Abstract
In the developing visual system of mammals, retinal axons from the two eyes compete for postsynaptic partners. After eye opening, this process is regulated in part by homeostatically constrained competition for synaptic connectivity with target neurons. However, prior to eye opening, the functional and synaptic basis of binocular map development is unclear. To examine the role of binocular interactions during early stages of visual map development, we performed in vitro patch-clamp recordings from the superior colliculus (SC) of neonatal mice. Using newly designed slice preparations, we compared retinocollicular synapse development in the medial SC, which receives binocular input, and the lateral SC, which is predominantly monocular. Surprisingly, we found that at P6–7, when eye-specific segregation has just emerged, retinocollicular synapses were stronger and more mature and dendritic arbors were more elaborate in the medial than the lateral SC. Furthermore, monocular enucleation of the ipsilateral eye at P0 selectively reduced synaptic strength and dendritic branching in the medial SC and abolished the differences normally observed between the two slices at P6–7. This specifically implicates binocular interactions in the development of retinocollicular connectivity prior to eye opening. Our findings contrast with the predictions of a constrained-connectivity model of binocular map development and suggest instead that binocular competition prior to eye opening enhances retinocollicular synaptic strength and the morphological development of retino-recipient neurons.
Keywords: visual map development, binocular competition, superior colliculus, monocular enucleation, morphological development
the brain organizes sensory information in maps. In the mammalian visual system, for instance, retinal ganglion cells (RGCs) extend their axons to the lateral geniculate nucleus (LGN) and the superior colliculus (SC), where they form spatially organized topographic representations of visual space. In addition, inputs from the two eyes terminate in spatially aligned, eye-specific domains of the LGN and SC to support binocular vision.
The emergence of this binocular organization is mediated, in part, through competition between retinal axons originating from the two eyes (Angelucci et al. 1997; Chalupa and Williams 1984; Colonnese and Constantine-Paton 2001; Crair et al. 1998; Koch et al. 2011; Rakic 1981; Shook and Chalupa 1986; Wiesel 1982). The mechanisms underlying binocular competition after eye opening, when the visual system is amenable to sensory manipulations, have been extensively studied (Katz and Crowley 2002). This competition involves homeostatic mechanisms that preserve the net drive to cortical neurons (Desai et al. 2002; Frenkel and Bear 2004; Kaneko et al. 2008; Mrsic-Flogel et al. 2007) and is mediated in part by TNF-α signaling, a molecular pathway involved in synaptic scaling (Kaneko et al. 2008; Stellwagen and Malenka 2006). Prior to eye opening, activity-mediated binocular competition supports segregation of retinal inputs into eye-specific domains and complements molecular factors involved in this process (Huberman 2007; Leamey et al. 2009). Retinal activity in these prevision stages is derived from spontaneously generated “retinal waves” (Wong 1999). Because waves are presumably generated independently in each eye, they could provide the pattern of activity necessary for supporting eye-specific segregation through binocular competition even prior to eye opening. Manipulations of relative activity levels between the two eyes, and more recently of neurotransmitter release from RGC axons, indicate that inputs from the two eyes compete for space in the target brain areas (Koch et al. 2011; Penn et al. 1998; Stellwagen and Shatz 2002). However, these anatomical studies were based on bulk-labeling of RGC axons, and therefore the synaptic and functional basis of binocular competition prior to eye opening remains unclear. Despite the generally presumed role of homeostatically constrained competition in this process, it is in fact unknown whether axons from the two eyes compete through a “capped” amount of connectivity to their target neurons or, alternatively, whether binocular interactions reduce or potentially even enhance the overall retinal synaptic input to postsynaptic neurons in the target. Interestingly, computational models of homeostatically constrained competition are very sensitive to modeling details, such as the specific form of the synaptic normalization rule or plasticity rule used (Elliott 2003; Miller and MacKay 1994), suggesting that this mode of competition is perhaps not the optimal strategy for early stages of visual map formation (see discussion).
We focus here on visual map development in the mouse SC because of the relatively precocious maturation of this map and its simple spatial organization (Godement et al. 1984; Grantyn et al. 2004; Mize and Salt 2004; Simon and O'Leary 1992; Wu et al. 2000). Retinal inputs from both eyes are present in the SC at birth, and by the time of eye opening [around postnatal day (P)14] the anatomical development of the visual map is virtually complete (Dhande et al. 2011; Godement et al. 1984). Initially, the ipsi- and contralateral inputs overlap, and subsequently, during the first and second postnatal weeks, the inputs segregate into eye-specific layers (SO and SGS, respectively). As this eye-specific segregation occurs, the ipsilateral retinal projection becomes progressively more restricted to the antero-medial crescent of the SC. Thus the effects of binocular competition are likely to be most prominent in the antero-medial portion of the SC during the first postnatal week.
Recent work from our lab shows that genetically altering retinal waves during the first postnatal week selectively impairs, at the anatomical level, eye segregation and retinotopic refinement in the antero-medial (“binocular”) portion of the SC (Xu et al. 2011). This impairment clearly depends on binocular interactions, because it is abolished by ipsilateral monocular enucleation at birth. This raises the intriguing possibility that map development differs between the binocular and monocular regions of the SC at the synaptic and functional level even in wild-type mice. To our knowledge, this has never been directly examined, likely because such regional differences within the SC are unexpected in a constrained-competition model of binocular competition.
Here we asked whether synapse maturation differs, during normal development, between the binocular and monocular SC. Moreover, if differences exist, do they stem from interactions between inputs from the two eyes? To examine these questions, we designed two new slice preparations that allowed us to record selectively from the medial (binocular) and lateral (mainly monocular) regions of the SC. We then performed patch-clamp recordings in the SC of both normally raised mice and mice that lack binocular competition through monocular enucleation at birth. Surprisingly, we found that by the end of the first postnatal week retinocollicular synapses were stronger and more mature and dendritic arbors were more elaborate in the medial than the lateral SC of normally raised mice. Ipsilateral monocular enucleation selectively affected the medial, normally binocular SC and abolished differences between the medial and lateral SC at P6–7. Thus, in contrast to the mechanisms operating after eye opening, binocular competition during early stages of map formation appears to enhance the overall connectivity between the eyes and their target neurons in the brain.
MATERIALS AND METHODS
Slice electrophysiology.
All procedures were reviewed and approved by the Yale Institutional Animal Care and Use Committee (IACUC) and carried out in compliance with US Department of Health and Human Services and institution guidelines. C57BL/6J mice pups of either sex were anesthetized on ice (P3–4) or through isoflurane inhalation (P6–P21) and decapitated. The brain was quickly removed and transferred to ice-cold (4°C) sucrose-based cutting solution containing (in mM) 190 sucrose, 25 glucose, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 CaCl, 5 MgCl, 4 Na-pyruvate, and 0.6 Na-ascorbate and saturated in 95% O2-5% CO2. Brains were mounted and sectioned into 350-μm-thick slices with a Leica VT1200 microtome (Leica Microsystems). Medial (“binocular”) slices were cut parasagittally with a 20° tilt toward the coronal plane. Lateral slices (“monocular”) were cut 40° from the parasagittal toward the horizontal plane. After sectioning, slices were transferred to room-temperature bubbled artificial cerebrospinal fluid (ACSF) and allowed 30- to 60-min recovery before recording. Experiments were performed at room temperature with bubbled ACSF perfused at a rate of 2–3 ml/min. ACSF contained (in mM) 124 NaCl, 5 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 2 CaCl2, 26 NaHCO3, and 11 glucose, pH 7.2, 290–310 mosM. In all experiments, bath ACSF contained 10 μM bicuculline (Tocris, Ellisville, MO) to eliminate GABA receptor-mediated currents. Only one cell per slice and one slice per animal were used.
Whole cell recording electrodes (2–5 MΩ) were pulled with a Sutter P-97 puller (Sutter Instruments). Internal solution contained (in mM) 100 cesium gluconate, 17.5 CsCl, 8 NaCl, 10 HEPES, 0.2 EGTA, 4 Mg-ATP, 0.3 GTP, 7 phosphocreatine, and 10 BAPTA, pH 7.2–7.5, 290–310 mosM. Whole cell voltage-clamp recordings were performed on cells in the SGS. Evoked responses were considered monosynaptic if they exhibited short and constant latency (3–5 ms).
Stimuli (one to five 0.1-ms-long pulses of 30–150 μA at 0.1-ms interstimulus interval) were delivered every 10–15 s through bipolar stimulating electrodes made from a pair of stainless steel or tungsten electrodes (FHC, Bowdoin, ME). The evoked responses in our recordings, as in previous studies using similar protocols (Hestrin 1992; Lee et al. 2001; Shi et al. 1997), are predominantly of retinal origin, for the following reasons. First, we rarely observed evoked responses unless the stimulating electrodes were placed in the optic tract and their tips oriented in parallel to retinal fibers. Second, at the ages examined, input to the SGS is dominated by retinal afferents. Cortico-collicular axons invade the SC only toward the end of the first postnatal week, and even by P14 the input from the visual cortex is quite immature (Phillips et al. 2011; Thong and Dreher 1986; Triplett et al. 2009). Nevertheless, some contribution from axons that do not originate in the retina cannot be completely ruled out.
Data were collected and analyzed with custom programs written in IgorPro (WaveMetrics, Lake Oswego, OR) and MATLAB (MathWorks). Input and series resistances were measured continuously to monitor the health of the cell, and data were discarded from analysis if these parameters drifted >20% over the course of the experiment.
Monocular enucleations.
P0 mice were anesthetized on ice. After the eye lid and the conjunctiva were cut, the eyeball was displaced from the socket, the extraocular muscles were sectioned, and the eyeball was removed. Antibiotic ointment was applied to the eye.
AMPA-to-NMDA ratios.
After a stable monosynaptic evoked AMPA response was recorded at a holding potential of −70 mV, AMPA receptors were blocked with 10 μM NBQX (Tocris), and the holding potential was switched to 40 mV to record NMDA receptor-mediated currents at the same stimulation strength. AMPA-to-NMDA ratios were computed based on the average peak current amplitudes of 10–20 sweeps at each holding potential.
Strontium evoked AMPA miniature events.
Stable monosynaptic evoked AMPA responses were recorded at a holding potential of −70 mV. Extracellular Ca2+ (2 mM) in the bath solution was then replaced with 3 mM Sr2+ to desynchronize synaptic vesicle release (Xu-Friedman and Regehr 1999). Evoked miniature events (AMPA-minis) were recorded in 1-s epochs every 10 s. Miniature events were analyzed off-line with Mini Analysis software (Synaptosoft, Decatur, GA). Amplitude thresholds were set at 2.5 times root mean square noise, and at least 50 events were used for analysis in each cell. Amplitude frequency histograms were generated with 4-pA bins and were normalized to the number of events in each experiment. Cumulative probability distributions were generated by integrating over the normalized frequency histograms.
Graded stimulation experiments.
Evoked AMPA responses were recorded at a holding potential of −70 mV. AMPA receptors were then blocked with 10 μM NBQX (Tocris), and the holding potential was switched to 40 mV to record NMDA receptor-mediated currents. Stimulation strength was adjusted until a mixture of subthreshold (failures) and suprathreshold (successes) responses were observed. Stimulation strength was then gradually increased to recruit more inputs until a saturating current response was achieved. Because response amplitudes at minimal stimulation strength were generally small, particularly in the lateral slice, we adopted an unbiased classification scheme to objectively classify responses into failures and successes. Specifically, we pooled all responses from stimulation strengths that yielded any nonresponse trials and defined success responses as those that peaked above 6 pA. The success (or single fiber) responses were then averaged, as were responses measured at the saturating stimulation strength. The number of inputs was estimated as the mean saturating responses divided by the mean single-fiber response.
Morphological reconstructions.
For cell staining, 60 μM Alexa Fluor 594 hydrazide was included in the internal solution. After recordings, slices were fixed in 4% paraformaldehyde (PFA), mounted on slides, and imaged with a Zeiss AxioImager Z2 microscope (Carl Zeiss). Reconstructions were carried out with Neurolucida and NeuroExplorer (MBF Bioscience). Fractal dimension was calculated with the box counting method.
Whole eye fill and retinal input analysis.
P5 mice were anesthetized on ice, and 1 μl of fluorescently labeled cholera toxin subunit B (CTB; Invitrogen) conjugated with Alexa 488/555 was injected with Nanoject (Drummond Scientific, Broomall, PA) into the vitreous of the right and left eyes, respectively. At P7, slices were prepared as described above (see Slice electrophysiology) and imaged with a Zeiss AxioImager Z2 microscope (Carl Zeiss). Images were background subtracted (based on a nonlabeled tissue region). The SC was divided into five segments along the antero-posterior axis, and for each segment the cumulative distribution of intensities in the ipsilateral eye channel was calculated. The maximum intensity was defined as the intensity value at 98% of the cumulative distribution. The quality of the ipsilateral whole eye fill, used for quantification, was verified by 1) robust and uniform labeling of the SGS in a slice containing the SC contralateral to that used for recordings and 2) robust labeling of pretectal nuclei.
Statistical analysis.
Data are presented as means ± SE. Differences in means were tested for significance as follows, unless otherwise indicated. Effects of age were tested with one-way ANOVA followed by pairwise comparisons using Tukey's honestly significant difference (HSD) criterion for multiple comparisons. Effect of slice was tested with Student's t-test with false discovery rate (FDR) procedure for multiple comparisons (Curran-Everett 2000; Curran-Everett and Benos 2004). Effect of enucleation on the medial/lateral slices at P6–7 was tested with two-way ANOVA with pairwise adjusted post hoc tests. Statistical analysis was done in MATLAB (MathWorks) with the statistics toolbox.
RESULTS
We performed patch-clamp recordings from neurons in the SC of neonatal mice to study the synaptic basis of visual map development in this brain area. The antero-medial crescent of the SC represents the binocular visual field and accordingly receives input from both eyes, whereas the lateral/posterior SC is dominated by monocular, contralateral retinal input (Hofbauer and Dräger 1985). To record selectively from the binocular or mainly monocular region of the SC, we developed two slice preparations (“medial” and “lateral”) that differ in their cutting orientations but preserve similar circuitry along the retinocollicular pathway (Fig. 1, A–C). To design the slices, we labeled retinal axons with whole eye injections of fluorescent tracer dyes and optimized cutting orientations in three dimensions that 1) target the desired parts of the SC and 2) minimize damage to retinal fibers and SC circuitry during slicing by cutting perpendicular to the surface of the SC. In the medial slice, the ipsilateral retinal input was substantially stronger and larger in area and extended more posteriorly than in the lateral slice (Fig. 1E; see Table 2), confirming the expected difference between the two slices based on their anatomical location.
Fig. 1.
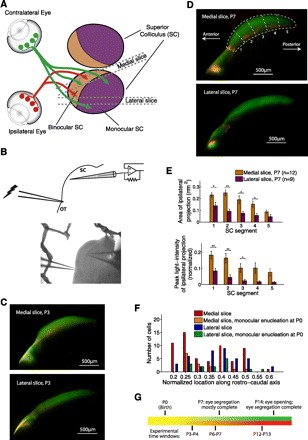
Brain slices for electrophysiological recordings from the medial and lateral superior colliculus (SC). A: schematic of retinal inputs to the mouse SC (dorsal view). The antero-medial crescent of the SC receives binocular retinal input, whereas the posterior-lateral SC receives input predominantly from the contralateral eye. We designed 2 brain slice preparations for electrophysiological recordings from the medial and lateral SC by optimizing cutting orientations in 3 dimensions, both slices cut approximately perpendicular to the surface of the hill-shaped SC to minimize damage to retinal fibers and to SC neurons during slicing (see materials and methods for cutting angles and further details). B, top: recording configuration: in both slices, we measured retinocollicular synaptic currents by performing patch-clamp recordings from neurons in the SGS layer of the SC while electrically stimulating retinal fibers passing through optic tract (OT). Bottom: DIC image of a postnatal day (P)7 medial slice. C: examples of medial (top) and lateral (bottom) slices prepared at P3 after whole-eye injections of tracer dyes at P1 (red/green for ipsilateral/contralateral eyes, respectively; see materials and methods). At this age, ipsi- and contralateral inputs are mixed in the SGS layer of the antero-medial SC. D: examples of medial and lateral slices prepared at P7 after whole eye injections of tracer dyes at P5. We divided the SC into 5 segments along the antero-posterior axis. All electrophysiological recordings were confined to segments 2 and 3 of the SC (20–60% along the antero-posterior axis; see F for distribution of recording locations). E: quantification of ipsilateral retinal input in the 2 slices at P7. Ipsilateral input was stronger, covered a larger area, and extended more posteriorly in the medial than the lateral slice, in accord with the anatomical location of the slices. Two-tailed Student's t-test with false discovery rate procedure for multiple-comparisons (data in this and following figures presented as means ± SE): *P < 0.05, **P < 0.01. F: recording locations along the antero-posterior axis. Mean recording locations for the medial slice (nonenucleated mice), medial slice after monocular enucleation at P0, lateral slice (nonenucleated mice), and lateral slice after monocular enucleation at P0 were 0.335 ± 0.015 (n = 59), 0.307 ± 0.021 (n = 14), 0.376 ± 0.016 (n = 40), and 0.363 ± 0.02 (n = 15), respectively. Recording locations did not differ significantly among the 4 groups [F(3,124) = 2.15, P = 0.098, 1-way ANOVA]. G: time line of eye-specific segregation in the SC and experimental time windows.
Table 2.
Ipsilateral retinal input and size of the SC in medial and lateral slices
| Property | Medial Slice (n = 12) | Lateral Slice (n = 9) | Relative Difference (medial vs. lateral slices) | P Value |
|---|---|---|---|---|
| Area of ipsilateral retinal input in 0.2–0.6 portion of SC along rostro-caudal axis, mm2 | 0.22 ± 0.06 | 0.08 ± 0.03 | 175% | 0.0013 |
| Max. brightness of ipsilateral retinal input in 0.2–0.6 portion of SC along rostro-caudal axis | 0.13 ± 0.06 | 0.03 ± 0.02 | 333% | 0.009 |
| Length of SC at P7, mm | 2.70 ± 0.11 | 2.48 ± 0.24 | 9% | 0.39 |
| Width of SGS, mm | 0.36 ± 0.02 | 0.33 ± 0.03 | 9% | 0.35 |
Data are presented as means ± SE. To quantify the amount of ipsilateral input in the medial and lateral slices, we divided the SC into 5 segments along the antero-posterior axis (Fig. 1D). All electrophysiological recordings were preformed in the 0.2–0.6 portion of the SC along the antero-posterior axis, that is, segments 2 and 3 (Fig. 1F). In these segments, the ipsilateral input was dramatically more prominent in the medial vs. the lateral slice: it covered a larger area and its intensity was higher (rows 1 and 2, 2-tailed Student's t-test). The difference in the area of the ipsilateral projection between the 2 slices cannot be accounted for by a difference in size of the SC itself. Even though the length and width of the SGS, as measured through labeling of the contralateral projection, were slightly (9%) smaller in the lateral compared to the medial slice, these differences were not statistically significant and can account for only a small fraction of the larger area of the ipsilateral projection in the medial slice. Ipsilateral enucleation at P0 did not affect the size of the SC (P > 0.05). Retinal input brightness values are in arbitrary units.
In both slices, we performed patch-clamp recordings from neurons in the SGS layer of the SC while electrically stimulating retinal fibers passing through the optic tract (see materials and methods). After eye segregation, the SGS layer receives input from the contralateral eye, so from P6–7 on our recordings reflect predominantly contralateral inputs. We recorded from a total of 128 neurons at ages P3–13, and of these we also labeled and morphologically reconstructed 65 cells. In addition, we labeled and reconstructed 12 cells at P19–21. All recordings were performed from neurons located between 20% and 60% of the anterior-posterior extent of the SC (segments 2 and 3 in Fig. 1, D and E; see Fig. 1F for recording locations).
Evoked retinocollicular synaptic responses are mediated predominantly by glutamatergic AMPA and NMDA receptors (Hestrin 1992; Shah and Crair 2008; Shi et al. 1997). We isolated AMPA-mediated responses by clamping the holding potential at −70 mV. At this holding potential, NMDA receptors are blocked by magnesium ions contained in the extracellular solution. Conversely, we isolated evoked NMDA-mediated responses by clamping the potential to +40 mV and simultaneously blocking AMPA currents with NBQX.
We examined three time points during development (Fig. 1G): 1) P3–4, when functional contacts from the retina to the colliculus have already formed but inputs from the two eyes are mixed in the SGS layer of the binocular SC (Godement et al. 1984; Wu et al. 2000); 2) P6–7, when inputs from the two eyes are mostly segregated into their corresponding layers; and 3) P12–13, shortly before eye opening and the onset of visual experience. Binocular interactions are likely to be most prominent in the antero-medial SC during the first postnatal week, when inputs from the two eyes are mixed in the SGS.
At P6–7, retinocollicular synapses are stronger and more mature in medial than lateral SC.
To examine the functional development of retinocollicular synapses, we first employed a miniature evoked postsynaptic current protocol (Fig. 2A). In these experiments, we detected a synchronous evoked AMPA response at −70 mV and then replaced the extracellular Ca2+ with Sr2+ to desynchronize vesicle release at stimulated axon terminals (Xu-Friedman and Regehr 1999). We found that AMPA-mini amplitudes varied with age in the medial but not the lateral SC (Fig. 2B). Specifically, AMPA-mini amplitudes increased from P3–4 to P6–7 in the medial but not the lateral slice and at P6–7 were significantly larger in the medial than the lateral slice. At P3–4 and P12–13, in contrast, AMPA-mini amplitudes were similar in the two slices. The difference between the two slices at P6–7 is surprising given that, anatomically, retinotopic refinement of retinocollicular axons is thought to happen uniformly across the SC (McLaughlin et al. 2003; Simon and O'Leary 1992).
Fig. 2.
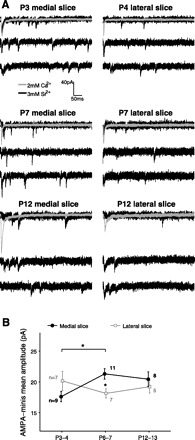
Developmental profiles of evoked AMPA miniature currents (“AMPA-minis”) in the medial and lateral SC. A: examples of evoked AMPA-minis recorded from medial (left) and lateral (right) slices at different ages. In these experiments, we first detected a stable evoked AMPA response using a Ca2+-ACSF bath (gray, average trace over 10–20 sweeps). We then replaced extracellular Ca2+ by Sr2+ to desynchronize synaptic release and recorded “miniature,” or evoked quantal AMPA currents (black). B: AMPA-mini amplitudes varied with age in medial but not lateral SC [medial slice: F(2, 25) = 4.18, P = 0.027, lateral slice: F(2, 16) = 0.76, P = 0.48; 1-way ANOVA]. Specifically, AMPA-mini amplitudes increased from P3–4 to P6–7 in the medial but not the lateral slice (P = 0.025, P = 0.45 for medial/lateral slices, respectively, Tukey's post hoc test). At P6–7, AMPA minis were significantly larger in the medial than in the lateral slice (P = 0.021; 2-tailed Student's t-test with false discovery rate procedure for multiple comparisons). Number of cells per group is indicated. Only 1 cell per slice and 1 slice per animal were used. *P < 0.05.
To further examine the functional development of retinocollicular synapses in the two slices, in a different pool of cells, after recording a synchronous AMPA response at a holding potential of −70 mV we switched to a holding potential of +40 mV to record NMDA-mediated currents while pharmacologically blocking AMPA-mediated transmission (Fig. 3A). One of the signatures of maturing synapses is a change in subunit composition of NMDA receptors and a corresponding shortening of the time constant of the postsynaptic response (Barth and Malenka 2001; Crair and Malenka 1995; Hestrin 1992; Lu et al. 2001; Monyer et al. 1994; Shi et al. 1997, 2000). Indeed, in both slices we observed a decrease of NMDA decay time constant (τNMDA) over development (Fig. 3B), but, interestingly, in the medial slice τNMDA decreased earlier and by P6–7 was significantly shorter than in the lateral slice, indicating more mature NMDA kinetics in the medial SC at P6–7. As with AMPA-minis, at P3–4 and P12–13 τNMDA was similar in the two slices.
Fig. 3.
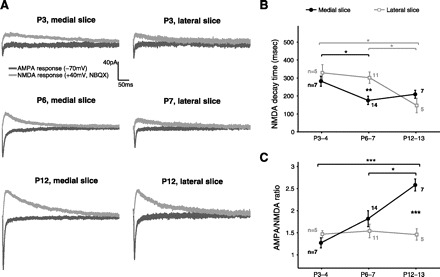
Developmental profiles of AMPA-to-NMDA ratios and NMDA kinetics in medial and lateral SC. A: examples of AMPA- and NMDA-mediated currents recorded in the 3 experimental age groups from neurons in medial (left) and lateral (right) slices. For each neuron, AMPA and NMDA responses were recorded at the same stimulation strength. Each trace is the average response calculated from 10–20 sweeps. B: developmental profiles of NMDA decay time (τNMDA) in medial and lateral slices. τNMDA decreased in the medial but not in the lateral slice from P3–4 to P6–7, resulting in shorter τNMDA and therefore more mature NMDA kinetics in the medial SC at P6–7 [effect of age: F(2, 25) = 4.39, P = 0.023 and F(2, 18) = 5.06, P = 0.018 for medial and lateral slices, respectively; effect of slice: P = 0.38, P = 0.0032, and P = 0.17 for P3–4, P6–7, and P12–13, respectively]. C: developmental profiles of AMPA-to-NMDA ratios in the medial and lateral SC. AMPA/NMDA were computed from the peak current amplitudes measured at the same stimulation strength. AMPA/NMDA increased from P3–4 to P12–13 in the medial but not the lateral slice, resulting in larger AMPA/NMDA in the medial than lateral slice by P12–13 [effect of age: F(2, 25) = 10.32, P = 0.0005 and F(2, 18) = 0.09, P = 0.92 for medial and lateral slices, respectively; effect of slice: P = 0.23, P = 0.27, and P = 0.00017 for P3–4, P6–7, and P12–13, respectively, 1-way ANOVA for effect of age, Tukey's post hoc test; 2-tailed Student's t-test for effect of slice with false discovery rate procedure for multiple comparisons]. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition to developmental changes in NMDA kinetics, immature glutamatergic synapses are typically dominated by NMDA receptors, whereas later in development AMPA receptors are trafficked into the synapses, resulting in an increased AMPA-to-NMDA current ratio (Crair and Malenka 1995; Lu et al. 2003; Malinow and Malenka 2002; Shah and Crair 2008; Shi et al. 2001; Takahashi et al. 2003). We observed a dramatic developmental increase in AMPA/NMDA in the medial slice but virtually no change in AMPA/NMDA in the lateral slice (Fig. 3C). AMPA/NMDA were similar in the two slices at P3–4 and P6–7 but significantly larger in the medial than the lateral slice at P12–13. Thus, unlike AMPA-mini amplitudes and NMDA kinetics, AMPA/NMDA were similar in the two slices through the end of the first postnatal week but diverged later in development. Notwithstanding the similar AMPA/NMDA at P6–7, the shorter NMDA kinetics and larger AMPA-mini amplitudes in the medial slice, as well as additional findings described below, suggest that retinocollicular synapses are stronger and more mature in the medial (binocular) than in the lateral (mainly monocular) SC at the end of the first postnatal week (Table 1).
Table 1.
Summary of electrophysiological and morphological properties in medial and lateral superior colliculus at P6–7
| Property | Medial SC (binocular) | Lateral SC (predominantly monocular) | Relative Difference (medial vs. lateral) | P Value |
|---|---|---|---|---|
| Quantal AMPA current amplitude, pA | 21.31 ± 0.86 (n = 11) | 18.15 ± 0.75 (n = 7) | +17% | 0.021* |
| NMDA decay time constant, ms | 176 ± 23 (n = 14) | 302 ± 32 (n = 11) | −42% | 0.0032† |
| AMPA-to-NMDA current ratio | 1.82 ± 0.18 (n = 14) | 1.54 ± 0.16 (n = 11) | +18% | 0.27 |
| NMDA single-fiber response amplitude, pA | 14.46 ± 1.15 (n = 10) | 10.23 ± 0.73 (n = 8) | +41% | 0.0098† |
| NMDA saturation response amplitude, pA | 59.90 ± 8.38 (n = 10) | 43.19 ± 7.87 (n = 8) | +39% | 0.17 |
| Number of retinal inputs | 4.31 ± 0.66 (n = 10) | 4.18 ± 0.68 (n = 8) | +3% | 0.89 |
| Input resistance, Ω | 1213 ± 169 (n = 28) | 1078 ± 152 (n = 18) | +12% | 0.58 |
| Number of dendritic nodes | 60.2 ± 12.5 (n = 10) | 26.8 ± 2.7 (n = 9) | +125% | 0.023* |
| Fractal dimensions of dendritic tree | 1.089 ± 0.017 (n = 10) | 1.059 ± 0.008 (n = 9) | +3% | 0.129 |
| Dendritic length, μm | 2203 ± 357 (n = 10) | 1445 ± 213 (n = 9) | +52% | 0.094 |
Data are presented as means ± SE. SC, superior colliculus. Percent difference between the slices defined as 100·(xm − xL)/xL, where xm and xL are the corresponding values in the medial and lateral slices, respectively. Differences in means tested with a 2-tailed Student's t-test: *P < 0.05,
P < 0.01. Synaptic/morphological properties that differed significantly between the 2 slices are in boldface.
As in other brain regions (e.g., Ramoa and McCormick 1994), we observed a gradual decrease in the input resistance of SC neurons with age in both slices (see Fig. 5A). However, this age-dependent decrease in input resistance did not differ significantly between the medial and lateral slices.
Fig. 5.
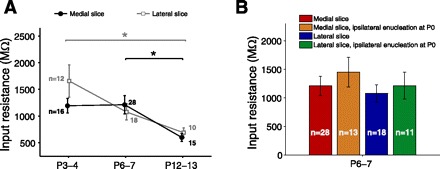
Developmental profiles of input resistance in medial and lateral slices. A: input resistance varied with age in both slices [medial: F(2, 56) = 4.23, P = 0.019; lateral: F(2, 37) = 4.88, P = 0.013; 1-way ANOVA]. Specifically, input resistance decreased from P6–7 to P12–13 (P = 0.022) in the medial slice and from P3–4 to P12–13 in the lateral slice (P = 0.011; Tukey's post hoc test; P > 0.05 in other comparisons). Input resistance did not differ significantly between the 2 slices at any age (P3–4: P = 0.14, P6–7: P = 0.58, P12–13: P = 0.46; 2-tailed Student's t-test with false discovery rate procedure for multiple comparisons). *P < 0.05. B: ipsilateral monocular enucleation at P0 had no statistically significant effect on input resistance at P6–7 (effect of slice: P = 0.96, effect of enucleation: P = 0.97, interaction: P = 0.93; 2-way ANOVA with factors encoding for slice and enucleation treatment).
Ipsilateral monocular enucleation at birth abolishes differences between medial and lateral SC at P6–7.
Why are retinocollicular synapses stronger and more mature in the medial than the lateral SC at P6–7? One possibility is that interactions between inputs from the two eyes enhance synapse development mainly in the medial SC, where binocular interactions are maximal. Alternatively, the differences in synapse development between the medial and lateral SC could stem from intrinsic regional difference across the SC, such as differential expression of a molecular signal involved in synapse strengthening and maturation, independently from interocular interactions. To distinguish between these alternatives, we eliminated interocular interactions by enucleating the ipsilateral eye at P0 and recorded synaptic properties at P6–7 from both slices. We then tested statistically for an interaction between the effects of enucleation (treatment/no treatment) and slice (medial/lateral) to determine whether enucleation affects selectively synaptic properties in one of the slices (see Nieuwenhuis et al. 2011).
For these experiments we focused on P6–7 animals, because 1) our previous protocols showed maximal differences between the medial and lateral SC at P6–7 and no noticeable differences at P3–4; 2) developmental differences related to binocular interactions are likely to be greatest around P6–7, just as inputs from the two eyes are segregating in the SC; and 3) on a methodological level, dissecting successful retinocollicular slices at younger ages is challenging, constraining the number of tests that can be performed in practice.
It is important to note that in the mouse input from the contralateral eye is dominant even in the binocular SC, whereas ipsilateral retinal axons constitute only a small fraction of the input to the SC. Accordingly, ipsilateral monocular enucleation has a relatively minor effect on the anatomical organization of contralateral retinal axons (SGS length and width after monocular enucleation at P0: medial slice, 2.59 ± 0.17 mm and 0.33 ± 0.03 mm, respectively; lateral slice, 2.50 ± 0.12 mm and 0.36 ± 0.03 mm; P > 0.05 relative to nonenucleated slices, see Table 2). Specifically, the contralateral axons innervate their usual territory in the SGS layer and also expand into the relatively small territory normally occupied by ipsilateral axons at the border between the SGS and the optic layer SO (Godement et al. 1980). In terms of timing, ipsilateral retinal axons invade the SC at about embryonic day (E)18–P0 (Edwards et al. 1986; Godement et al. 1984), about 3 days later than contralateral axons. Thus ipsilateral enucleation at P0 almost fully eliminates activity-mediated competition between axons of the two eyes.
We found that monocular ipsilateral enucleation at birth obliterated the difference in AMPA-mini amplitudes and τNMDA between the medial (normally binocular) slice and the lateral (predominantly monocular) slice at P6–7 (Fig. 4, A–D). For both AMPA-mini amplitudes and τNMDA there was a significant interaction between the effects of slice and enucleation, indicating that enucleation had a differential effect on the two slices. Specifically, enucleation 1) reduced AMPA-mini amplitudes in the medial but not the lateral slice and 2) increased τNMDA in the medial but not the lateral slice. There was a weak trend after monocular enucleation toward larger AMPA-mini amplitudes, larger NMDA single-fiber response amplitudes, and shorter τNMDA in the lateral slice, but these small effects were not statistically significant and they were opposite to those observed in the medial slice. This argues against a nonspecific reduction in synapse maturity as result of the enucleation. The possibility remains, however, that a larger sample size and/or more selective recording/stimulation techniques would reveal a small but significant effect of enucleation on the lateral SC. Such an effect, if it exists, could be related to the small ipsilateral input that is present in the lateral SC at young ages but mostly retracts to the antero-medial SC, or to some degree of reorganization of contralateral axons in response to the enucleation. Finally, monocular enucleation did not have a significant effect on AMPA-to-NMDA ratios or input resistance in either slice (Fig. 4E and Fig. 5B). In summary, monocular enucleation did not significantly affect those properties that were similar in the two slices to begin with (AMPA/NMDA, input resistance) and eliminated synaptic differences in the two slices that were apparent without enucleation (AMPA-minis, τNMDA).
Fig. 4.
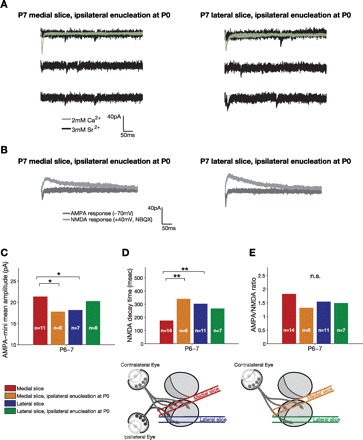
Effect of ipsilateral monocular enucleation at P0 on synaptic properties in the medial and lateral SC at P6–7. A: examples of AMPA-minis recorded at P7 from medial (left) and lateral (right) slices after ipsilateral monocular enucleation at P0. B: examples of AMPA- and NMDA-mediated currents at P7 after monocular enucleation. C–E: quantification of AMPA-mini amplitudes, NMDA decay time constant (τNMDA), and AMPA-to-NMDA ratios, respectively. Monocular enucleation selectively reduced AMPA-mini amplitudes and increased NMDA decay time constant in the medial but not lateral slice (effect of slice: P = 0.67, P = 0.29, P = 0.99; effect of enucleation: P = 0.50, P = 0.12, P = 0.10; interaction: P = 0.0055, P = 0.0081, P = 0.227 for AMPA-minis, τNMDA, and AMPA/NMDA, respectively; 2-way ANOVA with factors encoding for slice and enucleation treatment, followed by pairwise adjusted post hoc tests; nonenucleation data reproduced from Fig. 2). *P < 0.05, **P < 0.01. n.s., Not significant.
To further investigate differences between the medial and lateral SC at P6–7, we used a graded-stimulation protocol that allowed us to estimate both the number of retinal inputs to SC neurons and the response to single-fiber stimulation. In this protocol, we again recorded monosynaptic NMDA responses at a holding potential of +40 mV while pharmacologically blocking AMPA-mediated transmission. After detecting a stable NMDA response, we lowered the stimulus intensity to obtain a mixture of failures and successes. The mean amplitude of successful responses is an estimate of single-fiber response, which likely reflects synaptic input from a single RGC. We then increased the stimulation strength until a saturating response level was achieved (Fig. 6, A–D). To estimate the number of inputs, we divided the mean saturating response by the mean single-fiber response (Chandrasekaran et al. 2007; Chen and Regehr 2000; Hooks and Chen 2006). Importantly, because AMPA currents are blocked in this protocol, a wide range of stimulation strengths can be probed without the complication of intracollicular polysynaptic responses. We found that in normally raised mice at P6–7 the number of retinal inputs to SC neurons was comparable in the two slices. However, importantly, the NMDA response to single-fiber stimulation was substantially larger in the medial than the lateral slice. Saturating NMDA response amplitude was also somewhat larger in the medial slice, but this difference did not reach statistical significance (Fig. 6, E–G, compare red and blue histograms). The larger response amplitude to single retinal fiber stimulation is consistent with stronger retinocollicular connectivity in the medial than the lateral SC at the end of the first postnatal week.
Fig. 6.
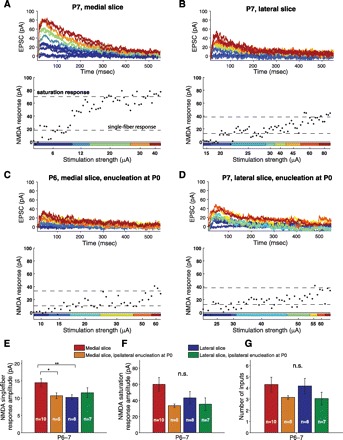
Number-of-inputs analysis in medial and lateral SC at P6–7 in normally raised mice and mice after ipsilateral monocular enucleation at birth. A–D: examples of graded stimulation experiments at P6–7 in medial and lateral slices of normally raised mice and mice after ipsilateral monocular enucleation at P0. In these experiments, we recorded NMDA-mediated responses for a range of stimulation intensities [excitatory postsynaptic current (EPSC), top] and then calculated the peak current amplitudes for each sweep (bottom). The lower stimulation strength in each experiment was adjusted to obtain a mixture of no-response trials (“failures”) and responses (“successes”). The mean amplitude of successful responses at the minimal stimulation strength is an estimate of the single-fiber response. We then gradually increased the stimulation intensity until saturation response amplitude was obtained. The saturation response divided by the single-fiber response is an estimate of the number of retinal inputs to the neuron. E–G: quantification of single-fiber response, saturation response, and number of inputs, respectively (effect of slice: P = 0.027, P = 0.11, P = 0.12; effect of enucleation: P = 0.29, P = 0.49, P = 0.83; interaction: P = 0.0135, P = 0.35, P = 0.70 for single-fiber response, saturation response, and number of inputs, respectively; 2-way ANOVA with pairwise adjusted post hoc tests). *P < 0.05, **P < 0.01.
Monocular enucleation at birth, like its effects on AMPA-minis and τNMDA, abolished the difference between the medial and lateral slices with regard to single-fiber response amplitudes (Fig. 6E). Specifically, enucleation decreased NMDA single-fiber response in the medial but not the lateral slice. In contrast, monocular enucleation had no statistically significant effect on the saturating NMDA response amplitude or number of retinal inputs (Fig. 6, F and G).
To summarize the enucleation experiments, ipsilateral enucleation selectively affected AMPA-mini amplitudes, NMDA kinetics, and single-fiber response amplitude in the medial slice and completely abolished the differences between the medial and lateral slices at P6–7. These findings suggest that the difference between medial and lateral SC in normally reared animals is not simply the result of a differentially expressed molecular signal along the medial-lateral axis of the SC. Such a mechanism should also promote synapse strengthening in contralateral axons of the medial SC in enucleated animals. Instead, our findings suggest that the stronger and more mature synapses in the medial than the lateral SC of normally reared mice stem, at least in large part, from interocular interactions among retinal inputs.
Binocular competition enhances dendritic branching of SC neurons.
We examined the morphological properties of some of the recorded neurons by including an Alexa dye in the whole cell recording solution and then reconstructing their morphology off-line (see materials and methods). Surprisingly in view of previous Golgi studies of the SC (Labriola and Laemle 1977; Tenório et al. 1996; Warton and Jones 1985), most neurons in our study exhibited complex dendritic morphology, including secondary and tertiary branches, as early as P3–4 (Fig. 7). The dendritic shape of roughly half of the reconstructed neurons could be classified into one of the previously described classes of dendritic morphologies in the upper layers of the SC (Langer and Lund 1974; May 2006; Mooney et al. 1992), whereas the remaining neurons had no clearly recognizable structure (Table 3). This is the first direct evidence, to our knowledge, that a diverse population of neuron types in the SGS receives functional retinal input as early as P3. Despite the heterogeneity in terms of dendritic shape, measures of overall dendritic branching showed, as described below, clear and consistent developmental trends. To quantify the complexity of the dendritic trees, we calculated for each neuron the number of dendritic nodes and the fractal dimension of the dendritic tree, which reflects its space-filling properties as one gradually zooms down to a finer scale. We also quantified the total dendritic length of each arbor (Fig. 8). We found that the number of nodes and dendritic length increased significantly from P3–4 to P6–7 in the medial but not the lateral slice. The fractal dimension of dendritic arbors showed similar trends, but these changes were not statistically significant. At P6–7, the number of nodes was significantly larger in the medial than in the lateral SC. Dendritic length were somewhat, but not significantly, larger in the medial slice. The increase in dendritic complexity in the medial SC at P6–7 appears to be transient, as it did not persist to P12–13. In sum, dendritic complexity as reflected by the number of dendritic nodes was significantly larger at P6–7 in the medial than lateral SC and thus, interestingly, recapitulates the overall difference between the two slices observed at the synaptic level.
Fig. 7.
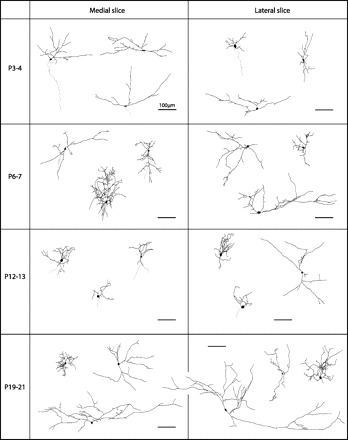
Examples of morphologically reconstructed neurons in medial and lateral SC. Shown are examples of neurons from medial (left) and lateral (right) slices reconstructed after cell labeling during electrophysiological recordings. Some of the reconstructed neurons could be easily categorized into previously reported morphological classes in the SGS layer of the SC (Table 3). All cells are oriented so that the pial surface is facing upward. Scale bar, 100 μm in all panels.
Table 3.
Breakdown of reconstructed neurons into morphological classes
| Wide Field Vertical | Narrow Field Vertical | Horizontal | Stellate | Not Classified | Total | |
|---|---|---|---|---|---|---|
| Medial slice, P3–4 | 2 (28.57%) | 0 (0.00%) | 1 (14.29%) | 1 (14.29%) | 3 (42.86%) | 7 |
| Medial slice, P6–7 | 0 (0.00%) | 3 (30.00%) | 0 (0.00%) | 1 (10.00%) | 6 (60.00%) | 10 |
| Medial slice, P12–13 | 2 (50.00%) | 1 (25.00%) | 0 (0.00%) | 0 (0.00%) | 1 (25.00%) | 4 |
| Medial slice, P19–21 | 0 (0.00%) | 2 (28.57%) | 1 (14.29%) | 1 (14.29%) | 3 (42.86%) | 7 |
| Lateral slice, P3–4 | 1 (14.29%) | 2 (28.57%) | 2 (28.57%) | 0 (0.00%) | 2 (28.57%) | 7 |
| Lateral slice, P6–7 | 1 (11.11%) | 2 (22.22%) | 0 (0.00%) | 0 (0.00%) | 6 (66.67%) | 9 |
| Lateral slice, P12–13 | 2 (28.57%) | 1 (14.29%) | 0 (0.00%) | 1 (14.29%) | 3 (42.86%) | 7 |
| Lateral slice, P19–21 | 1 (20.00%) | 2 (40.00%) | 0 (0.00%) | 0 (0.00%) | 2 (40.00%) | 5 |
| Medial slice, P6–7, ipsilateral enucleation at P6–7 | 0 (0.00%) | 1 (14.29%) | 1 (14.29%) | 1 (14.29%) | 4 (57.14%) | 7 |
| Lateral slice, P6–7, ipsilateral enucleation at P0 | 2 (14.29%) | 1 (7.14%) | 2 (14.29%) | 2 (14.29%) | 7 (50.00%) | 14 |
Data are number of cells and % of total cells (in parentheses) per group. At all ages examined, roughly half of the reconstructed neurons could be readily classified into one of the previously reported cell classes in the SGS layer of the SC.
Fig. 8.

Morphological developmental in the medial and lateral SC. A: number of dendritic nodes increased significantly from P3–4 to P6–7 in medial but not lateral SC, resulting in a larger number of nodes in the medial than the lateral slice at P6–7. Fractal dimension of the dendritic tree (B), and total dendritic length (C) showed a similar developmental trend, but differences between the two slices at P6–7 were not statistically significant (effect of age in medial slice: P = 0.025, P = 0.025, P = 0.082; in lateral slice: P = 0.40, P = 0.69, P = 0.41 for number of nodes, dendritic length, and fractal dimension, respectively, 1-way ANOVA with Tukey's post hoc test; comparison between the two slices at P6–7: P = 0.023, P = 0.094, P = 0.13 for number of nodes, dendritic length, and fractal dimension, respectively; Student's t-test with false discovery rate procedure for multiple comparisons). *P < 0.05.
We also examined cellular morphology in both slices at a later time point during development, P19–21 (Fig. 7), and found that both laterally and medially located neurons attained at this age a complex and elaborate dendritic structure, with similar measures of dendritic complexity in the two slices (number of nodes: 37.7 ± 5.1, 37.0 ± 8.0; dendritic length: 1,897 ± 308 μm, 2,147 ± 677 μm; fractal dimension: 1.063 ± 0.011, 1.069 ± 0.016: n = 7 and 5 for medial and lateral slices, respectively; P > 0.05, 2-tailed Student's t-test). This suggests that the transient differences between the two slices at P6–7 are unlikely to be due to overall persistent lower density of RGC inputs in the lateral SC, which would be expected to cause a lingering difference in dendritic structure between the medial and lateral SC.
To test whether the observed difference in dendritic complexity between the medial and lateral slices at P6–7 depends on binocular interactions or simply results from intrinsic regional differences within the SC, we repeated the morphological analysis of neurons in both slices for mice that were monocularly enucleated at P0 (Fig. 9). Strikingly, monocular enucleation selectively reduced dendritic branching in the medial slice and abolished the morphological difference between the medial and lateral slices at P6–7. Thus the morphological consequences of ipsilateral enucleation directly recapitulate the effects observed at the synaptic level and suggest that binocular interactions enhance not only retinocollicular synapse development but also branching of retino-recipient neurons in the SC.
Fig. 9.
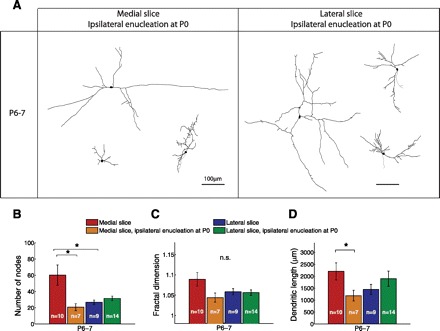
Effect of ipsilateral monocular enucleation at P0 on dendritic morphology at P6–7. A: examples of reconstructed neurons in the medial (left) and lateral (right) SC at P6–7 after ipsilateral monocular enucleation at P0 (cf. Fig 7, 2nd row). B: monocular enucleation selectively reduced the number of dendritic nodes in the medial (binocular) SC at P6–7 to a level comparable to that observed in the lateral (predominantly monocular) SC. C and D: effect of enucleation on fractal dimension of the dendritic tree and total dendritic length, respectively (effect of slice: P = 0.019, P = 0.36, P = 0.04; effect of enucleation: P = 0.11, P = 0.98, P = 0.45; interaction: P = 0.0045, P = 0.036, P = 0.09 for number of nodes, fractal dimension, and total dendritic length, respectively; 2-way ANOVA followed by pairwise adjusted post hoc tests). *P < 0.05.
DISCUSSION
We examined the synaptic basis of visual map development prior to eye opening using patch-clamp recordings and neuroanatomical techniques in the SC of neonatal mice. We found that retinocollicular synapse development differed between the medial SC, which receives binocular retinal input, and the lateral SC, which receives input predominantly from the contralateral eye. Specifically, at P6–7 retinocollicular synapses were stronger and more mature in the medial than in the lateral SC (Table 1). Furthermore, dendritic branching of SC neurons recapitulated the synaptic phenotype, displaying at P6–7 a larger number of nodes in the medial than in the lateral SC. To determine whether these differences stem from binocular interactions in the medial SC, or simply reflect intrinsic regional differences across the SC, we removed the ipsilateral eye at birth, thereby eliminating binocular competition during development. Notably, in the absence of competition from ipsilateral axons, the differences between the medial (normally binocular) SC over the lateral region at P6–7 were abolished (Figs. 4, 6, and 9). Together, these findings suggest a surprising form of binocular interaction during the first postnatal week of development in which competition among retinal axons enhances the strengthening and maturation of functional connectivity to target neurons in the SC.
Novel slice preparations.
An important methodological advance in our study is the design of two brain slice preparations to record selectively from the medial and lateral SC (Fig. 1). This approach enabled us to reveal unexpected differences in synapse development among subregions of the SC early during development when photoreceptors are not yet connected to RGCs, and therefore visual stimulation to differentially probe ipsilateral and contralateral functional connectivity is impractical. When interpreting regional differences between medial and lateral SC, it is important to note that the distinction between the “binocular” and “monocular” SC emerges gradually during development, rather than being clear-cut from the outset. Initially, the ipsilateral projection to the SC is widespread along both the medio-lateral and antero-posterior axes and then becomes progressively restricted to the antero-medial crescent of the SC during the first week after birth (Godement et al. 1984; Simon and O'Leary 1992). At the same time, the ipsi- and contralateral inputs are initially mixed and subsequently segregate into different eye-specific layers (cf. Fig. 1, C and D). Despite these caveats, functional binocular interactions throughout development are likely to be strongest in the medial slice cut from the anterio-medial SC, where the ipsilateral input is always strongest, particularly during the first postnatal week, when axons from the two eyes are physically mixed in the SGS.
Can the differences in synaptic properties between the medial and lateral SC at P6–7 stem from some methodological confound that differentiates between the two slices? It is possible, for instance, that a significantly larger number of retinal fibers is retained in one of the slices. The strongest argument against this possibility is that three of the properties we measured—AMPA-mini amplitudes, NMDA kinetics, and AMPA-to-NMDA ratios—are intrinsic to the synapses themselves, and therefore unlikely to be affected by the number of fibers preserved in the slice or other aspects of the slice preparation. Another argument against a methodological difference between the slices is that most synaptic properties at P3–4 and P12–13 were similar in the two slices. This would be hard to reconcile with a differential effect of slice preparation on the measurements.
Another potential experimental confound is stimulus selectivity. Electrical stimulation is nonselective in terms of which eye is being stimulated, and thus synaptic currents in the medial slice may reflect a mixture of ipsi- and contralateral inputs, whereas the lateral slice is predominantly contralateral. However, ipsilateral inputs should contribute at most a small fraction of the synaptic currents recorded at P6–7 in the medial slice. At this age, the SGS layer, where recordings were performed, receives almost exclusively contralateral input. Furthermore, ipsilateral inputs to the SC are likely to be less mature than contralateral inputs, as they arrive later, and even at P14–15 their axonal arbors are sparser than contralateral inputs (Dhande et al. 2011). In contrast, we observed stronger and more mature responses in the medial (binocular) SC, so that the differences between the two slices at this age are unlikely to stem simply from contribution of ipsilateral inputs. However, we cannot completely rule out a contribution of ipsilateral inputs to the recorded response properties, particularly at young ages (P3–4), and it would be useful to address this point in the future by developing selective stimulation protocols on top of the specialized slices presented here, for example, using optogenetic techniques (Zhang et al. 2011).
With regard to the enucleation experiments, it should also be noted that with fewer axons in the optic tract it is possible that the minimal stimulation protocol recruits different axons than in normal conditions. This could account, at least in part, for the effect of enucleation on single-fiber response amplitude in the medial slice (Fig. 6A), but, importantly, other measurements were performed under higher stimulation strengths and therefore are likely to reflect the properties of a mixed cohort of retinal axons. Since both minimal and stronger stimulation strengths yielded clear and consistent effects of enucleation in the medial slice, the effects of enucleation are unlikely to reflect just a stimulation bias resulting from a smaller number of axons in the optic tract.
Binocular interactions in functional development of visual maps.
Our results suggest that binocular interactions play an important role in the functional development of visual maps prior to eye opening. In mice, selectively stimulating one eye or manipulating its activity early in development is challenging. During the first postnatal week, photoreceptors are not yet fully functional, limiting the use of visual stimulation for this purpose. Also, genetic approaches are constrained by developmental delays in reaching effective protein expression levels. Previous work in the LGN, employing a notable slice preparation in which axons from either eye can be stimulated selectively, indicates that at P7–8 single LGN neurons receive binocular input whereas by P18–19 LGN neurons are virtually monocular (Jaubert-Miazza et al. 2005; Ziburkus and Guido 2006). Because the SC is further away from the eyes along the optic tract, employing this approach in the retinocollicular preparation is even more challenging. On the other hand, the simple geometry of the retinocollicular map in the SC, and its precocious development relative to the LGN (Dhande et al. 2011), make it a good candidate for examining the roles of binocular interactions early on. Recently, Koch et al. (2011) reduced glutamate release selectively from ipsilateral-projecting RGCs using a genetic approach. After this manipulation contralateral axons invaded the ipsilateral territory in the LGN, but interestingly the ipsilateral axons were still able to consolidate and maintain their normal territory. Glutamate release attenuation in these experiments starts at P5, and the resulting mapping phenotype was assayed, as in most studies so far, by bulk labeling of retinal axons. Two of the remaining questions to be examined in the future are 1) what the functional and synaptic correlates are of attenuating glutamate release in one eye and 2) whether binocular competition plays a similar role in the LGN and SC (cf. Reese 1986).
At P6–7, most synaptic properties that we examined indicated stronger and more mature synaptic connections in the medial than in the lateral SC (Table 1). One exception is AMPA-to-NMDA ratios, which were slightly, but not significantly larger in the medial SC and curiously increased toward P12–13 in the medial but not lateral slice. It is unclear why AMPA/NMDA are similar in the two slices at P6–7 but differ at P12–13. It is possible that the effect of binocular interactions on the AMPA/NMDA is delayed relative to its effect on other synaptic properties. For instance, the late increase in AMPA/NMDA in the medial slice may result from the elimination of NMDA-only “silent” retinocollicular synapses during the second postnatal week (see also Shah and Crair 2008). In any case, the difference in the developmental profiles of AMPA/NMDA vs. AMPA-minis and NMDA kinetics is intriguing, but it does not contradict our central claims, namely, that 1) retinocollicular connectivity is generally stronger and more mature at P6–7 in the medial than in the lateral SC and 2) this difference stems at least in large part from binocular interactions, as confirmed by the enucleation experiments.
Most synaptic and dendritic properties that we measured reached a similar level in the two slices at P12–13. In other words, differences between the binocular and monocular SC at P6–7 seem to be largely transient, and therefore should not affect normal vision after eye opening. The mechanisms that regulate this “leveling off” of synaptic properties across the retinotopic map during the second postnatal week are unclear but are likely to involve homeostatic regulation that is known to operate in the retinocollicular map at this developmental stage (Chandrasekaran et al. 2007). Differences between the binocular and monocular SC at P6–7 potentially reflect developmental processes that are beneficial during the first postnatal week but then give way to complementary forms of plasticity that better fit the constraints of later development.
Morphological development of SGS neurons.
We found that the dendritic arbors of neurons in the SGS of the SC were dramatically more complex during the first postnatal week than one would expect based on previous studies in the rat (Labriola and Laemle 1977; Tenório et al. 1996; Warton and Jones 1985). Species differences between mice and rats are unlikely to account for such a substantial difference in dendritic complexity, but other methodological differences may be important. In particular, cell filling in fresh tissue at young ages, as in our study, more efficiently labels thin and distant dendritic branches than fixed-tissue labeling methods (Ramoa et al. 1987). Apart from complex dendritic morphology, we also observed axons in many of our neurons that extended into the deeper layers of the SC or occasionally coursed anteriorly or posteriorly, as far as 1,500 μm from the soma, even at P3–4. This suggests that retino-recipient neurons of the SC are potentially capable of communicating with motor-related layers of the SC and other brain areas at least as early as P3–4. At P6–7, we observed dendritic arbors that were more elaborate in the medial than the lateral SC. Furthermore, ipsilateral monocular enucleation at P0 selectively reduced dendritic complexity in the medial SC. Together with the electrophysiological findings, this suggests a period of heightened plasticity during P3–6 (see also Shah and Crair 2008), when retinocollicular synapses and SC dendritic arbors are particularly sensitive to patterns of retinal input and the presence or absence of binocular innervation. Toward P12–13, dendritic complexity appeared to decrease in the medial SC. Thus there appears to be a transient increase in dendritic branching at P6–7 in the medial slice. These findings echo transient excessive dendritic branching previously observed in the cat retina (Ramoa et al. 1987, 1988) and in some regions of the rat brain (Rietzel and Friauf 1998; Rihn and Claiborne 1990).
At all ages examined we observed heterogeneous, and in many neurons difficult to categorize, dendritic morphologies. The morphological heterogeneity of neurons in the SGS is well established (Labriola and Laemle 1977; Ramón y Cajal 1995; Warton and Jones 1985), but it was not known which of the morphological classes receives functional retinal input, particularly early during development. Despite the cellular heterogeneity observed in our recordings, we did not note any salient correlations between synaptic properties and specific morphological classes of neurons. Interestingly, a recent study of the mouse LGN revealed a similar dissociation between electrophysiological and morphological properties (Krahe et al. 2011). Nonetheless, the possibility remains that a difference in the population of cells recorded in our two slices, either by chance or because of a systematic bias, contributed to the observed differences between the two slices at P6–7, particularly in terms of dendritic properties. The enucleation experiments argue to some extent against this possibility, as it is not clear why enucleation should affect cell type sampling in one slice (medial) but not the other (lateral). However, a contribution from differences in cell type sampling between the two slices cannot be completely ruled out, and efforts to record from identified subpopulations of neurons in the SC, e.g., with GFP reporter mice that selectively label particular classes of neurons in the SGS, would be particularly informative in this respect.
Models for binocular competition beyond homeostasis.
Two conceivable models for binocular competition, apart from homeostatically regulated competition, are a “dissipative” model and a “growth-promoting” model. In a “dissipative” model, axonal competition reduces the overall synaptic connectivity to the target neurons. This would be the case, for instance, if the competition “consumes” a resource that is required for synapse development. In a “growth-promoting” model, in contrast, axonal competition promotes an increase in the connectivity of the eventually “winning” axons due, for instance, to some cellular signaling process that enhances synapse development in response to the competition. Our findings support the idea of a “growth-promoting” type of competition among retinal axons in the SC during the first postnatal week. This conclusion is based on converging evidence from two sets of experiments: first, the comparison between the medial (binocular) and lateral (predominantly monocular) SC during normal development, indicating stronger and more mature synapses in the medial SC at P6–7, and second, the enucleation experiments, in which binocular interactions were eliminated during postnatal development and consequently the differences between the medial and lateral SC at P6–7 were abolished. The results of the enucleation experiments are surprising. One might expect that after ipsilateral enucleation the extra space available to the contralateral axons would allow them to form stronger, or at least normal, synaptic contacts to their targets. In contrast, we found that enucleation reduced synaptic strength as well as dendritic branching in the medial but not the lateral SC. We argue that these findings rule out a regional signal as the only reason for the differences between the medial and lateral SC in nonenucleated mice. However, our findings do not rule out a possible contribution from an unidentified signal that is 1) independent of binocular interactions and 2) expressed nonuniformly across the SC and 3) promotes synapse development in the medial SC (or inhibits it in the lateral SC).
Axonal competition has been reported in several parts of the developing nervous system (Cesa and Strata 2009; Kasthuri and Lichtman 2003), including competition among same-eye RGC axons in the zebrafish tectum (Fredj et al. 2010; Gosse et al. 2008; Hua et al. 2005). In mammals, RGC axons from the two eyes are thought to compete for space and synaptic connectivity with their postsynaptic target neurons. In particular, ocular dominance (OD) plasticity in the visual cortex is regulated in part through homeostatic mechanisms that preserve the net synaptic drive to cortical neurons (Desai et al. 2002; Frenkel and Bear 2004; Kaneko et al. 2008; Mrsic-Flogel et al. 2007). However, importantly, the critical period for OD plasticity occurs after the initial layout of the cortical map is well established (Crair et al. 1998). In contrast, early stages of map development occur while retinal axons are growing into their target areas, branching and beginning to form synapses with their postsynaptic partners (Dhande et al. 2011; Simon and O'Leary 1992). In these early stages, it is possible that homeostatic regulation may not fit the needs of the system (Ben-Ari and Spitzer 2010). Interestingly, theoretical models point out the potential limitations of homeostatically constrained competition. Mainly, models in which binocular inputs compete through constrained connectivity to the target are quite sensitive to details of model formulation. For example, whether a subtractive or divisive normalization rule is used for constraining the overall connectivity can determine whether an eye-segregated state is possible or not (Elliott 2003; Miller and MacKay 1994). This sensitivity reflects a relatively “weak” form of competition that may be appropriate during a gradual stage of experience-dependent plasticity but may not work for the rapid initial establishment of visual maps.
At the moment, we can only speculate on the cellular-molecular mechanisms that promote synapse strengthening through binocular interactions. Good candidates for mediating this process are neurotrophins, because of their roles in promoting axonal arborization and synapse development (Cantallops and Cline 2008; Hu et al. 2005; Ruthazer and Aizenman 2010; Vicario-Abejón et al. 2002) as well as in mediating activity-dependent axonal competition (Cao et al. 2007; Singh et al. 2008). An interesting possibility to be examined in the future is that during binocular map development competitive interactions among retinal axons elevate levels of neurotrophins, potentially via signaling pathways involving intracellular calcium levels, and thus promote branching and/or synapse formation in the winning axons.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.F. and M.C.C. conception and design of research; M.F. performed experiments; M.F. analyzed data; M.F. and M.C.C. interpreted results of experiments; M.F. prepared figures; M.F. drafted manuscript; M.F. and M.C.C. edited and revised manuscript; M.F. and M.C.C. approved final version of manuscript.
REFERENCES
- Angelucci A, Clascá F, Bricolo E, Cramer KS, Sur M. Experimentally induced retinal projections to the ferret auditory thalamus: development of clustered eye-specific patterns in a novel target. J Neurosci 17: 2040–2055, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci 4: 235–236, 2001 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Spitzer NC. Phenotypic checkpoints regulate neuronal development. Trends Neurosci 33: 485–492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantallops I, Cline HT. Rapid activity dependent delivery of the neurotrophic protein CPG15 to the axon surface of neurons in intact Xenopus tadpoles. Dev Neurobiol 68: 744–759, 2008 [DOI] [PubMed] [Google Scholar]
- Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason CA, Gogos JA. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol 17: 911–921, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa R, Strata P. Axonal competition in the synaptic wiring of the cerebellar cortex during development and in the mature cerebellum. Neuroscience 162: 624–632, 2009 [DOI] [PubMed] [Google Scholar]
- Chalupa L, Williams R. Organization of the cat's lateral geniculate nucleus following interruption of prenatal binocular competition. Hum Neurobiol 3: 103–107, 1984 [PubMed] [Google Scholar]
- Chandrasekaran A, Shah R, Crair M. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci 27: 1746–1755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955–966, 2000 [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Constantine-Paton M. Chronic NMDA receptor blockade from birth increases the sprouting capacity of ipsilateral retinocollicular axons without disrupting their early segregation. J Neurosci 21: 1557–1568, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair M, Gillespie D, Stryker M. The role of visual experience in the development of columns in cat visual cortex. Science 279: 566–570, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature 375: 325–328, 1995 [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol 279: R1–R8, 2000 [DOI] [PubMed] [Google Scholar]
- Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Heart Circ Physiol 287: H447–H449, 2004 [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5: 783–789, 2002 [DOI] [PubMed] [Google Scholar]
- Dhande O, Hua E, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer E, Feller M, Crair M. Development of single retinofugal axon arbors in normal and β2 knock-out mice. J Neurosci 31: 3384–3399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Schneider G, Caviness V. Development of the crossed retinocollicular projection in the mouse. J Comp Neurol 248: 410–421, 1986 [DOI] [PubMed] [Google Scholar]
- Elliott T. An analysis of synaptic normalization in a general class of Hebbian models. Neural Comput 15: 937–963, 2003 [DOI] [PubMed] [Google Scholar]
- Fredj NB, Hammond S, Otsuna H, Chien CB, Burrone J, Meyer MP. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J Neurosci 30: 10939–10951, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44: 917–923, 2004 [DOI] [PubMed] [Google Scholar]
- Godement P, Saillour P, Imbert M. The ipsilateral optic pathway to the dorsal lateral geniculate nucleus and superior colliculus in mice with prenatal or postnatal loss of one eye. J Comp Neurol 190: 611–626, 1980 [DOI] [PubMed] [Google Scholar]
- Godement P, Salaün J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol 230: 552–575, 1984 [DOI] [PubMed] [Google Scholar]
- Gosse NJ, Nevin LM, Baier H. Retinotopic order in the absence of axon competition. Nature 452: 892–895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R, Juttner R, Meier J. Development and use-dependent modification of synaptic connections in the visual layers of the rodent superior colliculus. In: The Superior Colliculus, edited by Hall WC, Moschovakis A. Boca Raton, FL: CRC, 2004, p. 173–210 [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357: 686–689, 1992 [DOI] [PubMed] [Google Scholar]
- Hofbauer A, Dräger UC. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J Comp Neurol 234: 465–474, 1985 [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52: 281–291, 2006 [DOI] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development 132: 4285–4298, 2005 [DOI] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature 434: 1022–1026, 2005 [DOI] [PubMed] [Google Scholar]
- Huberman A. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol 17: 73–80, 2007 [DOI] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22: 661–676, 2005 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka R, Stryker M. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58: 673–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature 424: 426–430, 2003 [DOI] [PubMed] [Google Scholar]
- Katz L, Crowley J. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci 3: 34–42, 2002 [DOI] [PubMed] [Google Scholar]
- Koch SM, Dela CCG, Hnasko TS, Edwards RH, Huberman AD, Ullian EM. Pathway-specific genetic attenuation of glutamate release alters select features of competition-based visual circuit refinement. Neuron 71: 235–242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, El-Danaf RN, Dilger EK, Henderson SC, Guido W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J Neurosci 31: 17437–17448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labriola AR, Laemle LK. Cellular morphology in the visual layer of the developing rat superior colliculus. Exp Neurol 55: 247–268, 1977 [DOI] [PubMed] [Google Scholar]
- Langer T, Lund R. The upper layers of the superior colliculus of the rat: a Golgi study. J Comp Neurol 158: 405–435, 1974 [DOI] [PubMed] [Google Scholar]
- Leamey C, Van Wart A, Sur M. Intrinsic patterning and experience-dependent mechanisms that generate eye-specific projections and binocular circuits in the visual pathway. Curr Opin Neurobiol 19: 181–187, 2009 [DOI] [PubMed] [Google Scholar]
- Lee PH, Schmidt M, Hall WC. Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J Neurosci 21: 8145–8153, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron 32: 619–634, 2001 [DOI] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical “barrel” map development. Nat Neurosci 6: 939–947, 2003 [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126, 2002 [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151: 321–378, 2006 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40: 1147–1160, 2003 [DOI] [PubMed] [Google Scholar]
- Miller KD, MacKay DJC. The role of constraints in Hebbian learning. Neural Comput 6: 100–126, 1994 [Google Scholar]
- Mize RR, Salt TE. Mechanisms underlying development of the retinocollicular pathway. In: The Superior Colliculus: New Approaches for Studying Sensorimotor Integration, edited by Hall WC, Moschovakis A. Boca Raton, FL: CRC, 2004, p. 211–240 [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994 [DOI] [PubMed] [Google Scholar]
- Mooney RD, Nikoletseas MM, King TD, Savage SV, Weaver MT, Rhoades RW. Structural and functional consequences of neonatal deafferentation in the superficial layers of the hamster's superior colliculus. J Comp Neurol 315: 398–412, 1992 [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel T, Hofer S, Ohki K, Reid R, Bonhoeffer T, Hübener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 54: 961–972, 2007 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14: 1105–1107, 2011 [DOI] [PubMed] [Google Scholar]
- Penn A, Riquelme P, Feller M, Shatz C. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279: 2108–2112, 1998 [DOI] [PubMed] [Google Scholar]
- Phillips MA, Colonnese MT, Goldberg J, Lewis LD, Brown EN, Constantine-Paton M. A synaptic strategy for consolidation of convergent visuotopic maps. Neuron 71: 710–724, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science 214: 928–931, 1981 [DOI] [PubMed] [Google Scholar]
- Ramoa A, Campbell G, Shatz C. Dendritic growth and remodeling of cat retinal ganglion cells during fetal and postnatal development. J Neurosci 8: 4239–4261, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci 14: 2089–2097, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS, Campbell G, Shatz CJ. Transient morphological features of identified ganglion cells in living fetal and neonatal retina. Science 237: 522–525, 1987 [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System of Man and Vertebrates. New York: Oxford Univ. Press, 1995 [Google Scholar]
- Reese B. The topography of expanded uncrossed retinal projections following neonatal enucleation of one eye: differing effects in dorsal lateral geniculate nucleus and superior colliculus. J Comp Neurol 250: 8–32, 1986 [DOI] [PubMed] [Google Scholar]
- Rietzel HJ, Friauf E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J Comp Neurol 390: 20–40, 1998 [PubMed] [Google Scholar]
- Rihn LL, Claiborne BJ. Dendritic growth and regression in rat dentate granule cells during late postnatal development. Dev Brain Res 54: 115–124, 1990 [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Aizenman CD. Learning to see: patterned visual activity and the development of visual function. Trends Neurosci 33:183–192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Crair M. Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves. J Neurosci 28: 292–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Aamodt SM, Constantine-Paton M. Temporal correlations between functional and molecular changes in NMDA receptors and GABA neurotransmission in the superior colliculus. J Neurosci 17: 6264–6276, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Townsend M, Constantine-Paton M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron 28: 103–114, 2000 [DOI] [PubMed] [Google Scholar]
- Shi J, Aamodt SM, Townsend M, Constantine-Paton M. Developmental depression of glutamate neurotransmission by chronic low-level activation of NMDA receptors. J Neurosci 21: 6233–6244, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BL, Chalupa LM. Organization of geniculocortical connections following prenatal interruption of binocular interactions. Brain Res 28: 47–62, 1986 [DOI] [PubMed] [Google Scholar]
- Simon DK, O'Leary D. Development of topographic order in the mammalian retinocollicular projection. J Neurosci 12: 1212–1232, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci 11: 649–658, 2008 [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz C. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33: 357–367, 2002 [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF. Nature 440: 1054–1059, 2006 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science 299: 1585–1588, 2003 [DOI] [PubMed] [Google Scholar]
- Tenório F, Giraldi-Guimarães A, Mendez-Otero R. Morphology of NADPH-diaphorase-positive cells in the retinoceptive layers of the developing rat superior colliculus. Int J Dev Neurosci 14: 1–10, 1996 [DOI] [PubMed] [Google Scholar]
- Thong IG, Dreher B. The development of the corticotectal pathway in the albino rat. Dev Brain Res 25: 227–238, 1986 [DOI] [PubMed] [Google Scholar]
- Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP, Feldheim DA. Retinal input instructs alignment of visual topographic maps. Cell 139: 175–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci 3: 965–974, 2002 [DOI] [PubMed] [Google Scholar]
- Warton S, Jones D. Postnatal development of the superficial layers in the rat superior colliculus: a study with Golgi-Cox and Klüver-Barrera techniques. Exp Brain Res 58: 490–502, 1985 [DOI] [PubMed] [Google Scholar]
- Wiesel TN. The postnatal development of the visual cortex and the influence of environment. Biosci Rep 2: 351–377, 1982 [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Neuroscience 22: 29–47, 1999 [DOI] [PubMed] [Google Scholar]
- Wu HH, Cork RJ, Mize RR. Normal development of the ipsilateral retinocollicular pathway and its disruption in double endothelial and neuronal nitric oxide synthase gene knockout mice. J Comp Neurol 426: 651–665, 2000 [DOI] [PubMed] [Google Scholar]
- Xu H, Furman M, Mineur YS, Chen H, King SL, Zenisek D. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 70: 1115–1127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Presynaptic strontium dynamics and synaptic transmission. Biophys J 76: 2029–2042, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ackman JB, Xu HP, Crair MC. Visual map development depends on the temporal pattern of binocular activity in mice. Nat Neurosci 22: 222–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol 96: 2775–2784, 2006 [DOI] [PubMed] [Google Scholar]


