Abstract
The extracellular matrix (ECM), important for maintaining tissue homeostasis, is abnormally expressed in mammary tumors and additionally plays a crucial role in angiogenesis. We hypothesize that breast cancer cells (BCCs) deposit ECM that supports unique patterns of vascular morphogenesis of endothelial cells (ECs). Evaluation of ECM expression revealed that a nontumorigenic cell line (MCF10A), a tumorigenic cell line (MCF7), and a metastatic cell line (MDA-MB-231) express collagens I and IV, fibronectin, and laminin, with tenascin-C limited to MCF10A and MCF7. The amount of ECM deposited by BCCs was found to be higher in MCF10A compared with MCF7 and MDA231, with all ECM differing in their gross structure but similar in mean fiber diameter. Nonetheless, deposition of ECM from BCC lines was overall difficult to detect and insufficient to support capillary-like structure (CLS) formation of ECs. Therefore, a coculture approach was undertaken in which individual BCC lines were cocultured with fibroblasts. Variation in abundance of deposited ECM, deposition of ECM proteins, such as absent collagen I deposition from MDA231-fibroblast cocultures, and fibril organization was found. Deposited ECM from fibroblasts and each coculture supported rapid CLS formation of ECs. Evaluation of capillary properties revealed that CLS grown on ECM deposited from MDA231-fibroblast cocultures possessed significantly larger lumen diameters, occupied the greatest percentage of area, expressed the highest levels of von Willebrand factor, and expressed the greatest amount of E-selectin, which was upregulated independent of exposure to TNF-α. To our knowledge, this is the first study to report tumor cell ECM-mediated differences in vascular capillary features, and thus offers the framework for future investigations interrogating the role of the tumor ECM in supporting vascular morphogenesis.
Keywords: endothelial cells, extracellular matrix
the extracellular matrix (ECM) is a noncellular entity, comprising a variety of macromolecules that together act as a scaffold to provide structural support to overlying cells and the tissues they make up. Locally resident cells, such as fibroblasts, secrete the majority of proteoglycans, nonproteoglycans, and fibrous proteins such as collagens, elastins, fibronectin, etc. (69), which collectively make up the ECM. While previously considered an inert filler substance, the ECM is known to actively influence numerous cellular activities including cell adhesion, proliferation, differentiation, self-renewal, survival, and migration in addition to providing mechanical support to overlying cells (36). In addition, the ECM sequesters numerous bioactive molecules, concentrating them locally and releasing them in response to proteolytic degradation, thereby indirectly influencing cellular decisions (64).
Alterations in ECM expression have been correlated to tumor development and progression. A well-known example is breast cancer, where increased mammographic density, recently attributed to alterations in stroma and ECM deposition (2, 26, 46), is associated with an increased risk for development of breast cancer (10, 11, 52). Provenzano et al. (60) specifically demonstrated that increased collagen deposition in mouse mammary tissues facilitated breast tumor initiation and progression. Additional ECM proteins implicated in breast tumor progression, invasion, and metastasis include tenascin-C (27, 34) and fibronectins (16, 49). While the aforementioned ECM proteins each contribute to some aspect of breast tumor progression, a direct, causal link between ECM protein expression and pathogenic angiogenesis, a necessary life force for breast as well as other solid tumor expansion, has not been addressed.
Angiogenesis, the generation of new blood vessels from the preexisting vasculature, occurs when endothelial cells (ECs) degrade their underlying basement membrane, proliferate, and invade the surrounding ECM, where they align, resynthesize their basement membrane, and form microvessels. In breast carcinomas, the extent of neovascularization in addition to the presence of high levels of proangiogenic molecules are correlated with poor breast cancer prognosis (24, 48, 51). While traditional antiangiogenesis therapies have had some success in the clinic, few patients are completely cured of their disease (29, 35), necessitating investigation into alternative means whereby the tumor vasculature may be targeted.
The ECM is a critical regulator of angiogenesis, sequestering proangiogenic cytokines, regulating EC survival, migration, and proliferation, and providing guidance cues to support capillary morphogenesis (17). Numerous ECM proteins have been shown to play unique and overlapping roles in the aforementioned steps of angiogenesis. Specifically, EC survival has been attributed to expression of fibronectin or laminin (18, 75); EC migration to tenascin-C or laminin (25, 82); EC proliferation to fibronectin and collagens I and IV (32, 74, 83); and formation and sprouting of vascular structures to a variety of ECM proteins (9, 14, 23, 50). In vitro evidence for the ability of the ECM as a whole to support capillary morphogenesis was first demonstrated by Soucy and Romer (65), who reported that a naturally derived, multicomponent ECM obtained from fibroblasts directed the growth of microvessels. Interestingly, another recent report documented that ECM derived from tumorigenic clones of telomerase-transfected mesenchymal stem cells possessed angiogenic potential, supporting in vitro formation of vascular structures (13). These studies provide initial evidence that the ECM is crucial for supporting capillary morphogenesis and additionally point to the ability of tumor-associated ECM to facilitate vascular formation.
In this study, we hypothesized that ECM deposited by breast cancer cells (BCCs) of different tumorigenic stages supports capillary-like structure (CLS) formation with specific kinetics and characteristics. For this, we explored three BCC lines including a nontumorigenic cell line (MCF10A), a tumorigenic cell line (MCF7), and a metastatic cell line (MDA-MB-231) to capture distinct stages of in vitro breast tumorigenesis. ECM expression and deposition by the epithelial BCC lines was determined and found to be unique for each of the BCC lines. However, ECM deposition by the BCCs was insufficient to support in vitro studies of capillary morphogenesis. Thus a coculture approach, whereby the BCC lines were individually cultured in direct contact with fibroblasts, was established to enable decellularization and in vitro studies of vascular network formation on deposited ECM.
MATERIALS AND METHODS
Cell lines and culture.
The BCC lines MCF10A and MDA231 were a gift from the Physical Sciences-Oncology Center (PSOC, National Institutes of Health, Bethesda, MD) and were obtained through the laboratory of Dr. Thea Tlsty (University of California, San Francisco, CA). The breast cancer cell line MCF7 was acquired through the American Type Culture Collection (ATCC, Manassas, VA). The human neonatal foreskin fibroblast (NuFF) cell line was obtained from Global Stem (Rockville, MD) at passage 9. Human umbilical vein endothelial cells (HUVECs) were obtained from Promocell (Heidelberg, Germany). MCF10A cells were cultured in Dulbecco's modified Eagle's medium-F-12 (DMEM-F-12; GIBCO, Carlsbad, CA) supplemented with 5% (vol/vol) horse serum (GIBCO), 0.5 μg/ml hydrocortisone, 20 ng/ml human epidermal growth factor, 10 μg/ml insulin (Sigma, Allentown, PA), 100 ng/ml cholera toxin (Sigma), and 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma). MCF7 cells were cultured in DMEM (GIBCO) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; GIBCO) and 100 U/ml penicillin and 100 μg/ml streptomycin. MDA231 cells were cultured in DMEM supplemented with 10% (vol/vol) FBS (Atlanta Biologicals). NuFF cells were cultured in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS (GIBCO), and HUVECs were cultured in EGM2 medium (Promocell) supplemented with 2% FBS (Promocell). Medium was exchanged every 2–3 days and passaged after reaching 80–90% confluence with 0.25% trypsin EDTA (Sigma) or 0.05% trypsin EDTA (for HUVECs). All cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2.
For coculture experiments, individual BCC lines and NuFF cells were seeded on the same day at a 1-to-1 ratio in medium comprising equal volumes of NuFF cell culture medium and medium specific to individual BCC lines (66, 67). Each well contained 4–5 × 104 cells/cm2 of NuFF cells and 4–5 × 104 cells/cm2 of individual BCCs. After a coculture period of 9 days, cells were lysed and ECM was isolated as described above.
ECM isolation and seeding of HUVECs.
ECM from individual cultures or cocultures was decellularized based on a previously established protocol (3). Briefly, a 50 mM NH4OH buffer containing either 0.4% or 2% Triton X, depending on experimental conditions, was utilized to lyse all overlying cells while leaving the ECM behind. Decellular ECM was gently washed two times with 1× PBS and utilized for subsequent analyses or for CLS formation assays. Prior to HUVEC seeding, ECM was incubated for 1 h at 37°C in 200 U/ml DNase I recombinant (RNase free) (Roche Scientific; Indianapolis, IN) to ensure complete removal of genomic DNA and thus any residual cells. After incubation, ECM was washed twice with 1× PBS and seeded with 4.0 × 105 HUVECs (65). HUVECs on ECM were cultured in the growth medium described above at 37°C in a humidified atmosphere containing 5% CO2 for 24 h prior to subsequent analyses.
Immunofluorescence staining and imaging of cells and deposited ECM.
Cell-containing samples were fixed with 3.7% paraformaldehyde for 30 min, followed by a 10-min incubation in 0.1% Triton X to permeabilize the fixed cells. Deposited ECM was fixed in 3.0% paraformaldehyde for 20 min (65). Samples were washed in 1× PBS and incubated for 1 h with primary antibody (Table 1), rinsed twice with PBS, and incubated with appropriate Cy3 or FITC secondary antibodies (Sigma). After being rinsed twice with PBS, in the case of CLS formation, cells were also incubated with FITC-conjugated phalloidin (1:40; Molecular Probes, Eugene, OR) for 1 h, rinsed with PBS, and incubated with DAPI (1:1,000; Roche Diagnostics, Basel, Switzerland) for an additional 10 min. The immunolabeled samples were examined by either fluorescence microscopy (Olympus BX60; Olympus, Center Valley, PA) or confocal microscopy (LSM 510 Meta; Carl Zeiss).
Table 1.
Antibodies used in study
| Antibody | Use | Concentration | Vendor |
|---|---|---|---|
| Fibronectin | IF; WB | 1:200; 1:400 | Sigma |
| Tenascin-C | IF; WB | 1:50; 1:150 | Abcam |
| Collagen I | IF; WB | 1:100; 1:500–1:1,000 | Abcam; Dr. Larry Fisher (NIH) |
| Collagen IV | IF; WB | 1:100; 1:500 | Santa Cruz; Abcam |
| Laminin | IF; WB | 1:200; 1:500–1:1,000 | Abcam |
| GAPDH | WB | 1:1,000 | Cell Signaling Technology |
| CD31 | IF | 1:200 | Dako |
| vWF | IF | 1:200 | Dako |
| VE-cadherin | IF | 1:200 | Santa Cruz |
IF, immunofluorescence; WB, Western blot; vWF, von Willebrand factor.
ECM quantification.
Decellularized ECM from respective cocultures or from NuFF cultured alone was collected and pooled from two six-well plates with previously established methods (65). Briefly, ECM was solubilized in 50 mM Tris, pH 7.6, 150 mM NaCl, 0.2% sodium azide, and 0.1% Triton X containing 1× protease inhibitor cocktail (Thermo Scientific). ECM was subjected to centrifugation at 1,000 RPM and 4°C for 10 min. The supernatants were collected and stored at −80°C. ECM (n = 3) was quantified with the detergent-compatible (DC) protein assay (Bio-Rad, Hercules, CA). Absorbances were read at 750 nm. ECM concentrations were determined with several known concentrations of bovine serum albumin (BSA) standards.
Western blot.
Whole cell lysates were prepared in either a Tris-Triton X buffer (1% Triton X, 150 mM NaCl, 50 mM Tris pH 7.5) or RIPA buffer (150 mM NaCl, 1.0% Triton X, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8.0) containing 1× protease inhibitor cocktail (Thermo-Pierce). Protein from either isolated ECM or whole cell lysates was quantified with the DC assay (Bio-Rad) and boiled at 95°C for 5 min in Laemmli buffer (Bio-Rad) with or without β-mercaptoethanol. A concentration of 50 μg of isolated protein from BCCs and 15 μg of isolated protein from NuFF- and BCC-NuFF-derived ECM was loaded per well into a 4–20% SDS PAGE gel (Bio-Rad). Proteins were transferred to nitrocellulose membranes, blocked for 1 h in 3% nonfat milk, and incubated overnight at 4°C and constant shaking with primary antibody (Table 1). Membranes were washed three times in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 15 min each and incubated for 2 h at room temperature and constant shaking with either anti-rabbit horseradish peroxidase (HRP) (1:1,000; Cell Signaling Technology) or anti-mouse HRP (1:3,000; Cell Signaling Technology). Membranes were washed three times in TBST, developed with enhanced chemiluminescence (Pierce), and visualized with the ChemiDoc XRS+ System (Bio-Rad). Images were acquired with Bio-Rad Quantity One software.
Scanning electron microscopy.
Decellularized ECM was fixed in glutaraldehyde-formaldehyde-containing buffer [3% (vol/vol) formaldehyde, 1.5% (vol/vol) glutaraldehyde, 0.1 M Na cacodylate, 5 mM MgCl2, 2.3 M sucrose, pH 7.4] for 20 min and washed three times with PBS. Samples were postfixed with 1% (vol/vol) osmium tetroxide for 20 min (Sigma), followed by a graded series of dehydration in ethanol. Samples were critical point dried (Tousimis 795) and coated with 2 nM platinum with a sputter coater (Anatech Hummer 6.2 Sputter Coater). ECM was visualized with a FEI Quanta 200 ESEM [Johns Hopkins Integrated Imaging Center (IIC)]. The neurofilament function in Imaris x64 7.2.1 (Bitplane) was utilized to evaluate fiber diameters in three nonoverlapping high-magnification images (40–60,000× magnification). All samples (n = 3) were evaluated in triplicate, with the exception of ECM derived from MDA231 cells, in which ECM was detectable from only two nonoverlapping areas in one sample.
Quantification of CLS, lumen dimension, and von Willebrand factor expression.
The mean capillary branch points were quantified as we previously described (1, 28, 71, 81). Briefly, we analyzed 27 images (×10 magnification) taken at different regions of each sample (n = 3; in triplicate) with the “Angiogenesis” tool of Metamorph software 6.1 (Universal Imaging, Downingtown, PA) or Image J [National Institutes of Health (NIH)]. The percent area occupied by CLS were evaluated with Image J (NIH). We evaluated 27 images (×10 magnification) taken at different regions of each sample (n = 3; in triplicate). Each image was thresholded, and the percent area occupied by CLS was assessed with the measurements function tool. Lumen dimensions were determined from the three-dimensional confocal images. Image J (NIH) was utilized to measure capillary lumen diameters from the short and long axes of each lumen. von Willebrand factor (vWF) expression was quantified (n = 2; in triplicate) with Image J as we previously described (19). Six nonoverlapping images per tested condition were evaluated. Images were separated to vWF- and DAPI-counterstained panels, and all pixels within the specified threshold were measured. Pixels of vWF panels that fell within the specified threshold were normalized to nuclear pixels to calculate the average number of vWF pixels per nucleus.
Transmission electron microscopy.
After 24 h in culture, vascular structures formed from HUVECs cultured on acellular ECM were fixed in glutaraldehyde-formaldehyde-containing buffer [3% (vol/vol) formaldehyde, 1.5% (vol/vol) glutaraldehyde, 0.1 M Na cacodylate, 5 mM calcium chloride, 2.3 M sucrose, pH 7.4] at room temperature for 1 h and subsequently washed in 0.1 M Na cacodylate-2.5% sucrose three times for 15 min each. Samples were postfixed with Palade's osmium tetroxide for 1 h on ice in the dark and then rinsed once with Kellenberger uranyl acetate and incubated in the dark at 4°C in Kellenberger uranyl acetate. The samples were processed conventionally through EPON embedding. Serial sections were cut and mounted on copper grids. Samples were viewed with a Philips EM410 transmission electron microscope (FEI, Hillsboro, OR). Images were captured with an FEI Eagle 2k camera.
Tumor necrosis factor-α activation.
To activate CLSs, HUVECs were seeded on acellular ECM for 12 h. After 12 h in culture, the cells were treated with medium containing a final concentration of 30 ng/ml tumor necrosis factor (TNF)-α (68). For controls, HUVECs on acellular ECM were given an equal volume of medium not containing TNF-α. After 12 h of treatment, samples were collected for real-time quantitative RT-PCR.
Real-time quantitative RT-PCR.
Two-step RT-PCR was performed on HUVECs cultured on acellular ECM, with or without TNF-α exposure. Total RNA was extracted with TRIzol (GIBCO, Invitrogen) according to the manufacturer's instructions. Total RNA was quantified by an ultraviolet spectrophotometer. The samples were validated for having no DNA contamination. RNA (1 μg per sample) was subjected to reverse transcriptase using Moloney murine leukemia virus (M-MLV; Promega, Madison, WI) and oligo(dT) primers (Promega), according to the manufacturer's instructions. The TaqMan Universal PCR Master Mix and Gene Expression Assay (Applied Biosystems, Foster City, CA) was used for E-selectin (ESEL) and ICAM and GAPDH, according to the manufacturer's instructions. The TaqMan PCR step was performed with an Applied Biosystems StepOne Real-Time PCR System (Applied Biosystems), following the manufacturer's instructions. The relative expression of ICAM and ESEL was normalized to the amount of GAPDH in the same cDNA by using the standard curve method described by the manufacturer. For each primer set, the comparative computerized tomography method (Applied Biosystems) was used to calculate amplification differences between the different samples. The values for experiments were averaged and graphed with standard deviations.
Statistical analyses.
All analyses were performed in triplicate for n = 3. vWF expression was analyzed in triplicate wells for n = 2 samples. Expression data of ESEL and ICAM were performed on triplicate samples. Statistical analysis was performed with GraphPad Prism 4.02 (GraphPad Software, La Jolla, CA). GraphPad Prism 4.02 was used to perform two-tailed t-tests, one-way ANOVA with Tukey's posttest, or nonparametric Friedman's test with Dunn's posttest, where appropriate. Significance levels were set at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001. Unless otherwise indicated, all graphical data are reported as ±SE.
RESULTS
Breast cancer cell ECM protein expression.
The BCC lines MCF10A, MCF7 and MDA231, representative of the in vitro stages of breast cancer, were assessed to determine whether patterns of ECM protein expression differ among the BCC lines along the culture period. Cells were cultured to confluence and examined after 6, 9, and 12 days. Fibronectin, a large 440-kDa glycoprotein, is frequently subjected to proteolytic degradation, resulting in the production of numerous smaller fragments (7). Similarly, we note the presence of several fibronectin fragments in each of the BCC lines along the culture period (Fig. 1A). While each of the fragments may have important implications for breast cancer progression, we focused specifically on the 110/120-kDa and 33/66-kDa fragments, of interest to the present study as these fragments have been shown to promote EC adhesion, spreading, and proliferation (30, 79). Here, we note the presence of the 110/120-kDa fragments in each of the BCCs along the culture period, with enriched expression of the 110/120-kDa fragments in MCF10A and MDA231 and the 33/66-kDa fragments in MCF7 (Fig. 1A). Evaluation of laminin revealed that it was confined to MCF10A, where greater abundance was observed at day 12, and MDA231, where abundances were similar along the culture period (Fig. 1B). For collagen I, reproducible immunoblots consistently demonstrated four bands, likely corresponding to, in order of smallest (i.e., most processed) to largest (i.e., least processed) molecular weight, α1(I), pro-α 1(I) N, pro-α1 C, and pro-α1(I) (5, 61, 65). All four collagen products were noted, with variable expression between the cell lines and along the culture period (Fig. 1C). For instance, in MCF10A, all four collagen products were absent at day 6, with pro-α1(I) absent at days 9 and 12 (Fig. 1C). The least processed of the collagen products, pro-α1(I), was constrained to MDA231 (Fig. 1C). For collagen IV, bands corresponding to 180 to >220 kDa were observed in each of the BCC lines, with the most pronounced expression observed for MCF7 (Fig. 1D). Tenascin-C was weakly expressed and limited to MCF10A and MCF7 (Fig. 1E).
Fig. 1.
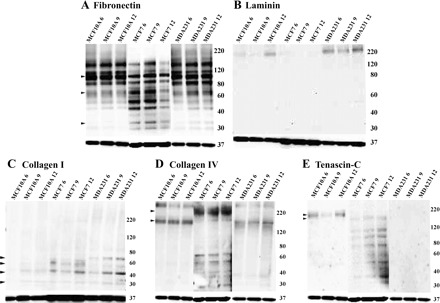
Extracellular matrix (ECM) expression in breast cancer cells (BCCs). Western blot analysis shows the expression of various ECM proteins along the culture period (6, 9, and 12 days) of BCC lines MCF10A, MCF7, and MDA231. A: fibronectin; arrows indicate 100- to 110-kDa cell binding fragments, enriched in MCF10A and MDA231, and 33/66-kDa heparin binding fragments, enriched in MCF7. B: laminin expressed in MCF10A and MDA231 cells. C: collagen I; 4 bands, likely corresponding to (in order of smallest to largest in molecular weight) α1(I), pro-α 1(I) N, pro-α1 C, and pro-α1(I), were noted with expression variable between the cell lines and along the culture period. D: collagen IV; bands corresponding from 180 to 220 kDa were observed in each of the BCC lines, with the most pronounced expression observed for MCF7. E: tenascin-C, expressed only in MCF10A (220 kDa) and in MCF7 (200 kDa). All samples were normalized to GAPDH, shown at bottom of each image.
Breast tumor cell ECM deposition.
To specifically study the effect of deposited ECM, BCCs were cultured for 6–9 days and lysed with a strong base, leaving the decellularized ECM. We chose to use this method as it was previously shown to generate decellularized fibroblast-derived ECM, which supported vascular tubulogenesis of HUVECs (65). Interestingly, MCF10A cells were found to produce significantly more ECM compared with MCF7 and MDA231 (Fig. 2A). However, while an abundant decellular ECM deposited by a control fibroblast cell line, human neonatal foreskin fibroblast (NuFF) cells, could be detected by fluorescence microscopy (Fig. 2B), ECM deposited by BCCs could not be robustly detected (Fig. 2B). To visualize the deposition of ECM from BCC lines, scanning electron microscopy (SEM) was utilized, which not only confirms that each of the BCC lines deposits ECM but further validates the limited ECM produced by these cells (Fig. 2C). It is important to note that while MCF10A shows ECM in the entirety of the image, this ECM was typically detected in a small section of the coverslip. After further analysis of high-resolution SEM images, we noted unique differences in the morphology of ECM. Specifically, we observed that MCF10A ECM has an overall organized, interconnected fiber morphology; MCF7 ECM has a less organized arrangement of fibers; MDA231 ECM has a thin, sparse fiber morphology; and NuFF has a copious monolayer of ECM containing both large and thin-diameter fibers (Fig. 2D). Given the unique differences in fiber morphologies, we sought to further investigate whether differences in fiber diameters exist between the ECM deposited by each of the NuFF and BCC lines. With high-resolution SEM images, all BCCs and NuFF were found to deposit ECM containing fibers with mean diameters ranging from 0.1 to 0.5 μm, with MDA231 cells depositing ECM containing slightly larger-mean diameter fibers than MCF10A, MCF7, and NuFF cells (Fig. 2E). It is important to note that ECM deposited from BCCs, and specifically from MDA231, were difficult to process and analyze compared with ECM deposited from NuFF.
Fig. 2.
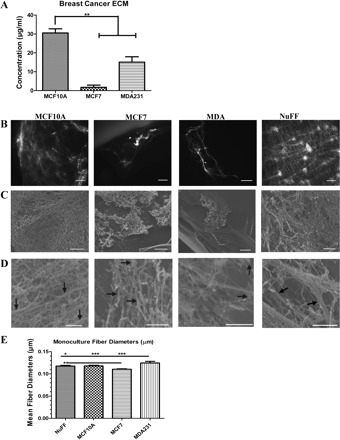
ECM deposition by BCCs. A: quantification of BCC-derived ECM revealed that MCF10A cells deposit significantly more ECM compared with MCF7 and MDA231. B and C: BCC-derived ECM was identified with fluorescence microscopy (B) and scanning electron microscopy (SEM) analysis (C). In B, scale bars are 50 µm and in C, scale bars are 5 µm. D: high-magnification SEM images revealed ECM fibers (0.1- to 0.5-μm diameter), indicated by arrows, deposited by all BCC lines. Scale bars are 1 μm. E: mean fiber diameters were found to be similar for ECM deposited by BCCs and neonatal foreskin fibroblast cells (NuFF). Significance levels were set at *P < 0.05, **P < 0.01, and ***P < 0.001. Values shown are means ± SE.
Decellular ECM from cocultures.
As our overall aim is to analyze the effect of cancerous ECM on CLS formation from ECs, we needed a robust method to generate an abundant decellularized ECM. Thus we sought to evaluate and characterize ECM deposited from coculturing individual BCC lines with fibroblast cells, reasoning that this is a desirable strategy as stromal cells are frequently recruited to tumors. Attempts to use primary human mammary fibroblasts failed, as their in vitro expansion is limited and inconsistent, and thus we utilized NuFF cells, a human dermal fibroblast cell line. BCCs were cocultured with NuFF cells for 9 days, with all overlying cells removed as described above, leaving the decellularized ECM behind. By SEM, the ultrastructure of coculture-derived ECM revealed an abundance of ECM deposited by each of the BCC lines cocultured with NuFF (Fig. 3A), a striking observation to the ECM deposited by BCC monocultures. Here as well, all BCC-NuFF cocultures and NuFF alone were found to deposit ECM containing fibers with mean diameters ranging from 0.1 to 0.5 μm, with BCC-NuFF-derived fibers having statistically significant larger-overall mean diameter fibers than NuFF-derived fibers (Fig. 3Bi). Within BCC-NuFF cocultures, it was found that MCF10A-NuFF and MCF7-NuFF produced significantly larger-mean diameter fibers than MDA231-NuFF (Fig. 3Bi). Interestingly, evaluation of large-diameter fibers revealed that fibers with a mean diameter ranging from 0.25 to 0.5 μm were not present in ECM deposited by NuFF (Fig. 3Bii).
Fig. 3.
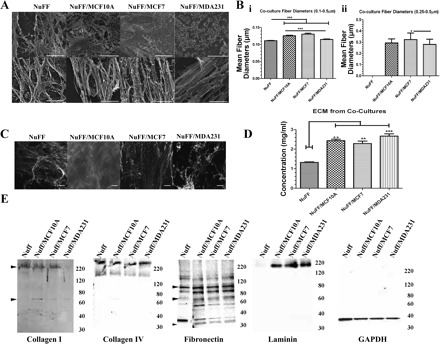
ECM deposition by BCC-NuFF cocultures. A: SEM analysis revealed an abundance of ECM deposited by NuFF alone and from MCF10A-NuFF, MCF7-NuFF, and MDA231-NuFF cocultures. Top: low magnification; scale bars are 10 μm; Bottom: high magnification; scale bars are 2 μm. B, i: mean fiber diameters were found to be significantly larger for ECM produced by each of the BCC-NuFF cocultures compared with ECM produced by NuFF. Within BCC-NuFF cocultures, MCF10A-NuFF and MCF7-NuFF produced significantly larger fibers than MDA231-NuFF. ii: Larger-mean diameter fibers (0.25–0.5 μm) were restricted to BCC-NuFF ECM and could not be detected in NuFF ECM. C: this ECM was easily detected with fluorescence microscopy. Scale bars are 50 µm. D: quantification revealed that cocultured BCC-NuFF cells deposited significantly more ECM compared with NuFF alone. E: Western blot demonstrated the presence of collagens I and IV, laminin, and fibronectin in ECM from all tested cases, with the exception of collagen I, which was not detected in MDA231-NuFF ECM, and laminin, which was negligibly expressed in NuFF ECM. Significance levels were set at *P < 0.05, **P < 0.01, and ***P < 0.001. Values shown are means ± SE.
The decellular ECM deposited by cocultures could be easily detected by fluorescence microscopy (Fig. 3C). Moreover, cocultures of all BCC lines with NuFF deposited significantly more ECM than NuFF alone, with similar quantities being deposited among the tested BCC lines (Fig. 3D). These observations suggest an additive effect of the coculture approach on the deposition of ECM.
We then determined the composition of the decellular ECM. Specifically, we investigated the presence of laminin, tenascin-C, collagens I and IV, and fibronectin. We did not observe differences in abundance of collagen IV from ECM obtained from NuFF and each of the BCC-NuFF cocultures (Fig. 3E). Similarly, we did not observe differences in the 110/120-kDa fibronectin fragments between each of the tested conditions; however, we do note that the smaller 33/36-kDa fragments were more abundantly expressed in ECM deposited from NuFF cells (Fig. 3E). The most notable difference in ECM protein expression was observed for collagen I and laminin. For collagen I, we reproducibly observed two products visualized at 200–250 kDa, potentially corresponding to the unprocessed pro-α1(I), and 75 kDa, possibly corresponding to the processed α1(I) (Fig. 3E). The larger 200- to 250-kDa collagen product was not observed from ECM deposited from MDA231-NuFF cocultures, while similar abundances were found in the remaining tested ECM (Fig. 3E). The smaller 75-kDa collagen product was barely visible in ECM deposited from each of the tested conditions (Fig. 3E). Evaluation of laminin revealed that it is absent in ECM deposited by NuFF cells but is found in similar quantities in ECM deposited from each of the BCC-NuFF cocultures (Fig. 3E). It should be noted that while immunofluorescence staining uncovered the presence of tenascin-C in all tested ECM (Fig. 4), it was not observed after several attempts at immunoblotting. Immunofluorescence staining verifies the presence of ECM proteins described above, further illustrating the limited collagen I deposition from MDA231-NuFF cocultures (Fig. 4). No significant differences could be observed in the organization of the deposited ECM proteins (Fig. 4).
Fig. 4.
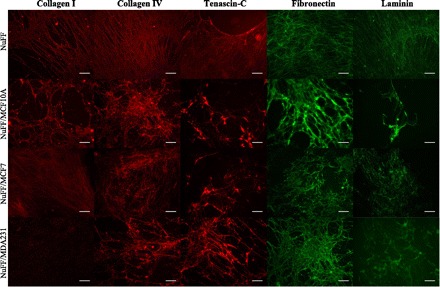
ECM deposition by cocultures. Immunofluorescence analysis shows the expression of various proteins examined in ECM deposited by NuFF-BCC cocultures and NuFF alone, with collagen I being negligibly expressed from ECM deposited by MDA231-NuFF cocultures. Scale bars are 50 μm.
Vascular morphogenesis on coculture-derived ECM.
To determine whether ECM deposited by the BCC lines cocultured with NuFF contributes to distinct differences in formation of CLS, we cultured HUVECs on decellularized ECM. We observed that HUVECs rapidly organized into CLS in all tested conditions within a time frame of 24 h (Fig. 5A). Exogenous growth factors were not added to the medium and CLS did not form in chamberslide controls (data not shown), suggesting that deposited ECM guided the formation of CLS. Moreover, differences in CLSs were evident among the different culture scenarios. CLS from each of the BCC-NuFF coculture conditions exclusively formed capillary branch points >1,500, numbers not observed for CLS grown on NuFF ECM (Fig. 5Bi). In addition, we observed that CLS grown on MDA231-NuFF ECM possessed capillaries that occupied the greatest percent area evaluated (Fig. 5Bii).
Fig. 5.
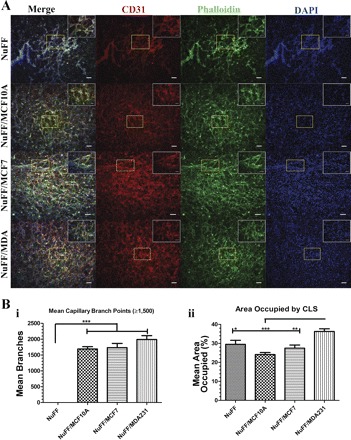
Vascular morphogenesis on ECM deposited by NuFF and cocultures of NuFF with BCCs. Capillary-like structures (CLSs) formed on deposited ECM were visualized with immunostaining for phalloidin (green), CD31 (PECAM; red), and nuclei (DAPI; blue) (A) and quantified for mean capillary branches (≥1,500; i) and % area occupied by capillaries (ii) (B). Scale bars are 100 μm and 50 μm for image insets. Significance levels were set at *P < 0.05, **P < 0.01, and ***P < 0.001. Values shown are means ± SE.
To verify the structures for tubular organization, cross sections of three-dimensional (3D) reconstructions of tubes with capillary lumens were identified in CLS grown on all tested ECM (Fig. 6A). The average lumen diameters for both short and long axes were largest for CLS grown on MDA231-NuFF ECM, with the long axes of lumens from these structures being significantly larger when evaluated against lumens from CLS grown on MCF10A-NuFF-, MCF7-NuFF-, and NuFF-derived ECM (Fig. 6B).
Fig. 6.
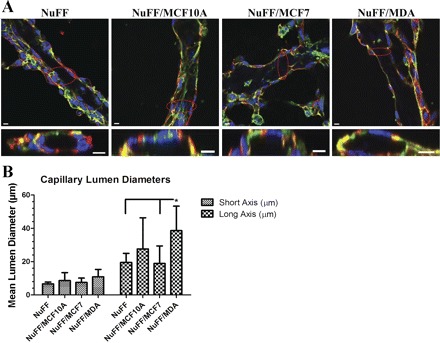
Characterization of capillary lumens from vascular structures. Tubular CLS formed on deposited ECM. A: 3-dimensional z-stack of confocal images (phalloidin in green, CD31 in red, and nuclei in blue) revealed lumens in CLSs grown on all tested ECM as demonstrated by the cross section image of the circled area. Scale bars are 10 μm for cross section images. B: average lumen diameter for the long axis of the tubes was significantly larger for CLSs grown on MDA231-NuFF-derived ECM. Significance levels were set at *P < 0.05. Values shown are means ± SE.
Characterization of CLS.
We next characterized the vascular structures formed on deposited ECM. First, the expression of vWF, a glycoprotein that plays a role in vascular hemostasis, was analyzed. vWF was expressed in CLS grown on NuFF ECM and each of the BCC-NuFF ECM. After quantification of vWF, we note that CLSs grown on BCC-NuFF ECM produce more vWF overall, compared with CLSs from NuFF ECM, with MDA231-NuFF expressing the most significant levels (Fig. 7Ai). Abundantly expressed vWF was detected by high-magnification confocal imaging of CLS formed on MDA231-NuFF ECM (Fig. 7Aii). Transmission electron microscopy (TEM) analyses revealed lengthened tubular structures with distinctive lumens and Weibel-Palade (WP) bodies, storage granules for vWF (72), within the cytoplasm of HUVECs (Fig. 7B).
Fig. 7.
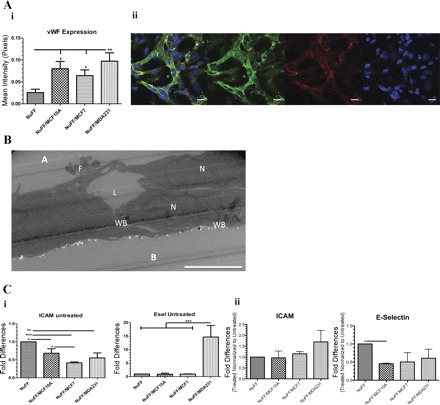
Characterization of CLSs. A, i: von Willebrand factor (vWF) expression was found in CLSs formed on all tested ECM, with significantly higher level in CLS formed on MDA231-NuFF ECM. ii: Confocal images (vWF in green, CD31 in red, and nuclei in blue) show abundant expression of vWF in CLSs grown on MDA231-NuFF ECM. Scale bar, 20 µm. B: transmission electron microscopy (TEM) analysis (shown here for CLS formed on MDA231-NuFF-deposited ECM) showed lengthened structures with lumen (L) between neighboring cells, as indicated by their nucleus (N). Weibel-Palade bodies (WB) were found in the cytoplasm, and filopodia (F) projected from the migrating human umbilical vein endothelial cells (HUVECs). Apical (A) and basal (B) sides are indicated. Scale bar, 5 μm. C, i: untreated CLS grown for 24 h on NuFF ECM were found to express more ICAM than CLS formed on BCC-NuFF (left), while CLS formed on MDA-NuFF ECM expressed more Esel (right). ii: In response to 12-h exposure to TNF-α, both ICAM (left) and Esel (right) were upregulated in similar levels in all CLS. Significance levels were set at *P < 0.05, **P < 0.01, and ***P < 0.001. Values shown are means ± SD.
Finally, we examined CLS response to stimulation by TNF-α, an inflammatory cytokine that has been shown to upregulate the expression of ICAM and E-selectin (Esel) in vascular cells (19, 68, 73). First, we found that after 24 h of HUVEC culture on decellularized ECM (without the addition of TNF-α), CLSs formed on NuFF ECM express more ICAM than CLSs formed on all BCC-NuFF ECM (Fig. 7Ci). For Esel, we found that it is highly expressed in CLS cultured on MDA231-NuFF ECM (Fig. 7Ci). Normalizing the expression levels of the treated samples to the expression levels of the untreated samples, we found similar responses to TNF-α of all CLS (Fig. 7Cii). Overall, exposure of CLS, previously in culture for 12 h, to TNF-α for an additional 12 h resulted in upregulation of both ICAM and Esel in CLS from all tested ECM (data not shown).
DISCUSSION
It is well known that angiogenesis is a hallmark of breast as well as other solid tumor malignancies. While several eloquent studies have elucidated the roles of various signaling molecules and microenvironmental conditions facilitating tumor angiogenesis, little attention has been given to the ECM, a known regulator of homeostatic angiogenesis. Since the ECM directs angiogenesis and is aberrantly expressed in breast carcinomas (34, 40–42), it is necessary to understand how the expression profiles of ECM proteins change during breast tumor progression and how this ECM potentiates tumor stage-associated changes in angiogenesis.
To study the effect of cancerous ECM on vascular networks in vitro, we utilized an approach in which decellular ECM, deposited by carefully selected BCC lines cocultured with fibroblasts, served as a scaffold for CLS formation. Given the propensity for tumor cells to recruit stromal cells to the tumor mass (6, 38, 63), we reasoned that such an approach will enable a systematic study of the formation, kinetics, and characteristics of the vascular networks in a cancerous ECM environment. For this, we explored three BCC lines including a nontumorigenic cell line (MCF10A), a tumorigenic cell line (MCF7), and a metastatic cell line (MDA-MB-231) to capture distinct stages of breast tumorigenesis. We recognize that while several mechanisms may be implicated in breast tumor-associated changes in ECM deposition, namely, direct cell-cell contact between BCCs and fibroblasts and BCC growth factor-induced changes in fibroblasts, attempts to differentiate these unique systems were beyond the scope of the present study.
First, we determined the expression of ECM proteins including collagens I and IV, fibronectin, tenascin-C, and laminin along the culture of BCC lines MCF10A, MCF7, and MDA231. We found that these ECM proteins, which are aberrantly expressed in breast carcinomas (15, 37, 45, 58–60) and additionally facilitate angiogenesis (31), are expressed in almost all of the BCC lines tested. ECM protein patterns have been demonstrated to be differentially expressed between breast tumor cells and nontumor cells (34, 40–42). Concordant with these studies, our results uncovered differences in ECM proteins expressed among the BCC lines tested. For instance, laminin was restricted to MCF10A and MDA231, tenascin-C to MCF10A and MCF7, and collagens I and IV to MDA231 cells. Several fibronectin fragments, a result of proteolytic degradation (7), were present in all BCCs. While we acknowledge that each of the fragments may play a unique role in breast cancer progression, we focused specifically on those fragments, namely, the 110/120-kDa and 33/66-kDa fragments, implicated in EC adhesion, spreading, and proliferation (30, 79). The 110/120-kDa and 33/66-kDa fibronectin fragments were present in each of the BCC lines, suggesting a potential role whereby these fragments, when deposited in the extracellular space, facilitate tumor angiogenesis.
After identification of differentially expressed ECM proteins in BCC lines, we sought to investigate whether BCC lines deposit ECM, which could be characterized in terms of ECM protein expression and abundance as well as its ability to support in vitro vascular morphogenesis. Recent studies have described the identification of ECM proteins in decellularized matrixes derived from fibroblasts (43, 65, 80). Similar to these reports, we document that NuFF cells deposit an extensive 3D matrix expressing collagens I and IV, fibronectin, tenascin-C, and laminin. Unlike NuFF cells, BCC lines did not deposit ECM that could be readily identified with immunofluorescence microscopy or immunofluorescence staining for the aforementioned ECM proteins. High-resolution analysis using SEM was necessary to validate that BCC lines deposit identifiable ECM. Moreover, this analysis further confirmed the limited quantity and uneven distribution of ECM deposited by BCC lines. These observations were further confirmed after quantification of total BCC-derived ECM. Overall, these analyses are the first, to our knowledge, to demonstrate that BCC lines do deposit identifiable ECM having morphological differences. Such differences may have important implications for breast tumor progression. However, since the goal of this study was to investigate CLS formation on decellularized ECM, a more robust system, reminiscent of the in vivo environment, was needed in order to conduct such studies.
Stromal cells are frequently recruited to tumors (6, 38, 63) and have been further shown to facilitate tumor progression (47, 57, 70). Thus, as the deposition of ECM from the epithelial BCCs was insufficient to support in vitro studies of capillary morphogenesis, we pursued a coculture approach in which fibroblast cells were individually cocultured with each of the BCC lines. This strategy allowed us to determine whether tumor stage-associated changes, represented by the nontumorigenic MCF10A, the tumorigenic MCF7, and the metastatic MDA231 BCC lines, differentially affect capillary morphogenesis on decellularized ECM. Earlier attempts to perform these studies with primary human mammary fibroblasts failed as their in vitro expansion is limited and inconsistent, and thus we utilized a human dermal fibroblast cell line, NuFF.
After isolation of ECM from NuFF and each of the BCC-NuFF cocultures, we observed significant increases in overall ECM deposited from each of the BCC lines cocultured with NuFF, illustrating an additive benefit a coculture approach has on ECM deposition. Additionally, we discovered differences in ECM organization. For example, we found that while mean fiber diameters were similar for ECM deposited by all cocultures and NuFF alone, larger-mean diameter fibers (0.25–5.0 μm) could not be detected in NuFF ECM. It is well known that mammographically dense tissue, attributed to alterations in stroma and ECM deposition (2, 26, 46), is associated with an increased risk for breast cancer (10, 11, 52). It would be interesting to pursue whether the large 0.25- to 5.0-μm fibers, exclusive to BCCs cocultured with NuFF, contribute to the mechanical stiffness characteristic of breast tumors.
Finally, differences in ECM protein expression were observed. Here, we report alterations in collagen I and laminin expression between ECM deposited from NuFF and each of the BCC-NuFF cocultures. Of interest is stromal collagen, its presence and abundance known to facilitate breast tumor initiation and invasion (59, 60). After evaluation of stromal collagen I in ECM deposited from NuFF and each of the BCC-NuFF cocultures, we found that the largest of the detectable products, potentially corresponding to the unprocessed pro-α1(I), was absent only in ECM deposited from MDA231-NuFF cocultures. On the other hand, the smallest of the products, potentially corresponding to the processed α1(I), was negligibly expressed in ECM from NuFF and each of the BCC-NuFF cocultures. The observation of the absence of collagen I deposition from MDA231-NuFF cocultures is striking, as both the fully unprocessed and processed collagen products were detected in cellular lysate from MDA231 cells. The absence of collagen I in ECM deposited from cocultures of metastatic MDA231 BCCs with NuFF may suggest that collagen I either is not deposited by the direct association of MDA231 BCCs with NuFF cells or is degraded by proteinases released by MDA231 cells. Further investigations are needed in order to address this observation. In addition, we note differences in the expression profiles for laminin, where we observed enriched abundance in ECM deposited from each of the BCCs cocultured with NuFF and negligible expression in ECM deposited from NuFF. These observations suggest that laminin, a structural component of the basement membrane of cells, is deposited predominantly when BCCs, irrespective of in vitro tumor grade, are directly cultured with NuFF cells. Whether these observations are due solely or in part to direct cell-to-cell interactions or to BCC-secreted growth factors is not understood. While previous studies have investigated differences in expression patterns of ECM proteins in breast tumor tissues (34, 40–42), findings that are concordant with our observations, we describe for the first time the isolation and characterization of ECM derived from BCC lines cultured with fibroblasts. Overall, we report unique differences in ECM protein expression, organization, and abundance among the ECM derived from the individual cocultures of BCCs with fibroblasts, observations that may have important implications for breast tumor initiation and progression.
Several groups not only have highlighted the differences in ECM protein expression and distribution in breast tumor tissues but have recently linked abnormal ECM architecture to breast tumor invasion and metastasis (4, 53). One means whereby an aberrant ECM framework in breast carcinomas may potentiate breast tumor progression is its ability to induce pathological angiogenesis. It is well known that individual ECM proteins have both distinct and overlapping roles in their abilities to promote and sustain angiogenesis (31). Recently, it was eloquently demonstrated that cellular-derived ECM, comprising a number of ECM proteins, efficiently supported EC vasculogenesis in vitro (65), while ECM derived from tumorigenic clones of telomerase-transfected mesenchymal stem cells was found to possess angiogenic potential, supporting in vitro formation of vascular structures (13). Similar to these studies, we found that ECM deposited by each of the BCC lines cocultured with fibroblasts supported the organization of vascular structures from mature ECs.
Aberrant vascular morphological features influence how tumors progress and respond to therapeutic agents (55). In breast cancer, angiogenesis is first evident at the preinvasive stage of ductal carcinoma in situ (21), with the extent of microvessel density being directly correlated with breast tumor size and metastatic potential (8, 20). While these studies have assessed the extent of tumor angiogenesis through the use of immunohistochemistry against the EC marker CD31, we sought to determine whether the various ECM induced unique patterns of 3D CLS formation. First, we found that CLS from each of the BCC-NuFF coculture conditions exclusively formed capillary branch points in numbers not observed for CLS grown on NuFF ECM. In addition, we observed that CLSs grown on MDA231-NuFF ECM have a complex structure and occupied the greatest percent area evaluated. Moreover, 3D z-stack confocal microscopy analysis revealed that the long axes of lumens from vascular networks formed on MDA231-NuFF ECM are significantly larger compared with all other BCC-NuFF cocultures and NuFF alone.
In an attempt to assess the angiogenic potential of CLS formed on the various ECM, we analyzed CLS for vWF, a large glycoprotein that not only participates in vascular hemostasis via binding of platelets to the subendothelial matrix but has been associated with tumor metastasis (22, 76). One important means whereby tumor cells metastasize is via their adhesion to the capillary wall prior to extravasation, a process mediated by platelet aggregation to tumor cells (54, 56). Utilizing interfering antibodies, Karpatkin et al. (39) found that metastases could be largely reduced via elimination of vWF binding with platelet-tumor cell aggregates, demonstrating the important role of vWF in tumor metastatic spread. In concordance with these studies, we report that CLSs grown on MDA231-NuFF ECM express the greatest level of vWF. In addition, we report the presence of WP bodies, detected through the use of TEM. WP bodies are EC-specific cytoplasmic organelles (33, 78) that serve as storage sites for vWF (72). The punctate presence of WP bodies in the cytoplasm of vascular structures further confirms vascular expression of vWF. Given that the present study utilized non-tumor-associated ECs, the enriched expression of vWF in CLS grown from MDA231-NuFF ECM is likely a result of an abnormal ECM. Therefore, it is conceivable that the ECM, localized to an environment composed of metastatic tumor cells, may be one mechanism driving increased vWF expression in capillaries and concomitant metastatic tumor spread.
TNF-α, an inflammatory cytokine implicated in breast tumorigenesis (44, 77), has been shown to upregulate the expression of downstream genes ICAM and Esel in both mature and progenitor vascular cells (19, 68, 73). Moreover, enriched Esel and ICAM expression in ECs have been shown to support tumor metastasis, a process mediated by EC TNF-α activation (12, 62). We found that without the addition of TNF-α, ICAM is suppressed in CLS grown on BCC-NuFF ECM compared with CLS grown on NuFF ECM. Esel, on the other hand, is upregulated significantly in CLS grown on MDA231-NuFF ECM. All CLSs, regardless of their culture on the various tested ECM, responded similarly to the external addition of TNF-α, confirming prior reports (19, 68, 73). Our results, demonstrating different expression levels of ICAM and Esel in CLS grown in NuFF and MDA231-NuFF ECM environments, respectively, may suggest that the ECM sequesters cell-produced TNF-α, which in turn activates the overlying vascular cells.
Overall, our results indicate that ECM derived from fibroblast ECM and from cocultures of BCCs with fibroblasts promotes rapid formation of CLS, suggesting that deposited ECM from each of the tested conditions is sufficient in its protein expression and abundance to elicit vasculogenesis. Coculturing the metastatic MDA231 BCCs with fibroblasts gave rise to ECM, which supported the development of CLS having statistically significant differences in capillary lumen diameters, percent area occupied by vascular structures, vWF expression, and response to TNF-α stimulation. To our knowledge, these studies are the first of their kind to provide important insights into the means whereby cocultures of stromal fibroblasts with BCC lines, each representing the in vitro stages of breast tumorigenesis, differentially deposit ECM that supports unique patterns of vasculogenesis. These studies provide the framework for future investigations aimed at interrogating the role of the tumor ECM in supporting vascular morphogenesis, inquiries that have the potential to uncover novel targets for antiangiogenic treatments.
GRANTS
Funding for this work was supported by the National Cancer Institute Physical Sciences-Oncology Network (U54CA143868).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.C.H. and S.G. conception and design of research; A.C.H. and C.Q. performed experiments; A.C.H., C.Q., and S.G. analyzed data; A.C.H. and S.G. interpreted results of experiments; A.C.H. and S.G. prepared figures; A.C.H. and S.G. edited and revised manuscript; S.G. drafted manuscript; S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael McCaffery for his assistance with confocal microscopy and TEM analysis and Jacqueline Trivero and Jimmy Su from Johns Hopkins University for assisting with vascular architecture analyses. We also gratefully thank Dr. Larry Fisher of the National Institutes of Health for provision of the LF68 antibody and Dr. Thea Tlsty and the National Cancer Institute Physical Sciences-Oncology Network for provision of MCF10A and MDA231 breast cancer cell lines.
REFERENCES
- 1. Abaci HE, Truitt R, Tan S, Gerecht S. Unforeseen decreases in dissolved oxygen levels affect tube formation kinetics in collagen gels. Am J Physiol Cell Physiol 301: C431–C440, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res 5: R129–R135, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol Chapter 10: Unit 10.9, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bemis LT, Schedin P. Reproductive state of rat mammary gland stroma modulates human breast cancer cell migration and invasion. Cancer Res 60: 3414–3418, 2000 [PubMed] [Google Scholar]
- 5. Bernstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, Robey FA, Lakkakorpi J, Uitto J. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol 34: 209–218, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev 15: 97–101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197–250, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res 25: 365–372, 2006 [PubMed] [Google Scholar]
- 9. Bonanno E, Iurlaro M, Madri JA, Nicosia RF. Type IV collagen modulates angiogenesis and neovessel survival in the rat aorta model. In Vitro Cell Dev Biol Anim 36: 336–340, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7: 1133–1144, 1998 [PubMed] [Google Scholar]
- 11. Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep 3: 314–321, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer 71: 612–619, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Burns JS, Kristiansen M, Kristensen LP, Larsen KH, Nielsen MO, Christiansen H, Nehlin J, Andersen JS, Kassem M. Decellularized matrix from tumorigenic human mesenchymal stem cells promotes neovascularization with galectin-1 dependent endothelial interaction. PLoS One 6: e21888, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci 108: 797–809, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Chia J, Kusuma N, Anderson R, Parker B, Bidwell B, Zamurs L, Nice E, Pouliot N. Evidence for a role of tumor-derived laminin-511 in the metastatic progression of breast cancer. Am J Pathol 170: 2135–2148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das S, Banerji A, Frei E, Chatterjee A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci 82: 467–476, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97: 1093–1107, 2005 [DOI] [PubMed] [Google Scholar]
- 18. DeHahn KC, Gonzales M, Gonzalez AM, Hopkinson SB, Chandel NS, Brunelle JK, Jones JC. The alpha4 laminin subunit regulates endothelial cell survival. Exp Cell Res 294: 281–289, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Dickinson LE, Moura ME, Gerecht S. Guiding endothelial progenitor cell tube formation using patterned fibronectin surfaces. Soft Matter 6: 5109–5119, 2010 [Google Scholar]
- 20. El-Gohary YM, Metwally G, Saad RS, Robinson MJ, Mesko T, Poppiti RJ. Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. Am J Clin Pathol 129: 578–586, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL. Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J Pathol 181: 207–212, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Eppert K, Wunder JS, Aneliunas V, Kandel R, Andrulis IL. von Willebrand factor expression in osteosarcoma metastasis. Mod Pathol 18: 388–397, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Fouser L, Iruela-Arispe L, Bornstein P, Sage EH. Transcriptional activity of the alpha 1(I)-collagen promoter is correlated with the formation of capillary-like structures by endothelial cells in vitro. J Biol Chem 266: 18345–18351, 1991 [PubMed] [Google Scholar]
- 24. Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol 13: 765–782, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JC. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell 12: 85–100, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev 10: 243–248, 2001 [PubMed] [Google Scholar]
- 27. Guttery DS, Hancox RA, Mulligan KT, Hughes S, Lambe SM, Pringle JH, Walker RA, Jones JL, Shaw JA. Association of invasion-promoting tenascin-C additional domains with breast cancers in young women. Breast Cancer Res 12: R57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanjaya-Putra D, Yee J, Ceci D, Truitt R, Yee D, Gerecht S. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J Cell Mol Med 14: 2436–2447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs 23: 289–304, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Huebsch JC, McCarthy JB, Diglio CA, Mooradian DL. Endothelial cell interactions with synthetic peptides from the carboxyl-terminal heparin-binding domains of fibronectin. Circ Res 77: 43–53, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost 5, Suppl 1: 32–40, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci USA 87: 3579–3583, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaffe E, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. J Clin Invest 52: 2745–2756, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jahkola T, Toivonen T, Virtanen I, von Smitten K, Nordling S, von Boguslawski K, Haglund C, Nevanlinna H, Blomqvist C. Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer 78: 1507–1513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer 8: 309–316, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev 61: 198–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Ignatoski KM, Ethier SP, Livant DL. Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res 64: 8674–8681, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 6: 392–401, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 81: 1012–1019, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kharaishvili G, Cizkova M, Bouchalova K, Mgebrishvili G, Kolar Z, Bouchal J. Collagen triple helix repeat containing 1 protein, periostin and versican in primary and metastatic breast cancer: an immunohistochemical study. J Clin Pathol 64: 977–982, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int J Cancer 126: 640–650, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Koukoulis GK, Howeedy AA, Korhonen M, Virtanen I, Gould VE. Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic and neoplastic breast. J Submicrosc Cytol Pathol 25: 285–295, 1993 [PubMed] [Google Scholar]
- 43. Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol 25: 1468–1475, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Lee PP, Hwang JJ, Murphy G, Ip MM. Functional significance of MMP-9 in tumor necrosis factor-induced proliferation and branching morphogenesis of mammary epithelial cells. Endocrinology 141: 3764–3773, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev 14: 343–349, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One 4: e7965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol 18: 1423–1431, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Maity G, Choudhury PR, Sen T, Ganguly KK, Sil H, Chatterjee A. Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity. Tumour Biol 32: 129–138, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman HK, Ponce ML. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J 13: 53–62, 1999 [PubMed] [Google Scholar]
- 51. Martin TA, Watkins G, Lane J, Jiang WG. Assessing microvessels and angiogenesis in human breast cancer, using VE-cadherin. Histopathology 46: 422–430, 2005 [DOI] [PubMed] [Google Scholar]
- 52. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 1159–1169, 2006 [DOI] [PubMed] [Google Scholar]
- 53. McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, Schedin P. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol 168: 608–620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood 63: 55–63, 1984 [PubMed] [Google Scholar]
- 55. Munn LL. Aberrant vascular architecture in tumors and its importance in drug-based therapies. Drug Discov Today 8: 396–403, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost 74: 282–290, 1995 [PubMed] [Google Scholar]
- 57. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4: 38, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med 6: 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rocnik EF, van der Veer E, Cao H, Hegele RA, Pickering JG. Functional linkage between the endoplasmic reticulum protein Hsp47 and procollagen expression in human vascular smooth muscle cells. J Biol Chem 277: 38571–38578, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Roland CL, Harken AH, Sarr MG, Barnett CC., Jr ICAM-1 expression determines malignant potential of cancer. Surgery 141: 705–707, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76: 69–125, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17: 153–162, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Soucy PA, Romer LH. Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol 28: 273–283, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Stuelten CH, Busch JI, Tang B, Flanders KC, Oshima A, Sutton E, Karpova TS, Roberts AB, Wakefield LM, Niederhuber JE. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-beta mediated mechanism in a mouse xenograft model of breast cancer. PLoS One 5: e9832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stuelten CH, DaCosta Byfield S, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. J Cell Sci 118: 2143–2153, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Suarez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol 184: 21–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Teti A. Regulation of cellular functions by extracellular matrix. J Am Soc Nephrol 2: S83–S87, 1992 [DOI] [PubMed] [Google Scholar]
- 70. Tyan SW, Kuo WH, Huang CK, Pan CC, Shew JY, Chang KJ, Lee EY, Lee WH. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS One 6: e15313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vo E, Hanjaya-Putra D, Zha Y, Kusuma S, Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev 6: 237–247, 2010 [DOI] [PubMed] [Google Scholar]
- 72. Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol 95: 355–360, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Waldorf HA, Walsh LJ, Schechter NM, Murphy GF. Early cellular events in evolving cutaneous delayed hypersensitivity in humans. Am J Pathol 138: 477–486, 1991 [PMC free article] [PubMed] [Google Scholar]
- 74. Wang H, Su Y. Collagen IV contributes to nitric oxide-induced angiogenesis of lung endothelial cells. Am J Physiol Cell Physiol 300: C979–C988, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through alpha5beta1 and alphavbeta3 integrins via MAP kinase signalling. J Neurochem 96: 148–159, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, Chen PM. Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol 11: 2166–2170, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Warren MA, Shoemaker SF, Shealy DJ, Bshar W, Ip MM. Tumor necrosis factor deficiency inhibits mammary tumorigenesis and a tumor necrosis factor neutralizing antibody decreases mammary tumor growth in neu/erbB2 transgenic mice. Mol Cancer Ther 8: 2655–2663, 2009 [DOI] [PubMed] [Google Scholar]
- 78. Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol 23: 101–112, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wilson SH, Ljubimov AV, Morla AO, Caballero S, Shaw LC, Spoerri PE, Tarnuzzer RW, Grant MB. Fibronectin fragments promote human retinal endothelial cell adhesion and proliferation and ERK activation through alpha5beta1 integrin and PI 3-kinase. Invest Ophthalmol Vis Sci 44: 1704–1715, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Wolchok JC, Tresco PA. The isolation of cell derived extracellular matrix constructs using sacrificial open-cell foams. Biomaterials 31: 9595–9603, 2010 [DOI] [PubMed] [Google Scholar]
- 81. Yee D, Hanjaya-Putra D, Bose V, Luong E, Gerecht S. Hyaluronic acid hydrogels support cord-like structures from endothelial colony-forming cells. Tissue Eng Part A 17: 1351–1361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zagzag D, Shiff B, Jallo GI, Greco MA, Blanco C, Cohen H, Hukin J, Allen JC, Friedlander DR. Tenascin-C promotes microvascular cell migration and phosphorylation of focal adhesion kinase. Cancer Res 62: 2660–2668, 2002 [PubMed] [Google Scholar]
- 83. Zhou L, Isenberg JS, Cao Z, Roberts DD. Type I collagen is a molecular target for inhibition of angiogenesis by endogenous thrombospondin-1. Oncogene 25: 536–545, 2006 [DOI] [PubMed] [Google Scholar]


