Abstract
Plasma contains a variety of long-chain fatty acids (FAs), such that about 35% are saturated and 65% are unsaturated. There are countless examples that show how different FAs impart specific and unique effects, or even opposing actions, on cellular function. Despite these differing effects, palmitate (C16:0) is regularly used to represent “FAs” in cell based experiments. Although palmitate can be useful to induce and study stress effects in cultured cells, these effects in isolation are not physiologically relevant to dietary manipulations, obesity, or the consequences of physiological concentrations of FAs. Hence, authors should avoid conclusions that generalize about “FAs” or “saturated FAs” or “high-fat diet” effects if only a single FA was used in the reported experiments.
fatty acids (FAs) derived from the lipolysis of adipose tissue triacylglycerol are released into the circulation, where they can be taken up by cells to be used as an energy substrate or to form other lipids that are essential for survival. However, there is now a compelling body of evidence that chronic oversupply of FAs to nonadipocytes can result in cellular dysfunction and even apoptotic cell death. Right? Well, almost. Although on face value this statement appears to be accurate, as a generalization, the statement overlooks and even misrepresents the distinct roles of specific FAs in cellular processes and physiological functions.
Plasma contains a variety of long-chain FAs, such that about 35% are saturated and 65% are unsaturated. There are countless examples that show how different FAs impart specific and unique effects, or even opposing actions, on cellular function. Despite these differing effects, palmitate (C16:0) is regularly used to represent “FAs” in cell based experiments. This approach is problematic because palmitate often induces cytotoxic responses that are at variance with most other FAs. In fact, it is observed in many cases that the addition of an equal concentration of oleate prevents the adverse effects of palmitate. Thus, it is incorrect to incubate cells with palmitate in the absence of other unsaturated FAs and infer that the outcome represents a physiological effect of “FAs” or of “saturated FAs.” In skeletal muscle, for example, palmitate induces diacylglycerol and ceramide accumulation (1, 2), stress kinase activation (13, 18), endoplasmic reticulum stress (9), proinflammatory signaling and cytokine production (4, 5, 16), mitochondrial reactive species production (18), and apoptosis (15) (Fig. 1). By contrast, oleate has little or no effect on these processes and even prevents the stress or the toxic effects of palmitate when they are coincubated (2, 7, 15). Similar reports exist for almost all cell types. For example, long-chain saturated FAs (C14:0–C18:0) activate Toll-like receptor 4 signaling in macrophages, but preincubation with polyunsaturated FAs attenuates this response (12). Similarly, palmitate activates the NLRP3 inflammasome in macrophages, whereas oleate has no effect (17). Differences between species are also observed when radiolabeled FAs are used to measure rates of FA oxidation and storage. The choice of a representative FA influences the interpretation of such experiments (3, 11), as does the addition to the incubating medium of l-carnitine, which permits normal rates of mitochondrial uptake and oxidation of long-chain FA (data not shown). However, it is appropriate to use a single FA, including palmitate, to examine relative amounts of uptake, oxidation, and/or incorporation in cells treated acutely under specific conditions.
Fig. 1.
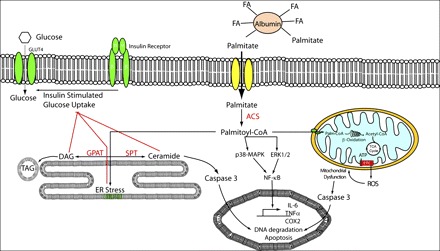
Schematic outlining cellular events induced by palmitate (C16:0) treatment and prevented by coincubation with oleate (C18:1). These processes include (L→R) ceramide accumulation, the induction of endoplasmic reticulum (ER) stress, proinflammatory response, apoptosis, and mitochondrial dysfunction. ACSL, long-chain acyl-CoA synthetase; COX, cyclooxygenase; DAG, diacylglycerol; ERK, extracellular regulated kinase; ETC, electron transport chain; FA, fatty acid; GLUT, glucose transporter; GPAT, glycerol-3-phosphate acyltransferase; IL-6, interleukin 6; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κ/light-chain enhancer of activated B cells; ROS, reactive oxygen species; SPT, serine palmitoyl transferase; TAG, triacylglycerol; TCA, tricarboxylic acid; TNF, tumor necrosis factor.
Although palmitate can be useful to induce and study stress effects in cultured cells (6), these effects in isolation are not physiologically relevant to dietary manipulations, obesity, or the consequences of physiological concentrations of FAs. To avoid mistaken interpretations, we recommend that FAs should be used at physiological concentrations (e.g., 50–750 μM), that FAs should be complexed to albumin at an approriate molar ratio (0.5:3 fatty acid/albumin), and that the interpretation of an experiment should be limited to the experimental condition employed. Investigators should not infer that the effect of a single FA tells one something about “FA effects” in general, particularly if coincubation with an equal amount of oleate rescues normal cellular function. Where possible, investigators should endeavor to replicate key experiments with a mixture of saturated and unsaturated FAs delivered in a molar ratio and in concentrations that represent those found in blood (e.g., 1:1 or 1:2 palmitate/oleate; 1:2:1 palmitate/oleate/linoleate) (8). Such studies should be construed as crucial controls that provide a minimum standard to judge the general role of “FAs” in a physiological context (Table 1). Although this stance might be considered hypercritical or bordering on just plain finicky, the devil is often in the details; accurate reporting and careful, inclusive experiments can change the interpretation of a data set and, ultimately, the conclusions relating to biological function.
Table 1.
General recommendations for examining and reporting fatty acid effects in cell culture and isolated tissue experiments
| Variable | Recommendation |
|---|---|
| Fatty acid concentration | Mixture: 50–1,500 μM; individual: 50–750 μM |
| Fatty acid mixtures | 1:1 or 1:2 Palmitate/oleate; 1:2:1 palmitate/oleate/linoleate (or any mixture at physiological ratios) |
| Albumin concentration | 0.5–3:1 Fatty acid/albumin molar ratio |
| l-carnitine | 1 mM |
| Reporting | List the fatty acid species in the title if only 1 fatty acid is used |
These interpretations apply in vivo. To take one example, Obici et al. (8) and Ross et al. (10) conducted a seminal series of studies to examine the effects of central FA administration on hepatic glucose metabolism and feeding. In the first study (8), the authors showed that infusing oleate (C18:1), but not octanoate (C8:0), into the third cerebral ventricle enhanced insulin action, inhibited hepatic glucose, production and decreased food intake. Those authors accurately titled the study “Central Administration of Oleic Acid Inhibits Glucose Production and Food Intake”; however, they inaccurately concluded that “This is the first demonstration that fatty acids can signal nutrient availability to the CNS.” Later studies published in AJP-Endocrinology and Metabolism (10) confirmed that hepatic insulin action is enhanced by central oleate administration but also showed that palmitate replicated these effects, albeit with considerably less potency than oleate, and that linoleate (18:2ω6) had no effect. In this case, the title “Differential effects of hypothalamic long-chain fatty acid infusions on suppression of hepatic glucose production” describes the effects more precisely than does the original publication.
Thus, to ensure accurate reporting and interpretation, we recommend that authors identify the specific FA used in the title (if a single FA is used), that they provide the FA and albumin concentrations in the methods sections of their articles, that they use a mixture of saturated and unsaturated FAs where possible, and, importantly, that they avoid conclusions that generalize about “FAs” or “saturated FA”s or “high-fat diet” effects if only a single FA was used in the reported experiments.
GRANTS
M. J. Watt and A. J. Hoy were supported by funding from the National Health and Medical Research Council of Australia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.H. prepared the figure; M.J.W. drafted the manuscript; M.J.W., A.J.H., D.M.M., and R.A.C. edited and revised the manuscript; M.J.W., A.J.H., D.M.M., and R.A.C. approved the final version of the manuscript.
REFERENCES
- 1. Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419: 101–109, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283: 11107–11116, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Gaster M, Rustan AC, Beck-Nielsen H. Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes 54: 648–656, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Jové M, Planavila A, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology 147: 552–561, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Kadotani A, Tsuchiya Y, Hatakeyama H, Katagiri H, Kanzaki M. Different impacts of saturated and unsaturated free fatty acids on COX-2 expression in C2C12 myotubes. Am J Physiol Endocrinol Metab 297: E1291–E1303, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta 1801: 246–251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100: 3077–3082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Peng G, Li L, Liu Y, Pu J, Zhang S, Yu J, Zhao J, Liu P. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology 152: 2206–2218, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Ross RA, Rossetti L, Lam TK, Schwartz GJ. Differential effects of hypothalamic long-chain fatty acid infusions on suppression of hepatic glucose production. Am J Physiol Endocrinol Metab 299: E633–E639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res 40: 2322–2332, 1999 [PubMed] [Google Scholar]
- 12. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinha S, Perdomo G, Brown NF, O'Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279: 41294–41301, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 291: E1341–E1350, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem 279: 23942–23952, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12: 408–415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 299: E1096–E1105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


