Abstract
Neurons in the rodent midline thalamic paraventricular nucleus (PVT) receive inputs from brain stem and hypothalamic sites known to participate in sleep-wake and circadian rhythms. To evaluate possible diurnal changes in their excitability, we used patch-clamp techniques to record and examine the properties of neurons in anterior PVT (aPVT) in coronal rat brain slices prepared at zeitgeber time (ZT) 2–6 vs. ZT 14–18 and recorded at ZT 8.4 ± 0.2 (day) vs. ZT 21.2 ± 0.2 (night), the subjective quiet vs. aroused states, respectively. Compared with neurons recorded during the day, neurons from the night period were significantly more depolarized and exhibited a lower membrane conductance that in part reflected loss of a potassium-mediated conductance. Furthermore, these neurons were also significantly more active, with tonic and burst firing patterns. Neurons from each ZT period were assessed for amplitudes of two conductances known to contribute to bursting behavior, i.e., low-threshold-activated Ca2+ currents (IT) and hyperpolarization-activated cation currents (Ih). Data revealed that amplitudes of both IT and Ih were significantly larger during the night period. In addition, biopsy samples from the night period revealed a significant increase in mRNA for Cav3.1 and Cav3.3 low-threshold Ca2+ channel subtypes. Neurons recorded from the night period also displayed a comparative enhancement in spontaneous bursting at membrane potentials of approximately −60 mV and in burst firing consequent to hyperpolarization-induced low-threshold currents and depolarization-induced current pulses. These novel in vitro observations reveal that midline thalamic neurons undergo diurnal changes in their IT, Ih, and undefined potassium conductances. The underlying mechanisms remain to be characterized.
Keywords: midline thalamus, day-night, burst firing patterns, brain slice preparations
the thalamic midline and intralaminar nuclei have long been regarded as relays in a “nonspecific” thalamocortical arousing system. Increasingly, these regions of thalamus are being recognized as having specific afferent and efferent connectivity with select areas of cortex and striatum, and as participants in distinct functions that include arousal and vigilance, motivated behaviors, cognition, stress, nociception, and memory (Groenewegen and Berendse 1994; Sewards and Sewards 2003; Shyu and Vogt 2009; Van der Werf et al. 2002). Aberrant function in midline thalamus may also contribute to limbic epilepsy, sleep disorders, and various psychopathologies (reviewed in Bennaroch 2008; Rajasekaran et al. 2009).
Of emerging interest is the thalamic paraventricular nucleus (PVT), the most dorsal component of the midline cell group. In the rodent and monkey brain, PVT is reported to be one of the few extrahypothalamic targets for efferents from the suprachiasmatic nucleus (SCN), an innervation confirmed with various tracing techniques and shown to contain several coexisting neurotransmitter molecules (reviewed in Price and Drevets 2010). PVT is also a unique thalamic converging site for axons originating from lateral hypothalamic neurons that synthesize the arousal and orexigenic orexin (hypocretin) neuropeptides (de Lecea et al. 1998; Peyron et al. 1998; Sakurai et al. 1998) and from brain stem catecholaminergic cell groups engaged in sleep-waking cycles (e.g., Otake and Ruggerio 1995). Distinct from the predominantly cortical and thalamic reticular nucleus connectivity that is characteristic of neurons in the lateral and ventrobasal thalamus, PVT neurons are part of a neural circuitry that engages limbic brain regions, i.e., amygdala, bed nucleus of the stria terminalis, accumbens, and prefrontal cortex (Groenewegen and Berendse 1994; Li and Kirouac 2008; Moga et al. 1995). In addition, whereas lateral and ventrobasal thalamic neurons are traditionally considered to mediate somatosensory information to cortex and are subject to modulation over the sleep-wake cycle (for review, see McCormick and Bal 1997), neurons in PVT and other midline thalamic nuclei appear to be involved in mood and motivated behaviors, often as a response to various stressors (reviewed in Price and Drevets 2010; Sewards and Sewards 2003; Van der Werf et al. 2002). These latter functions require vigilance and arousal. However, comparatively little is known about the excitability of midline thalamic neurons, in particular during periods of arousal vs. inactive/sleep behaviors. One in vivo study in the cat noted that intralaminar centralis lateralis-paracentralis neurons displayed a prevalence of tonic firing during wakefulness, and also during electroencephalographic (EEG) desynchronization and rapid eye movement (REM) sleep, contrasting with membrane hyperpolarization and a burst firing mode during EEG synchronization and slow-wave sleep (Glenn and Steriade 1982; Steriade et al. 1993). In rodents, immediate early gene expression is selectively enhanced in PVT neurons during the subjective waking (lights off) period (e.g., Novak and Nunez 1998; Peng et al. 1995). To gain further insight and test the hypothesis that PVT neurons are indeed more active during times of subjective arousal, we applied patch-clamp recording techniques to sample the behavior and properties of anterior PVT neurons in vitro in rat brain slices prepared at zeitgeber times (ZT) corresponding to the animal's inactive (lights on) vs. active (lights off) periods. In tissue samples from midline thalamus, we also assessed mRNA expression for low-threshold Ca2+ channel and hyperpolarization-activated cyclic nucleotide-gated (HCN) subtypes.
MATERIALS AND METHODS
Slice preparation.
Experimental protocols conformed to the Canadian Council for Animal Care guidelines and were approved by the Ottawa Hospital Research Institute Animal Care and Use Committee. Recordings were obtained from neurons in the anterior part of the PVT (aPVT; bregma −1 to −2 mm) in acutely prepared brain slice preparations from Wistar rats weighing 50–120 g (both sexes; 21–35 days old). Animals were housed singly or in pairs in a temperature-controlled (22–24°C) environment with a 12:12-h light-dark (LD) cycle with food and water ad libitum. To evaluate diurnal variations, animals were housed in separate rooms and acclimatized for at least 2 wk to LD cycles where lights on (ZT 0) was adjusted so that the brain could be harvested at either ZT 2–6 (during the subjective quiet-day period) or ZT 14–18 (during the subjective active-night period; see Fig. 1B). Animals were euthanized by guillotine, and the brain was quickly removed and immersed in oxygenated (95% O2-5% CO2), cooled (<4°C) artificial cerebrospinal fluid (ACSF) of the following composition (mM): 127 NaCl, 3.1 KCl, 1.3 MgCl2, 2.4 CaCl2, 26 NaHCO3, and 10 glucose (pH 7.3, osmolality 300–310 mosmol/kg H2O). With the use of a vibrating blade microtome (Leica VT1000S; Leica, Nussloch, Germany), two 400- to 450-μm coronal slices were obtained through the most anterior part of the PVT. In an earlier study (Zhang et al. 2006b), we reported that the most rostral slice always contained the SCN and that electrical stimulation in SCN could elicit monosynaptic postsynaptic potentials in aPVT neurons. In the present study, we confirmed in 69 aPVT neurons that electrical stimulation in SCN evoked monosynaptic postsynaptic potentials, implying that the pathway from SCN to aPVT was functionally intact and therefore that neurotransmitters in these SCN efferents could potentially influence excitability in these aPVT neurons. Slices were incubated in gassed ACSF for >1 h at room temperature and then transferred to a submerged chamber and superfused (2–4 ml/min) with oxygenated ACSF at 22–24°C.
Fig. 1.

Enhanced activity is a characteristic of midline anterior thalamic paraventricular nucleus (aPVT) neurons from the night period. In this and subsequent figures, red symbols, traces, and histograms pertain to data on aPVT neurons in slices prepared during the subjective day period [zeitgeber time (ZT) 2–6], and black symbols, traces, and histograms pertain to data on neurons in slices prepared during the night period (ZT 14–18). A: representative whole cell current-clamp traces from 3 different aPVT neurons illustrate the different spontaneous patterns (silent, bursting, and tonic) observed during the initial 5 min of recordings, with corresponding ratemeter records below. Corresponding resting membrane potentials were −65 mV (silent cell; day period), −63 mV (burst firing; night period), and −50 mV (tonic firing cell; night period). Inset depicts higher temporal resolution (500 ms) of a typical low-threshold spike (LTS) crowned with sodium spikes. B: horizontal bars along the ZT axis (bottom) correspond to times for brain slice preparation (ZT 2–6, day or ZT 14–18, night). Dots in the modes of activity graph (top) represent the actual ZT times for whole cell recordings for all aPVT neurons. C: histograms illustrate the percentile distribution of silent and active neurons from both ZT periods, with numbers of cells indicated within the bars. Statistical comparisons were performed using the χ2 test.
Electrophysiology.
Individual slices were transferred to a submerged recording chamber and superfused (2–4 ml/min) with oxygenated ACSF at room temperature. Data from aPVT neurons were obtained with borosilicate thin-walled micropipettes filled with (mM) 130 K-gluconate, 10 KCl, 2 MgCl2, 10 HEPES, 1 EGTA, 2 Mg-ATP, and 0.3 Na-GTP (pH adjusted with KOH to 7.3). Lucifer yellow (2 mM) was included in the pipette solution to facilitate identification of cell location and to generate a profile of the morphology of aPVT neurons (cf. Richter et al. 2006; Zhang et al. 2006a, 2006b). Pipettes had resistances of 4–7 MΩ. Access resistance <15 MΩ was considered acceptable. Correction for liquid junction potential (approximately −10 mV) was applied to recorded membrane currents and voltages. Whole cell current-clamp and voltage-clamp recordings were obtained using an Axopatch 1D amplifier (MDS Analytical Technologies, Sunnydale, CA). Data were filtered at 2 kHz and continuously monitored and stored on disk. A Digidata 1200B interface with Clampex software (pClamp 9; MDS Analytical Technologies) was used to generate current and voltage commands and to store data. Input resistances/conductances were determined from the linear slope (between −60 and −80 mV) of the current-voltage (I-V) relationship (holding potential = −60 mV; 600-ms-duration voltage pulses stepped from −110 to −50 mV in 10-mV decrements) obtained immediately after the whole cell configuration was established. For cells displaying tonic firing, observations in current clamp mode during the first 60 s were used to calculate spontaneous mean and highest frequencies. For cells displaying spontaneous bursting activity at the beginning of recording, data from the initial 5 min were used to calculate number of bursts, interburst intervals, and burst frequencies (mean and highest, measured between first 2 spikes in a burst). In cell-attached recordings, focal application of 500 μM glutamate from a local micropipette was used to trigger firing and verify recordings from otherwise silent neurons.
Statistical analysis.
Off-line analyses were performed using Clampfit version 9 (Molecular Devices). Comparisons between control and experimental values (P < 0.05 or better) were determined using appropriate statistical tests (SigmaStat 3.0; paired and unpaired t-test, 1-way ANOVA, ANOVA on ranks, Pearson correlation). To compare distribution of neurons between day and night periods, we used the χ2 test. All results are means ± SE. Drugs were bath applied at the concentrations indicated. Drugs and reagents were purchased from Sigma Chemical (St. Louis, MO) with the exception of tetrodotoxin (TTX), which was obtained from Alomone Labs (Jerusalem, Israel).
Total RNA extraction and reverse transcription.
At times that corresponded with the slice preparations for electrophysiology (see above), another set of animals (n = 5) was euthanized, and coronal brain slices (500 μm thick) were taken for punch biopsy of tissue samples of the area of thalamus that contained aPVT. Samples were immediately frozen in dry ice and shipped overnight to Oregon. Total RNA was extracted from each sample using the RNaqueous Micro kit (Ambion, Austin, TX), quantified on a spectrophotometer (Nanodrop Technologies, Wilmington, DE), and the RNA quality measured at the ratio of 260/280 nm in a 1-μl sample. Total RNA was DNase I treated (DNAfree; Ambion) at 37°C for 30 min. cDNA synthesis was performed on 200 ng of total RNA including the following reagents: 50 units of MuLV reverse transcriptase (RT; Applied Biosystems, Foster City, CA), 10 mM Tris·HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 5 mM MgCl2, 0.625 μM dNTPs, 15 units of RNasin (Promega, Madison, WI), 10 mM DTT, and 100 ng of random hexamers, brought to a final volume of 20 μl with DEPC-treated water (Ambion). Reverse transcription was carried out at 42°C for 60 min, denatured at 99°C for 5 min, and cooled on ice for 5 min. Two hundred nanograms of total RNA from additional PVT tissue samples were processed as positive and negative controls; these samples were also reverse transcribed according to the same protocol, removing the RT from the negative controls. cDNA was diluted 1:20 with nuclease-free water (Ambion).
Quantitative real-time PCR (qPCR) was performed using the TaqMan Universal PCR master mix (Applied Biosystems) with predesigned TaqMan Gene Expression Assays (Applied Biosystems) that include both primers and probes on the 7500 Fast Real-time PCR System (Applied Biosystems). The rat-specific target probes (Cav3.1, assay ID Rn00581051_m1; Cav3.2, assay ID Rn01460335_g1; Cav3.3, assay ID Rn00571684_m1; HCN1, assay ID Rn00584498_m1; HCN2, assay ID Rn01408575_gH; HCN3, assay ID Rn00586666_m1; HCN4, assay ID Rn00572232_m1) and control probe (β-actin, assay ID 4352340) were designed to span introns. The target probes contained a 6-carboxyfluorescein phosphoramidite (FAM dye) at the 5′ end, whereas the endogenous control probe (β-actin) contained a VIC dye label at the 5′ end of the gene. A multiplex qPCR reaction contained 10 μl of 2× master mix, 1 μl of 20× probe and primer for the target gene, 1 μl of 20× probe and primer for the control gene, 4 μl of cDNA, and nuclease-free water to a 20-μl final volume. qPCR was performed on samples in triplicate under the following conditions: 95°C, 10 min; 40 cycles of amplification at 95°C, 15 s and 60°C, 1 min.
Each gene assay was tested to determine and compare the efficiencies of the target and control gene amplifications. cDNA from rat hippocampus (Cav) or PVT (HCN) was used to construct standard curves, and the efficiency was calculated according to the following formula: E = 10(−1/m) − 1, where m = slope (Livak and Schmittgen 2001). Efficiencies were as follows: Cav3.1/β-actin, 94%/96%; Cav3.2/β-actin, 96%/97%; Cav3.3/β-actin, 96%/97%, HCN1/β-actin, 93%/92%; HCN2/β-actin, 91%/94%; HCN3/β-actin, 100%/99%; HCN4/β-actin, 93%/96%. The similar efficiencies allowed us to use the ΔΔCT method to determine differences in our treatment groups (Livak and Schmittgen 2001; Pfaffl 2001). A calibrator cDNA sample from the hippocampus was used as a reference to which all samples were compared for Cav3.1, Cav3.2, and Cav3.3. We used the hippocampus cDNA for Cav3.1–3.3 because the target genes were expressed equally in these tissues. The mean ΔCT value for HCN1 in ZT 2–6 PVT samples was used as a calibrator for calculating HCN2, HCN3, and HCN4 expression at both time points as well as HCN1 in ZT 14–18 samples. The relative target gene expression was calculated using 2(−ΔΔCT), where ΔCT = target cycle threshold (CT) − control CT and ΔΔCT = ΔCT of target − ΔCT of calibrator (Livak and Schmittgen 2001). Therefore, all transcription data are expressed as an n-fold difference relative to the calibrator. The n-fold difference was averaged for each Cav3 subunit and each HCN isoform at each time point and was analyzed using a two-tailed Student's t-test or ANOVA (P < 0.05).
RESULTS
Spontaneous activity patterns.
Whole cell recordings were obtained from a total of 217 aPVT neurons. Of the 144 neurons recorded in slices prepared during the day period (mean ZT recording time 8.4 ± 0.2 h; red traces, symbols, and bars in Fig. 1), the majority (92% ; n = 132) lacked spontaneous firing and were silent at rest. The remaining cells (8%; n = 12) displayed either tonic firing at 3.8 ± 0.4 Hz (n = 5) or burst firing (n = 7), where several action potentials at high frequency (74 ± 22 Hz) were present on the crest of low-threshold spikes (LTS). The mean interburst interval was 5.5 ± 0.9 s. By contrast, data from a population of 73 neurons recorded in slices prepared from rats during the night period (mean ZT recording time 21.2 ± 0.2 h; black traces, symbols, and bars in Fig. 1) revealed significantly fewer silent neurons (48%; n = 35) and more neurons with spontaneous activity (52%; n = 38) manifested as either tonic firing (2.8 ± 0.3 Hz; n = 21) or burst firing (105 ± 10 Hz on the peak of LTS; n = 17) with an interburst interval of 7.3 ± 0.7 s. Although the numbers of active neurons were significantly larger in the night period, the ratio of tonic vs. burst firing cells between both groups was similar (5 and 7 for day vs. 21 and 17 for night; χ2 test, P = 0.624), as were the interburst intervals (ANOVA, F = 2.021, P = 0.174).
A possibility that these results might be influenced by cell dialysis associated with the whole cell patch-clamp configuration led us to perform additional recordings utilizing the cell-attached configuration. Results again revealed a significant difference in terms of numbers of active vs. silent cells: in the day period, only 6 neurons were active whereas 22 neurons were silent; in the night period, 14 neurons were active whereas 5 were silent (χ2 test, χ2 = 10.597, P = 0.001), confirming the validity of the data obtained with the whole cell patch-clamp technique. Collectively, these observations indicated that there was a significant increase in the number of “active” neurons in slices prepared during the night period, when rodents typically would have exhibited behavioral arousal in vivo.
Intrinsic properties.
A comparison of intrinsic membrane properties of neurons recorded in slices from the different ZT windows revealed two significant differences: in slices from the night period, neurons were more depolarized (−59 ± 1 vs. −69 ± 1 mV, ANOVA on ranks, H = 43.556, P < 0.001); they also had a lower resting membrane conductance (1.19 ± 0.06 vs. 1.55 ± 0.06 nS, ANOVA on ranks, H = 13.241, P < 0.001; Fig. 2A). To assess whether these two sets of intrinsic properties were different independently of data distribution, we applied the nonparametric and distribution-free Kolmogorov-Smirnov test. Results shown in Fig. 2B reveal significant differences in both resting membrane potentials and conductances across all values, implying that neurons from the night period were significantly more depolarized as a result of lower resting conductances.
Fig. 2.

Neurons from the night period display membrane depolarization and reduced conductance. A: graphs depict the significantly more depolarized resting membrane potential (RMP; left) and lower resting conductance (right) in aPVT neurons recorded from slices prepared during the night period (ZT 14–18; n = 73) vs. the day period (ZT 2–6; n = 144). Data are means ± SE for both axes. B: graphs are cumulative plots for RMP (left) and conductance (right) for all aPVT neurons included in Fig. 1, from both ZT periods. Statistical comparisons were performed using the Kolmogorov-Smirnov (KS) test. C: graph of the averaged (mean ± SE) current-voltage (I-V) relationships for all cells reveals a difference between data from neurons recorded in slices prepared during each ZT period. The net difference current (gray plot) calculated by subtraction (data from day period minus data from night period) reflects the reduction in membrane conductance recorded in neurons from the night period, with a net reversal potential approximating −110 mV. D: plots of the RMPs vs. resting conductances for neurons recorded from the day period (red; left) and night period (black; right). The gray lines, representing linear regressions, reveal a significant correlation only in the data from neurons prepared during the day period (Pearson correlation test; P = 0.008).
Subtraction of mean I-V plots for these two populations of neurons yielded a net inward current with a reversal potential near −110 mV (Fig. 2C). The estimated potassium equilibrium potential (EK) in these preparations was −97 mV. Neurons recorded in slices from the day period exhibited a correlation between the resting membrane potential and the magnitude of the resting membrane conductance (Pearson correlation test; P < 0.05). The loss of this correlation (P > 0.05; Fig. 2D) together with I-V plot changes (Fig. 2C) observed in neurons recorded from the night period are consistent with the notion that a reduction in one or more potassium conductances could be one of the contributing factors to the membrane depolarization in neurons from the night period. The preceding comparisons based on activity patterns were assessed according to the ZT period. We next compared resting membrane potentials and conductances from spontaneously active vs. silent neurons within each ZT period. As listed in Table 1, active neurons in both ZT periods were significantly more depolarized and had lower conductances than silent neurons. Although the properties of active neurons (comparing tonic vs. burst firing neurons separately) between the two ZT periods did not differ significantly (P > 0.05), the silent neurons recorded from slices prepared in the night period did have a significantly more depolarized resting membrane potential and lower resting membrane conductance compared with silent neurons recorded in slices prepared in the day period (for membrane potential: ANOVA on ranks, H = 10.240, P < 0.001; for conductance: ANOVA on ranks, H = 4.635, P < 0.05). These data suggest that reduction of conductances that contribute to membrane potentials was a major factor contributing to membrane depolarization and increased spontaneous activity in aPVT neurons in slices from the night period.
Table 1.
Basic intrinsic properties of aPVT neurons recorded from slices prepared in 2 different ZT periods vs. firing status
| Silent |
Active |
Tonic |
Bursts |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recording Time | RMP, mV | Conductance, nS | n | RMP, mV | Conductance, nS | n | RMP, mV | Conductance, nS | n | RMP, mV | Conductance, nS | n |
| ZT 8.40 ± 0.2 | −70 ± 1 | 1.6 ± 0.1 | 132 | −57 ± 3b | 1.1 ± 0.1a | 12 | −50 ± 1 | 1.2 ± 0.1 | 5 | −62 ± 4c | 1.0 ± 0.2 | 7 |
| ZT 21.20 ± 0.2 | −64 ± 1f | 1.3 ± 0.1e | 35 | −55 ± 1b | 1.1 ± 0.1a | 38 | −51 ± 1 | 1.2 ± 0.1 | 21 | −59 ± 1d | 1.0 ± 0.1 | 17 |
Values are means ± SE for resting membrane potential (RMP) and conductance measured in anterior thalamic paraventricular (aPVT) neurons. All statistical evaluations were done using ANOVA.
P < 0.05;
P < 0.001, silent vs. active neurons within the same zeitgeber time (ZT) period.
P < 0.05;
P < 0.001, tonic vs. burst-displaying neurons within the same ZT period.
P < 0.05;
P < 0.001, comparison between ZT periods.
T-type Ca2+ channels.
Previous investigations in thalamocortical neurons, and in other CNS neurons, revealed that low-threshold T-type Ca2+ channels are major contributors to rhythmic oscillatory behavior (for reviews, see Destexhe and Sejnowski 2003; Perez-Reyes 2003). T-type Ca2+ channels in various central nervous system (CNS) neurons are selectively blocked by low concentrations of nickel (reviewed in Huguenard 1996). In aPVT, we noted that 3/3 neurons that displayed spontaneous burst firing ceased firing on addition of 200 μM nickel to the bath (not shown), consistent with a role of T-type Ca2+ channels in modulating this pattern of neuronal activity. We previously reported that PVT neurons possess an abundance of low-threshold T-type Ca2+ channels (Richter et al. 2005, 2006). We hypothesized that an increase in the availability of T-type Ca2+ channels together with a decrease in membrane conductance could be major contributors to the rise in rhythmic oscillatory behavior observed in aPVT neurons in the night period. As an initial approach, we assessed the magnitude of low-threshold activated Ca2+ currents (IT) in voltage-clamp mode in ACSF containing 1 μM TTX. After a 1-s voltage prepulse to −110 mV to remove steady-state inactivation of IT, 2-s depolarizing voltage increments were applied from −50 to −90 mV to elicit IT in neurons sampled from slices in each ZT group. As shown in Fig. 3A, at levels more depolarized than −60 mV, IT amplitudes were significantly increased in neurons examined in the night period compared with those from the day period. In the population of neurons we evaluated, the mean resting membrane conductances between neurons recorded at a mean ZT of 7.3 ± 0.2 (n = 23; day period) and a mean ZT of 19.6 ± 0.2 (n = 11; night period) were not significantly different (P = 0.852) and lacked any correlation between resting conductance and the size of IT (P = 0.362), suggesting that an intrinsic mechanism contributed to the observed IT amplification.
Fig. 3.

Amplitude of T-type low-voltage-activated Ca2+ currents (IT) is enhanced in slices from the night period. A: plots and traces reflect amplitudes and voltage dependence of IT for neurons in slices from each ZT period. For all neurons, holding potential was −70 mV. The protocol used a 1-s voltage prepulse to −110 mV to remove steady-state inactivation of IT, followed by 2-s depolarizing voltage increments from −50 to −90 mV. The graph (left) is a summary of the mean IT amplitudes plotted against test membrane potentials. At levels more depolarized than −60 mV (vertical arrow), IT amplitudes were significantly increased in the neurons recorded in the night period. *P < 0.05 (1-way ANOVA). At right, representative voltage traces of IT in a neuron from each ZT period reflect the increase in peak IT amplitude (arrows) typically observed in neurons from the night period. B: summary histograms (note the different y-axis scaling) reflect relative mRNA expression for T-type low-voltage-activated Ca2+ channel subtypes (Cav3.1–3.3) in thalamic tissue biopsies of aPVT. Note the prevalence for Cav3.1 and Cav3.3 and their significant increase (*P < 0.05) in samples obtained during the night period.
We also used RT-PCR to assess mRNA expression of the individual T-type Ca2+ channel isoforms in punch biopsies from the aPVT area in slice preparations obtained at ZT 2–6 (n = 4; day period) vs. ZT 14–18 (n = 4; night period). The mammalian genome encodes genes for three distinct isoforms of the T-type Ca2+ channel, Cav3.1 (or α1G), Cav3.2 (or α1H), and Cav3.3 (or α1I) (reviewed in Perez-Reyes 2003). Our analysis revealed that the major subunit in this area of thalamus was Cav3.1, followed by Cav3.3, with almost no Cav3.2 (Fig. 3B; cf. Talley et al. 1999). Interestingly, in tissue obtained from the night period, there was a significant increase of 32% and 39% in mRNAs for both Cav3.1 and Cav3.3 isoforms, respectively (Fig. 3B; P < 0.05). Thus data from both RT-PCR analysis and from electrophysiology imply a diurnal change in IT in aPVT neurons. Although further investigations are required to assess possible mechanisms underlying these changes, here we applied additional protocols to assess T-type channel contributions to spontaneous and evoked activity (see Figs. 5 and 6 below).
Fig. 5.

Membrane potential-dependent spontaneous firing patterns. A and B: samples of current-clamp traces from 2 different neurons recorded in slices from the day period (A; red traces; ZT 7.6 for this neuron) or the night period (B; black traces; ZT 20.3 for this neuron). In this protocol, cells were manually clamped at 3 different negative membrane potentials starting at −50 mV where all neurons from both ZT periods exhibited tonic firing. At −60 mV, the majority of neurons (20/26) from the day period were silent, contrasting with 19/34 neurons from the night period, where a −10-mV hyperpolarization resulted in spontaneous bursting. At −70 mV, neurons from both ZTs were silent. C: middle histograms illustrate a significantly different percentile distribution (χ2 test) of silent and burst-firing neurons at RMP = −60 mV from both ZT periods (numbers of cells indicated within bars). There were no differences in the distribution of neurons at RMP = −50 and −70 mV.
Fig. 6.
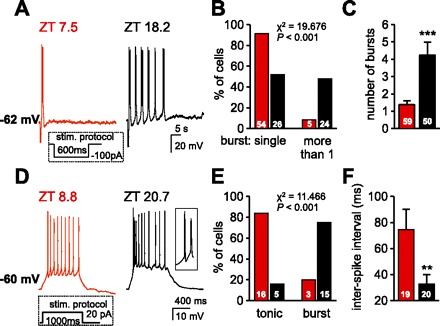
Hyperpolarization- and depolarization-induced burst firing patterns. A: representative current-clamp traces of the LTS induced on return to RMP following a 600-ms hyperpolarizing pulse of −100 pA. The trace from a neuron recorded in the day period (ZT 7.5 for this neuron) revealed only a single LTS, the most commonly observed response. By contrast, the trace from a neuron recorded in the night period (ZT 18.2 for this neuron) illustrates that the initial LTS was followed by several LTS-initiated bursts. In the cells sampled, aside from membrane conductance, no significant differences were apparent between these 2 groups of neurons in terms of RMPs (day period, −62.1 ± 0.4 mV vs. night period, −61.7 ± 0.4 mV, P > 0.05), number of action potentials crowning the first LTS (day period, 7.7 ± 1.3 vs. night period, 6.6 ± 1.1, P > 0.05), and initial frequency between the first 2 spikes in the first LTS (day period, 94 ± 8 Hz vs. night period, 99 ± 9 Hz; P > 0.05). B: summary histogram reflects the percentile distribution of neurons in slices from each ZT period that displayed a single LTS or recurrent bursting with this protocol. Cell numbers are indicated within bars. Statistical comparisons were performed using the χ2 test. C: histogram illustrates a significant increase in number of bursts in neurons from the night period with numbers of cells indicated within bars. ***P < 0.001. D: representative current-clamp recordings reflect membrane depolarization and firing patterns in response to a 20-pA depolarizing current pulse lasting 1,000 ms from a holding potential of approximately −60 mV (day period at −60.7 ± 0.4 mV, n = 19; night period at −60.5 ± 0.2 mV, n = 20). Note that the neuron recorded in the day period (red trace; ZT 8.8 for this neuron) responded with tonic firing, whereas the neuron from the night period (black trace; ZT 20.7 for this neuron) responded with an initial LTS and high-frequency firing, measured as the interval between the first 2 spikes (see inset at right). E: summary histogram illustrates the percentile distribution of the depolarizing step-induced tonic or burst firing patterns recorded from neurons in slice preparations from each ZT period. The number of cells is indicated within bars. Statistical comparisons were performed using the χ2 test. F: histogram illustrates a significant decrease in interspike intervals between the first 2 spikes in the response to a depolarizing pulse observed in cells during the night period. **P < 0.01. Stim, stimulus.
Hyperpolarization-activated cation currents.
In thalamocortical neurons, another significant intrinsic contributor to both resting membrane potentials and oscillatory and bursting activity is the hyperpolarization-activated cation current (Ih), also known as the pacemaker current (e.g., McCormick and Pape 1990a). Since we have previously reported that midline thalamic neurons do express features consistent with an Ih type of conductance (Zhang et al. 2006a), we briefly examined the contribution of Ih to resting membrane potential and to spontaneous LTS bursts in a sample of aPVT neurons.
We first tested the effects of bath applied ZD7288, a selective inhibitor of Ih-type conductances. When we compared the effect of ZD7288 (50 μM for 7–10 min) on resting membrane potentials in neurons from both ZT periods under similar conditions (maintaining membrane potential at −60 mV for all cells), we noted a significantly larger hyperpolarization in neurons from the night period (20.0 ± 5.1 mV, n = 4) compared with neurons from the day period (5.0 ± 0.5 mV, n = 6; ANOVA, F = 13.516, P < 0.01; Fig. 4A, inset). Of interest are reports that ZD7288 at concentrations used in this study can suppress T-type Ca2+ currents (Felix et al. 2003; Sanchez-Alonso et al. 2008). However, T-type Ca2+ currents were not significantly changed (from 501 ± 78 to 378 ± 54 pA; P = 0.114) in a sample of five aPVT neurons evaluated. Therefore, we can assume that the membrane hyperpolarization and decrease in oscillatory behavior illustrated in Fig. 4A are most likely the result of ZD7288 suppression of an Ih type of conductance, and that an Ih type of conductance contributes to resting membrane potential and to the generation of recurring oscillatory behavior in aPVT neurons.
Fig. 4.

Neurons from the night period display enhanced hyperpolarization-activated cation current (Ih) types of conductance. A: a typical current-clamp trace from a spontaneously bursting aPVT neuron recorded in a slice during the night period (ZT 20.4 for this cell) illustrates a gradual membrane hyperpolarization, reduction in interburst frequency, and their eventual cessation during continuous (blue line) bath application of ZD7288 alone. At right, the inset histogram illustrates a significantly larger membrane hyperpolarization induced by bath application of 50 μM ZD7288 in aPVT neurons from slices prepared from the night period (black bar; n = 4) vs. the day period (red bar; n = 6). These data were derived from a set of experiments where ZD7288 was bath applied for 10 min in the presence of 1 μM TTX at membrane potentials near −60 mV. **P < 0.01. B: 2 typical I-V relationships, with the protocol shown below, demonstrate the increase in Ih amplitude and faster activation in aPVT neurons from the night period (black traces; ZT 20.7 for this cell) vs. recordings from the day period neuron (red traces; ZT 8.6 for this cell). All recordings to assess Ih properties were done using a modified artificial cerebrospinal fluid (see text). C: summary I-V plots display Ih amplitudes (means ± SE) plotted against membrane potentials for neurons sampled in slices from each ZT period. Ih amplitudes were obtained by subtraction of the instantaneous currents (circles) measured at the end of capacitive current transients (∼20 ms after pulse onset) from steady-state currents at the end of 2-s voltage steps (triangles). Note the significant increase in Ih amplitudes in the neuron population recorded in slices from the night period. D: summary histograms reflect relative mRNA expression for Ih-type channel subtypes (HCN1–4) in thalamic tissue biopsies of aPVT. Any differences in samples obtained during the day vs. the night periods lacked significance.
We next assessed Ih expression under voltage-clamp conditions using ACSF that contained tetraethylammonium (20 mM) to block the delayed rectifier K+ current, barium (1 mM) to block inward rectifier K+ currents, 4-aminopyridine (2 mM) to block any A-type transient K+ current, TTX (1 μM) to block voltage-dependent Na+ channels, and nickel (0.5 mM) to block T-type Ca2+ channels (cf. Funahashi et al. 2003). Under these conditions, no significant difference was detected in resting membrane conductance between aPVT neurons recorded in slices prepared during the day period (1.14 ± 0.06 nS; n = 13) and the night period (1.26 ± 0.16 nS; n = 11; P = 0.463). Figure 4B illustrates the protocol applied and traces typical of Ih as detected in neurons evaluated at a mean ZT of 9.6 ± 0.3 (day period) and neurons similarly evaluated at a mean ZT of 21.3 ± 0.5 (night period). For both populations of neurons, Ih became visible at membrane potentials more hyperpolarized than −80 mV, beyond which Ih amplitudes increased significantly (from −90 mV) in the neuron population recorded from the night period (Fig. 4C, right; ANOVA, F from 4.468 to 6.146, P < 0.05). Thus, as with IT above, these observations suggest that there is also a diurnal change in an Ih type of conductance in aPVT neurons.
We also used RT-PCR to assess mRNA expression of the individual Ih-type channel isoforms in punch biopsies from the aPVT area in slice preparations obtained at ZT 2–6 (n = 4; day period) vs. ZT 14–18 (n = 5; night period; Fig. 4D). The mammalian genome encodes genes for four distinct isoforms of the Ih-type channels, HCN 1–4 (reviewed in Biel et al. 2009). Our analysis revealed that the major subunit in this area of thalamus was HCN2, followed by HCN4 > HCN3 > HCN1 (Fig. 4D). However, by contrast with the data from assessment of the mRNA for T-type Ca2+ channels (see above), there were no significant differences in relative mRNA expression for any isoform of HCN channels between the day and night periods (Fig. 4D). These data suggest that diurnal changes in both IT and Ih likely result from different mechanism(s). Although we did not pursue possible mechanisms in the present study, the data do imply that diurnal changes in both IT and Ih are likely to influence neuronal behavior at the different ZT periods examined in this study.
Membrane potential-dependent spontaneous firing patterns.
In thalamic neurons, burst and tonic firing patterns are closely related to membrane potential. We therefore applied a simple protocol to evaluate how neurons from slices in each ZT period behaved in response to an induced −10-mV change in membrane potential from a membrane potential of −50 mV, a level where we observed that aPVT neurons would display tonic low-frequency firing. As illustrated in Fig. 5, we noted a significant difference in neuronal behavior between the ZT groups when neurons were stepped to −60 mV: 19/33 neurons from the night period displayed a significant increase in the number of cells that exhibited burst firing; by contrast, only 6/26 neurons in slices from the day period exhibited the same behavior (Fig. 5C). All neurons in both ZT groups were silent at a membrane potential of −70 mV and tonically active at −50 mV (Fig. 5C).
Hyperpolarization-induced firing patterns.
The preceding observations suggested that neurons recorded from the different ZT periods might also behave differently in response to current-induced firing, especially at membrane potentials around −60 mV. Therefore, we assessed cell behavior on return to resting membrane potentials (approximately −60 mV) following a 600-ms membrane hyperpolarization, of sufficient duration to allow T-type Ca2+ channels to recover from inactivation. As illustrated in Fig. 6A, aPVT neurons typically responded to this protocol with an LTS that triggered a burst of action potentials (cf. Richter et al. 2005). Not surprisingly, the LTS was completely eliminated in the presence of nickel (200 μM; n = 3; not shown). However, the addition of 400 μM cadmium had little effect on these neurons (n = 3; not shown), suggesting that high-voltage-activated Ca2+ channels had little or no contribution to this bursting behavior.
In neurons recorded in slices from the day period, the majority (54/59; 92%) responded to this protocol with only a single LTS (Fig. 6, A and B, red trace and histograms). By contrast, 24/50 (48%) neurons recorded in slices from the night period responded with recurring bursts (range 2–20) following the initial LTS (Fig. 6, A and B, black trace and histograms). In summary (Fig. 6C), neurons from slices prepared during the night period were more likely to display an increase in the number of bursts triggered by a hyperpolarizing pulse (1.4 ± 0.2, day period vs. 4.4 ± 0.8, night period; ANOVA on ranks, H = 22.215, P < 0.001). In addition, in the night period, the 24 cells with recurring bursts (more than 1) had a resting membrane conductance of 1 ± 0.1 nS, significantly lower than the membrane conductance of 1.3 ± 0.1 nS in the 26 neurons that displayed a single LTS from the same period (ANOVA, F = 6.345, P < 0.05). Similar differences applied for the 5 cells with recurring bursts vs. the 54 neurons with a single burst recorded in slices from the day period (0.9 ± 0.2 vs. 1.4 ± 0.1 nS; ANOVA on ranks, H = 4.623, P < 0.05). Moreover, we noted that neurons with recurrent LTS bursts possessed, in addition to a decrease in conductance, a significantly larger Ih (−16.4 ± 3.5 pA; n = 24) compared with measurements in neurons with a single LTS (−8.6 ± 2.2 pA; n = 27; P < 0.05). As expected for involvement of Ih in the generation of slow rhythmicity (cf. Lüthi et al. 1998), ZD7288 significantly decreased the number of hyperpolarization-induced LTS-dependent recurrent bursts (from to 8 ± 2 to 2 ± 1; n = 4; paired t-test, P < 0.05; not shown).
Depolarization-induced firing patterns.
We next assessed firing patterns (tonic vs. burst mode) in response to a 20-pA depolarizing current pulse applied from holding potentials (approximately −60 mV) similar to those used for hyperpolarization-induced firing. Here, in slices from the day period, most neurons (16/19; 84%) responded with tonic firing (Fig. 6, D–F, red trace and histograms), whereas only 3/19 neurons displayed an initial burst of high-frequency firing followed by variable degrees of spike frequency adaptation. By contrast, in slices from the night period, most neurons (15/20; 75%) responded with an initial burst of high-frequency firing followed by variable degrees of adaptation (Fig. 6D, black trace and histograms). Neurons within this latter population (night period) collectively exhibited a significantly lower membrane conductance (1.1 ± 0.1 nS) than those from the day period (1.5 ± 0.1 nS; ANOVA, F = 7.489, P < 0.01), suggesting membrane conductance could be a contributing factor to the observed differences.
DISCUSSION
Whereas many investigations have addressed the properties of neurons in the so-called specific thalamic relay nuclei over the day-night cycle, comparatively few have focused on the characteristics of neurons in the midline thalamus. As noted earlier, in both the rodent and monkey brains, the midline aPVT exhibits a unique anatomic connectivity with brain stem and hypothalamic regions known to contribute to sleep and wakefulness. In this study we provide for the first time an initial evaluation of the properties of aPVT neurons in rat brain slices prepared at ZT 2–6 vs. ZT 14–18 and recorded at ZT 8.4 ± 0.2 vs. ZT 21.2 ± 0.2 (day vs. night period), times that approximate their rest/sleep vs. arousal/feeding periods, respectively. Comparisons between the data from these different ZT periods revealed that neurons in slices from the night period were significantly more depolarized, had reduced membrane conductance, exhibited burst and tonic modes of firing, and were more responsive to LTS induction and oscillatory behavior. Consistent with the latter, these neurons also exhibited augmented amplitudes of both IT and Ih currents. These data are in agreement with studies in rodents where Fos-related protein expression in PVT was found to be increased during the hours of darkness, when the rats are awake, and dropping significantly during the hours of light, when rodents typically sleep (Novak and Nunez 1998; Peng et al. 1995).
Reasons for a significant increase in the amplitude of IT in aPVT neurons in slices prepared during the night period remain to be investigated. Part of the explanation may be inferred from our RT-PCR data, which revealed a significant increase in the expression of mRNA for Cav3.1 and Cav3.3 Ca2+ channel subtypes in tissue samples containing aPVT from the night period. Of note, our data are in agreement with previous reports that the Cav3.1 Ca2+ channel subtype was most dominant in PVT (McKay et al. 2006; Talley et al. 1999). Interestingly, a diurnal pattern of gene expression for T-type Ca2+ channels has been reported in mouse thalamus (Nordskog et al. 2006) and rat SCN and cerebellum (Nahm et al. 2005). In the mouse thalamus, whereas the expression of Cav3.1 peaks late at night, corresponding to the early sleep period, Cav3.2 and Cav3.3 expression peak during early night, when the mice are in their late inactive phase. Similarly, in rat SCN, gene expression of T-type Ca2+ channels is greatest during the transition period from day to night, correlating with late sleep period. In this regard, our data correspond most closely to the situation in cerebellum, where expression of T-type Ca2+ channels peaks in the middle of the night, when rodents are most active.
Although there was a significant difference in resting membrane conductance between neurons recorded in slices from the two ZT groups, our analysis of IT involved populations of neurons where membrane conductances were similar, suggesting that membrane conductance was unrelated to the changes in IT amplitude. T-type Ca2+ channels are subject to modulation by many neurotransmitters and hormones, phosphorylation, G proteins, and protein kinases (Chemin et al. 2006; Leresche et al. 2004; Perez-Reyes 2003). Recently, it was shown that IT potentiation is a strong candidate for the occurrence of high-frequency LTS-dependent firing observed in thalamic neurons of awake animals (Bessaih et al. 2008). It is therefore plausible that such factors as neurotransmitters active during the waking state (e.g., glutamate) contribute to the observed increase in T-type current amplitudes. One example might be a functional coupling with metabotropic glutamate receptors, recently reported to potentiate Cav3.1 T-type Ca2+ channel-mediated transients in cerebellar Purkinje neurons (Hildebrand et al. 2009).
IK-leak and Ih, ionic conductances active below firing threshold, are important determinants of resting membrane potentials (reviewed in Biel et al. 2009; Goldstein et al. 2001). In this study, aPVT neurons from the night period showed a reduction in membrane K+ conductance, implied from the net conductance change that reversed close to EK and from the loss of a correlation between resting membrane potential and conductance present in neurons in slices from the day period. Among the major contributors to IK-leak are products of the K2P gene family, also known as “two-pore-domain channels,” a family of K+ channels that are constitutively active at rest (reviewed in Goldstein et al. 2001; Patel and Honoré 2001). Two-pore-domain transcripts are differentially expressed throughout the thalamus (Talley et al. 2001), and their modulation by transmitters and neuropeptides is a key mechanism governing neuronal excitability (Talley et al. 2000, 2001). In geniculate thalamocortical neurons, both two-pore-domain TASK-type K+ channels and Ih channels contribute to resting membrane potentials (Meuth et al. 2006). Similar conditions may apply in aPVT neurons, since 1) acid-sensitive TASK-like K+ channels contribute to resting membrane potentials, and their suppression by the arousal-promoting orexin neuropeptides contributes to membrane depolarization (Doroshenko and Renaud 2009; Kolaj et al. 2007); and 2) exposure to the selective Ih blocker ZD7288 induces membrane hyperpolarization (see Fig. 4A).
Ih is mediated by a family of hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels with four members, HCN 1–4, with a wide CNS expression (Biel et al. 2009; Monteggia et al. 2000; Notomi and Shigemoto 2004). In aPVT neurons from the night period, we found that Ih currents were significantly increased in amplitude (Fig. 4C). A larger Ih would be expected to contribute to resting membrane potential and likely to burst firing. Thus upregulation of Ih appears to be an integral part of the increased bursting patterns observed in these neurons, consistent with the suppressed oscillations induced by ZD7288. Mechanisms contributing to this diurnal shift in Ih remain to be defined. The possibility that upregulation in HCN subunit composition plays a role in any increase of Ih currents seems less likely, because we detected no significant change in their mRNA expression throughout the day-night cycle. However, HCN channels are tightly regulated by both voltage and ligands, including cAMP and phosphatidylinositol 4,5-bisphosphate (PIP2) (for review, see Biel et al. 2009). Thus any neurotransmitter that enhances either cAMP or PIP2 could potentially promote an increase in the amplitude of Ih currents, as noted in thalamocortical neurons exposed to catecholamines (McCormick and Pape 1990b) or the neuropeptides VIP and pituitary adenylate cyclase-activating peptide (PACAP) (Sun et al. 2003). In addition, a host of other transmembrane and cytosolic proteins are known or proposed to interact with HCN channels (for review, see Biel et al. 2009). For the moment, one can only speculate that diurnal changes in Ih currents are a reflection of HCN subunit coupling with these proteins and/or manifestation of neurotransmitter-induced interactions.
Thalamic relay neurons display two distinct modes of firing: at depolarized membrane potentials, cells display tonic firing and single action potentials that faithfully relay inputs to the cortex; at hyperpolarized membrane potentials, burst-firing mode prevails and neurons are deemed less faithful followers of incoming stimuli (for review, see Sherman 2001a). In sensory thalamocortical neurons, burst firing has traditionally been thought to occur only during times of inattention, drowsiness, and slow-wave sleep, when membrane hyperpolarizations are of sufficient duration to permit deinactivation of T-type Ca2+ channels (reviewed in Llinas and Steriade 2006; McCormick and Bal 1997). By contrast, tonic mode of firing was originally thought to be associated with behavioral states of arousal or REM sleep. However, an increasing number of observations in vivo support the occurrence of T-type Ca2+ channel-induced high-frequency bursts of action potentials in thalamus during different states of arousal (e.g., Faselow et al. 2001; Guido and Weyand 1995; Ramcharan et al. 2000). Because T-type Ca2+ channels can activate with small depolarizations of the membrane, they may be an important mechanism to boost weak synaptic signals, thereby playing an important role in synaptic integration (e.g., Markram and Sakmann 1994). As an entity of cellular communication, burst firing can also have a distinct presynaptic impact not only on transmitter release but also to enhance postsynaptic signal-to-noise ratios, features that clearly have potential relevance to synaptic plasticity and information processing during the aroused state (Lisman 1997; Sherman 2001b; Swadlow and Gusev 2001). In the midline thalamus, one might speculate that burst firing is pertinent to specific information transfer from aPVT to target neurons in prefrontal cortex, amygdala, and/or limbic brain regions. Upregulation of T-type Ca2+ as well as and HCN-type channels could be one mechanism contributing to the enhanced bursting noted here during the night period, when rats are generally aroused and feeding.
What molecular mechanisms might underlie the changes in cellular characteristics we observed in aPVT neurons within the circadian cycle? Many CNS neurons, including PVT, are now known to contain “clock” genes similar to those initially discovered in SCN, and their expression displays cyclic changes in mRNA and protein expression, although not necessarily in phase with that in SCN (Abe et al. 2002; Feillet et al. 2008; Shieh 2003). Rhythmic diurnal expression of the products of these clock-controlled genes might be a means whereby neurons govern their membrane excitability, conductance, and activity patterns across different ZT periods. For example, in SCN, diurnal changes have now been reported in a variety of K+ conductances, notably a fast delayed rectifier (Itri et al. 2005), BK type of Ca2+-activated K+ channels (Meredith et al. 2006; Pitts et al. 2006), and A-type K+ currents (Itri et al. 2010). By varying the expression of these (and other) channels, SCN (and other CNS) neurons can effect changes in their activity patterns and excitability over the circadian cycle (Kuhlman and McMahon 2006). Interestingly, in SCN, neuronal activity peaks during the light phase of the circadian cycle, coincident with animal inactivity and sleep, and is lowest during the dark phase when animal locomotion is at its peak (Meijer et al. 1998; Nakamura et al. 2008; Yamazaki et al. 1998). By contrast, recordings from areas outside SCN, including the adjacent subparaventricular zone (SPZ), indicate the opposite pattern of activity, with highest levels during the dark phase of the cycle (Meijer et al. 1998; Nakamura et al. 2008; Yamazaki et al. 1998). Our observations indicate that aPVT neurons are also more active during the dark phase of the day-night cycle. Given that both SPZ and aPVT are directly targeted by SCN axons, one might anticipate that some form of communication from SCN, either via neural circuitry or by some diffusible factor(s) (see Dibner et al. 2010), could have a role in determining diurnal changes in their excitability. Thus it will be important to compare the current data with observations from other midline and intralaminar thalamic neurons that do not have an anatomical association with SCN.
To conclude, this initial in vitro study evaluated and compared firing patterns and intrinsic properties in PVT neurons in brain slices prepared at ZT times that corresponded with quiet/sleeping (ZT 2–6) vs. aroused/feeding times (ZT 14–18). PVT in rodents and lower primates appears to represent a unique midline thalamic nucleus by virtue of its connectivity with brain stem and lateral hypothalamic neurons known to participate in feeding and sleep-arousal behaviors, and with prefrontal and limbic brain regions associated with motivation and cognitive functions. The observations presented in this study reveal for the first time that PVT neurons in slices from the night period are significantly more active with tonic and bursting activity, with depolarized membrane potentials and lower membrane conductances. Contributing to this enhanced activity is an associated increase in cell Ih and IT. Associated with the latter is an upregulation in mRNA for T-type Cav3.1 and Cav3.3 Ca2+ channel isoforms in aPVT tissue samples from the night period.
GRANTS
This work was supported by Canadian Institutes of Health Research (CIHR) Grant MOP-77745 and Ontario Heart and Stroke Foundation Grant T 5643 (to L. P. Renaud) and by National Institute of Neurological Disorders and Stroke Grant NS43330 (to O. K. Rønnekleiv). L. P. Renaud holds the GSK/CIHR/J. David Grimes Research Chair at the University of Ottawa.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.K., L.Z., and L.P.R. conception and design of research; M.K., L.Z., and O.K.R. analyzed data; M.K., L.Z., and L.P.R. interpreted results of experiments; M.K., L.Z., and O.K.R. prepared figures; M.K. drafted manuscript; M.K., O.K.R., and L.P.R. edited and revised manuscript; M.K., L.Z., and L.P.R. approved final version of manuscript; L.Z. and O.K.R. performed experiments.
ACKNOWLEDGMENTS
We thank Martha A. Bosch for excellent technical assistance with the channel mRNA expression analysis, and Michael Hermes for comments on an earlier version of this manuscript.
REFERENCES
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci 22: 350–356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The midline and intralaminar thalamic nuclei. Anatomic and functional specificity and implications in neurologic disease. Neurology 71: 944–949, 2008 [DOI] [PubMed] [Google Scholar]
- Bessaïh T, Leresche N, Lambert RC. T current potentiation increases the occurrence and temporal fidelity of synaptically evoked burst firing in sensory thalamic neurons. Proc Natl Acad Sci USA 105: 11376–11381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885, 2009 [DOI] [PubMed] [Google Scholar]
- Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium 40: 121–134, 2006 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski TJ. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol Rev 83: 1401–1453, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010 [DOI] [PubMed] [Google Scholar]
- Doroshenko P, Renaud LP. Acid-sensitive TASK-like K+ conductances contribute to resting membrane potential and to orexin-induced membrane depolarization in rat thalamic paraventricular nucleus neurons. Neuroscience 158: 1560–1570, 2009 [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA 98: 15330–15335, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feillet CA, Mendoza J, Albrecht U, Pévet P, Challet E. Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci 37: 209–221, 2008 [DOI] [PubMed] [Google Scholar]
- Felix R, Sandoval A, Sánchez D, Gómora JC, De la Vega-Beltrán JL, Treviño Darszon A. ZD7288 inhibits low-threshold Ca2+ channel activity and regulates sperm function. Biochem Biophys Res Commun 311: 187–192, 2003 [DOI] [PubMed] [Google Scholar]
- Funahashi M, Mitoh Y, Kohjitani A, Matsuo R. Role of the hyperpolarization-activated cation current (Ih) in pacemaker activity in area postrema neurons of rat brain slices. J Physiol 552: 135–148, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn LL, Steriade M. Discharge rates and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci 2: 1387–1404, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SAN, Brockenhauer D, O'Kelly I, Zilberg N. Potassium leak channels and the KCNK family of two-p-domain subunits. Nat Rev Neurosci 2: 175–184, 2001 [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci 17: 52–57, 1994 [DOI] [PubMed] [Google Scholar]
- Guido W, Weyland T. Burst responses in thalamic relay cells of the awake behaving cat. J Neurophysiol 74: 1782–1786, 1995 [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, Isope P, Miyazaki T, Nakaya T, Garcia E, Feltz A, Schneider T, Hescheler J, Kano M, Sakimura K, Watanabe M, Dieudonné S, Snutch TP. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines. J Neurosci 29: 9668–9682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR. Low threshold calcium currents in central nervous system neurons. Annu Rev Physiol 58: 329–348, 1996 [DOI] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci 8: 650–656, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol 103: 632–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Doroshenko P, Cao XY, Coderre E, Renaud LP. Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience 147: 1066–1075, 2007 [DOI] [PubMed] [Google Scholar]
- Kuhlman S, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms 21: 470–481, 2006 [DOI] [PubMed] [Google Scholar]
- Leresche N, Hering J, Lambert RC. Paradoxical potentiation of neuronal T-type Ca2+ current by ATP at resting membrane potential. J Neurosci 24: 5592–5602, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506: 263–287, 2008 [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38–43, 1997 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TW. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95: 3297–3308, 2006 [DOI] [PubMed] [Google Scholar]
- Lüthi A, Bal T, McCormick DA. Periodicity of thalamic spindle waves is abolished by ZD7288, a blocker of Ih. J Neurophysiol 79: 3284–3289, 1998 [DOI] [PubMed] [Google Scholar]
- Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci USA 94: 5207–5211, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20: 185–215, 1997 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurons. J Physiol 431: 291–318, 1990a [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurons. J Physiol 431: 319–342, 1990b [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. CaV3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci 24: 2582–2594, 2006 [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Détári L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci 18: 9078–9087, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci 9: 1041–1049, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth SG, Kanyshkova T, Meuth P, Landgraf P, Munsch T, Ludwig A, Hofmann F, Pape HC, Budde T. Membrane resting potential of thalamocortical relay neurons is shaped by the interaction among TASK3 and HCN2 channels. J Neurophysiol 96: 1517–1529, 2006 [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359: 221–238, 1995 [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res Mol Brain Res 81: 129–139, 2000 [DOI] [PubMed] [Google Scholar]
- Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci 25: 9304–9308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD, Takumi T. In vivo monitoring of circadian timing in freely moving mice. Curr Biol 18: 381–385, 2008 [DOI] [PubMed] [Google Scholar]
- Nordskog BK, Hammarback JA, Godwin DW. Diurnal gene expression patterns of T-type calcium channels and their modulation by ethanol. Neuroscience 141: 1365–1373, 2006 [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol 471: 241–276, 2004 [DOI] [PubMed] [Google Scholar]
- Novak CM, Nunez AA. Daily rhythms in Fos activity in the rat ventrolateral preoptic area and midline thalamic nuclei. Am J Physiol Regul Integr Comp Physiol 275: R1620–R1626, 1998 [DOI] [PubMed] [Google Scholar]
- Otake K, Ruggerio DA. Monoamines and nitric oxide are employed by afferents engaged in midline thalamic regulation. J Neurosci 15: 1891–1911, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci 24: 339–346, 2001 [DOI] [PubMed] [Google Scholar]
- Peng ZC, Zucconi GG, Bentivoglio M. Fos-related protein expression in the thalamic midline paraventricular nucleus of the rat thalamus: basal oscillation and relationship with limbic afferents. Exp Brain Res 104: 21–29, 1995 [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83: 117–161, 2003 [DOI] [PubMed] [Google Scholar]
- Peyron C, Tight DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res 1071: 54–62, 2006 [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacol Rev 35: 192–216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran K, Sun C, Bertram EH. Altered pharmacology and GABA-A receptor subunit expression in dorsal midline thalamic neurons in limbic epilepsy. Neurobiol Dis 33: 119–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci 17: 55–62, 2000 [DOI] [PubMed] [Google Scholar]
- Richter TA, Kolaj M, Renaud LP. Low voltage-activated Ca2+ channels are coupled to Ca2+-induced Ca2+ release in rat thalamic midline neurons. J Neurosci 25: 8267–8271, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Kolaj M, Renaud LP. Heterogeneity in low voltage-activated Ca2+ channel-evoked Ca2+ responses within neurons of the thalamic paraventricular nucleus. Eur J Neurosci 24: 1316–1324, 2006 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998 [DOI] [PubMed] [Google Scholar]
- Sanchez-Alonso JL, Halliwell JV, Colino A. ZD 7288 inhibits T-type calcium current in rat hippocampal pyramidal cells. Neurosci Lett 439: 275–280, 2008 [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull 61: 25–49, 2003 [DOI] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24: 122–126, 2001a [DOI] [PubMed] [Google Scholar]
- Sherman SM. A wake-up call from the thalamus. Nat Neurosci 4: 344–346, 2001b [DOI] [PubMed] [Google Scholar]
- Shieh KR. Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience 118: 831–843, 2003 [DOI] [PubMed] [Google Scholar]
- Shyu BC, Vogt BA. Short-term plasticity in the nociceptive thalamic-anterior cingulate pathway. Mol Pain 5: 51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curró Dossi R, Contreras D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (∼40 HZ) spike-bursts at ∼1000 HZ during waking and rapid eye movement sleep. Neuroscience 56: 1–9, 1993 [DOI] [PubMed] [Google Scholar]
- Sun QQ, Prince DA, Huguenard JR. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. J Neurosci 23: 2751–2758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci 4: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19: 1895–1911, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39: 107–140, 2002 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci 18: 10709–10723, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Doroshenko P, Cao XY, Irfan N, Coderre E, Kolaj M, Renaud LP. Vasopressin induces depolarization and state-dependent firing patterns in rat thalamic paraventricular nucleus neurons in vitro. Am J Physiol Regul Integr Comp Physiol 290: R1226–R1232, 2006a [DOI] [PubMed] [Google Scholar]
- Zhang L, Kolaj M, Renaud LP. Suprachiasmatic nucleus communicates with anterior thalamic paraventricular nucleus neurons via rapid glutamatergic and GABAergic neurotransmission: state-dependent response patterns observed in vitro. Neuroscience 141: 2059–2066, 2006b [DOI] [PubMed] [Google Scholar]


