107:915–923. doi: 10.1016/0016-5085(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Mostov K. Transepithelial transport of immunoglobulins. Annual Review of Immunology. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Neutra M, Mantis N, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissue. Nature Immunology. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- Neutra M, Phillips T, Mayer E, Fishkind D. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer’s patch. Cell and tissue research. 1987;247:537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, Yi A, Fernandez-Prada C, Guzman M, Leon-Barua R, et al. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59:2341–2350. doi: 10.1128/iai.59.7.2341-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cell alpha-defensin synthesis and function. Curr Top Microbiol Immunol. 2006;306:1–25. doi: 10.1007/3-540-29916-5_1. [DOI] [PubMed] [Google Scholar]
- Owen R, Pierce N, Apple R, Cray W., Jr. M Cell Transport of Vibrio cholerae from the Intestinal Lumen into Peyer’s Patches: A Mechanism for Antigen Sampling and for Microbial Transepithelial Migration. The Journal of Infectious Diseases. 1986;153:1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Pappo J, Ermak TH. Uptake and translocation of fluorescent latex particles by rabbit Peyer’s patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989;76:144–148. [PMC free article] [PubMed] [Google Scholar]
- Perrier C, Sprenger N, Corthesy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem. 2006;281:14280–14287. doi: 10.1074/jbc.M512958200. [DOI] [PubMed] [Google Scholar]
- Petri WA, Jr., Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- Phalipon A, Corthesy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends in Immunology. 2003;24:55–58. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- Phalipon A, Kaufmann M, Michetti P, Cavaillon JM, Huerre M, Sansonetti P, Kraehenbuhl JP. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. Journal of Experimental Medicine. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalipon A, Michetti P, Kaufmann M, Cavaillon JM, Huerre M, Kraehenbuhl JP, Sansonetti PJ. Protection against invasion of the mouse pulmonary epithelium by a monoclonal IgA directed against Shigella flexneri lipopolysaccharide. Annals of the New York Academy of Sciences. 1994;730:356–358. doi: 10.1111/j.1749-6632.1994.tb44291.x. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell host & microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. Journal of Biological Chemistry. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326:624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Arondel J, Cantey JR, Prevost MC, Huerre M. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infection & Immunity. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroten H, Stapper C, Plogmann R, Kohler H, Hacker J, Hanisch FG. Fab-independent antiadhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infection & Immunity. 1998;66:3971–3973. doi: 10.1128/iai.66.8.3971-3973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Rowinski J, Warchol JB, Jarzabek Z, Gut W, Szczygiel B, Bielecki K, Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990;98:56–58. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer’s patches. J Virol. 2001;75:10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes CR, Soothill JF, Turner MW. Immune exclusion is a function of IgA. Nature. 1975;255:745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- Stubbe H, Berdoz J, Kraehenbuhl JP, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. Journal of Immunology. 2000;164:1952–1960. doi: 10.4049/jimmunol.164.4.1952. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2:468–471. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- Tam MA, Rydstrom A, Sundquist M, Wick MJ. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev. 2008;225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- Tohno M, Shimosato T, Kitazawa H, Katoh S, Iliev ID, Kimura T, Kawai Y, Watanabe K, Aso H, Yamaguchi T, et al. Toll-like receptor 2 is expressed on the intestinal M cells in swine. Biochem Biophys Res Commun. 2005;330:547–554. doi: 10.1016/j.bbrc.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Tomana M, Niedermeier W, Mestecky J, Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976;13:325–328. doi: 10.1016/0019-2791(76)90342-6. [DOI] [PubMed] [Google Scholar]
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. EurJImmunol. 2005;35:180–188. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- Van de Verg L, Herrington DA, Murphy JR, Wasserman SS, Formal SB, Levine MM. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990;58:2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Wallace GS, Cheng-Mayer C, Schito ML, Fletcher P, Miller Jenkins LM, Hayashi R, Neurath AR, Appella E, Shattock RJ. Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection. J Virol. 2009;83:9175–9182. doi: 10.1128/JVI.00820-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin R, Lucia-Jandris P, Michetti P, Fields BN, Kraehenbuhl JP, Neutra MR. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. JCell Biol. 1989;108:1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RC, Gibbons RJ. Inhibition of bacterial adherence by secretory immunoglobulin A: A mechanism of antigen disposal. Science. 1972;172:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Wolbank S, Kunert R, Stiegler G, Katinger H. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J Virol. 2003;77:4095–4103. doi: 10.1128/JVI.77.7.4095-4103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold AE, Mestecky J, Tomona M, Kobata A, Ohbayashi H, Endo T, Eden CS. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Rubin D, Finberg R, Kauffman R, Sharpe A, Trier J, Fields B. Intestinal M cells: A pathway for entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]