Abstract
Large increases in systemic oxygen content cause substantial reductions in exercising forearm blood flow (FBF) due to increased vascular resistance. We hypothesized that 1) functional sympatholysis (blunting of sympathetic α-adrenergic vasoconstriction) would be attenuated during hyperoxic exercise and 2) α-adrenergic blockade would limit vasoconstriction during hyperoxia and increase FBF to levels observed under normoxic conditions. Nine male subjects (age 28 ± 1 yr) performed forearm exercise (20% of maximum) under normoxic and hyperoxic conditions. Studies were performed in a hyperbaric chamber at 1 atmosphere absolute (ATA; sea level) while breathing 21% O2 and at 2.82 ATA while breathing 100% O2 (estimated change in arterial O2 content ∼6 ml O2/100 ml). FBF (ml/min) was measured using Doppler ultrasound. Forearm vascular conductance (FVC) was calculated from FBF and blood pressure (arterial catheter). Vasoconstrictor responsiveness was determined using intra-arterial tyramine. FBF and FVC were substantially lower during hyperoxic exercise than normoxic exercise (∼20–25%; P < 0.01). At rest, vasoconstriction to tyramine (% decrease from pretyramine values) did not differ between normoxia and hyperoxia (P > 0.05). During exercise, vasoconstrictor responsiveness was slightly greater during hyperoxia than normoxia (−22 ± 3 vs. −17 ± 2%; P < 0.05). However, during α-adrenergic blockade, hyperoxic exercise FBF and FVC remained lower than during normoxia (P < 0.01). Therefore, our data suggest that although the vasoconstrictor responsiveness during hyperoxic exercise was slightly greater, it likely does not explain the majority of the large reductions in FBF and FVC (∼20–25%) during hyperbaric hyperoxic exercise.
Keywords: α-adrenergic, vasoconstriction, skeletal muscle blood flow
it is well documented that blood flow regulation during exercise is matched to the metabolic demands of contracting skeletal muscle and ensures adequate delivery of oxygen (O2). Changes in O2 availability often observed under different environmental conditions, disease conditions, or both can alter the matching of muscle blood flow and metabolism and, ultimately, exercise capacity. Along these lines, both increases and decreases in O2 availability bring about several cardiovascular adjustments and can have profound influences on the circulation (42). This is particularly apparent in the skeletal muscle circulation during exercise. Under conditions of increased O2 availability (e.g., during hyperoxia), vascular conductance is reduced in contracting muscles. Indeed, reductions in skeletal muscle blood flow have been observed during exercise in response to both small and large increases in arterial O2 content while breathing 100% O2 under normobaric and hyperbaric environments, respectively (7, 20, 49). Conversely, acute reductions in O2 availability (i.e., hypoxia) elicit a compensatory vasodilation and an augmented blood flow in contracting muscles relative to the same level of exercise under normoxic conditions (8, 38, 41, 50). This enhanced, hypoxia-dependent vasodilation is proportional to the hypoxia-induced fall in arterial O2 content, thus preserving muscle O2 delivery and ensuring it is matched to demand (21, 38, 41).
Although the mechanisms for compensatory vasodilation during hypoxic exercise have been investigated and described in detail (6), the mechanisms for increased vascular resistance and lower blood flow in contracting muscle during hyperoxic exercise remain unclear. Under normoxic conditions sympathetic vasoconstrictor responses are blunted in the vascular beds of contracting limbs (14, 36), a phenomenon referred to as functional sympatholysis. This blunting of sympathetic vasoconstriction is believed to optimize blood flow distribution and O2 delivery within the active muscle and is potentially modulated by various substances from the muscle, vascular endothelium, or both (46). Theoretically, the increased vascular resistance and reduced blood flow during hyperoxic exercise may be attributed to an enhanced sympathetic restraint via an attenuated functional sympatholysis or a greater α-adrenergic–mediated vasoconstriction. Along these lines, it has been suggested that alterations in O2 availability can potentially influence the degree in which sympathetic α-adrenergic vasoconstriction is blunted in contracting muscle (i.e., functional sympatholysis) (23). Additionally, greater sympathetic responses to isometric handgrip exercise have been demonstrated during hyperoxic conditions compared with normoxic conditions (26).
Although the influence of reduced O2 availability on α-adrenergic vasoconstriction in contracting muscle have been investigated by our group (50) and others (23), we are unaware of any studies that have directly assessed the role of increased O2 availability on vasoconstrictor responsiveness during exercise. Because skeletal muscle blood flow is a key determinant of exercise capacity and is highly dependent on O2 availability, it is crucial to understand the mechanisms governing exercise hyperemia across a wide range of O2 content. With this information as background, we aimed to test the hypothesis that the blunting of sympathetic α-adrenergic vasoconstriction would be less in contracting muscles during hyperoxic exercise than during normoxic exercise. Additionally, we examined whether α-adrenergic blockade would limit vasoconstriction during hyperoxia and increase forearm blood flow to similar levels observed under normoxic conditions.
METHODS
Ethical approval.
A total of 10 young healthy male subjects volunteered to participate in the study. Subjects completed written informed consent. They were not obese (BMI <28 kg/m2), nonsmokers, and were not taking any medications. A detailed history and physical examination directed toward hyperbaric oxygen therapy risks were performed by a board-certified Undersea and Hyperbaric Medicine Physician (P.L.C.) prior to each subject's study day. Additionally, chest X-rays and 12-lead electrocardiographic recordings were performed as part of the standard screening procedures for exposure to hyperbaric oxygenation. Studies were performed after an overnight fast and the subjects refrained from exercise and caffeine for at least 24 h prior to the study. All study protocols were approved by the Mayo Clinic Institutional Review Board and were performed according to the Declaration of Helsinki.
Forearm exercise.
Subjects performed rhythmic forearm exercise with a handgrip device using the right arm at 20% of each subject's maximal voluntary contraction (MVC, mean 55 ± 3 kg; range, 38–68 kg), determined at the beginning of each experiment. The weight was lifted 4–5 cm over a pulley at a duty cycle of 1 s contraction and 2 s relaxation (20 contractions per minute) using a metronome to ensure correct timing. The average weight used for forearm exercise was 10.9 ± 0.7 kg.
Arterial catheterization.
A 20-gauge, 5-cm (Model RA-04020; Arrow International, Reading, PA) catheter was placed in the brachial artery of the exercising arm under aseptic conditions after local anesthesia (2% lidocaine) for measurement of arterial pressure and administration of study drugs and was continuously flushed (3 ml/h) with heparinized saline.
Heart rate and systemic blood pressure.
Heart rate (HR) was recorded via continuous three-lead electrocardiogram. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure (Cardiocap/5; Datex-Ohmeda, Louisville, CO).
Forearm blood flow.
Brachial artery mean blood velocity was determined with a 4 MHz pulsed Doppler probe (Model 500 V; Multigon Industries, Mt Vernon, NY) proximal to the catheter insertion site. A linear, 15 MHz Doppler ultrasound probe (M-Turbo; SonoSite, Bothell, WA) was placed immediately proximal to the velocity probe to measure brachial artery diameter. Brachial artery blood velocity was measured throughout each condition with a probe insonation angle of 60°. Brachial artery diameter measurements were obtained at end diastole between contractions during steady-state conditions. Forearm blood flow (FBF) was calculated as the product of mean blood velocity (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min).
Pharmacological infusions.
Tyramine was infused via brachial catheter at 8 μg (deciliter forearm volume)/min to elicit endogenous norepinephrine release. Care was taken to normalize the concentration of tyramine in the blood perfusing the forearm by adjusting the infusions on the basis of forearm blood flow and forearm volume (15, 50). Tyramine evokes norepinephrine release from sympathetic nerve endings (19), eliciting vasoconstriction that is abolished by nonselective α-adrenergic receptor blockade in humans (5, 13). Tyramine does not have any direct vasoconstrictor effects in skeletal muscle (19).
To control for the potential influence of increased blood flow on vasoconstrictor responsiveness, sodium nitroprusside (NTP) was used to elevate resting FBF to values similar to those observed during exercise and served as a high-flow control condition. NTP was infused at 2.0 μg (deciliter forearm volume)/min.
Phentolamine, a nonselective α-adrenergic receptor antagonist, was administered to the experimental forearm via brachial artery catheter as a loading dose [10 μg (deciliter forearm volume)/min for 5 min] followed by a continuous maintenance dose (25 μg/min). This dose of phentolamine has been shown to effectively inhibit α-adrenergic receptor vasoconstriction.(17).
Experimental protocol.
A schematic diagram of the general experimental timeline is illustrated in Fig. 1. All studies, consisting of seven separate trials, were performed in a hyperbaric chamber at ∼340 m above sea level with the subjects positioned supine. Although ambient temperature varied during compression and decompression, the temperature in the hyperbaric chamber during the study trials was maintained at approximately thermoneutral with a climate control system.
Fig. 1.
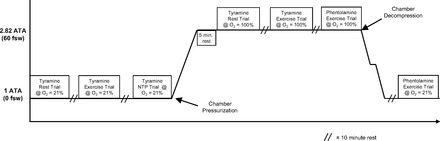
Detailed study timeline. Hyperbaric exposure is shown in atmospheres absolute pressure (ATA) units. A total of seven separate trials were performed. Five trials were performed with tyramine to investigate whether blunting of sympathetic α-adrenergic vasoconstriction (functional sympatholysis) would be less in contracting muscles during hyperoxic exercise than during normoxic exercise. Two trials were performed with phentolamine to examine the effects of α-adrenergic blockade on forearm blood flow and vascular conductance under normoxic and hyperoxic conditions.
Each subject completed a resting baseline trial and two separate exercise trials under normoxic and hyperoxic conditions, as well as a high-flow control trial (i.e., NTP) under normoxic conditions. The first series of trials (rest, exercise, and NTP) were performed at 1 atmosphere absolute (ATA) (unpressurized chamber) while breathing normoxic gas (air) (21% O2; PiO2 ≈150 mmHg). The hyperbaric chamber was then pressurized to 2.82 ATA over 5–10 min. The next series of trials (rest, exercise, and exercise with phentolamine) were performed at 2.82 ATA while breathing hyperoxic gas (100% O2; PiO2 ≈2,100 mmHg). Inspired gas (air and 100% O2) was supplied at ambient pressure via a tight-fitting, non-rebreathing, silicone oronasal mask connected to a demand regulator. The fit of the mask was adjusted by tightening the mask until no air leaks were apparent to the subject during inhalation against a closed valve.
The total time of the hyperbaric exposure was limited to 60 min to prevent the need for decompression for study personnel who breathed air throughout the study. Additionally, 2.82 ATA was selected for chamber pressurization on the basis of the time required (Fig. 1) to complete the exercise trials without the need for decompression for study personnel. The hyperbaric chamber was depressurized at a rate of 0.9 ATA/min while all personnel breathed 100% O2 with a 2-min safety (decompression) stop at 1.6 ATA. Following decompression a final exercise trial was performed during phentolamine administration while breathing air at 1 ATA (normoxic exercise with phentolamine).
Tyramine was administered for the final 3 min at rest and during exercise in all trials except for those performed with phentolamine (Fig. 2, A and B). Forearm vasoconstriction to endogenous norepinephrine was assessed at rest and during exercise under both normoxic and hyperoxic conditions. The dose of tyramine was calculated on the basis of forearm volume and blood flow obtained between minute 2 and minute 3 of rest and minute 3 and minute 4 of exercise, respectively. After completion of the normoxic rest and exercise trials, NTP was administered under normoxic conditions for 6 min. As with the rest and exercise trials, tyramine was administered for the final 3 min of NTP infusion and the tyramine dose was calculated on the basis of forearm volume and blood flow obtained between minute 2 and minute 3 of NTP infusion (Fig. 2C).
Fig. 2.
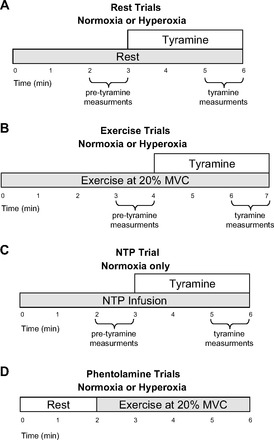
Schematic diagram of each experimental trial. Subjects completed resting (A) and exercise (B) trials under normoxic and hyperoxic conditions. A high-flow control trial using sodium nitroprusside (NTP) was performed under normoxic conditions (C). Tyramine was infused for the final 3 min of each of these trials. The phentolamine trials (D) were performed under hyperoxic and normoxic conditions and consisted of 2 min of rest followed by 4 min of exercise.
The phentolamine trials were performed under hyperoxic and normoxic conditions (Fig. 1). Each trial consisted of 2 min of rest followed by 4 min of exercise (Fig. 2D). The phentolamine trials were always performed last due to the drug's half-life.
Data analysis and statistics.
Data were collected at 250 Hz, stored on a computer, and analyzed off-line with signal processing software (WinDaq; DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was determined from the brachial artery pressure waveform and HR was determined from the electrocardiographic recordings. Vasoconstrictor effects caused by endogenous norepinephrine release were determined by averaging FBF values from the final 30 s of tyramine administration. Forearm vascular conductance (FVC) was calculated as (FBF/arterial pressure) × 100 and expressed as ml/min (100 mmHg). The percent reduction in FBF during tyramine infusion was calculated as:
This calculation was also used to determine the percent reduction in FVC during tyramine administration. FBF, arterial pressure, and HR during phentolamine trials were determined by averaging values from the second minute of rest and the fourth minute of exercise.
Repeated measures ANOVA were performed to assess differences in the vasoconstrictor effects of endogenous norepinephrine release at rest and during exercise between conditions (normoxia vs. hyperoxia). Hemodynamic variables were compared via repeated measures ANOVA to detect differences between responses during hyperoxia at rest and during exercise. Finally, repeated measures ANOVA was used to compare FBF and FVC during exercise with phentolamine under normoxic and hyperoxic conditions. Tukey's post hoc analysis determined where statistical differences occurred. All values are expressed as means ± SE. Statistical difference was set a priori at P < 0.05.
Estimated arterial oxygen content (CaO2) was calculated under each condition as:
where SaO2 is arterial saturation; PaO2 is the partial pressure of arterial oxygen, 1.36 is the oxygen capacity of hemoglobin; and 0.003 is the solubility of oxygen in plasma. A hemoglobin value of 14 g/dl was used across trials and is based on average values observed in young male subjects from our previous studies (4, 8, 9). PaO2 values were derived from the Alveolar Air Equation:
where FiO2 is the fraction of inspired air, PB is the barometric pressure, PH2O is the water vapor pressure in the airways (47 mmHg), PaCO2 is the partial pressure of arterial CO2 (estimated to be 40 mmHg), and 0.8 represents the assumed respiratory quotient. The average PB on the study days was 736 ± 2 mmHg (range 728–744) and this was used in the calculations of PaO2 for the normobaric trial. PB for the hyperbaric trials was consistent among all trials (2.82 ATA or 2,143 mmHg). The PaO2 was estimated from PaO2 based on a previously reported arterial-to-alveolar PO2 ratio (a/A ratio) of 0.9 (47). It is important to note that the a/A ratio did not vary between normobaric normoxia and various levels of hyperbaric hyperoxia (1.2–3 ATA) in that study.
RESULTS
Nine of the ten subjects completed the first aim (i.e., tyramine trials) of the study protocol. One subject did not complete either aim of the protocol due to ear pain associated with the pressurization of the chamber. Due to a national shortage of phentolamine mesylate, α-adrenergic blockade trials were not performed in one of the nine subjects. The nine subjects completing the first aim of the study were 28 ± 1 yr of age, 180 ± 2 cm in height, and weighed 82 ± 3 kg (body mass index, 25 ± 1 kg/m2).
Hemodynamic responses during normoxic and hyperoxic exercise.
HR and MAP responses during the exercise trials are presented in Table 1. HR did not differ at baseline (rest) between normoxia and hyperoxia and was unchanged during exercise under both conditions (P > 0.05 for both) for all exercise trials (tyramine and phentolamine). However, HR during exercise with concurrent administration of tyramine was greater than baseline during the normoxic trial and greater than baseline and exercise (without tyramine) during the hyperoxic trial. MAP at rest and during exercise was higher at 2.82 ATA than at 1 ATA. However, the increase in MAP (from rest to exercise) was similar between normoxic and hyperoxic conditions (P > 0.05). Infusion of tyramine increased exercising MAP during the normoxic and hyperoxic trials. Baseline and exercise MAP during the phentolamine did not differ between the normoxia and hyperoxia trials (P > 0.05 for both).
Table 1.
Hemodynamic responses during exercise
| Normoxia |
Hyperoxia |
|||||
|---|---|---|---|---|---|---|
| Baseline (rest) | Exercise | Exercise + Tyramine | Baseline (rest) | Exercise | Exercise + Tyramine | |
| Exercise trials with tyramine | ||||||
| HR, beats/min | 66 ± 2 | 68 ± 2 | 68 ± 1† | 65 ± 2 | 65 ± 2 | 67 ± 1‡ |
| MAP, mmHg | 92 ± 2 | 101 ± 2† | 105 ± 2‡ | 101 ± 2* | 109 ± 2*† | 113 ± 2*‡ |
| Exercise trials with phentolamine | ||||||
| HR, beats/min | 63 ± 2 | 65 ± 2† | 64 ± 2 | 65 ± 2 | ||
| MAP, mmHg | 101 ± 2 | 109 ± 3† | 101 ± 2 | 108 ± 2† | ||
Values are mean ± SE. HR, heart rate; MAP, mean arterial pressure.
P < 0.05 vs. normoxia;
P < 0.05 vs. baseline;
P < 0.05 vs. exercise.
Forearm blood flow and conductance during hyperoxia.
Baseline (resting) FBF and FVC were similar under normoxic and hyperoxic conditions. FBF and FVC were substantially lower (∼20%) during hyperoxic exercise than during normoxic exercise (P < 0.01; Fig. 3, A and B).
Fig. 3.
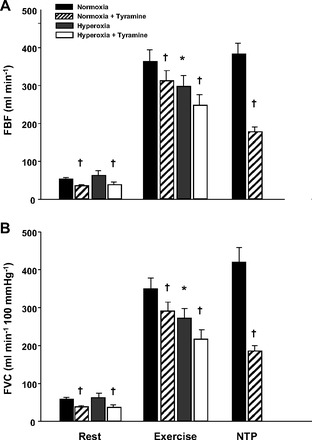
Effects of hyperbaric hyperoxia on forearm blood flow (FBF) (A) and forearm vascular conductance (FVC) (B) at rest and during exercise. Hyperoxia did not change resting FBF or FVC compared with normoxia. However, FBF and FVC during hyperoxic exercise were substantially reduced compared with normoxic exercise. FBF and FVC were lower with tyramine infusion at rest, during exercise (normoxia and hyperoxia), and during NTP infusion (normoxia only). *P < 0.01 vs. normoxia. †Main effect of tyramine, P < 0.01.
Forearm vasoconstrictor responses to endogenous norepinephrine release.
The absolute decrease in FBF to tyramine infusion was similar between normoxia and hyperoxia at rest (−18 ± 2 vs. −25 ± 7 ml/min; P = 0.37) and during exercise (−50 ± 12 vs. −50 ± 10 ml/min; P = 0.97; Fig. 3A). Similarly, the absolute decrease in FVC to tyramine infusion was similar between normoxia and hyperoxia at rest [−20 ± 3 vs. −25 ± 7 ml/min (100 mmHg); P = 0.52] and during exercise [−59 ± 11 vs. −56 ± 6 ml/min (100 mmHg); P = 0.70; Fig. 3B]. As shown in Fig. 4, the vasoconstrictor responsiveness to tyramine, expressed as a percent decrease in FBF and FVC, was blunted during exercise compared with rest during both normoxia and hyperoxia (P < 0.01). The vasoconstrictor responsiveness (percent change) did not differ between normoxia and hyperoxia at rest (P = 0.85 for FBF and 0.83 for FVC). However, the vasoconstrictor responsiveness was slightly greater during hyperoxic than during normoxic exercise (P < 0.05; Fig. 4, A and B). The absolute and relative reductions in FBF and FVC to tyramine infusion were substantially greater during NTP (i.e., high-flow control trial) than during both normoxic and hyperoxic exercise trials (Figs. 3 and 4). This demonstrated that increasing blood flow under resting conditions via an exogenous vasodilator does not blunt vasoconstrictor responsiveness and is consistent with previous findings (14, 50).
Fig. 4.
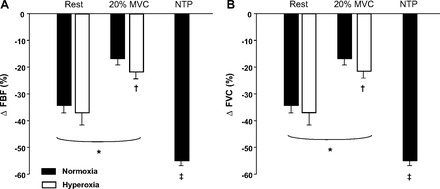
Effects of hyperoxia, hyperoxic exercise, and NTP infusion on vasoconstrictor responsiveness to endogenous norepinephrine release via tyramine. The change (Δ) in forearm blood flow (FBF) (A) and forearm vascular conductance (FVC) (B) (% decrease) was blunted with exercise compared with rest (normoxia and hyperoxia). The Δ in FBF and FVC was slightly greater during hyperoxic exercise than during normoxic exercise. The Δ in FBF and FVC during NTP infusion was greater than both normoxic and hyperoxic exercise. *P < 0.01 vs. rest. †P < 0.05 vs. normoxic exercise. ‡P <0.01 vs. normoxic and hyperoxic exercise.
Influence of α-adrenergic blockade on FBF and FBV during normoxic and hyperoxic exercise.
Infusion of the nonspecific α-adrenergic antagonist phentolamine increased FBF and FVC approximately threefold over values obtained at rest during nonphentolamine trials (P < 0.001). However, baseline FBF and FVC values were not different between normoxia and hyperoxia during phentolamine administration (P > 0.05 for both; Fig. 5, A and B). Of particular interest to the current study, FBF and FVC were substantially lower during hyperoxic than during normoxic exercise in the phentolamine trials (P < 0.01; Fig. 5, A and B).
Fig. 5.
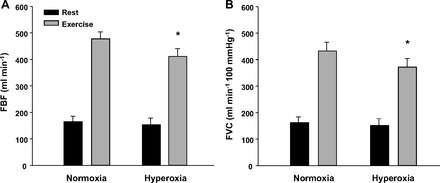
Effects of α-adrenergic blockade via phentolamine infusion on forearm blood flow (FBF) (A) and forearm vascular conductance (FVC) (B) at rest and during exercise under normoxic and hyperoxic conditions (n = 8). FBF and FVC were similar between normoxia and hyperoxia at rest; however, FBF and FVC were blunted during hyperoxic compared with normoxic exercise. *P < 0.01 vs. normoxic exercise.
Estimated arterial oxygen content.
The estimated PaO2 during the hyperbaric hyperoxia trials (1,773 mmHg) was substantially greater than during the normobaric normoxia trials (87 mmHg). The PaO2 during hyperbaric hyperoxia would result in a ∼25% increase in the estimated CaO2 compared with the normobaric normoxia trials (24.4 vs. 18.9 ml O2/100 ml).
DISCUSSION
In the present study we sought to determine the role of sympathetic vasoconstriction in the reduced blood flow observed during hyperoxic exercise. We directly assessed whether functional sympatholysis was attenuated in contracting skeletal muscle under hyperoxic conditions. When examining the vasoconstrictor responsiveness as an absolute change it appears that the magnitude of functional sympatholysis is similar during hyperoxic and normoxic exercise. However, expressing the vasoconstrictor responsiveness as a relative (%) change revealed slight, yet significant differences during exercise between normoxic and hyperoxic conditions (Fig. 4). That is, functional sympatholysis was attenuated slightly under conditions of elevated O2 availability. Controversy exists over the appropriate way to express vasoconstrictor responsiveness (absolute vs. relative), especially when blood flow varies between conditions prior to the vasoconstrictor stimulus. In the present study, the exercise FBF and FVC prior to tyramine infusion were significantly greater during normoxic conditions compared with hyperoxic conditions. It should be noted that the majority of studies using similar approaches have relied on the relative changes to quantify functional sympatholysis.
Therefore, using relative changes to quantify vasoconstrictor responsiveness in the current study might suggest that increases in O2 availability via hyperbaric hyperoxia contribute in part to the attenuated exercise blood flow and vasodilation. However, the degree to which a blunted functional sympatholysis contributes to substantial reductions in flow during hyperoxic exercise is likely minimal; that is, the small yet significant difference in relative vasoconstrictor responsiveness between normoxic and hyperoxic exercise (∼5%) likely explains only a portion of the large reductions in blood flow and vasodilation (∼20–25%) in contracting muscle under hyperbaric hyperoxic conditions observed in the current study and previously published work (7). Moreover, removal of α-adrenergic vasoconstriction did not increase hyperoxic exercise blood flow to similar levels of normoxic exercise in the current study. If functional sympatholysis during hyperoxic exercise is a major cause of reduced exercise hyperemia during hyperbaric hyperoxia, then, α-adrenergic blockade should have restored blood flow to levels similar to those observed during normoxic exercise.
Despite evidence for reduced muscle sympathetic nerve activity in response to acute exposure to hyperoxia at rest in humans (26, 43, 53), it may be possible that sympathetic responses are greater during hyperoxic exercise than during normoxic exercise. To our knowledge, only two studies have directly examined the impact of hyperoxia on muscle sympathetic activity during exercise. Seals and colleagues (43) demonstrated that hyperoxia does not appear to enhance the magnitude of change in sympathetic nerve activity to nonactive skeletal muscle during dynamic exercise. Conversely, others have demonstrated enhanced metaboreflex sensitivity during hyperoxic isometric forearm exercise, which results in a greater sympathetic reactivity (26). These findings are in agreement with data in experimental animals in which hyperoxia increases the activation of sensory endings in skeletal muscle and enhances the discharge of group IV muscle afferents (1).
Although these studies may suggest an enhanced sympathetic outflow to the exercising muscle during hyperoxia, the magnitude of vasoconstriction and influence on blood flow were not determined. Our current data from the phentolamine trials demonstrate that α-adrenergic–mediated vasoconstriction does not restrict blood flow to a greater extent during exercise under hyperoxic conditions. This is evidenced by the fact that α-adrenergic blockade did not restore FBF and FVC to levels observed during normoxic exercise (Fig. 5).
Potential causes for hyperoxia-induced vasoconstriction during exercise.
The increase in vascular resistance under hyperoxic exercise conditions may be a direct vasoconstrictor action of O2 on the arterial and arteriolar wall. Along these lines, some evidence suggests that the vascular response (i.e., constriction) to hyperoxia in the hind limbs of dogs is not mediated by the autonomic nervous system but rather by direct action of high arterial O2 tension (3). Additionally, an enhanced release of the potent vasoconstrictor endothelin-1 (ET-1) has been observed from cultured endothelial cells in vitro when exposed to hyperoxia (25). Moreover, hyperoxia-induced increases in ET-1 are associated with decreased blood flow in the cerebral and retinal circulations (2, 12). Important to the present study, Wray and colleagues (52) demonstrated a significant metabolic attenuation of ET-1–mediated vasoconstriction during dynamic leg exercise under normoxic conditions. Whether the magnitude of ET-1 vasoconstriction is altered in contracting muscle under hyperoxic conditions is unknown.
A decreased release or responsiveness to local vasodilators [i.e., nitric oxide (NO), prostaglandins, or adenosine] within the contracting muscle under hyperoxic conditions could also promote a shift in the vasoconstrictor/vasodilator balance and consequently alter vascular tone. In this context, prostaglandin-mediated vasodilation following isometric exercise is reduced during hyperoxia (51). A decreased synthesis of vasodilator prostaglandins has also been suggested as a contributor to hyperoxia-induced skin vasoconstriction in humans (40, 54).
We have previously demonstrated that NO-mediated mechanisms contribute to the compensatory vasodilation in skeletal muscle during incremental hypoxic exercise (8). Whether elevated levels of O2 alter NO bioavailability and consequently contribute to the vasoconstriction in contracting muscle during hyperoxic exercise has not been directly assessed. Recent evidence suggests that NO metabolites are unaffected in humans during normobaric hyperoxic exercise (16). However, hyperoxic-induced vasoconstriction in human skin is partly due to the decreased activity of functional NO synthase (NOS) (54). Additionally, vasoconstriction via a reduction in basal NO production has been observed in porcine coronary arteries exposed to elevated levels of O2 (34). Hyperoxia has also been associated with increased production of reactive oxygen species (ROS) (28). Theoretically, increases in ROS may reduce NO bioavailability directly by affecting NOS or through scavenging of NO. In this context, an increase in free radical production (i.e., superoxide anions) during hyperbaric oxygenation causes vasoconstriction in the cerebral circulation of rats via inactivation of NO (56). Interestingly, elevated ROS can also disrupt the normal NO-dependent attenuation of sympathetic vasoconstriction (55). Moreover, administration of vitamin C reverses hyperoxic vasoconstriction in the human forearm (31) and coronary (33) circulation under resting conditions.
Finally, accumulating evidence suggests that erythrocytes modulate vascular tone in response to changes in oxygen (via release of ATP, NO, or both), thus leading to appropriate changes in blood flow and matching oxygen delivery with metabolic need (18). Under conditions of hemoglobin desaturation and mechanical deformation ATP is released from erythrocytes and is believed to contribute to the augmented blood flow during hypoxic exercise (27, 44, 45). Therefore, an attenuated release of ATP in response to large increases in oxygen may have blunted the blood flow response to exercise via these or related mechanisms.
Experimental considerations.
In the present study, tyramine was infused to elicit norepinephrine release from sympathetic nerve terminals during normoxia and hyperoxia to examine postjunctional responsiveness of α-adrenergic receptor–mediated vasoconstriction during hyperoxic exercise. We took careful measures to normalize the concentration of tyramine in the blood perfusing the experimental forearm between trials by adjusting the infusions on the basis of FBF and forearm volume (15, 39, 50). With this approach we cannot exclude the possibility that the attenuated FBF and FVC observed during hyperoxic exercise is due to an enhanced sympathetic outflow (26), or to an enhanced norepinephrine release from sympathetic nerve terminals, or both. However, Seals et al. (43) demonstrated similar increases in muscle sympathetic nerve activity and norepinephrine during normobaric hyperoxic exercise compared with normoxic exercise. Measurements of plasma norepinephrine were not performed in any of the trials in the current study and it is unclear whether tyramine-induced norepinephrine release was affected differentially between O2 conditions. However, we have previously demonstrated that tyramine-evoked norepinephrine release was similar between normoxic and hypoxic exercise (50).
The findings of the present study are limited to data derived from young men. There is strong evidence that sex-specific differences exist in neurovascular control at rest (22, 24, 29) and potentially during exercise (35) in young adults. Therefore, it is possible that the influence of α-adrenergic vasoconstriction during hyperoxic exercise may be different between young men and women. However, recent data demonstrate that the degree of functional sympatholysis of α1- and α2-adrenergic–mediated vasoconstriction is similar in young men and women (30).
Similar to our previous hyperbaric study (7), we were unable to directly measure arterial and venous blood gases due to maximal PO2 limits (999 mmHg) of our commercially available arterial blood gas analyzers. Additionally, the blood gas analyzers are not able to be used in the hyperbaric chamber, and blood samples obtained under pressure are subject to supersaturation and bubbling of O2 upon decompression for analysis, which can result in significant reading errors (10). Therefore, our estimated increase (∼25%) in arterial oxygen content during hyperoxia is based on calculated values of arterial PO2 as described in Methods. The estimated oxygen tensions from the current study are similar to those previously reported using direct measurements of PaO2 (i.e., micro-oxygen electrode or arterial blood sample) in subjects exposed to hyperbaric oxygen (11, 32, 47, 48). Additionally, we were not able to calculate or determine whether oxygen consumption in the active forearm was different between trials. However, previous studies have demonstrated that oxygen consumption of an active limb is not different between normoxic and hyperoxic conditions (37, 49).
Conclusions.
This study demonstrates that functional sympatholysis is impaired slightly in contracting muscles under conditions of increased O2 availability. However, when considered in the context of the substantial reductions in skeletal muscle blood flow and vasodilation (∼20–25%) observed during hyperbaric hyperoxic exercise, the contribution of the exaggerated vasoconstrictor responsiveness (∼5% difference compared with normoxia) does not appear to be a major contributor. This idea is further supported by the fact that muscle blood flow remains substantially reduced (∼15%) during hyperoxic exercise compared with normoxic exercise with α-adrenergic blockade. Identifying other potential mechanisms and determining how they interact during hyperoxic exercise warrants further study.
GRANTS
This research was supported by National Institutes of Health Grants HL-46493 to M.J.J., HL-105467 to D.P.C., and by Mayo Clinic Center for Translational Science Activities Grant UL1 TR000135. The Mayo Clinic Department of Anesthesiology and Division of Preventive, Occupational, and Aerospace Medicine and the Caywood Professorship via the Mayo Foundation also supported this research.
ACKNOWLEDGMENTS
The authors are grateful to the study volunteers for their participation. We also thank Branton Walker, Essa Mohamed, Christopher Johnson, Nancy Meyer, Maureen Bigelow, William Fuqua, Jamie Campos, Jon Balgeman, Joseph Halleland, and the Hyperbaric & Altitude Medicine staff for their technical assistance.
REFERENCES
- 1. Arbogast S, Vassilakopoulos T, Darques JL, Duvauchelle JB, Jammes Y. Influence of oxygen supply on activation of group IV muscle afferents after low-frequency muscle stimulation. Muscle Nerve 23: 1187–1193, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Armstead WM. Endothelin-1 contributes to normocapnic hyperoxic pial artery vasoconstriction. Brain Res 842: 252–255, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Bachofen M, Gage A, Bachofen H. Vascular response to changes in blood oxygen tension under various blood flow rates. Am J Physiol 220: 1786–1792, 1971 [DOI] [PubMed] [Google Scholar]
- 4. Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide–mediated vasodilation becomes independent of β-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol 110: 687–694, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey DP, Joyner MJ. α-Adrenergic blockade unmasks a greater compensatory vasodilation in hypoperfused contracting muscle. Front Physiol 3: 271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol 111: 1527–1538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casey DP, Joyner MJ, Claus PL, Curry TB. Hyperbaric hyperoxia reduces exercising forearm blood flow in humans. Am J Physiol Heart Circ Physiol 300: H1892–H1897, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol 107: 1128–1137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherry AD, Forkner IF, Frederick HJ, Natoli MJ, Schinazi EA, Longphre JP, Conard JL, White WD, Freiberger JJ, Stolp BW, Pollock NW, Doar PO, Boso AE, Alford EL, Walker AJ, Ma AC, Rhodes MA, Moon RE. Predictors of increased PaCO2 during immersed prone exercise at 4.7 ATA. J Appl Physiol 106: 316–325, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Clark JM, Lambertsen CJ. Alveolar-arterial O2 differences in man at 0.2, 10, 20, and 35 ATA inspired PO2. J Appl Physiol 30: 753–763, 1971 [DOI] [PubMed] [Google Scholar]
- 12. Dallinger S, Dorner GT, Wenzel R, Graselli U, Findl O, Eichler HG, Wolzt M, Schmetterer L. Endothelin-1 contributes to hyperoxia-induced vasoconstriction in the human retina. Invest Ophthalmol Vis Sci 41: 864–869, 2000 [PubMed] [Google Scholar]
- 13. Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol 540: 1103–1110, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, González-Alonso J. Erythrocyte-dependent regulation of human skeletal muscle blood flow: role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin, and ATP. Am J Physiol Heart Circ Physiol 299: H1936–H1946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eklund B, Kaijser L. Effect of regional alpha- and beta-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol 262: 39–50, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frewin DB, Whelan RF. The mechanism of action of tyramine on the blood vessels of the forearm in man. Br J Pharmacol Chemother 33: 105–116, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 21. González-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hachiya T, Hashimoto I, Saito M, Blaber AP. Peripheral vascular responses of men and women to LBNP. Aviat Space Environ Med 83: 118–124, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol 527 Pt 2: 387–396, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol 589: 5285–5297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins RD, Hendricks-Munoz KD, Caines VV, Gerrets RP, Rifkin DB. Hyperoxia stimulates endothelin-1 secretion from endothelial cells; modulation by captopril and nifedipine. Curr Eye Res 17: 487–493, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Houssière A, Najem B, Cuylits N, Cuypers S, Naeije R, van de Borne P. Hyperoxia enhances metaboreflex sensitivity during static exercise in humans. Am J Physiol Heart Circ Physiol 291: H210–H215, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Jamieson D. Oxygen toxicity and reactive oxygen metabolites in mammals. Free Radic Biol Med 7: 87–108, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol 109: 1360–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol 282: H2414–H2421, 2002 [DOI] [PubMed] [Google Scholar]
- 32. McDowall DG, Ledingham IM, Tindal S. Alveolar-arterial gradients for oxygen at 1, 2, and 3 atmospheres absolute. J Appl Physiol 24: 324–329, 1968 [DOI] [PubMed] [Google Scholar]
- 33. McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, Sinoway LI. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol 102: 2040–2045, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Pasgaard T, Stankevicius E, J̸orgensen MM, Ostergaard L, Simonsen U, Fr̸obert O. Hyperoxia reduces basal release of nitric oxide and contracts porcine coronary arteries. Acta Physiol (Oxf) 191: 285–296, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962 [DOI] [PubMed] [Google Scholar]
- 37. Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent V̇o2 max in the exercise-trained human quadriceps. J Appl Physiol 86: 1048–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. alpha1- and alpha2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol 547: 971–976, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rousseau A, Tesselaar E, Henricson J, Sjöberg F. Prostaglandins and radical oxygen species are involved in microvascular effects of hyperoxia. J Vasc Res 47: 441–450, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986 [DOI] [PubMed] [Google Scholar]
- 42. Rowell LB, Blackmon JR. Human cardiovascular adjustments to acute hypoxaemia. Clin Physiol 7: 349–376, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Seals DR, Johnson DG, Fregosi RF. Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol Regul Integr Comp Physiol 260: R873–R878, 1991 [DOI] [PubMed] [Google Scholar]
- 44. Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 97: 731–738, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Weaver LK, Howe S. Normobaric measurement of arterial oxygen tension in subjects exposed to hyperbaric oxygen. Chest 102: 1175–1181, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Weaver LK, Howe S, Snow GL, Deru K. Arterial and pulmonary arterial hemodynamics and oxygen delivery/extraction in normal humans exposed to hyperbaric air and oxygen. J Appl Physiol 107: 336–345, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977 [DOI] [PubMed] [Google Scholar]
- 50. Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Win TS, Marshall JM. Contribution of prostaglandins to the dilation that follows isometric forearm contraction in human subjects: effects of aspirin and hyperoxia. J Appl Physiol 99: 45–52, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Yamauchi K, Tsutsui Y, Endo Y, Sagawa S, Yamazaki F, Shiraki K. Sympathetic nervous and hemodynamic responses to lower body negative pressure in hyperbaria in men. Am J Physiol Regul Integr Comp Physiol 282: R38–R45, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Yamazaki F, Takahara K, Sone R, Johnson JM. Influence of hyperoxia on skin vasomotor control in normothermic and heat-stressed humans. J Appl Physiol 103: 2026–2033, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W, Thomas GD. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension 48: 637–643, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Zhilyaev SY, Moskvin AN, Platonova TF, Gutsaeva DR, Churilina IV, Demchenko IT. Hyperoxic vasoconstriction in the brain is mediated by inactivation of nitric oxide by superoxide anions. Neurosci Behav Physiol 33: 783–787, 2003 [DOI] [PubMed] [Google Scholar]


