Abstract
Merkel cells have been proposed to play a role in mechanical transduction of light touch in mammals. In the present study, Merkel cells were prepared from upper segments of rat vibrissal hair follicles and maintained in culture. Reponses of these cells to shear mechanical forces were examined by Ca2+ imaging technique. Shear forces of ≥0.8 dyn/cm2 that were delivered to the cells by the application of normal bath solution significantly increased intracellular Ca2+ levels ([Ca2+]i) in some of these cells, and up to 30% cells responded to 1.6 dyn/cm2 shear force applied for 20 s. Gd3+ (100 μM), a compound widely used to inhibit mechanically activated channels, abolished shear force-induced increases of [Ca2+]i in these cells. Reduction of extracellular Ca2+ concentration from 2 mM to 0.2 mM also abolished shear force-induced increases of [Ca2+]i in these cells. In addition to shear force, we found that many shear force-responding cells also responded to hypotonic solution. However, the response to hypotonic solution was not abolished by Gd3+ (100 μM). We also found that all shear force-responding cells responded to ATP (100 μM) with large increases of [Ca2+]i. The responses to ATP remained in the presence of Gd3+. Taken together, our results suggest that Merkel cells in culture are sensitive to shear force stress, osmotic, and chemical stimuli and that shear force-induced increases of [Ca2+]i may be mediated by the activation of mechanically activated channels.
Keywords: shear stress, mechanical stimulation, mechanotransduction, mechanoreceptors, ion channel
merkel cells were first identified and described by Friedrich Sigmund Merkel in 1875 and were thought to be “touch cells” (Merkel 1875). These cells are found at touch-sensitive areas of glabrous and hairy skin as well as the inner cell layer of the superior enlargement of hair follicles of mammals. Merkel cells are innervated by large myelinated (Aβ) axonal neurites, and their contact points morphologically resemble chemical synapses and are called Merkel cell-neurite complexes (Halata et al. 2003). Merkel cell-neurite complexes are proposed to be functional units for transduction of slowly adapting (SA) mechanical stimuli such as light touch and hair movement (Halata et al. 2003; Iggo and Muir 1969). However, how Merkel cells sense mechanical stimuli remains uncertain.
It has been hypothesized that Merkel cells are sensory transducer cells directly sensing mechanical stimuli, which in turn triggers the release of chemical messengers from Merkel cells onto Aβ fiber neurites to transmit mechanical stimuli into sensory nerve impulses. Consistent with this hypothesis, deletion of Merkel cells from animals by different approaches such as irradiation of quinacrine-loaded Merkel cells or chemical damage of Merkel cells by chloroquine (Ikeda et al. 1994; Senok et al. 1996) or genetic deletion of the transcription factor Atoh1 and Merkel cells (Maricich et al. 2009) resulted in the loss of the characteristic neurophysiological responses to light touch. However, conflicting results were obtained in other studies. For example, in a different study selective elimination of the Merkel cells from rat touch domes by irradiation of quinacrine-loaded Merkel cells showed little change in mechanosensory function of these touch domes (Mills and Diamond 1995). In neurotrophin receptor p75 knockout mice in which Merkel cells were progressively lost, neither the survival nor the mechanical sensitivity of slowly adapting mechanoreceptive Aβ fibers was severely affected (Kinkelin et al. 1999). The transducer function of Merkel cells was also challenged by the extremely fast mechanical responses recorded from Merkel cell-neurite complexes, which suggested that mechanical transducers might be located on the neurites of Merkel cell-neurite complexes (Gottschaldt and Vahle-Hinz 1981). In agreement with this idea, several types of mechanically activated currents have been recorded from primary afferent neurons (Cho et al. 2002, 2006; Drew et al. 2002; Drew and Wood 2007).
Merkel cells contain dense-cored vesicles in which a number of transmitter substances such as VIP, substance P, enkephalin, CGRP, and 5-HT have been identified (English et al. 1992; Garcia-Caballero et al. 1989; Tachibana and Nawa 2005; Zaccone 1986; Zaccone et al. 1995). These substances were thought to be releasable and necessary for the characteristic slowly adapting response of SA type I (SAI) mechanoreceptors (Findlater et al. 1987). Agonists and/or antagonists for 5-HT (He et al. 2003) and ionotropic (Fagan and Cahusac 2001) and metabotropic (Cahusac and Senok 2006) glutamate receptors were found to have effects on SAI response, and therefore 5-HT and glutamate were suggested to be either transmitters or modulators of Merkel cell-neurite complexes. Molecular profiling revealed that Merkel cells have neuronal synaptic release machinery including presynaptic molecules, vesicular glutamate transporter 2, voltage-gated Ca2+ channels, and K+ channels (Haeberle et al. 2004). Merkel cells in the intact skin were specifically labeled by antibodies against voltage-activated Ca2+ channels (CaV2.1) and voltage- and Ca2+-activated K+ (BKCa) channels (Piskorowski et al. 2008). Voltage-clamp recordings from acutely dissociated Merkel cells revealed voltage-gated Ca2+ channel currents and K+ channel currents (Piskorowski et al. 2008; Yamashita et al. 1992). Ca2+ entry through voltage-gated Ca2+ channels produced small intracellular Ca2+ transients, but the signal was amplified severalfold by Ca2+-induced Ca2+ release (Piskorowski et al. 2008). Voltage-activated K+ currents in Merkel cells were carried predominantly by BKCa channels (Piskorowski et al. 2008). These properties suggest that Merkel cells have neuronal-like excitability, although recent studies suggested that they were originated from epidermal lineage (Morrison et al. 2009).
Attempts have been made to determine whether Merkel cells respond directly to different types of mechanical stimulation. A previous study using patch-clamp recording technique failed to show any mechanical response in Merkel cells that were acutely dissociated from rat footpad epidermis (Yamashita et al. 1992). In a sinus hair preparation, mechanical stimulation of Merkel cells with fine glass rods resulted in small transient increases in intracellular Ca2+ levels in the group of Merkel cells around the stimulating probes (Chan et al. 1996). In acutely dissociated Merkel cells, swelling of Merkel cells by a hypotonic solution triggered cytoplasmic Ca2+ transients. These Ca2+ signals appeared to arise from swelling-activated Ca2+-permeable ion channels since Ca2+ transients were abolished in extracellular Ca2+-free solution (Boulais et al. 2009; Haeberle et al. 2008). Using the same preparation, a recent study showed a sustained inward current that was evoked by the application of a high negative pressure through patch-clamp electrodes (Boulais et al. 2009). The study also used the Ca2+ imaging technique to show that swelling Merkel cells with hypotonic solution increased intracellular Ca2+ levels in Merkel cells. Although both negative pressure and cell swelling could elicit responses in Merkel cells, the mechanotransduction and osmoreception were suggested to be mediated by different mechanisms (Boulais et al. 2009).
Merkel cells have been successfully cultured under different conditions (Fukuda 1996; Haeberle et al. 2008; Shimohira-Yamasaki et al. 2006). Merkel cells usually have a fibroblast-like morphology with some fine processes when they are grown in serum-containing culture medium (Fukuda 1996; Haeberle et al. 2008). When Merkel cells and nerve cells were cultured together, both nerve fibers and cytoplasmic processes of Merkel cells outgrew and cooperatively organized synapselike structures at their contact points (Shimohira-Yamasaki et al. 2006). Cultured Merkel cells may provide an important system to investigate molecular mechanisms by which mechanical stimuli are detected by Merkel cells and subsequently transmitted to sensory nerve fibers. Only recently has a study demonstrated that cultured Merkel cells respond to hypotonic solution with increases of intracellular Ca2+ levels (Haeberle et al. 2008). Whether Merkel cells in culture may respond to other types of mechanical stimulation remains to be determined. In the present study, we used cultured Merkel cells isolated from vibrissal hair follicles of rat whisker pads and calcium imaging technique to characterize the sensitivity of these cells to shear force, cell swelling, and ATP.
MATERIALS AND METHODS
Animal care and use conformed to National Institutes of Health guidelines for care and use of experimental animals. Experimental protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Sprague-Dawley rats at the age of 14–21 days were deeply anesthetized with isoflurane and killed by decapitation. Whisker pads were removed and placed in Leibovitz L-15 medium (Cellgro, Manassas, VA), and vibrissal hair follicles were dissected out under a light microscope. Free follicles were truncated to obtain enlargement segments where Merkel cells are located (Fig. 1). The Merkel cell-containing segments were transferred to a tube containing 4 mg of collagenase (Sigma, St. Louis, MO) and 10 mg of dispase (Roche Applied Science, Penzberg, Germany) in 2 ml of MEM (HyClone Lab, Logan, UT) and incubated at 37°C for 1 h on an orbital shaker. Enzyme activity was ceased by washing the follicle segments three times with MEM. Merkel cells were then dissociated from the follicle segments by repeated trituration with a Pasteur pipette. The dissociated cells were plated on coverslips precoated with poly-d-lysine and maintained in a MEM culture medium that also contained 5% heat-inactivated horse serum (JRH Biosciences, Lenexa, KS), uridine/5-fluoro-2′-deoxyuridine (10 μM; Sigma), 8 mg/ml glucose, and 1% vitamin solution (Invitrogen, Eugene, OR). The cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 for 24 h, and then the culture medium was replaced with a pure MEM culture medium. The cultures remained in the incubator at 37°C with 95% air and 5% CO2. Cells were used within 72 h in culture.
Fig. 1.
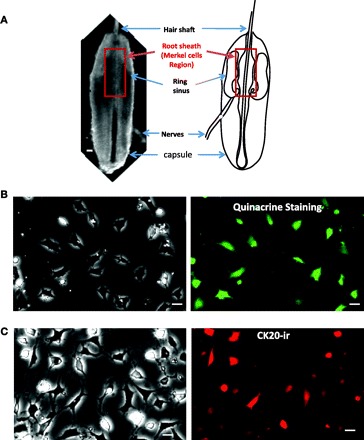
Quinacrine staining and cytokeratin 20 (CK20) immunoreactivity (ir) of cultured Merkel cells dissociated from vibrissal hair follicles of rat whisker pads. A, left: an intact vibrissal hair follicle dissected out from a rat whisker pad and viewed under a light microscope. Right: schematic diagram illustrates the structures of a vibrissal hair follicle. Highlighted region in both panels is the root sheath of upper hair follicle segment where Merkel cells are present at a high density. B: cultured cells labeled with quinacrine. Left: viewed under a light microscope. Right: viewed under a fluorescent microscope. C: cells after immunostaining with an antibody against CK20. Left: viewed under a light microscope. Right: viewed under a fluorescent microscope. In both B and C cells were in culture for 48 h before staining. Scale bars, 50 μm in A and 10 μm in B and C.
To stain Merkel cells with quinacrine, a coverslip with cultured cells was mounted on a perfusion chamber. Cells were incubated 1 h with 10 nM quinacrine in normal bath solution at room temperature. Normal bath solution contained (in mM) 150 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.3 adjusted with NaOH and osmolarity 320 mosM adjusted with sucrose. Cells were then perfused with the normal bath solution at 1 ml/min. Quinacrine-stained cells were examined under an inverted fluorescent microscope with an FITC filter set (Olympus IX70 microscope, Lake Success, NY). To immunostain cells against cytokeratin 20 (CK20), cells in culture dishes were fixed with a fixation solution that contained 4% paraformaldehyde and 0.4% Triton X-100 overnight at 4°C. Cells were rinsed five times with distilled water and then incubated with 2% Triton X-100 for 1.5 h at room temperature. Cells were further rinsed three times with a phosphate-buffered saline (PBS) solution that contained 1% goat serum and 2% Triton X-100 (1% GS-PBS-T). Cells were then exposed to a 1:30 goat serum GS-PBS-T solution for 1 h at room temperature and incubated with a monoclonal anti-mouse CK20 antibody overnight at 4°C. After being rinsed with 1% GS-PBS-T three times, the cells were exposed to 1:100 goat anti-mouse IgG conjugated with Alexa 594 (Invitrogen) for 3 h at room temperature, washed three times with 1% GS-PBS-T, and mounted on coverglasses. CK20 immunoreactivity (CK20-ir) was examined under a ×10 objective with a Texas red filter set. Fluorescent images for quinacrine staining and CK20-ir were taken by a Peltier-cooled charge-coupled device (CCD) camera (PentaMAX-III System, Roper Scientific, Trenton, NJ) under a ×10 objective with 100-ms exposure time, and positively stained cells were counted off-line.
For calcium imaging experiments, the calcium indicator fluo-3 was loaded into Merkel cells on coverslips by incubation of cells with 5 μM fluo-3 AM (Invitrogen) in normal bath solution at 37°C for 1 h. After dye loading, a coverslip was mounted on a perfusion chamber (width of 20 mm and height of 2 mm) and the chamber was then placed on the stage of an inverted Olympus IX70 microscope. Cells on the coverslip were continuously perfused with normal bath solution flowing at 0.5 ml/min. At this perfusion flow rate, the shear force at the center of the chamber was 0.0024 dyn/cm2, estimated based on the equation S = 3μQ/wh2, where μ is fluid viscosity of the bath solution (0.0077 P), Q is the flow rate (ml/s), w is the width of the perfusion chamber, and h is the height of the chamber (Jacobs et al. 1995). Fluo-3 was excited at 450 nm with a mercury lamp; fluorescence emission was collected at 550 nm, and the wave lengths of excitation and emission were achieved by a fluorescence filter set. Fluo-3 fluorescence in the cells was detected with a Peltier-cooled CCD camera (PentaMAX-III System) under a ×10 objective. Images were acquired at one frame every 5 s, 100-ms exposure time per frame, with MetaFluor Imaging System software (Molecular Devices, Sunnyvale, CA). To test shear force, normal bath solution was applied to cells at the center of the chamber through a glass tube (1-mm ID) positioned 1.0 mm away from cells. At flow rates of 0.3, 0.6, 1.2, and 2.4 ml/min, the shear stress force was 0.4, 0.8, 1.6, and 3.2 dyn/cm2, estimated based on the equation S = 4μQ/πr3, where μ is fluid viscosity (0.0077 P), Q is the flow rate (ml/s), and r is the internal radius of the tube (Jacobs et al. 1995; Olesen et al. 1988). Unless otherwise indicated, the time interval was at least 15 min between each test. In some experiments, Gd3+ (100 μM) was preapplied through the bath and then coapplied during the application of shear force. To test the hypotonic solution effect, a modified bath solution with 224 mosM was applied through the bath. To test the effect of ATP, ATP at a concentration of 100 μM was applied to cells through the above glass tube in the presence of Gd3+. All experiments were carried out at room temperature of ∼24°C.
Relative fluorescence intensity (ΔF/F0) was used as response to stimuli, and cells with ΔF/F0 values of ≥0.15 (i.e., equal to or above 15% of increase in fluorescence intensity) were designated as responsive cells. Sample sizes are numbers of cells in each experimental group. Percentages of responding cells were calculated in each dish, and sample sizes are numbers of dishes in each experimental group. Unless otherwise specified, data are presented as means ± SE. Analysis of variance (ANOVA, 1 way) was used for statistical analyses of data sets of multiple groups, followed by Student-Newman-Keuls post hoc test. Student's t-test was used to evaluate the significance of changes in mean values between two groups. Statistical significance was considered at the level of P < 0.05.
RESULTS
Intact vibrissal hair follicles dissected out from rat whisker pads have the following structures when viewed under a light microscope: hair shaft, root sheath, ring sinus, attached nerves, and capsule (Fig. 1A). Merkel cells have been found to be present at high density in root sheath of upper hair follicle segment (Fukuda 1996). Enzyme treatment of the upper hair follicle segments and subsequent mechanical dissociation yielded high-density Merkel cells in culture dishes as evidenced by staining with quinacrine (Fig. 1B) or CK20-ir (Fig. 1C), two often-used markers for Merkel cell identification (Fukuda et al. 2003). The sizes of these cells were ∼10–20 μm. Quinacrine-labeled cells and CK20-ir-positive cells were 69.8 ± 4.2% (n = 14 dishes, total 233/335 cells, 3 dishes) and 60.8 ± 4.4% (n = 19 dishes, total 203/340 cells, 4 dishes), respectively. Merkel cells were round in shape when immediately plated in dishes (not shown), and they extended some processes in culture overnight (Fig. 1, B and C).
Figure 2A is a schematic diagram that shows the experimental setup for the application of shear force onto cells. In these experiments, cells were continuously perfused with normal bath solution (perfusion bath) at a constant flow rate of 0.5 ml/min. At this flow rate in our recording chamber, the perfusion bath only produced 0.0024 dyn/cm2 shear force on the cells at the center of the recording chamber, and no cell responded to this baseline shear force (Fig. 2B, top left). Accordingly, only those cells at the center field of the recording chamber were tested, in order to keep baseline shear force constant. To test cell mechanical sensitivity, a shear force bath (same as normal bath solution) was applied from a tube whose opening was located near the center of the recording chamber (Fig. 2A). Applications of shear forces could elicit a rapid increase of intracellular Ca2+ levels in some cells, as indicated by the increase of fluo-3 fluorescence intensity (Fig. 2B, bottom).
Fig. 2.
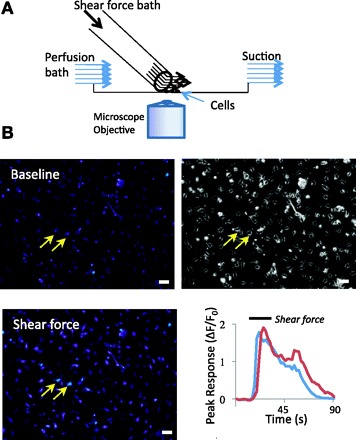
Experimental setup for delivering shear force and measuring cell responses. A: schematic diagram illustrates the experimental setup for delivering shear force to cells. Shear forces were generated by delivering a shear force bath at different flow rates from a glass tube positioned very close to the center of the recording chamber. The shear force bath had chemical composition identical to the perfusion bath in control experiments. B: an example shows Ca2+ imaging obtained under baseline conditions (top left) and after the application of 1.6 dyn/cm2 shear force (bottom left). Cells in the recording chamber were continuously perfused with perfusion bath flowing at 0.5 ml/min (baseline), which yielded negligible shear force on the cells at the center of the recording chamber. Top right: cells viewed under light microscope (×10 objective). Scale bars, 50 μm in each panel. Bottom right: 2 traces show the increases of intracellular Ca2+ levels in the 2 cells indicated by 2 arrows in the images. ΔF/F0, relative fluorescence intensity.
We examined cell responses to different magnitudes of shear forces. In this experiment, shear forces were applied to cells for 20 s with a time interval of 20 min between each application (Fig. 3, A–D). At the shear force of 0.4 dyn/cm2, no cell was found to have any response (not shown). When shear force increased to 0.8 dyn/cm2, 3.2 ± 0.9% cells (n = 13 dishes, 64/1,997 cells) showed responses, and the peak responses (ΔF/F0) were 0.38 ± 0.05 (n = 64 cells). A further increase of shear force to 1.6 dyn/cm2 resulted in significant increase in the percentage of responding cells to 29.9 ± 3.6% (n = 13 dishes, 574/1,997 cells; P < 0.05) and also increases in peak responses (ΔF/F0) to 0.55 ± 0.02 (n = 574 cells, 13 dishes; P < 0.05). There was no further increase in the cell numbers and peak responses when shear force increased to 3.2 dyn/cm2 (not shown). While shear force of 1.6 dyn/cm2 elicited responses in the cells that morphologically resemble Merkel cells, it had no effect on the cells that morphologically resemble keratinocytes (Fig. 3, E–G). Of 26 keratinocytes tested with a shear force of 1.6 dyn/cm2, none responded to the shear force. This is in sharp contrast to Merkel cells (n = 26; Fig. 3G).
Fig. 3.
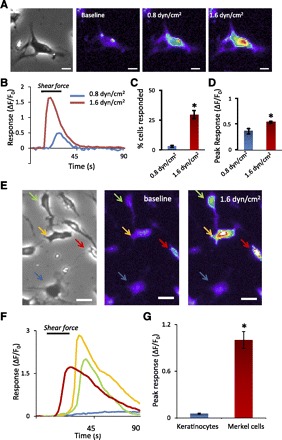
Increases of intracellular Ca2+ levels following the application of shear force. A: images from left to right show a cultured cell viewed under light microscope (1st panel), baseline fluo-3 fluorescence (2nd panel), and increases of fluo-3 fluorescence intensity following the application of 0.8 dyn/cm2 (3rd panel) and 1.6 dyn/cm2 (4th panel) shear forces. Scale bars, 10 μm. B: 2 traces show the responses of the cell shown in A to 0.8 dyn/cm2 and 1.6 dyn/cm2 shear forces. The horizontal bar above the traces indicates the duration (20 s) of shear force application. C: summary of % of responding cells to 0.8 dyn/cm2 (n = 13 dishes, total 64/1,997 cells) and 1.6 dyn/cm2 (n = 13 dishes, total 574/1,997 cells) shear forces. D: summary of peak responses to 0.8 dyn/cm2 (n = 64 cells) and 1.6 dyn/cm2 (n = 574 cells) shear forces. E: images from left to right are bright field (1st panel) and fluo-3 fluorescence images at baseline (2nd panel) and after the application of 1.6 dyn/cm2 (3rd panel) shear force. Scale bars, 20 μm. In the image field there were several Merkel cells, and 3 of them responded to 1.6 dyn/cm2+ shear force (indicated by red, orange, and green arrows). There was a keratinocyte (a big cell indicated by a blue arrow) that had no response to the shear force. F: time courses of fluo-3 fluorescence intensity in the cells indicated by arrows. The horizontal bar above the traces indicates shear force application. G: peak fluo-3 fluorescence intensity in keratinocytes (n = 26) and Merkel cells (n = 26). Data represent means ± SE. *P < 0.05.
We next examined effects of shear force duration on cell responses. At the same shear force of 1.6 dyn/cm2, the peak responses (ΔF/F0) were 0.22 ± 0.02 (n = 43 cells, 5 dishes), 0.30 ± 0.03 (n = 81, 5 dishes), 0.36 ± 0.02 (n = 94, 5 dishes), and 0.38 ± 0.02 (n = 145, 5 dishes) when shear force stimulation duration was 2, 5, 10, and 20 s, respectively (Fig. 4, A and B). Responses evoked by 1.6 dyn/cm2 shear force at stimulation durations of 10 and 20 s were significantly larger than the responses evoked by 2-s stimulation (P < 0.05).
Fig. 4.
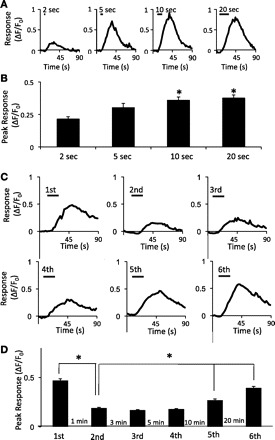
Responses to shear force at different stimulation durations and to repeated shear force stimulation. A: sample traces show responses of a cell to 1.6 dyn/cm2 shear force at durations of 2, 5, 10, and 20 s. The horizontal bar above each trace indicates the duration of shear force application. B: summary of peak responses of cells to 1.6 dyn/cm2 shear force at durations of 2, 5, 10, and 20 s. C: sample traces show responses of a cell to repeated shear force stimulation. The intensity of each stimulus was 1.6 dyn/cm2 and the duration 20 s. The time intervals between each stimulus are shown in D. D: bar graph shows summary of peak responses of cells to repeated shear force stimulation at different intervals. Data represent means ± SE. *P < 0.05.
Shear force-evoked increases of intracellular Ca2+ levels showed some degree of adaptation to consecutive stimulation. As shown in Fig. 4, C and D, the peak responses to the first stimulation were 0.47 ± 0.02 (n = 543 cells, 7 dishes) and were significantly reduced to 0.19 ± 0.01 (n = 543, 7 dishes; P < 0.05) in the subsequent stimulation at the time interval of 1 min. As the time intervals between shear force stimulation increased to 3, 5, 10, and 20 min, responses were 0.17 ± 0.05 (n = 543, 7 dishes), 0.18 ± 0.01 (n = 543, 7 dishes), 0.27 ± 0.01 (n = 543, 7 dishes), and 0.39 ± 0.02 (n = 543, 7 dishes), respectively (Fig. 4D). Peak responses at the intervals of 10 and 20 min showed significant recovery from the adaptation (P < 0.05).
Increases of intracellular Ca2+ level responding to shear force stimulation could be due to Ca2+ entry from the extracellular side or Ca2+ release from intracellular Ca2+ stores. To test these possibilities, we performed experiments in either normal bath solution that contained 2 mM Ca2+ or a low-Ca2+ bath solution that contained 0.2 mM Ca2+ and examined shear force effect under these two conditions (Fig. 5). Cell responses (ΔF/F0) to 1.6 dyn/cm2 shear force were 0.98 ± 0.05 (n = 157 cells, 6 dishes) in normal bath solution and significantly reduced to 0.05 ± 0.002 (n = 157, 6 dishes, P < 0.05) in the low-Ca2+ bath solution (Fig. 5). After return to normal Ca2+ bath solution, responses to the shear force recovered to 0.60 ± 0.04 (n = 157 cells, 6 dishes).
Fig. 5.
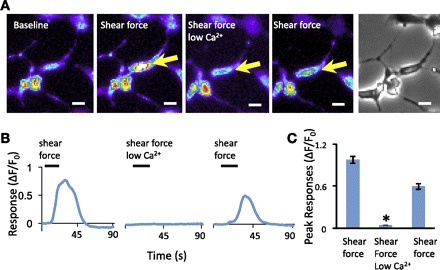
Extracellular Ca2+ dependence of shear force-induced responses. A: sample images show responses of a cell (indicated by arrow) to shear force in normal bath solution with 2 mM Ca2+ (2nd and 4th panels) and in a bath solution with low Ca2+ (0.2 mM, 3rd panel). The images in the 1st and 5th panels are baseline and bright field, respectively. Scale bars, 10 μm. B: traces show the responses of the cell indicated in A. C: bar graph shows summary of peak responses under each condition described in A (n = 157 cells). In all experiments, 1.6 dyn/cm2 shear force was applied for 20 s and the time interval between each test was 15 min. Data represent means ± SE. *P < 0.05.
Shear force-evoked Ca2+ entry may be due to the activation of mechanically activated channels expressed on the cells of our Merkel cell preparation. To test this possibility, we examined whether shear force-induced responses could be blocked by Gd3+, a widely used blocker for mechanically activated channels (Boulais et al. 2009). As shown in Fig. 6, peak responses to 1.6 dyn/cm2 shear force were 0.66 ± 0.04 (n = 219, 5 dishes) in the absence of Gd3+ and were significantly reduced to 0.03 ± 0.002 in the presence of 100 μM Gd3+ (n = 219, 5 dishes; P < 0.05). Thus, in cells in which intracellular Ca2+ levels were increased by shear force, the effect was almost completely abolished in the presence of 100 μM Gd3+. The blockage by Gd3+ was reversed (peak response: 0.75 ± 0.06, n = 219, 5 dishes) after 20-min wash in baseline normal bath solution (Fig. 6).
Fig. 6.
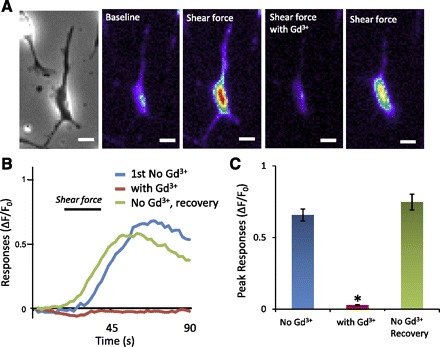
Inhibition of cell responses to shear force by Gd3+. A: from left to right, a bright field image (1st panel) and fluorescent images taken under baseline conditions (2nd panel) and after the application of 1.6 dyn/cm2 shear force with normal bath solution (3rd panel), the application of 1.6 dyn/cm2 shear force with bath solution that also contained Gd3+ (100 μM, 4th panel), and the application of 1.6 dyn/cm2 shear force after washing out of Gd3+ (5th panel). Scale bars, 10 μm. B: traces show the responses of the cell illustrated in A under different conditions. The duration of shear force application is indicated by a bar above the traces. C: bar graph shows the summary of peak responses and inhibition by Gd3+ (n = 219 cells). In each experiment, 1.6 dyn/cm2 shear force was applied for 20 s and the time interval between each test was 15 min. Data represent means ± SE. *P < 0.05.
Many cells that responded to shear force also responded to hypotonic solution, and the effect of hypotonic solution remained in the presence of 100 μM Gd3+ (Fig. 7). In this set of experiments, the percentage of cells that responded to 1.6 dyn/cm2 shear force was 18.5 ± 2.8% (n = 7 dishes, 196/1,143 cells) and the percentage responding to hypotonic solution was 19.0 ± 6.0% (n = 7 dishes, 189/1,143 cells; Fig. 7C, left). Of all 196 cells that responded to the shear force, 59 (30%) also responded to hypotonic solution that contained 100 μM Gd3+. The peak responses to the shear force were 0.75 ± 0.03 (n = 196, 7 dishes), significantly higher than the responses induced by hypotonic solution (0.46 ± 0.03, n = 189, 7 dishes, P < 0.05; Fig. 7C, right).
Fig. 7.
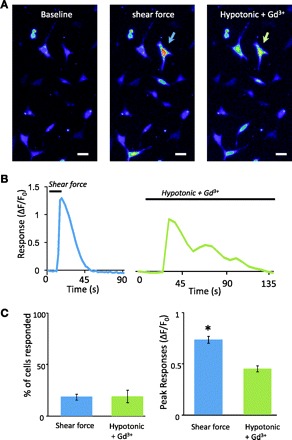
Responses induced by hypotonic solution. A: images show Fluo-3 fluorescence intensity in cells before (1st panel) and after the application of 1.6 dyn/cm2 shear force (2nd panel) or the application of a hypotonic solution (3rd panel). The hypotonic solution was applied in the presence of 100 μM Gd3+. A cell that responded to both shear force and hypotonic solution is indicated by arrows in 2nd and 3rd panels. Scale bars, 20 μm. B: traces are the responses of the cell indicated in A to 1.6 dyn/cm2 shear force (left) and the hypotonic solution (right). The horizontal bar above each trace indicates the stimulation duration. C, left: summary of the percentage of the cells that responded to shear force (n = 7 dishes, total 196/1,143 cells) and hypotonic solution (n = 7 dishes, 189/1,143 cells). Right: peak responses to the shear force (n = 196 cells) and the hypotonic solution (n = 189). In each experiment, the hypotonic solution was applied in the presence of 100 μM Gd3+. Data represent means ± SE. *P < 0.05.
The cells that responded to shear force also responded to 100 μM ATP (Fig. 8). However, more cells responded to ATP than to shear force. For a shear force of 1.6 dyn/cm2, 24.8 ± 1.2% (n = 9 dishes, 588/2,407 cells) cells responded (Fig. 8C). The percentage of cells that responded to 100 μM ATP was 86.7 ± 2.5% (n = 9 dishes, 2,096/2,407 cells) in the absence of Gd3+ and 84.3 ± 2.2% (n = 9 dishes, 2,044/2,407 cells) in the presence of Gd3+, significantly higher than the percentage of cells responding to shear force (P < 0.05). Peak responses to 100 μM ATP in the absence and presence of 100 μM Gd3+ were 1.26 ± 0.06 (n = 205) and 1.04 ± 0.06 (n = 205) respectively, significantly higher than shear force-induced responses (0.83 ± 0.04, n = 205, P < 0.05; Fig. 8D).
Fig. 8.
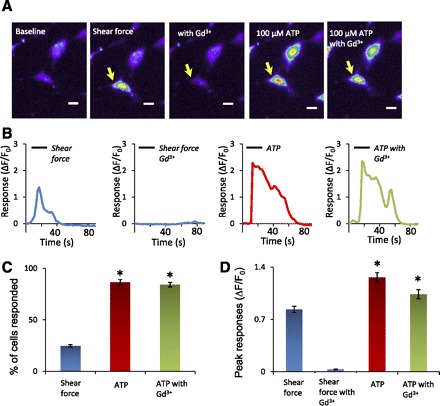
Responses induced by ATP. A: images from left to right show responses of a cell (indicated by arrow) to 1.6 dyn/cm2 shear force in the absence (2nd panel) and presence (3rd panel) of 100 μM Gd3+ and to 100 μM ATP in the absence (4th panel) and presence (5th panel) of 100 μM Gd3+. Scale bars, 10 μm. B: traces show the time course of the responses of the cell indicated in A. The horizontal bar over each trace indicates the application of shear force or ATP. C: summary of the percentage of responding cells in the experiments illustrated in A (n = 9 dishes in each group, total 2,407 cells). D: summary of the peak responses in the experiments illustrated in A (n = 205 cells in each group). Data represent means ± SE. *P < 0.05.
DISCUSSION
This is the first study to use shear force to demonstrate mechanical sensitivity of Merkel cells grown in culture. In this study, we isolated cells from the upper segment of rat vibrissal hair follicles. Our preparation yielded ∼70% cells that were stained with quinacrine, a chemical marker often used for identifying Merkel cells (Fukuda et al. 2003). There were ∼61% of cells in our preparation that were immunoreactive to CK20, a specific immunochemical marker for Merkel cells (Fukuda et al. 2003). The percentage of Merkel cells in our preparation as based on the staining with quinacrine and CK20-ir were lower than that of a previous study using the same cell preparation with quinacrine staining (Fukuda 1996). In that study, >90% of cells were labeled by quinacrine and considered to be Merkel cells (Fukuda 1996). The discrepancy between the previous study and ours could be because we excluded weak-staining cells as positive cells and therefore may underestimate the percentage of Merkel cells in our Merkel cell preparation. Although most cells (61–70%) in our preparation were defined as Merkel cells by the staining methods, shear force-sensitive cells were only up to 30%. This indicated that not all cultured Merkel cells were sensitive to shear force under our experimental condition.
Previous studies have demonstrated mechanical sensitivity of noncultured Merkel cells with several other stimulation methods including probing Merkel cell membrane with fine glass rods (Chan et al. 1996), swelling Merkel cells with hypotonic solution (Boulais et al. 2009; Haeberle et al. 2008), and application of negative pressures into cells with patch-clamp electrodes (Boulais et al. 2009). None of these approaches is a physiological stimulus for Merkel cells in vivo. Shear force is also not a physiological stimulus for Merkel cells. However, using shear force as a method to mechanically stimulate Merkel cells may have certain advantages over other approaches when experiments are performed with cultured Merkel cells. For example, probing cell membranes with fine glass rods may potentially damage cell membranes. This may be particularly a problem for cultured Merkel cells because they are small and flat in culture. On the other hand, this is not a concern when shear force is used to stress cell membranes. The application of negative pressure into cells through patch-clamp electrodes may change intracellular environment due to intracellular dialysis. This is also not a concern when shear force is used to apply mechanical force onto Merkel cells. Although both cell swelling and shear force can cause mechanical stress on cell membranes, the effect of hypotonic solution is slow-on and slow-off. On the other hand, membrane stress induced by shear force has a short delay in both on-time and off-time of mechanical force, thereby probably better mimicking natural stimuli experienced by Merkel cells in vivo.
We showed that shear force-induced increases of intracellular Ca2+ levels depended on Ca2+ entry from the extracellular side. This could be due to a direct entry of Ca2+ through a mechanically activated channel. Other mechanisms including voltage-gated Ca2+ channels and intracellular stores might also contribute to the increases of intracellular Ca2+ levels following shear force stimulation. Voltage-gated Ca2+ channels have been found to be expressed in Merkel cells, and depolarization of Merkel cell membranes by high concentrations of potassium chloride could result in the increases of intracellular Ca2+ levels due to the activation of voltage-gated Ca2+ channels (Haeberle et al. 2008; Piskorowski et al. 2008; Yamashita et al. 1992). The initial Ca2+ signals induced by mechanical stimulation could be further amplified and prolonged through calcium-induced calcium release in Merkel cells (Haeberle et al. 2008; Piskorowski et al. 2008). There has been evidence indicating the presence of caffeine- and ryanodine-sensitive calcium release stores in Merkel cells isolated from rat vibrissal hair follicles (Senok and Baumann 1997). Release of Ca2+ from intracellular Ca2+ stores may also be the mechanism by which ATP increased intracellular Ca2+ levels in Merkel cells shown in the present study. This suggestion is based on a previous study showing that ATP-induced responses in Merkel cells were mediated by P2Y receptors (Chan et al. 1996).
A previous study suggested that mechanical responses induced by intracellular negative pressure and hypotonic solution may be mediated by two different types of transducer molecules (Boulais et al. 2009). This suggestion was based on the observation that Gd3+, a widely used blocker of mechanically activated channels/receptors, abolished mechanical responses induced by intracellular negative pressure but not by hypotonic solution (Boulais et al. 2009). Consistent with this previous work, we found that Gd3+ abolished shear force-induced responses but not hypotonic solution-induced response. The differential effects of Gd3+ in our study raised a possibility that responses to shear force and hypotonic solution have different underlying mechanisms. Alternatively, both shear force and hypotonic solution may act on the same stretch-activated channel but extracellular Gd3+ may only block the channel when stretch force is applied extracellularly by shear force. The clear answers to these questions will need highly specific blockers of mechanically activated ion channels, but such blockers are currently not available.
Molecular identities of mechanically activated channels/receptors remain unclear in Merkel cells and other somatosensory tissues in mammals. Other types of cells such as endothelial cells have been shown to respond to shear stress, and the responses in these cells could be blocked by Gd3+ (Lansman et al. 1987; Naruse and Sokabe 1993). Shear force-induced responses in these cells were thought to be mediated by stretch-activated ion channels whose molecular identities remain poorly defined (Sachs 2010). It is possible that the ion channels mediating shear force responses in Merkel cells are the same stretch-activated ion channels that mediate shear force responses in other cell types. Alternatively, the shear force-induced response in Merkel cells was mediated by different mechanically activated ion channels. Transcripts of several TRP channels have been identified in Merkel cells, including PKD1, PKD2, and TRPC1, and they were considered to be good candidates of mechanically activated channels in Merkel cells (Haeberle et al. 2008). A previous study indicated that TRPC1 and TRPC6 channels cooperate with TRPV4 channels to mediate mechanical hyperalgesia and primary afferent nociceptor sensitization (Alessandri-Haber et al. 2009). More recently, two new molecules, Piezo1 and Piezo2, have been described to play a broad role in mechanotransduction in different forms of life, and Piezo2 is suggested to be involved in rapidly adapting mechanically activated currents in somatosensory neurons (Coste et al. 2010). Of interest, Piezo1 but not Piezo2 was found to be expressed in a high amount in mouse skin (Coste et al. 2010), making Piezo1 one of the candidates for mechanical transducers in Merkel cells. The establishment of shear force-induced mechanical sensitivity in cultured Merkel cells will allow the use of a cell-based knockdown strategy to screen candidates of mechanically activated channels/receptors in Merkel cells. It may also be useful in studying potential transmitters released by Merkel cells in responding to mechanical stimulation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci 29: 6217–6228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulais N, Pennec JP, Lebonvallet N, Pereira U, Rougier N, Dorange G, Chesne C, Misery L. Rat Merkel cells are mechanoreceptors and osmoreceptors. PLoS One 4: e7759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahusac PM, Senok SS. Metabotropic glutamate receptor antagonists selectively enhance responses of slowly adapting type I mechanoreceptors. Synapse 59: 235–242, 2006 [DOI] [PubMed] [Google Scholar]
- Chan E, Yung WH, Baumann KI. Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Exp Brain Res 108: 357–366, 1996 [DOI] [PubMed] [Google Scholar]
- Cho H, Koo JY, Kim S, Park SP, Yang Y, Oh U. A novel mechanosensitive channel identified in sensory neurons. Eur J Neurosci 23: 2543–2550, 2006 [DOI] [PubMed] [Google Scholar]
- Cho H, Shin J, Shin CY, Lee SY, Oh U. Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci 22: 1238–1247, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Wood JN. FM1–43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol Pain 3: 1, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Wood JN, Cesare P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci 22: RC228, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English KB, Wang ZZ, Stayner N, Stensaas LJ, Martin H, Tuckett RP. Serotonin-like immunoreactivity in Merkel cells and their afferent neurons in touch domes from the hairy skin of rats. Anat Rec 232: 112–120, 1992 [DOI] [PubMed] [Google Scholar]
- Fagan BM, Cahusac PM. Evidence for glutamate receptor mediated transmission at mechanoreceptors in the skin. Neuroreport 12: 341–347, 2001 [DOI] [PubMed] [Google Scholar]
- Findlater GS, Cooksey EJ, Anand A, Paintal AS, Iggo A. The effects of hypoxia on slowly adapting type I (SAI) cutaneous mechanoreceptors in the cat and rat. Somatosens Res 5: 1–17, 1987 [DOI] [PubMed] [Google Scholar]
- Fukuda J. A pure, monolayer culture of Merkel cells from sinus hair follicles of the rat. Neurosci Lett 216: 73–76, 1996 [DOI] [PubMed] [Google Scholar]
- Fukuda J, Ishimine H, Masaki Y. Long-term staining of live Merkel cells with FM dyes. Cell Tissue Res 311: 325–332, 2003 [DOI] [PubMed] [Google Scholar]
- Garcia-Caballero T, Gallego R, Roson E, Basanta D, Morel G, Beiras A. Localization of serotonin-like immunoreactivity in the Merkel cells of pig snout skin. Anat Rec 225: 267–271, 1989 [DOI] [PubMed] [Google Scholar]
- Gottschaldt KM, Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science 214: 183–186, 1981 [DOI] [PubMed] [Google Scholar]
- Haeberle H, Bryan LA, Vadakkan TJ, Dickinson ME, Lumpkin EA. Swelling-activated Ca2+ channels trigger Ca2+ signals in Merkel cells. PLoS One 3: e1750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J, Lumpkin EA. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci USA 101: 14503–14508, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 271: 225–239, 2003 [DOI] [PubMed] [Google Scholar]
- He L, Tuckett RP, English KB. 5-HT2 and 3 receptor antagonists suppress the response of rat type I slowly adapting mechanoreceptor: an in vitro study. Brain Res 969: 230–236, 2003 [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200: 763–796, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda I, Yamashita Y, Ono T, Ogawa H. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. J Physiol 479: 247–256, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ER, Cheliakine C, Gebremedhin D, Birks EK, Davies PF, Harder DR. Shear activated channels in cell-attached patches of cultured bovine aortic endothelial cells. Pflügers Arch 431: 129–131, 1995 [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. Eur J Neurosci 11: 3963–3969, 1999 [DOI] [PubMed] [Google Scholar]
- Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature 325: 811–813, 1987 [DOI] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science 324: 1580–1582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel F. Tastzellen and Tastkoerperchen bei den Hausthieren und beim Menschen. Arch Mikrosk Anat 11: 636–652, 1875 [Google Scholar]
- Mills LR, Diamond J. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. J Neurocytol 24: 117–134, 1995 [DOI] [PubMed] [Google Scholar]
- Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol 336: 76–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Sokabe M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol Cell Physiol 264: C1037–C1044, 1993 [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331: 168–170, 1988 [DOI] [PubMed] [Google Scholar]
- Piskorowski R, Haeberle H, Panditrao MV, Lumpkin EA. Voltage-activated ion channels and Ca2+-induced Ca2+ release shape Ca2+ signaling in Merkel cells. Pflügers Arch 457: 197–209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Stretch-activated ion channels: what are they? Physiology (Bethesda) 25: 50–56, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senok SS, Baumann KI. Functional evidence for calcium-induced calcium release in isolated rat vibrissal Merkel cell mechanoreceptors. J Physiol 500: 29–37, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senok SS, Halata Z, Baumann KI. Chloroquine specifically impairs Merkel cell mechanoreceptor function in isolated rat sinus hairs. Neurosci Lett 214: 167–170, 1996 [DOI] [PubMed] [Google Scholar]
- Shimohira-Yamasaki M, Toda S, Narisawa Y, Sugihara H. Merkel cell-nerve cell interaction undergoes formation of a synapse-like structure in a primary culture. Cell Struct Funct 31: 39–45, 2006 [DOI] [PubMed] [Google Scholar]
- Tachibana T, Nawa T. Immunohistochemical reactions of receptors to met-enkephalin, VIP, substance P, and CGRP located on Merkel cells in the rat sinus hair follicle. Arch Histol Cytol 68: 383–391, 2005 [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Akaike N, Wakamori M, Ikeda I, Ogawa H. Voltage-dependent currents in isolated single Merkel cells of rats. J Physiol 450: 143–162, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccone G. Neuron-specific enolase and serotonin in the Merkel cells of conger-eel (Conger conger) epidermis. An immunohistochemical study. Histochemistry 85: 29–34, 1986 [DOI] [PubMed] [Google Scholar]
- Zaccone G, Fasulo S, Ainis L, Mauceri A, Licata A, Lauriano ER. Enkephalin immunoreactivity in the paraneurons of the tiger salamander (Ambystoma tigrinum) tongue. Neuropeptides 28: 257–260, 1995 [DOI] [PubMed] [Google Scholar]


