Abstract
In uninjured humans, it is well established that voluntary contraction of muscles on one side of the body can facilitate transmission in the contralateral corticospinal pathway. This crossed facilitatory effect may favor interlimb coordination and motor performance. Whether this aspect of corticospinal function is preserved after chronic spinal cord injury (SCI) is unknown. Here, using transcranial magnetic stimulation, we show in patients with chronic cervical SCI (C5–C8) that the size of motor evoked potentials (MEPs) in a resting intrinsic hand muscle remained unchanged during increasing levels of voluntary contraction with a contralateral distal or proximal arm muscle. In contrast, MEP size in a resting hand muscle was increased during the same motor tasks in healthy control subjects. The magnitude of voluntary electromyography was negatively correlated with MEP size after chronic cervical SCI and positively correlated in healthy control subjects. To examine the mechanisms contributing to MEP crossed facilitation we examined short-interval intracortical inhibition (SICI), interhemispheric inhibition (IHI), and motoneuronal behavior by testing F waves and cervicomedullary MEPs (CMEPs). During strong voluntary contractions SICI was unchanged after cervical SCI and decreased in healthy control subjects compared with rest. F-wave amplitude and persistence and CMEP size remained unchanged after cervical SCI and increased in healthy control subjects compared with rest. In addition, during strong voluntary contractions IHI was unchanged in cervical SCI compared with rest. Our results indicate that GABAergic intracortical circuits, interhemispheric glutamatergic projections between motor cortices, and excitability of index finger motoneurons are neural mechanisms underlying, at least in part, the lack of crossed corticospinal facilitation observed after SCI. Our data point to the spinal motoneurons as a critical site for modulating corticospinal transmission after chronic cervical SCI.
Keywords: primary motor cortex, force, corticospinal drive, spinal motoneurons, sensorimotor cortex
the corticospinal pathway undergoes reorganization after a spinal cord injury (SCI) (for review see Raineteau and Schwab 2001). Animal studies have shown that corticospinal neurons exhibit an extensive capacity for spontaneous sprouting (Fouad et al. 2001; Rosenzweig et al. 2010) and that axotomized axons can be incorporated into the circuitry of intact body segments (Ghosh et al. 2009). Human studies in patients with SCI demonstrated a decreased output and conduction delays in corticospinal axons as revealed by changes in the size and latencies of motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS; Barthélemy et al. 2010; Ellaway et al. 2007; Thomas and Gorassini 2005). A decrease has also been documented in the common corticospinal synaptic drive to a set of muscles (Hansen et al. 2005; Norton and Gorassini 2006). A better understanding of how the reorganized corticospinal pathway responds to voluntary activity during a motor task is especially important for elucidating its role in recovery after SCI.
The corticospinal tract is a predominantly crossed pathway with prominent interactions between both hemispheres (Brus-Ramer et al. 2009; Ferbert et al. 1992; Lemon 2008). In uninjured humans, it is well established that the size of MEPs in resting arm muscles is facilitated by strong isometric contractions of contralateral arm muscles (Hortobagyi et al. 2003; Muellbacher et al. 2000; Perez and Cohen 2008, 2009; Stedman et al. 1998). This pronounced crossed facilitation of the corticospinal pathway involves changes in transmission at the cortical and spinal cord levels (Meyer et al. 1995; Muellbacher et al. 2000; Perez and Cohen 2008). Crossed facilitation may contribute to interlimb coordination during unimanual and bimanual actions (Carson et al. 2004, 2008) and to enhanced motor performance after repeated training (Lee et al. 2010; Perez et al. 2007).
Whether crossed facilitation of the corticospinal pathway during unilateral actions is preserved after chronic cervical SCI remains unknown. Electrophysiological studies have demonstrated that recruitment of corticospinal drive during increasing levels of voluntary contraction of hand muscles is impaired in patients with SCI (Davey et al. 1998), suggesting that crossed facilitatory effects in corticospinal drive (Perez and Cohen 2008, 2009) may be affected in these patients. Changes in intracortical inhibitory circuits and spinal motoneurons (Muellbacher et al. 2000; Perez and Cohen 2008) contribute to the control of crossed corticospinal facilitation in healthy control subjects, and transmission in these pathways is altered after SCI (Butler and Thomas 2003; Davey et al. 1998; Norton et al. 2008; Roy et al. 2011). Therefore, we hypothesized that crossed corticospinal facilitation in an intrinsic finger muscle will be altered in humans with chronic cervical SCI most likely because of deficits in intracortical inhibition and motoneuronal excitability.
We examined intracortical inhibition and interhemispheric inhibition (IHI) by using a paired-pulse TMS protocol (Ferbert et al. 1992; Kujirai et al. 1993) and the behavior of index finger motoneurons by testing F-wave persistence and amplitude (Butler and Thomas 2003) via peripheral nerve stimulation and MEPs evoked by electrical stimulation at the cervicomedullary junction (CMEPs; Taylor and Gandevia 2004; Ugawa et al. 1992).
METHODS
The study was performed in accordance with the Declaration of Helsinki. All subjects gave their informed consent to the experimental procedures, which were approved by the local ethics committee at the University of Pittsburgh. Patients were recruited from the Department of Physical Medicine and Rehabilitation research registry at the University of Pittsburgh.
Subjects.
Fourteen patients with SCI (mean age 42.2 ± 12.6 yr, 12 men, 2 women; Table 1) and 10 age-matched healthy control subjects (mean age 32.9 ± 16.2 yr, 3 men, 7 women) participated in the study. All patients had a chronic (≥1 yr) cervical traumatic injury (C5–C8), an intact (score = 2) or impaired (score = 1) but not absent innervation in dermatome C6 during light touch and pin prick stimulus using the ASIA (American Spinal Cord Injury Association) sensory scores, and residual hand and arm motor function. Six of 14 patients were categorized as ASIA A (complete injury) because of the lack of sacral sparing (Marino et al. 2003) despite the fact that they were able to elicit voluntary force with hand and arm muscles. The other eight patients were classified as incomplete ASIA C and D. Participants were able to exert maximal voluntary contraction (MVC) isometric forces into index finger abduction (healthy control 19.9 ± 6.7 N, cervical SCI 11.6 ± 8.7 N; P = 0.03) and elbow flexion (healthy control 125.4 ± 30.9 N, cervical SCI 139.3 ± 56.2 N; P = 0.5). Only two patients with cervical SCI were unable to generate index finger voluntary force (Table 1, patients 4 and 8), and they were only tested for the elbow flexion task. We also conducted additional analysis on a subset (7/14) of patients with good motor recovery in whom index finger abduction force was comparable to that in the healthy control group (healthy control 19.9 ± 6.7 N, cervical SCI 16.9 ± 8.0 N; P = 0.4). Thus forces exerted during both index finger abduction and elbow flexion tasks tested were matched across groups.
Table 1.
SCI patient characteristics
| Patient | Age, yr | Sex | ASIA Score | Level | Etiology | Time, yr | Index Finger Abduction MVC, N | Elbow Flexion MVC, N |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | M | A | C7 | T | 17 | 1.3 | 138.5 |
| 2 | 57 | F | D | C5 | T | 11 | 7.0 | 118.6 |
| 3 | 51 | M | C | C7 | T | 10 | 27.0 | 239.4 |
| 4 | 27 | M | A | C6 | T | 13 | n/a | 179.3 |
| 5 | 42 | M | A | C7 | T | 13 | 1.5 | 187.7 |
| 6 | 29 | M | A | C6 | T | 5 | 21.4 | 144.7 |
| 7 | 45 | M | D | C7 | T | 6 | 4.4 | 144.6 |
| 8 | 61 | M | A | C5 | T | 10 | n/a | 12.5 |
| 9 | 23 | M | D | C5 | T | 7 | 6.9 | 88.5 |
| 10 | 26 | M | A | C5 | T | 11 | 1.5 | 195.6 |
| 11 | 45 | M | C | C8 | T | 9 | 24.9 | 168.5 |
| 12 | 50 | M | D | C5 | T | 1 | 14.4 | 136.1 |
| 13 | 42 | F | C | C7 | T | 20 | 11.9 | 81.7 |
| 14 | 58 | M | D | C7 | T | 2 | 16.7 | 113.9 |
SCI, spinal cord injury; M, male; F, female; T, traumatic; MVC, maximum voluntary contraction; n/a, not applicable (patient completed elbow flexion only).
Recordings.
Electromyography (EMG) was recorded bilaterally from the first dorsal interosseous (FDI), biceps, and triceps brachii by surface electrodes secured to the skin over the belly of each muscle (Ag-AgCl, 10-mm diameter). The signals were amplified, filtered (20–1,000 Hz), and sampled at 2 kHz for off-line analysis (CED 1401 with Signal software, Cambridge Electronic Design, Cambridge, UK). Forces exerted at the proximal interphalangeal joint of the index finger and at the elbow were measured by load cells (Honeywell; range ± 498.1 N, voltage ± 5 V, high-sensitivity transducer 0.045 V/N). Force was sampled at 200 Hz and stored on a computer for off-line analysis.
Experimental setup.
Subjects were seated in an armchair with both arms flexed at the elbow by 90° with the forearm pronated and the wrist and forearm restrained by straps. In this position, index fingers were attached to a custom two-axis load cell (Honeywell), which measures finger abduction force. Testing was also completed with one arm maintained in the position described above while the contralateral shoulder was flexed by 90° and the elbow flexed by 90° with the forearm supinated and the wrist restrained by straps. A custom device was used to maintain the position of the arm with a two-axis load cell (Honeywell) attached to measure elbow flexion forces. At the start of the experiment subjects performed three brief MVCs (3–5 s) into index finger abduction or elbow flexion, separated by 30 s. The maximal forces were used to set targets for subsequent submaximal contractions. During testing, subjects were instructed to remain at rest while the contralateral side remained at rest or performed 30% or 70% of MVC into index finger abduction (“index finger abduction” task) or elbow flexion (“elbow flexion” task) force. Patients performed increasing levels of MVC with the less affected arm, and healthy control subjects used the dominant arm. Custom software (LabVIEW) was written to acquire signals from the load cell and to display visual feedback corresponding to rest and MVC levels in real time. Subjects were instructed to move a cursor to a target box presented on a computer monitor by performing index finger abduction or elbow flexion. A familiarization trial was completed at the beginning of each experiment to ensure that subjects were able to complete the task. EMG from the resting index finger was displayed continuously on an oscilloscope, and verbal feedback was provided to the subjects to ensure that physiological measurements in the FDI were acquired at rest at all times. To ensure that the same background activity was present in both conditions, trials in which mean rectified EMG activity in the resting FDI exceeded 2 SD of the mean resting EMG, measured 100 ms before the stimulus artifact, were excluded from further analysis (13.1% of trials were excluded from analysis consistent with previous reports in related tasks; Hortobagyi et al. 2003; Muellbacher et al. 2000; Perez and Cohen 2008).
TMS.
Transcranial magnetic stimuli were delivered from a Magstim 200 stimulator (Magstim) through a figure-eight coil (loop diameter, 7 cm; type no. SP15560) with a monophasic current waveform. TMS was delivered to the optimal scalp position for activation of the left or right FDI muscle. To identify the optimal scalp position for the FDI, the coil was held tangential to the scalp with the handle pointing backward and 45° away from the midline. With this coil position the induced current in the brain flowed in an anterior-medial direction and probably produced D- and early I-wave activation of corticospinal neurons (Di Lazzaro et al. 2004). The TMS coil was held to the head of the subject with a custom coil holder while the head was firmly secured to a headrest by straps to limit head movements. TMS measurements included MEPs, resting motor threshold (RMT), maximal MEP size (MEP-max), short-interval intracortical inhibition (SICI), and IHI.
MEPs.
RMT was defined as the minimal stimulus intensity required to induce MEPs >50 μV peak-to-peak amplitude in at least three of five consecutive trials in the relaxed muscle (Rothwell et al. 1999). MEP-max was defined in all participants at rest by increasing stimulus intensities in 5% steps of maximal device output until the MEP amplitude did not show additional increases (healthy control 6.1 ± 2.9 mV, cervical SCI 2.8 ± 3.0 mV; P = 0.01). TMS intensity used to elicit MEPs ∼50% of MEP-max (healthy control 119.9 ± 4.6%, cervical SCI 117.2 ± 4.5% of RMT; P = 0.2) and RMT (healthy control 42.4 ± 7.7%, cervical SCI 53.1 ± 16.6% of stimulator output; P = 0.1) was similar across groups. Single TMS pulses were delivered at 4-s intervals in sets of three and separated by resting periods as needed. Thirty MEPs were averaged in each condition. TMS pulses were given when subjects were at rest or performed 30% and 70% of MVC during index finger abduction or elbow flexion in a randomized order.
SICI.
SICI was tested by a previously described method (Kujirai et al. 1993). A conditioning stimulus (CS) was set at an intensity that elicited a conditioned MEP that was ∼50% of the test MEP at rest. The same stimulation intensity was used for the CS across conditions. The test stimulus (TS) was adjusted to produce an MEP ∼50% of the MEP-max. The intensity used for the CS and the TS was similar across groups (see Table 2). The TS was delivered 2 ms after the CS. Because the size of the MEP in the resting hand increased during increasing levels of contralateral MVC, SICI was also tested by adjusting the size of the test MEP. SICI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP [(conditioned MEP × 100)/(test MEP)]. Twenty test MEPs and twenty conditioned MEPs were tested in each condition. Measurements were repeated three times at rest until a consistent baseline was established.
Table 2.
Short-interval intracortical inhibition stimulation parameters
| Rest | Index Finger Abduction 70% MVC | Rest | Elbow Flexion 70% MVC | P Values | |
|---|---|---|---|---|---|
| Healthy control | |||||
| Unadjusted | |||||
| TS, % | 58.6 ± 11.0 | 57.6 ± 10.6 | 0.76 | ||
| CS, % | 36.5 ± 9.6 | 37.4 ± 9.2 | 0.35 | ||
| Test MEP, mV | 1.2 ± 0.6 | 2.4 ± 1.6 | 1.2 ± 0.6 | 1.5 ± 0.8 | 0.01 |
| Adjusted | |||||
| TS, % | 53.0 ± 9.6 | 56.2 ± 10.8 | 0.06 | ||
| Test MEP, mV | 1.3 ± 0.5 | 1.2 ± 0.6 | 0.98 | ||
| Cervical SCI | |||||
| Unadjusted | |||||
| TS, % | 62.7 ± 14.0 | 67.0 ± 17.9 | 0.20 | ||
| CS, % | 41.3 ± 11.3 | 40.4 ± 11.5 | 0.22 | ||
| Test MEP, mV | 1.2 ± 1.4 | 1.3 ± 1.5 | 0.9 ± 1.3 | 0.9 ± 1.5 | 0.60 |
Values are mean ± SD stimulus intensity used for the test stimulus (TS) and the conditioning stimulus (CS) during short-interval intracortical inhibition (SICI) measurements. In both groups measurements were completed without adjusting (Unadjusted) the size of the test motor evoked potential (MEP) in the resting first dorsal interosseous (FDI) while the contralateral side performed 70% of MVC during index finger abduction or elbow flexion. Note that the same stimulus intensity was used across conditions. SICI was also measured by adjusting (Adjusted) the size of the test MEP in healthy control subjects. Here, the intensity of the TS was reduced to acquire a similar test MEP size across conditions. Note that in the Adjusted condition there were no differences in the size of the test MEP across conditions. P values represent t-tests and ANOVAs performed on test and conditioning MEP.
IHI.
Previous studies have demonstrated that IHI measured in an upper limb muscle at rest is increased during contralateral isometric voluntary contractions in healthy control subjects (Ferbert et al. 1992; Hinder et al. 2010; Perez and Cohen 2008; Talelli et al. 2008; Vercauteren et al. 2008). Here, IHI was tested in patients from the motor cortex controlling the less affected to the most affected arm with a randomized conditioning-test design reported previously (Ferbert et al. 1992). Testing was completed at a conditioning-test interval of 10 ms, the time between the CS and the TS. The CS was delivered to the optimal scalp position for activating the corresponding FDI muscle. At rest, a suprathreshold CS was set at an intensity that elicited a conditioned MEP that was ∼50% of the test MEP at rest (mean = 73.9 ± 21.5% of stimulator output). The same stimulation intensity was used for the CS during 70% of contralateral MVC with FDI or biceps muscle. The intensity of the TS was adjusted for each patient to elicit a test MEP in the FDI of ∼50% of the MEP-max (mean = 68.7 ± 24.7% of stimulator output). The TS was always delivered to the optimal scalp position for activation of the more affected FDI muscle. IHI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP [(conditioned MEP × 100)/(test MEP)]. A total of 20 test MEPs and 20 conditioned MEPs were tested in each condition.
F waves.
Although there are some limitations in the use of F waves in motor control experimental paradigms (Espiritu et al. 2003; Hultborn and Nielsen 1995; Lin and Floeter 2004), several studies have suggested that changes in F-wave amplitude and persistence can detect valuable information about changes in motoneuronal excitability in patients with SCI (Butler and Thomas 2003; Kim et al. 2007) and healthy control subjects (Fierro et al. 1990; Muellbacher et al. 2000; Panayiotopoulos and Chroni 1996). Motoneuronal excitability (reflected by F-wave amplitude and persistence) was measured with the use of supramaximum stimulus intensity to the ulnar nerve at the wrist (200-μs pulse duration; DS7A, Digitimer). The anode and cathode were 3 cm apart and 1 cm in diameter, with the cathode positioned proximally. The stimuli were delivered at 1 Hz at an intensity of 120% of the maximal motor response (M-max). For each trial we quantified peak-to-peak amplitude (expressed relative to M-max) and F-wave persistence (number of F waves present in each set). If the F wave was not present, an amplitude of zero was included in the mean (Butler and Thomas 2003). We observed the same result in all groups whether the zero F-wave amplitudes were included in the analysis or not. While 20 trials are considered to be clinically adequate for testing (Butler and Thomas 2003; Curt et al. 1997; Panayiotopoulos and Chroni 1996), it has also been reported that to achieve measurements within 50% of the true value ∼40 F waves are needed (Lin and Floeter 2004). In our study, 20 F waves were recorded for each condition and repeated twice. Therefore, a total of 40 F waves were averaged at rest and 40 F waves were averaged during 70% of MVC on each motor task.
Cervicomedullary MEPs.
The corticospinal tract was stimulated at the cervicomedullary level by a high-voltage electrical current (100-μs duration; DS7AH Digitimer) passed between adhesive Ag-AgCl electrodes fixed to the skin behind the mastoid process (Taylor and Gandevia 2004; Ugawa et al. 1992). The stimulation intensity (396 ± 83.8 mA) was set to elicit a CMEP of ∼5% of the M-max at rest in the FDI muscle. Twenty CMEPs were tested at rest and twenty CMEPs were acquired during contralateral 70% of MVC in the index finger abduction and elbow flexion tasks. We monitored that the stimulation was below the intensity required to activate the axons of the motoneurons directly. Because of the higher intensities required to elicit CMEPs in finger muscles compared with proximal arm muscles (Taylor and Gandevia 2004), the test was completed in one participant from each group tested.
Data analysis.
Normal distribution was tested by the Shapiro-Wilk test and homogeneity of variances by the Brown-Forsythe test. Repeated-measures ANOVAs were performed to determine the effect of MVC level (rest, 30% of MVC, and 70% of MVC), task (index finger abduction and elbow flexion), and group (healthy control and cervical SCI) on MEP size, SICI, F-wave persistence and amplitude, and mean rectified EMG. The same analysis was also completed on each group separately. Additionally, repeated-measures ANOVA was performed to determine the effect of group (good vs. poor recovery) and MVC level (rest, 30% of MVC, and 70% of MVC) on MEP size. A Bonferroni post hoc test was used to test for significant comparisons. Independent t-tests were used to compare RMT, stimulator output intensity, MEP-max, mean rectified EMG, force, MEP size, M-max, IHI, TS and CS intensity, and CMEPs. Significance was set at P < 0.05. Group data are presented as means ± SD in the text. Pearson correlation analysis was used as needed.
RESULTS
MEPs.
Figure 1, A and B, illustrate MEPs in a representative healthy control subject and a patient with chronic cervical SCI recorded from the resting FDI during the index finger abduction and elbow flexion tasks; note the increase in FDI MEP size during 30% and 70% of MVC during both motor tasks in the healthy control subject but not in the patient.
Fig. 1.
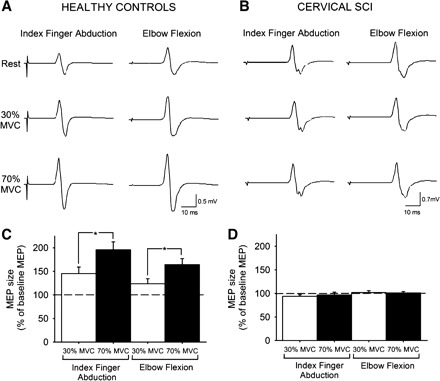
Motor evoked potentials (MEPs). A and B: MEPs recorded from the resting first dorsal interosseous (FDI) of a representative healthy control subject (A) and in a patient with cervical spinal cord injury (SCI) (B) while the other side remained at rest or performed 30% or 70% maximal voluntary contraction (MVC) during index finger abduction or elbow flexion. C and D: group data for healthy control subjects (n = 10, C) and cervical SCI patients (n = 14, D). The x-axis shows the MVC levels tested. The y-axis shows the size of the FDI MEP as % of the baseline FDI MEP. Note the increase in FDI MEP size during contralateral index finger abduction and elbow flexion in healthy control subjects but not in patients with cervical SCI. Error bars indicate SE. *P < 0.05.
When both groups were compared [healthy control (n = 10) and cervical SCI (n = 14); Table 3], we found significant group [F(1,20) = 52.6, P < 0.001] and MVC level [F(2,40) = 24.5, P < 0.001] effects on resting FDI MEP size but not task [F(1,20) = 3.2, P = 0.1]. There was a significant interaction between group and MVC level [F(2,40) = 25.7, P < 0.001] and between group and task [F(1,20) = 9.0, P = 0.01]. These results indicate that healthy control subjects showed an increase in FDI MEP size with increasing MVC levels (Fig. 1C) that was not found in cervical SCI patients (Fig. 1D). In the healthy control group, the increase in FDI MEP size was larger at 70% (10/10) compared with 30% (7/10) of MVC (P = 0.01) and it was similar during the index finger abduction and elbow flexion tasks (P = 0.1). In contrast, patients with cervical SCI showed no changes in MEP sizes during increasing levels of MVC [F(2,22) = 0.1, P = 0.9; Table 2] in either motor task [F(1,11) = 3.8, P = 0.1]. Mean background rectified EMG activity in the resting FDI was similar across conditions [F(1,20) = 0.8, P = 0.4] and groups [F(1,20) = 0.01, P = 0.8]. Mean background rectified EMG activity and force in the arm performing increasing MVC levels are reported in Table 3.
Table 3.
Motor evoked potentials, mean rectified EMG, and force
| Healthy Control | Cervical SCI | P Values | |
|---|---|---|---|
| MEP, % | |||
| Index finger abduction | |||
| 30% MVC | 151.0 ± 49.0 | 93.9 ± 11.8 | <0.001 |
| 70% MVC | 231.3 ± 78.8 | 96.6 ± 23.0 | <0.001 |
| Elbow flexion | |||
| 30% MVC | 117.3 ± 33.4 | 101.7 ± 15.4 | <0.05 |
| 70% MVC | 177.1 ± 41.6 | 101.0 ± 11.4 | <0.001 |
| EMG, mV | |||
| Index finger abduction | |||
| 30% MVC | 0.20 ± 0.07 [1.06 ± 0.43%] | 0.09 ± 0.07 [1.05 ± 0.83%] | <0.01 [0.97] |
| 70% MVC | 0.38 ± 0.15 [2.22 ± 1.03%] | 0.13 ± 0.09 [1.49 ± 1.24%] | <0.001 [0.17] |
| Elbow flexion | |||
| 30% MVC | 0.08 ± 0.07 | 0.12 ± 0.15 | 0.51 |
| 70% MVC | 0.23 ± 0.17 | 0.32 ± 0.29 | 0.57 |
| Force, N | |||
| Index finger abduction | |||
| 30% MVC | 4.88 ± 1.00 [25.71 ± 5.15%] | 2.02 ± 1.69 [23.13 ± 15.37%] | <0.001 [0.62] |
| 70% MVC | 11.02 ± 1.60 [58.47 ± 11.74%] | 4.69 ± 4.00 [54.56 ± 37.34%] | <0.001 [0.76] |
| Elbow flexion | |||
| 30% MVC | 30.17 ± 7.48 | 29.94 ± 13.12 | 0.96 |
| 70% MVC | 66.12 ± 14.85 | 61.09 ± 27.48 | 0.61 |
Values are mean ± SD FDI MEP size, mean rectified EMG activity, and force; values in brackets are expressed as % of resting FDI maximal motor response (M-max). FDI MEP size is reported in the resting arm, while mean rectified EMG activity and force are reported in the arm performing 30% and 70% of MVC during index finger abduction and elbow flexion in patients and healthy control subjects. MEP size is expressed as % of baseline MEP size. Note that resting FDI MEP size increased in healthy control subjects but not after cervical SCI. Also note that EMG and force were significantly lower in patients during index finger abduction compared with control subjects and that when EMG activity and force were expressed as % of the FDI M-max (bracketed values) there was no difference across groups. Both groups generated similar EMG and force during elbow flexion. P values represent independent t-tests performed on MEP size, EMG, and force during increasing levels of contraction.
We conducted additional analysis on a subset (7/14) of patients in whom index finger abduction force, MEP size tested, and M-max were comparable to those in the healthy group [n = 7; index finger abduction = 16.9 ± 8.0 N (P = 0.4); FDI MEP size tested = 1.3 ± 1.0 mV (P = 0.6); FDI M-max = 16.6 ± 5.8 mV (P = 0.3)]. In line with the previous result, the subset of patients also showed no changes in MEP size during increasing levels of MVC [F(2,12) = 0.3, P = 0.8] in both motor tasks [F(1,6) = 0.9, P = 0.4]. A comparison between these patients with good recovery and those with larger motor deficits (poor recovery, n = 7) showed differences in the amount of changes in MEP size during increasing levels of MVC [F(1,12) = 4.8, P = 0.049]. The MEP size was larger in the better-recovered group (good recovery 102.6 ± 13.6%; poor recovery 93.8 ± 16.9%). Overall, these results show that during increasing levels of unilateral index finger abduction or elbow flexion isometric force healthy control subjects increase contralateral FDI MEP while in patients with chronic cervical SCI, with less or equal force as the healthy control subjects, neither motor task modulated contralateral FDI MEP size.
SICI.
We examined the contribution of intracortical pathways to the changes in MEP size by measuring SICI in the resting FDI muscle during contralateral index finger abduction and elbow flexion. The effects on MEP size were stronger at 70% of MVC; therefore, we selected this force level to examine this mechanism. SICI was similar at rest across groups [healthy control 48.9 ± 8.3 (n = 8), cervical SCI 39.4 ± 15.6% (n = 8; good recovery, n = 4); P = 0.1]. Figure 2, A and B, illustrate that SICI was decreased during 70% of MVC in the index finger abduction and elbow flexion tasks in a representative healthy control subject but not in a patient with cervical SCI. A group comparison revealed a significant interaction between group and MVC level [F(1,12) = 15.2, P < 0.01]. This result indicates that during 70% of MVC SICI was decreased compared with rest in healthy control subjects [by 69.9 ± 35.2% (range 44.3–129.2%) during index finger abduction and by 29.9 ± 22.2% (range 6.0–78.3%) during elbow flexion, 7/8; Fig. 2C] but remained unchanged in either task in patients (Fig. 2D).
Fig. 2.
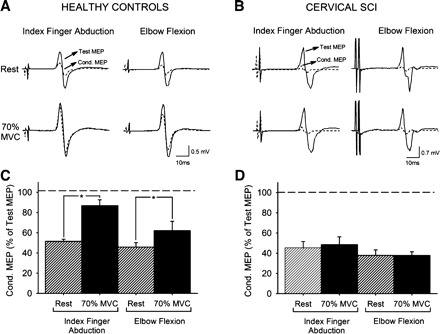
Short-interval intracortical inhibition (SICI). A and B: SICI recorded from the resting FDI of a representative healthy control (A) and a patient with cervical SCI (B). The test MEP (solid traces) and conditioned MEP (Cond. MEP, dashed traces) are indicated by arrows. C and D: group data for healthy control subjects (n = 8, C) and cervical SCI patients (n = 8, D). The x-axis shows all conditions tested. The y-axis shows the magnitude of the conditioned MEP expressed as % of the test MEP. The horizontal dashed line represents the size of the test MEP. Note that SICI decreased during index finger abduction and elbow flexion in healthy control subjects but not after cervical SCI. Error bars indicate SE. *P < 0.05.
Because MEP size increased during 70% of MVC in healthy control subjects but not in patients, we also tested SICI by adjusting the size of the test stimulus (Table 3). Here, SICI was decreased (P < 0.01) during 70% of MVC in the index finger abduction (by 44.4 ± 61.9%) and elbow flexion (by 65.9 ± 49.2%) tasks compared with rest.
IHI.
We examined IHI in the resting FDI muscle during contralateral index finger abduction and elbow flexion at 70% of MVC (n = 7; good recovery, n = 5). The size of the TS remained similar at rest and during 70% of MVC in the index finger abduction (P = 0.18) and elbow flexion (P = 0.2) tasks. The size of the MEP elicited by the CS remained unchanged at rest and during 70% of MVC in the index finger abduction (P = 0.19) and elbow flexion (P = 0.08) tasks. IHI was similar at rest and during contralateral index finger abduction (rest 49.6 ± 12.9%, 70% of MVC 36.2 ± 12.8%; P = 0.2) and elbow flexion (rest 57.9 ± 12.8%, 70% of MVC 53.6 ± 29.9%; P = 0.27).
F waves.
Motoneuronal excitability could also contribute to the observed changes in MEP size. We examined the excitability of index finger motoneurons by assessing the amplitude and persistence of F waves in the resting FDI during contralateral index finger abduction and elbow flexion at 70% of MVC (healthy controls, n = 8; SCI, n = 9; good recovery, n = 3). The maximal M wave was larger in healthy control subjects (20.1 ± 4.1 mV) compared with patients with cervical SCI (10.7 ± 8.4 mV; P < 0.01) and remained consistent across conditions (P = 0.6).
Figure 3, A and B, illustrate changes in the amplitude and persistence of F waves in the resting FDI during 70% of MVC during both motor tasks in representative subjects. F-wave persistence (calculated as the proportion of trials in which an F wave was present in a sample of 40 consecutive stimuli) at rest was similar in healthy control subjects (51.2 ± 20.3%, n = 8) and patients (71.0 ± 28.0%, n = 9; P = 0.3). There was a significant interaction between group and MVC level [F(1,14) = 9.1, P < 0.01]. This result shows that F-wave persistence was increased during 70% of MVC during index finger abduction (by 43.1 ± 30.5%) and elbow flexion (by 32.6 ± 28.2%) tasks in healthy control subjects (7/8, P < 0.001; Fig. 3C) but not in patients (1/9, P = 0.17; Fig. 3D). Mean F-wave amplitude at rest was higher in patients (3.8 ± 2.6% M-max) compared with healthy control subjects (1.1 ± 0.5% M-max; P = 0.01). There was a significant interaction between group and MVC level [F(1,14) = 10.2, P < 0.01]. We found that during 70% of MVC in both tasks mean F-wave amplitude increased in healthy control subjects [index finger abduction by 40.1 ± 25.1% (P < 0.01), elbow flexion by 36.3 ± 35.2%, 7/8 (P < 0.01); Fig. 3C] but not in patients (1/9, P = 0.6; Fig. 3D) compared with rest.
Fig. 3.
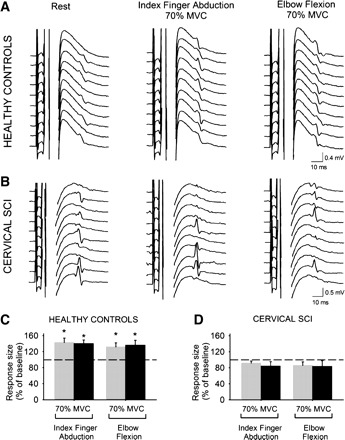
F waves. A and B: M waves and F waves recorded from the resting FDI of a representative healthy control subject (A) and a patient with cervical SCI (B) during index finger abduction and elbow flexion. C and D: group data for healthy control subjects (n = 8, C) and cervical SCI patients (n = 9, D). The x-axis shows all conditions tested [F-wave persistence (gray bars) and F-wave mean amplitude (black bars) during 70% of MVC of index finger abduction and elbow flexion]. The y-axis shows F-wave persistence (% of F waves present on each set) and F-wave amplitude [% of maximal motor response (M-max)]. Note that F-wave persistence and mean amplitude increased during index finger abduction and elbow flexion in healthy control subjects but not after cervical SCI. Error bars indicate SE. *P < 0.05.
CMEPs.
CMEPs are not affected by changes in cortical excitability and are sensitive to motoneuron excitability. Figure 4, A and B, illustrate changes in the amplitude of CMEPs in the resting FDI during both motor tasks in the subjects tested. Note that in the healthy control subject the amplitude of the FDI CMEPs increased during 70% of MVC compared with rest [index finger abduction by 45.7 ± 27.2% (P < 0.01), elbow flexion by 37.7 ± 24.8% (P = 0.01); Fig. 4C]. In contrast, in the patient with cervical SCI CMEP amplitude remained the same during 70% of MVC compared with rest [index finger abduction (P = 0.3), elbow flexion (P = 0.3); Fig. 4D]. Mean background rectified EMG activity in the resting FDI was similar across conditions and subjects tested (P = 0.7).
Fig. 4.
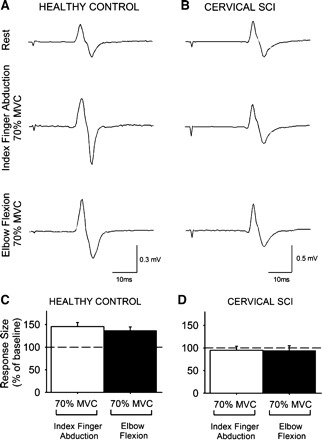
Cervicomedullary motor evoked potentials (CMEPs). A and B: CMEPs recorded from the resting FDI of a healthy control subject (A) and a patient with cervical SCI (B) while the other side remained at rest or performed 70% of MVC during index finger abduction or elbow flexion. C and D: x-axis shows the conditions tested (70% of MVC during index finger abduction, white bars; 70% of MVC during elbow flexion, black bars), and y-axis shows the size of the FDI CMEP as % of the baseline FDI CMEP. Note the increase in FDI MEP size during contralateral index finger abduction and elbow flexion in the healthy control subject (C) but not in the patient with cervical SCI (D). Error bars indicate SE.
Correlation analysis.
A positive correlation was found between changes in FDI MEP and F-wave persistence [(F-wave persistence at 70% of MVC × 100)/(F-wave persistence at rest)] (r = 0.78, P < 0.0001; Fig. 5A) but not SICI [(SICI at 70% of MVC × 100)/(SICI at rest)] (r = 0.26, P = 0.18; Fig. 5B) in both groups tested. In healthy control subjects, the changes in resting FDI MEP size correlated positively with changes in contralateral EMG (r = 0.53, P < 0.001) and force (r = 0.59, P < 0.001) across conditions (Fig. 6A). In patients with cervical SCI, a negative correlation was found between resting FDI MEP size and contralateral EMG (r = −0.52, P < 0.001; Fig. 6B) but not with force (r = −0.21, P = 0.2).
Fig. 5.
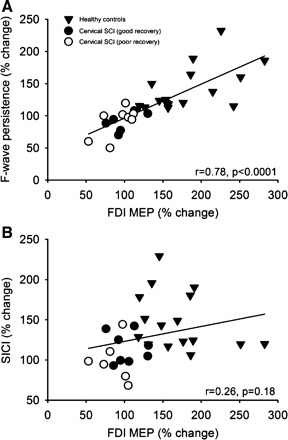
Correlation analysis between the changes in FDI MEP size during 70% of MVC during the index finger abduction and elbow flexion motor tasks and F-wave persistence (A) and SICI (B) in all subjects tested. In both graphs, the x-axis shows the size of the FDI MEP during 70% of MVC expressed as % of the FDI MEP size at rest. The y-axis shows the F-wave persistence and SICI during 70% of MVC expressed as % of the same measurements taken at rest. Note that larger changes in FDI MEP size were associated with larger F-wave persistence but not with changes in SICI.
Fig. 6.
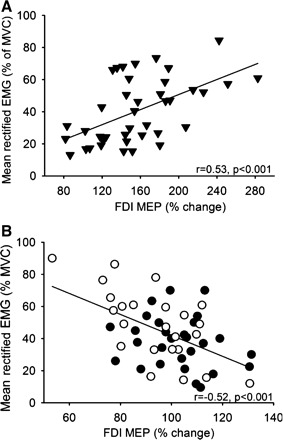
Correlation analysis between the changes in FDI MEP size during 30% and 70% of MVC during the index finger abduction and elbow flexion motor tasks and mean rectified EMG activity exerted during the same motor tasks in healthy control subjects (A, ▾) and patients with cervical SCI with poor (B, ○) and good (B, ●) recovery. In both graphs, x-axis shows the size of the FDI MEP during 30% and 70% of MVC expressed as % of the FDI MEP size at rest and y-axis shows the mean rectified EMG activity in the FDI expressed as % of the MVC. Note that larger changes in mean rectified EMG activity in the FDI muscle were associated with larger FDI MEP size in healthy control subjects and smaller FDI MEP size in patients with SCI.
DISCUSSION
The present study investigated whether a voluntary contraction by one arm can influence cortical and spinal processes in the contralateral resting hand after chronic cervical SCI. We showed that in patients the size of MEPs in a resting finger muscle remained unchanged during increasing levels of voluntary contractions with a contralateral intrinsic finger or an elbow flexor muscle. In contrast, MEP size was increased in healthy control subjects during the same motor tasks. Our data indicate that impaired SICI, IHI, and spinal motoneuron excitability contribute, at least in part, to the lack of crossed corticospinal facilitation after SCI. The correlation between changes in MEP size and F-wave persistence point to the spinal motoneurons as a critical site contributing to crossed interactions between distal and proximal arm muscles during unilateral voluntary activity in humans with chronic cervical SCI.
Effects of changing contraction strength on contralateral resting MEP size.
Our results in healthy control subjects are consistent with previous studies showing that increasing levels of voluntary activity with arm muscles increase the size of MEPs in the contralateral resting arm (Hortobagyi et al. 2003; Muellbacher et al. 2000; Perez and Cohen 2008, 2009; Stedman et al. 1998). The positive correlation found between the amount of MEP facilitation and contralateral voluntary activity also agrees with previous results (Perez and Cohen 2008, 2009). An intriguing question is why the crossed facilitatory effect was not present in patients, considering that all had remaining voluntary control of index finger and elbow flexor muscles and that a subset of patients were able to perform the same level of voluntary force as control subjects. First, we will discuss how an injury to the cervical spinal cord may have contributed to our results since the FDI segmental representation is close to the injury zone. On one side the injury may have resulted in spinal motoneuronal damage/loss, which is indicated by the smaller FDI M-max observed in patients. However, a lack of MEP crossed facilitation was also present in a subset of patients (good recovery) with a FDI M-max similar to that of the control group. Previous reports have also demonstrated that after cervical SCI some properties of motoneurons that innervate partially paralyzed muscles remain similar to those in control subjects (Butler and Thomas 2003; Thomas et al. 1997). These observations together suggest that it is less likely that significant damage/loss of spinal motoneurons was the main feature underlying our findings. Another possibility is that the magnitude of force exerted during testing affected our results since patients produced weaker forces during the finger abduction task, but when force values were normalized to the M-max (Table 3) the results were comparable across groups, suggesting that a similar proportion of the motoneuronal pool was activated during the task in both groups tested. Although we cannot exclude the possibility that motoneurons that are more affected by the injury were more involved in crossed facilitatory effects, we still found a lack of MEP crossed facilitation in patients who exerted index finger force comparable to that in the control group. Also, in the elbow flexion task where force was similar in both groups MEP crossed facilitation was present in healthy control subjects and absent in patients. As background EMG activity in the tested hand was similar across conditions, it is less likely that differences in subthreshold or spontaneous muscle activity (Chardon et al. 2010; Mottram et al. 2010) contributed to our results. If motoneurons were closer to their discharge threshold after injury one might expect an increase in MEP size, while no changes were observed in our patients. Therefore, taking these considerations together it seems more likely that the lack of MEP crossed facilitation after chronic cervical SCI relates to changes in mechanisms contributing to the control of corticospinal output.
Mechanisms contributing to impaired crossed corticospinal effects after cervical SCI.
Since the size of an MEP can be influenced by changes at multiple levels within the CNS we assessed changes in SICI, IHI, and spinal motoneurons during the same motor tasks. We found that SICI was unchanged during 70% of MVC during both motor tasks in patients and decreased in healthy control subjects. These results in healthy control subjects are in agreement with previous studies showing that SICI decreases in the hemisphere ipsilateral to the contracting arm during increasing levels of unilateral force (Muellbacher et al. 2000; Perez and Cohen 2008). This effect on SICI appears widespread, because it was evoked by activating contralateral distal and proximal arm muscles. This widespread effect is in agreement with our MEP results (Fig. 1) and supports the existence of crossed interactions between bilateral distal and proximal arm muscles during voluntary activity (Sohn et al. 2003; Soteropoulos and Perez 2011). Moreover, this effect is supported by the wide distribution of callosal projections (Gould et al. 1986), which may contribute to the functional specialization of upper limb movements.
Previous studies have demonstrated that although the magnitude of SICI in hand muscles is reduced at rest after SCI compared with controls (Roy et al. 2011; Saturno et al. 2008) the relative excitability profile of cortical inhibitory circuits is unchanged (Roy et al. 2011). SICI can be influenced by proprioceptive afferent input (Aimonetti and Nielsen 2001; Ridding and Rothwell 1999), opening the possibility that sensory deficits observed after cervical SCI contributed, at least in part, to our results. Indeed, proprioceptive afferent input has shown to decrease SICI in upper limb muscles (Aimonetti and Nielsen 2001; Ridding and Rothwell 1999). However, as proprioceptive afferent input arrives to the contralateral sensorimotor cortex it is likely that other pathways were involved in the present effects observed in the ipsilateral motor cortex. As the intensity of the CS to elicit SICI was similar across groups, it is also less likely that spinal inhibitory circuits caudal to the injury were accessed by the CS and may have contributed to SICI results. We would like to propose that the lack of changes in SICI may be in part related to the lack of changes in IHI observed in our patients. This is in agreement with previous evidence demonstrating that during a similar motor task the magnitude of SICI in one hemisphere is modulated by IHI from the contralateral hemisphere (Perez and Cohen 2008). This possibility is also supported by our findings of a less pronounced change in the MEP elicited by the CS and by the decreased recruitment in corticospinal drive during stronger levels of unilateral force after cervical SCI (Davey et al. 1999), which are factors contributing to modulate transcallosal inhibition from a contracting to a resting limb (Perez and Cohen 2008).
We found that the F-wave amplitude and persistence measured in a resting hand muscle remained unchanged during both motor tasks in patients and increased in healthy control subjects. A similar increase was found in CMEP amplitude, suggesting that MEP crossed facilitation might have also occurred subcortically, including changes in the excitability of the motoneuronal pool. In agreement with this, such an increase in motoneuronal excitability has also been reported in healthy control subjects in a similar testing protocol (Muellbacher et al. 2000; Stedman et al. 1998). Several changes may occur in motoneurons of partially paralyzed muscles, including receiving new inputs from nearby neurons (Calancie et al. 2000), increased excitability (Bennett et al. 2004), and increase in synaptic inputs (Norton et al. 2008). Therefore, the lack of changes in motoneuronal excitability observed after cervical SCI during our motor tasks was unexpected. Since some actions of pyramidal tract neurons on ipsilateral spinal motoneurons are evoked via crossed spinal pathways (Jankowska et al. 2006) or via branched corticospinal projections (Rosenzweig et al. 2009), it is possible that damage of these crossed structures by the injury contributed to our results. This is in agreement with results by Zidjewind and collaborators (2011) showing that in patients with chronic cervical SCI a strong voluntary contraction exerted with hand muscles did not change the behavior of motoneurons in the contralateral hand, whereas additional inputs applied to the same limb successfully increased their firing rate. This is also supported by our results showing that patients who were able to exert levels of EMG and force similar to those of control subjects did not show crossed facilitation, suggesting that the recovery of motor function and crossed facilitatory effects during the same unilateral task may be differentially affected by the injury. The similarities in F-wave persistence in both groups at baseline suggest that the intrinsic threshold for antidromic excitation of motoneurons remained similar after SCI and are consistent with previous findings (Butler and Thomas 2003). These results indicate that although aspects of motoneuronal excitability at rest may be differentially affected by the injury, the overall responsiveness of motoneurons to contralateral voluntary drive after chronic cervical SCI is deficient.
In summary, our results show that after chronic cervical SCI cortical and subcortical sites contribute to the absence of crossed facilitatory effects. This is in agreement with previous studies showing cortical and subcortical changes after SCI in humans and nonhuman primates (Nishimura and Isa 2011; Norton et al. 2008; Roy et al. 2011). Our data support the view that the lack of changes in SICI may be partially explained by the lack of interaction with IHI mechanisms (Perez and Cohen 2008), while the lack of changes in F waves and CMEPs may be in part related to damage of crossed structures at the spinal level. We cannot exclude the possibility that other descending pathways such as propriospinal neurons contributed to our results, since these pathways are located at midcervical levels and receive peripheral as well as corticospinal inputs (Pierrot-Deseilligny and Burke 2005). Although ipsilateral corticospinal projections appear to play a less prominent role in crossed facilitatory effects in healthy control subjects (Lee et al. 2010; Zidjewind et al. 2006), these pathways have not been explored after SCI and may provide a substrate for these effects and for the recovery of motor function (Jankowska et al. 2005).
Functional considerations.
Several lines of evidence have suggested that crossed facilitatory effects might be beneficial in situations in which the less affected limb can be used to induce neural adaptations that enhance motor output in the most affected limb in patients with motor disorders (Renner et al. 2005; Stromberg 1986; Woldag et al. 2004). Crossed facilitatory effects may also be used as an adjunct procedure to optimize motor output (Carson et al. 2008; Kennedy and Carson 2008) and motor learning procedures (Lee et al. 2010; Perez et al. 2007) after SCI (Kowalczewski et al. 2011). Since in many patients with SCI one limb is more affected than the other, it is possible that principles of crossed facilitation may be also beneficial in this patient population. Indeed, our results showed that deficits in crossed facilitation were more pronounced in patients with poor motor recovery compared with patients with good recovery, suggesting that this phenomenon, at least to some extent, may be sensitive to variations in motor function. However, the contribution of these effects to the pathophysiology of recovery of strength after SCI remains to be tested.
The positive correlation between changes in MEP size and F-wave persistence but not SICI (Fig. 5) suggests that spinal motoneurons provide a more direct contribution to crossed facilitatory effects after injury. These findings support a role for spinal motoneurons in driving corticospinal excitability (Taylor and Martin 2009) after chronic cervical SCI. The inverse correlation between the amount of crossed facilitation and EMG activity (Fig. 6) suggests that strategies aiming to enhance corticospinal output in a resting limb after cervical SCI may benefit from low levels of contralateral voluntary activity.
GRANTS
This work was funded by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (Grant R01-NS-076589-1 to M. A. Perez) and by the Paralyzed Veterans of America (Grant 2821 to K. L. Bunday).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.L.B. and M.A.P. conception and design of research; K.L.B. and M.A.P. performed experiments; K.L.B. and M.A.P. analyzed data; K.L.B. and M.A.P. interpreted results of experiments; K.L.B. and M.A.P. prepared figures; K.L.B. and M.A.P. drafted manuscript; K.L.B. and M.A.P. edited and revised manuscript; K.L.B. and M.A.P. approved final version of manuscript.
REFERENCES
- Aimonetti and Nielsen, 2001. Aimonetti JM, Nielsen JB. Changes in intracortical excitability induced by stimulation of wrist afferents in man. J Physiol 534: 891–902, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélemy et al., 2010. Barthélemy D, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering-Sørensen F, Nielsen JB. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol 104: 1167–1176, 2010 [DOI] [PubMed] [Google Scholar]
- Bennett et al., 2004. Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Brus-Ramer et al., 2009. Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci 29: 6196–6206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler and Thomas, 2003. Butler JE, Thomas CK. Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. J Appl Physiol 94: 567–575, 2003 [DOI] [PubMed] [Google Scholar]
- Calancie et al., 2000. Calancie B, Molano MR, Broton JG. Neural plasticity as revealed by the natural progression of movement expression—both voluntary and involuntary—in humans after spinal cord injury. Prog Brain Res 128: 71–88, 2000 [DOI] [PubMed] [Google Scholar]
- Carson et al., 2008. Carson RG, Kennedy NC, Linden MA, Britton L. Muscle-specific variations in use-dependent crossed-facilitation of corticospinal pathways mediated by transcranial direct current (DC) stimulation. Neurosci Lett 441: 153–157, 2008 [DOI] [PubMed] [Google Scholar]
- Carson et al., 2004. Carson RG, Riek S, Mackey DC, Meichenbaum DP, Willms K, Forner M, Byblow WD. Excitability changes in human forearm corticospinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol 560: 929–940, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardon et al., 2010. Chardon MK, Suresh NL, Rymer WZ. An evaluation of passive properties of spastic muscles in hemiparetic stroke survivors. Conf Proc IEEE Eng Med Biol Soc 2010: 2993–2996, 2010 [DOI] [PubMed] [Google Scholar]
- Curt et al., 1997. Curt A, Keck ME, Dietz V. Clinical value of F-wave recordings in traumatic cervical spinal cord injury. Electroencephalogr Clin Neurophysiol 105: 189–193, 1997 [DOI] [PubMed] [Google Scholar]
- Davey et al., 1998. Davey NJ, Smith HC, Wells E, Maskill DW, Savic G, Ellaway PH, Frankel HL. Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in patients with incomplete spinal cord injury. J Neurol Neurosurg Psychiatry 65: 80–87, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey et al., 1999. Davey NJ, Smith HC, Savic G, Maskill DW, Ellaway PH, Frankel HL. Comparison of input-output patterns in the corticospinal system of normal subjects and incomplete spinal cord injured patients. Exp Brain Res 127: 382–390, 1999 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al., 2004. Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115: 255–266, 2004 [DOI] [PubMed] [Google Scholar]
- Ellaway et al., 2007. Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL, Jamous A, Savic G. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev 44: 69–76, 2007 [DOI] [PubMed] [Google Scholar]
- Espiritu et al., 2003. Espiritu MG, Lin CS, Burke D. Motoneuron excitability and the F wave. Muscle Nerve 27: 720–727, 2003 [DOI] [PubMed] [Google Scholar]
- Ferbert et al., 1992. Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro et al., 1990. Fierro B, Raimondo D, Modica A. F-response assessment in healthy control subjects. Electromyogr Clin Neurophysiol 30: 501–508, 1990 [PubMed] [Google Scholar]
- Fouad et al., 2001. Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001 [DOI] [PubMed] [Google Scholar]
- Ghosh et al., 2009. Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zörner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci 29: 12210–12219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould et al., 1986. Gould HJ, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 247: 297–325, 1986 [DOI] [PubMed] [Google Scholar]
- Hansen et al., 2005. Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sørensen F, Nielsen JB. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol 94: 934–942, 2005 [DOI] [PubMed] [Google Scholar]
- Hinder et al., 2010. Hinder MR, Schmidt MW, Garry MI, Summers JJ. Unilateral contractions modulate interhemispheric inhibition most strongly and most adaptively in the homologous muscle of the contralateral limb. Exp Brain Res 205: 423–433, 2010 [DOI] [PubMed] [Google Scholar]
- Hortobagyi et al., 2003. Hortobagyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90: 2451–2459, 2003 [DOI] [PubMed] [Google Scholar]
- Hultborn and Nielsen, 1995. Hultborn H, Nielsen JB. H-reflexes and F-responses are not equally sensitive to changes in motoneuronal excitability. Muscle Nerve 18: 1471–1474, 1995 [DOI] [PubMed] [Google Scholar]
- Jankowska et al., 2005. Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurons. J Neurosci 25: 7401–745, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska et al., 2006. Jankowska E, Stecina K, Cabaj A, Pettersson LG, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol 575: 527–541, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy and Carson, 2008. Kennedy NC, Carson RG. The effect of simultaneous contractions of ipsilateral muscles on changes in corticospinal excitability induced by paired associative stimulation (PAS). Neurosci Lett 445: 7–11, 2008 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2007. Kim Y, Aoki T, Ito H. Evaluation of parameters of serially monitored F-wave in acute cervical spinal cord injury. J Nippon Med Sch 74: 106–113, 2007 [DOI] [PubMed] [Google Scholar]
- Kowalczewski et al., 2011. Kowalczewski J, Chong SL, Galea M, Prochazka A. In-home tele-rehabilitation improves tetraplegic hand function. Neurorehabil Neural Repair 25: 412–422, 2011 [DOI] [PubMed] [Google Scholar]
- Kujirai et al., 1993. Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2010. Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol 588: 201–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon, 2008. Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008 [DOI] [PubMed] [Google Scholar]
- Lin and Floeter, 2004. Lin JZ, Floeter MK. Do F-wave measurements detect changes in motor neuron excitability? Muscle Nerve 30: 289–294, 2004 [DOI] [PubMed] [Google Scholar]
- Marino et al., 2003. Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 26: 50–56, 2003 [DOI] [PubMed] [Google Scholar]
- Meyer et al., 1995. Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118: 429–440, 1995 [DOI] [PubMed] [Google Scholar]
- Mottram et al., 2010. Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Origins of spontaneous firing of motor units in the spastic-paretic biceps brachii muscle of stroke survivors. J Neurophysiol 104: 3168–3179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher et al., 2000. Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111: 344–349, 2000 [DOI] [PubMed] [Google Scholar]
- Nishimura and Isa, 2011. Nishimura Y, Isa T. Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys. Exp Neurol (August 22, 2011). doi:10.1016/j.expneurol.2011.08.013 [DOI] [PubMed] [Google Scholar]
- Norton et al., 2008. Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton and Gorassini, 2006. Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol 95: 2580–2589, 2006 [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos and Chroni, 1996. Panayiotopoulos CP, Chroni E. F-waves in clinical neurophysiology: a review, methodological issues and overall value in peripheral neuropathies. Electroencephalogr Clin Neurophysiol 101: 365–374, 1996 [PubMed] [Google Scholar]
- Perez and Cohen, 2008. Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez and Cohen, 2009. Perez MA, Cohen LG. Scaling of motor cortical excitability during unimanual force generation. Cortex 45: 1065–1071, 2009 [DOI] [PubMed] [Google Scholar]
- Perez et al., 2007. Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci 27: 1045–1053, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny and Burke, 2005. Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Cambridge, UK: Cambridge Univ. Press, 2005, chapter 10, p. 452–510 [Google Scholar]
- Raineteau and Schwab, 2001. Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2: 263–273, 2001 [DOI] [PubMed] [Google Scholar]
- Renner et al., 2005. Renner CIE, Woldag H, Atanasova R, Hummelsheim H. Change of facilitation during voluntary bilateral hand activation after stroke. J Neurol Sci 239: 25–30, 2005 [DOI] [PubMed] [Google Scholar]
- Ridding and Rothwell, 1999. Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res 126: 536–544, 1999 [DOI] [PubMed] [Google Scholar]
- Rosenzweig et al., 2009. Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, Havton LA, Tuszynski MH. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol 513: 151–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig et al., 2010. Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci 13: 1505–1510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell et al., 1999. Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52: 97–103, 1999 [PubMed] [Google Scholar]
- Roy et al., 2011. Roy FD, Zewdie ET, Gorassini MA. Short-interval intracortical inhibition with incomplete spinal cord injury. Clin Neurophysiol 122: 1387–1395, 2011 [DOI] [PubMed] [Google Scholar]
- Saturno et al., 2008. Saturno E, Bonato C, Miniussi C, Lazzaro V, Callea L. Motor cortex changes in spinal cord injury: a TMS study. Neurol Res 30: 1084–1085, 2008 [DOI] [PubMed] [Google Scholar]
- Sohn et al., 2003. Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res 148: 176–185, 2003 [DOI] [PubMed] [Google Scholar]
- Soteropoulos and Perez, 2011. Soteropoulos DS, Perez MA. Physiological changes underlying bilateral isometric arm voluntary contractions in healthy humans. J Neurophysiol 105: 1594–1602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman et al., 1998. Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve 21: 1033–1039, 1998 [DOI] [PubMed] [Google Scholar]
- Stromberg, 1986. Stromberg BV. Contralateral therapy in upper extremity rehabilitation. Am J Phys Med 65: 135–143, 1986 [PubMed] [Google Scholar]
- Talelli et al., 2008. Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 186: 59–66, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor and Martin, 2009. Taylor JL, Martin PG. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci 29: 11708–11716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor and Gandevia, 2004. Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol 96: 1496–1503, 2004 [DOI] [PubMed] [Google Scholar]
- Thomas and Gorassini, 2005. Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 94: 2844–2855, 2005 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 1997. Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland-Ritchie BR. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp Neurol 148: 414–23, 1997 [DOI] [PubMed] [Google Scholar]
- Ugawa et al., 1992. Ugawa Y, Genba K, Mannen T, Kanazawa I. Stimulation of corticospinal pathways at the level of the pyramidal decussation in neurological disorders. Brain 115: 1947–1961, 1992 [DOI] [PubMed] [Google Scholar]
- Vercauteren et al., 2008. Vercauteren K, Pleysier T, Van Belle L, Swinnen SP, Wenderoth N. Unimanual muscle activation increases interhemispheric inhibition from the active to the resting hemisphere. Neurosci Lett 445: 209–213, 2008 [DOI] [PubMed] [Google Scholar]
- Woldag et al., 2004. Woldag H, Lukhaup S, Renner C, Hummelsheim H. Enhanced motor cortex excitability during ipsilateral voluntary hand activation in healthy subjects and stroke patients. Stroke 35: 2556–2559, 2004 [DOI] [PubMed] [Google Scholar]
- Zijdewind et al., 2006. Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res 175: 526–535, 2006 [DOI] [PubMed] [Google Scholar]
- Zijdewind et al., 2011. Zijdewind I, Gant K, Bakels R, Thomas CK. Do additional inputs change maximal voluntary motor unit firing rates after spinal cord injury? Neurorehabil Neural Repair 26: 58–67, 2011 [DOI] [PubMed] [Google Scholar]


