Summary
Patients with advanced solid malignancies were enrolled to an open-label, single-arm, dose-escalation study, in which CRLX101 was administered intravenously over 60 min among two dosing schedules, initially weekly at 6, 12, and 18 mg/m2 and later bi-weekly at 12, 15, and 18 mg/m2. The maximum tolerated dose (MTD) was determined at 15 mg/m2 bi-weekly, and an expansion phase 2a study was completed. Patient samples were obtained for pharmacokinetic (PK) and pharmacodynamic (PD) assessments. Response was evaluated per RECIST criteria v1.0 every 8 weeks. Sixty-two patients (31 male; median age 63 years, range 39-79) received treatment. Bi-weekly dosing was generally well tolerated with myelosuppression being the dose-limiting toxicity. Among all phase 1/2a patients receiving the MTD (n=44), most common grade 3/4 adverse events were neutropenia and fatigue. Evidence of systemic plasma exposure to both the polymer-conjugated and unconjugated CPT was observed in all treated patients. Mean elimination unconjugated CPT Tmax values ranged from 17.7 to 24.5 h, and maximum plasma concentrations and areas under the curve were generally proportional to dose for both polymer-conjugated and unconjugated CPT. Best overall response was stable disease in 28 patients (64 %) treated at the MTD and 16 (73 %) of a subset of NSCLC patients. Median progression-free survival (PFS) for patients treated at the MTD was 3.7 months and for the subset of NSCLC patients was 4.4 months. These combined phase 1/2a data demonstrate encouraging safety, pharmacokinetic, and efficacy results. Multinational phase 2 clinical development of CRLX101 across multiple tumor types is ongoing.
Keywords: Nanopharmaceutical, Polymer conjugate camptothecin, Phase 1/2a, Solid tumor
Introduction
Camptothecin derivatives such as irinotecan (Camptosar®, Pfizer Inc, New York, NY) and topotecan (Hycamtin®, GlaxoSmithKline, Research Triangle Park, NC) demonstrate clinical utility for the treatment of advanced solid tumors. Binding of CPT to its primary target, the Topo 1-DNA cleavage complex, inhibits Topo 1-mediated unwinding and subsequent DNA repair [3]. Exposure of cancer cells to CPT leads to replication-mediated accumulation of DNA double-strand breaks and apoptosis [4, 5]. However, interaction of CPT with DNA is non-covalent and reverses within minutes of drug removal. More recently, CPT and prolonged low-dose topotecan have been shown to induce topoisomerase-independent down-regulation of HIF-1α that is associated with angiogenesis, metastasis, and resistance to VEGF inhibition [6, 7]. Both mechanisms of action have important pharmacologic implications that favor sustained exposure of tumors to active concentrations of these compounds. However, both CPT derivatives are associated with considerable toxicity, including diarrhea and myelosuppression.
CRLX101 is a dynamically tumor targeted nanopharmaceutical that contains a cyclodextrin-containing polymer (CDP) conjugated to camptothecin (CPT) that self-assembles into 30 to 40-nm diameter nanoparticles (Fig. 1) [1, 2, 8-15]. Conjugation of CPT to CDP increases its solubility by roughly 3 orders of magnitude and prevents inactivation through spontaneous lactone ring opening, which can occur rapidly at physiologic pH [9]. In preclinical studies, systemically administered CRLX101 circulated and distributed as intact nanoparticles demonstrated less rapid renal clearance than CPT, and had a prolonged plasma half-life compared with small-molecule CPT analogues [1, 2, 10, 14]. Similarly, in human tumor xenograft models, intact CRLX101 nanoparticles and sustained levels of CPT localize preferentially to tumor cells [1, 2, 10, 14]. It is hypothesized that selective targeting of tumor cells by CRLX101 occurs because of the enhanced permeability of tumor neo-vasculature compared with normal tissue [16]. Prolonged release of active CPT from CRLX101 nanoparticles, resulting in extended Topo 1 and HIF-1α inhibition, and increased antitumor activity compared with irinotecan and topotecan, has been reported in a variety of tumor tissues and multiple human tumor xenograft models (lymphoma, breast, ovarian, lung, and colon) [1, 2, 8, 10-14].
Fig. 1.
a Characterization of CRLX101 - self assembly of CDP-CPT conjugates into nanoparticles. b Characterization of CRLX101 -enhanced tumor permeability
Patients and methods
Patients
Patients ≥ 18 years old with histologically or cytologically confirmed metastatic or unresectable solid tumor malignancies, refractory to standard therapy, or for which no standard curative therapy is available were eligible. Additional eligibility requirements included Eastern Cooperative Oncology Group (ECOG) performance status ≤2, acceptable organ and bone marrow function, no evidence of clinically significant cardiac conduction abnormalities or ischemia, and cardiac ejection fraction ≥ 45 %. Previous chemotherapy, radiotherapy, or other investigational therapy needed to be completed at least 4 weeks prior to treatment. Patients who received prior treatment with a Topo 1 inhibitor or high-dose chemotherapy with autologous stem-cell transplantation were not eligible.
The study was approved by the institutional review board or ethics committee at each participating center (City of Hope Comprehensive Cancer Center, Duarte, CA; San Juan Oncology Associates, Farmington, NM; and Virginia G. Piper Cancer Center Clinical Trials at Scottsdale Healthcare, Scottsdale, AZ) and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained from all patients prior to study entry.
Study design
This was a phase 1/2a, open-label, multicenter, dose-escalation study of CRLX101 in previously treated patients with advanced solid tumor malignancies. In phase 1, CRLX101 was infused intravenously initially over 60-min weekly on Days 1, 8, and 15 of each 28-day cycle. Dose escalations starting at 6 mg/m2 were implemented using an accelerated Simon design with a modified Fibonacci dose escalation schema [17]. No intrapatient dose escalation was permitted. Based on PK data suggesting that a biweekly dosing schedule would be better tolerated, the Day 8 infusion was eliminated per protocol amendment. Dose escalation continued at 12 mg/m2 with every other week dosing on Days 1 and 15 of a 28-day cycle.
Dose-limiting toxicity (DLT) was defined as any treatment-emergent related adverse event (AE) that occurred during the first cycle and included any combination of the following: grade ≥ 3 non-hematologic toxicity with the exclusion of alopecia; absolute neutrophil count (ANC) ≤500 cells/uL without growth factor support; or any febrile neutropenia with ANC ≤500 cells/uL; platelet count ≤ 50,000 cells/uL without transfusion; hemoglobin <8 g/dL or other persisting toxicity of any grade delaying treatment by ≥ 14 days. The MTD was defined as the highest administered dose at which ≤1 of 6 patients in a dose group experienced a drug-related DLT during Cycle 1.
Patients who experienced severe or life-threatening non-hematologic toxicity at any time during the study were discontinued. Patients who experienced a non-life-threatening, rapidly resolving, non-hematologic toxicity, or any hematologic toxicity were dose de-escalated to a lower dose at their subsequent treatment visit. Treatment was delayed for patients who experienced grade ≥ 2 non-hematologic toxicity except for grade 2 fatigue or anorexia.
The protocol was amended so that after determination of the MTD, additional patients would be enrolled in the phase 2a MTD expansion cohort, with a focused selection of cancers that historically have demonstrated sensitivity to topoisomerase I inhibitors, including a minimum of 12 patients with non-small cell lung cancer (NSCLC). In the phase 2a cohort, CRLX101 treatment continued until disease progression (determined by Response Evaluation Criteria in Solid Tumors, RECIST version 1.0) [18], patient withdrawal, excessive toxicity, or AE delaying treatment for 28 days or resulting in death. Patients continuing CRLX101 treatment also received additional supportive care including: 1 l of clinically suitable intravenous hydration both pre- and post-treatment to minimize risk of cystitis; and pre-treatment anti-inflammatory medications including corticosteroid, antihistamine, and H2 antagonist to minimize risk of hypersensitivity reaction.
Assessments
Whole blood samples (5 mL) were collected from patients on days 1, 8, and 15 of Cycle 1 for assessment of polymer-conjugated and unconjugated CPT plasma pharmacokinetics. CRLX101 plasma concentration versus time data were analyzed using the WinNonlin, version 5.2.1 software (Pharsight Inc., Mountain View, CA) using a non-compartmental model and Microsoft Office Excel 2003 maintained at Seventh Wave Laboratories LLC (Chesterfield, MO). Results were summarized using descriptive statistics available within WinNonLin.
Total urine was collected either at 8, 24, and 48 h following Cycle 1 Day 1 dosing or 24-h collection prior to dosing on Day 1 of Cycle 1 to 5, Day 8 of Cycle 6, and Day 15 of Cycle 1 to 6 for determination of urinary excretion of polymer-conjugated and unconjugated CPT over 48 or 24 h, respectively. Samples were analyzed by LC-MS/MS using an 1100 series HPLC system (Agilent, Palo Alto, CA) coupled to a Micromass Quattro Ultima Triple Quadrupole Mass Spectrometer (Micromass, Inc., Beverly, MA). MassLynx version 3.5 software was used for data acquisition and processing.
Safety evaluations were conducted at baseline and prior to each dose. All patients had a complete blood count with differential and serum chemistries evaluated. Urinalysis was completed at baseline and at the start of each cycle. During the study per a protocol amendment, an additional urinalysis time point was added, prior to the Day 15 dose of each cycle. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version (CTCAE) 3.0. Baseline radiographic CT scans were obtained and patients were evaluated for tumor response based on RECIST criteria version 1.0 every 2 cycles (8 weeks). The overall best tumor response was recorded from the start of treatment until disease progression, taking as reference for tumor progression the smallest measurements recorded since the treatment started.
To be assigned a best tumor response status of partial (PR) or complete response (CR), confirmation was required with a repeat scan performed no less than 4 weeks after the criteria for response was first met. In the case of stable disease (SD), follow-up measurements must have met the SD criteria at least once after study entry at a minimum interval of 8 weeks.
Exploratory analyses
As part of the initial study consent, patients were given the opportunity to consent to an optional tumor biopsy for exploratory analyses. Tissue was only collected if deemed feasible and of minimal risk to the patient. Ascites fluid from a patient with ovarian cancer was collected before treatment and 2 days following treatment with 6 mg/m2 CRLX101. Formalin fixed cells were paraffin embedded, and immunohistochemical staining was performed on 5-μm thick sections using an anti-Topo 1 rabbit polyclonal antibody (Abcam, Cambridge, MA). Lysates containing total cellular protein from frozen ascites cells [19] were used to determine the catalytic activity of Topo 1 (TopoGEN, Port Orange, FL) following the manufacturer’s instructions.
Confocal fluorescence microscopy
Detection of CRLX101 and CPT was assessed using fixed and sectioned (10 μM) tumor samples stained with polyethyleneglycolylated (PEGylated) and adamantane-modified gold nanoparticles (Au-PEG-AD) and mounted with Mowiol 4–88 and glycerol, as described previously for animal tissues [14]. Confocal scanning fluorescence microscopy was used to detect both Au-PEG-AD-stained CRLX101 and CPT (which has natural fluorescence).
Results
Patient demographics
From June 2006 to April 2010, the phase 1 portion of the study enrolled 24 patients; and from April 2010 to January 2011, the phase 2a portion enrolled 38 patients for a total of 62 patients (median age 63 years; range, 39-79 years). The demographic and clinical characteristics were similar among the phase 1 and phase 2a patients and between those treated at MTD and those at other dose levels (Table 1). All 62 patients were included in the safety population with 44 patients receiving the MTD. Sixty patients were included in the pharmacokinetic analysis. A total of 22 patients with NSCLC were enrolled in the MTD dose group and are presented below as a subset. Subsequent to a protocol amendment, 27 of the 62 patients received the additional supportive care of pre- and post-treatment IV hydration. Mean duration on treatment was 109 days (111 days in the MTD group) with a median duration of 62 days (99 days in the MTD group). Mean number of cycles completed was 4, with median of 3 cycles overall (4 cycles in the MTD group). During the study, 54 (87 %) patients had at least one post-treatment scan and were considered evaluable for tumor response and progression-free survival by RECIST v1.0. The primary reason for discontinuation from the study was progressive disease in 39 of the 62 patients as evidenced by increase in tumor size or tumor markers (CA19-9). Patient disposition is summarized in Table 2.
Table 1.
Patient demographics and baseline clinical characteristics
| Phase 1 |
Phase 2A | MTDa | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weekly |
Bi-Weekly |
|||||||||
| Characteristic | Statistic | 6 mg/m2 (n=6) |
12 mg/m2 (n=3) |
18 mg/m2 (n=3) |
12 mg/m2 (n=3) |
15 mg/m2 (n=6) |
18 mg/m2 (n=3) |
15 mg/m2 (n=38) |
15 mg/m2 (n=44) |
(n=62) |
| Age | Mean | 54.8 | 58.7 | 64.3 | 60.3 | 65.5 | 56.7 | 62.8 | 63.2 | 61.8 |
| SD | 7.8 | 4.5 | 11.6 | 7.4 | 11.0 | 8.7 | 9.1 | 9.3 | 9.1 | |
| Median | 52.0 | 59.0 | 70.0 | 63.0 | 63.0 | 59.0 | 65.0 | 65.0 | 63.0 | |
| Range | 46–65 | 54–63 | 51–72 | 52–66 | 54–79 | 47–64 | 39–76 | 39–79 | 39–79 | |
| BSA (m2) | Mean | 1.8 | 2.2 | 1.7 | 1.9 | 1.9 | 2.0 | 1.8 | 1.8 | 1.8 |
| Gender | ||||||||||
| Male | n (%) | 3 (50.0) | 3 (100.0) | 1 (33.3) | 2 (66.7) | 2 (33.3) | 2 (66.7) | 18 (47.4) | 20 (45.5) | 31 (50.0) |
| Female | n (%) | 3 (50.0) | 0 | 2 (66.7) | 1 (33.3) | 4 (66.7) | 1 (33.3) | 20 (52.6) | 24 (54.5) | 31 (50.0) |
| Race | ||||||||||
| White | n (%) | 5 (83.3) | 3 (100.0) | 1 (33.3) | 0 | 5 (83.3) | 2 (66.7) | 29 (76.3) | 34 (77.3) | 45 (72.6) |
| Asian | n (%) | 1 (16.7) | 0 | 1 (33.3) | 2 (66.7) | 1 (16.7) | 1 (33.3) | 5 (13.2) | 6 (13.6) | 11 (17.7) |
| American Indian or Alaska Native | n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7.9) | 3 (6.8) | 3 (4.8) |
| Other | n (%) | 0 | 0 | 1 (33.3) | 1 (33.3) | 0 | 0 | 1 (2.6) | 1 (2.3) | 3 (4.8) |
| ECOG | ||||||||||
| 0 | n (%) | 3 (50.0) | 1 (33.3) | 0 | 2 (66.7) | 4 (66.7) | 1 (33.3) | 16 (42.1) | 20 (45.5) | 27 (43.5) |
| 1 | n (%) | 3 (50.0) | 2 (66.7) | 3 (100.0) | 1 (33.3) | 1 (16.7) | 2 (66.7) | 21 (55.3) | 22 (50.0) | 33 (53.2) |
| 2 | n (%) | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (2.6) | 2 (4.5) | 2 (3.2) |
| Cancer Type | ||||||||||
| Lung | n (%) | 6 (25.0) | 21 (55.3) | 22 (50.0) | 27 (43.5) | |||||
| Pancreas | n (%) | 5 (20.8) | 6 (15.8) | 4 (15.9) | 11 (17.7) | |||||
| Head and Neck | n (%) | 1 (4.2) | 2 (5.3) | 2 (4.6) | 3 (4.8) | |||||
| Renal | n (%) | 2 (8.3) | 2 (5.3) | 3 (6.8) | 4 (6.5) | |||||
| Ovarian | n (%) | 1 (4.2) | 2 (5.3) | 2 (4.6) | 3 (4.8) | |||||
| Breast | n (%) | 3 (12.5) | 0 | 2 (4.6) | 3 (4.8) | |||||
| Bile Duct | n (%) | 1 (4.2) | 1 (2.6) | 1 (2.3) | 2 (3.2) | |||||
| Uterine | n (%) | 1 (4.2) | 1 (2.6) | 2 (4.6) | 2 (3.2) | |||||
| Otherb | n (%) | 4 (16.7) | 3 (7.9) | 3 (6.8) | 7 (11.3) | |||||
| Prior Therapies | Median (Mean) | 3 (3.6) | 4 (3.5) | 4 (3.6) | 3 (3.5) | |||||
Includes all patients in Phase 1 and 2a treated at the MTD dose
Other cancers include: ampullary, colon, intestinal, gastric, liver, thyroid, urethral (n=l for each)
Table 2.
Patient disposition
| Phase 1 |
Phase 2A | MTDa | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weekly |
Bi-weekly |
|||||||||
| Statistic | 6 mg/m2 (n=6) |
12 mg/m2 (n=3) |
18 mg/m2 (n=3) |
12 mg/m2 (n=3) |
15 mg/m2 (n=6) |
18 mg/m2 (n=3) |
15 mg/m2 (n=38) |
15 mg/m2 (n=44) |
(n=62) | |
| Safety Population | n | 6 | 3 | 3 | 3 | 6 | 3 | 38 | 44 | 62 |
| MTD Population | n (%) | 0 | 0 | 0 | 0 | 6 (100.0) | 0 | 38 (100.0) | 44 (100.0) | 44 (71.0) |
| NSCLC Population | n (%) | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 21 (55.3) | 22 (50.0) | 22 (35.5) |
| Primary Reason for Discontinuation | ||||||||||
| Progressive Disease | n (%) | 5 (83.3) | 1 (33.3) | 1 (33.3) | 2 (66.7) | 5 (83.3) | 1 (33.3) | 21 (55.3) | 26 (59.1) | 36 (58.1) |
| Adverse Event | n (%) | 1 (16.7) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 1 (16.7) | 0 | 8 (21.1) | 9 (20.5) | 14 (22.6) |
| Withdrew Consent | n (%) | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (2.6) | 1 (2.3) | 2 (3.2) |
| Investigator Discretion | n (%) | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 3 (7.9) | 3 (6.8) | 4 (6.5) |
| Symptomatic Deterioration | n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7.9) | 3 (6.8) | 3 (4.8) |
| Increase Tumor Marker (CA19-9) | n (%) | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 2 (5.3) | 2 (4.5) | 3 (4.8) |
| Duration of Treatment | ||||||||||
| Days | Mean | 132.8 | 94.0 | 115.3 | 76.3 | 130.0 | 78.3 | 107.4 | 110.5 | 108.9 |
| SD | 254.7 | 107.4 | 139.0 | 45.4 | 132.0 | 92.1 | 100.7 | 104.0 | 120.3 | |
| Median | 36.0 | 35 | 57.0 | 58.0 | 97.5 | 36.0 | 98.5 | 98.5 | 62.0 | |
| Range | 15-652 | 29-218 | 15-274 | 43-128 | 43-393 | 15-184 | 1^122 | 1-422 | 1-652 | |
| Cycles | Mean | 4.5 | 4.0 | 4.3 | 3.0 | 4.5 | 2.3 | 4.1 | 4.2 | 4.1 |
| SD | 7.1 | 3.5 | 4.9 | 1.0 | 3.8 | 2.3 | 3.3 | 3.3 | 3.7 | |
| Median | 2.0 | 2.0 | 2.0 | 3.0 | 3.5 | 1.0 | 4.0 | 4.0 | 3.0 | |
| Range | 1-19 | 2-8 | 1-10 | 2-4 | 2-12 | 1-5 | 1-14 | 1-14 | 1-19 | |
MTD maximum tolerated dose; SD standard deviation
Includes all patients from Phase 1 to 2a treated at the MTD dos(
Dose-limiting toxicities and maximum tolerated dose
In Cycle 1 during dose escalation, a total of 3 patients experienced DLTs, two in the weekly 18 mg/m2 dose cohort and one patient in the biweekly 18 mg/m2 dose cohort. In the weekly 18 mg/m2 dose this included: a patient with relapsed metastatic breast cancer, with metastatic disease to liver, lung, and bone, heavily pretreated with 6 prior therapies, developed grade 4 neutropenia accompanied by grade 4 thrombocytopenia and was discontinued from study. The second DLT occurred in a patient with advanced stage NSCLC, heavily pretreated with 4 prior therapies, who developed grade 3 anemia, neutropenia, and leukopenia, and continued treatment with a dose reduction to 9 mg/m2. In the bi-weekly dosing cohort, a patient with advanced pancreatic cancer with metastatic disease to the lungs and heavily pretreated with 5 prior therapies, experienced grade 4 thrombocytopenia and continued treatment with an initial dose reduction to 15 mg/m2 and subsequent second dose reduction to 12 mg/m2. These hematologic events were not unexpected for this study drug.
Based on these dose ranging studies, the MTD and recommended phase 2 dose (RP2D) of CRLX101 was determined to be 15 mg/m2 administered bi-weekly by intravenous infusion, and a phase 2a expansion cohort was added. A total of 44 patients (6 in phase 1, 38 in phase 2a) received CRLX101 at the MTD of 15 mg/m2 bi-weekly.
Safety profile
The most common treatment-emergent related AEs (≥15 %) of any grade overall were fatigue (n=23), cystitis (n=17), anemia (n=16), neutropenia (n=13), nausea (n=12), dysuria (n=11), hematuria (n=11) and leukopenia (n=10). Grade 3/4 related AEs occurring in multiple patients included neutropenia (n=12), fatigue (n=7), leukopenia (n=4), thrombocytopenia (n=4), anemia (n=3), dehydration (n=2) and hematuria (n=2). Grade 3/4 related AEs associated with the bladder included cystitis, hemorrhagic cystitis, and dysuria (n=1 each). Grade 3/4 related AEs at time of infusion included hypersensitivity, infusion-related reaction and cytokine release syndrome (n=1 each). Other grade 3/4 related AEs included pelvic pain, penile pain, dyspnea, acute respiratory distress and rash (n=1 each). Related treatment-emergent AEs occurring in ≥5 % are summarized in Table 3.
Table 3.
Treatment-emergent related adverse events in ≥ 5 % across all dose groups, any grade (grade ≥ 3)
| Phase 1 Weekly |
Phase 1 Bi-Weekly |
Phase 2A | MTDa | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 mg/m2 | 12 mg/m2 | 18 mg/m2 | 12 mg/m2 | 15 mg/m2 | 18 mg/m2 | 15 mg/m2 | 15 mg/m2 | |||
| (n=6) | (n=3) | (n=3) | (n=3) | (n=3) | (n=38) | (n=44) | (n=62) | |||
| Statistic | n (n) | n (n) | n (n) | n (n) | n (n) | n (n) | n (n) | n (n) | n (n) | % |
| Hematologic | ||||||||||
| Anemia | 1 | 1 | 3 (1) | 1 | 2 | 2 (1) | 6 (1) | 8 (1) | 16 (3 ) | 26 % |
| Neutropenia | 0 | 0 | 3 (3 ) | 0 | 1 (1) | 2 (2 ) | 7 (6) | 8 (7) | 13 (12) | 21 % |
| Leukopenia | 0 | 1 | 3 (2 ) | 0 | 2 | 1 (1) | 3 (1) | 5 (1) | 10 (4) | 16 % |
| Thrombocytopenia | 0 | 0 | 2 (2 ) | 0 | 0 | 2 (2 ) | 0 | 0 | 4 (4) | 6 % |
| Non-hematologic | ||||||||||
| Fatigue | 3 (2 ) | 2 | 2 (1) | 0 | 2 | 1 | 13 (4) | 15 (4) | 23 (7) | 37 % |
| Nausea | 0 | 0 | 1 | 1 | 4 | 0 | 6 | 10 | 12 | 19 % |
| Diarrhea | 0 | 0 | 0 | 0 | 1 (1) | 2 | 4 | 5 (1) | 7 (1) | 11 % |
| Vomiting | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 3 | 6 | 10 % |
| Alopecia | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 4 | 5 | 8 % |
| Decreased appetite | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 5 | 8 % |
| Urinary tract infection | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 3 | 5 | 8 % |
| Constipation | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 4 | 6 % |
| Dehydration | 1 | 1 (1) | 0 | 0 | 1 | 0 | 1 (1) | 2 (1) | 4 (2) | 6 % |
| Neuropathy peripheral | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 4 | 4 | 6 % |
| Non-hematologic (renal) | ||||||||||
| Cystitis | 1 | 3 | 1 | 0 | 1 | 1 | 10 (1) | 11 (1) | 17 (1) | 27 % |
| Dysuria | 3 (1) | 1 | 1 | 0 | 0 | 0 | 6 | 6 | 11(1) | 18 % |
| Hematuria | 2 | 3 | 2 (1) | 0 | 0 | 0 | 4 (1) | 4 (1) | 11(2) | 18 % |
| Leukocyturia | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 6 % |
| Micturition urgency | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 3 | 5 % |
| Cystitis, hemorrhagicb | 1 | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1) | 3 % |
NA not applicable, Gr. Grade, MTD maximum tolerated dose
Includes all patients from Phase 1 to 2a at the MTD dose
Values <5 % are included for this AE given its relevance to the study drug
Among the 44 patients receiving the MTD, the most common treatment-emergent related grade 3/4 AEs occurring in ≥ 5 % of patients were neutropenia (n=7) and fatigue (n=4). Four treatment-emergent serious AEs considered related to study drug were observed among 5 (11 %) patients treated at the MTD: grade 2 hypotension accompanied by a grade 2 pyrexia; grade 3 acute respiratory distress syndrome (ARDS) resulting in dose interruption; grade 3 cytokine release syndrome resulting in study drug discontinuation; and two serious AEs for cystitis, one reported as grade 2 accompanied by grade 3 dehydration and another reported as grade 3 accompanied by grade 3 hematuria. Both serious AEs with cystitis symptoms occurred in patients receiving pre-medication and pre-hydration after multiple cycles of study drug, the first on post-treatment Day 11 during Cycle 9, and the other on post-treatment Day 10 during Cycle 11; both resulted in a dose delay and dose reduction to 12 mg/m2.
Among all treated patients, 31 experienced a total of 50 dose delays secondary to AEs and 8 patients experienced a total of 9 AE-related dose reductions. Of note here, however, is that of the 31 patients with dose delays, only 21 of these patients were in the MTD group, and of these, only 8 of the 21 occurred as the result of severe (grade 3 or 4) AEs that were at least possibly related to CRLX101. Sixteen (26 %) patients overall, 11 treated in the MTD and 5 in non-MTD dosing cohorts, discontinued from the study due to an adverse event, of which 4 in the MTD and 4 in non-MTD dosing cohorts were related to study drug. Two of the 4 discontinuations in the MTD group were the result of infusion reactions which occurred prior to a protocol amendment which instituted the administration of pre-medications for potential hypersensitivity reactions.
Pharmacokinetic analyses
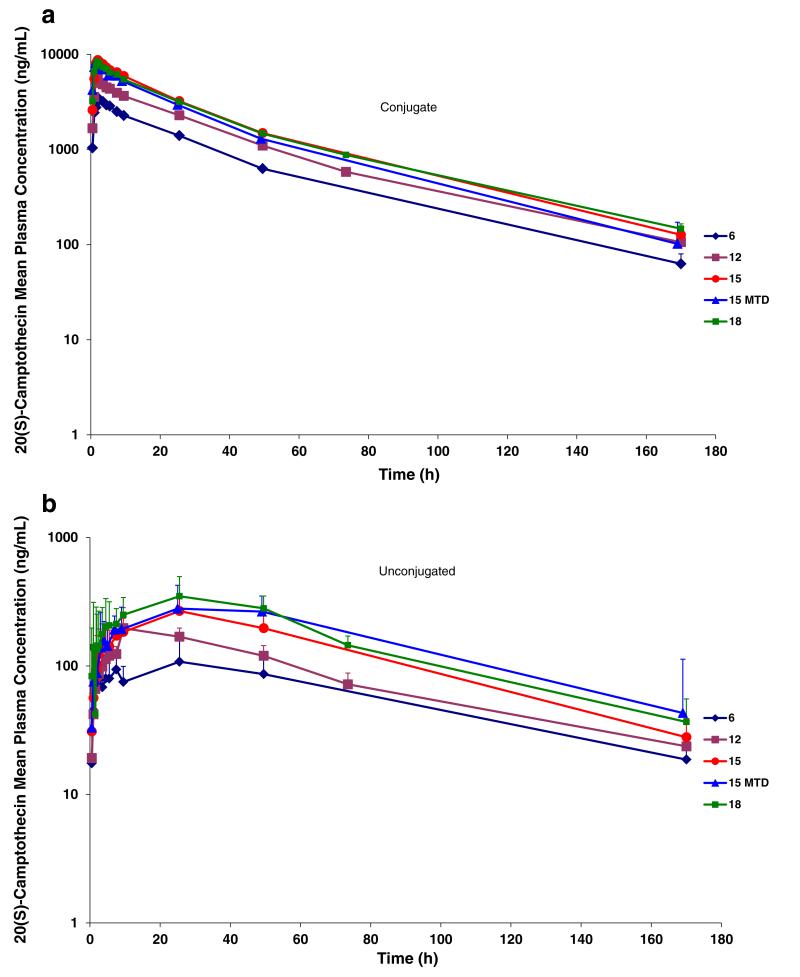
Evidence of systemic plasma exposure to both polymer-conjugated and unconjugated CPT was observed in all treated patients (Fig. 2). Polymer-conjugated CPT plasma concentrations increased sharply following IV infusion of CRLX101 with mean Cmax values ranging from 3580 to 8780 ng/mL over the dose range evaluated. The Cmax for conjugated CPT was reached within the first 2 h for most patients. Polymer-unconjugated CPT plasma concentrations increased gradually following IV infusion of CRLX101 with mean Cmax values ranging from 116 to 351 ng/mL over the dose range evaluated. Mean unconjugated CPT Cmax values ranged from 17.7 to 24.5 h over the dose range evaluated. These prolonged Cmax values are consistent with a gradual and slow release of CPT from the polymer conjugate.
Fig. 2.
a Mean plasma concentration versus time for polymer-conjugated camptothecin (CPT) following administration of CRLX101 by dose. Abbreviations MTD maximum tolerated dose. b Mean plasma concentration versus time for polymer-unconjugated camptothecin (CPT) following administration of CRLX101 by dose. Abbreviations MTD maximum tolerated dose. Error Bars indicate standard deviations
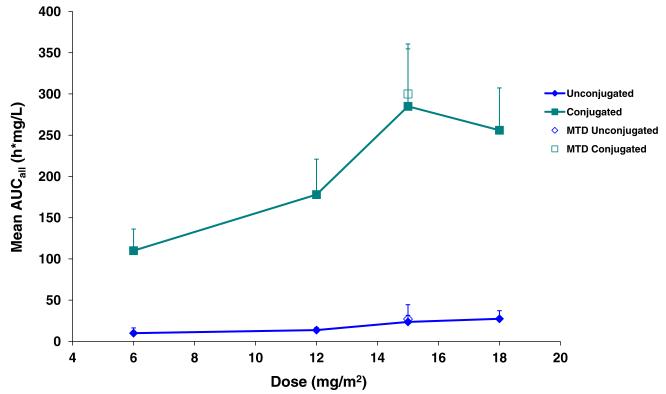
Polymer-conjugated and unconjugated CPT exposure (as assessed by mean Cmax and AUCall) increased in a dose-related fashion over the 6 to 15 mg/m2 dose range. Only polymer-unconjugated CPT exposure further increased over the 15 to 18 mg/m2 dose range. Across the full dose range, observed increases in Cmax and AUCall were reasonably proportional to dose for polymer-conjugated and polymer-unconjugated CPT. Comparison of polymer-conjugated to unconjugated CPT exposure (as assessed by AUCall) revealed an ~11-fold increase in conjugated CPT exposure relative to unconjugated CPT suggesting selective release of CPT payload in tumor cells (Fig. 3).
Fig. 3.
Mean AUCall for polymer-conjugated and unconjugated camptothecin by dose. Abbreviations AUC area under the curve, MTD maximum tolerated dose. Error bars indicate standard deviations
Mean clearance and volume of distribution values for the conjugated CPT over the dose range evaluated were dose-independent and ranged from 0.0914 to 0.132 L/h and 2.33 to 4.63 L; respectively. Volume of distribution values for the conjugated CPT suggests this material was retained within the vasculature and highly perfused tissues. A summary of pharmacokinetic parameters for CRLX101 is shown in Table 4.
Table 4.
Pharmacokinetic Parameters for CRLX101
| Cmax, μg/L |
Tmax, hours | AUCall, h-mg/L |
AUC∞, h-mg/L |
CI, L/h | VSS,L | T1/2, hours |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conj | Un | Un | Conj | Un | Conj | Un | Conj | Conj | Conj | Un | ||
| Dose | n | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| 6 mg/m2 | 6 | 3580(457) | 116 (61.1) | 17.7 (10.3) | 110 (26.2) | 9.98 (6.32) | 116 (22.5) | 13.2 (7.04) | 0.099 (0.022) | 3.34 (0.59) | 28.9 (4.99) | 48.4 (16.1) |
| 12 mg/m2 | 6a | 5620 (872) | 203 (62.6) | 19.3 (7.47) | 178 (42.9) | 13.7 (3.05) | 188 (38.2) | 16.6 (3.06) | 0.132 (0.024) | 4.63 (1.07) | 30.2 (5.20) | 50.1 (30.6) |
| 15 mg/m2 | 6 | 7190(1800) | 268 (92.5) | 24.5 (1.14) | 323 (128) | 23.6 (7.94) | 335 (122) | 25.4 (8.47) | 0.094 (0.041) | 2.33 (1.25) | 31.5 (1.50) | 43.3 (7.86) |
| 15 mg/m2 | 36b | 8260(1300) | 306(160) | 23.6 (9.16) | 300 (60.6) | 27.1 (17.4) | 306 (57.8) | 32.4 (11.5) | 0.091 (0.023) | 2.42 (0.70) | 27.9 (2.93) | 46.5 (12.8) |
| 18 mg/m2 | 6 | 8770(1830) | 351(146) | 22.8 (6.47) | 285 (69.7) | 27.4 (9.82) | 291 (71.1) | 31.5 (10.9) | 0.103 (0.045) | 3.52 (1.60) | 33.5 (6.92) | 41.9 (10.6) |
Cmax maximum plasma concentration; Tmax time of maximum concentration; AUCall area under the curve between the time of dose and the last timepoint at 24 h; AUC∞ area under the concentration-time curve from 0 to infinity; CI clearance; Vss steady-state volume of distribution; T1,2 elimination half-life; Conj conjugated CPT; Un unconjugated CPT
Combined data for weekly and bi-weekly administration at this dosing level
Phase 2a patients Cycle 1 PK data
Excretion
Urinary excretion across patients was variable with a mean of 21 % of the total administered dose of CRLX101 excreted as CPT in the urine within the first 48 h (Fig. 4). The majority of excreted CPT was in the polymer-conjugated form with a mean value of 16.2 % of the total administered dose of CRLX101 compared to an unconjugated mean value of 4.4 % of the total administered dose of CRLX101. Considerable time dependency on urinary excretion of the polymer-conjugated CPT was observed with the majority excreted during the initial 24 h, followed by a noteworthy decline in the 24- to 48-h collection period. In addition, a subset of patients who performed a 0-8 h urine collection revealed the majority of the conjugated CPTwas cleared during the first 8 h following dosing. Urinary clearance of unconjugated CPTwas notably higher than that for polymer-conjugated CPT over all observation periods. Urinary clearance of unconjugated CPT in the first 48 h post administration was independent of dose or creatinine clearance.
Fig. 4.
a Urinary camptothecin (CPT) analysis by collection time period. b Urinary camptothecin (CPT) analysis by dosing cohort. Error Bars indicate standard deviations
The majority of patients receiving CRLX101 at 15 mg/m2 or 18 mg/m2 on a bi-weekly schedule demonstrated measureable levels of unconjugated CPT in plasma at 14 days post administration; however, these values represent less than 3.1 % of the respective mean Cmax values for each dose level. The lower dose group (12 mg/m2) on a bi-weekly schedule did not have any measurable levels of unconjugated CPT in their plasma by 14 days post administration. These results indicate that in order to avoid significant carry-over of unconjugated plasma CPT from one dose to the next, a dosing interval greater than 1 week is required. No polymer-conjugated CPT was detectable in plasma at 14 days post administration of CRLX101 for the 18 mg/m2, 15 mg/m2, or 12 mg/m2 dose groups on a bi-weekly schedule during the dose escalation phase.
Efficacy
Median progression free survival (PFS) for patients treated at the MTD was 3.7 months (Table 5). The best response per RECIST v1.0 criteria reported by the Investigators was stable disease (SD) in 28 patients (64 %) treated at MTD of which 15 (34 %) had confirmed stable disease at subsequent evaluations.
Table 5.
Best response and progression free survival
| MTDa (n=44) n (%) | NSCLC (n=22) n (%) | |
|---|---|---|
| Evaluablec | 39 (89) | 19 (86) |
| Stable disease | 28 (64) | 16 (73) |
| Confirmedb | 15 (34) | 8 (36) |
| Unconfirmedc | 13 (30) | 8 (36) |
| Progressive disease | 10 (23) | 4(18) |
| Unable to determine | 6(14) | 2 (9) |
| Median PFS, months | 3.7 | 4.4 |
MTD maximum tolerated dose; PFS progression-free survival
Includes all patients from Phase 1 to 2a at the MTD dose
Confirmed are patients with ≥1 repeat scan every 8 weeks with continued stable disease
Unconfirmed are patients without repeat scan at 8 weeks demonstrat-ing stable disease
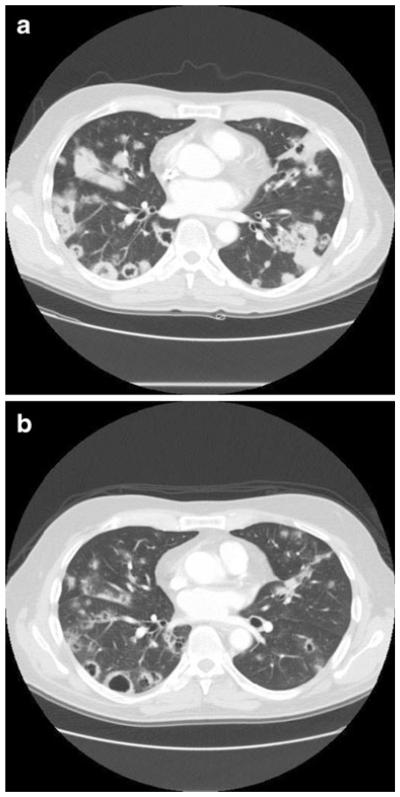
Six patients went on to receive > 6 months of treatment with CRLX101, of which one patient with pancreatic cancer with liver and lung metastases experienced stable disease and received a total of 24 cycles of CRLX101 at 6 mg/m2 weekly dosing prior to discontinuing for progressive disease (Fig. 5). In a subset of 22 patients with NSCLC median PFS was 4.4 months (4.8 months among patients with non-Squamous histology only), with stable disease reported in 16 patients (73 %) of which 8 had confirmed stable disease at subsequent evaluations.
Fig. 5.
Computed tomography scans of a patient with metastatic pancreatic cancer a before treatment and b after 6 months of treatment with 3-weekly doses of 6 mg/m2 CRLX101
Exploratory pharmacodynamic analyses
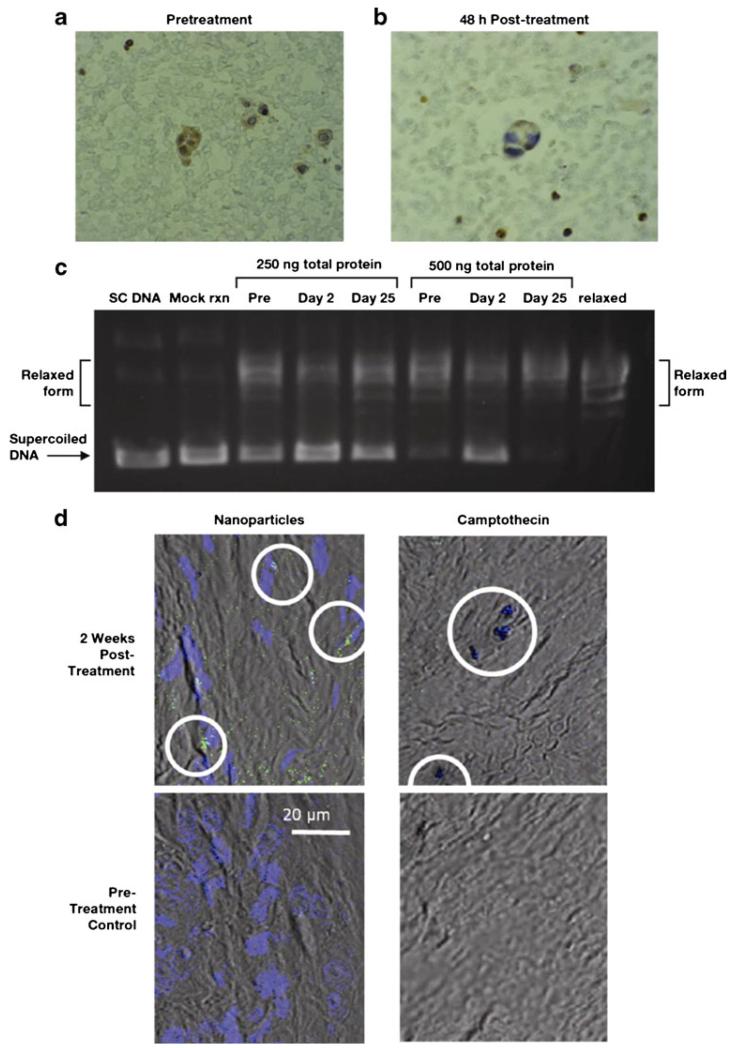
Ascites cells were collected from one patient with ovarian cancer receiving weekly 6 mg/m2 CRLX101 before treatment and on Days 2 and 25 after treatment. Additionally, polymer-conjugated CPT and unconjugated CPT were assessed in ascites fluid pretreatment and on Day 2. Concentrations detected at Day 2 were 47 μg/L for polymer-conjugated CPT and 20 μg/L for unconjugated CPT. Immunohistochemical analyses revealed a 30 % decrease in Topo 1 staining in the nucleus of ovarian cancer cells isolated from ascites fluid 2 days following treatment compared with pretreatment levels (Fig. 6 a,b). Topo 1 unwinding activity in tumor cells was lower on Day 2 following treatment compared with activity prior to treatment or on Day 25 following treatment (Fig. 6c).
Fig. 6.
Immunohistochemical analysis of tumor topoisomerase-1 expression levels a before treatment and b on day 2 after infusion with CRLX101 in a patient with ovarian cancer. c Tumor cell topoisomerase-1 activity in whole-cell lysates before treatment and on day 2 and day 25 of treatment with CRLX101 in a patient with ovarian cancer. d Confocal fluorescence microscopy of tumor-localized CRLX101 nanoparticles modified with Au-PEG-AD (green) and DAPI-stained nuclei (blue; left panels, 507-nm emission) and camptothecin (right panels, 440-nm emission) in a patient with triple-negative breast cancer after 2 weeks (top panels) or before (bottom panels) CRLX101 treatment. Abbreviations: Au-PEG-AD, PEG-adamantane-modified gold; DAPI, 4′,6-diamidino-2-phenylindole; PEG, polyethyleneglycol
Correlative studies of CRLX101 and CPT in tumor tissue biopsies
Tumor biopsies from a patient with triple-negative breast cancer receiving biweekly 15 mg/m2 CRLX101 were obtained prior to treatment and on Cycle 1 Day 14. CRLX101 nanoparticles, detected via fluorescence of Au-PEG-AD-modified nanoparticle staining and unconjugated CPT detected via its natural fluorescence were observed in post-treatment, but not pretreatment tumor tissue (Fig. 6d).
Discussion
Early clinical trials with Camptothecin (CPT) demonstrated a high incidence of hemorrhagic cystitis and severe bone-marrow suppression [20, 21]. Severe bladder toxicity was attributed to the high urinary excretion of the inactive carboxylate form of CPT and its subsequent conversion to the active lactone form under acidic conditions found in urine. These factors led to discontinuation of the drug’s development despite signals of strong antitumor activity. Subsequently, derivatives of CPT were synthesized to address these safety issues and two less potent small-molecule analogues (topotecan and irinotecan) are currently approved for use across multiple indications. While these compounds achieve notable improvements over CPT, these are still not well tolerated and there exists continued need for the improved differential tumor delivery and decreased systemic exposure and associated toxicity of Topo 1 inhibitors [3, 22, 23].
CRLX101 consists of 30 to 40-nm diameter nanoparticles with a near-neutral surface charge and high solubility. These properties enable this nanopharmaceutical to achieve extended circulation times that minimize single-pass renal clearance and allow preferential accumulation in tumor tissues presumably due to increased permeability of tumor neo-vasculature. CRLX101 achieves prolonged intratumoral release of active CPT from nanoparticles while residing intracellularly [1, 2, 12]. Across multiple preclinical animal models, CRLX101 demonstrates strong activity compared to small-molecule analogues of CPT and has been combined successfully with multiple agents [1, 2, 9-14]. Furthermore, the ability of the carrier molecule to deliver CPT directly into tumor cells greatly decreases CPT systemic exposure and associated toxicity.
This first-in-human combined phase 1/2a study assessed the tolerability, safety profile, and PK of CRLX101 in patients with advanced solid tumor malignancies. Of note here is the heavily pre-treated nature of this patient population with an average of 3.5 prior regimens of therapy. Following dose-escalation in phase 1, an MTD/RP2D and dosing schedule were identified and a phase 2a dose expansion study in 38 patients was completed. In the combined phase 1/2a study presented here, 62 patients were treated, including 44 patients treated at the MTD of 15 mg/m2 bi-weekly dosing, with the finding that bi-weekly dosing appears to be well-tolerated and the cyclodextrin backbone does not appear to contribute to toxicity. Six patients received long-term treatment (>6 months), with one patient on study up to 22 months with no evidence of incremental toxicity, further demonstrating the favorable long-term safety profile of this drug with mitigation of well-characterized CPT associated toxicities such as cystitis. PK studies presented here reveal exposure that is generally proportional to dose and prolonged plasma exposure that is heavily weighted to nanoparticle-conjugated CPT.
With regard to this study’s secondary objective of assessing preliminary signals of efficacy, we are encouraged by the 6 patients in this study who received >6 months of treatment with CRLX101 as well as by the subset of 22 patients with NSCLC among whom median PFS of 4.4 months (4.8 months among patients with non-Squamous histology only) was observed. We take note here of a recent ASCO reference [24] to a 0 % response rate in 4th line NSCLC and beyond.
As described in the background section of this manuscript, CRLX101 exploits the pores and fenestrations of tumor neo-vasculature in order to selectively deliver its cytotoxic payload to cancer cells. For this reason, it is anticipated that the drug’s primary activity occurs along the vascular periphery of tumors. Drug effect in this manner is hypothesized to manifest itself most readily in the type of long term disease stabilization investigators observed in this phase 2a study. For these reasons, follow-up clinical trials evaluating CRLX101 have been designed around overall survival and progression free survival endpoints.
We further take note of mounting evidence suggesting that the pharmacology of Topo 1 inhibitors is both concentration and schedule-dependent. Specifically, preclinical and clinical data demonstrate improved activity of these agents when lower doses are administered more frequently [6, 23, 25]. These data suggest that prolonged exposure of cancer cells to CPT is required in order to allow cellular replication and transcription machinery sufficient time to convert reversible complexes into permanent DNA damage [26]. Furthermore, topo-1-mediated inhibition of HIF-1α, which is associated with both drug resistance and more aggressive cancer phenotypes, appears to be triggered only with prolonged, metronomic dosing schedules of Topo 1 inhibitors [6, 27]. For these reasons, the sustained intratumoral release of CPT from CRLX101 suggested by PK data presented here is particularly encouraging [2, 12]. A study evaluating the combination of CRLX101 and the VEGF-A monoclonal antibody, bevacizumab, (Avastin®, Genentech/Roche, San Francisco, CA) in renal cell carcinoma is currently enrolling patients and it is hypothesized that this drug combination may further harness the potential of CRLX101-mediated modulation of HIF-1α expression.
In summary, phase 1/2a data presented here demonstrate encouraging safety, pharmacokinetics, and efficacy results in patients treated with CRLX101, setting the stage for multi-national clinical development of CRLX101 across several tumor types that is now underway. Specifically, promising anti-tumor activity in NSCLC patients led to a randomized phase 2 trial evaluating CRLX101 in 2nd/3rd line treatment for patients with advanced, treatment-refractory NSCLC (www.clinicaltrials.gov identifier NCT01380769). Additional phase 1b and phase 2 clinical trials of CRLX101 as a single-agent and in combination are currently underway in other cancers including small-cell lung cancer, gastric cancer, renal cell carcinoma (in combination with bevacizumab), and ovarian cancer.
Translational relevance.
Selective delivery of cytotoxic chemotherapy with minimization of toxicity remains an overarching goal of cancer therapeutics. Camptothecin (CPT) exerts potent anti-cancer activity through the inhibition of topoisomerase I (Topo 1) and hypoxia-inducible factor 1, alpha subunit (HIF-1α) [1]. Hemorrhagic cystitis and severe bone-marrow suppression has limited the use of CPT to its chemical derivatives irinotecan and topotecan. We report here a first in-human phase 1/2a trial of CRLX101, formerly IT-101, (Cerulean Pharma, Inc., Cambridge, MA). Preclinical studies demonstrate that nanoparticles exhibit less rapid renal clearance than CPT and prolonged plasma half-life compared with CPT derivatives [2]. We conducted exploratory pharmacodynamic analyses in ovarian cancer cells isolated from a patient with malignant ascites which demonstrated a decrease in Topo 1 activity following treatment. On-treatment tumor biopsies were obtained for confocal immunofluorescence microscopy and these exhibited preferential nanoparticle and CPT uptake similar to xenograft models.
Acknowledgments
The authors would like to thank all participating patients and their families, as well as the investigators, study coordinators, and operations staff. The authors also want to thank Dr. Marcia M. Miller and Dr. Mariana Tihova, Electron Microscopy Core; Dr. Shu Mi, Clinical Immunobiology Correlative Studies Laboratory (CICSL); and Yafan Wang, Translational Research Laboratory, City of Hope Comprehensive Cancer Center.
Acknowledgement of Research Support for the Study Financial support for this study was provided by Cerulean Pharma, Inc., and by a grant from the National Cancer Institute (CA U54119347). Financial support for medical editorial assistance was provided by Cerulean Pharma, Inc.
Footnotes
Previous Presentation Presented in part at the 22nd EORTC-NCI-AACR Symposium; November 16-19, 2010; Berlin, Germany. Presented in part at the AACR 102nd Annual Meeting; April 2-6, 2011; Orlando, Florida.
Final Phase1/2a results presented at AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; November 12-16, 2011; San Francisco, CA.
Author Disclosures DLK, EG, JH, JLR, and JJP are/were employees at Cerulean Pharma, Inc.
MED has stock in and is a paid consultant to Cerulean Pharma, Inc.
TS is a paid consultant for Cerulean Pharma, Inc. and employee at Calando Pharmaceuticals.
Contributor Information
Glen J. Weiss, Virginia G. Piper Cancer Center Clinical Trials at Scottsdale Healthcare/TGen, Scottsdale, AZ, USA
Joseph Chao, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Jeffrey D. Neidhart, San Juan Oncology Associates, Farmington, NM, USA
Ramesh K. Ramanathan, Virginia G. Piper Cancer Center Clinical Trials at Scottsdale Healthcare/TGen, Scottsdale, AZ, USA
Dawn Bassett, Virginia G. Piper Cancer Center Clinical Trials at Scottsdale Healthcare/TGen, Scottsdale, AZ, USA.
James A. Neidhart, San Juan Oncology Associates, Farmington, NM, USA
Chung Hang J. Choi, California Institute of Technology, Pasadena, CA, USA
Warren Chow, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Vincent Chung, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Stephen J. Forman, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
Edward Garmey, Cerulean Pharma Inc., Cambridge, MA, USA.
Jungyeon Hwang, Cerulean Pharma Inc., Cambridge, MA, USA.
D. Lynn Kalinoski, Cerulean Pharma Inc., Cambridge, MA, USA; Calando Pharmaceuticals, Pasadena, CA, USA.
Marianna Koczywas, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Jeffrey Longmate, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Roger J. Melton, Seventh Wave, Chesterfield, MO, USA
Robert Morgan, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Jamie Oliver, Peptagen, Inc., Raleigh, NC, USA.
Joanna J. Peterkin, Cerulean Pharma Inc., Cambridge, MA, USA
John L. Ryan, Cerulean Pharma Inc., Cambridge, MA, USA
Thomas Schluep, Calando Pharmaceuticals, Pasadena, CA, USA.
Timothy W. Synold, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
Przemyslaw Twardowski, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Mark E. Davis, California Institute of Technology, Pasadena, CA, USA
Yun Yen, City of Hope Comprehensive Cancer Center, Duarte, CA, USA; Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, CA 91010, USA.
References
- 1.Gaur S, Chen L, Yen T, et al. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin for the treatment of gastric cancer. Nanomedicine. 2012;8:721–730. doi: 10.1016/j.nano.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Schluep T, Cheng J, Khin KT, et al. Pharmacokinetics and biodistribution of the camptothecin-polymer conjugate IT-101 in rats and tumor-bearing mice. Cancer Chemother Pharmacol. 2006;57:654–662. doi: 10.1007/s00280-005-0091-7. [DOI] [PubMed] [Google Scholar]
- 3.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 4.Han Z, Wei W, Dunaway S, et al. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem. 2002;277:17154–17160. doi: 10.1074/jbc.M112401200. [DOI] [PubMed] [Google Scholar]
- 5.Magrini R, Bhonde MR, Hanski ML, et al. Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLH1 status. Int J Cancer. 2002;101:23–31. doi: 10.1002/ijc.10565. [DOI] [PubMed] [Google Scholar]
- 6.Kummar S, Raffeld M, Juwara L, et al. Multihistology, target-driven pilot trial of oral topotecan as an inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha) in advanced solid tumors. Clin Cancer Res. 2011;17:5123–5131. doi: 10.1158/1078-0432.CCR-11-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou JJ, Chua YL, Chew EH, et al. Inhibition of hypoxia-inducible factor-1 alpha (HIF-1alpha) protein synthesis by DNA damage inducing agents. PLoS One. 2010;5:e10522. doi: 10.1371/journal.pone.0010522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Khin KT, Davis ME. Antitumor activity of beta-cyclodextrin polymer-camptothecin conjugates. Mol Pharm. 2004;1:183–193. doi: 10.1021/mp049966y. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Khin KT, Jensen GS, et al. Synthesis of linear, beta-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjug Chem. 2003;14:1007–1017. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME. Design and development of IT-101, a cyclodextrin-containing polymer conjugate of camptothecin. Adv Drug Deliv Rev. 2009;61:1189–1192. doi: 10.1016/j.addr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Jensen G, Hwang J, Schluep T. Antitumor activity of IT-101, a cyclodextrin-containing polymer-camptothecin nanoparticle, in combination with various anticancer agents in human ovarian cancer xenografts. AACR Meeting Abstracts. 2008:767. [Google Scholar]
- 12.Numbenjapon T, Wang J, Colcher D, et al. Preclinical results of camptothecin-polymer conjugate (IT-101) in multiple human lymphoma xenograft models. Clin Cancer Res. 2009;15:4365–4373. doi: 10.1158/1078-0432.CCR-08-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluep T, Hwang J, Cheng J, et al. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin Cancer Res. 2006;12:1606–1614. doi: 10.1158/1078-0432.CCR-05-1566. [DOI] [PubMed] [Google Scholar]
- 14.Schluep T, Hwang J, Hildebrandt IJ, et al. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc Natl Acad Sci U S A. 2009;106:11394–11399. doi: 10.1073/pnas.0905487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svenson S, Wolfgang M, Hwang J, et al. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release. 2011;153:49–55. doi: 10.1016/j.jconrel.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 17.Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Minagawa Y, Kigawa J, Irie T, et al. Enhanced topoisomerase I activity and increased topoisomerase II alpha content in cisplatin-resistant cancer cell lines. Jpn J Cancer Res. 1997;88:1218–1223. doi: 10.1111/j.1349-7006.1997.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creaven PJ, Allen LM, Muggia FM. Plasma camptothecin (NSC-100880) levels during a 5-day course of treatment: relation to dose and toxicity. Cancer Chemother Rep. 1972;56:573–578. [PubMed] [Google Scholar]
- 21.Muggia FM, Creaven PJ, Hansen HH, et al. Phase I clinical trial of weekly and daily treatment with camptothecin (NSC-100880): correlation with preclinical studies. Cancer Chemother Rep. 1972;56:515–521. [PubMed] [Google Scholar]
- 22.Ikegami T, Ha L, Arimori K, et al. Intestinal alkalization as a possible preventive mechanism in irinotecan (CPT-11)-induced diarrhea. Cancer Res. 2002;62:179–187. [PubMed] [Google Scholar]
- 23.Morris R, Munkarah A. Alternate dosing schedules for topotecan in the treatment of recurrent ovarian cancer. Oncologist. 2002;7(Suppl 5):29–35. doi: 10.1634/theoncologist.7-suppl_5-29. [DOI] [PubMed] [Google Scholar]
- 24.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 25.Hochster H, Wadler S, Runowicz C, et al. Activity and pharmacodynamics of 21-Day topotecan infusion in patients with ovarian cancer previously treated with platinum-based chemotherapy. New York Gynecologic Oncology Group. J Clin Oncol. 1999;17:2553–2561. doi: 10.1200/JCO.1999.17.8.2553. [DOI] [PubMed] [Google Scholar]
- 26.Pommier Y. Camptothecins and topoisomerase I: a foot in the door. Targeting the genome beyond topoisomerase I with camptothecins and novel anticancer drugs: importance of DNA replication, repair and cell cycle checkpoints. Curr Med Chem Anticancer Agents. 2004;4:429–434. doi: 10.2174/1568011043352777. [DOI] [PubMed] [Google Scholar]
- 27.Choi YJ, Rho JK, Lee SJ, et al. HIF-1alpha modulation by topoisomerase inhibitors in non-small cell lung cancer cell lines. J Cancer Res Clin Oncol. 2009;135:1047–1053. doi: 10.1007/s00432-009-0543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]