Abstract
The regulation of forces is integral to motor control. However, it is unclear how information from sense organs that detect forces at individual muscles or joints is incorporated into a frame of reference for motor control. Campaniform sensilla are receptors that monitor forces by cuticular strains. We studied how loads and muscle forces are encoded by trochanteral campaniform sensilla in stick insects. Forces were applied to the middle leg to emulate loading and/or muscle contractions. Selective sensory ablations limited activities recorded in the main leg nerve to specific receptor groups. The trochanteral campaniform sensilla consist of four discrete groups. We found that the dorsal groups (Groups 3 and 4) encoded force increases and decreases in the plane of movement of the coxo-trochanteral joint. Group 3 receptors discharged to increases in dorsal loading and decreases in ventral load. Group 4 showed the reverse directional sensitivities. Vigorous, directional responses also occurred to contractions of the trochanteral depressor muscle and to forces applied at the muscle insertion. All sensory discharges encoded the amplitude and rate of loading or muscle force. Stimulation of the receptors produced reflex effects in the depressor motoneurons that could reverse in sign during active movements. These data, in conjunction with findings of previous studies, support a model in which the trochanteral receptors function as an array that can detect forces in all directions relative to the intrinsic plane of leg movement. The array could provide requisite information about forces and simplify the control and adaptation of posture and walking.
Keywords: sensory encoding, load, posture, walking
the detection and regulation of forces are both integral and adaptive processes in pattern generation and motor control (Pearson 2008; Rossignol et al. 2006). Signals of forces and load act as cues in walking that can affect the timing of phase transitions and coordination of leg movements (Büschges et al. 2008; Duysens et al. 2000). Receptors that detect forces also provide feedback that adjusts motor output to load during the stance phase (Klint et al. 2010). In addition, loading has similar temporal and scalar effects when the direction of walking is changed, even though different groups of leg muscles must be activated (Pang and Yang 2000; Rosenbaum et al. 2010).
Force regulation has also been suggested as an organizing principle in the generation of motor outputs by the nervous system (Roh et al. 2011). Some premotor interneurons in vertebrates can function as “motor primitives” that activate leg muscles to produce specific force vectors (Giszter et al. 2007; Hart and Giszter 2010). In postural perturbations, muscles are recruited as synergists according to the forces they produce in maintaining the position of the center of mass (Torres-Oviedo and Ting 2007; Welch and Ting 2009). Similar synergies may underlie muscle activation patterns in walking (Chvatal et al. 2011; Neptune et al. 2009). The regulation of forces has also been successfully adopted in simulations of walking and in the control of legged robots (Ekeberg and Pearson 2005; Schneider and Schmucker 2006). In control systems and models, forces are often defined as vectors within a reference frame (Raptis et al. 2010). For example, in a body reference frame, gravity acts as a vertical force in upright posture, while propulsive and lateral forces occur as vectors perpendicular to the plane of the substrate (Ting and Macpherson 2004).
However, little is known about the overall neuronal organization or frame of reference underlying processing of sensory inputs that signal force and load in animals (Jankowska and Edgley 2010). In vertebrates, forces and loads are monitored at individual muscles by Golgi tendon organs (Jami 1992), but it is unclear how this information is integrated into more global mechanisms of control (Gossard et al. 1994), as occurs in force regulation when the direction of walking is changed (Mugge et al. 2010; Pang and Yang 2002).
Campaniform sensilla are mechanoreceptors that detect forces as strains in the exoskeleton of insects (Schmitz 1993; Zill et al. 2004). In insect legs, the receptors are located in groups with similar directional sensitivities and response characteristics (Zill et al. 1999, 2011). In insects, campaniform sensilla are found on all leg segments distal to the coxa but are highly concentrated on the trochanter, a small segment in the proximal leg (Frantsevich and Wang 2009; Petryszak and Fudalewicz-Niemczyk 1994). Earlier experiments demonstrated that the trochanteral receptors are particularly important in walking. After removal of the trochanter sensors (by amputation or cutting of leg nerves), animals do not use the leg forcefully and show multiple stepping in single cycles of movement (Noah et al. 2004; Wendler 1966), similar to findings in cats in which individual legs were deafferented by dorsal rhizotomy (Wetzel et al. 1976). Recent studies have also shown that trochanteral campaniform sensilla have potent effects on pattern generation in walking: sensory signals can reset the rhythm of muscle bursts (Akay et al. 2004) and have determining effects on the pattern of leg coordination (Borgmann et al. 2009). In addition, effects of some campaniform sensilla can change and reverse according to the direction of progression (Akay et al. 2007).
Despite their early identification and characterization (Pringle 1938a, 1938b), there has been no overall understanding of how the trochanteral receptors encode forces or whether these signals are related to a reference frame. Furthermore, it has not been clear why forces are measured at that location rather than at the feet (tarsi), which would more closely reflect ground reaction forces similar to a load cell or force plate (Djuric et al. 2010). Modeling studies using techniques of finite-element analysis have indicated that the campaniform sensilla in cockroaches signal external loads and muscle forces as an array (Kaliyamoorthy et al. 2006). However, those studies were complicated by the mobility of the trochanter-femur joint in cockroaches (Watson et al. 2002).
We have recorded the responses of the trochanteral campaniform sensilla in stick insects to both loads and muscle contractions. The stick insect is advantageous for these studies because of the immobility of the trochantero-femoral joint, which permits application of forces to the femur in a number of directions (Schindler 1979). There are four groups of campaniform sensilla on the stick insect trochanter, and previous studies have shown that some sensilla (Groups 1 and 2) encode loads that occur in an anterior-posterior direction, orthogonal to the plane of joint movement (Hofmann and Bässler, 1982, 1986; Schmitz 1993). The response properties of other groups have not been determined. In addition, responses of campaniform sensilla to muscle forces have been clearly demonstrated (Delcomyn 1991; Zill et al. 2011; Zill and Moran 1981), but their effects on load detection are unclear. However, the presence of muscle contractions or stiffness is necessary for force detection by the receptors (Zill et al. 2004; Zill and Moran 1981).
The present study shows that the trochanteral campaniform sensilla provide specific information about loads and muscle forces, similar to Golgi tendon organs. Furthermore, forces detected at the trochanter occur within a frame of reference, the common plane of movement of most intrinsic leg joints (Cruse and Bartling 1995). The signals provided by the array of trochanteral campaniform sensilla may simplify leg control and the recruitment of synergist muscles.
METHODS
All experiments were performed on adult female stick insects (Carausius morosus) raised in animal colonies at the University of Bielefeld or the University of Cologne.
Morphological Studies
For studies using confocal and light microscopy, the trochanter of the middle leg was removed and split (with a small piece of razor blade) into either anterior/posterior or dorsal/ventral halves. For detailed study of Groups 3 and 4, the cuticle of the dorsal trochanter was further dissected and isolated. Specimens were then treated with 1 M potassium hydroxide for a minimum of 1 h. Preparations were fixed in 4% formalin and placed in Conray (a radiopaque dye that can be used as a clearing agent for insect cuticle; Zill et al. 2010) and then viewed and imaged by standard light photomicrographic techniques. Specimens were also imaged by confocal microscopy with a Leica TCS SP5 II microscope at the Marshall University microscopy facility. Three-dimensional reconstructions were assembled from image series in Voxblast (Vaytek) or ImageJ (National Institutes of Health) software.
Physiological Studies
Sensory recordings.
Physiological studies were performed on the left middle legs (N = 63). Animals (intact or after sensory ablation) were first securely restrained on a platform with staples made of bent insect pins. The coxa of the middle leg was firmly fixed with cyanoacrylate adhesive to small staples placed above and below the segment to prevent movements at the body-coxa (thoraco-coxal) joint. Care was taken so that adhesive did not spread to the coxo-trochanteral joint so that it remained freely movable when forces were not resisted. The coxa was carefully oriented so that the leg plane was parallel to the upper surface of the platform. The distal leg was amputated in the distal femur, proximal to the femoro-tibial joint, and a mixture of Vaseline and paraffin oil was placed over the end of the femur to prevent desiccation. The proximal leg segments were not dissected and remained attached to the thorax to ensure normal ventilation through the animal's tracheal system.
In recordings of sensory activities, the main leg nerve (nervus cruris) was then exposed through a window made in the thorax proximal to the coxa (Bässler 1983). The nerve was crushed with a pair of fine forceps either close to the mesothoracic ganglion or distal to the branch point of the nerve to the coxal depressor (nerve C2). Recordings were taken with an oil-hook electrode with a reference electrode placed in the thorax (Schmitz et al. 1988). We were able to isolate a greater length of the nervus cruris when it was crushed at the mesothoracic ganglion and motor branches were not preserved. The amplitude of potentials recorded from the nervus cruris was higher, and classes of units could be distinguished according to the size of the potentials in those preparations. Signal-to-noise ratios were also improved by placing petroleum jelly around the nervus cruris.
Ablation of sense organs.
Sense organs at the coxo-trochanteral joint were ablated as follows: the anterior and posterior groups of trochanteral (Groups 1 and 2) and the femoral campaniform sensilla (Group 5) were destroyed with a fine insect pin (Schmitz 1993). The trochanteral hair plate was ablated with a very small section of razor blade (Dean and Schmitz 1992; Schmitz 1986). The damage to cuticle was kept to a minimum, and sensory responses recorded from these preparations could remain stable for hours (maximum tested 7 h).
In addition, other signals from sense organs at the thoraco-coxal and coxo-trochanteral joints were eliminated by cutting their nerves near the ganglion. For example, inputs from internal levator stretch receptors (levSR) were eliminated by cutting the nl3 nerve (Tatar 1976). Signals from the femoral chordotonal organ were eliminated by cutting its apodeme (when the leg was severed in the distal femur) and by carefully removing the apodeme with the attached sensory neurons.
Mechanical stimulation.
Forces were imposed on the femur with a probe with strain gauges mounted on a piezoelectric crystal (Zill et al. 2010). Voltage waveforms were generated from prerecorded sequences with a CED laboratory interface (power 1401mkII, CED, Cambridge, UK). At the start of the tests, the force probe was brought into sustained contact with the leg (as indicated by a small rise in the output of the strain gauges). This point was taken as a zero value. Data were discarded if the probe did not maintain contact with the femur throughout a series (as indicated by the gauge output). In ramp and hold tests, the force levels could reach a peak and then decline during the hold phase while the voltage to the piezoelectric crystal was held constant. This was also seen in a previous study of the tibial campaniform sensilla and may reflect viscoelasticity of the cuticle in stick insects (Zill et al. 2011).
To apply forces at the depressor muscle insertion, the head of a minuten pin was inserted into the cuticle distal to the attachment of the depressor muscle tendon. The proximal, ventral part of the trochanter is reinforced by an internal cuticular buttress (see Fig. 1E and below) that creates a small compartment distal to the muscle insertion. The tip of the pin was inserted through ventral cuticle into this compartment. Forces were applied to the pin and insertion point to mimic depressor muscle contractions driven by a computer-controlled linear motor (Hellekes et al. 2012). Voltage waveforms were applied to the motor from the CED interface. The plane of pull of the motor was carefully adjusted so that it produced smooth depression movements when unopposed by the force probe at the femur. No sensory discharges occurred during these tests (see results), indicating that the pin did not act to directly compress the cuticular caps of the campaniform sensilla. We did not test application of forces at other points on the trochanter, as firing of campaniform sensilla can occur when mechanical stresses are applied immediately adjacent to the receptor caps (Chapman et al. 1973). The pin was also used to resist loads applied to the femur in all directions, although forces were only generated by the motor and applied to the pin in the direction of joint depression.
Fig. 1.
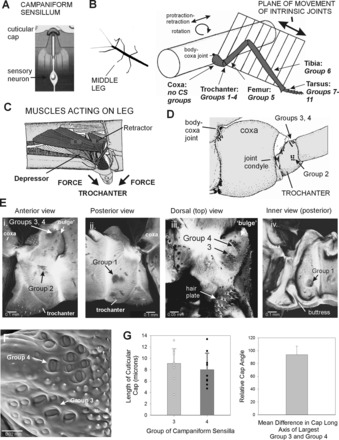
Structure of leg and trochanteral campaniform sensilla. A: campaniform sensilla monitor strains in the exoskeleton through the dendritic insertion of the sensory neuron to a cuticular cap. B: drawing of middle leg and locations of campaniform sensilla (redrawn after Cruse and Bartling 1995). The leg is segmented and attached to the body at the basal segment (coxa). The body-coxa joint has considerable freedom of movement. The more distal leg articulations are intrinsic hinge joints that act in a single plane. The distribution of groups of campaniform sensilla is not uniform. No groups are found on the coxa, many groups (Groups 1–4) are concentrated on the trochanter, and single groups are found on the distal segments or subsegments (Groups 5–11). CS, campaniform sensilla. C: interior view of the thorax and muscles that act on the leg (redrawn after Marquardt 1940). The largest muscles that move the leg (retractor, protractor, levator, and depressor muscles) are located in the thorax and insert onto the coxa (Cx) and trochanter (Tr). D: drawing of anterior side of the coxa and trochanter. The groups of campaniform sensilla are located distal to the coxo-trochanteral joint condyle and are therefore in the leg plane. E: reconstructions of confocal images of the exoskeleton. i: Anterior view of trochanter—Group 2 sensilla are located on the anterior side of the trochanter opposite the anterior condyle of the coxo-trochanteral hinge joint. ii: Posterior view—Group 1 receptors are situated on the posterior side and have a similar orientation relative to the posterior joint condyle. iii: Dorsal view—Groups 3 and 4 are located on the dorsal side of the trochanter adjacent to the joint with the femur. iv: Interior view of the posterior half of the trochanter—a thick internal projection (buttress) reinforces the trochanter adjacent to the insertion of the trochanteral depressor muscle. F: scanning electron micrograph showing cuticular caps of Groups 3 and 4. G: histograms of sizes and orientation of sensilla. Each group contains cuticular caps of different sizes (left), and the largest caps of Groups 3 and 4 are mutually perpendicular (right) [mean difference between Groups 3 and 4 = 93.8 ± 13.4° (SD), N = 8].
Stimulation and ablation of caps of campaniform sensilla.
The caps of single sensilla were mechanically stimulated with a fine tungsten wire that was etched to a tip size of ∼1 μm and mounted to a separate piezoelectric crystal (method of Chapman et al. 1973). The cuticular caps were viewed at high power in the dissecting microscope with a small first surface mirror mounted on the platform adjacent to the leg. In other experiments, the cuticular caps were ablated with a minuten pin either hand held or mounted to a micromanipulator. We also developed a method that permitted selective ablation of Group 4 sensilla with an insect pin that was electrochemically etched to a fine point and mounted in a micromanipulator. The pin was carefully advanced parallel to the femur until it made contact with and barely penetrated the distal end of the rounded cuticular elevation (“bulge,” see results), which bears the Group 4 receptors. The site of ablation was confirmed by subsequent visualization of specimens as whole mount preparations. In all experiments, responses to mechanical stimuli were retested after the ablations.
Recordings of depressor motoneuron activities.
The motor innervation of the depressor muscle, as a whole, is derived from two sources: nl4 innervates the part of the muscle that arises in the thorax, while nerve C2 provides motor axons to the coxal part of the depressor (Cruse et al. 1993; Schmitz 1986). Bursts in nl4 were often of large units that produced cross talk on the sensory recording from the nervus cruris. Activities of the trochanteral depressor were therefore monitored through muscle recordings (with 50-μm silver wires insulated to their tips placed in the coxal part of the muscle) or nerve recordings from branch C2 (Akay et al. 2001; Rosenbaum et al. 2010). The slow excitatory depressor motoneuron was identified by its pattern of activity and responses to sensory inputs (Schmitz 1986). In some studies, the effects of muscle forces on sensory activities were studied in intact animals. Muscle contractions occurred spontaneously or were evoked by touching the animal with a small brush. We were also able to obtain recordings of trochanteral campaniform sensilla in some preparations when C2 arose proximally, allowing the nervus cruris to be crushed distal to the branching point while leaving the innervation of the muscle intact. In all studies, forces generated by the depressor muscle were resisted only by the probe adjacent to the femur (in the midrange of the coxo-trochanteral joint angle). Movements at the joint were not impeded when the probe was moved away from the femur.
Data Storage and Analysis
All neurophysiological and force data were recorded with a CED laboratory interface. Recordings were analyzed with Spike 2 scripts and plotted in SigmaPlot.
Data on the response properties of Group 3 and 4 sensilla were plotted from experiments in which forces were applied at low levels and recordings had high signal-to-noise ratios. These recordings permitted sorting of multiunit activities into classes of potentials with templates identified by stimulation of cuticular caps of individual sensilla. This method was found to be essential in determining receptor discharge frequencies for analysis of sensitivities to rate of force application and the summation of the effects of muscle force and loads. The resulting plots of force sensitivities, although based upon multiple tests from a limited number of preparations, were similar to those obtained in previous studies of responses of campaniform sensilla (Ridgel et al. 2000). In addition, the basic characteristics of discharges of the trochanteral campaniform sensilla that we report, such as sensitivities to force direction and discharges to force increases/decreases, were apparent in all recordings. However, spike collisions and summations prevented analysis of classes of units according to spike amplitude.
RESULTS
Leg Structure and Anatomy of Groups of Campaniform Sensilla
Campaniform sensilla are located in groups at discrete locations in the leg that are related to the types of joint movements and the insertions of groups of muscles (Fig. 1, A–C). The middle legs of stick insects, like those of other arthropods, are segmented (Bässler and Büschges 1998): the most proximal segment, the coxa, is attached to the body by a series of flexible articulations that permit considerable freedom of movement (Fig. 1, B and C). The more distal segments, the trochanter and tibia, are linked by more restrictive hinge joints whose axes of rotation are nearly parallel and form a common plane, the “leg plane” (Cruse and Bartling 1995). The trochanter-femur joint is considered to be completely fused in stick insects, and it is the site at which autotomy occurs (Schindler 1979). The most distal segment, the tarsus, is composed of subsegments that are linked by flexible membranes.
The most proximal segment, the coxa, has no groups of campaniform sensilla (Fig. 1, D and E). This is also true in all other insects that have been studied (Petryszak and Fudalewicz-Niemczyk 1994), although isolated cuticular caps may be seen in some preparations (S. N. Zill, personal observation). In contrast, four prominent groups (Groups 1–4) are present on the trochanter, and one group is found on each of the more distal segments (femur, tibia) or tarsal subsegments (Hofmann and Bässler 1982; Pringle 1938b). Thus all campaniform sensilla are located within segments that form the plane of leg movement (or are distal to that plane).
The factors underlying the concentration of receptors on the trochanter have been unclear, but it is important to note that the largest muscles that move the leg take origin in the body (Fig. 1C). These muscles (protractors-retractors, levators-depressors, and coxal rotators) insert on the coxa, trochanter, or small cuticular plates (trochantins, etc.) that attach the leg to the body wall (Marquardt 1940). The forces generated by all these muscles are transmitted to the distal leg through the trochanter. The trochanter thus forms a final common pathway for forces that act on and within the plane of leg movement (because the trochanter is located distal to the coxo-trochanteral joint).
The four groups of campaniform sensilla are located on the anterior, posterior, and dorsal sides of the trochanter (Fig. 1, D and E). The caps are oval shaped. The long axes of the cuticular caps of the anterior (Group 1) and posterior (Group 2) groups are oriented perpendicular to the condyles of the coxo-trochanteral hinge joint on each side (Delcomyn 1991; although Group 2 also has a subgroup of another orientation, Hofmann and Bässler 1982). The dorsal groups (Groups 3 and 4) are on the surface of a prominent lobular projection [termed the “bulge” (Wulst) by Schindler 1979] adjacent to the joint between the trochanter and femur. Group 3 sensilla are generally perpendicular to the leg long axis (Fig. 1F). Measurements of the lengths and orientations of the caps of sensilla of Groups 3 and 4 in whole mount preparations showed that they had a range of sizes and that the largest sensilla of Groups 3 and 4 were mutually perpendicular in orientation (Fig. 1G).
While the outer surface of the trochanter is relatively smooth, the inner surface has a prominent internal projection that forms a buttress spanning the ventral surface at the insertion of the trochanteral depressor muscle (Fig. 1Eiv). Similar internal structures are found in cockroaches (Zill et al. 2000), and studies using finite-element analysis suggest that they serve mainly as structural reinforcements, as their removal leads to higher levels but qualitatively similar types of strain (Flannigan 1998). In the present study, the buttress permitted application of forces to the muscle insertion to mimic depressor contractions.
Sensory Responses Recorded from All Groups Simultaneously
To gain an overview of the types of information provided by the trochanteral campaniform sensilla, we recorded sensory responses in the main leg nerve (nervus cruris) to forces applied to the femur in different directions in preparations with all groups of campaniform sensilla intact (Fig. 2, A and B). These tests also served as controls to ensure that further ablations did not grossly alter sensory responses. In these preparations, the trochanteral hair plate was carefully shaved, the femoral chordotonal was removed (see methods), and the leg was severed proximal to the femoro-tibial joint. Figure 2C shows recordings of tests in which forces were applied in the direction of depression or levation of the coxo-trochanteral joint and in directions perpendicular to the plane of joint movement (anterior, posterior). Movement of the trochanter was prevented by the pin placed at the depressor muscle insertion. Vigorous, multiunit discharges occurred to forces applied in each direction that were often sustained for the duration of force application. [Rapid repetitive bursting or sudden response cessation, which occurs in overstimulation or injury discharges of cuticular mechanoreceptors, was not seen in any test (Chapman 1965)]. Figure 2D shows histograms of the mean responses recorded in the nervus cruris to different directions of forces (levate n = 22, depress n = 12, anterior n = 23, posterior n = 18; N = 3 animals) applied at similar rates and magnitudes in animals in which no groups of sensilla were ablated. Large discharges occur during the rising phase of the stimulus that adapts to a variable extent during the hold phase. In addition, consistent responses were obtained in all directions when applied forces were decreased. Thus the trochanteral receptors can apparently encode force increases or decreases in all directions.
Fig. 2.
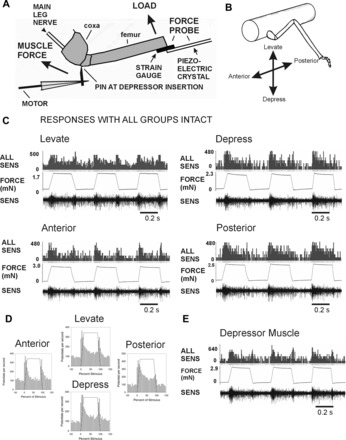
Preparation and responses of sensilla with all groups intact. A: preparation. Loads were applied to the femur with a force probe linked to a piezoelectric crystal. The probe contained strain gauges and was used to monitor forces in all experiments. Movement of the leg was resisted by a pin inserted into a small hole adjacent to the depressor muscle insertion. In experiments emulating the effects of muscle contractions, forces were applied to the pin via a computer-controlled linear motor. Sensory activities were recorded in the main leg nerve (nervus cruris) proximal to the coxa; the nerve was crushed close to the mesothoracic ganglion. B: orientation of forces relative to the leg plane. Loads were applied in different directions (levation, depression, anterior, posterior) by rotating the micromanipulator that held the force probe. C: recordings during load application with all groups of campaniform sensilla intact (other receptors ablated). Applying loads via the probe with ramp and hold functions produced sensory discharges in all directions. D: mean sensory discharges: histograms plotting the mean sensory discharges during application of forces in each direction. Discharges occurred during the rising phase that rapidly adapted to lower levels during the hold phase. Prominent phasic discharges occurred to decreasing forces in all directions. E: response to forces applied at the depressor muscle insertion. Intense firing at similar amplitudes occurred when movements of the femur were blocked by the force probe. Bursts were also present during force decreases.
We also tested responses to forces applied at the insertion of the trochanteral depressor muscle (Fig. 2E) that were resisted by the probe at the end of the femur. Strong discharges occurred both to force application and release, and the size of the recorded potentials was similar to that seen to loads imposed as forces to the distal femur. Thus trochanteral sensilla can encode both external loads and strains resulting from resisted muscle contractions.
Responses of Groups 3 and 4 to Loads Applied to Femur
We characterized the specific responses of Groups 3 and 4 by first carefully ablating all other groups of campaniform sensilla (Groups 1 and 2 on the trochanter and Group 5 on the femur). Forces were also applied at low amplitudes to decrease the effects of spike collisions. Vigorous sensory discharges were recorded in the nervus cruris when forces were applied to the femur in a dorsal direction (levation of the CTr joint) using half-sine waveforms (Fig. 3A; as in Keller et al. 2007). The largest units typically fired phasically during the rising phase of the stimulus. There were also discrete bursts in all preparations during the declining phase that was typically smaller in extracellularly recorded amplitude (although this was not invariant). Discharges also occurred when forces were applied ventrally (in the direction of depression of the coxo-trochanteral joint) (Fig. 3B). In many recordings, the pattern of activity to depression force was the reverse of that seen to levation, with a discharge of smaller amplitude to force increases and firing of large amplitude to force decreases. The constancy of discharges to force increases and decreases is also apparent in the cumulative histograms in Fig. 3C (levation: n = 102 tests, N = 3 animals; depression: n = 121 tests from same animals).
Fig. 3.
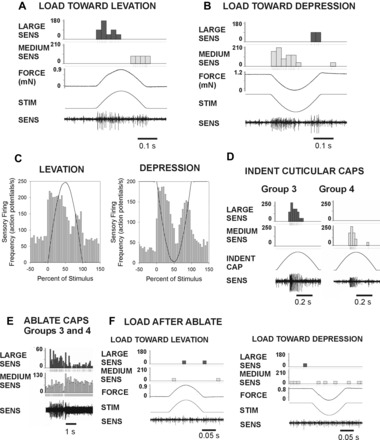
Responses of trochanteral campaniform sensilla Groups 3 and 4. All sensilla except Groups 3 and 4 were first ablated. Forces of small amplitude were applied via the probe and resisted by the pin. A: force in the direction of levation produced large-amplitude spikes during force increase and a burst of smaller amplitude during force decrement. B: loads applied toward levation produced the opposite pattern, with smaller units firing during the rise phase and larger units during the force decline. C: histogram of mean firing during levation and depression. Firing rose rapidly after the stimulus onset. Discharges during force decreases were consistently observed in both levation and depression. D: indentation of cuticular caps. Mechanical stimulation of the caps of individual Group 3 and Group 4 sensilla produced unitary sensory discharges of amplitude similar to those seen following force application to the leg. E: ablation of Groups 3 and 4 produced intense discharges of similar amplitudes. F: sensory bursts did not occur upon application of forces toward levation or depression after cap ablation.
Subsequent stimulation of individual caps of Group 3 and 4 sensilla produced discrete unit discharges in the nervus cruris recording. The potentials recorded during cap stimulation were of amplitude equivalent to firing produced by leg loading. The spikes of Group 3 sensilla had the largest amplitude in all experiments, while smaller potentials typically were produced by indentation of the Group 4 receptors (Fig. 3D). In most experiments, the individual sensilla could not be selectively ablated (because of technical limitations). However, ablation of all receptors of Groups 3 and 4 produced injury discharges in the recordings of similar amplitudes (Fig. 3E) and eliminated the responses to both dorsal and ventral loads in subsequent tests (Fig. 3F).
Selective Ablation of Group 4 Sensilla and Directional Tuning
Selective ablation of individual groups proved difficult because of limited separation of Groups 3 and 4. However, we did develop a method of ablating only Group 4 receptors that was successful in some preparations (N = 3; see methods). Figure 4A shows a recording in which responses were first tested with Groups 3 and 4 intact (all other sense organs had previously been ablated). Forces applied in the direction of joint levation and depression elicited sensory discharge to both increasing and decreasing forces. Group 4 receptors were then selectively ablated by insertion of a sharpened minuten pin. After the ablation the response to increasing forces to levation remained, but the activity to decreasing forces was eliminated (Fig. 4B). In contrast, the opposite pattern was seen when forces were applied in the direction of depression: firing to increasing forces was eliminated, while the discharge to decreasing force remained intact. Subsequent ablation of Group 3 eliminated sensory bursts following force application (Fig. 4C). These tests demonstrated that Group 4 encodes increasing forces in the direction of joint depression and decreasing forces in levation, while Group 3 encodes increases in levation and decreases in depression forces.
Fig. 4.
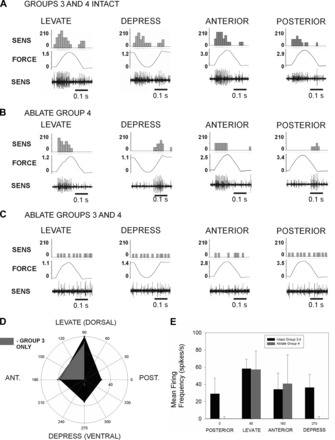
Selective ablation of sensilla and directional tuning. The specificity of the responses of the dorsal groups was tested by applying forces in different directions and by selective ablation of Group 4. A: Groups 3 and 4 Intact: recordings of tests in which half-sine waveforms were applied to the probe in the joint plane (levation, depression) and perpendicular to the plane of joint movement (anterior, posterior). Discharges were obtained to both force increases and decreases in all directions. B: Ablate Group 4 sensilla: specific components of the responses were eliminated by ablation of Group 4. Discharges still occurred to force increases in the direction of levation and anterior force application and to force decreases in depression and to posterior forces. Firing to other directions was eliminated. C: Ablate Groups 3 and 4: subsequent ablation of Group 3 caps eliminated all bursting upon force application or release. D: polar plot of mean discharge rates during force increases before and after ablation of Group 4. With both groups intact (dark area), the highest rates of firing occurred in the plane of joint movement. After ablation of Group 4 (lighter gray), only discharges to levation and anterior force persisted. F: histogram of mean firing frequencies and standard deviations. Ablation eliminates responses to depression and posterior forces.
We also examined the specificity and directional sensitivities of the sensory discharges to load by applying forces perpendicular to the plane of joint movement (Fig. 4, A–C). The dorsal groups of receptors showed responses to forces applied anteriorly and posteriorly that were generally at lower levels than those seen to forces in the plane of joint movement. After ablation of Group 4, the responses to increases in anteriorly directed forces were retained while the discharges to posterior forces were eliminated.
Figure 4D is a polar plot of the mean discharges obtained to forces applied dorsally, ventrally, anteriorly, and posteriorly (N = 3 animals in which all directions were tested). This plot compares tests in which forces were applied at approximately equivalent levels and rates in each direction. In animals with Groups 1, 2, and 5 ablated, Groups 3 and 4 showed responses to all directions tested, although the mean discharges were higher in the plane of joint movement. After ablation of Group 4, the discharges to dorsal (levation) and anterior forces remained largely unchanged while firing to ventral (depression) and posterior forces was greatly decreased. Similar results were obtained in three other animals, but small discharges remained, mostly likely because incomplete ablations. The histogram in Fig. 4E compares the mean change in firing rates after the onset of the stimulus for two animals with tests at closely matched amplitudes (total 111 tests, mean force = 0.94 mN). Although there is variability in the magnitude of the response (due to spike counting), the differences in responses in ventral and posterior directions are apparent and statistically significant after ablation of Group 4 sensilla (t-test, P = < 0.01). These findings support the idea that Groups 3 and 4 encode different forces consistent with the orientation of their cuticular caps.
Amplitude and Rate Sensitivity to Imposed Loading
Encoding of force magnitude and rate was more precisely characterized by applying sequential ramp and hold functions to the force probe. In these tests, the magnitude of applied forces was again kept at low levels to facilitate spike classification in the multiunit recordings. Figure 5A shows tests from a series of stimuli that were applied with a constant rate of rise but variable amplitude. Application of forces over a range of increasing levels in the directions of levation (Fig. 5A, top) and depression (Fig. 5A, bottom) produced graded increments in sensory discharge frequencies of Group 3 and 4 sensilla. Figure 5B, top, shows a plot of sensillum firing to forces applied toward joint levation (6 repetitions of a series of ramp and hold forces at 5 levels in a single preparation; r2 = 0.95, slope = 132.4; pooled data n = 22 repetitions, N = 3 preparations; r2 = 0.96, slope = 87.4). Figure 5B, bottom, shows comparable tests in the direction of joint flexion (10 repetitions of 5 levels in the same preparation r2 = 0.93, slope = 41.1; pooled data n = 20 repetitions in the same 3 preparations; r2 = 0.97, slope = 45.4). These data reflect an apparently greater sensitivity to forces applied toward levation than to depression (see also Ridgel et al. 2000).
Fig. 5.
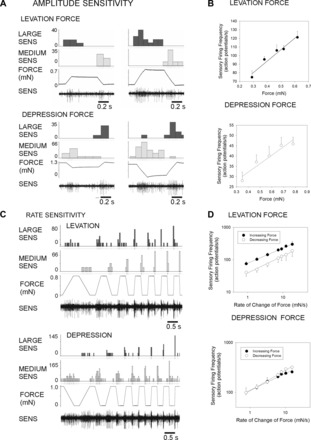
Rate and amplitude sensitivity. A: amplitude sensitivity: recordings of responses to ramp and hold waveforms of variable amplitude (constant rate of change). Firing of sensilla of the largest extracellular amplitude was sustained in tests applied toward levation, while firing of receptors of smaller amplitude was increased to forces applied toward depression. B: plots of mean discharge frequencies during the hold phase show that sensilla could encode force amplitude. C: sensory activities during application of ramp and hold waveforms of variable rate of change but constant amplitude. All sensilla showed strong rate sensitivities to both force increases and decreases. D: plots of mean discharge frequencies during forces increases and decreases. Receptors effectively encoded the rate of change of force. When forces were applied toward levation, sensory response frequencies to force increases were higher than force decreases. Responses were more closely equivalent for forces applied toward depression.
All recordings showed strong sensitivities to force dynamics when tests were applied using ramp and hold functions with the same low amplitude but different rates of rise and decline (Fig. 5C). Discharges to both increases and decreases in force were highly rate dependent. In general, large spike units fired predominantly during the ramp phase, while smaller units were active in both the ramp and hold phases. Spike collisions generally prevented analysis of firing of smaller units. Figure 5D, top, shows a plot of the average firing rates during the ramp rise of larger units force in the direction of joint levation and during the ramp decrease for the same tests (n = 35 repetitions of 7 rates, N = 3 preparations, mean force = 0.92 mN). Figure 5D, bottom, contains a similar plot of responses during tests in which forces were applied in the direction of joint depression (n = 13 repetitions of 7 rates, N = 2 preparations, mean force = 0.78 mN). These data demonstrate that trochanteral campaniform sensilla can accurately encode the rate of change of increasing or decreasing forces even when force amplitudes are small.
Responses to Depressor Muscle Contractions Generated by the Animal
Tests of sensory responses to forces generated by spontaneous contractions of the trochanteral depressor muscle also proved to be technically challenging but could be obtained in some preparations (see methods). In most preparations, sensory firing occurred to depressor contractions but the magnitude and duration of muscle bursts were variable. The recording shown in Fig. 6A was obtained in a preparation that showed tonic activity in the slow depressor muscle that was modulated and burst at the rate of abdominal respiratory pumping (∼0.3 Hz) (Bässler and Wegner 1983). Bursting in the slow depressor was initiated at a low level and then accelerated in frequency. With leg movements resisted by the probe, force was developed only with iterative activity, as is characteristic of slow motoneuron firing at moderate rates (Schmitz 1986). Sensory discharges from the nervus cruris (crushed proximally) were not coincident with muscle discharges but instead followed the development of force by the muscle. This is also apparent in the cumulative histograms in Fig. 6B, which plot the mean sensory discharges, muscle activities, and force (averaged by sampling channels for 1.1 s after the start of the muscle bursts: n = 102 bursts resisted, n = 47 bursts not resisted, N = 2 preparations). To confirm that these discharges reflected the forces, we moved the probe and allowed the leg to extend freely. No sensory discharges occurred during these tests, but they were present when the probe was again moved to resist the muscle contraction. In one preparation, we also successfully ablated the Group 3 and 4 sensilla, which eliminated the sensory discharge. These tests confirmed that the trochanteral campaniform sensilla encoded the strains resulting from muscle contractions and were not activated during unopposed movements of the leg.
Fig. 6.
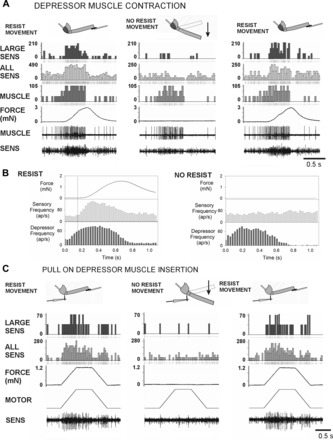
Responses to spontaneous depressor muscle contractions and forces applied at the muscle insertion. A: spontaneous contractions of the trochanteral depressor muscle in a preparation in which all sensilla were ablated except for Groups 3 and 4. Activities of the slow depressor motoneuron were recorded myographically (MUSCLE). Sensory activities (SENS, bottom) were recorded from nervus cruris, which was crushed distal to the depressor motor nerve branch. Muscle contractions were first resisted (RESIST MOVEMENT) by the probe that registered the resultant forces on the femur (FORCE). The probe was then moved to allow the femur to move freely (NO RESIST MOVEMENT) and then returned to the contact with the femur. Firing of sensory units with large extracellular amplitudes occurred when movement was resisted. This firing did not occur when the resistance was removed but returned after the probe again blocked leg movements. B: histograms of sensory discharges during resisted and unresisted muscle contractions. The increase in sensory firing was concurrent with the force increase, not at the time of initiation of muscle bursting. The rise in sensory frequency did not occur when the probe was moved away and the leg moved freely toward depression. ap, Action potential. C: forces imposed at the depressor muscle insertion. Preparation similar to A but the depressor nerve branch was cut, permitting more complete exposure of the nervus cruris and better discrimination of action potentials. Vigorous responses occurred to forces applied at the insertion of the trochanteral depressor muscle. Discrete responses also occurred during force decreases when the ramp rate was sufficiently high. Sensillum did not fire when the probe was removed and the leg moved freely but returned if the probe was repositioned to resist the force.
Responses to Forces Applied to Depressor Muscle Insertion
To study the effects of muscle contractions on sensory discharges more extensively and precisely, we applied forces to the depressor insertion, using the computer-controlled motor (Fig. 6C, Fig. 7). Figure 6C shows a recording in which forces were applied to the depressor insertion with the use of ramp and hold functions (all sense organs ablated except Groups 3 and 4). Strong multiunit sensory discharges occurred when forces were resisted by the probe at the femur. Firing occurred to both force increases and decreases at this rate of force application. When the probe was removed, the forces applied at the muscle insertion produced substantial movements of the femur in the plane of the coxo-trochanteral joint (∼20–40° changes in joint angle) similar to those that occurred spontaneously when the muscle innervation was intact and motoneurons were activated by the animal. However, no discharge of campaniform sensilla occurred to unresisted movement. Afferent firing readily returned when the force probe was repositioned and provided resistance to movement. Thus these tests were similar to responses obtained from forces generated by muscle contractions in intact animals and support the idea that the trochanteral receptors only encode muscle forces when movement is resisted.
Fig. 7.
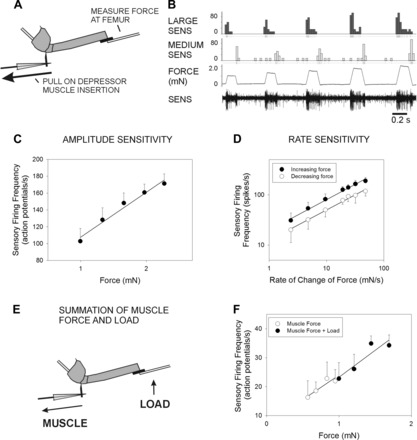
Rate and amplitude sensitivity to forces applied at the depressor muscle insertion. A: preparation. Sensory activities were recorded in preparations in which all sensilla were ablated except for Groups 3 and 4. Forces that mimicked depressor contractions were applied through a pin placed at the depressor muscle insertion. Movement was resisted and forces monitored by a probe placed against the femur. B: amplitude sensitivity. Response discharges increased in frequency to increases in the amplitude of the stimulus (same animal as in Fig. 3). C: plot of mean discharge in the hold phase. Sensilla effectively encode the force amplitude. D: plot of mean discharge frequencies to forces applied at different rates. Sensory firing to force increases and decreases show rate sensitivity. E: summation of muscle forces and load. Sensory activity was first recorded when force was applied to the depressor insertion, and the probe was only used to monitor the forces (as in A). The same force was then applied at the depressor, and a small force was also applied simultaneously by activating the piezoelectric crystal that held the force probe. F: plot of sensory discharge to muscle forces and to muscle forces and small loads in a single preparation. The combination of muscle forces and loads acted like a simple summation, and the sensory discharges were merely shifted to a higher range.
We tested responses to force applied at the muscle insertion, using the same waveforms that were used to characterize responses to loads (Fig. 7A). Figure 7B shows a test in which forces were applied to the muscle insertion at increasing amplitudes but the same rate of rise and decline. Discharges occurred during both rising and falling phases of the stimulus, and in many experiments the amplitudes of potentials were similar to firing recorded during tests of loading in the direction of levation: bursts during the rising phase were larger than those seen in the falling phase. Figure 7C is a plot of the mean firing at increasing stimulus amplitudes (plotted as in Fig. 3A: all potentials from stimulus onset to end of hold phase; mean of n = 18 tests of 5 levels, N = 3 preparations). The discharge effectively encodes the amplitude of muscle force (r2 = 0.97). We also tested rate sensitivities to forces applied at the muscle insertion. Figure 7D plots the mean discharge during the ramp rise and decline (n = 18 series of 7 levels, N = 3 preparations). Discharges are higher to increasing forces than to decreasing force, but both effectively encode the rate of change of force (for both plots r2 = 0.97). In most experiments, responses to muscle forces were completely eliminated by ablation of the caps of the Group 3 and 4 sensilla.
Simultaneous Application of Muscle Forces and Loads
In many experiments, discharges of classes of units of similar amplitudes occurred to resisted muscle contractions and to loads applied to the femur (compare Fig. 3 and Fig. 7). In a number of experiments (N = 22) we also tested the effects of simultaneous application of load and muscle forces by applying the same waveform to the piezoelectric crystal and to the motor that pulled on the depressor muscle insertion (Fig. 7E). Force was first applied only at the muscle insertion. We then also applied the same waveform to the motor linked to the pin at the muscle insertion and to the piezoelectric crystal that held the force probe, increasing the load. Simultaneous application of both stimuli produced a slightly larger force measured at the piezoelectric crystal. In both tests units of similar size were apparently activated. The plot in Fig. 7F shows the effects of slight increase in load (5 tests muscles only, 5 tests muscle and load at 4 different amplitudes). Increasing load increased the sensory discharge but merely shifted the firing rate to a higher range, as higher total forces were applied. These results are also consistent with the idea that similar units are activated to muscle force and load.
Effects on Depressor Motoneuron Firing
The effects of Group 3 and 4 trochanteral campaniform sensilla on activities of the trochanteral depressor muscle were tested both by stimulation of the cuticular caps of individual receptors and by applying forces to the femur. Stimulation of caps of trochanteral campaniform sensilla with a fine wire probe was highly effective in producing reflex effects in the slow motoneuron to the trochanteral depressor muscles in preparations showing low levels of tonic firing in motoneurons. The sign of these reflexes differed for Group 3 and Group 4 sensilla. Indentation of the cap of Group 4 sensilla could elicit spiking activities in the trochanteral depressor (Fig. 8A), while stimulation of Group 3 receptors produced inhibition of ongoing firing (Fig. 8C). The histograms in Fig. 8, B and D, plot the mean firing frequencies of the depressor before, during, and after cap stimulation (Fig. 8B: n = 120 tests, N = 3 preparations; Fig. 8D: n = 88 tests in the same preparations). Motor firing is significantly increased in the first bin (12.5 ms) following the onset of Group 4 stimulation. The differences in the motor effects of Group 3 and Group 4 are consistent with their differential responses to load.
Fig. 8.
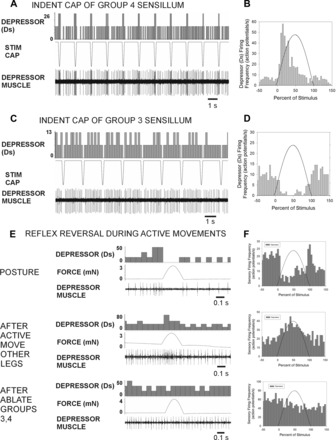
Effects of trochanteral campaniform sensilla on activity in the trochanteral depressor muscle. A: recording of effects of repetitive indentation of the cuticular caps of Group 4 campaniform sensilla on tonic activities of the slow trochanteral depressor motoneuron (Ds). Each mechanical stimulus produced a transient increase in the depressor firing frequency. B: histogram of tests from 3 preparations shows consistent depressor excitation at short latency from Group 4 stimulation. C: effects of stimulation of the cap of a Group 3 sensillum on depressor firing. Depressor firing was completely inhibited by sensillum activation. D: histogram of tests (from the same 3 preparations) shows consistent inhibition following indentation of Group 3 sensilla. E and F: effects of forces applied in the direction of levation in a preparation with only Group 3 and 4 sensilla intact. In preparations showing tonic postural activity, forces applied toward levation produced inhibition of depressor firing (E, top; F, top). Large active muscle contractions could be evoked after stimulation of the abdomen or cercus. Forces applied toward levation then produced excitation of the depressor (E, middle; F, middle). Both the inhibitory and excitatory effects were eliminated by ablation of the trochanteral campaniform sensilla (E, bottom; F, bottom).
We also tested the reflex effects of Group 3 sensilla by applying forces to the femur in preparations in which all other groups of campaniform sensilla had been ablated. As previous studies have indicated that the motor effects of the trochanteral campaniform can show plasticity (Akay et al. 2007), we applied stimuli in preparations both at “rest” and after leg movements were elicited by touching the abdomen or cerci (Bässler 1988). The effects of forces imposed toward levation depended upon the state and level of activity of the preparation (movement was resisted by the pin at the depressor muscle insertion). In resting preparations (with low to moderate levels of activity) forces applied to the femur in the direction of levation produced inhibition of the slow depressor, consistent with the tests using cap stimulation of Group 3 sensilla (Fig. 8, E and F, top; histogram mean of n = 107 tests in N = 3 preparations). These effects reversed in sign in preparations that were made “active,” and leg movements were elicited. When “active,” these preparations showed vigorous bursts in the depressor with apparent reciprocal bursting in the levator. These bouts often were terminated with a large burst in the depressor as the animal pushed against the restraints and force probe. Forces applied to the femur in the direction of levation could then produce excitation of the depressor (Fig. 8, E and F, middle; histogram mean of n = 53 tests in the same 3 preparations). The occurrence of reversals was not sustained and declined rapidly (within the first minute) after the active muscle contractions. Ablation of the Group 3 and 4 receptors eliminated the excitatory responses (Fig. 8, E and F, bottom; histogram 54 tests in 2 of the same preparations). These findings indicate that the sign of effects of the Group 3 sensilla can apparently reverse when the animal becomes active and generates prolonged, large contractions of the depressor muscle.
DISCUSSION
This study has sought to 1) characterize the responses of stick insect trochanteral campaniform sensilla to forces resulting from muscle contractions and external loads and 2) gain insight into why many groups of receptors are concentrated on the trochanter. We have shown that the dorsal groups of receptors encode forces from resisted contractions of the trochanteral depressor muscle but do not discharge during unresisted leg movements. The sensilla also provide detailed information about increases and decreases in load in the plane of movement of the coxo-trochanteral joint. The trochanteral sensilla can therefore effectively encode loads as the resistance to muscle contractions, similar to vertebrate Golgi tendon organs (Stuart et al. 1970). These findings suggest that many campaniform sensilla are concentrated on the trochanter because that segment acts as a focal point for forces generated by a number of leg and body muscles (Marquardt 1940). As discussed below, the trochanter also provides an anatomical frame of reference for the integration of muscle forces and loads because the trochanteral sensilla are located within the plane of leg movement (Cruse and Bartling 1995).
Design of the Trochanter and Groups of Campaniform Sensilla
The arrangement of the trochanteral campaniform sensilla in four discrete groups in C. morosus is homologous to that found in the stick insect Cuniculina (Hoffmann and Bässler 1982) and in cockroaches (Pringle 1938b). Multiple groups of trochanteral sensilla, often at similar locations, are present on the trochanter in locusts (Hustert et al. 1981; Knyazeva 1974), crickets (Knyazeva et al. 1975), moths (Kent and Griffen 1990), and fruit flies and beetles (Petryszak and Fudalewicz-Niemczyk 1994). These arrangements may reflect common mechanisms underlying the distribution of cuticular strains. Campaniform sensilla of insect legs respond directionally to compressive strains that act perpendicular to the long axis of the ovoid cuticular caps (Chapman et al. 1973). The trochanter is cylindrical (Frantsevich and Wang 2009; Schindler 1979), and, despite many irregularities, the segments of insect legs transmit strains like simple cylinders (Cocatre-Zilgien and Delcomyn 1999; Skordos et al. 2002; Zill and Moran 1981). Bending forces produce increases in length (longitudinal tensions) on the side of force application and decreases in length (longitudinal compressions) on the opposite side. The long axes of most of the cuticular caps of Groups 1 (posterior) and 2 (anterior) are oriented perpendicular to the condyles of the coxo-trochanteral joint. Previous studies have demonstrated that these groups show differential sensitivities to bending in an anterior-posterior plane (Schmitz 1993). The present study has shown that cuticular caps of Groups 3 and 4 (located dorsally) are oriented perpendicular to each other [as is also found in Cuniculina (Hoffman and Bässler 1982) and in blow flies (Merritt and Murphy 1992)]. Mutually perpendicular orientations are associated with differential responses to bending forces as longitudinal tensions are accompanied by transverse compressions (Zill and Moran 1981). Similar analysis of strain distribution has also been applied to other groups of campaniform sensilla (receptors on the halteres of flies, Fox 2010; Fox et al. 2010).
Encoding Properties of Trochanteral Campaniform Sensilla in Stick Insects
External loads within the joint plane.
Although activities monitored in the main leg nerve were multiunit recordings, application of forces at low levels permitted identification of classes of units. The amplitudes of these potentials matched the sizes of spikes recorded by indentation of the cuticular caps of individual sensilla. In these tests, receptors showed phasico-tonic discharges to force increases and transient bursts to force decreases. All responses were strongly rate sensitive, and tonic discharges effectively encoded force magnitude (Hofmann and Bässler 1982). Ablation studies demonstrated that responses of individual groups were not bidirectional, consistent with single-unit recordings obtained by intracellular techniques (Hofmann and Bässler 1986). These basic response characteristics are similar to those found in multiunit recordings of trochanteral campaniform sensilla of cockroaches (Zill et al. 1999) and in recordings of identified receptors in tibial sensilla of stick insects (Zill et al. 2010), cockroaches (Ridgel et al. 2000), and locusts (Burrows and Pflüger 1988). In addition, discharges to decreases in force have been extensively studied in campaniform sensilla in cockroaches (Keller et al. 2007; Ridgel et al. 1999) and locusts (Newland and Emptage 1996) and in receptors of vertebrates (Trulsson 2001). Signals of force decreases are important cues at the end of the stance phase of walking and can indicate leg slipping and loss of substrate adherence (Duysens et al. 2000).
Previous studies have demonstrated that trochanteral sensilla in Groups 1 and 2 encoded forces in an anterior-posterior plane (Delcomyn 1991; Schmitz 1993). Our studies have, for the first time, established that the campaniform sensilla in stick insects can encode forces within the plane of movement of the coxo-trochanteral joint (confirming observations of Akay 2002; Akay et al., 2001; Borgmann et al. 2011). Responses in this plane could readily signal the forces needed to support body weight (Zill et al. 1999). In previous models of motor control in stick insects, the coxo-trochanteral joint was considered to counter body load by controlling body height through negative feedback from receptors that encode kinematic variables (Dürr et al. 2004). The present study suggests that body weight could be monitored directly and contribute to motor control at the coxo-trochanteral joint.
Loads applied outside the joint plane.
The trochanteral campaniform sensilla also showed responses to bending forces applied in directions outside the plane of joint movement. Sensillum response frequencies were lower than firing to forces in the coxo-trochanteral joint plane. The range of directions was specific for individual groups: for example, Group 3 sensilla responded to anterior bending but did not discharge to posterior bending (which activated Group 4 receptors). Similar “response clouds” have been demonstrated for the tibial campaniform sensilla in cockroach (Zill and Moran 1981) and stick insect (S. N. Zill, unpublished observation) legs, halteres of flies (Fox 2010; Fox and Daniel 2008), as well as cuticular mechanoreceptors of spiders (Hößl et al. 2009).
These types of responses are also predicted if the sensilla are modeled as strain gauges on a cylinder (Cocatre-Zilgien and Delcomyn 1999; Zill and Moran 1981). In a cylinder or beam, strains are maximal to bending forces applied in the plane in which the gauge is located. The magnitude of strains from force application in other directions is a function of the vectoral component of the force that is perpendicular to the strain gauge.
Applied to the present results, this analysis suggests that, in the stick insect trochanter, Group 3 and 4 sensilla detect the vectoral components of forces that could be regulated by muscles acting in the joint plane. However, in a cylinder, forces applied in perpendicular planes produce zero strain (the “neutral” plane) and are symmetrically distributed. In contrast, the sensilla show discharges (albeit at lower frequencies) to forces applied anterior and posterior to the plane of movement of the coxo-trochanteral joint. Some responsiveness may be due to the location of the receptors, which are offset from the plane of joint movement (Zill and Moran 1981). Variations in the thickness and mechanical properties of the cuticle could also alter response sensitivities (Chapman et al. 1973).
Responses to muscle contractions.
Our experiments have provided the first quantitative data on the sensitivities of campaniform sensilla to strains generated by muscle contractions. The dorsal groups of campaniform sensilla showed vigorous responses to strains produced by spontaneous depressor muscle contractions and to forces applied at the muscle insertion when they were resisted by the force probe. Responses of Groups 3 and 4 were directional and showed amplitude and rate sensitivities to forces applied at the muscle insertion that were similar to those seen in responses to external loads. Directional responses to muscle contractions have previously been demonstrated in tibial campaniform sensilla of stick insects (Zill et al. 2011) and cockroaches (Zill and Moran 1981) and to forces applied at muscle insertions (trochanteral sensilla, Delcomyn 1991; tarsal sensilla, Zill et al. 2010).
We also showed that the trochanteral sensilla did not fire to spontaneous muscle contractions or forces imposed at the muscle insertion when movements were not resisted (similar to observations in cockroach tibial and tarsal campaniform sensilla: Zill and Moran 1981; Zill et al. 2010). This finding is consistent with experiments in which the activities of campaniform sensilla have been recorded in freely walking animals: sensory discharges were limited to the stance phase when muscle contractions were resisted by the substrate (Noah et al. 2004). Direct recordings of strains in the legs of walking locusts also show maximal values in the stance phase (Newland and Emptage 1996).
Do insect campaniform sensilla only detect loads as the resistance to active contractions of individual muscles? Many previous studies have noted that resistance to leg movement (presumed to occur through muscle tensions) is a necessary prerequisite for discharges of the campaniform sensilla to external loads (Delcomyn 1991). However, it is important to note that campaniform sensilla can still indicate changes in load, even in the absence of motoneuron firing, if the muscle tonus is sufficiently high to resist movement (Zill and Moran 1981). Activities of campaniform sensilla may also result from more complex patterns of motor activation, as discharges of some receptors occur during cocontractions of antagonist muscles in the absence of joint movements (Burrows and Pflüger 1988).
Why Are So Many Campaniform Sensilla Concentrated on the Trochanter? A Model of Force Detection and Control of Walking Direction
Recordings with all groups of trochanteral campaniform sensilla intact showed discharges to loads applied as bending forces in all directions (Fig. 2 and Fig. 9). Many older studies utilizing ablations of leg segments have suggested that the trochanter acts as a center for monitoring forces in walking. In the “peg leg experiments,” animals showed active use and coordination after ablations distal to the trochanter but did not use the leg forcefully if the trochanteral segment was removed or denervated (Noah et al. 2004; Wendler 1966). One rationale for sensing forces in the proximal leg segments, which is reflected in those experiments, is that damage to the distal leg segments is common in older insects (Ridgel et al. 2003). One adaptive advantage provided in the location of the force sensors on the trochanter, instead of the tarsi (feet), is that it permits leg use when the distal segments are damaged.
Fig. 9.
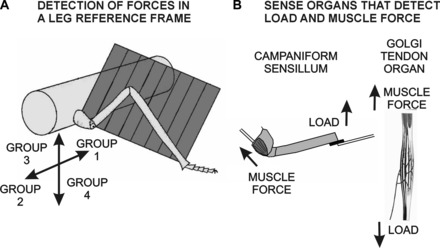
Model of force detection and control. A: frame of reference. Our findings suggest that the trochanteral sensilla are organized to supply information about the vectorial direction of loads relative to the plane of leg movement. Trochanteral Group 3 receptors show the largest discharge to forces applied dorsally in the leg plane, while Group 4 sensilla respond to ventral forces. Previous tests have demonstrated that the Group 2 receptors encode forces in an anterior direction, while Group 1 discharge to posterior forces. Thus the plane of joint movement forms a frame of reference for information about forces acting on the leg. B: campaniform sensilla share properties of other force receptors. Both campaniform sensilla and Golgi tendon organs encode muscle forces. Detection of load depends upon the presence of muscle tensions.
Furthermore, our anatomical and physiological studies provide insight into why many sensilla are specifically concentrated on the trochanteral segment (rather than the coxa, femur, or tibia). They support the hypothesis that, in the stick insect, force information may be integrated by trochanteral campaniform sensilla within the frame of reference of the leg plane. The evidence to support this hypothesis is discussed below.
The receptors are specifically concentrated at the proximal end of the leg plane.
Structurally, the trochanter is unique as a location for monitoring and integrating forces. While the joint between the body and coxa allows for considerable freedom of movement, there are no groups of campaniform sensilla on the coxa (Fig. 2). The more distal leg articulations (coxo-trochanteral and femoro-tibial joints) are hinge joints that act in a common plane, termed the leg plane (Cruse and Bartling 1995). The groups of campaniform sensilla that are concentrated on the trochanter are located distal to the coxo-trochanteral joint and are, therefore, at the proximal end of the leg plane.
Responses of the dorsal sensilla to load are maximal in the plane of leg movement.
Our selective ablations have demonstrated that the dorsal groups (Groups 3 and 4) show the maximal discharge to loads applied in the leg plane. Previous studies have shown that Groups 1 and 2 effectively encode forces applied in anterior and posterior directions relative to the leg plane (Hoffman and Bässler 1982, 1986; Schmitz 1993). These findings suggest that individual groups of sensilla can preferentially monitor different ranges of force vectors acting within and upon the leg plane.
The dorsal receptors respond vigorously to the trochanteral depressor muscle, which acts within the leg plane.
The present study has also shown that the trochanteral sensilla encode muscle forces. We have demonstrated that the dorsal groups of receptors can most effectively encode forces generated by the trochanteral depressor. This muscle acts in a dorsal-ventral direction that is coincident with the leg plane.
However, because campaniform sensilla are located on the cuticle, the receptors are sensitive to strains generated by muscles that act upon the adjacent joint and by muscles acting at other joints. The largest muscles that move the leg are located in the thorax and insert on the coxa and trochanter. The demonstrated sensitivities of Groups 1 and 2 strongly suggest that they could monitor forces produced by promotor and remotor muscles (Schmitz 1993). The trochanteral sensilla are also positioned to monitor the net forces produced by a number of other muscles located in the thorax including the coxal rotators and adductors (Marquardt 1940). The forces generated by all these muscles upon the leg plane would also be monitored by the trochanteral campaniform sensilla.
The ability of campaniform sensilla to provide such integrated information was also recognized by Pringle in his early studies of campaniform sensilla: “[campaniform] sensilla serve as proprioceptors not for any one muscle, but for the appendage as a whole. This provides another contrast to the vertebrate arrangement, for it means that in the insect the behaviour of the limb is automatically integrated before being reported back to the central nervous system” (Pringle 1938b, p. 130).
An alternative hypothesis that accounts for our results is that the sensilla are simply encoding forces relative to the plane of movement of the adjacent coxo-trochanteral joint. However, the idea that the plane of leg movement is used as a reference frame for force regulation gains support from a number of previous experiments on the mechanisms underlying changes in walking direction. Rosenbaum et al. (2010) studied the pattern of activities in leg muscles during forward and backward walking in stick insects and found that activities of the protractor and retractor muscles, which move the leg as a whole, were altered in phase when the walking direction was changed. The intrinsic leg muscles (depressor/levator, extensor/flexor) that lie within the leg plane maintained the same general pattern in both directions. Those findings are consistent with the idea that changes in walking direction are effected by altering the forces generated in propulsion while similar forces providing support and stability are produced within the leg plane. The central mechanisms that could underlie changes in walking direction in a single leg have been recently been described in an elegant model by Tóth et al. (2012) in which a kinematic signal of joint angle acts as the switch from protraction to retraction. Previous experiments by Akay et al. (2007) have also shown that changes in walking direction are accompanied by changes in the effects of the trochanteral campaniform sensilla that detect forces in an anterior-posterior plane. Our results suggest that changes in the direction of progression may be accompanied by specific changes in force feedback from muscles that move the leg plane while the same functional synergies occur in leg muscles at different joints within the leg plane. These findings complement and can augment the kinematic model, as the nervous system most probably utilizes convergent feedback from receptors monitoring forces and sense organs that detect kinematic variables (Schmitz and Stein 2000; Stein and Schmitz 1999).
Movements of the leg plane may also underlie adaptation of leg use in generating substrate grip and in responses to postural perturbations. Many insects maintain adhesion by generating forces along the long axis of the leg (Niederegger and Gorb 2003). During walking on vertical or inverted surfaces, the legs are used in a strategy known as distributed inward grip, in which the long axes of opposite legs are aligned across the body to maintain substrate contact (Wile et al. 2008). By positioning pairs of legs in opposition, each leg can develop axial forces that effectively counter the forces from the opposite leg. This allows greater forces to be developed at the tarsi, effectively increasing adhesion while pulling the body toward the point of attachment. In addition, during perturbation tests in which the substrate is repeatedly moved, cockroaches tend to align the long axis of the leg parallel to the direction of displacement (Ridgel et al. 2001; S. N. Zill, unpublished observation). These behaviors may represent optimization strategies as the intrinsic muscles of the leg generate maximal forces in the leg plane.
The legs may also be used adaptively in climbing by altering the direction of action of intrinsic leg muscles. Watson et al. (2002) studied motor activities in cockroaches that occur during climbing over a block. They found that the same basic pattern of synergistic activation of the intrinsic (tibial and trochanteral) muscles was used in both walking and climbing, although the burst durations were altered. During climbing, the angle of the coxa-body joint was changed so that the middle leg pushed the animal up from the substrate rather than propelling it forward. It is important to note that in cockroaches the trochanter-femur joint is not fused but shows limited mobility (as in some other insects; Frantsevich and Wang 2009). The small muscle (reductor) that acts on the joint probably does not generate substantial forces, but joint movement in swing can serve to expand the range of foot placement during walking and climbing, when the reductor is used in conjunction with muscles of the body wall (Bender et al. 2010). The net effect of trochanter-femur joint movements is apparently to change the direction of the vector produced by contractions of intrinsic leg muscles, but it is unclear how this would affect the accuracy of force signaling by the trochanteral campaniform sensilla.
Reflex Effects on Motoneurons
We have also demonstrated that stimulation of the caps of individual campaniform sensilla has strong effects on activities of the trochanteral depressor muscle. These effects are consistent with their cap orientation and response to muscle forces and load. In quiescent animals, indentation of caps of individual Group 4 receptors produced depressor activation while stimulation of Group 3 caps produced inhibition. These reflexes could provide negative feedback control, as Group 3 receptors are excited by contractions of the depressor muscle and their discharge would reflexively decrease firing in the slow depressor motoneuron. Similar reflex effects of Groups 1 and 2 sensilla have previously been demonstrated in the protractor and retractor motoneurons and could serve to limit the magnitude of muscle contractions (Schmitz 1993). Other recent studies in stick insects have shown that activities of the depressor can be modulated by bending forces in semirestrained preparations (Akay 2002; Borgmann 2006; Borgmann et al. 2011). Trochanteral sensilla have also been shown to produce short-latency activation of the depressor muscle in cockroaches (Pearson 1972) and locusts (Höltje and Hustert 2003).
We also showed that the reflex effects of the campaniform sensilla, elicited by applying bending forces to the femur without joint movement, could reverse when animals made active movements. Force applied to the femur that opposed depressor muscle contractions produced reflex excitation of depressor motor neurons. These reflex effects would constitute positive force feedback (Burrows and Pflüger 1988). Reversals of the motor effects of the trochanteral campaniform sensilla have recently been shown to occur during walking when the direction of progression is changed (Akay et al. 2007). Earlier studies demonstrated that stick insect campaniform sensilla can facilitate (Akay and Büschges 2006) or support (Bässler 1988) reflex reversals associated with flexion signals from the femoral chordotonal organ (“active reactions”). Increased load can also enhance depressor firing in walking animals (Rosenbaum et al. 2010). Motor effects of receptors that monitor forces have been shown to be task specific in a number of vertebrates and invertebrates (Duysens et al. 2000).
Comparison with Tendon Organs and Force Detection in Vertebrates
The present studies have shown that properties and effects of campaniform sensilla are remarkably similar to those of Golgi tendon organs (Fig. 9B). Even though the mechanisms of force transduction differ, both tendon organs and campaniform sensilla 1) encode the rate and amplitude of forces from muscle contractions and loads; 2) show summation when external and muscle-generated forces are applied simultaneously (Houk and Henneman 1967; Mileusnic and Loeb 2009; Wilkinson and Fukami 1983); and 3) have direct effects on the magnitude and timing of motor activities in walking through inputs to pattern-generating interneurons (Borgmann et al. 2011; Gossard et al. 1994). The available evidence also suggests that, in walking, discharges of Golgi tendon organs and campaniform sensilla are limited to the stance phase, when muscle contractions and movements are resisted (Noah et al. 2004; Prochazka and Gorassini 1998).
However, there is only a limited understanding in any system of how sensory information about forces is processed and integrated into more global mechanisms of control (Gossard et al. 1994), such as those utilized in force regulation when the direction of walking is changed (Pang and Yang 2002). While our results suggest that information is provided by campaniform sensilla within the frame of reference of the leg plane, little information is available about the organization of interneurons that receive inputs from campaniform sensilla within the central nervous system (Stein and Schmitz 1999). In vertebrates, inputs from Golgi tendon organs show considerable convergence at the level of both spinal motoneurons (Eccles et al. 1957) and interneurons (Jankowska and Edgley 2010). Many of the interneurons that receive force inputs are multimodal, although these connections may be task specific and altered by filtering through presynaptic inhibition (Krutki et al. 2011).
This problem is relevant to the design of simulations and models of posture and locomotion, many of which utilize calculations of forces relative to the center of mass or pressure (Maus et al. 2011). It is not clear how the nervous system calculates the location of the center of mass or how force inputs could be related to it. Furthermore, the mechanisms that match inputs from tendon organs to the muscle synergies that underlie motor primitives (Giszter et al. 2007) and responses to postural perturbations (Ting and Macpherson 2004) are presently unknown. Calculation of forces based upon anatomical synergies may provide an intermediate step in these calculations (Neptune et al. 2009). In the simulation of cockroach walking of Nelson and Quinn (1999), for example, the forces generated by synergist muscles within the leg plane were calculated and the force vector exerted upon the center of mass was determined by using measurements of the joint between the leg and body.
However, in both tendon organs and campaniform sensilla, the signals of load depend upon muscle contractions or tonus (Stuart et al. 1970). Utilization of force signals apparently requires comparator elements to differentiate external loads from self-generated forces (Flanders 2011). Premotor interneurons that could provide this information via efference copy have been identified in cats (Krutki et al. 2011). Understanding those mechanisms will provide insight into how the nervous system uses information about forces to change the walking direction or adapt other behaviors in both vertebrates and invertebrates. Such mechanisms could be used to similar advantage in controlling walking machines.
GRANTS
This work was supported by a DFG grant to A. Büschges (BU 857/10). J. Schmitz was supported by an EU-FP7 grant (ICT-6-2.1 270182). Some earlier morphological studies of the trochanteral sensilla were supported by National Science Foundation Grant IBN-0235997 (to S. N. Zill).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.N.Z., J.S., S.C., and A.B. conception and design of research; S.N.Z., J.S., S.C., and A.B. performed experiments; S.N.Z., J.S., S.C., and A.B. analyzed data; S.N.Z., J.S., S.C., and A.B. interpreted results of experiments; S.N.Z., J.S., and A.B. prepared figures; S.N.Z., J.S., and A.B. drafted manuscript; S.N.Z., J.S., S.C., and A.B. edited and revised manuscript; S.N.Z., J.S., S.C., and A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Simon Giszter and Lena Ting for helpful discussions and gratefully acknowledge the excellent technical assistance of Annelie Exter, Michael Duebbert, and David Neff.
REFERENCES
- Akay T. The Role of Sensory Signals for Interjoint Coordination in Stick Insect Legs (Carausius morosus and Cuniculina impigra) (PhD thesis). Cologne, Germany: University of Cologne, 2002 [Google Scholar]
- Akay T, Bässler U, Gerharz P, Büschges A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol 85: 594–604, 2001 [DOI] [PubMed] [Google Scholar]
- Akay T, Büschges A. Load signals assist the generation of movement-dependent reflex reversal in the femur-tibia joint of stick insects. J Neurophysiol 96: 3532–3537, 2006 [DOI] [PubMed] [Google Scholar]
- Akay T, Haehn S, Schmitz J, Büschges A. Signals from load sensors underlie interjoint coordination during stepping movements of the stick insect leg. J Neurophysiol 92: 42–51, 2004 [DOI] [PubMed] [Google Scholar]
- Akay T, Ludwar BC, Göritz ML, Schmitz J, Büschges A. Segment specificity of load signal processing depends on walking direction in the stick insect leg muscle control system. J Neurosci 27: 3285–3294, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bässler U. Neural Basis of Elementary Behavior in Stick Insects. Berlin: Springer, 1983 [Google Scholar]
- Bässler U. Functional principles of pattern generation for walking movements of stick insect forelegs: the role of the femoral chordotonal organ afferences. J Exp Biol 136: 125–147, 1988 [Google Scholar]
- Bässler U, Büschges A. Pattern generation for stick insect walking movements: multisensory control of a locomotor program. Brain Res Brain Res Rev 27: 65–88, 1998 [DOI] [PubMed] [Google Scholar]
- Bässler U, Wegner U. Motor output of the denervated thoracic ventral nerve cord in the stick insect Carausius morosus. J Exp Biol 105: 127–145, 1983 [Google Scholar]
- Bender JA, Simpson EM, Ritzmann RE. Computer-assisted 3D kinematic analysis of all leg joints in walking insects. PLoS ONE 5: e13617, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann A. Intersegmental Influences Contributing to Coordination in a Walking Insect (PhD thesis). Cologne, Germany: University of Cologne, 2006 [Google Scholar]
- Borgmann A, Hooper SL, Büschges A. Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. J Neurosci 29: 2972–2983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann A, Toth TI, Gruhn M, Daun-Gruhn S, Büschges A. Dominance of local sensory signals over inter-segmental effects in a motor system: experiments. Biol Cybern 105: 399–411, 2011 [DOI] [PubMed] [Google Scholar]
- Burrows M, Pflüger HJ. Positive feedback from proprioceptors involved in leg movements of the locust. J Comp Physiol A 163: 425–440, 1988 [Google Scholar]
- Büschges A, Akay T, Gabriel JP, Schmidt J. Organizing network action for locomotion: insights from studying insect walking. Brain Res Rev 57: 162–171, 2008 [DOI] [PubMed] [Google Scholar]
- Chapman KM. Campaniform sensilla on the tactile spines of the legs of the cockroach. J Exp Biol 42: 191–203, 1965 [DOI] [PubMed] [Google Scholar]
- Chapman KM, Duckrow RB, Moran DT. Form and role of deformation in excitation of an insect mechanoreceptor. Nature 244: 453–454, 1973 [DOI] [PubMed] [Google Scholar]
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol 106: 999–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocatre-Zilgien JH, Delcomyn F. Modeling stress and strain in an insect leg for simulation of campaniform sensilla responses to external forces. Biol Cybern 2: 149–160, 1999 [Google Scholar]
- Cruse H, Bartling C. Movement of joint angles in the legs of a walking insect, Carausius morosus. J Insect Physiol 41: 761–771, 1995 [Google Scholar]
- Cruse H, Schmitz J, Braun U, Schweins A. Control of body height in a stick insect walking on a treadwheel. J Exp Biol 181: 141–155, 1993 [Google Scholar]
- Dean J, Schmitz J. The two groups of sensillae in the ventral coxal hairplate of Carausius morosus have different roles during walking. Physiol Entomol 17: 331–341, 1992 [Google Scholar]
- Delcomyn F. Activity and directional sensitivity of leg campaniform sensillae in a stick insect. J Comp Physiol A 168: 113–119, 1991 [DOI] [PubMed] [Google Scholar]
- Djuric SM, Nagy LF, Damnjanovic MS. Detection of ground reaction force using a miniaturized inductive displacement sensor. In: Power Electronics and Motion Control Conference (EPE/PEMC), 2010, p. T15-7–T15-12 [Google Scholar]
- Dürr V, Schmitz J, Cruse H. Behaviour-based modeling of hexapod locomotion: linking biology and technical application. Arthropod Struct Dev 33: 237–250, 2004 [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol 138: 227–252, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeberg O, Pearson K. Computer simulation of stepping the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition. J Neurophysiol 94: 4256–4268, 2005 [DOI] [PubMed] [Google Scholar]
- Flanders M. What is the biological basis of sensorimotor integration? Biol Cybern 104: 1–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannigan C. Finite Element Modeling of Arthropod Exoskeleton (Master's thesis). Cleveland, OH: Case Western Reserve University, 1998 [Google Scholar]
- Fox JL, Daniel TL. A neural basis for gyroscopic force measurement in the halteres of Holorusia. J Comp Physiol A 194: 887–897, 2008 [DOI] [PubMed] [Google Scholar]
- Fox JL, Fairhall AL, Daniel TL. Encoding properties of haltere neurons enable motion feature detection in a biological gyroscope. Proc Natl Acad Sci USA 107: 3840–3845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL. Information Processing in the Halteres of Dipteran Insects (PhD dissertation). Seattle, WA: University of Washington, 2010 [Google Scholar]
- Frantsevich L, Wang W. Gimbals in insect legs. Arthropod Struct Dev 38: 16–30, 2009 [DOI] [PubMed] [Google Scholar]
- Giszter SF, Patil V, Hart CB. Primitives, premotor drives and pattern generation: combined computational and neuroethological perspective. Prog Brain Res 165: 323–246, 2007 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994 [DOI] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. A neural basis for motor primitives in the spinal cord. J Neurosci 30: 1322–1336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellekes K, Blincow E, Hoffmann J, Büschges A. Control of reflex reversal in stick insect walking: effects of intersegmental signals, changes in direction, and optomotor-induced turning. J Neurophysiol 107: 239–249, 2012 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Bässler U. Anatomy and physiology of trochanteral campaniform sensilla in the stick insect, Cuniculina impigra. Physiol Entomol 7: 413–426, 1982 [Google Scholar]
- Hofmann T, Bässler U. Response characteristics of single trochanteral campaniform sensillae in the stick insect, Cuniculina impigra. Physiol Entomol 11: 17–21, 1986 [Google Scholar]
- Höltje M, Hustert R. Rapid mechano-sensory pathways code leg impact and elicit very rapid reflexes in insects. J Exp Biol 206: 2713–2724, 2003 [DOI] [PubMed] [Google Scholar]
- Hößl B, Böhm HJ, Schaber CF, Rammerstorfer FG, Barth FG. Finite element modeling of arachnid slit sensilla. II. Actual lyriform organs and the face deformations of the individual slits. J Comp Physiol A 195: 881–894, 2009 [DOI] [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol 30: 466–481, 1967 [DOI] [PubMed] [Google Scholar]
- Hustert R, Pflüger JH, Bräunig P. Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. Cell Tissue Res 216: 97–111, 1981 [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72: 623–666, 1992 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliyamoorthy S, Zill SN, Quinn RD. Force sensors in hexapod locomotion. In Mobile Robotics: Moving Intelligence, edited by Kordic V, Laznica A, Merdan M. Vienna: Advanced Robotic Systems, 2006, p. 495–512 [Google Scholar]
- Keller BR, Duke EF, Amer AS, Zill SN. Tuning posture to body load: decreases in load produce discrete sensory signals in the legs of freely standing cockroaches. J Comp Physiol A 193: 881–891, 2007 [DOI] [PubMed] [Google Scholar]
- Kent KS, Griffin LM. Sensory organs of the thoracic legs of the moth Manduca sexta. Cell Tissue Res 259: 209–223, 1990 [DOI] [PubMed] [Google Scholar]
- Klint R, Mazzarol N, Nielsen J, Sinkjaer T, Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010 [DOI] [PubMed] [Google Scholar]
- Knyazeva NI. Campaniform sensilla of Locusta migratoria, L. (Orthoptera, Acrididae). Ent Rev 53: 62–66, 1974 [Google Scholar]
- Knyazeva NI, Fudalewicz-Niemczyk W, Rosciszewska M. Proprioceptors of the house cricket (Gryllus domesticus, L.) (Orthoptera). Acta Biol Cracoviensia 18: 33–44, 1975 [Google Scholar]
- Krutki P, Jeleni S, Jankowska E. Do premotor interneurons act in parallel on spinal motoneurons and on dorsal horn spinocerebellar and spinocervical tract neurons in the cat? J Neurophysiol 105: 1581–1593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt F. Beiträge zur Anatomie der Muskulatur und der peripheren Nerven von Carausius (Dixippus) morosus. Zool Jahrb Anat 66: 63–128, 1940 [Google Scholar]
- Maus HM, Seyfarth A, Grimmer S. Combining forces and kinematics for calculating consistent centre of mass trajectories. J Exp Biol 214: 3511–3517, 2011 [DOI] [PubMed] [Google Scholar]
- Merritt DJ, Murphy RK. Projections of leg proprioceptors within the CNS of the fly phormia in relation to the generalized insect ganglion. J Comp Neurol 322: 16–34, 1992 [DOI] [PubMed] [Google Scholar]
- Mileusnic MP, Loeb GE. Force estimation from ensembles of Golgi tendon organs. J Neural Eng 6: 1–14, 2009 [DOI] [PubMed] [Google Scholar]
- Mugge W, Abbink DA, Schouten AC, Dewald JPA, van der Helm FCT. A rigorous model of reflex function indicates that position and force feedback are flexibly tuned to position and force tasks. Exp Brain Res 200: 325–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GM, Quinn RD. Posture control of a cockroach-like robot. IEEE Control Syst 19: 9–14, 1999 [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: a simulation study. J Biomech 42: 1282–1287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland PL, Emptage NJ. The central connection and actions during walking of tibial campaniform sensilla in the locust. J Comp Physiol A 178: 749–762, 1996 [DOI] [PubMed] [Google Scholar]
- Niederegger S, Gorb S. Tarsal movements in flies during leg attachment and detachment on a smooth substrate. J Insect Physiol 49: 611–620, 2003 [DOI] [PubMed] [Google Scholar]
- Noah JA, Quimby L, Frazier SF, Zill SN. Walking on a “peg leg”: extensor muscle activities and sensory feedback after distal leg denervation in cockroaches. J Comp Physiol A 190: 217–231, 2004 [DOI] [PubMed] [Google Scholar]
- Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol 528: 389–404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MY, Yang JF. Sensory gating for the initiation of the swing phase in different directions of human infant stepping. J Neurosci 22: 5734–40, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Central programming and reflex control of walking in the cockroach. J Exp Biol 56: 173–193, 1972 [Google Scholar]
- Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Rev 57: 222–227, 2008 [DOI] [PubMed] [Google Scholar]
- Petryszak A, Fudalewicz-Niemczyk A. External proprioceptors on the legs of insects of higher order. Acta Biol Cracoviensia Ser Zool 36: 13–22, 1994 [Google Scholar]
- Pringle JWS. Proprioception in insects. I. A new type of mechanical receptors from the palps of the cockroach. J Exp Biol 15: 101–113, 1938a [Google Scholar]
- Pringle JWS. Proprioception in insects. II. The action of the campaniform sensilla on the legs. J Exp Biol 15: 114–131, 1938b [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol 507: 293–304, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis H, Burtet L, Forget R, Feldman AG. Control of wrist position and muscle relaxation by shifting spatial frames of reference for motoneuronal recruitment: possible involvement of corticospinal pathways. J Physiol 588: 1551–1570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel A, DiCaprio R, Frazier S, Zill S. Active signaling of leg loading and unloading in the cockroach. J Neurophysiol 81: 1432–1437, 1999 [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Frazier SF, DiCaprio RA, Zill SN. Encoding of forces by cockroach tibial campaniform sensilla: implications in dynamic control of posture and locomotion. J Comp Physiol A 186: 359–374, 2000 [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Frazier SF, Zill SN. Dynamic responses of tibial campaniform sensilla studied by substrate displacement in freely moving cockroaches. J Comp Physiol A 187: 405–420, 2001 [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Ritzmann RE, Schaefer PL. Effects of aging on behavior and leg kinematics during locomotion in two species of cockroach. J Exp Biol 206: 4453–4465, 2003 [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VC, Bizzi E. Modules in the brain stem and spinal cord underlying motor behaviors. J Neurophysiol 106: 1363–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum P, Wosnitza A, Büschges A, Gruhn M. Activity patterns and timing of muscle activity in the forward walking and backward walking stick insect Carausius morosus. J Neurophysiol 104: 1681–1695, 2010 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006 [DOI] [PubMed] [Google Scholar]
- Schindler G. Funktionsmorphologische Untersuchungen zur Autotomie der Stabheuschrecke Carausius morosus Br. (Insecta: Phasmida). Zool Anzeiger 203: 316–326, 1979 [Google Scholar]
- Schmitz J. The depressor trochanteris motoneurones and their role in the coxo-trochanteral feedback loop in the stick insect Carausius morosus. Biol Cybern 55: 25–34, 1986 [Google Scholar]
- Schmitz J. Load-compensating reactions in the proximal leg joints of stick insects during standing and walking. J Exp Biol 183: 15–33, 1993 [Google Scholar]
- Schmitz J, Büschges A, Delcomyn F. An improved electrode design for en passant recording from small nerves. Comp Biochem Physiol 91: 769–772, 1988 [DOI] [PubMed] [Google Scholar]
- Schmitz J, Stein W. Convergence of load and movement information onto leg motoneurons in insects. J Neurobiol 42: 424–436, 2000 [DOI] [PubMed] [Google Scholar]
- Schneider A, Schmucker U. Force sensing for multi-legged walking robots: theory and experiments—Part 1: overview and force sensing. In: Mobile Robots, Moving Intelligence, edited by Buchli J. Mammendorf, Germany: ARS/pIV, 2006, p. 447–470 [Google Scholar]
- Skordos A, Chan PH, Vincent JFV, Jeronimidis G. A novel strain sensor based on the campaniform sensillum of insects. Philos Trans R Soc Lond A Math Phys Sci 360: 239–253, 2002 [DOI] [PubMed] [Google Scholar]
- Stein W, Schmitz J. Multimodal convergence of presynaptic afferent inhibition in insect proprioceptors. J Neurophysiol 82: 512–514, 1999 [DOI] [PubMed] [Google Scholar]
- Stuart DG, Goslow GE, Mosher CG, Reinking RM. Stretch responsiveness of Golgi tendon organs. Exp Brain Res 10: 463–476, 1970 [DOI] [PubMed] [Google Scholar]
- Tatar G. Mechanische Sinnesorgane an den Beinen der Stabheuschrecke Carausius morosus (diploma thesis). Cologne, Germany: University of Cologne, 1976 [Google Scholar]
- Ting LH, Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. J Neurophysiol 92: 808–823, 2004 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol 98: 2144–2156, 2007 [DOI] [PubMed] [Google Scholar]
- Tóth TI, Knops S, Daun-Gruhn S. A neuro-mechanical model explaining forward and backward stepping in the stick insect. J Neurophysiol 107: 3267–3280, 2012 [DOI] [PubMed] [Google Scholar]
- Trulsson M. Mechanoreceptive afferents in the human sural nerve. Exp Brain Res 137: 111–116, 2001 [DOI] [PubMed] [Google Scholar]
- Watson JT, Ritzmann RE, Zill SN, Pollack AJ. Control of obstacle climbing in the cockroach, Blaberus discoidalis. I. Kinematics. J Comp Physiol A 188: 39–53, 2002 [DOI] [PubMed] [Google Scholar]
- Welch TDJ, Ting LH. A feedback model explains the differential scaling of human postural responses to perturbation acceleration and velocity. J Neurophysiol 101: 3294–3309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler G. The co-ordination of walking movements in arthropods. Symp Soc Exp Biol 20: 229–249, 1966 [PubMed] [Google Scholar]
- Wetzel MC, Atwater AE, Wait JV, Stuart DG. Kinematics of locomotion by cats with a single hindlimb deafferented. J Neurophysiol 39: 667–678, 1976 [DOI] [PubMed] [Google Scholar]
- Wile GD, Daltorio KA, Diller ED, Palmer LR, Gorb SN, Ritzmann RE, Quinn RD. Screenbot: walking inverted using distributed inward gripping. In: IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS'08), 2008, p. 1513–1518 [Google Scholar]
- Wilkinson RS, Fukami Y. Responses of isolated Golgi tendon organs of cat to sinusoidal stretch. J Neurophysiol 49: 976–988, 1983 [DOI] [PubMed] [Google Scholar]
- Zill SN, Frazier SF, Neff D, Quimby L, Carney M, DiCaprio R, Thuma J, Norton M. Three dimensional graphic reconstruction of the insect exoskeleton through confocal imaging of endogenous fluorescence. Microsc Res Tech 48: 367–384, 2000 [DOI] [PubMed] [Google Scholar]
- Zill S, Büschges A, Schmitz J. Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J Comp Physiol A 197: 851–867, 2011 [DOI] [PubMed] [Google Scholar]
- Zill SN, Keller BR, Chaudhry S, Duke ER, Neff D, Quinn R, Flannigan C. Detecting substrate engagement: responses of tarsal campaniform sensilla in cockroaches. J Comp Physiol A 196: 407–420, 2010 [DOI] [PubMed] [Google Scholar]
- Zill SN, Moran DT. The exoskeleton and insect proprioception. I. Responses of tibial campaniform sensilla to external and muscle regenerated forces in the American cockroach, Periplaneta americana. J Exp Biol 91: 1–24, 1981 [Google Scholar]
- Zill SN, Ridgel AL, DiCaprio RA, Frazier SF. Load signalling by cockroach trochanteral campaniform sensilla. Brain Res 822: 271–275, 1999 [DOI] [PubMed] [Google Scholar]
- Zill S, Schmitz J, Büschges A. Load sensing and control of posture and locomotion. Arthropod Struct Dev 33: 273–286, 2004 [DOI] [PubMed] [Google Scholar]


