Abstract
Given the strong link between visceral adiposity and (hepatic) insulin resistance as well as liver steatosis, it is crucial to characterize obesity-associated alterations in adipocyte function, particularly in fat depots drained to the liver. Yet these adipose tissues are not easily accessible in humans, and the most frequently studied depot in rodents is the perigonadal, which is drained systemically. In the present study, we aimed to study alterations in lipolysis between mesenteric and perigonadal adipocytes in mice. Basal free fatty acid and glycerol release was significantly lower in perigonadal compared with mesenteric adipocytes isolated from chow-fed C57BL/6J mice. However, this difference completely vanished in high-fat diet-fed mice. Consistently, protein levels of the G0/G1 switch gene 2 (G0S2), which were previously found to be inversely related to basal lipolysis, were significantly lower in mesenteric compared with perigonadal fat of chow-fed mice. Similarly, perilipin was differently expressed between the two depots. In addition, adipocyte-specific overexpression of G0S2 led to significantly decreased basal lipolysis in mesenteric adipose tissue of chow-fed mice. In conclusion, lipolysis is differently regulated between perigonadal and mesenteric adipocytes, and these depot-specific differences might be explained by altered regulation of G0S2 and/or perilipin.
Keywords: intra-abdominal adipose tissue, visceral fat, portal hypothesis, obesity, insulin resistance
many studies point toward a critical role of visceral adipose tissue in the development of obesity-associated insulin resistance and ultimately type 2 diabetes mellitus. True visceral adipose tissue (i.e., adipose tissue that is in close proximity to the intra-abdominal viscera) is characterized and defined by its drainage via the portal vein into the liver. In obesity, exaggerated portal/hepatic delivery of free fatty acids (FFAs) and proinflammatory cytokines results in hepatic insulin resistance and metabolic deterioration constituting the “portal theory” (2, 6, 8, 9, 18). However, in many studies involving rodents mainly perigonadal (epididymidal) fat pads are assessed to determine biological function of adipose tissue due to its easy accessibility and anatomic landmark. Although perigonadal white adipose tissue (WAT) is located intra-abdominally, it is drained systemically via the inferior vena cava, whereas mesenteric WAT is drained via the portal vein (19). Nevertheless, perigonadal adipose tissue is often categorized as visceral adipose tissue, thereby implying similar function and regulation (3, 7, 12, 16).
Lipolysis is controlled by a complex of different lipases and regulatory proteins interacting with lipase activity (1). Among the latter perilipin, comparative gene identification-58 and lately the protein product of the G0/G1 switch gene 2 (G0S2) were identified. G0S2 interacts directly with the adipose tissue triglyceride lipase (ATGL) to attenuate triglyceride hydrolase activity (23). Interestingly, G0S2 expression was reduced significantly in perigonadal adipose tissue of db/db and high-fat diet (HFD)-fed wild-type mice, and overexpression of G0S2 in perigonadal fat reduced basal lipolysis. Thus, G0S2 may contribute to ATGL-mediated lipolysis in perigonadal WAT.
In contrast with previous studies where metabolic functions of different systemically drained fat depots were compared (perigonadal vs. inguinal; Refs. 3, 7, and 12), the aim of the present study was to better characterize lipolysis and the role of G0S2 in portally drained mesenteric fat tissue and to compare it with the perigonadal (epididymidal) fat depot in mice fed either a standard chow or a HFD.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old male C57BL6JOlaHsd mice were fed ad libitum with standard rodent diet (chow) or HFD (D12331; Research Diets, New Brunswick, NJ) for 8 wk. HFD consisted of 56% calories derived from fat, 28% from carbohydrate, and 16% from protein. Body weight of the two groups was similar at initiation of the HFD [21.2 ± 0.3 (chow) vs. 20.8 ± 0.4 g (HFD), P = 0.4]. Mice were fasted for 5 h prior to euthanization. All protocols conformed to Swiss animal protection laws and were approved by the Cantonal Veterinary Office in Zurich, Switzerland.
To generate transgenic mice overexpressing G0S2 specifically in adipose tissue, the murine G0S2 cDNA sequence (Genbank NM_008059) was subcloned into a pBluescript II SK(+) vector containing a 5.4-kB adipocyte fatty acid binding protein (aP2) promoter and a poly(A) tail (Addgene). G0S2 was PCR-amplified to add a 5′ Kozak sequence along with NotI and SmaI restriction enzyme cut sites on the 5′ and 3′ ends, respectively. The Kozak-G0S2 sequence was inserted downstream of the aP2 promoter and upstream of the poly(A) tail using the NotI and SmaI restriction sites. The completed aP2-G0S2-poly(A) construct was confirmed by sequencing. Through the University of Michigan Transgenic Animal Model Core, the transgene fragment was released by SalI digestion, purified, and microinjected into fertilized eggs of C57BL/6J mice. Tail DNA genotyping revealed that seven independent transgenic founder lines were obtained, of which five lines underwent successful germline transmission.
Intraperitoneal glucose and insulin tolerance test.
Mice were injected intraperitoneally with 2 g/kg body wt glucose after overnight fasting or 1 IU/kg body wt human insulin after a 3-h fast, as described previously (11). Blood glucose concentration was measured with a Glucometer (Accu-Check Aviva; Roche Diagnostics, Rotkreuz, Switzerland) with blood from tail-tip bleedings. Area under the curve (AUC) was calculated as changes from zero.
Determination of plasma leptin and insulin.
Blood was sampled in overnight-fasted (14 h) animals. Plasma leptin levels were determined using a mouse LINCOplex kit from Linco Research (Labodia, Yens, Switzerland). Plasma insulin levels were measured as described previously (11).
Viability assessment and cell size determination.
Adipocytes were isolated, and viability was determined with an LDH assay, as described previously (21), with the following adaptation: mesenteric adipocytes were isolated by collagenase digestion for 30 min (digestion period for mesenteric adipose tissue was reduced by 15 min since it has an increased surface/volume ratio compared with perigonadal adipose tissue) and perigonadal adipocytes for 45 min. Aliquots of isolated adipocytes were used to determine mean cell diameters. Photographs of isolated adipocytes were taken in the haematocytometer, and images were analyzed using ImageJ software for quantification (National Institutes of Health, Bethesda, MD). At least 100 adipocytes per animal were analyzed.
Lipolysis assays.
Lipolysis was assessed in isolated adipocytes or fat explants as indicated. Isolated adipocytes were incubated in the absence or presence of 100 nM insulin or 1 μM isoproterenol (Sigma, Buchs, Switzerland) for 1 h. FFA levels were measured using the ACS-ACOD-MEHA method from Wako Chemicals (Neuss, Germany). Glycerol content of the incubation medium was determined using a colorimetric assay, as described (22). FFA and glycerol were determined in portal and systemic blood sampled from mice fasted for 5 h.
Determination of cytokines.
Isolated adipocytes were incubated for 1 h, as described above. Cytokines released into the incubation medium were determined with a Milliplex MAP kit (Millipore, Zug, Switzerland).
Western blotting.
Cell lysates and tissue samples were homogenized, and Western blotting was performed as described previously (22). The following primary antibodies were used: anti-perilipin A was purchased from MBL (Woburn, MA), and anti-ATGL, anti-hormone-sensitive lipase (HSL), and anti-phospho-HSL were purchased from Cell Signaling Technology (Beverly, MA). Anti-G0S2 was raised as described previously (23). Membranes were exposed in an Image Reader and analyzed with Image Analyzer (FujiFilm, Dielsdorf, Switzerland). One of the perigonadal samples (chow-fed) was loaded on every gel and was used as a reference band for quantification; i.e., all bands on a membrane were normalized to the expression levels of this sample.
RNA extraction and quantitative RT-PCR.
Total RNA was extracted using RNeasy Lipid Tissue Mini Kit (Qiagen, Basel, Switzerland), and concentration was determined with a nanodrop (spectrophotometer); 0.5 μg of RNA was reverse transcribed with Superscript III Reverse Transcriptase (Invitrogen, Basel, Switzerland) using random hexamer primer (Invitrogen). Taqman (Applied Biosystems, Rotkreuz, Switzerland) was used for real-time PCR amplification. The following PCR primers (Applied Biosystems) were used: ATGL Mm00503040_m1, HSL Mm00495359_m1, and perilipin Mm00558672_m1. Relative gene expression was obtained after normalization to 18s RNA (Applied Biosystems), using the formula 2−ΔΔCp (17).
Total liver lipid determination.
Liver tissue (20–30 mg) was homogenized in PBS, and lipids were extracted in a chloroform-methanol (2:1) mixture. Total liver lipids were determined by a sulfophosphovanillin reaction, as described previously (10).
Data analysis.
Statistical analyses were performed using Student's t-test or by analysis of variance with a Tukey correction for multiple group comparisons. P values <0.05 were considered significant.
RESULTS
HFD-induced increase in fat pad weight differs among perigonadal and mesenteric fat depots.
Eight weeks of HFD led to significant changes in metabolic parameters and total liver lipid levels in male C57BL6J mice compared with chow-fed animals (Table 1) as well as significantly impaired glucose and insulin tolerance, as assessed by an intraperitoneal glucose and insulin tolerance test [AUC intraperitoneal glucose tolerance test chow (1,547 ± 70 mmol·l−1·min−1) vs. AUC HFD (2,146 ± 82 mmol·l−1·min−1), P < 0.0001; AUC intraperitoneal insulin tolerance test chow (267 ± 15 mmol·l−1·min−1) vs. AUC HFD (465 ± 26 mmol·l−1·min−1), P < 0.001; Fig. 1A]. Under HFD, fat pad weight was higher in both depots analyzed; however, relative weight increase was significantly higher in perigonadal compared with mesenteric fat pads (Fig. 1B). Similarly, mean adipocyte diameter increased in both depots (Fig. 1, C and D), but again, increase was lower in adipocytes of mesenteric compared with adipocytes of perigonadal origin (Fig. 1D). However, HFD did not affect adipocyte number in the two examined fat pads (Fig. 1E), suggesting that HFD initiated a predominantly hypertrophic expansion of adipose tissue that was less pronounced in mesenteric compared with perigonadal fat.
Table 1.
Phenotypic characteristics of chow- and HFD-fed C57BL/6J mice (overnight-starved mice)
| Chow Fed | HFD Fed | |
|---|---|---|
| Body weight, g | 24.4 ± 0.8 | 30.2 ± 0.5*** |
| Blood glucose, mmol/l | 5.1 ± 0.3 | 6.3 ± 0.2** |
| Insulin, pmol/l | 52.2 ± 9.6 | 85.8 ± 11.7 |
| HOMA-IR | 1.6 ± 0.3 | 3.4 ± 0.6* |
| Leptin, pg/ml | 135 ± 34 | 7,764 ± 1,789** |
| Total liver lipids, μg/mg | 42.8 ± 2.9 | 66.8 ± 4.0** |
Results are means ± SE of 5 mice/group.
HFD, high-fat diet; HOMA-IR, homeostatic model assessment of insulin resistance.
P < 0.05,
P < 0.01,
P < 0.001 (Student's t-test).
Fig. 1.
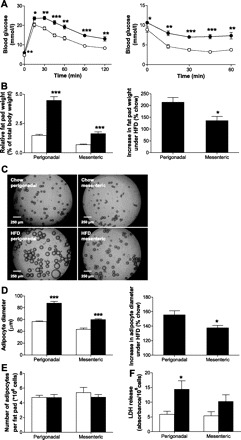
Increase in mean adipocyte diameter is significantly less pronounced in adipocytes isolated from mesenteric fat pads. A: intraperitoneal glucose (2 g/kg body wt) and insulin (1 IU/kg body wt) tolerance test was performed in chow-fed (○) and high-fat diet (HFD)-fed (•) mice. Results are means ± SE of 5–6 animals/group. B: relative fat pad weight (%total body wt) was determined in mice fed either a regular chow diet (open bars) or a HFD (black bars). Results are means ± SE of 5–6 animals/group. C: representative photographs of adipocytes isolated from respective fat pads of chow- and HFD-fed mice are shown (scale bar, 250 μm). D: mean adipocyte diameter (left) in both fat pads was assessed in chow- (open bars) or HFD-fed (black bars) mice. Relative increase in adipocyte diameter of HFD-fed mice is depicted (right). Results represent the mean ± SE of 4–8 mice. E: adipocyte number per fat pad was calculated by dividing fat pad weight (mg) by average cell volume (μm3) and an assumptive adipose tissue density of 0.9 kg/l. Results are means ± SE of 4–8 independent experiments. F: viability of isolated adipocytes was determined by an LDH release assay. Results are means ± SE of 4–9 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t-test).
To verify that the lower increase in cell size in mesenteric fat was not a consequence of a higher artifactual loss of large mesenteric adipocytes during isolation, we assessed release of lactate dehydrogenase (LDH) to the medium. Whereas higher LDH release was observed in adipocytes isolated from HFD-fed compared with chow-fed mice, no difference was found between mesenteric and perigonadal adipocytes. In fact, LDH release tended to be lower in adipocytes isolated from mesenteric fat pads (Fig. 1F).
Increased basal lipolysis in mesenteric compared with perigonadal adipocytes isolated from chow-fed mice.
To evaluate lipolysis, concentrations of FFA and glycerol were determined in the incubation medium. In chow-fed mice, basal FFA release per cell was significantly higher in mesenteric compared with perigonadal adipocytes (Fig. 2A). Similar results were obtained for glycerol release (Fig. 2A). As expected, 8 wk of HFD led to an increase in basal lipolysis in perigonadal adipocytes. Interestingly, however, there was no increase in basal FFA and glycerol release in adipocytes isolated from mesenteric fat pads of HFD- compared with chow-fed mice (Fig. 2A). To have a functional readout for stimulated lipolysis, we assessed the ability of isoproterenol to induce release of FFAs. In chow-fed mice, stimulation with isoproterenol led to a higher fold increase in FFA release in perigonadal compared with mesenteric adipocytes (Fig. 2B), whereas under HFD, lipolysis upon β-adrenergic stimulation was similar in both fat depots (Fig. 2B). Similar results were obtained for glycerol release (Fig. 2B). Basal lipolysis values obtained in these experiments are as shown in Fig. 2A [FFA release: for perigonadal adipocytes 0.89 ± 0.11 (chow) and 1.68 ± 0.23 μmol·106 cells−1·60 min−1 (HFD), for mesenteric adipocytes 5.00 ± 1.86 (chow) and 2.64 ± 0.40 μmol·106 cells−1·60 min−1 (HFD); glycerol release: for perigonadal adipocytes 0.73 ± 0.08 (chow) and 1.76 ± 0.24 μmol·106 cells−1·60 min−1 (HFD), for mesenteric adipocytes 2.23 ± 0.96 (chow) and 2.00 ± 0.22 μmol·106 cells−1·60 min−1 (HFD)].
Fig. 2.
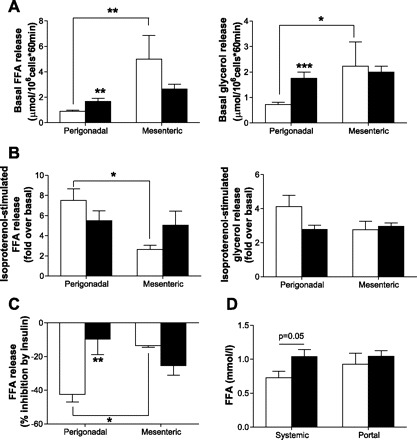
Increased basal lipolysis in mesenteric compared with perigonadal adipocytes in chow-fed mice. A: basal free fatty acid (FFA; left) and glycerol (right) release from adipocytes of chow- (open bars) and HFD-fed (black bars) mice is depicted. Results are means ± SE of 4–9 independent experiments. Isoproterenol-stimulated FFA and glycerol (B) and insulin-inhibited FFA release (C) from adipocytes of chow- (open bars) and HFD-fed (black bars) mice is depicted. Results are means ± SE of 3–9 independent experiments. D: FFA concentration was determined in systemic and portal plasma samples of chow- (open bars) and HFD-fed (black bars) mice fasted for 5 h. Results are means ± SE of 5–7 mice. *P < 0.05, **P < 0.01, ***P < 0.001 (Student's t-test, ANOVA).
In adipocytes isolated from chow-fed animals, the effect of insulin to inhibit lipolysis was significantly higher in perigonadal compared with mesenteric adipocytes (Fig. 2C). Moreover, the ability of insulin to inhibit lipolysis was blunted under HFD in perigonadal adipocytes, whereas there was no further deterioration of the antilipolytic effect in mesenteric adipocytes (Fig. 2C). Thus, our results suggest that the inhibitory effect of insulin on lipolysis varies between the two fat depots. Moreover, lipolysis is differently regulated between the differentially drained perigonadal and mesenteric fat depots in chow- and HFD-fed mice.
Similar FFA concentrations in portal blood of chow- and HFD-fed mice.
To have an in vivo readout for basal lipolysis as observed in Fig. 2A, FFA levels were determined in systemic and portal blood of chow- and HFD-fed mice. Whereas high-fat feeding led to an ∼45% increase in FFA levels in systemic circulation, FFA levels in portal blood were comparable between chow- and HFD-fed mice (Fig. 2D). Although systemic FFA levels are influenced by both the release and uptake of fatty acids from different fat depots and tissues, it is mentionable that changes in circulating FFA levels under HFD are paralleled by changes in basal lipolysis, i.e., increased basal lipolysis in systemically drained perigonadal fat pads compared with unchanged lipolysis in portally drained mesenteric fat tissue.
Increased expression of perilipin and G0S2 in perigonadal compared with mesenteric adipose tissue of chow-fed mice.
To better understand differences in basal lipolysis, expression of lipases and regulatory proteins was determined in mesenteric and perigonadal WAT. In chow-fed mice, protein levels and mRNA expression of ATGL and HSL (Fig. 3, A and B) were slightly decreased in mesenteric compared with perigonadal WAT. Moreover, phosphorylation of HSL at Ser563 (a residue that is phosphorylated by protein kinase A and therefore leads to increased lipolysis) was reduced significantly in mesenteric compared with perigonadal WAT of chow-fed mice (Fig. 3C), whereas HFD reduced phosphorylation of HSL at Ser563 in perigonadal WAT, confirming previous findings (3). Besides Ser563, PKA phosphorylates HSL at Ser659 and Ser660, a mechanism that is required for the translocation of HSL from the cytosol to the lipid droplet (4). Although phosphorylation of HSL at the latter two sites was not assessed in this study, our data suggest that increased basal lipolysis in mesenteric compared with perigonadal adipocytes of chow-fed mice cannot be explained by increased lipase expression/activation.
Fig. 3.
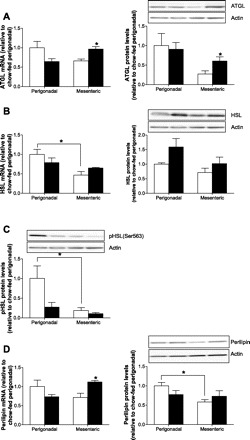
Increased perilipin protein levels in perigonadal compared with mesenteric fat depots of chow-fed mice. A: adipose tissue triglyceride lipase (ATGL) mRNA expression (left) and protein levels (right) in adipose tissue of chow- (open bars) and HFD-fed (black bars) mice is depicted. Results are means ± SE of 3–4 independent experiments (Student's t-test). B: mRNA expression (left) and protein levels (right) of hormone-sensitive lipase (HSL) in adipose tissue of chow- (open bars) and HFD-fed (black bars) mice are shown. Results are means ± SE of 3–4 independent experiments (ANOVA). C: protein levels of p-Ser563 HSL in adipose tissue of chow- (open bars) and HFD-fed (black bars) mice are depicted. Results are means ± SE of 4 independent experiments (ANOVA). The actin blots presented in A–C are the same since the presented membrane was exposed to p-HSL, ATGL, and actin and then stripped and exposed to HSL. D: mRNA expression (left) and protein levels (right) of perilipin in adipose tissue of chow- (open bars) and HFD-fed mice (black bars) are shown. Results are means ± SE of 3–8 independent mice (Student's t-test, ANOVA). *P < 0.05.
Expression/content of the lipid droplet-coating protein perilipin is inversely correlated with lipolysis (20). Both perilipin mRNA expression and perilipin protein content were lower in mesenteric compared with perigonadal WAT of chow-fed mice, although the difference was significant only in the latter (Fig. 3D).
Recently, G0S2 was identified to directly interact with ATGL to attenuate its triglyceride hydrolase activity (23). In perigonadal WAT, there was a trend toward reduced G0S2 protein levels due to HFD (Fig. 4A), as was reported previously (23). In contrast, protein content of G0S2 was increased in mesenteric WAT of obese and insulin-resistant mice (Fig. 4A). Moreover, in chow-fed animals, G0S2 protein levels were significantly higher in perigonadal compared with mesenteric WAT. Thus, basal lipolysis in mesenteric adipocytes is negatively associated with G0S2 protein content. To support a role of G0S2 expression in the regulation of basal lipolysis in mesenteric adipose tissue, adipocyte-specific G0S2-overexpressing mice were used. These mice are ∼15% heavier than wild-type control mice due to a 2.1-fold increased fat mass, as assessed by EchoMRI examination. Moreover, they have improved glucose tolerance and insulin sensitivity, as revealed by glucose tolerance test, insulin tolerance test, and metabolic chamber studies. In addition, overall triglyceride lipase activity is reduced by 30–40% in fat extracts. As shown in Fig. 4B, G0S2 protein levels were increased in mesenteric adipose tissue of these mice. Moreover, basal glycerol release from mesenteric adipose tissue of G0S2-overexpressing mice was significantly reduced compared with wild-type mice (Fig. 4C), further supporting a role for G0S2 in the regulation of basal lipolysis in mesenteric adipose tissue.
Fig. 4.
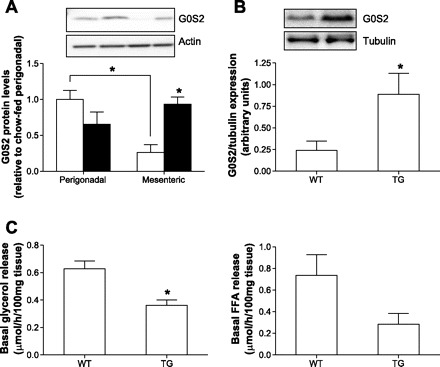
Increased protein levels of G0/G1 switch gene 2 (G0S2) in mesenteric fat depots of HFD- compared with chow-fed mice. A: protein levels of G0S2 in adipose tissue of chow- (open bars) and HFD-fed mice (black bars) are depicted. Results are means ± SE of 3–8 independent experiments (ANOVA). B, top: a representative blot of G0S2 protein levels in mesenteric adipose tissue of wild-type and G0S2-overexpressing mice is depicted. B, bottom: a quantification of G0S2 overexpression in mesenteric adipose tissue. The results represent means ± SE of 3 independent experiments (Student's t-test). C: basal glycerol (left) and FFA release (right) from mesenteric fat explants of wild-type and G0S2-overexpressing mice [transgene (TG)] are depicted. Results are means ± SE of 3 independent experiments (Student's t-test). *P < 0.05.
DISCUSSION
In contrast with previous studies, which assessed mainly differences between perigonadal and inguinal fat depots, the aim of the present study was to compare lipolysis between two different intra-abdominal fat depots, namely between the systemically drained perigonadal and the portally drained mesenteric adipose tissue. Of note, differences in basal lipolysis were most obvious in lean mice and probably reflected differences in metabolic turnover between the two depots. As expected, lipolysis increased in perigonadal adipocytes under HFD. In contrast, FFA and glycerol release from mesenteric adipocytes, as well as portal FFA concentration, was not significantly different between lean and obese mice. At first glance, such observation seems to contradict the “portal theory” (2, 6, 8, 9). However, increased release of proinflammatory cytokines into portal circulation may play a more crucial role in the development of hepatic insulin resistance than increased FFA release (2, 22). Indeed, published evidence supports an important role for portal IL-6 as well as IL-1β in the induction of hepatic insulin resistance (15, 18). Likewise, IL-6 release from isolated mesenteric but not perigonadal adipocytes of HFD- compared with chow-fed mice was increased significantly (Fig. 5A), whereas IL-1β concentrations were below the detection limit. In addition, there was a trend toward increased proinflammatory monocyte chemoattractant protein-1 and decreased anti-inflammatory IL-10 release from mesenteric adipocytes isolated from HFD-fed mice (Fig. 5, B and C).
Fig. 5.
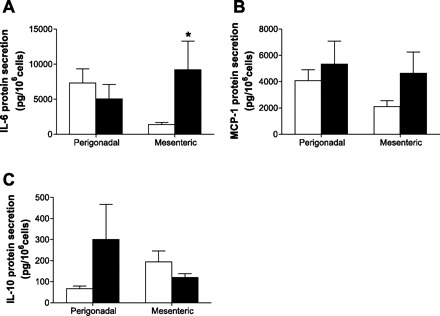
Increased IL-6 release from HFD- compared with chow-fed mesenteric adipocytes. Release of IL-6 (A), monocyte chemoattractant protein-1 (MCP-1; B), and IL-10 (C) from isolated adipocytes of chow- (open bars) and HFD-fed mice (black bars) is depicted. Results are means ± SE of 5–9 mice. *P < 0.05 (Student's t-test).
Recently, G0S2 was identified to inhibit lipolysis via direct interaction with the triglyceride hydrolase activity of ATGL (23). Consequently, G0S2 protein levels correlated negatively with lipolytic activity. Herein, we could confirm such correlation, since decreased G0S2 protein levels in mesenteric compared with perigonadal WAT from chow-fed mice paralleled higher basal lipolysis in mesenteric adipocytes. Interestingly, high-fat feeding led to an increase in G0S2 levels in mesenteric adipose tissue, whereas they were decreased in perigonadal adipose tissue, as was reported previously for the latter (23). Consistent with increased G0S2 protein expression, basal lipolysis in mesenteric adipocytes did not increase in mice fed a fat-enriched diet despite increased ATGL mRNA expression and protein levels. The latter finding is in accord with the notion that FFA concentration was similar in portal plasma samples of chow- and HFD-fed mice despite increased mesenteric fat pad weight. Moreover, overexpression of G0S2 in mesenteric WAT reduced basal lipolysis in chow-fed mice, supporting a role of G0S2 in the regulation of basal lipolysis in mesenteric adipose tissue.
The lipid droplet-coating protein perilipin is an important regulator of triacylglycerol hydrolysis, and its absence in adipocytes led to elevated basal lipolysis, whereas adrenergic-stimulated lipolysis was attenuated (20). As expected, perilipin protein levels in adipocytes of chow-fed mice were negatively associated with basal lipolysis; i.e., increased basal lipolysis in mesenteric adipocytes was paralleled by decreased perilipin content. Similar to a recent study showing a significant downregulation of perilipin protein levels in perigonadal adipocytes after 8 wk of HFD (3), we observed a decrease in perilipin protein levels in perigonadal WAT in HFD-fed mice, which was paralleled by increased lipolysis. On the other hand, HFD led to a slight increase in perilipin expression and protein levels in mesenteric adipocytes, matching decreased FFA release. Therefore, different regulation of perilipin in examined intra-abdominal fat depots may be at least partly responsible for observed differences in lipolysis. Of note, observed differences in lipolysis and gene expression/protein content of adipose tissue were assessed after a 5-h fasting period. Possibly, different time points and/or durations of fasting might have affected obtained results (5, 14).
Interestingly, most of the observed differences between perigonadal and mesenteric adipose tissue in chow-fed mice (basal lipolysis, insulin-inhibited lipolysis, perilipin and G0S2 protein levels) diminished under HFD. Hence, although perigonadal adipocytes are not indicative for HFD-induced changes in mesenteric adipocytes, they might nevertheless be good representatives of portally drained adipocytes in the HFD state for the parameters assessed, i.e., lipolysis. Although there is mesenteric adipose tissue in humans (visceral fat), no fat depot paralleling perigonadal WAT exists in men, and hence, the uniqueness of perigonadal fat in rodents was highlighted (19). The results presented here further support such uniqueness; lipolysis and cytokine secretion were different between perigonadal and mesenteric adipocytes. However, it is difficult to compare differences observed in this study to human studies, where most often metabolism of subcutaneous and visceral adipocytes is investigated. Nevertheless, similar to the results presented in this study, human visceral adipocytes were shown to be less sensitive to the antilipolytic effect of insulin compared with retroperitoneal adipocytes (13). In contrast, basal lipolysis increased in human visceral adipose tissue under obesity (24), whereas it did not change in murine visceral adipocytes between chow- and high fat-fed mice.
In conclusion, lipolysis is differently regulated between systemically drained perigonadal and portally drained mesenteric fat pads. These depot-specific differences in intra-abdominal fat might be explained by altered regulation of G0S2 and/or perilipin content.
GRANTS
This work was supported by research grants from the Swiss National Science Foundation (no. 310000-112275) and the European Foundation for the Study of Diabetes (both to D. Konrad).
DISCLOSURES
No conflicts of interest exist for any of the authors.
AUTHOR CONTRIBUTIONS
S.W. and D.K. did the conception and design of the research; S.W. and X.Y. performed the experiments; S.W., X.Y., J.L., and D.K. analyzed the data; S.W., X.Y., J.L., and D.K. interpreted the results of the experiments; S.W., X.Y., J.L., and D.K. prepared the figures; S.W. and D.K. drafted the manuscript; S.W., X.Y., J.L., E.J.S., and D.K. approved the final version of the manuscript; E.J.S. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Prof. Giatgen Spinas for continuous support.
REFERENCES
- 1. Brasaemle DL. Lipolysis control: the plot thickens. Cell Metab 11: 173–174, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Catalano KJ, Stefanovski D, Bergman RN. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 59: 1416–1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 298: C961–C971, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31: 1120–1124, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr 86: s867–s871, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93: S57–S63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 27: 2563–2570, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab 288: E454–E461, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R; Association for Weight Management and Obesity Prevention; NAASO; Obesity Society; American Society for Nutrition; American Diabetes Association Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 30: 1647–1652, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Knight JA, Anderson S, Rawle JM. Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin Chem 18: 199–202, 1972 [PubMed] [Google Scholar]
- 11. Konrad D, Rudich A, Schoenle EJ. Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia 50: 833–839, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58: 803–812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mårin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjöström L, Björntorp P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 41: 1242–1248, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Miles JM, Wooldridge D, Grellner WJ, Windsor S, Isley WL, Klein S, Harris WS. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 52: 675–681, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Nov O, Kohl A, Lewis EC, Bashan N, Dvir I, Ben-Shlomo S, Fishman S, Wueest S, Konrad D, Rudich A. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology 151: 4247–4256, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Okamoto Y, Higashiyama H, Rong JX, McVey MJ, Kinoshita M, Asano S, Hansen MK. Comparison of mitochondrial and macrophage content between subcutaneous and visceral fat in db/db mice. Exp Mol Pathol 83: 73–83, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes 60: 56–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab 293: E1012–E1020, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98: 6494–6499, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 52: 541–546, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, Chervonsky AV, Rudich A, Schoenle EJ, Donath MY, Konrad D. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest 120: 191–202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11: 194–205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang X, Smith U. Adipose tissue distribution and risk of metabolic disease: does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia 50: 1127–1139, 2007 [DOI] [PubMed] [Google Scholar]


