Abstract
Background
Increased brain tumour incidence over recent decades may reflect improved diagnostic methods and clinical practice, but remain unexplained. Although estimated doses are low a relationship between radon and brain tumours may exist.
Objective
To investigate the long-term effect of exposure to residential radon on the risk of primary brain tumour in a prospective Danish cohort.
Methods
During 1993–1997 we recruited 57,053 persons. We followed each cohort member for cancer occurrence from enrolment until 31 December 2009, identifying 121 primary brain tumour cases. We traced residential addresses from 1 January 1971 until 31 December 2009 and calculated radon concentrations at each address using information from central databases regarding geology and house construction. Cox proportional hazards models were used to estimate incidence rate-ratios (IRR) and 95% confidence intervals (CI) for the risk of primary brain tumours associated with residential radon exposure with adjustment for age, sex, occupation, fruit and vegetable consumption and traffic-related air pollution. Effect modification by air pollution was assessed.
Results
Median estimated radon was 40.5 Bq/m3. The adjusted IRR for primary brain tumour associated with each 100 Bq/m3 increment in average residential radon levels was 1.96 (95% CI: 1.07; 3.58) and this was exposure-dependently higher over the four radon exposure quartiles. This association was not modified by air pollution.
Conclusions
We found significant associations and exposure-response patterns between long-term residential radon exposure radon in a general population and risk of primary brain tumours, adding new knowledge to this field. This finding could be chance and needs to be challenged in future studies.
Introduction
Brain tumours are rare; however incidence rates in Nordic countries have increased during the past few decades in both men and women [1], [2]. The increased incidence rates may partially be explained by improved diagnostic methods and clinical practice [3], but remain largely unknown. Epidemiological studies have investigated many potential risk factors for brain tumour over the past several decades, but the only established cause is ionizing radiation given in therapeutic [4]–[7] and diagnostic doses [8] and data from atomic bomb survivors support this [9], [10]. Residential radon is responsible for the majority of exposure to ionizing radiation in the general population, and although doses are several orders of magnitude lower than doses from therapeutic treatments, the same mechanisms of damage to the brain are expected.
Exposure to radon and alpha emitters polonium-218 and polonium-214 [11] has been classified as a human carcinogen [12]. Radon-222 gas arises from the radioactive decay of radium-226, present throughout the earth’s crust and in many building materials. Radon-222 has a 3.8-day half-life, and builds up indoors where most exposure to the general population occurs. The airways and lungs are the primary target organs, but dose calculations predict that inhaled radon gas and radon progeny can pass the blood-brain-barrier [13] and although estimated brain doses are low, a relationship between residential radon and brain tumours may exist. Yet, little attention has been given to this possibility; three studies of miners exposed to elevated occupational levels of radon have investigated mortality, but report conflicting results, with one showing increased brain cancer mortality related to radon exposure [14] and the others showing reduced mortality [15], [16], all statistically insignificant. A more recent study of uranium miners including 14 deaths due to brain tumours found an excess risk associated with radon exposure although with no dose-response relationship and expressing caution of possible diagnostic misclassification [17]. No study to date has investigated the association between incidence of brain tumour and exposure to residential radon in the general population.
A recent cohort study suggested that the risk of brain tumour could be associated with air pollution at the residential address [18]. Traffic related particulate matter (PM) in ambient air penetrates homes and contributes significantly to indoor PM [19]. Presence of indoor PM may modify the association between residential radon and brain tumours. Unattached radon progeny with an aerodynamic diameter around 1 nm have a high extrathoracic deposition, including the nasal cavity [13]; whilst radon progeny easily attach to PM in the air [20], [21]. The attachment of radon progeny to aerosols in the air reduces the fraction of the so-called unattached radon decay products and increases the airborne concentration of attached radon decay products due to a significant reduction in the plate out on indoor surfaces [22]. Attachment furthermore significantly influences the deposition pattern in the lungs due to the altered size distribution of the radon decay products [23] and experimental evidence supports the theory that ultrafine PM can reach the brain both via the systemic circulation through the blood-brain barrier and via the olfactory neuronal pathway [24]–[26]. Also, radon uptake from airways might be enhanced by exposure to traffic airway irritants including nitrogen dioxide (NO2) which is also a marker of PM from traffic. We have previously reported a non-significant pattern of stronger associations between radon and leukaemia among children living at streets with high traffic density [27]; indicating that residential radon and indoor PM might operate together and influence risk.
The “Diet, Cancer and Health” cohort is a large prospective study with detailed information on potential confounders collected at baseline with little potential for recall bias and the radon regression model has been successfully validated [28] and applied in three previous epidemiological studies [27], [29], [30]. Our purpose was to investigate the association between predicted levels of residential radon at the 168,624 residencies of the cohort members, over a period of 39 years and the risk for primary brain tumour in Denmark and to examine the potential modifying effects of air pollution.
Methods
Design
Between December 1993 and May 1997, 57,053 persons aged 50 to 64 years were enrolled in the prospective study “Diet, Cancer and Health”. The participants had to be born in Denmark, live in Copenhagen or Aarhus, and cancer free at the time of inclusion [31]. The baseline examination included a self-administered questionnaire on diet including fruit and vegetable consumption, occupational history, including occupation in the chemical industry as well as other items related to health, lifestyle and socio-economic status.
Since establishment of the Danish Civil Registration System (CRS) [32] in 1968, all citizens of Denmark have been given a unique personal identification number, which allows accurate linkage between registers. The CRS is continuously updated regarding many person variables including vital status, place of residence and information on emigration. We traced the date of death, emigration or disappearance of cohort members in the CRS by use of the personal identification number. We retrieved the unique past and present addresses of each participant from 1 January 1971 until 30 December 2009 from the CRS, thus including 39 years of address history dating back to when these cohort members were in their 20 to 40′s. Addresses were identified according to municipality, town, postal code, street, building number, and floor.
We followed each cohort member for occurrence of any cancer from enrolment until 30 December 2009 in the Danish Cancer Registry, which provides accurate and virtually complete nationwide ascertainment of cancers since 1943, including benign tumours [33], by use of the unique personal identification number. Cancers were classified according to ICD-10 (international classification of diseases, 10th revision).
The Scientific Ethics Committee for Copenhagen and Frederiksberg and The Danish Data Protection Agency approved the study, and written informed consent was obtained from all participants prior to enrolment.
Exposure assessment
Residential radon concentrations at each address of all participants were predicted with a validated regression model [28]. The model uses nine explanatory variables, including geographic location, geology (soil types) and dwelling characteristics including type of house, floor level, total number of floors, fraction of inhabitable space in top floor, basement and building materials. All explanatory variables are available from central Danish databases.
Geographical coordinates were identified by the Danish Geodata Agency by linking the identified unique addresses for all cohort members to the cadastral register, which is a database of all official addresses and their geoordinates in Denmark. The overall goal of the Danish Geodata Agency is to supply and insure reliable and accurate maps and geographical coordinates on all parts of the Realm. Geographical coordinates were obtained for 94% of all the addresses the cohort members had lived in.
The Geological Survey of Denmark and Greenland identified the local soil from digital soil maps using geographical coordinates for each address. House construction data were obtained from the Building and Housing Register [34]. Model predictions were corrected for seasonal variation. The model predicts low level residential radon with great certainty and detects differences in groups well and a comparison with independent radon test data shows that the model makes sound predictions (R2 = 0.5) and that errors of radon predictions are only weakly correlated with the estimates themselves [28].
Two radon exposures were calculated for each cohort member from 1 January 1971 onwards. The first was a time-weighted average exposure and the second was a cumulated radon exposure. Both were calculated with and without a 10-year latency period that is relevant for brain tumours. These concentrations were entered into their respective statistical cancer risk models as time-dependent variables; thus recalculating exposure for non-censored persons at the time of each censor.
Information on traffic has previously been collected for the entire study population and traffic-related air pollution has been significantly linked to brain tumours [18]. We estimated the concentration of nitrogen oxides (NOx) which correlates strongly with concentrations of ultrafine particles in Danish streets through a wide range of particle sizes (R2>0.83) [35] and also includes the airway irritant NO2. The average concentrations of NOx at the front door of each dwelling during the period that the participants occupied the address were estimated by use of the Danish air pollution dispersion modeling system, with high temporal and spatial resolution (R2>0.75) [36] and including the state-of-the-art Operational Street Pollution Model, currently used in over 17 countries worldwide [37]. We calculated the time-weighted average NOx concentrations at each cohort member’s residential addresses from 1 January 1971 onwards.
Statistical methods
The end-point for the risk analyses was primary brain tumours, including benign tumours (ICD-10 C71, D330–D332 and D430–D432). Incidence rate-ratios (IRRs) were estimated by a Cox proportional hazards model with age as the underlying time scale ensuring risk estimates were based on individuals at exactly the same age [38]. We calculated two-sided 95% confidence intervals (CIs) on the basis of the Wald test statistic for regression parameters in Cox regression models with the PHREG procedure in SAS (version 9.2; SAS Institute, Cary, NC). Analyses were corrected for delayed entry at the time of enrolment, so that persons were considered under risk from time of enrolment into the cohort. People diagnosed with cancer before enrolment into cohort (except non-melanoma skin cancer) were excluded from the analyses. Censoring occurred at the time of death, emigration or disappearance, cancer diagnosis, or 30 December 2009 (end of follow-up), whichever came first.
Data were analyzed with and without adjustment for a-priori determined variables. The crude model was adjusted for age (underlying time scale) and sex. The second model was further adjusted for individual variables with confounding potential, based on previous literature including: consumption of fruit and vegetables (linear, g/day) [39]–[43], a dichotomous variable indicating employment for at least one year in the chemical industry [12], [44], [45] and NOx at residencies since 1971 (linear, µg/m3) [18]. Consumption of fruit and vegetables [39]–[43] and occupation in the chemical industry [12], [44], [45] have been linked to brain tumour; whilst smoking, alcohol and body mass index have consistently been reported to have no effect, despite their association with many other cancer types [4], [7]. The third explorative model was further adjusted for socio-economic variables known to be risk factors for many other cancers, including length of school attendance (<8, 8–10 and >10 years), marital status (single, married/de facto relationship, divorced and widowed) and occupational status (employed versus unemployed). Cohort members that had a missing value for any covariate were excluded, thus ensuring the same number of persons in crude and adjusted analyses.
The assumption of linearity for the continuous variables (residential radon, fruit and vegetable consumption and NOx in relation to brain tumour was evaluated graphically using linear splines with boundaries placed at the nine deciles among all participants as well as by a numerical likelihood ratio test statistic to compare the model assuming linearity with the linear spline model. None of these co-variates deviated significantly from linearity.
We formed four intervals for exposure to residential radon using the 25th, 50th and 75th percentiles for all participants as the cut-off points and estimated the IRRs for primary brain tumour for the higher exposure ranges compared with the lowest exposure range. IRRs were also estimated as linear trends in residential radon concentrations. The possible effect modification by traffic-related air pollution was evaluated by introducing interaction terms into the adjusted model and using the Wald’s test.
Exposure-response curves with 95% confidence limits were visualized using a restricted cubic spline in R (library Survival and Design, version 2.13.1), adjusting for age, sex, consumption of fruit and vegetables, employment for at least one year in the chemical industry and NOx at residencies since 1971 [46].
Results
Among the 57,053 cohort members, we excluded 571 due to a cancer diagnosis before enrolment, 2 because of uncertain date of cancer diagnosis, 960 for which address history was not available in the CRS or their baseline address could not be geocoded, 1,603 because of missing data in potential confounders, and 2,243 because radon or NOx exposure was assessed for less than 80% of the time from 1 January 1971 until diagnosis or censoring. The 51,674 included cohort members had lived in a total of 168,684 addresses and were followed up for cancer for an average of 12.6 years (total person years at risk was 652,028). We identified 121 primary brain tumour cases, corresponding to an overall incidence rate of 18.6 per 100,000 person-years.
Table 1 shows the characteristics of the cohort members and the primary brain tumour cases. Sex distribution and marital status were similar among cases and cohort members. The proportion of participants with employment and long school attendance was slightly lower among cases than among the cohort members and cases consumed slightly less fruit and vegetables. The median predicted residential radon concentration was slightly higher for cases (41.8 Bq/m3) than the whole cohort (40.5 Bq/m3) and median NOx concentrations were similar for cases and the cohort members and the 95 percentile values for both radon and NOx exposure was higher for cases. Table 1 also shows that those living at addresses with high radon tended to: be men, be employed, have longer school attendance, be married or live in de facto relationships and be exposed to lower NOx levels.
Table 1. Characteristics of all study participants, cases and those with low and high levels of radon at the residences.
| Characteristic | Cohort | Cases | Radone,c <67.6 Bq/m3 | Radone,c <67.6 Bq/m3 | ||||
| No. (%) | Median (5–95 percentile) | No. (%) | Median (5–95 percentile) | No. (%) | Median (5–95 percentile) | No. (%) | Median (5–95 percentile) | |
| All participants | 51 674 (100) | 121* (100) | 38 763 (75.0) | 12 911 (25.0) | ||||
| Age at enrolment | 56.1 (50.7–64.2) | 57.2 (50.9–64.2) | 56.1 (50.7–64.2) | 56.5 (50.9–64.2) | ||||
| Sex | ||||||||
| Male | 24 533 (47.5) | 56 (46.3) | 18 067 (46.6) | 6 466 (50.1) | ||||
| Female | 27 141 (52.5) | 65 (53.7) | 20 696 (53.4) | 6 445 (49.9) | ||||
| Occupational status | ||||||||
| Employed | 40 314 (78.0) | 89 (73.5) | 30 010 (77.4) | 10 306 (79.8) | ||||
| Unemployed | 11 360 (22.0) | 32 (26.5) | 8 753 (22.6) | 2 605 (20.2) | ||||
| School attendance (years) | ||||||||
| <8 | 16 924 (33.0) | 54 (44.6) | 13 130 (33.9) | 3 794 (29.4) | ||||
| ≥8 | 34 750 (67.0) | 67 (55.4) | 25 633 (66.1) | 9 117 (70.6) | ||||
| Marital status | ||||||||
| Single | 3 028 (5.9) | 10 (8.3) | 2 832 (7.3) | 196 (1.5) | ||||
| Married/living de facto | 37 225 (72.0) | 87 (71.9) | 26 180 (67.5) | 11 045 (85.6) | ||||
| Divorced | 8 581 (16.6) | 17 (14.1) | 7 510 (19.4) | 1 071 (8.3) | ||||
| Widowed | 2 840 (5.5) | 7 (5.8) | 2 241 (5.8) | 599 (4.6) | ||||
| Fruit and vegetable intake (g/day) | 312 (96–735) | 282 (75–617) | 308 (91–744) | 322 (115–713) | ||||
| Enployment in chemical industrya | ||||||||
| No | 51 446 (99.6) | 119 (98.4) | 38 587 (99.6) | 12 859 (99.6) | ||||
| Yes | 228 (0.4) | 2 (1.6) | 176 (0.4) | 52 (0.4) | ||||
| Radon at the addressb,c (Bq/m3) | 40.5 (9.1–91.0) | 41.8 (8.8–92.2) | ||||||
| NOx at front doord (µg/m3) | 21.6 (14.8–67.7) | 22.0 (15.2–92.9) | 23.1 (15.3–76.6) | 17.5 (14.4–31.8) | ||||
aEver employed in the chemical industry for at least 1 year.
Time-weighted average radon.
For the period 1 January 1971, to censoring date with inclusion of a 10-year latency period relevant for brain tumour.
Time-weighted average NOx, we focused on the concentration of NOx as an indicator for particular matter (PM) from traffic because NOx correlates strongly with ultrafine particles in Danish streets [35].
Cut-off based on the 75th percentile for time-weighted average radon concentrations.
Including 11 cases of benign tumours.
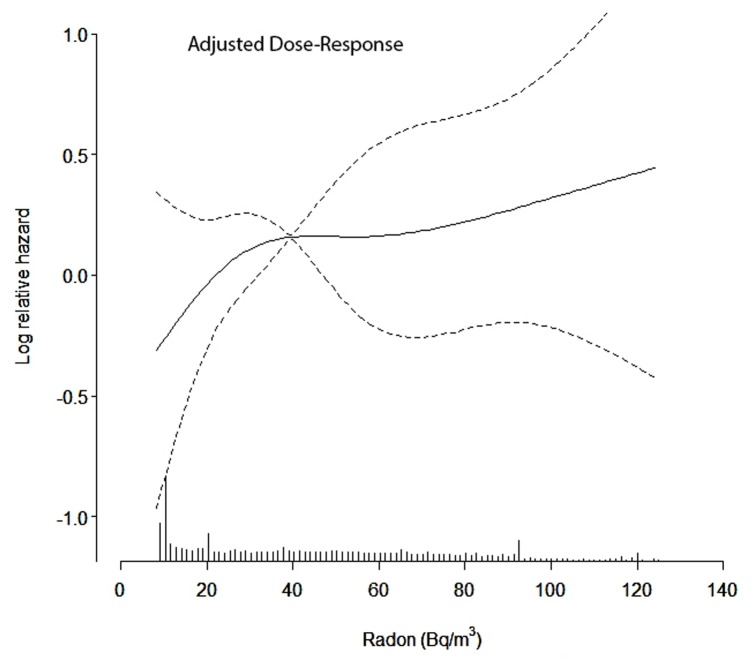
Overall the adjusted IRR associated with each 100 Bq/m3 increment increase in average radon levels was 1.96 (95% CI: 1.07; 3.58) and the adjusted IRR associated with a 103 Bq/m3-years increment increase in cumulated radon was 1.37 (95% CI: 1.03; 1.82). The IRRs for both average and cumulated radon exposure were exposure-dependently higher over the four radon exposure quartiles (Table 2). The unadjusted results showed lower IRR associated with radon levels but the IRRs were also dose-dependently higher over the four-radon quartiles (Table 2). Traffic related air pollution was the most important co-variate for the change in the estimated association between radon and brain tumour risk in model 2 and the crude model. When exploring the effects of co-variates related to socio-economic status in an extended model we found IRRs for the association increased further, primarily due to adjustment for length of schooling (Table 2). These risks estimated were only affected to a small extent by exclusion of the 10-year latency period for radon exposure (results not shown). Figure 1 shows the adjusted exposure-response between average residential radon concentrations and primary brain tumour risk shown in Table 2. There was no evidence that the association between radon and risk of primary brain tumour was modified by traffic-related air pollution, although the point estimate of the IRR was lower among subjects with high levels of NOx at their residence (p-value for interaction = 0.15) (Table 3).
Table 2. Incidence rate ratios (95% CI) for primary brain tumour risk associated with the residential radon concentrations.
| Radon | Cases, n | IRR (95% CI)b | ||
| Crudec | Model 2c,d | Model 3c,d.e | ||
| Time weighted average (Bq/m3)a | ||||
| <17.2 | 28 | 1.00 | 1.00 | 1.00 |
| 17.2–40.5 | 29 | 1.20 (0.69–2.08) | 1.44 (0.82–2.54) | 1.53 (0.87–2.71) |
| 40.5–67.6 | 31 | 1.27 (0.74–2.20) | 1.68 (0.94–3.01) | 1.86 (1.03–3.38) |
| >67.6 | 33 | 1.38 (0.82–2.36) | 1.90 (1.07–3.39) | 2.09 (1.16–3.79) |
| Linear trend per 100 Bq/m3 | 121 | 1.43 (0.81–2.55) | 1.96 (1.07–3.58) | 2.15 (1.16–4.01) |
| Cumulated exposure (Bq/m3-years)a | ||||
| <377 | 29 | 1.00 | 1.00 | 1.00 |
| 377–895 | 31 | 1.14 (0.69–1.97) | 1.40 (0.81–2.42) | 1.50 (0.86–2.60) |
| 895–1506 | 30 | 1.16 (0.67–1.94) | 1.49 (0.84–2.63) | 1.64 (0.92–2.94) |
| >1506 | 31 | 1.41 (0.83–2.39) | 1.92 (1.08–3.40) | 2.11 (1.17–3.81) |
| Linear trend per 103Bq/m3years | 121 | 1.19 (0.90–1.56) | 1.37 (1.03–1.82) | 1.44 (1.07–1.93) |
From 1 January 1971 until censoring, with inclusion of a 10 year latency period. The cut-off points between exposure groups were the 25th, 50th and 75th percentiles for all participants.
Analyses based on 51,674 cohort members and 121 brain tumours.
Adjusted for age by using it as the underlying time scale in the Cox model and sex.
Adjusted for consumption of fruit and vegetables, employment in the chemical industry for at least one year and traffic (time-weighted average NOx exposure between 1971 and the censoring date).
Adjusted for employment status, schooling and marital status. Due to exclusion of cohort members with missing value in any covariate, the number of persons is identical in the crude and the adjusted analyses.
Figure 1. The spline function is adjusted for age, sex, consumption of fruit and vegetables, employment in the chemical industry for at least one year and traffic-related air pollution.
The exposure distribution of average residential radon is marked on the x-axis. The spline function can be interpreted as the exposure-response association. The difference between two points on the y-axis on the curve is interpreted as the difference in loge(IRR) for the corresponding difference in exposure, which can be read on the x-axis between the same two points.
Table 3. Adjusteda incidence rate ratios for primary brain tumour in association with a 100 Bq/m3 increase in domestic radonb within strata of NOx at the residential address.
| Potential effect modifier | Cases, n | IRR (95% CI) | Pc |
| NOx at front door (µg/m3)d | |||
| <21.6 | 58 | 2.53 (1.06–6.04) | 0.15 |
| ≥21.6 | 63 | 0.98 (0.39–2.50) |
We adjusted the analyses for age (underlying time scale), sex, employment in the chemical industry for at least one year and consumption of fruit and vegetables.
Radon exposure was entered as a continuous variable in all models as the time-weighted average concentration at residences from 1. January 1971 until censoring.with inclusion of a 10 year latency period.
Test of the null hypothesis that the linear trends are identical, for Wald test for interaction.
Time-weighted average concentration for NOx.
Discussion
We found significant associations and exposure-response patterns between long-term exposure to residential radon in a general Danish population and primary brain tumour risk.
The strengths of this study include a prospective follow-up where information on potential confounding factors was collected at enrolment without potential for recall bias. Complete follow-up for cancer, vital status as well as address history from 1971 onwards was ensured by use of reliable population-based Danish registries. The use of a recently developed regression model facilitated estimation of residential radon in as many as 168,624 homes, over almost four decades. The model has been applied in three previous epidemiological studies [27], [29], [30] and successfully validated against independent radon measurements [28]. Model-based estimation of radon is inevitably associated with uncertainty [28] and it is clear that measurements in homes would provide a more accurate assessment of radon concentrations. But use of measurements in epidemiological studies may imply disadvantages such as a limited number of measurements due to economy constraints and exposure misclassification when reconstructing past residential radon exposures. The advantage of our model-based estimation of radon levels is the facilitation of a larger study with historical estimates of radon exposure since 1971, at reasonable costs. Limitations of our study include the limited number of cases, which prevented analyses of association for specific neuroepithelial/astrocytic tumours. Also, the possibilities of therapeutic exposures at large doses were not considered here. Finally, the exposure of cohort members before 1971 could not be estimated, as residential histories before that date were unknown. Therefore, we were unable to assess early-life radon exposure which is an important limitation as early life environmental exposures might be most significant for cancer risk.
In the present study we show an exposure-response association between residential radon and a risk of primary brain tumour that was almost doubled per each 100 Bq/m3 increment in average long-term residential radon exposure. This adds novel information to this field as no study to date has been conducted on the relationship between brain tumour risk and residential radon exposure of the general population.
Models predicting the dose possibly reaching the brain after alveolar uptake from radon exposure indicate that this is equivalent to less than 0.15 mSv per year from 200 Bq/m3 [13]. The risk we found associated with residential radon exposures for up to 39 years cannot be explained by a cumulative dose to the brain from transport through the blood and a simple dose-response extrapolation from high-level exposures. An alternative explanation might be that especially unattached radon progeny with aerodynamic diameter around 1 nm and a high extrathoracic deposition including the nasal cavity could reach the brain via the olfactory neuronal pathway [25], [47] resulting in local intense exposure. If this is the explanation for our finding one could, however, have expended a more clear association between the higher radon exposure of miners and risk for brain tumours than found in the four previous studies of miners, although the different particle size distribution in the mines compared to residences could make the olfactory neuronal pathway less relevant in the mines. However, this is hypothetical and our findings could be a result of chance and should be challenged by more studies.
We hypothesised that the presence of PM modifies the association between residential radon and risk of brain tumour and tested the hypothesis with respect to outdoor traffic-related air pollution at the residence which can penetrate indoors [19] as a marker of indoor PM, but our results did not support this hypothesis. In fact we found a stronger association between radon and brain tumour at low outdoor NOx concentrations, although the effect modification was not significant. Hypothetically, presence of traffic emission particles mainly in the size range from around 20 nm, could reduce the availability of unattached radon progeny, which would reduce upper airway deposition and could thus be of importance if the olfactorial neuronal pathway is of relevance for brain exposure.
Conclusion
We found significant association and exposure-response patterns between long-term exposure to residential radon in a general Danish population and risk of primary brain tumour. Our findings could be a result of chance and need to be challenged by future studies.
Funding Statement
This work was supported by a Research Grant from The Danish Medical Research Council (Grant number: 09 064754). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lonn S, Klaeboe L, Hall P, Mathiesen T, Auvinen A, et al. (2004) Incidence trends of adult primary intracerebral tumors in four Nordic countries. Int J Cancer 108: 450–455. [DOI] [PubMed] [Google Scholar]
- 2. Deltour I, Johansen C, Auvinen A, Feychting M, Klaeboe L, et al. (2009) Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. J Natl Cancer Inst 101: 1721–1724. [DOI] [PubMed] [Google Scholar]
- 3. Muir CS, Storm HH, Polednak A (1994) Brain and other nervous system tumours. Cancer Surv 19–20: 369–392. [PubMed] [Google Scholar]
- 4. McKinney PA (2004) Brain tumours: incidence, survival, and aetiology. J Neurol Neurosurg Psychiatry 75 Suppl 2ii12–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2: 494–503. [DOI] [PubMed] [Google Scholar]
- 6. Davis FS (2007) Epidemiology of brain tumors. Expert Rev Anticancer Ther 7: S3–S6. [DOI] [PubMed] [Google Scholar]
- 7. Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, et al. (2008) Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer 113: 1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, et al. (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, et al. (2002) Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst 94: 1555–1563. [DOI] [PubMed] [Google Scholar]
- 10. Braganza MZ, Kitahara CM, Berrington de GA, Inskip PD, Johnson KJ, et al. (2012) Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol 14: 1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darby S, Hill D, Doll R (2001) Radon: a likely carcinogen at all exposures. Ann Oncol 12: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 12.International Agency for Research on Cancer (IARC) (1988) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 43, Man-made Mineral Fibres and Radon. Lyon France: International Agency for Research on Cancer 1988.
- 13. Kendall GM, Smith TJ (2002) Doses to organs and tissues from radon and its decay products. J Radiol Prot 22: 389–406. [DOI] [PubMed] [Google Scholar]
- 14. Darby SC, Radford EP, Whitley E (1995) Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 103 Suppl 245–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomasek L, Darby SC, Swerdlow AJ, Placek V, Kunz E (1993) Radon exposure and cancers other than lung cancer among uranium miners in West Bohemia. Lancet 341: 919–923. [DOI] [PubMed] [Google Scholar]
- 16. Kreuzer M, Grosche B, Schnelzer M, Tschense A, Dufey F, et al. (2010) Radon and risk of death from cancer and cardiovascular diseases in the German uranium miners cohort study: follow-up 1946–2003. Radiat Environ Biophys 49: 177–185. [DOI] [PubMed] [Google Scholar]
- 17. Vacquier B, Rage E, Leuraud K, Caer-Lorho S, Houot J, et al. (2011) D (2011) The Influence of Multiple Types of Occupational Exposure to Radon, Gamma Rays and Long-Lived Radionuclides on Mortality Risk in the French “post-55” Sub-cohort of Uranium Miners: 1956–1999. Radiat Res 176: 796–806. [DOI] [PubMed] [Google Scholar]
- 18. Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, et al. (2011) Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneider T, Jensen KA, Clausen PA, Afshari A, Gunnarsen L, et al. (2004) Prediction of indoor concentration of 0.5–4mm particles of outdoor origin in an uninhabited apartment. Atmospheric Environment 38: 6349–6359. [Google Scholar]
- 20. Tokonami S (2000) Experimental verification of the attachment theory of radon progeny onto ambient aerosols. Health Phys 78: 74–79. [DOI] [PubMed] [Google Scholar]
- 21. Yu KN, Wong BTY, Law JYP, Lau BMF, Nikezic D (2001) Indoor Dose Conversion Coefficients for Radon Progeny for Different Ambient Environments. Environmental Science & Technology 35: 2136–2140. [DOI] [PubMed] [Google Scholar]
- 22. Abu-Jarad F (1997) Indoor cigarette smoking: Uranium contents and carrier of indoor radon products. Radiation Measurements 28: 579–584. [Google Scholar]
- 23. Bair WJ (1995) The ICRP Human Respiratory Tract Model for Radiological Protection. Radiat Prot Dosimetry 60: 307–310. [Google Scholar]
- 24. Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, et al. (2002) Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65: 1531–1543. [DOI] [PubMed] [Google Scholar]
- 25. Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, et al. (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16: 437–445. [DOI] [PubMed] [Google Scholar]
- 26. Lucchini RG, Dorman DC, Elder A, Veronesi B (2012) Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 33: 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bräuner EV, Andersen CE, Andersen HP, Gravesen P, Lind M, et al. (2010) Is there any interaction between domestic radon exposure and air pollution from traffic in relation to childhood leukemia risk? Cancer Causes Control 21: 1961–1964. [DOI] [PubMed] [Google Scholar]
- 28. Andersen CE, Raaschou-Nielsen O, Andersen HP, Lind M, Gravesen P, et al. (2007) Prediction of 222Rn in Danish dwellings using geology and house construction information from central databases. Radiat Prot Dosimetry 123: 83–94. [DOI] [PubMed] [Google Scholar]
- 29. Raaschou-Nielsen O, Andersen CE, Andersen HP, Gravesen P, Lind M, et al. (2008) Domestic Radon and Childhood Cancer in Denmark. Epidemiology 19: 536–543. [DOI] [PubMed] [Google Scholar]
- 30. Bräuner EV, Andersen CE, Sørensen M, Andersen ZJ, Gravesen P, et al. (2012) Residential radon and lung cancer incidence in a Danish cohort. Environ Res 118: 130–136. [DOI] [PubMed] [Google Scholar]
- 31. Tjønneland A, Olsen A, Boll K, Stripp C, Christensen J, et al. (2007) Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health 35: 432–441. [DOI] [PubMed] [Google Scholar]
- 32. Pedersen CB (2011) The Danish Civil Registration System. Scand J Public Health 39: 22–25. [DOI] [PubMed] [Google Scholar]
- 33. Gjerstorff ML (2011) The Danish Cancer Registry. Scand J Public Health 39: 42–45. [DOI] [PubMed] [Google Scholar]
- 34. Christensen G (2011) TheBuilding and Housing Register. Scand J Public Health 39: 106–108. [DOI] [PubMed] [Google Scholar]
- 35. Ketzel M, Wahlin P, Berkowicz R, Palmgren F (2003) Particle and trace gas emission factors under urban driving conditions in Copenhagen based on street and roof-level observations. Atmospheric Environment 37: 2735–2749. [Google Scholar]
- 36. Jensen SS, Berkowicz R, Hansen HS, Hertel O (2001) A Danish decision-support GIS tool for management of urban air quality and human exposures. Transportation Research Part D-Transport and Environment 6: 229–241. [Google Scholar]
- 37. Kakosimos KE, Hertel O, Ketzel M, Berkowicz R (2010) Operational Street Pollution Model (OSPM) A review of performed application and validation studies, and future prospects. Environ Chem 7: 485–503. [Google Scholar]
- 38. Thiebaut AC, Benichou J (2004) Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med 23: 3803–3820. [DOI] [PubMed] [Google Scholar]
- 39. Hu J, La VC, Negri E, Chatenoud L, Bosetti C, et al. (1999) Diet and brain cancer in adults: a case-control study in northeast China. Int J Cancer 81: 20–23. [DOI] [PubMed] [Google Scholar]
- 40. Preston-Martin S, Mack W, Henderson BE (1989) Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res 49: 6137–6143. [PubMed] [Google Scholar]
- 41. Burch JD, Craib KJ, Choi BC, Miller AB, Risch HA, et al. (1987) An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst 78: 601–609. [PubMed] [Google Scholar]
- 42. Giles GG, McNeil JJ, Donnan G, Webley C, Staples MP, et al. (1994) Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer 59: 357–362. [DOI] [PubMed] [Google Scholar]
- 43. Blowers L, Preston-Martin S, Mack WJ (1997) Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA). Cancer Causes Control 8: 5–12. [DOI] [PubMed] [Google Scholar]
- 44. Brownson RC, Reif JS, Chang JC, Davis JR (1990) An analysis of occupational risks for brain cancer. Am J Public Health 80: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lynge E, Thygesen L (1990) Occupational cancer in Denmark. Cancer incidence in the 1970 census population. Scand J Work Environ Health 16 Suppl 23–35. [PubMed] [Google Scholar]
- 46.Harrel FE (2001) Regression modeling strategies. Springer, New york.
- 47. Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, et al. (2006) Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect 114: 1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]